Abstract
The response performances of the crystalline organic fluorescence probe are highly dependent on the long-range ordered arrangement of crystalline structure. Herein, a novel organic crystalline fluorescent probe with a high quantum yield was established through the rapid self-assembly of 1,2,4,5-Tetrakis (4-carboxyphenyl) benzene (H4TCPB) and DMF molecules. Each H4TCPB, which connects to four DMF molecules through hydrogen bonds, acts as the structural unit. The building units are packed by π–π, lone pair···π, and lone pair···lone pair interactions to form solid-state crystalline materials. H4TCPB·4DMF exhibits distinct blue fluorescent under UV light, while the quantum yield is as high as 89.02% and the fluorescence lifetime is 1.95 ns. The H4TCPB·4DMF nanocrystal exhibits a specific fluorescence quench sensibility to tetracycline (TC), compared with the common chemicals and ions in environmental water. Moreover, the test results can be obtained quickly and are easily visible to the naked eye. The limit of detection for TC is as low as 12 nM in an aqueous solution. Spectral analysis and density functional theory (DFT) theoretical calculations were used to explain the fluorescence quenching mechanism of H4TCPB·4DMF toward TC, which could be attributed to the photoinduced electron transfer occurring from H4TCPB·4DMF to TC. Our work enriches the database of crystalline luminescent materials and provides theoretical support for the design and mechanical studies of organic fluorescent probes.
1. Introduction
Fluorescent materials have attracted significant attention owing to their great potential in optoelectronic science, fluorescence sensing, bioimaging, and environmental monitoring [1,2,3,4]. Fluorescence detection is widely used to detect environmental pollutants due to its high sensitivity, rapid detection, easy operation, and low cost [5,6]. Photoinduced electron transfer (PET) plays a significant role in fluorescence detection and many other photochemical scenarios. Particularly, PET, which is the process of electron transfer through photoexcitation, is considered to be responsible for fluorescence quenching in the field of fluorescence detection. Chemists have made great efforts to design, synthesize, and develop many luminescent materials for practical application and have made great progress. For instance, the concept of aggregation-induced emission (AIE) materials proposed by Tang and coworkers [7,8], in which molecular aggregates exhibit stronger emission than single molecules. Many new sensors have been developed based on the photoluminescence properties of organic functional molecules. They are usually manufactured from organic or inorganic photoluminescent materials. Compared to inorganic photoluminescent materials, which are often composed of toxic heavy metals or rare earth elements, nontoxic organic photoluminescent fluorescent probes have a more comprehensive range of application scenarios [9,10]. Organic fluorescence probes include crystalline organic fluorescence probes and amorphous organic fluorescence probes. They have unique physical–chemical properties due to the diversity of organic ligands and rich functional groups. The long-range ordered arrangement of crystalline organic fluorescent probes restricts intramolecular rotation and enhances the emission [11], facilitating specific and selective identification and the modification of their fluorescence properties when interacting with captured analytes. Solid-state crystal luminescent materials can generate different responses via different stimuli, including light [12], vapor [13], pH [14], and temperature [15], which do not change the chemical structure of the material. The detected substance may interact with the probe physically/chemically, resulting in a change in its luminescence state, while the change in fluorescence intensity (a decrease or increase) is one of the most easily observed phenomena in most sensing studies [16,17].
Tetracycline (TC) is a broad-spectrum antibiotic that is used to treat bacterial infections because of its low price and powerful function [18,19,20]. At present, TC is widely used in animal husbandry and aquaculture, resulting in a high level of residual TC in soil and water environments [21,22]. Moreover, accumulated TC enters the human body through the food chain and even endangers health in severe cases [23]. Therefore, it is necessary to detect and analyze the trace tetracycline in the sample quickly and selectively. Traditionally, such tests have been performed using large or expensive techniques such as mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), and surface-enhanced Raman scattering (SERS) [24,25,26,27]. These kinds of methods are complicated and expensive, and their results are not immediately available. Hence, it is of great significance to develop a method with low cost, simple operation, low detection limit, noticeable, and rapid results.
In this work, we prepared a nanocrystalline organic fluorescent probe using an organic molecule, 1,2,4,5-Tetrakis (4-carboxyphenyl) benzene (H4TCPB), based on a solvent temperature-controlled rapidly crystallized method for detecting TC in water via a photoinduced electron transfer (PET) fluorescence quenching mechanism. H4TCPB has five benzene rings, one at the center and four on the side, resulting from the benzene ring and the relatively fixed X-shaped space stretch; it is partially flexible as the angle between the central benzene ring and the side benzene rings are rotatable before crystallization (Figure 1). H4TCPB and DMF were cocrystallized in a solvent to form molecular crystals of H4TCPB·4DMF. The bulk crystal was synthesized to investigate the microstructure of the molecular crystals, and the nanosized crystals were prepared using a rapid method to develop the nanofluorescence probe. Hydrogen bonding, π–π, lone pair···π, and lone pair···lone pair interactions were confirmed in the H4TCPB·4DMF crystal structure. H4TCPB·4DMF presents a significant blue fluorescence under UV light and shows a significant quenching effect by TC compared with other irons and chemicals. Hence, a rapid fluorescence detection method for TC was developed and the detection performances, including selectivity and sensitivity, were evaluated. Moreover, the sensing mechanism was investigated by combining spectral analysis and density functional theory (DFT) simulation.
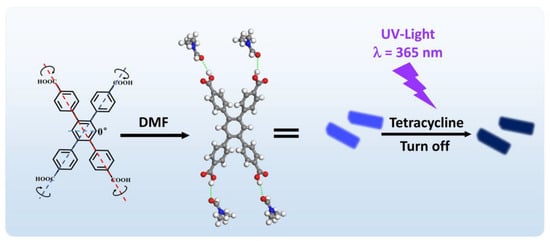
Figure 1.
Schematic diagrams of the structure of H4TCPB·4DMF and the fluorescence quenching response toward tetracycline (TC).
2. Results and Discussion
2.1. Crystalline Structure of H4TCPB·4DMF
The single-crystal X-ray diffraction data of H4TCPB·4DMF have been mentioned in our previous work (HOF-TCPB-298) [28]. Here, they are described in more detail. The structure of the H4TCPB·4DMF molecule consists of five benzene rings and four carboxyl groups. One benzene ring is in the center, and four attached carboxyl phenyl groups can theoretically serve as the hydrogen bond donors. Also, in the crystal structure of H4TCPB·4DMF, each H4TCPB connected with four DMF molecules by hydrogen bonding (Figure 2a) which is considered to be a structure unit. The hydrogen-bonding distances between the two O atoms (dO−H···O) are 2.616 Å and 2.597 Å, and the corresponding angles (θO−H···O) are 156.314° and 171.708°, which confirms the substantial hydrogen bonds. Such structure units are packed through π–π interaction and hydrogen bonds. The stacking view along the (100) direction is shown in Figure 2b, where some interactions are marked. As shown in Figure 2c,d, the distance between the rings in locations where π–π stacking interactions occured was calculated. The plane distance between phenyl rings at the center of each H4TCPB molecule is 5.380 Å, and the center distance of the phenyl ring is 6.255 Å. Moreover, the diagonally positioned phenyl rings of H4TCPB are located in the same plane, and the phenyl rings are in the same position as adjacent H4TCPB linkers. The distance between the center of the corresponding phenyl rings is 4.409 Å, while the distance between planes is only 3.487 Å. Figure 2e presents the lone pair···π contact between the nitrogen atom of the DMF molecule, which was linked to H4TCPB through hydrogen bonds, and the benzene ring. The distance between the nitrogen atom and the plane of the benzene ring is 3.528 Å. Moreover, there are lone pair···lone pair contacts between the nitrogen atoms in adjacent DMF molecules and the distance between them is 3.746 Å (Figure 2f). Based on these interactions, the building units of H4TCPB·4DMF stack and grow to the long-range ordered crystal materials.
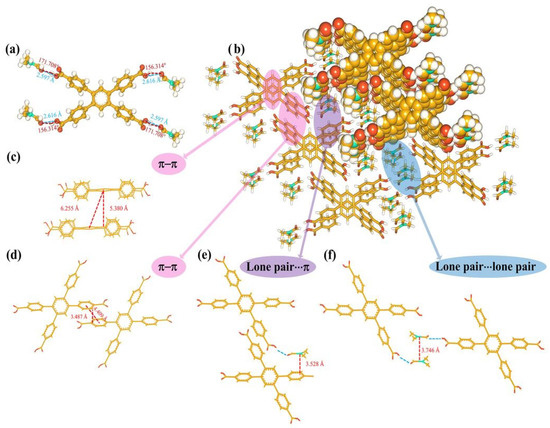
Figure 2.
Crystal structure of H4TCPB·4DMF: (a) bond lengths and bond angles of hydrogen bonds between H4TCPB and DMF molecules; (b) stacking view of H4TCPB·4DMF along the (100) direction; (c) the π–π interaction of center benzene rings; (d) the π–π interaction of benzene rings on the side; (e) schematic representing the modes of lone pair···π contacts; (f) schematic representing the modes of lone pair···lone pair.
2.2. Characterization of H4TCPB·4DMF
The experimental PXRD patterns of both the bulk crystal and nanocrystal of H4TCPB·4DMF are consistent with the simulated ones, indicating that the crystalline phase is pure. Moreover, the broadening of the PXRD peaks of the nanocrystal could be attributed to the small size effect (Figure 3a). The FT−IR spectra of H4TCPB and H4TCPB·4DMF were measured, and the results are displayed in Figure 3b. The peaks at 1650, 1178, 1103, and 1007 cm−1 are significantly enhanced in the FT−IR spectra of H4TCPB·4DMF compared to H4TCPB, which can be attributed to the C=O and C–N bonds of DMF. The regular arrangement of the highly crystallized H4TCPB·4DMF results in a significant enhancement of the broadband representing O–H belonging to the carboxyl located at 2700−3300 cm−1. The SEM images for both the bulk crystal and nanocrystal of H4TCPB·4DMF present rectangular sheets (Figure 3c,d). The bulk single crystal exhibits micron-size flake morphology, while the plan view size of the nanocrystalline is about 200 nm × 800 nm. The TGA of H4TCPB·4DMF was carried out in an argon atmosphere. As shown in Figure S1, H4TCPB·4DMF does not lose weight below 100 °C; with further heating, the hydrogen bonding between DMF molecules and H4TCPB will be gradually destroyed, and a significant weight loss can be observed owing to the departure of DMF; when the temperature reaches above 400 °C, H4TCPB begins to decompose.
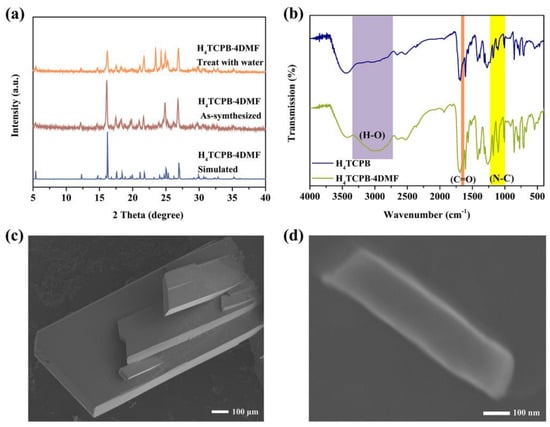
Figure 3.
Characterizations of H4TCPB·4DMF: (a) FT−IR spectra; (b) PXRD patterns; (c) SEM image for the bulk crystals; (d) SEM image for the nanocrystal.
2.3. Fluorescence Properties of H4TCPB·4DMF
The fluorescence properties of H4TCPB·4DMF were investigated at room temperature. As shown in the optical photos of the bulk crystals and the nanocrystal sample (Figure 4a), the changes in the color of H4TCPB·4DMF under different lights are instantly visible to the naked eye. It is colorless in daylight and blue-purple in UV light (λ = 365 nm). This indicates that H4TCPB·4DMF can undergo fluorescence excitation with UV light, which should be attributed to the fact that the long range ordered arrangement in the crystal structure limits the rotation and vibration of the benzene rings, thus reducing nonradiative transition and providing better quantum yield [29]. This was further investigated using fluorescence spectral analysis. As shown in Figure 4b, the solid-state sample of H4TCPB·4DMF shows a width excitation band from 250 nm to 375 nm with a peak at 350 nm, and the emission spectra with the excitation wavelength of 350 nm peak at 400 nm. The fluorescence emission lifetime of the solid sample is τ = 1.9448 ns (Figure 4c), which exhibits the fluorescence behavior of H4TCPB·4DMF. The quantum yield is as high as 89.02% (Figure S2). Furthermore, the ultraviolet absorption, fluorescence excitation, and emission spectral analysis of the H4TCPB·4DMF aqueous suspension were performed (Figure 4d). The wavelength coverage of fluorescence excitation is consistent with that of ultraviolet absorption. The peaks of the fluorescence excitation and emission spectra are 318 nm and 400 nm, respectively.
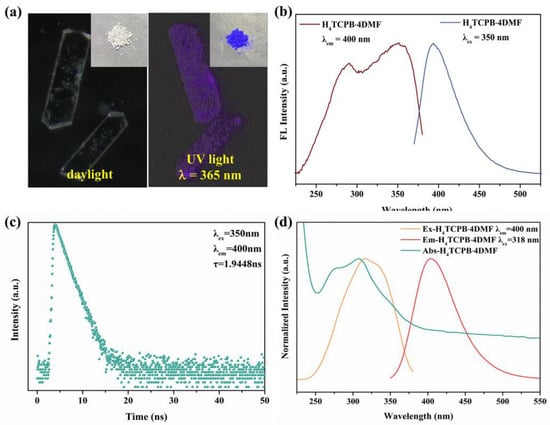
Figure 4.
Fluorescence characteristics of H4TCPB·4DMF: (a) optical photographs of bulk crystal and nanocrystal samples (inset) under daylight and UV light of 365 nm; (b) solid fluorescence spectrum of nanocrystalline H4TCPB·4DMF; (c) decay curve of nanocrystalline H4TCPB·4DMF; (d) fluorescence and absorbance spectra of nanocrystalline H4TCPB·4DMF in aqueous suspension.
2.4. Sensing of TC in Water
The flexible torsion between the benzene rings; a mass of hydrogen bonds; and π–π, lone pair···π, and lone pair···lone pair interactions, as well as the highly ordered crystalline state and its own photochemical properties, encourage us to develop H4TCPB·4DMF nanocrystals as fluorescence probes [16]. At the same concentration, when comparing different solvents, it can be seen that the fluorescence intensity of H4TCPB·4DMF in water is the strongest, and luminescence quenching can be observed by the naked eye under a laboratory 365 nm UV lamp, resulting in the most apparent quenching effect (Figure S3). The photoluminescence intensity of the suspension has a direct association with both aqueous solution and polar organic solvents [30]. The largest PL intensity in H2O can be attributed to the highest polarity. Moreover, using water as the dispersing agent of the probe without introducing other organic solvents is more environmentally friendly. Hence, we chose water as the dispersing solution of the probe.
The fluorescence behavior of the H4TCPB·4DMF nanofluorescence probe was investigated with the presence of some common chemicals and metal ions in TC environments, such as TC, penicillin sodium (PNC), salicylic acid (BHA), norfloxacin (NFX), diuron, disulfiram (TETD), Fe3+, Co2+, Ca2+, Na+, Ti4+, Zn2+, and K+. As shown in Figure 5a, the fluorescence intensity of H4TCPB·4DMF is quenched if TC is contained in the water. However, there is no significant change in fluorescence intensity and wavelength when adding other chemicals and metal ions into the aqueous solution. The luminescent quenching effect can be quantitatively rationalized using the Stern–Volmer equation:
where Ksv is the quenching constant, [Q] is the concentration of the analyte (100 mg/L), and I0 and I are the luminescence intensities in the absence and presence of the analyte [31]. The Ksv value is determined as 64,413 M−1, which is two to four orders of magnitude higher than those of the remaining ions and chemicals (Table 1). The large Ksv value reveals the strong quenching effect of TC on the luminescence of H4TCPB·4DMF, as well as the high sensitivity of H4TCPB·4DMF for TC detection. When the initial concentration of TC is 100 mg/L, the fluorescence intensity of aqueous suspensions of H4TCPB·4DMF is quenched in an instant and remains stable after that (Figure 5b), indicating a rapid response time. Subsequently, the functional relation between the fluorescence intensity of H4TCPB·4DMF and TC concentration was studied. According to the fluorescence spectra and the optical photographs in Figure 5c, the fluorescence intensity of H4TCPB·4DMF gradually decreases as the concentration of TC increases. In addition, the natural logarithm of fluorescence intensity (Ln(I/I0)) of H4TCPB·4DMF) is linearly dependent on the concentration of TC in the concentration range of 0 to 0.4 mg/L and 0.4 to 100 mg/L with a slope value (S) of 0.428 and 0.0274, respectively (Figure 5d). The linear fitting correlation coefficient (R2) reached 0.9916 in the high concentration range, indicating that the fluorescence probe has a broad detection range. Such a good linear relationship, with the linear correlation reaching 0.9973, allows for the accurate quantification of TC over a range from 0 to 0.4 mg/L. The limit of detection (LOD) of H4TCPB·4DMF toward TC was calculated to be 12 nM based on 3σ/S (Figure S4), where σ is the standard deviation of the blank solution [21]. The detection limits of tetracycline with similar types of fluorescent probes are listed and compared in Table 2.
I0/I =1+Ksv × [Q]
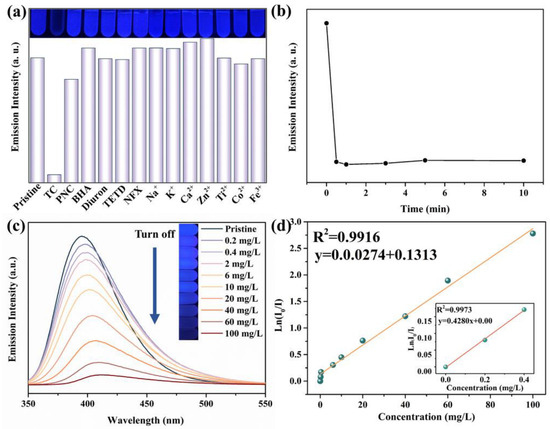
Figure 5.
Fluorescence response performances of the H4TCPB·4DMF aqueous suspension for TC: (a) changes in the emission intensity determined by fluorescence spectra upon the addition of different substances (100 mg/L); the inset is the optical photographs under UV light, λ = 356 nm; (b) changes in emission intensity with time when TC is added, C0 = 100 mg/L; (c) fluorescence spectra with different TC concentrations; the inset is the color change observed in the optical photographs under UV light, λ = 356 nm; (d) linear relationship between Ln(I0/I) and TC concentration in the range of 0.4–100 mg/L; the inset is the linear relationship between Ln(I0/I) and TC concentration in the range of 0–0.4 mg/L.

Table 1.
Quenching constants (Ksv) of various species.

Table 2.
A comparison of the limit of detection (LOD) of various fluorescence probes for TC detection in water at room temperature (298 K).
2.5. Sensing Mechanism Exploration
The mechanism of H4TCPB·4DMF sensing TC in aqueous solution was explored. First, the fluorescence excitation spectrum of H4TCPB·4DMF, as well as the UV–vis absorption spectra of H4TCPB·4DMF and TC, are compared in Figure 6a, and it can be seen that they partly overlap in the wavelength range of 250–400 nm. Moreover, the fluorescence spectrum of H4TCPB·4DMF and UV–vis absorption spectrum of TC are shown in Figure S5. It can be seen that there is little overlap between the fluorescence emission of H4TCPB·4DMF and the ultraviolet absorption of TC. This rules out the possibility that the fluorescence emission is absorbed, which results in fluorescence quenching. Therefore, the luminescence quenching of H4TCPB·4DMF toward TC in the aqueous solution might be attributed to PET and the competition of energy absorption [39]. PET is a process of exciting electron transfer from the photoexcited donor (H4TCPB·4DMF) to the lowest unoccupied molecular orbital (LUMO) of the acceptor (TC) [40]. Based on the confirmed crystal structure of H4TCPB·4DMF, the DFT calculation was implemented to further prove the PET mechanism. As the diagram in Figure 6b reveals, the HOMO energy levels of H4TCPB·4DMF and TC are −0.6545 eV and −6.1981 eV, respectively, while the LUMO energy level of H4TCPB·4DMF is 2.1802 eV, which is higher than that of TC (−2.2859 eV). In addition, the functional groups on TC have a strong electron-withdrawing capacity under the combined effect of conjugation and induction [41]. As a result, the luminescence quenching of H4TCPB·4DMF in the solution toward TC might be due to the PET process from H4TCPB·4DMF to TC. Moreover, the binding of DMF molecules enhances the van der Waals forces around H4TCPB, and enhances the photostability and photochemical activities during the process quenching C [42].
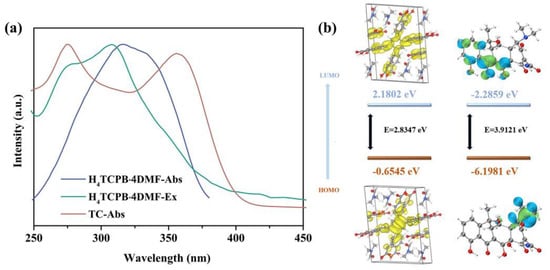
Figure 6.
(a) UV–vis absorption of H4TCPB·4DMF and TC and fluorescence excitation spectrum of H4TCPB·4DMF in aqueous solution; (b) calculated HOMO and LUMO energies for H4TCPB·4DMF and TC.
3. Materials and Methods
3.1. Materials
1,2,4,5-Tetrakis (4-carboxyphenyl) benzene (H4TCPB, 98%) was supplied by Jilin Chinese Academy of Science-Yanshen Technology Co., Ltd (Changchun, China). Hydrochloric acid (HCl, 36-38%) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai China) N, N-dimethylformamide (DMF, 99.5%), ethanol (99.7%), methanol (99.5%), acetone (99.5%), tetrahydrofuran (99.5%) nitric acid (HNO3, 65%), NaNO3 (99%), KNO3 (99%), Ca(NO3)2 (99%), Ti(NO3)4 (99%), Zn(NO3)2 (99%), Co(NO3)2, (99%), and Fe(NO3)3 (99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai China) A wide range of antibiotics and chemicals, i.e., tetracycline, penicillin sodium, salicylic acid, diuron, disulfiram, and norfloxacin, were purchased from Shanghai Maclin Biochemical Technology Co., Ltd. (Shanghai China).
3.2. Analytical Conditions
Powder X-ray diffraction (PXRD) data were collected on an UltimaIV diffractometer from Japan Tokyo. The single-crystal X-ray diffraction (SC-XRD) data collection was carried out on a Bruker AXS D8-VENTURE diffractometer (Mo Kα radiation, λ = 0.71073 Å) (Karlsruhe, Germany). Morphologies were observed using a scanning electron microscope (SEM, JSM-7610F, Tokyo, Japan). Thermogravimetric analysis (TGA) was performed using STA449F5 (Netzsch, Selb Germany). An F-4700 fluorescence spectrophotometer (HITACHI, Tokyo, Japan) was used for the collection of luminescence and excitation spectra. Decay curves and PL quantum yields were collected on an FLS 1000 spectrofluorometer (Edinburgh Instruments, Edinburgh, UK). The ultraviolet-visible (UV–vis) absorption spectra were collected on a UV 2600 (Tianmei, Shanghai, China) spectrophotometer.
3.3. Preparation of H4TCPB·4DMF
Synthesis of Bulk Crystals: Briefly, 10 mg of H4TCPB was dissolved in DMF (1 mL) within a glass vial. Then, the uncapped vial was placed inside a 100 mL beaker containing 10 mL of deionized water. Lastly, the beaker was tightly sealed and allowed to stand at room temperature. After seven days, the colorless sheet crystals of H4TCPB·4DMF could be obtained.
Synthesis of Nanocrystals: First, 100 mg of H4TCPB was dissolved in 5 mL of DMF. The filtered clear solution was heated at 60 °C for 30 min. Then, 5 mL of H2O (room temperature) was added to the above DMF solution dropwise (60 °C). The nanocrystals were separated immediately. The solid products were obtained via centrifugation at 10,000 rpm for 5 min and subsequently purified twice with ethanol.
3.4. Fluorescence Measurements
The solid excitation spectra, emission spectra, fluorescence lifetime, and quantum yield of H4TCPB·4DMF were collected using Edinburgh FLS1000 (England, Edinburgh). The analytes selected for TC sensing in this work were distributed in H2O. The ground powder sample of H4TCPB·4DMF (1 mg) was immersed in 10 mL of deionized water and ultrasonicated for about 10 min to form a stable turbid suspension. Spectra were collected immediately after ultrasonic treatment until the suspension was evenly mixed, recording the excitation and emission spectra of the detection system. Then, the H4TCPB·4DMF suspension with a concentration of 100 mg/L was used as a fluorescence probe to detect TC in the concentration range of 0.2 mg/L to 100 mg/L at their maximum excitation wavelengths. To study the selectivity ability of H4TCPB·4DMF to detect TC, the probe was used for the spectral acquisition of TC. The emission spectra of penicillin sodium (PNC), salicylic acid (BHA), diuron, disulfiram (TETD), norfloxacin (NFX), Na+, K+, Ca2+, Zn2+, Ti4+, Co2+, and Fe3+ (100 mg/L) were collected at the maximum excitation wavelength.
3.5. Theoretical Simulations
We used first principles to perform all spin-polarized DFT to calculate the orbital energy level of H4TCPB·4DMF [43]. The Perdew–Burke–Ernzerhof (PBE) formulation was used for generalized gradient approximation (GGA) [44]. The projected augmented wave (PAW) potential was chosen to describe the ionic core [45,46]. We used a plane-wave basis to take valence electrons into account. The partial occupancy of the Kohn–Sham orbit was facilitated using Gaussian smearing. When the energy changes within a certain range, the electron energy is self-consistent, and the geometric optimization is considered convergent. The Brillouin region is integrated with the Monkhorst–Pack 4×2×2 K-point grid structure. The weak interactions were described using the empirically modified DFT+D3 method in grime format [47].
TC calculations were performed using Gauss 16 software as well as the B3LYP functional and 6-311G(d) basis set. SMD implicit solvation model was used to explain the solvation effect [48,49]. The DFT with gradient corrections and a contracted Gaussian set were used. Bj-damped DFT−D3 was used to improve the calculation accuracy, and the weak interaction was corrected [47]. A Multiwfn software was used to analyze the orbital energy level analysis [50]. The TC track was visualized with VMD-1. 9. 4 software. The specific parameters can be found in the Supporting Information.
4. Conclusions
In summary, we successfully assembled a nanocrystalline fluorescent probe of H4TCPB·4DMF based on its excellent luminescence properties. It has a strong fluorescence emission and a high quantum yield owing to the highly ordered crystal structure, a large number of hydrogen bonds, as well as π–π, lone pair···π, and lone pair···lone pair interactions. The likely mechanism was elucidated indicating that the PET effect occurs between H4TCPB·4DMF and TC, leading to a fluorescence quenching effect. Hence, H4TCPB·4DMF nanocrystals are good candidates as nanofluorescence probes for TC. This work enriches the library of crystalline organic fluorescent sensors and provides theoretical support for the design and mechanical studies of organic fluorescent sensors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28196774/s1, Figure S1: TGA curve of H4TCPB·4DMF; Figure S2: Quantum yield measurement of H4TCPB·4DMF; Figure S3: Luminescence spectra of TC, which were added into different solutions of H4TCPB·4DMF; Figure S4: Emission spectra of deionized water. Figure S5. Fluorescence spectra of H4TCPB 4DMF and UV–vis absorption of TC.
Author Contributions
X.Z.: experimental operations, data collection, and writing—original draft preparation; W.L.: data analysis; M.Y.: reviewing and funding acquisition; Z.L.: writing—review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21771056) and the Natural Science Foundation of Shandong Province (ZR2022QD128, ZR2023MB113).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Not applicable.
References
- Kim, K.S.; Noh, S.B.; Katsuda, T.; Ito, S.; Osuka, A.; Kim, D. Charge transfer induced enhancement of near-IR two-photon absorption of 5,15-bis(azulenylethynyl) zinc(II) porphyrins. Chem. Commun. 2007, 24, 2479–2481. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Ooyama, Y.; Matsugasako, A.; Oka, K.; Nagano, T.; Sumomogi, M.; Komaguchi, K.; Imae, I.; Harima, Y. Fluorescence PET (photo-induced electron transfer) sensors for water based on anthracene-boronic acid ester. Chem. Commun. 2011, 47, 4448–4850. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, S.; Michail, E.; Rauch, F.; Ogasawara, H.; Wang, C.; Sato, Y.; Edkins, R.M.; Zhang, Z.; Taki, M.; Lambert, C.; et al. The Effect of Branching on the One- and Two-Photon Absorption, Cell Viability, and Localization of Cationic Triarylborane Chromophores with Dipolar versus Octupolar Charge Distributions for Cellular Imaging. Chem. Eur. J. 2019, 25, 13164–13175. [Google Scholar] [CrossRef] [PubMed]
- Strobl, M.; Walcher, A.; Mayr, T.; Klimant, I.; Borisov, S.M. Trace Ammonia Sensors Based on Fluorescent Near-Infrared-Emitting aza-BODIPY Dyes. Anal. Chem. 2017, 89, 2859–2865. [Google Scholar] [CrossRef]
- Hu, F.; Li, J.; Zhang, Z.; Li, M.; Zhao, S.; Li, Z.; Peng, N. Smartphone-Based Droplet Digital LAMP Device with Rapid Nucleic Acid Isolation for Highly Sensitive Point-of-Care Detection. Anal. Chem. 2020, 92, 2258–2265. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B. Zinc (II) and AIEgens: The “Clip Approach” for a Novel Fluorophore Family. A Review. Molecules 2021, 26, 4176. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Costanzo, L.d.; Gentile, F.S.; Panunzi, B. Colorimetric recognition of multiple first-row transition metals: A single water-soluble chemosensor in acidic and basic conditions. Dyes Pigments 2021, 184, 108832. [Google Scholar] [CrossRef]
- Erstling, J.A.; Hinckley, J.A.; Bag, N.; Hersh, J.; Feuer, G.B.; Lee, R.; Malarkey, H.F.; Yu, F.; Ma, K.; Baird, B.A.; et al. Ultrasmall, Bright, and Photostable Fluorescent Core-Shell Aluminosilicate Nanoparticles for Live-Cell Optical Super-Resolution Microscopy. Adv. Mater. 2021, 33, e2006829. [Google Scholar] [CrossRef]
- Tian, X.; Murfin, L.C.; Wu, L.; Lewis, S.E.; James, T.D. Fluorescent small organic probes for biosensing. Chem. Sci. 2021, 12, 3406–3426. [Google Scholar] [CrossRef]
- Fang, Y.; Meng, Y.; Yuan, C.; Du, C.; Wang, K.P.; Chen, S.; Hu, Z.Q. Efficient deep blue emission by 4-styrylbenzonitrile derivatives in solid state: Synthesis, aggregation induced emission characteristics and crystal structures. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 267, 120575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Han, H.H.; Zang, Y.; Li, J.; He, X.P.; Feringa, B.L.; Tian, H. Remote light-controlled intracellular target recognition by photochromic fluorescent glycoprobes. Nat. Commun. 2017, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-Based Covalent Organic Frameworks for Acid Vapor Sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Kong, Y.; You, J.; Zhang, C.; Xian, Y. Rational Synthesis of Imine-Linked Fluorescent Covalent Organic Frameworks with Different pK(a) for pH Sensing In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2021, 13, 51351–51361. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, C.; Wang, S.; Wu, L.; Zhang, X. Light-harvesting metal-organic framework nanoprobes for ratiometric fluorescence energy transfer-based determination of pH values and temperature. Mikrochim. Acta 2019, 186, 476. [Google Scholar] [CrossRef]
- Wang, B.; He, R.; Xie, L.-H.; Lin, Z.-J.; Zhang, X.; Wang, J.; Huang, H.; Zhang, Z.; Schanze, K.S.; Zhang, J.; et al. Microporous Hydrogen-Bonded Organic Framework for Highly Efficient Turn-Up Fluorescent Sensing of Aniline. J. Am. Chem. Soc. 2020, 142, 12478–12485. [Google Scholar] [CrossRef]
- Yadav, S.; Choudhary, N.; Ranjan Dash, M.; Ranjan Paital, A. High surface area dendritic silica pairing with anthraquinone derivative: A promising single platform for dual applications of detection and remediation of nitroaromatics and copper ion. Chem. Eng. J. 2022, 450, 138042. [Google Scholar] [CrossRef]
- Qin, J.; Xie, L.; Ying, Y. Feasibility of terahertz time-domain spectroscopy to detect tetracyclines hydrochloride in infant milk powder. Anal. Chem. 2014, 86, 11750–11757. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sensor. Actuat. B-Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Li, W.-T.; Wang, J.-S.; Pang, M.; Li, Y.; Ruan, W.-J. Fluorescent sensor array for tetracyclines discrimination using a single Dye@MOF composite sensor. Sensor. Actuat. B-Chem. 2023, 381, 133375. [Google Scholar] [CrossRef]
- Bu, D.; Song, H.; Li, Z.; Wei, L.; Zhang, H.; Yu, M. Carbon-dot-based ratiometric fluorescent probe of intracellular zinc ion and persulfate ion with low dark toxicity. Luminescence 2020, 35, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.; Fernandes, C.; Silva, L.H.M.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food. Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef]
- Han, S.; Yang, L.; Wen, Z.; Chu, S.; Wang, M.; Wang, Z.; Jiang, C. A dual-response ratiometric fluorescent sensor by europium-doped CdTe quantum dots for visual and colorimetric detection of tetracycline. J. Hazard. Mater. 2020, 398, 122894. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, D.; Hamed, A.M.; Gilbert-Lopez, B.; Gamiz-Gracia, L.; Garcia-Campana, A.M. Evaluation of a multiresidue capillary electrophoresis-quadrupole-time-of-flight mass spectrometry method for the determination of antibiotics in milk samples. J. Chromatogr. A 2017, 1510, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Yan, B.; Yao, G.; Chao, K.; Zhu, C.; Huang, Q. Surface-Enhanced Raman Spectroscopy for Trace Detection of Tetracycline and Dicyandiamide in Milk Using Transparent Substrate of Ag Nanoparticle Arrays. ACS Appl. Nano Mater. 2020, 3, 7066–7075. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Jiang, L.; Wang, Y.; Liu, W.; Li, Z. Temperature-Dependent Preparation of Hydrogen-Bond Organic Frameworks: Ultrathin and Stable Nanosheets for Fluorescent Sensing toward Uranyl. Cryst. Grow. Des. 2023, 23, 1840–1847. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Liu, J.; Cheng, X.; Sun, J.Z.; Qin, A.; Tang, B.Z. A novel pyridinium modified tetraphenylethene: AIE-activity, mechanochromism, DNA detection and mitochondrial imaging. J. Mater. Chem. B 2018, 6, 1279–1285. [Google Scholar] [CrossRef]
- Hoshino, A.; Omata, K.; Takami, S.; Adschiri, T.; Terada, N.; Funatsu, T.; Yasuhara, M.; Yamamoto, K. Fluorescence millisecond oscillation in polar solvents regulates fluorescence intensity of colloidal quantum dots’ solution. J. Nanophotonics 2007, 1, 013516. [Google Scholar] [CrossRef]
- Lei, M.; Jia, Y.; Zhang, W.; Xie, J.; Xu, Z.; Wang, Y.; Du, W.; Liu, W. Ultrasensitive and Selective Detection of Uranium by a Luminescent Terbium−Organic Framework Ultrasensitive and Selective Detection of Uranium by a Luminescent Terbium−Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 51086–51094. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, D.; Vidal, E.; Canals, A.; Domini, C.E.; Garrido, M. Simple-to-use and portable device for free chlorine determination based on microwave-assisted synthesized carbon dots and smartphone images. Talanta 2021, 229, 122298. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Qi, H.; Ma, R.; Sun, Z.; Xiao, L.; Wei, G.; Huang, Z.; Liu, S.; Li, J.; Dong, M.; et al. N,S-self-doped carbon quantum dots from fungus fibers for sensing tetracyclines and for bioimaging cancer cells. Mater. Sci. Eng. C 2019, 105, 110132. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Bu, T.; Sun, X.; Liu, Y.; Liu, J.; Wang, Q.; Shui, Y.; Guo, S.; Wang, L. A sensitive and selective approach for detection of tetracyclines using fluorescent molybdenum disulfide nanoplates. Food. Chem. 2019, 297, 124969. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, C.; Yu, X.; Li, J.; Wang, Z.; Zhang, Z.; Liu, B. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J. Mater. Chem. C 2018, 6, 9636–9641. [Google Scholar] [CrossRef]
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y. A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens. Bioelectron. 2020, 148, 111791. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.; Xie, G.; Ren, Y.; Zheng, Y.Q. Ratiometric system based on graphene quantum dots and Eu(3+) for selective detection of tetracyclines. Anal. Chim. Acta 2018, 1022, 131–137. [Google Scholar] [CrossRef]
- Tan, H.; Ma, C.; Song, Y.; Xu, F.; Chen, S.; Wang, L. Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. Biosens. Bioelectron. 2013, 50, 447–452. [Google Scholar] [CrossRef]
- Li, C.-P.; Long, W.-W.; Lei, Z.; Guo, L.; Xie, M.-J.; Lü, J.; Zhu, X.-D. Anionic metal–organic framework as a unique turn-on fluorescent chemical sensor for ultra-sensitive detection of antibiotics. Chem. Commun. 2020, 56, 12403–12406. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Joarder, B.; Chaudhari, A.K.; Mukherjee, S.; Ghosh, S.K. Highly selective detection of nitro explosives by a luminescent metal-organic framework. Angew. Chem. Int. Ed. Engl. 2013, 52, 2881–2885. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, L.; Mao, S.; Wu, D.; Fan, J. The role of structural elements and its oxidative products on the surface of ferrous sulfide in reducing the electron-withdrawing groups of tetracycline. Chem. Eng. J. 2019, 378, 122195. [Google Scholar] [CrossRef]
- He, X.; Luo, Y.; Zheng, Z.; Wang, C.; Wang, J.; Hong, D.; Zhai, L.; Guo, L.; Sun, B. Porphyrin-Based Hydrogen-Bonded Organic Frameworks for the Photocatalytic Degradation of 9,10-Diphenylanthracene. ACS Appl. Nano Mater. 2019, 2, 7719–7727. [Google Scholar] [CrossRef]
- Kressee, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, J. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).