Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils
Abstract
1. Introduction
2. Results
2.1. USAE Trials
2.2. Characterization of Oil Preparations
Quality Criteria
2.3. Storage Studies of Flavored Oil Preparations
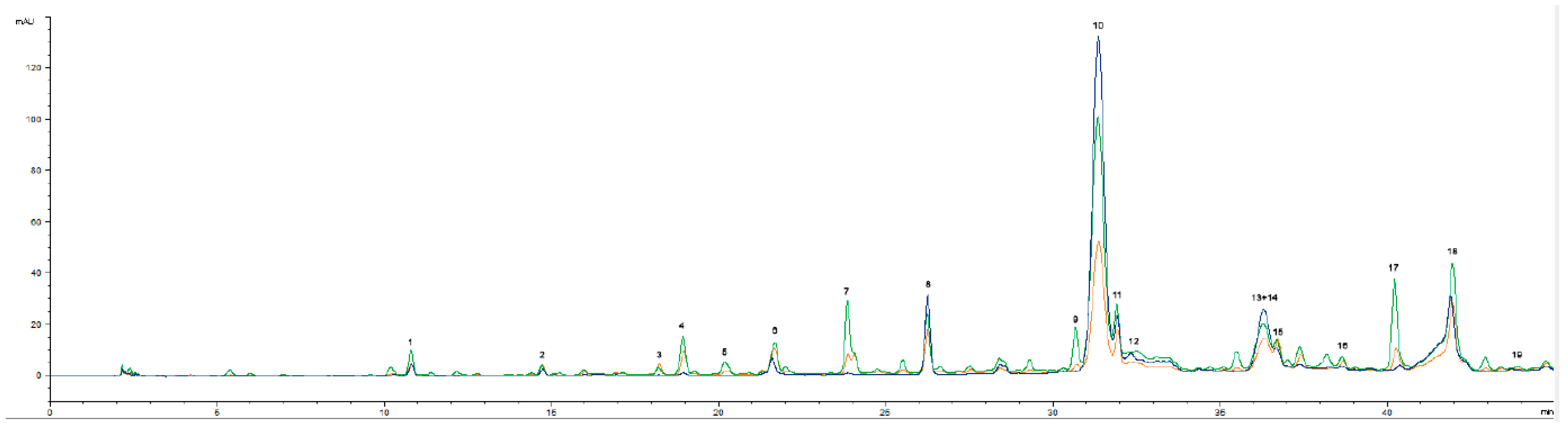
2.3.1. Fatty Acid and Phenolic Compositions
2.3.2. Chlorophyll Pigments
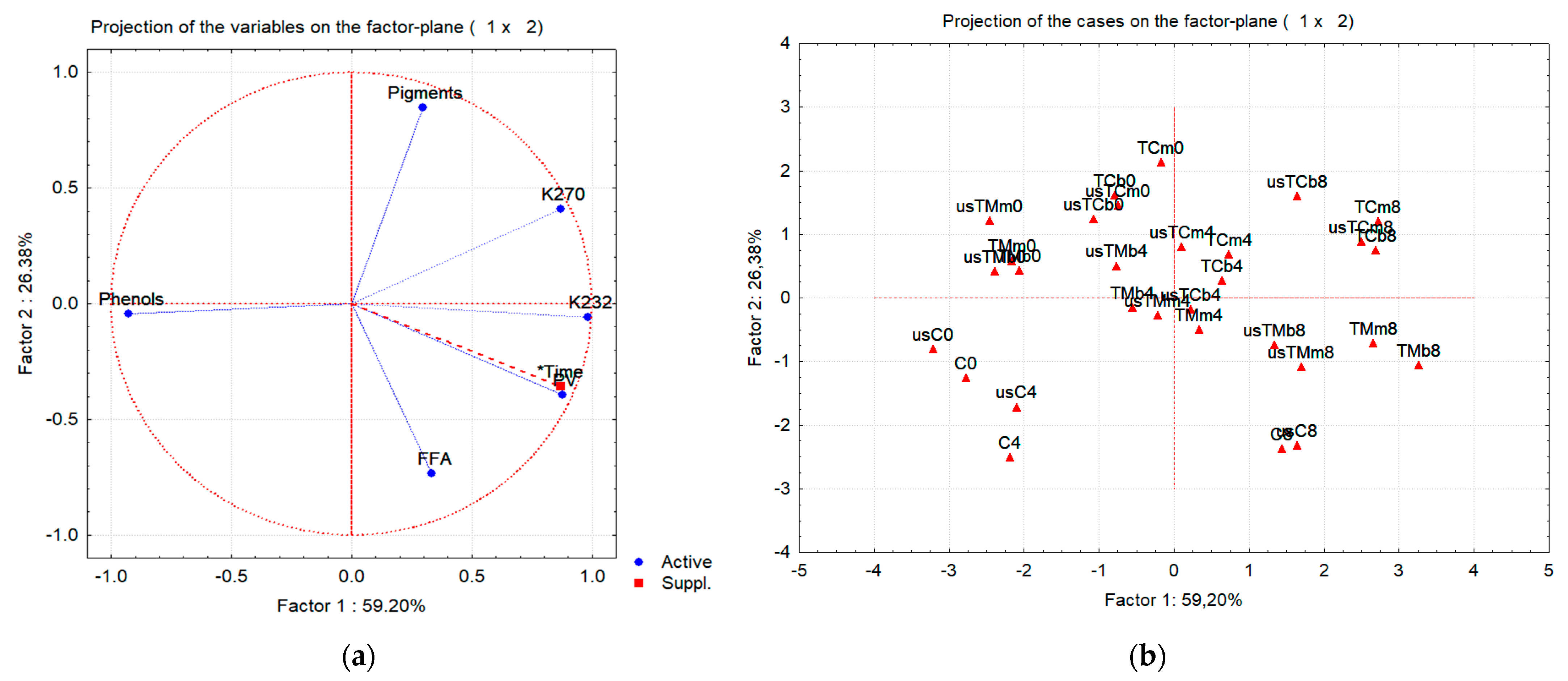
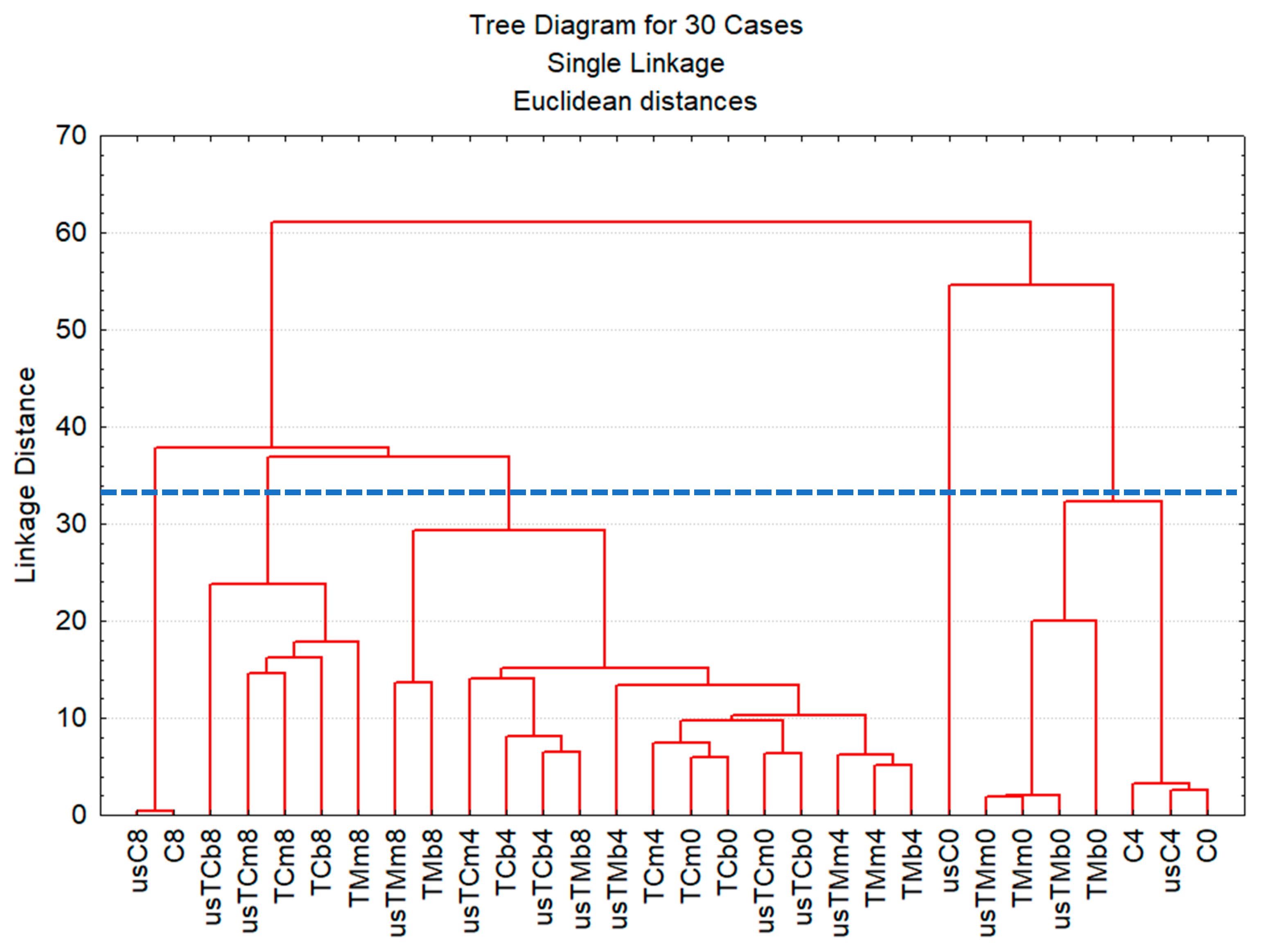
2.3.3. Multivariate Data Analysis of Flavored Oil Preparations after Storage
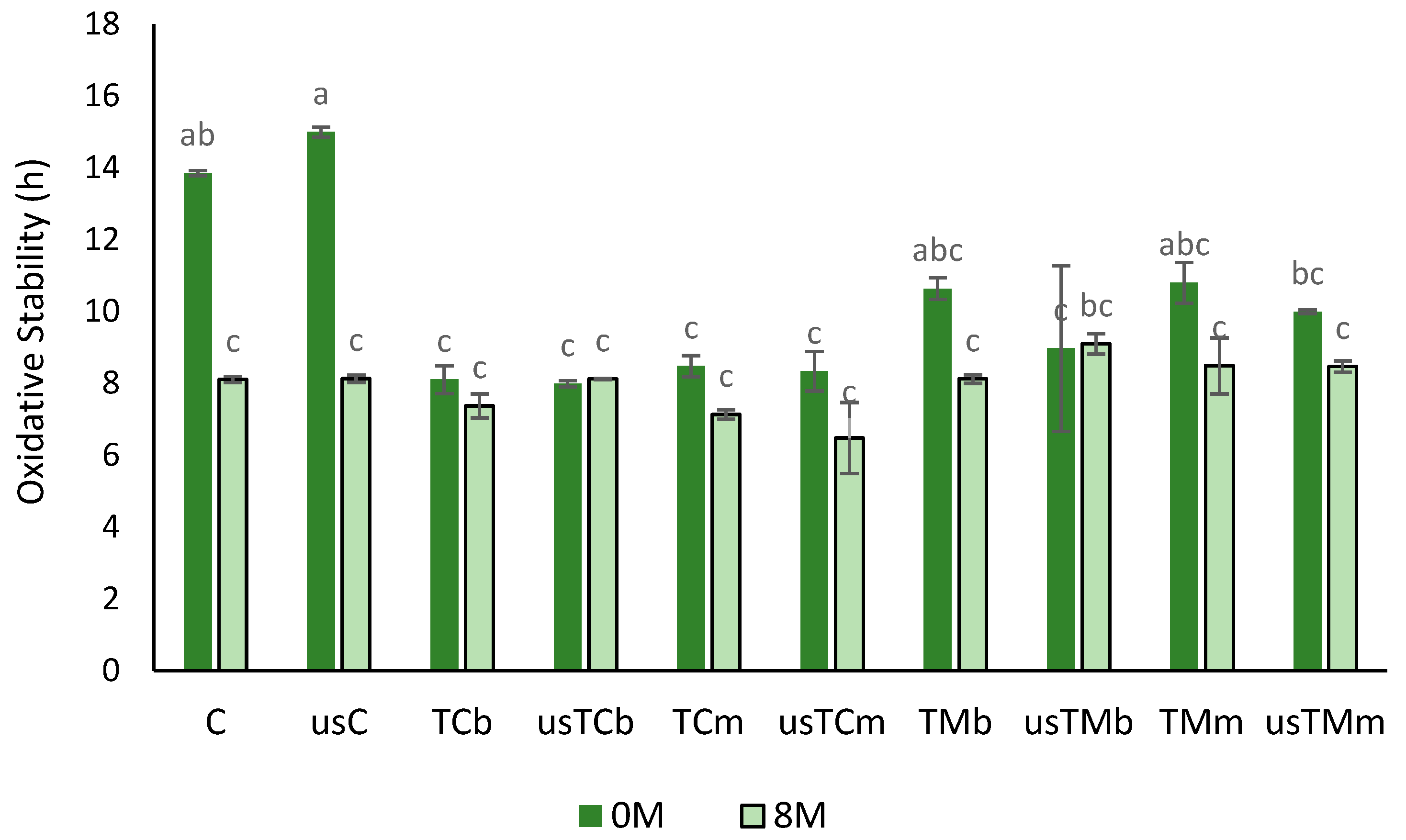
2.3.4. Oxidative Stability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Coextraction and US Assisted Trials
4.2.2. Characterization of Olive Oils
4.2.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IOC. 2023. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/12/HO-W901-13-12-2022-P.pdf (accessed on 2 January 2023).
- Caporaso, N.; Paduano, A.; Nicoletti, G.; Sacchi, R. Capsaicinoids, antioxidant activity, and volatile compounds in olive oil flavored with dried chili pepper (Capsicum annuum). Eur. J. Lipid Sci. Technol. 2013, 115, 1434–1442. [Google Scholar] [CrossRef]
- Benkhoud, H.; M’Rabet, Y.; Gara Ali, M.; Mezni, M.; Hosni, K. Essential oils as flavoring and preservative agents: Impact on volatile profile, sensory attributes, and the oxidative stability of flavored extra virgin olive oil. J. Food Process. Preserv. 2022, 46, e15379. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.A.; Zaghdoudi, K.l.; Pereira, J.A.; Peres, A.M. Kinetic-thermodynamic study of the oxidative stability of Arbequina olive oils flavored with lemon verbena essential oil. LWT 2021, 140, 110711. [Google Scholar] [CrossRef]
- Benmoussa, H.; Elfalleh, W.; Farhat, A.; Servili, M.; Algabr, M.; Romdhane, M. Impact of Microwave Assisted Infusion on the Quality and Volatile Composition of Olive Oil Aromatized with Rosemary Leaves. J. Am. Oil Chem. Soc. 2016, 93, 921–928. [Google Scholar] [CrossRef]
- Peres, F.; Roldão, M.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Co-Processed Olive Oils with Thymus mastichina L.—New Product Optimization. Life 2021, 11, 1048. [Google Scholar]
- Cecchi, L.; Balli, D.; Urciuoli, S.; Urciuolo, A.; Bordiga, M.; Travaglia, F.; Zanoni, B.; Mulinacci, N. Co-milling of sound olives with fresh chili peppers improves the volatile compound, capsaicinoid and sensory profiles of flavoured olive oil with respect to the typical infusion. Food Chem. 2023, 404, 134696. [Google Scholar] [CrossRef]
- Caponio, F.; Durante, V.; Varva, G.; Silletti, R.; Previtali, M.A.; Viggiani, I.; Squeo, G.; Summo, C.; Pasqualone, A.; Gomes, T.; et al. Effect of infusion of spices into the oil vs. combined malaxation of olive paste and spices on quality of naturally flavoured virgin olive oils. Food Chem. 2016, 202, 221–228. [Google Scholar] [CrossRef]
- AFIDOL. 2018. Available online: https://afidol.org/wp-content/uploads/2018/06/InfolexOlea_2018-01_Huiles_aromatisees.pdf (accessed on 2 January 2023).
- Andreou, V.; Kourmbeti, E.; Dimopoulos, G.; Psarianos, M.; Katsaros, G.; Taoukis, P. Optimization of Virgin Olive Oil Yield and Quality Applying Nonthermal Processing. Food Bioprocess Technol. 2022, 15, 891–903. [Google Scholar] [CrossRef]
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, A.C. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioprocess Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez de Marañón, I. Olive oil pilot-production assisted by pulsed electric field: Impact on extraction yield, chemical parameters and sensory properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Bejaoui, M.A.; Beltrán, G.; Sánchez-Ortiz, A.; Sánchez, S.; Jiménez, A. Continuous high power ultrasound treatment before malaxation, a laboratory scale approach: Effect on virgin olive oil quality criteria and yield. Eur. J. Lipid Sci. Technol. 2016, 118, 332–336. [Google Scholar] [CrossRef]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Beltrán, G.; Uceda, M. High-power ultrasound in olive paste pretreatment. Effect on process yield and virgin olive oil characteristics. Ultrason. Sonochem. 2007, 14, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Durante, V.; La Notte, D.; Punzi, R.; Gambacorta, G. Ultrasound-assisted extraction of virgin olive oil to improve the process efficiency. Eur. J. Lipid Sci. Technol. 2013, 115, 1062–1069. [Google Scholar] [CrossRef]
- Moustakime, Y.; Hazzoumi, Z.; Amrani Joutei, K. Aromatization of virgin olive oil by seeds of Pimpinella anisum using three different methods: Physico-chemical change and thermal stability of flavored oils. Grain Oil Sci. Technol. 2021, 4, 108–124. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B.; Gozzi, M.; Meireles, M.Á.A.; Wang, S.; Guamis, B.; Pan, Z.; Ramaswamy, H.; Sastry, S.; et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2022, 62, 5925–5949. [Google Scholar] [CrossRef]
- Manganiello, R.; Pagano, M.; Nucciarelli, D.; Ciccoritti, R.; Tomasone, R.; Di Serio, M.G.; Giansante, L.; Del Re, P.; Servili, M.; Veneziani, G. Effects of Ultrasound Technology on the Qualitative Properties of Italian Extra Virgin Olive Oil. Foods 2021, 10, 2884. [Google Scholar] [CrossRef]
- Assami, K.; Chemat, S.; Meklati, B.Y.; Chemat, F. Ultrasound-Assisted Aromatisation with Condiments as an Enabling Technique for Olive Oil Flavouring and Shelf Life Enhancement. Food Anal. Methods 2016, 9, 982–990. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Crupi, P.; Durante, V.; Pesce, V.; Maiellaro, I.; Lovece, A.; Mercurio, A.; Laghezza, A.; Corbo, F.; et al. Comparison between Different Flavored Olive Oil Production Techniques: Healthy Value and Process Efficiency. Plant Foods Hum. Nutr. 2016, 71, 81–87. [Google Scholar] [CrossRef]
- Baiano, A.; Previtali, M.A.; Viggiani, I.; Varva, G.; Squeo, G.; Paradiso, V.M.; Summo, C.; Gomes, T.; Caponio, F. As oil blending affects physical, chemical, and sensory characteristics of flavoured olive oils. Eur. Food Res. Technol. 2016, 242, 1693–1708. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Gambacorta, G.; Notte, E.L. Changes in Quality Indices, Phenolic Content and Antioxidant Activity of Flavored Olive Oils during Storage. J. Am. Oil Chem. Soc. 2009, 86, 1083. [Google Scholar] [CrossRef]
- Sacchi, R.; Della Medaglia, D.; Paduano, A.; Caporaso, N.; Genovese, A. Characterisation of lemon-flavoured olive oils. LWT-Food Sci. Technol. 2017, 79, 326–332. [Google Scholar] [CrossRef]
- Cert, A.; Alba, J.; Leon Camacho, M.; Moreda, W.; Perez-Camino, M.C. Effects of talc addition and operating mode on the quality and oxidative stability of virgin olive oils obtained by centrifugation. J. Agric. Food Chem. 1996, 44, 3930–3934. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of Extra Virgin Olive Oil: Adulteration, Oxidative Stability, and Antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. From famine plants to tasty and fragrant spices: Three Lamiaceae of general dietary relevance in traditional cuisine of Trás-os-Montes (Portugal). LWT-Food Sci. Technol. 2011, 44, 543–548. [Google Scholar] [CrossRef][Green Version]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality. A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Pinho, T.; Cruz, R.; Baptista, P.; Martín, H.; Asensio-S.-Manzanera, M.C.; Peres, A.M.; Pereira, J.A. Olive oil characteristics of eleven cultivars produced in a high-density grove in Valladolid province (Spain). Eur. Food Res. Technol. 2021, 247, 3113–3122. [Google Scholar] [CrossRef]
- Peres, F.; Gouveia, C.; Salgueiro, C.; Anjos, O.; Lourenço, C. Caracterização Química e Nutricional de Azeites de Montanha. In Desenvolvimento e Inovação de um Produto Endógeno. O caso dos Azeites de Montanha; Peres, F., Paiva, T., Raposo, D., Eds.; IPCB: Castelo Branco, Portugal, 2019; pp. 56–67. [Google Scholar]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL–Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), Anti–Inflammatory Properties (ID 1882), Contributes to the Upper Respiratory Tract Health (ID 3468), Can Help to Maintain a Normal Function of Gastrointestinal Tract (3779), and Contributes to Body Defences against External Agents (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006; European Food Safety Authority (EFSA): Parma, Italy, 2011.
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Chemical Characterization and Bioactivity of Extracts from Thymus mastichina: A Thymus with a Distinct Salvianolic Acid Composition. Antioxidants 2020, 9, 34. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Sponza, S.; Asensio-S-Manzanera, M.C.; Novak, J. Contribution of the main polyphenols of Thymus mastichina subsp. mastichina to its antioxidant properties. Ind. Crops Prod. 2015, 66, 291–298. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Laboratory-scale optimization of olive oil extraction: Simultaneous addition of enzymes and microtalc improves the yield. Eur. J. Lipid Sci. Technol. 2014, 116, 1054–1062. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of Olive Oil Antioxidants between Oil and Water Phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L. Industrial Ultrasound Applications in the Extra-Virgin Olive Oil Extraction Process: History, Approaches, and Key Questions. Foods 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Advances and innovations associated with the use of acoustic energy in food processing: An updated review. Innov. Food Sci. Emerg. Technol. 2021, 74, 102863. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical Strategies to Increase Nutritional and Sensory Quality of Virgin Olive Oil by Modulating the Endogenous Enzyme Activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef]
- Peres, F.; Jeleń, H.H.; Majcher, M.M.; Arraias, M.; Martins, L.L.; Ferreira-Dias, S. Characterization of aroma compounds in Portuguese extra virgin olive oils from Galega Vulgar and Cobrançosa cultivars using GC–O and GCxGC–ToFMS. Food Res. Int. 2013, 54, 1979–1986. [Google Scholar] [CrossRef]
- Pokorny, J.; Kalinová, L.; Dysseler, P. Determination of Chlorophyll pigments in Crude Vegetable Oils. Pure Appl. Chem. 1995, 67, 1781–1787. [Google Scholar]
- IOC. Determination of Biophenols in Olive Oils by HPLC; COI/T20/DOC.29; IOC: Madrid, Spain, 2009. [Google Scholar]
- Pirisi, F.M.; Cabras, P.; Cao, C.F.; Migliorini, M.; Muggelli, M. Phenolic Compounds in Virgin Olive Oil. 2. Reappraisal of the Extraction, HPLC Separation, and Quantification Procedures. J. Agric. Food Chem. 2000, 48, 1191–1196. [Google Scholar] [CrossRef]
- Alvin, C.R. Methods of Multivariate Analysis; John Wiley & Sons Inc.: New York, NY, USA, 2002. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry; Horwood: Chichester, UK, 1993. [Google Scholar]


| Quality Criteria | Olive Oils | |
|---|---|---|
| C | usC | |
| Acidity (% oleic acid) | 0.17 ± 0.00 | 0.16 ± 0.01 |
| Peroxide value (meq O2 kg−1) | 5.35 ± 0.39 | 5.39 ± 0.28 |
| K270 | 0.14 ± 0.01 | 0.15 ± 0.01 |
| K232 | 2.01 ± 0.00 | 1.97 ± 0.00 |
| Median of defect | 0 | 0 |
| Olive ripe fruity | 1.95 ± 0.21 | 2.2 ± 0.14 |
| Bitter | 1.50 ± 0.71 | 2.0 ± 0.71 |
| Pungent | 3.25 ± 0.35 | 3.75 ± 0.36 |
| Quality Criteria | TCb | usTCb | TCm | usTCm | TMb | usTMb | TMm | usTMm |
|---|---|---|---|---|---|---|---|---|
| Acidity (% oleic acid) | 0.16 | 0.16 | 0.14 | 0.15 | 0.17 | 0.17 | 0.16 | 0.14 |
| Peroxide value (meq O2 kg−1) | 4.68 | 4.75 | 6.17 | 5.54 | 4.97 | 4.47 | 4.79 | 4.97 |
| K270 | 0.18 | 0.17 | 0.20 | 0.19 | 0.16 | 0.14 | 0.16 | 0.16 |
| K232 | 2.14 | 2.12 | 2.16 | 2.14 | 2.04 | 2.03 | 2.03 | 1.98 |
| Median of defect | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thyme flavor | 3.5 | 1.5 | 3.0 | 2.5 | 4.7 | 3.75 | 4.9 | 4.45 |
| Bitter | 1.0 | 0.85 | 1.25 | 1.5 | 0.7 | 1.05 | 1.6 | 1.5 |
| Pungent | 3.25 | 2.25 | 1.75 | 2.65 | 3.6 | 2.6 | 4.0 | 1.2 |
| Fatty Acids | C | TCb | usTCb | TCm | usTCm | TMb | usTMb | TMm | usTMm |
|---|---|---|---|---|---|---|---|---|---|
| Oleic acid (C18:1) | 68.3 | 68.2 | 68.3 | 68.2 | 68.4 | 69.3 | 69.1 | 69.3 | 69.3 |
| Linoleic acid (C18:2) | 15.9 | 15.9 | 15.9 | 15.8 | 15.8 | 15.1 | 15.0 | 15.1 | 14.9 |
| Palmitic acid (C16:0) | 9.7 | 9.5 | 9.5 | 9.6 | 9.6 | 10.1 | 10.1 | 10.0 | 10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, F.; Marques, M.P.; Mourato, M.; Martins, L.L.; Ferreira-Dias, S. Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules 2023, 28, 6898. https://doi.org/10.3390/molecules28196898
Peres F, Marques MP, Mourato M, Martins LL, Ferreira-Dias S. Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules. 2023; 28(19):6898. https://doi.org/10.3390/molecules28196898
Chicago/Turabian StylePeres, Fátima, Madalena Pinho Marques, Miguel Mourato, Luisa L. Martins, and Suzana Ferreira-Dias. 2023. "Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils" Molecules 28, no. 19: 6898. https://doi.org/10.3390/molecules28196898
APA StylePeres, F., Marques, M. P., Mourato, M., Martins, L. L., & Ferreira-Dias, S. (2023). Ultrasound Assisted Coextraction of Cornicabra Olives and Thyme to Obtain Flavored Olive Oils. Molecules, 28(19), 6898. https://doi.org/10.3390/molecules28196898











