Design and Synthesis of Novel Helix Mimetics Based on the Covalent H-Bond Replacement and Amide Surrogate
Abstract
1. Introduction
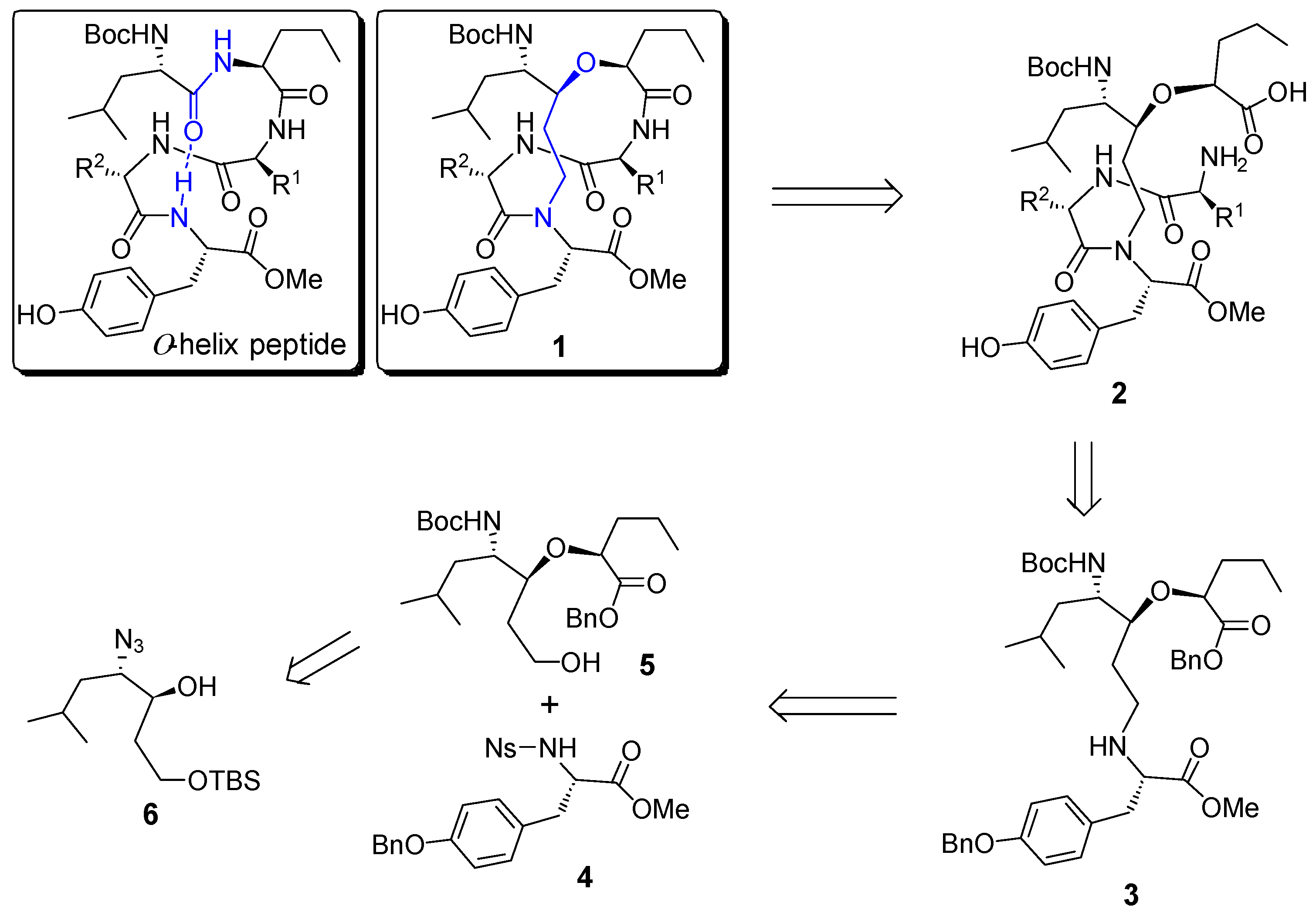
2. Results and Discussion
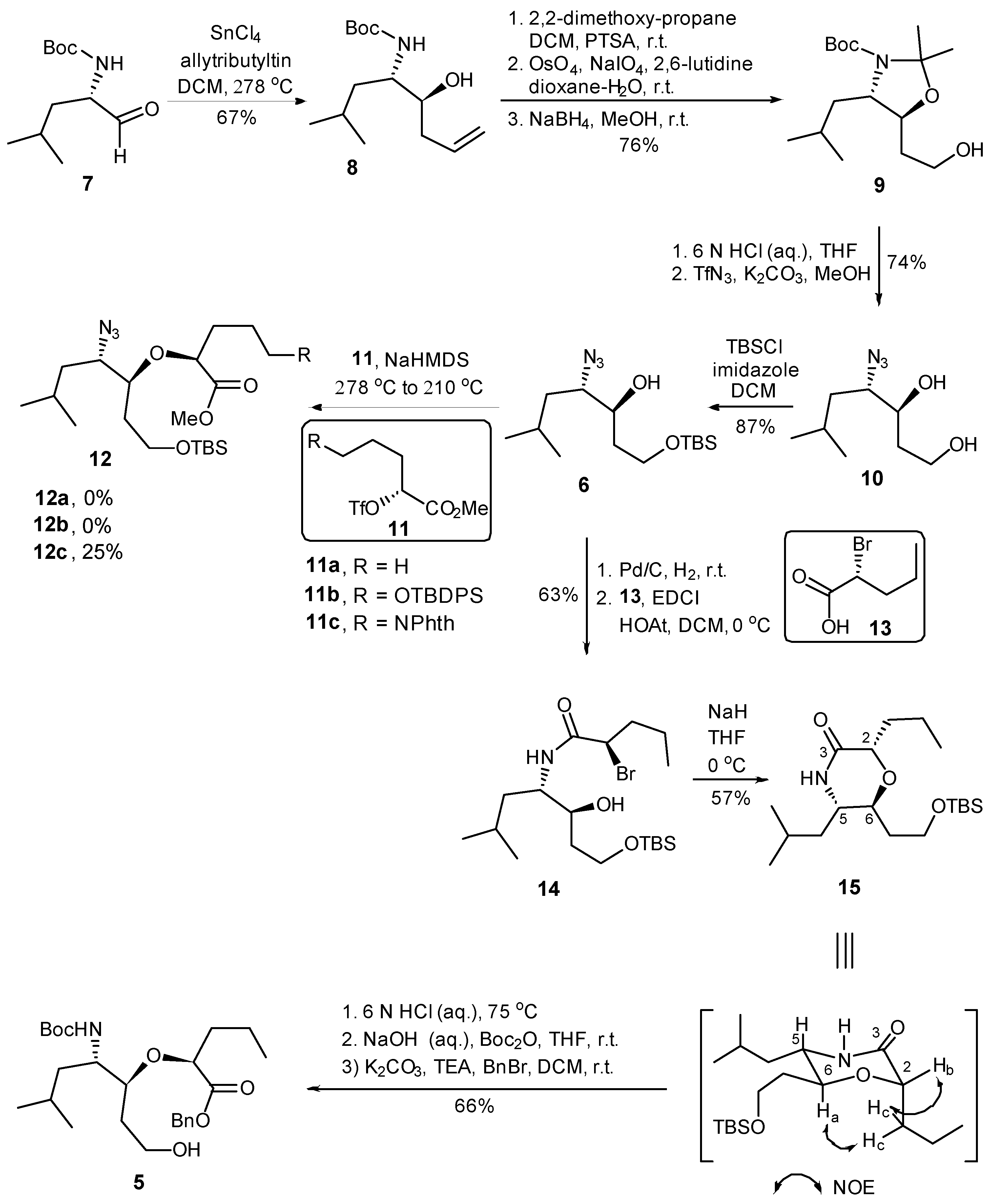
2.1. Synthesis of Helix Mimetic Template
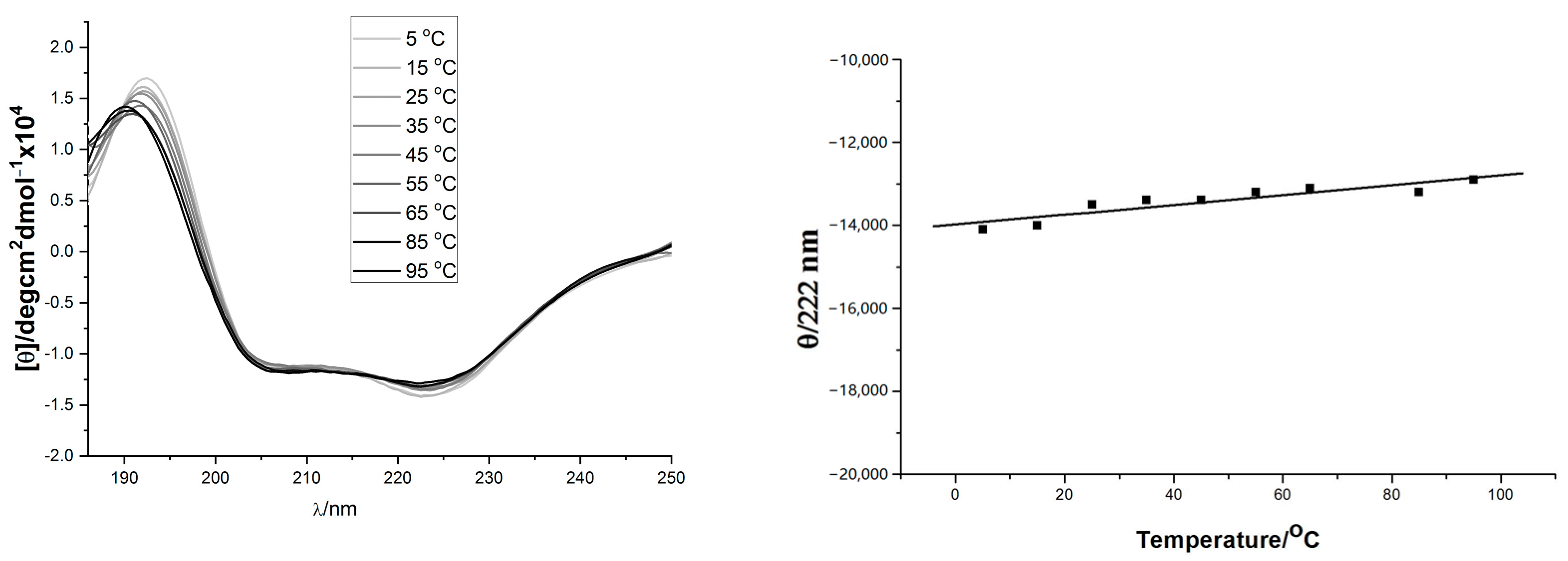
2.2. CD Spectra of Helix Templates
3. Materials and Methods
3.1. General Experimental Details
3.2. Procedures and Analytical Description of Compounds
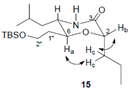
3.3. Two-Dimensional COSY and NOESY Analysis of Compound 15
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Watkins, A.M.; Wuo, M.G.; Arora, P.S. Protein–Protein Interactions Mediated by Helical Tertiary Structure Motifs. J. Am. Chem. Soc. 2015, 137, 11622–11630. [Google Scholar] [CrossRef] [PubMed]
- Kussie, P.H.; Gorina, S.; Marechal, V.; Elenbaas, B.; Moreau, J.; Levine, A.J.; Pavletich, N.P. Structure of the MDM2 Oncoprotein Bound to the p53 Tumor Suppressor Transactivation Domain. Science 1996, 274, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Sable, R.; Jois, S. Surfing the Protein-Protein Interaction Surface Using Docking Methods: Application to the Design of PPI Inhibitors. Molecules 2015, 20, 11569–11603. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Dong, G.; Miao, Z.; Zhang, W.; Wang, W. State-of-the-art strategies for targeting protein–protein interactions by small-molecule inhibitors. Chem. Soc. Rev. 2015, 44, 8238–8259. [Google Scholar] [CrossRef]
- Milroy, L.-G.; Grossmann, T.N.; Hennig, S.; Brunsveld, L.; Ottmann, C. Modulators of Protein–Protein Interactions. Chem. Rev. 2014, 114, 4695–4748. [Google Scholar] [CrossRef]
- Wilson, A.J. Inhibition of protein–protein interactions using designed molecules. Chem. Soc. Rev. 2009, 38, 3289–3300. [Google Scholar] [CrossRef]
- Robertson, N.S.; Spring, D.R. Using Peptidomimetics and Constrained Peptides as Valuable Tools for Inhibiting Protein–Protein Interactions. Molecules 2018, 23, 959. [Google Scholar] [CrossRef]
- Sawyer, N.; Watkins, A.M.; Arora, P.S. Protein Domain Mimics as Modulators of Protein–Protein Interactions. Acc. Chem. Res. 2017, 50, 1313–1322. [Google Scholar] [CrossRef]
- Pelay-Gimeno, M.; Glas, A.; Koch, O.; Grossmann, T.N. Structure-Based Design of Inhibitors of Protein–Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. 2015, 54, 8896–8927. [Google Scholar] [CrossRef]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of α-helix-mediated protein–protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Q.-S.; Geng, H.; Tian, Y.; Cheng, M.; Jiang, Y.-H.; Xie, M.-S.; Niu, X.-G.; Jiang, F.; Zhang, Y.-O.; et al. Crosslinked Aspartic Acids as Helix-Nucleating Templates. Angew. Chem. Int. Ed. 2016, 55, 12088–12093. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Wang, D.; Li, Z. Design and Synthetic Strategies for Helical Peptides. In Cyclic Peptide Design; Goetz, G., Ed.; Springer: New York, NY, USA, 2019; pp. 107–131. [Google Scholar]
- Andrews, M.J.I.; Tabor, A.B. Forming stable helical peptides using natural and artificial amino acids. Tetrahedron 1999, 55, 11711–11743. [Google Scholar] [CrossRef]
- Garner, J.; Harding, M.M. Design and synthesis of α-helical peptides and mimetics. Org. Biomol. Chem. 2007, 5, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, H.E.; Grubbs, R.H. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew. Chem. Int. Ed. 1998, 37, 3281–3284. [Google Scholar] [CrossRef]
- Schafmeister, C.E.; Po, J.; Verdine, G.L. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J. Am. Chem. Soc. 2000, 122, 5891–5892. [Google Scholar] [CrossRef]
- Cabezas, E.; Satterthwait, A.C. The Hydrogen Bond Mimic Approach: Solid-Phase Synthesis of a Peptide Stabilized as an α-Helix with a Hydrazone Link. J. Am. Chem. Soc. 1999, 121, 3862–3875. [Google Scholar] [CrossRef]
- Patgiri, A.; Jochim, A.L.; Arora, P.S. A Hydrogen Bond Surrogate Approach for Stabilization of Short Peptide Sequences in α-Helical Conformation. Acc. Chem. Res. 2008, 41, 1289–1300. [Google Scholar] [CrossRef]
- Chapman, R.N.; Dimartino, G.; Arora, P.S. A Highly Stable Short α-Helix Constrained by a Main-Chain Hydrogen-Bond Surrogate. J. Am. Chem. Soc. 2004, 126, 12252–12253. [Google Scholar] [CrossRef]
- Koo, N.; Sharma, A.K.; Narayan, S. Therapeutics Targeting p53-MDM2 Interaction to Induce Cancer Cell Death. Int. J. Mol. Sci. 2022, 23, 5005. [Google Scholar] [CrossRef]
- Shangary, S.; Wang, S. Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction to Reactivate p53 Function: A Novel Approach for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 223–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Aguilar, A.; Bernard, D.; Wang, S. Small-Molecule Inhibitors of the MDM2–p53 Protein–Protein Interaction (MDM2 Inhibitors) in Clinical Trials for Cancer Treatment. J. Med. Chem. 2015, 58, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.; Wu, M.; Higa, L.A.; Gavrilova, N.; Quan, J.; Ye, T.; Kobayashi, R.; Sun, H.; Zhang, H. L2DTL/CDT2 and PCNA Interact with p53 and Regulate p53 Polyubiquitination and Protein Stability through MDM2 and CUL4A/DDB1 Complexes. Cell Cycle 2006, 5, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Wang, B.; Ye, T. Cyp11B, Cyp17, and/or Cyp21 Inhibitors. WO 2012083112 A2 2012-06-21, WO 2011088160 A2 2011-07-21, US 2010216699 A1 2010-08-26, WO 2010065174 A1 2010-06-10, GB2464617 A 2010-04-28. Available online: https://patents.google.com/patent/US20130252930 (accessed on 9 January 2023).
- Liu, J.; Chen, Y.; Luesch, H.; Ye, T. Total Synthesis of des-Thiomethyllooekeyolide A. Org. Lett. 2022, 24, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yu, J.; Meng, J.; Guo, Y.; Ye, T. Total Synthesis of Pagoamide A. Molecules 2021, 26, 4224. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Gao, B.; Zhao, M.; Yan, J.-L.; Xu, Z.; Choi, S.; Ye, T. Total Synthesis of Hoiamide A Using an Evans–Tishchenko Reaction as a Key Step. Org. Lett. 2019, 21, 5471–5474. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, B.; Xu, Z.; Ye, T. Total Synthesis and Stereochemical Assignment of Callyspongiolide. J. Am. Chem. Soc. 2016, 138, 6948–6951. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhang, J.; Xu, Z.; Ye, T. The total synthesis and stereochemical assignment of scytonemin A. Chem. Commun. 2016, 52, 1002–1005. [Google Scholar] [CrossRef]
- Liao, L.; Zhou, J.; Xu, Z.; Ye, T. Concise Total Synthesis of Nannocystin A. Angew. Chem. Int. Ed. 2016, 55, 13263–13266. [Google Scholar] [CrossRef]
- Qu, S.; Chen, Y.; Wang, X.; Chen, S.; Xu, Z.; Ye, T. Total synthesis of largamide B. Chem. Commun. 2015, 51, 2510–2513. [Google Scholar] [CrossRef]
- Lei, H.; Yan, J.; Yu, J.; Liu, Y.; Wang, Z.; Xu, Z.; Ye, T. Total Synthesis and Stereochemical Reassignment of Mandelalide A. Angew. Chem. Int. Ed. 2014, 53, 6533–6537. [Google Scholar] [CrossRef]
- Wang, D.; Chen, K.; Kulp, J.L.; Arora, P.S. Evaluation of Biologically Relevant Short α-Helices Stabilized by a Main-Chain Hydrogen-Bond Surrogate. J. Am. Chem. Soc. 2006, 128, 9248–9256. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.B.; Arora, P.S. Design, synthesis and protein-targeting properties of thioether-linked hydrogen bond surrogate helices. Chem. Commun. 2012, 48, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Rittle, K.E.; Homnick, C.F.; Ponticello, G.S.; Evans, B.E. A synthesis of statine utilizing an oxidative route to chiral .alpha.-amino aldehydes. J. Org. Chem. 1982, 47, 3016–3018. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bischoff, A.; Cappiello, J. Asymmetric Total Synthesis of the Gastroprotective Microbial Agent AI-77-B. Eur. J. Org. Chem. 2003, 2003, 821–832. [Google Scholar] [CrossRef]
- Vara Prasad, J.V.N.; Rich, D.H. Addition of Allylic Metals to α-Aminoaldehydes. Application to the Synthesis of Statine, Ketomethylene and Hydroxyethylene Dipeptide Isosteres. Tetrahedron Lett. 1990, 31, 1803–1806. [Google Scholar] [CrossRef]
- Qabar, M.N.; Meara, J.P.; Ferguson, M.D.; Lum, C.; Kim, H.-O.; Kahn, M. Synthesis of 3-alkoxyazetidin-2-ones: Dipeptide mimics. Tetrahedron Lett. 1998, 39, 5895–5898. [Google Scholar] [CrossRef]
- Philippe, C.; Milcent, T.; Crousse, B.; Bonnet-Delpon, D. Non Lewis acid catalysed epoxide ring opening with amino acid esters. Org. Biomol. Chem. 2009, 7, 2026–2028. [Google Scholar] [CrossRef]
- Snider, B.B.; Lin, H. Biomimetic Total Syntheses of (−)-TAN1251A, (+)-TAN1251B, (+)-TAN1251C, and (+)-TAN1251D. Org. Lett. 2000, 2, 643–646. [Google Scholar] [CrossRef]
- Wünsch, E.; Fries, G.; Zwick, A. Beiträge zur Peptidsynthese, V. Darstellung und peptidsynthetische Verwendung von O-Benzyl-L-tyrosin. Chem. Ber. 1958, 91, 542–547. [Google Scholar] [CrossRef]
- Fukuyama, T.; Jow, C.-K.; Cheung, M. 2- and 4-Nitrobenzenesulfonamides: Exceptionally versatile means for preparation of secondary amines and protection of amines. Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar] [CrossRef]
- Holzwarth, G.; Doty, P. The Ultraviolet Circular Dichroism of Polypeptides. J. Am. Chem. Soc. 1965, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Sears, D.W.B.S. Physical Principles and Techniques of Protein Chemistry; Academic Press: New York, NY, USA, 1973; pp. 460–466. [Google Scholar]
- Patgiri, A.; Joy, S.T.; Arora, P.S. Nucleation Effects in Peptide Foldamers. J. Am. Chem. Soc. 2012, 134, 11495–11502. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tang, S.; Yan, J.-L.; Ye, T. Design and Synthesis of Novel Helix Mimetics Based on the Covalent H-Bond Replacement and Amide Surrogate. Molecules 2023, 28, 780. https://doi.org/10.3390/molecules28020780
Liu J, Tang S, Yan J-L, Ye T. Design and Synthesis of Novel Helix Mimetics Based on the Covalent H-Bond Replacement and Amide Surrogate. Molecules. 2023; 28(2):780. https://doi.org/10.3390/molecules28020780
Chicago/Turabian StyleLiu, Junyang, Shoubin Tang, Jia-Lei Yan, and Tao Ye. 2023. "Design and Synthesis of Novel Helix Mimetics Based on the Covalent H-Bond Replacement and Amide Surrogate" Molecules 28, no. 2: 780. https://doi.org/10.3390/molecules28020780
APA StyleLiu, J., Tang, S., Yan, J.-L., & Ye, T. (2023). Design and Synthesis of Novel Helix Mimetics Based on the Covalent H-Bond Replacement and Amide Surrogate. Molecules, 28(2), 780. https://doi.org/10.3390/molecules28020780




