Abstract
Higher plants possess the ability to synthesize a great number of compounds with many different functions, known as secondary metabolites. Polyphenols, a class of flavonoids, are secondary metabolites that play a crucial role in plant adaptation to both biotic and abiotic environments, including UV radiation, high light intensity, low/high temperatures, and attacks from pathogens, among others. One of the compounds that has received great attention over the last few years is luteolin. The objective of the current paper is to review the extraction and detection methods of luteolin in plants of the Greek flora, as well as their luteolin content. Furthermore, plant species, crop management and environmental factors can affect luteolin content and/or its derivatives. Luteolin exhibits various biological activities, such as cytotoxic, anti-inflammatory, antioxidant and antibacterial ones. As a result, luteolin has been employed as a bioactive molecule in numerous applications within the food industry and the biomedical field. Among the different available options for managing periodontitis, dental care products containing herbal compounds have been in the spotlight owing to the beneficial pharmacological properties of the bioactive ingredients. In this context, luteolin’s anti-inflammatory activity has been harnessed to combat periodontal disease and promote the restoration of damaged bone tissue.
1. Introduction
Higher plants produce, through the photosynthesis process, all the necessary substances for their growth and also for all the other life forms of nature. These are called ‘primary metabolites’. In addition, plants have the ability to biosynthesize a large number of substances that have specific functions and are known as secondary metabolites. Polyphenols are one of the most common groups of secondary metabolites widely distributed in all plant species [1]. The content of polyphenols increases in response to various factors, such as ultraviolet (UV) radiation, high light intensity, low/high temperature, salinity, drought, etc. These conditions cause the creation of free oxygen and nitrogen radicals due to the stress they cause to the plants. One of the functions of polyphenols is the reduction of the effect caused by the presence of free radicals [2]. A class of polyphenols are flavonoids, which are the largest group of phenolic compounds, accounting for more than 5000 different compounds present in plant species [3]. In the last decade, flavonoids have been studied systematically due to experimental and clinical research as they are used as anti-cancer, antioxidant, anti-inflammatory, and antiviral compounds. They also act as cardioprotective, neuroprotective and chemoprotective agents [4].
Flavonoids are polyhydroxy–phenolic compounds produced through the phenylpropanoid biosynthetic pathway in plants [5]. They have 15 carbon atoms (phenolic compounds of the type C6–C3–C6) with the structure of two benzene rings joined by a heterocyclic oxygen-centered ring. Flavonoids are divided into seven major subclasses: flavan-3-ols, flavones, flavonols, flavanones, anthocyanins, chalcones and isoflavonoids (Figure 1). Flavonoids include, in particular, flavones, such as luteolin and tetramethoxyluteolin. Flavones are structurally characterized by a double bond and an oxygen atom in the heterocyclic ring C of the flavonoid skeleton. Flavones, such as apigenin and luteolin, can be found in plants showing a wide range of substitutions, including methylations, hydroxylations, acylations, and glycosylations leading mainly to O- or C-glycosides [6].
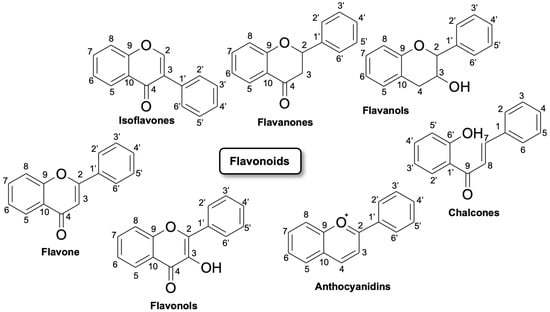
Figure 1.
Flavonoids classification and their possible chemical structure [7].
Luteolin (3′,4′,5,7-tetrahydroxyflavone) belongs to a group of naturally occurring compounds called flavonoids, which are naturally found in several plant species. Because of their abundance in foods, e.g., vegetables, fruits, and medicinal plants, flavonoids are common compounds that act as antioxidants, estrogenic regulators, and antimicrobial agents [8]. Chemically, luteolin has a C6–C3–C6 structure that contains two benzene rings and one oxygen-containing ring with a C2–C3 carbon double bond (Figure 2). Structure-activity relationship studies have shown that the presence of hydroxyl moieties at carbons 5, 7, 3′ and 4′ positions of the luteolin structure and the presence of the 2−3 double bond are responsible for its multiple pharmacological effects [9]. The hydroxyl moieties and the 2–3 double bonds are important structural features in luteolin that are associated with its biochemical and biological activities (anti-cancer, antioxidant, anti-inflammatory, neuroprotective, etc.) [10]. As in other flavonoids, luteolin is often glycosylated in plants, and the glycoside is hydrolyzed to free luteolin during absorption [11].
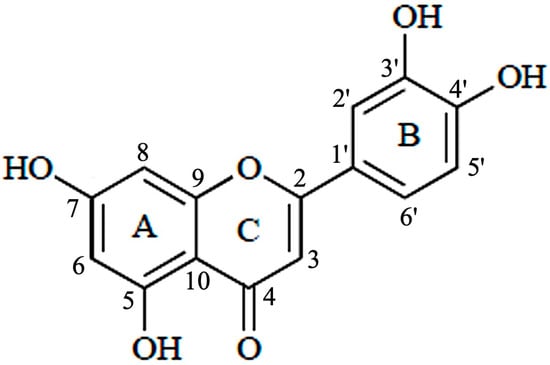
Figure 2.
Structure of luteolin.
In this review, we focus on the plant species of the Mediterranean area (especially the Greek flora where luteolin has been reported to be present), exploring whether cultivation techniques can affect its content. In addition, the methods for isolating luteolin along with its mechanism of action in the treatment of periodontitis are reviewed.
2. Determination of Luteolin Content in Plants of the Greek Flora
Areas that have favorable climate conditions for plant species [12], such as Greece, are ideal for the cultivation of aromatic and medicinal plants [13]. Aromatic and medicinal plants are important for the protection of the environment. In particular, since ancient times, they have been used as fresh, frozen or dry essential oils, originally for the food, pharmaceutical and cosmetic industries [12]. It is estimated that 50,000–70,000 species of higher plants may be used in traditional and modern medicinal systems throughout the world, and about 3000 belong to the group of medicinal and aromatic plants [14]. Aromatic plants contain chemical substances, such as essential oils, polyphenols, glycosides, quinones, flavonols/flavonoids, terpenes, alkaloids, polypeptides or their oxygen-substituted derivatives [15,16]. Some bioactive compounds have therapeutic value, such as antioxidant and antiseptic activities and may reduce the risk of cancer or cardiovascular diseases and treatment of respiratory diseases, stomach or inflammatory disorders [17,18]. Luteolin is a common flavonoid that belongs to the subclass of flavones and exists in many types of plants, including fruits, vegetables, and medicinal herbs [9]. Luteolin, in medicinal plants, is most commonly found in leaves, having been isolated from many plants [19]. The major natural sources of luteolin, based on the literature, are celery, thyme, dandelion, clover flower, chamomile, carrots, peppers, olive oil, peppermint, thyme, rosemary, oregano, and parsley [20,21]. Luteolin is a powerful antioxidant with anti-inflammatory properties that can be used to treat diseases such as periodontitis [22,23]. Table 1 summarizes the major plant species of the Greek flora that contain luteolin and its derivatives.

Table 1.
Content of luteolin and/or its derivative(s) in different plant species of the Greek flora.
The olive tree (Olea europaea, Oleaceae), along with its fruit (olive) and leaves, is considered to be very important, exhibiting high nutritional and medicinal value. Besides the main bioactive compound oleuropein, olive drupes and leaves have been found to contain luteolin and its derivatives, among other compounds. Blekas and co-workers (2002) studied different samples of table olives obtained from the retail market that were representative of the main Greek cultivars Conservolea (Amfissa), Nychati (Kalamata) and Chalkidiki. The analyses—using a high-performance liquid chromatography system coupled with diode array detector (HPLC-DAD) and reference compounds—showed that luteolin was among the most abundant phenols in all samples, yet Kalamata olives contained the highest quantity [36]. Luteolin was also detected in olive oils that were extracted from various Greek cultivars Koroneiki, Tsounati, Adramitini, Throubolia, Native from Zakynthos, Lianolia, Asprolia, and Thiaki [35,37,39]. The identification of luteolin was performed either using NMR [35,37] or HPLC-UV and LC-DAD-mass spectrometry (MS) [39]. Moreover, luteolin and luteolin 7-O-glucoside were determined in fresh olive fruits, as well as in Greek-style and Spanish-style processed olives from the Kalamon cultivar. Luteolin 7-O-glucoside was identified and quantified by means of HPLC-UV [42], as well as HPLC-DAD and LC-(ESI)-MS/MS [41]. In another study, olive leaves from the Greek cultivars Koroneiki, Megaritiki and Kalamon were examined and found to contain luteolin 7-O-glucoside. LC-(ESI)-MS/MS method was employed to determine glycoside content in the olive leaves [38]. 4-O-glucoside and 7-O-glucoside of luteolin were present in the leaves and drupes of samples collected from several major Greek olive varieties, such as Koroneiki, Lianolia Kerkyras, Mastoidis, Adramytini, Megaritiki, Gaidourelia, Kalamata, Konservolia, and Chalkidiki. The two metabolites were identified and quantified by using reference compounds and HPLC-UV analysis [40].
The Lamiaceae family constitutes one of the most significant botanical families, including a large number of aromatic and medicinal plants, such as Origanum vulgare, Rosmarinus officinalis, Salvia officinalis and Thymus vulgaris, among others. Luteolin and/or its derivatives have been identified in various plant species of the Lamiaceae family. As mentioned above, luteolin was detected in samples collected from different sites in Greece regarding the following species: O. vulgare [32,43], R. officinalis [46], S. officinalis [46], S. fruticosa [43], and Thymus vulgaris [46]. The above analyses were performed by means of HPLC-DAD and using reference compounds. Additionally, luteolin was present in Mentha spicata [32], Nepeta cataria and N. sibthorpii [33,51], Teucrium chamaedrys [46] and T. polium [27]. Other studies have reported that various luteolin derivatives have also been detected in species belonging to the Lamiaceae family. Specifically, 6-hydroxy luteolin 7-O-glucoside, luteolin 7-O-glucuronide, luteolin 7-O-rutinoside, luteolin diglucuronide, 6-methoxyluteolin 7-O-glucoside, and 6-methoxyluteolin derivative were tentatively identified via HPLC-DAD and LC-(ESI)-MS/MS methods in Salvia fruticosa and S. pomifera [48,49]. Choulitoudi et al. reported that Satureja thymbra was found to contain luteolin 7,4′-di-O-glucuronide, 6-OH luteolin 7,3′-dimethyl ether and 6-OH luteolin 7,3′,4′-trimethyl ether. The above components were identified through HPLC-DAD-ESI-MS/MS analysis [50].
Members of the Asteraceae family were analyzed through NMR and MS methods for their flavonoid content, and it was reported that luteolin was identified in Crepis incana [26] and Cynara species [29]. Moreover, the derivatives luteolin 7-O-rutinoside, luteolin 7-O-glucoside, luteolin 7-O-malonylglycoside, luteolin 7-O-β-d-glucoside, luteolin 7-O-β-d-rutinoside, and luteolin 7-O-β-gentiobioside, were also present in Cynara cardunculus, Cynara humilis and Cynara cornigera [28,29].
Different research groups have detected luteolin and its derivatives (luteolin 7-O-glucoside, 3′-methyl-luteolin) in Cuminum cyminum [27], Petroselinum crispum [45] and Petroselinum sativum [46]. Other sources of luteolin that have been described in the literature are Asphodelus ramosus [25], Juglans regia [30], Laurus nobilis [31], Pistacia lentiscus [47], and Spartium junceum [27]. Finally, luteolin and its derivatives luteolin 3′-O-β-d-glucoside and luteolin 3′,4′-di-O-β-d-glucopyranoside were identified by ultra-high-performance LC-high resolution MS (UHPLC-HRMS) analysis in Paeonia clusii [44].
3. Effect of Crop Management on Luteolin Content
Luteolin’s concentration, as well as the quantitative and qualitative composition of the products of secondary metabolites, depends on the genotype of the plant, the crop management (fertilization, irrigation), the growth stage of the plant, soil and climate conditions such as ultraviolet (UV) radiation, strong light, low/high temperature, drought, etc. [52]. These conditions can cause the creation of free radicals due to the stress they cause to the plants, and one of the functions of flavonoids in plants is the reduction of the effect caused by the presence of reactive oxygen species (ROS) [2]. It is possible that luteolin can protect plants from abiotic and biotic stresses. Also, it was proposed that flavonoids, where luteolin belongs, can function as signal molecules, phytoalexins, allopathic compounds, detoxifying agents, antimicrobial defensive compounds and UV filters [53]. They also protect plants from drought, heat and freezing [54,55].
Fertilization is a factor that can affect the concentration of flavonoids. For example, luteolin-7-O-glucoside was found to be higher at the first growth stage and also at the 150 kg N ha−1 treatment (Table 2) [52]. In addition, crop management, such as organic and conventional, can affect luteolin content. Particularly, luteolin concentration was higher by 51% in the organic system compared with the conventional one [56]. In the same study, luteolin content was increased by 39% with the application of 20 kg N ha−1 (1.741 mg 100 g−1 dry weight) compared to 0 kg N ha−1 (1.383 mg 100 g−1 dry weight). Regarding the effect of N fertilization on the organic management system, it was reported that there were no significant changes [56]. Moreover, in another work, the effect of genotype, the ripening stage and their interactions in different growing systems (organic and conventional) were studied in different species of Capsicum. It was observed that the ripening stage significantly affected the concentration of luteolin; specifically, luteolin content appeared higher in ripening fruits, as well as in the organic system [57].

Table 2.
Effect of Nitrogen Fertilization and Harvest Time on Luteolin-7-O-glucoside in Leaves of Stevia rebaudiana.
In a field experiment carried out in Pisa, Italy, different amounts of nitrogen fertilizer were applied to Stevia rebaudiana: N0 (without N fertilization), N50 (50 kg N ha−1), N150 (150 kg N ha−1), N300 (300 kg N ha−1) and Norg (150 kg N ha−1, as organic nitrogen from Nutex N7 based on wool wastes, poultry manure, blood, and pomace with 7% organic N and 35% organic carbon). In addition, leaf samplings were accomplished at three vegetative stages (H1, H2, and H3), where H1 and H2 were during the vegetative growth and H3 at the flowering stage. The experiment showed that luteolin-7-O-glucoside was significantly affected by the amount of nitrogen fertilization (N), harvest time (H), and NxH interaction. Luteolin-7-O-glucoside was higher in the H1 compared to the other harvests with 150 kg N ha−1 (Table 2). In H2, a decrease in Luteolin-7-O-glucoside was recorded compared to H1, while in H3, the amount of this compound increased in each N treatment. Values similar in H1 were recorded in the N0 and Norg treatments [52].
Abiotic stress can affect the concentration of luteolin as it was found that water stress was among the main factors that increase the content of secondary metabolites, especially polyphenols/flavonoids [58,59,60]. Water stress increased the concentration of luteolin in Chrysanthemum plants [60]. In addition, another study conducted with Lactuca sativa plants found that drought stress and UV-B affected the flavonoid content, such as quercetin, luteolin and anthocyanin, as there was an increase in luteolin content under water stress [61].
One of the most common abiotic stresses is salinity, as it affects many agricultural areas worldwide (Vafadar et al., 2020). Also, salinity stress affects the secondary metabolism, and it was found that the concentration of phenolic and flavonoid compounds increased [62,63,64]. In addition, it was found that the concentration of luteolin was increased by up to 75 mM NaCl in Dracocephalum kotschyi [65].
Agati et al. [66] reported that salinity and UV radiation significantly enhanced the biosynthesis of luteolin 7-O-glycosides. Other researchers studied the effect of salt stress using Solanum nigrum seedlings, and the results of the experiment showed increased flavonoid accumulation and decreased root and leaf dry biomass in the treatment with the highest salt concentration [67]. Similar results were reached by other researchers [68,69].
The growth stage of the plants can affect the flavonoid content and especially the content of luteolin, as it was found by several studies in Origanum majorana. Specifically, the highest content is found at the bud stage, as well as in the early vegetative stages and at full bloom [70,71,72].
4. Methods of Luteolin Extraction from Different Plant Species
There are various extraction techniques that are reported in the literature and that have been used by many research groups in order to efficiently acquire luteolin and/or luteolin-enriched extracts. Both conventional and modern methods have been applied for extracting luteolin from different plant sources. From maceration and Soxhlet extraction to ultrasound-assisted and microwave-assisted extraction, each technique offers several advantages while exhibiting certain limitations. The choice of the extraction method is highly dependent on the quantity and characteristics of the plant material, as well as the properties of the bioactive compound(s) to be extracted.
4.1. Conventional Extraction Methods
Blekas et al. performed the extraction of luteolin from freeze-dried table olive fruits while using the maceration technique and 80% (v/v) ethanol (containing 0.5% v/v sodium metabisulfite) as a solvent. The extraction process lasted 20 min and was repeated three times. Luteolin was found to be present at concentrations ranging from 1 to 74 mg/kg of fresh weight in the different table olive samples [36]. Mitsopoulos and co-workers collected samples of leaves and drupes of several olive varieties (e.g., Koroneiki, Kalamata, Konservolia, Chalkidiki) and extracted them using methanol by means of mechanical homogenization. The chromatographic analysis of the extracts showed the presence of 4-O-glucoside and 7-O-glucoside of luteolin at concentrations of 0.07–1.60 mg/g and 0.11–2.03 mg/g of fresh weight, respectively [40]. In another study, different solvents of increasing polarity (petroleum ether, dichloromethane, methanol and methanol/water 60:40 v/v) were used to extract samples of olive tree leaves of the Greek cultivars Koroneiki, Megaritiki, and Kalamon that were collected from Thessaloniki, Greece. Luteolin 7-O-glucoside was among the main constituents that were detected in the leaf extracts [38]. Samples of virgin olive oil from Southern Greece were extracted with a mixture of ethanol/water (80:20 v/v), resulting in extracts containing luteolin (0.31–1.29 mmol/100 g of virgin oil) [37]. Kotsiou and Tasioula-Margari [39] performed a liquid–liquid extraction by mixing samples of extra virgin olive oil that were collected from areas of Western Greece with methanol. The analysis of the extracts demonstrated the presence of luteolin at a concentration range of 0.11–1.69 mg/kg olive oil.
Samples of C. cardunculus leaves were extracted with methanol/water (80:20 v/v) under mild stirring at room temperature. A series of luteolin derivatives were identified in the extract, namely luteolin 7-O-rutinoside, luteolin 7-O-glucoside and luteolin 7-O-malonylglycoside [28]. Similarly, Cynara humilis and C. cornigera leaves were extracted with ether at room temperature, yielding luteolin and a series of derivatives, such as luteolin 7-O-β-d-glucoside and luteolin 7-O-β-d-rutinoside [29].
Miceli et al. performed the extraction of flowers and leaves of Nepeta sibthorpii by using 95% (v/v) methanol. The resulting data showed that the methanolic extract contained luteolin at a concentration of 0.387 mg/g of dry weight [33].
Luteolin (0.5 mg/g of dry weight) was also detected in the flowers of Asphodelus ramosus that were collected from the Campus of the University of Athens, Greece. In specific, the dried flowers were macerated under agitation with a mixture of methanol/water (5:1 v/v) [25].
Barda et al. collected the aerial parts of Crepis incana from Central Greece and extracted them by using two types of solvents successively. A mixture of cyclohexane/diethyl ether/water (1:1:1 v/v/v) and a mixture of methanol/water (5:1 v/v) were used as solvents. Luteolin (0.13 mg/g of extract) and luteolin 7-O-β-d-glucopyranoside (0.22 mg/g of extract) were detected in the extract [26].
Samples of Laurus nobilis leaves that were collected from Northern Greece were macerated with methanol under agitation, leading to a methanolic extract containing luteolin at a quantity of 393.4 μg/g of dry weight [31].
Samples of Mentha spicata and Origanum vulgare leaves were collected from Southern Greece and ground before extracting them via maceration with a mixture of ethanol/water (10:90 v/v) at room temperature for 14 days. Luteolin was detected in both plant species, with its content being 19.89 μg/mL of extract and 25.97 μg/mL of extract for M. spicata and O. vulgare, respectively [32].
Different luteolin derivatives (luteolin 3′-O-β-d-glucoside, luteolin 3′,4′-di-O-β-d-glucopyranoside) along with the parent compound were identified in a methanolic extract of Paeonia clusii. The extract was acquired by macerating P. clusii seeds with methanol at room temperature for 24 h. The content of luteolin and its two derivatives was between 0.33 and 0.69 mg/g of dry weight [44].
Chinou and Harvala proceeded to the extraction of C. humilis and C. cornigera leaves with a Soxhlet apparatus by successively using 95% (v/v) and 50% (v/v) ethanol. The analysis showed that luteolin 7-O-β-gentiobioside was detected in both species [29].
Luteolin was also identified in the extracts of O. vulgare and S. fruticosa. Both plants were extracted via Soxhlet extraction, while ethanol and acetone were selected as extracting solvents. However, the quantity of luteolin was below the quantitative detection limit based on HPLC/DAD analysis [43].
In another study, fresh and dried Pistacia lentiscus leaves were extracted for 3–4 h with a Soxhlet apparatus and ethanol or water as solvents. Soxhlet extraction of fresh leaves with water exhibited the highest yield (31.99%) compared to the dried leaves, as well as to the ethanol extract [47].
Choulitoudi and co-workers employed a Soxhlet apparatus for performing the extraction of Satureja thymbra leaves. Ethyl acetate and ethanol were used as solvents during the successive extractions. The obtained extracts contained different luteolin derivatives, such as luteolin 7,4′-di-O-glucuronide (15.6 g/kg of dry extract), 6-OH luteolin 7,3′-dimethyl ether (11.9–13.5 g/kg of dry extract) and 6-OH luteolin 7,3′,4′-trimethyl ether (11.9–13.3 g/kg of dry extract) [50].
Various Greek aromatic plants were extracted with the heat reflux method. Specifically, dried samples of Cuminum cyminum, Nepeta cataria, Petroselinum sativum, Rosmarinus officinalis, Salvia officinalis, Spartium junceum, Teucrium chamaedrys, Teucrium polium and Thymus vulgaris were mixed with methanol/water (62.5:37:5 v/v), containing 1 g/L butylated hydroxytoluene, and then refluxed in a water bath at 90 °C for 2 h. Luteolin was detected in all plant extracts, with T. vulgaris demonstrating the highest concentration (36 mg/100 g of dry weight) [27,46].
4.2. Modern Extraction Methods
Cvetkovikj et al. [49] performed an ultrasound-assisted extraction of Greek Salvia fruticosa and Salvia pomifera species. Particularly, milled leaves were mixed with 70% (v/v) methanol and sonicated at 40 °C for 20 min. The chromatographic analyses indicated that the methanolic extract of S. fruticosa was abundant in luteolin 7-O-rutinoside (4.84 mg/g of dry weight) and luteolin diglucuronide (6.18 mg/g of dry weight), while this was not the case for S. pomifera, where the respective values for the luteolin derivatives were <1.00 mg/g of dry weight. S. fruticosa was also extracted through a technique combining ultrasonication pretreatment and stirred-tank extraction. Aerial parts of S. fruticosa were acquired from a retail store in Southern Greece, mixed with glycerol/water (60:40 v/v) and sonicated in an ultrasonic bath at 50 °C for 40 min. Next, the mixture was placed in an oil bath at 50 °C for 150 min, under stirring. For comparison reasons, the stirred-tank extraction was also performed at 80 °C and with aqueous solutions containing methyl β-cyclodextrin as a booster for extracting polyphenols. The results showed that luteolin 7-O-glucuronide was among the most abundant polyphenols with quantities of 5.51 mg/g of dry weight (60% v/v glycerol) and 6.96 mg/g of dry weight (methyl β-cyclodextrin). Other luteolin derivatives (6-hydroxy luteolin 7-O-glucoside, luteolin 7-O-rutinoside, 6-methoxyluteolin 7-O-glucoside, 6-methoxyluteolin derivative and 6-methoxyluteolin derivative) were tentatively identified as well, yet their concentrations were significantly low [48].
Another research group extracted freeze-dried olives (Kalamon cultivar) three times by using 80% (v/v) acetone and an ultrasonic ice bath for 15 min. The extract contained luteolin (0.04 mg/g of dry weight) and its 7-O-glucoside (0.21 mg/g of dry weight) [42]. Similarly, Salis et al. [41] used fresh Kalamon olives for extracting luteolin. The extraction process involved the mechanical homogenization of olives with hexane, followed by sonication in a supersonic bath with 80% (v/v) methanol for 30 min. The sonication step was repeated four times in total. The HPLC analysis of the extracts revealed that luteolin content ranged between 92.4 and 118.16 μg/g of fresh weight. Luteolin was also extracted via sonication from Juglans regia septa and Petroselinum crispum leaves. Powdered J. regia septa were mixed with methanol/water (60:40 v/v) containing 0.05% (v/v) trifluoroacetic acid and sonicated in an ultrasonic bath for 10 min at 25 °C. On the other hand, P. crispum leaves were freeze-dried, powdered and mixed with 80% (v/v) methanol for 3 h at room temperature prior to sonication (30 min). Once sonication was completed, the extraction continued via maceration overnight at 4 °C in the dark. The content of luteolin in J. regia septa was 2.4–3.4 μg/g of dry weight, while in P. crispum leaves 0.13–0.15 mg/100 g of dry weight [30,45]. In addition to the above, Boutsika et al. detected luteolin 7-O-glucoside (0.27–0.45 mg/100 g of dry weight) and 3′-methyl-luteolin (0.22–0.32 mg/100 g of dry weight) in P. crispum extract [45].
5. Pharmacological Activities and Applications of Luteolin
The different pharmacological activities of luteolin have been well described in the literature. Luteolin exhibits a series of biological activities, such as cytotoxic, anti-inflammatory, antioxidant and antibacterial ones. Some of the possible mechanisms through which luteolin exerts its therapeutic effects are the following: preventing DNA alterations in oncogene and tumor-suppressor genes, decreasing vascular endothelial growth factor (VEGF), inhibiting the elevation of reactive oxygen species (ROS), and downregulating inflammatory cytokines (TNF-α) and transcription factors (Nf-κB, STAT3) [21]. Moreover, luteolin, as an anti-inflammatory agent, acts by inhibiting the activation of mast cells and T cells in cases of neuroinflammation and allergic inflammation [73,74,75,76].
In a recent study, luteolin was also found to inhibit in vitro and in vivo (C57BL/6J mice) the activation of NOD-, LRR- and pyrin domain-containing protein 3(NLRP3) inflammasome, therefore supporting luteolin’s role as an anti-inflammatory agent [77]. Moreover, Wang and co-workers demonstrated that luteolin could modify the M1/M2 polarization of macrophages and exert its anti-inflammatory role by decreasing p-STAT3 and increasing p-STAT6 [78]. Alternatively, luteolin reduced the inflammation in an acute gouty arthritis rat model by negatively regulating the TLR/MyD88/NF-κB pathway [79]. Researchers also reported that luteolin protected retinal pigment epithelium cells from increasing interleukin levels –and thereby, inflammation– through inhibition of MAPK and NF-kΒ [80]. Last but not least, luteolin protects ulcerative colitis rats by reducing inflammation and enhancing the composition and diversity of gut microbiota [81]. In a different animal model (TG-AD mice), luteolin was found to exert a protective effect against Alzheimer’s disease through the inhibition of endoplasmic reticulum stress-associated neuroinflammation [82]. A common luteolin derivative, luteolin 7-O-glucoside, showed promising in vitro anti-inflammatory properties by suppressing ROS and STAT3 activation in HUVEC cells [55].
The antioxidant and antibacterial activities of luteolin have been recently reported by different groups, demonstrating the ability of this molecule to reduce ochratoxin-stimulated oxidative stress in vitro through Nrf2 and HIF-1α pathways and suppressing the growth of Trueperella pyogenes by disrupting cell wall and cell membrane integrity, hindering the synthesis of nucleic acids, altering metabolism and modulating protein expression [83,84].
Due to its biological potential, luteolin has been employed as a bioactive molecule in various applications within the food industry and the biomedical field. For instance, luteolin has been incorporated into the active packaging system of different foods as well as an additive [85]. Bi et al. [86] demonstrated that luteolin nanoemulsions displayed potent antioxidant properties, therefore protecting fat-containing foods, such as beef, poultry and fish (Figure 3). Additionally, luteolin acted as a food preservative when added to minced meat by hindering the development of Listeria monocytogenes [87]. Also, a commercially available dietary supplement that contains luteolin, rutin and quercetin was shown to exert an antioxidant effect. Other therapeutic applications of luteolin include the employment of different delivery platforms (micelles, liposomes, nanoemulsions, amorphous solid dispersions) with the aim to increase the active molecule’s bioavailability and its overall therapeutic efficacy [88,89,90,91]. Luteolin and luteolin-containing drug delivery systems have been studied for the treatment of numerous diseases, such as neurological disorders [92], rheumatoid arthritis [93] and different types of cancers [94,95,96] (Figure 3).
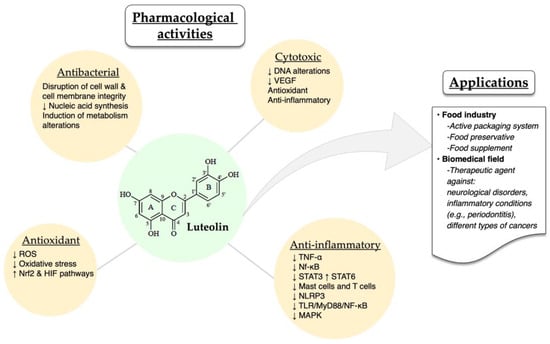
Figure 3.
Overview of the pharmacological activities and applications of luteolin. The up and down arrows indicate increases and decreases, respectively.
6. The Effect of Luteolin in the Prevention and Treatment of Periodontal Disease
Among the various available options for managing periodontitis, dental care products containing herbal compounds have been in the spotlight owing to the beneficial pharmacological properties of the bioactive ingredients [97]. In this context, the anti-inflammatory activity of luteolin has been harnessed in order to combat periodontal disease and promote the restoration of damaged bone tissue. Gutiérrez-Venegas and co-workers demonstrated that luteolin could hinder the inflammation in lipopolysaccharide-stimulated human gingival fibroblasts by downregulating a series of mitogen-activated protein kinase family members [98]. In a different study, Wistar rats were used as a model for assessing the use of luteolin in periodontitis prevention. The results suggested that luteolin succeeded in reducing the inflammation and potentially induced osteoblast differentiation [99]. Moreover, it was found that luteolin could facilitate osteogenic differentiation in human periodontal ligament cells through the activation of the Wnt/β–catenin signaling pathway [100]. Casili et al. confirmed that luteolin could alleviate periodontitis symptoms in Sprague-Dawley rats by reducing inflammation through the reduction of TNF-α and IL-6 expression [23]. In relation to the anti-inflammatory activity of luteolin, which is relevant in periodontitis treatment, both in vitro and in vivo studies have shown that luteolin inhibits many pro-inflammatory cytokines (like TNF-α), as well as modulate NF-κB pathway [101].
Luteolin’s antimicrobial properties have been well documented. In some studies, it was attempted to isolate the bioactive compounds of several flavonoids while examining their antimicrobial activities against oral bacteria related to the establishment of periodontitis. Luteolin appeared to exhibit great antimicrobial activity against the oral microbes tested (including Porphyromonas gingivalis) due to the presence of hydroxyl group at the third position [102]. More specifically, it inhibited bacterial growth, leading to a reduction in the total count of bacteria. Taken together, our collected data suggest that luteolin has the potential, as a useful adjunct agent, to regulate both prevention and treatment of periodontal diseases.
7. Conclusions
Flavonoids are a class of naturally occurring compounds that are present in a plethora of aromatic plants. More specifically, they are a group of low molecular weight polyphenols being produced as secondary metabolites in plants. These compounds have attracted a great amount of interest because of their important biological activities and their beneficial effect on human health. Luteolin is a bioactive flavonoid that also belongs to the subgroup of flavones and can be found in several plants, such as the genera Cynara, Origanum and Salvia, among others. Greek flora is abundant with a vast number of aromatic plants that may present a good source of luteolin and/or its derivatives. This review provided a comprehensive overview of the different luteolin-containing Greek plant species, the employed conventional and modern extraction techniques for obtaining luteolin and/or its derivatives, and the analytical methods for detecting these bioactive compounds. Additionally, the up-to-date literature data concerning crop management (e.g., fertilization, irrigation) in relation to the production of luteolin (or its derivatives) in plants has been discussed. Overall, the findings of the current review suggest that Greek plants could be used for isolating adequate quantities of luteolin and further utilizing it for developing oral formulations against periodontal disease.
Funding
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: Τ2EDK-01627).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural Diversity of Polyphenols and Distribution in Foods. Diet. Polyphen. Their Metab. Health Eff. 2020, 1–29. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Gurung, R.B.; Pandey, R.P.; Sohng, J.K. Apigenin and Naringenin: Natural Sources, Pharmacology and Role in Cancer Prevention. Chapter: Role of Apigenin in Cancer Prevention. Nova Sci. 2015. [Google Scholar]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Chan, T.S.; Galati, G.; Pannala, A.S.; Rice-Evans, C.; O’Brien, P.J. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidase system. Free. Radic. Res. 2003, 37, 787–794. [Google Scholar] [CrossRef]
- Hempel, J.; Pforte, H.; Raab, B.; Engst, W.; Bohm, H.; Jacobasch, G. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung 1999, 43, 201–204. [Google Scholar] [CrossRef]
- Bogers, R.J.; Craker, L.E.; Lange, D. Medicinal and Aromatic Plants: Agricultural, Commercial, Ecological, Legal, Pharmacological and Social Aspects (Wageningen UR Frontis Series); Springer: Berlin, Germany, 2006; Volume 17, 309p. [Google Scholar]
- Giannoulis, K.; Evangelopoulos, V.; Gougoulias, N.; Wogiatzi, E. Could bio-stimulators affect flower, essential oil yield, and its composition in organic lavender (Lavandula angustifolia) cultivation? Ind. Crops Prod. 2020, 154, 112611. [Google Scholar] [CrossRef]
- Leaman, D.J. The International Standard for Sustainable Wild Collection of Medicinal and Aromatic Plants (ISSC-MAP): Elements of ISSC-MAP Resource Assessment Guidance Relevant to Cites NDF; International Expert Workshop on CITES Non-Detriment Findings Perennial Plant Working Group (Ornamentals, Medicinal and Aromatic Plants Cancun, Mexico): Cancun, Mexico, 2008. [Google Scholar]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Kadri, A.; Zarai, Z.; Chobba, I.B.; Bekir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical constituents and antioxidant properties of Rosmarinus officinalis L. essential oil cultivated from South-Western Tunisia. J. Med. Plants Res. 2011, 5, 5999–6004. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Nur, A.; Mi-Yeon, K.; Jae, Y.C. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Kostić, Μ.; Kitić, D.; Petrović, M.B.; Jevtović-Stoimenov, T.; Jović, M.; Petrović, A.; Živanović, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59. [Google Scholar] [CrossRef]
- Casili, G.; Ardizzone, A.; Lanza, M.; Gugliandolo, E.; Portelli, M.; Militi, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Treatment with Luteolin Improves Lipopolysaccharide-Induced Periodontal Diseases in Rats. Biomedicines 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Dretcanu, G.; Stirbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stanila, A.; Farcas, A.; Borda, I.M.; Iuhas, C.; Diaconeasa, Z. Chemical structure, sources and role of bioactive flavonoids in cancer prevention: A Review. Plants 2022, 11, 1117. [Google Scholar] [CrossRef]
- Chimona, C.; Karioti, A.; Skaltsa, H.; Rhizopoulou, S. Occurrence of secondary metabolites in tepals of Asphodelus ramosus L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 31–34. [Google Scholar]
- Barda, C.; Ciric, A.; Soković, M.; Tsoukalas, M.; Skaltsa, H. Phytochemical investigation of Crepis incana Sm. (Asteraceae) endemic to southern Greece. Biochem. Syst. Ecol. 2018, 80, 59–62. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017, 8, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.; Harvala, C. Polyphenolic Constituents from the Leaves of two Cynara Species Growing in Greece. Planta Medica 1997, 63, 469–470. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Samanidou, V.F. A validated ultrasound-assisted extraction coupled with spe-hplc-dad for the determination of flavonoids in by-products of plant origin: An application study for the valorization of the walnut septum membrane. Molecules 2021, 26, 6418. [Google Scholar] [CrossRef]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef]
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373. [Google Scholar] [CrossRef]
- Miceli, N.; Taviano, M.F.; Giuffrida, D.; Trovato, A.; Tzakou, O.; Galati, E.M. Anti-inflammatory activity of extract and fractions from Nepeta sibthorpii Bentham. J. Ethnopharmacol. 2005, 97, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Patras, A.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N. Application of principal component and hierarchical cluster analysis to classify different spices based on in vitro antioxidant activity and individual polyphenolic antioxidant compounds. J. Funct. Foods 2011, 3, 179–189. [Google Scholar] [CrossRef]
- Agiomyrgianaki, A.; Petrakis, P.V.; Dais, P. Influence of harvest year, cultivar and geographical origin on Greek extra virgin olive oils composition: A study by NMR spectroscopy and biometric analysis. Food Chem. 2012, 135, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in table olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Christophoridou, S.; Dais, P. Detection and quantification of phenolic compounds in olive oil by high resolution 1H nuclear magnetic resonance spectroscopy. Anal. Chim. Acta 2009, 633, 283–292. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Kotsiou, K.; Tasioula-Margari, M. Monitoring the phenolic compounds of Greek extra-virgin olive oils during storage. Food Chem. 2016, 200, 255–262. [Google Scholar] [CrossRef]
- Mitsopoulos, G.; Papageorgiou, V.; Komaitis, M.; Hagidimitriou, M. Phenolic Profile of leaves and drupes in major Greek olive varieties. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 162–166. [Google Scholar] [CrossRef][Green Version]
- Salis, C.; Papadakis, I.E.; Hagidimitriou, M. Identification and Quantification of Phenolic Compounds in Fresh and Processed Table Olives of Cv. ‘kalamata’. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 1–13. [Google Scholar] [CrossRef]
- Tsantili, E. Quality attributes and their relations in fresh black ripe “Kalamon” olives (Olea europaea L.) for table use—Phenolic compounds and total antioxidant capacity. Int. J. Food Sci. Technol. 2014, 49, 657–665. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Klontza, V.; Graikou, K.; Cheilari, A.; Kasapis, V.; Ganos, C.; Aligiannis, N.; Chinou, I. Phytochemical Study on Seeds of Paeonia clusii subsp. rhodia—Antioxidant and Anti-Tyrosinase Properties. Int. J. Mol. Sci. 2023, 24, 4935. [Google Scholar] [CrossRef] [PubMed]
- Boutsika, A.; Sarrou, E.; Cook, C.M.; Mellidou, I.; Avramidou, E.; Angeli, A.; Martens, S.; Ralli, P.; Letsiou, S.; Selini, A.; et al. Evaluation of parsley (Petroselinum crispum) germplasm diversity from the Greek Gene Bank using morphological, molecular and metabolic markers. Ind. Crops Prod. 2021, 170, 113767. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Bampouli, A.; Kyriakopoulou, K.; Papaefstathiou, G.; Louli, V.; Aligiannis, N.; Magoulas, K.; Krokida, M. Evaluation of total antioxidant potential of Pistacia lentiscus var. chia leaves extracts using UHPLC–HRMS. J. Food Eng 2015, 167, 25–31. [Google Scholar] [CrossRef]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Hydroglycerolic solvent and ultrasonication pretreatment: A green blend for high-efficiency extraction of Salvia fruticosa polyphenols. Sustainability 2020, 12, 4840. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Xristou, M.; Tsimogiannis, D.; Oreopoulou, V. The effect of temperature on the phenolic content and oxidative stability of o/w emulsions enriched with natural extracts from Satureja thymbra. Food Chem. 2021, 349, 129206. [Google Scholar] [CrossRef]
- Proestos, C.; Sereli, D.; Komaitis, M. Determination of phenolic compounds in aromatic plants by RP-HPLC and GC-MS. Food Chem. 2006, 95, 44–52. [Google Scholar] [CrossRef]
- Tavarini, S.; Sgherri, C.; Ranieri, A.M.; Angelini, L.G. Effect of nitrogen fertilization and harvest time on steviol glycosides, flavonoid composition and antioxidant properties in Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 31, 7041–7050. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. The significance of the study about the biological effects of solar ultraviolet radiation using the exposed facility on the international space station. Biol. Sci. Space 2004, 18, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Salata, A.; Nurzynska-Wierdak, R.; Kalisz, A.; Kunicki, E.; Ibáñez-Asensio, S.; Moreno-Ramón, H. Effects of organic cropping on phenolic compounds and antioxidant capacity of globe artichoke herbs. Agronomy 2022, 12, 192. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Adalid, A.M.; Raigon, M.D.; Hellin, P.; Fita, A.; Rodríguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Hodaei, Μ.; Rahimmalek, Μ.; Arzania, A.; Talebib, Μ. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Rajabbeigi, E.; Eichholz, I.; Beesk, N.; Ulrichs, C.; Kroh, L.W.; Rohn, S.; Huyskens-Keil, S. Interaction of drought stress and UV-B radiation—Impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). J. Appl. Bot. Food Qual. 2013, 86, 190–197. [Google Scholar]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Shafeiee, M.; Ehsanzadeh, P. Physiological and biochemical mechanisms of salinity tolerance in several fennel genotypes: Existence of clearly-expressed genotypic variations. Ind. Crops Prod. 2019, 132, 311–318. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M. Salinity stress alters ion homeostasis, antioxidant activities and the production of rosmarinic acid, luteolin and apigenin in Dracocephalum kotschyi Boiss. Biologia 2020, 75, 2147–2158. [Google Scholar] [CrossRef]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.B.; Aung, B.; Amyot, L.; Lalin, I.; Lachaal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- El-Shafey, N.M.; AbdElgawad, H. Luteolin, a bioactive flavone compound extracted from Cichorium endivia L. subsp. divaricatum alleviates the harmful effect of salinity on maize. Acta Physiol. Plant. 2012, 34, 2165–2177. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Mallouchos, A.; Komaitis, M. Investigation of the antioxidant behavior of air- and freeze-dried aromatic plant materials in relation to their phenolic content and vegetative cycle. J. Agric. Food Chem. 2008, 56, 5743–5752. [Google Scholar] [CrossRef]
- Skoula, M.; Grayer, R.J.; Kite, G.C.; Veitch, N.C. Exudate flavones and flavanones in Origanum species and their interspecific variation. Biochem. Syst. Ecol. 2008, 36, 646–654. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoran (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Iliopoulou, B.P. Mast cells, T cells, and inhibition by luteolin: Implications for the pathogenesis and treatment of multiple sclerosis. Immune-Mediat. Dis. Theory Ther. 2007, 601, 423–430. [Google Scholar]
- Kempuraj, D.; Tagen, M.; Iliopoulou, B.P.; Clemons, A.; Vasiadi, M.; Boucher, W.; House, M.; Wolfberg, A.; Theoharides, T.C. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br. J. Pharmacol. 2008, 155, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Luteolin as a therapeutic option for multiple sclerosis. J. Neuroinflammation 2009, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Kritas, S.K.; Saggini, A.; Varvara, G.; Murmura, G.; Caraffa, A.; Antinolfi, P.; Toniato, E.; Pantalone, A.; Neri, G.; Frydas, S.; et al. Luteolin inhibits mast cell-mediated allergic inflammation. J. Biol. Regul. Homeost. Agents 2013, 27, 955–959. [Google Scholar]
- Lee, M.N.; Lee, Y.; Wu, D.; Pae, M. Luteolin inhibits NLRP3 inflammasome activation via blocking ASC oligomerization. J. Nutr. Biochem. 2021, 92, 108614. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Xu, S.; Shi, J.; Mao, X.; Yao, X.; Liu, C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation 2020, 43, 95–108. [Google Scholar] [CrossRef]
- Shen, R.; Ma, L.; Zheng, Y. Anti-inflammatory effects of luteolin on acute gouty arthritis rats via TLR/MyD88/NF-kappaB pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 115–122. [Google Scholar]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Hsiao, C.Y.; Wu, S.J. Luteolin Attenuates IL-1beta-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-kappaB and MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9421340. [Google Scholar] [CrossRef]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; et al. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1alpha pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020, 141, 111436. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The Antibacterial Activity and Mechanism of Action of Luteolin Against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef]
- Tan, L.; Liang, C.; Wang, Y.; Jiang, Y.; Zeng, S.; Tan, R. Pharmacodynamic effect of luteolin micelles on alleviating cerebral ischemia reperfusion injury. Pharmaceutics 2018, 10, 248. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and evaluations of transdermally delivered luteolin loaded cationic nanoemulsion: In vitro and ex vivo evaluations. Pharmaceutics 2021, 13, 1218. [Google Scholar] [CrossRef]
- Koromili, M.; Kapourani, A.; Barmpalexis, P. Preparation and Evaluation of Amorphous Solid Dispersions for Enhancing Luteolin’s Solubility in Simulated Saliva. Polymers 2023, 15, 169. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Pang, J.; Yang, F.; Zhang, Z.; Yang, W.; Li, Y.; Xu, H. The role of luteolin nanocomposites in rheumatoid arthritis treatment. Mater. Express 2021, 11, 303–309. [Google Scholar]
- Dia, V.P.; Pangloli, P. Epithelial-to-mesenchymal transition in paclitaxel-resistant ovarian cancer cells is downregulated by luteolin. J. Cell. Physiol. 2017, 232, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.S.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Bin-Jumah, M.; Rizwanullah, M.; Imam, S.S.; Imtiyaz, K.; Alshehri, S.; Rizvi, M.M.A. Chitosan coated luteolin nanostructured lipid carriers: Optimization, In vitro-Ex vivo assessments and cytotoxicity study in breast cancer cells. Coatings 2021, 11, 158. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Karakostas, P.; Kavakloglou, S.; Assimopoulou, A.; Barmpalexis, P.; Tsalikis, L. Clinical Effectiveness of Herbal Oral Care Products in Periodontitis Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10061. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Kawasaki-Cárdenas, P.; Maldonado-Frías, S. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. Eur. J. Pharmacol. 2006, 541, 95–105. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Toker, H.; Yildirim, A.; Tekin, M.B.; Gevrek, F.; Altunbas, N. The effect of luteolin in prevention of periodontal disease in Wistar rats. J. Periodontol. 2019, 90, 1481–1489. [Google Scholar] [CrossRef]
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid Med. Cell Longev. 2022, 6, 9966750. [Google Scholar] [CrossRef]
- Kariu, T.; Hamada, N.; Lakshmyya, K. Luteolin inhibits Porphyromonas gingivalis growth and alleviates alveolar bone destruction in experimental murine periodontitis. Biosci. Biotechnol. Biochem. 2023, zbad137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).