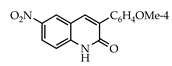
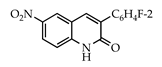
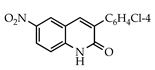
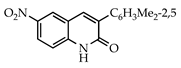
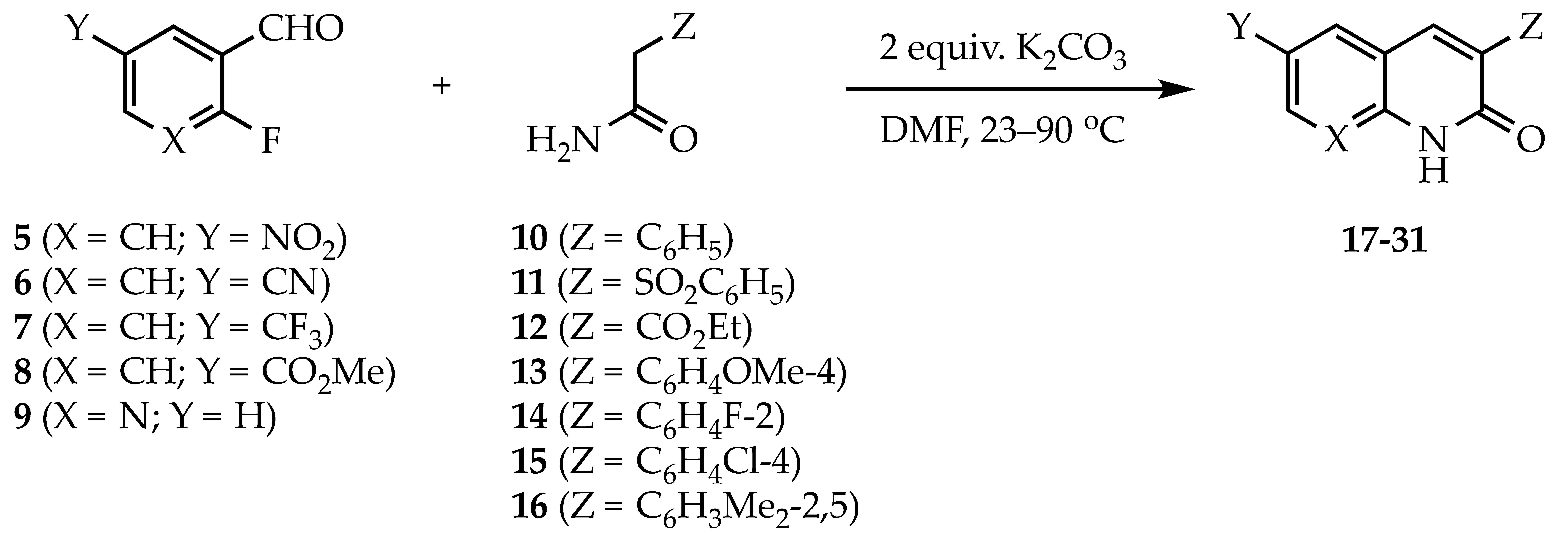Abstract
A domino aldol-SNAr-dehydration [3+3] annulation strategy has been utilized to fuse six-membered cyclic amides onto aromatic substrates. 2-Arylacetamides have been reacted with 2-fluorobenzaldehyde derivatives activated toward SNAr reaction by an electron-withdrawing substituent (NO2, CN, CF3, CO2Me) at C5 to prepare 3,6-disubstituted quinolin-2(1H)-ones. Additionally, 3-substituted 1,8-naphthyridin-2(1H)-ones have been similarly derived from 2-fluoronicotinaldehyde. Fifteen examples are reported, and two possible mechanistic scenarios are presented and discussed.
1. Introduction
Over the past 15 years, our group has reported numerous cases of domino reactions involving the SNAr reaction to fuse heterocycles to pre-existing aromatic frameworks. Our early contributions in this area have been reviewed [1]. More recent projects have broadened the scope of these processes. One study described an imine addition-SNAr reaction to tert-butyl (2-fluoro-5-nitrobenzoyl)acetate to give 4-oxo-1,2,3,4-tetrahydroquinoline-3-carboxylic esters [2]. Further use of domino procedures on Morita-Baylis-Hillman acetates afforded efficient access to naphthalenes and quinolines [3] as well as dihydroquinolines, dihydronaphthyridines and quinolin-4(1H)-ones [4]. Additionally, we have also reported a domino Michael-SNAr-heteroaromatization sequence using SNAr-activated 2-fluoroaryl-acrylate esters to prepare highly substituted 1H-indole-3-carboxylate esters [5].
An earlier study from this laboratory described a synthetic approach to 4H-1-benzo-pyrans involving a domino SN2 alkylation of a β-ketoester by 2-fluoro-5-nitrobenzyl bromide followed by SNAr ring closure through the enolate oxygen of the alkylated product [6]. This annulation utilized a [3+3] strategy combining a double nucleophile (a β-ketoester) and a double electrophile (a benzyl bromide aryl-activated for SNAr addition). The reaction was originally performed in acetone using an eight-fold excess of K2CO3, but subsequent experiments revealed that it could proceed with less base (four equiv.) in DMF solvent.
Since this report, many additional studies of [3+3] cyclizations to prepare heterocycles have been advanced. Two acid-catalyzed aza-annulations have been reported by the Hsung lab as strategies for the synthesis of alkaloids. The first described an attempt to assemble propyleine via intramolecular reaction of a ring-embedded vinylogous amide with a vinyliminium salt (Reaction (1)), but only modest results were achieved [7]. On the other hand, a chiral auxiliary was successfully used in an intermolecular variant of this reaction to generate a common precursor to two stereochemical scaffolds indigenous to the Lepadin family of alkaloids (Reaction (2)) [8].
| (1) | 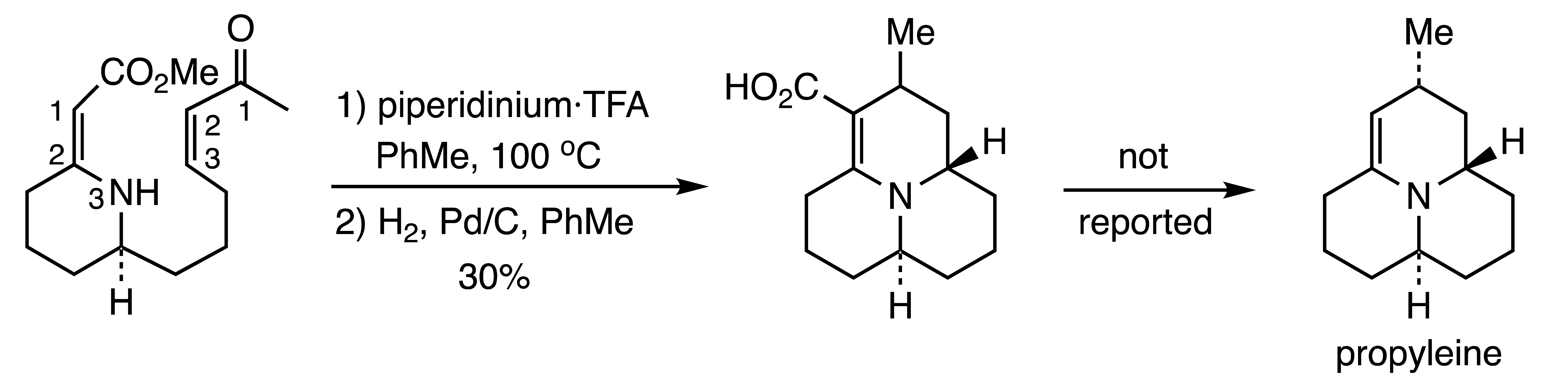 |
| (2) | 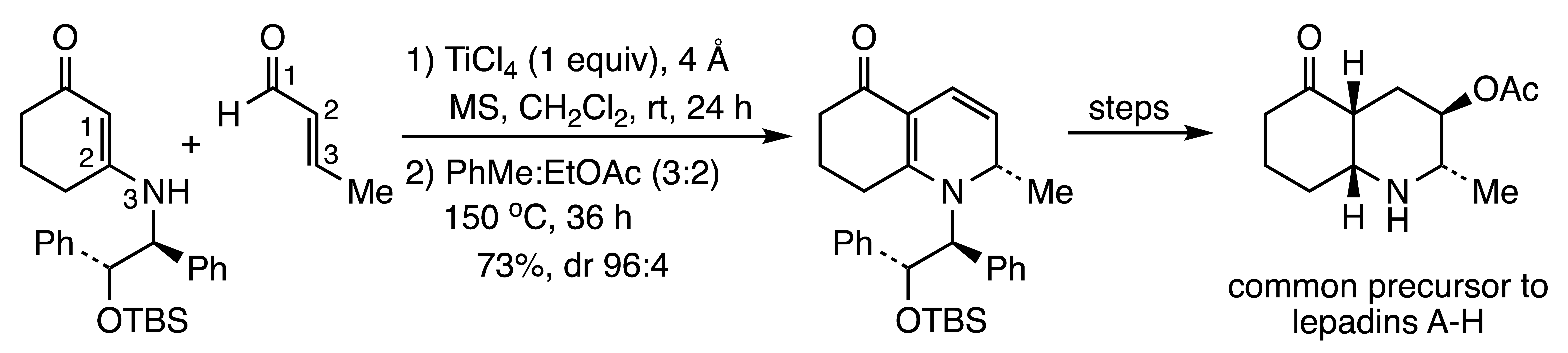 |
Four additional papers explored additions of nitrogen heterocycles to allylic esters. Tong and co-workers reported access to thiopyrano[2,3-b]indoles by addition of indoline-2-thiones to β-acetoxy allenoates (Reaction (3)) [9]. A related study by the Swamy group described the preparation of α-carbolines from β-acetoxy allenoates with iminoindolines [10] (Reaction (4)). A further investigation by the Guo lab enantioselectively produced substituted spirocyclohexenes by reaction of Morita-Baylis-Hillman carbonates with α-arylidene pyrazolinones in the presence of a chiral dihydroquinidine catalyst (Reaction (5)) [11]. The fourth investigation by Satham and Namboothiri presented a synthesis of 2-aryl terephthalates from nitroallylic acetates with stabilized sulfur ylides (Reaction (6)) [12].
| (3) | 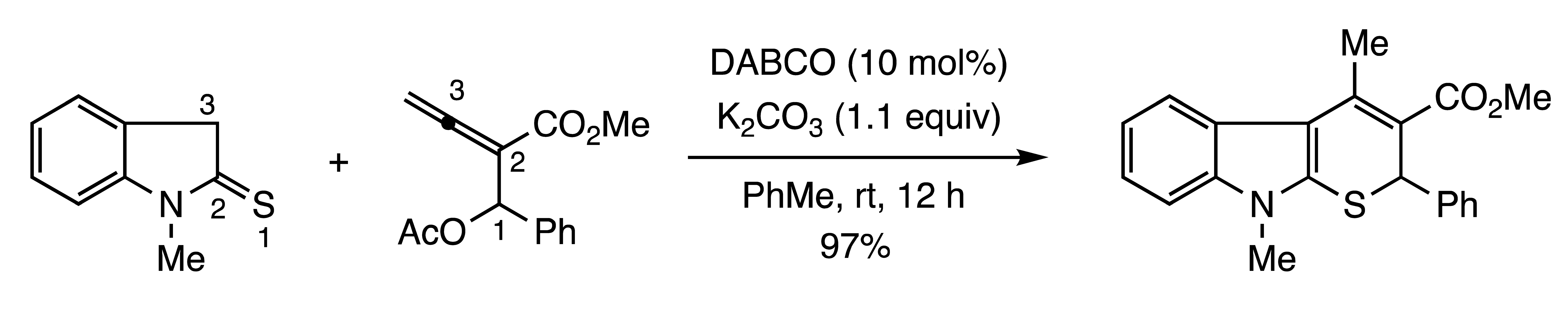 |
| (4) | 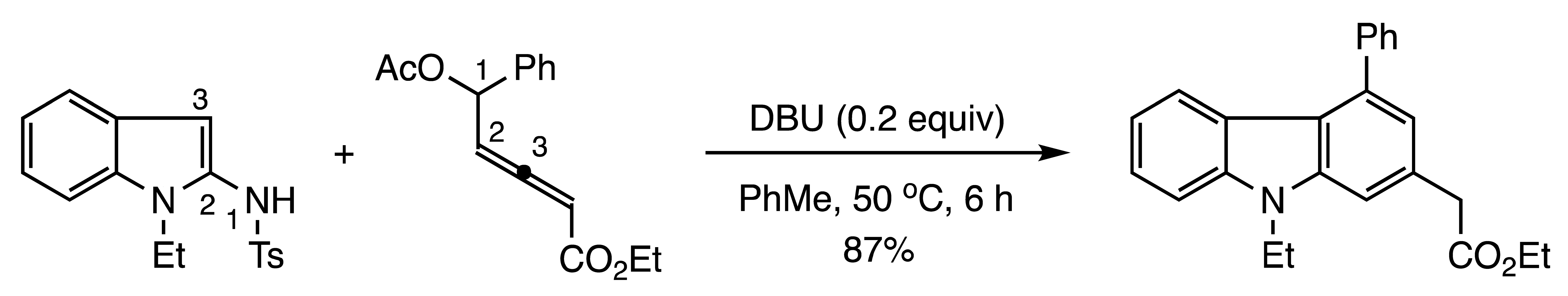 |
| (5) | 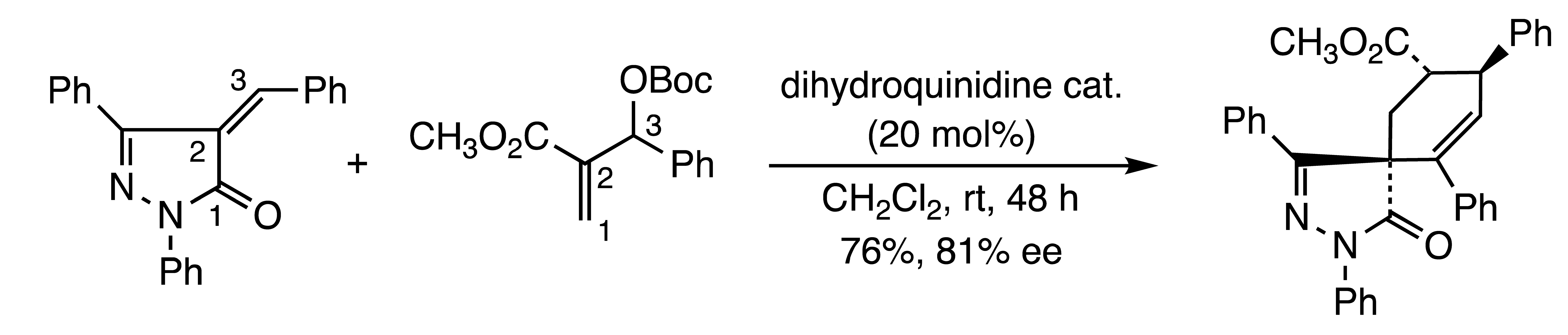 |
| (6) | 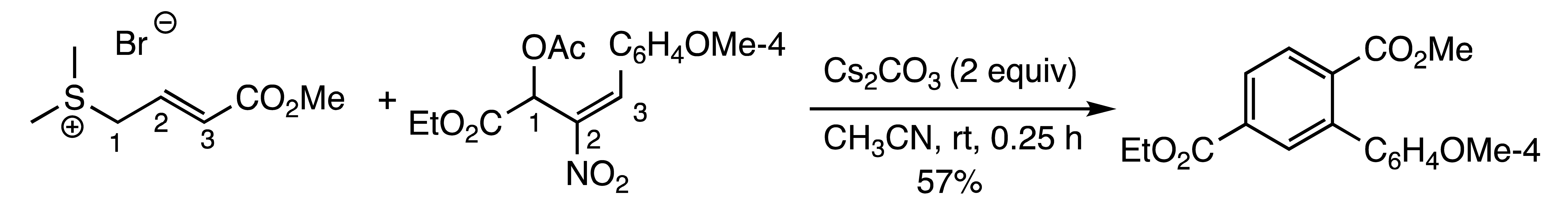 |
Four other articles have appeared describing metal- and organocatalyzed fusion of heterocycles to pre-existing rings. Huang and co-workers disclosed a copper-promoted synthesis of quinolines from aryl ketoximes and 2-fluorobenzaldehydes (Reaction (7)) [13]. Kanchupalli et al. unveiled a chemodivergent Rh(III)-catalyzed annulation involving indoles and iodonium carbenes to generate tri- and tetracyclic nitrogen heterocycles (Reaction (8)) [14]. The Wang group developed a zinc-catalyzed enantioselective ring formation to prepare chiral spiro[indoline-3,4′-thiopyrano[2,3-b]indole] derivatives [15] (Reaction (9)). Finally, the chemical synthesis team at the Sichuan Key Laboratory published a route to several chiral spiro-δ-lactam oxindoles from 3-carboxamides and β,γ-unsaturated-α-ketoesters using bifunctional urea and squaramide organocatalysts (Reaction (10)) [16]. Numerous other [3+3] annulations are detailed in a review by Feng and Liu [17].
| (7) | 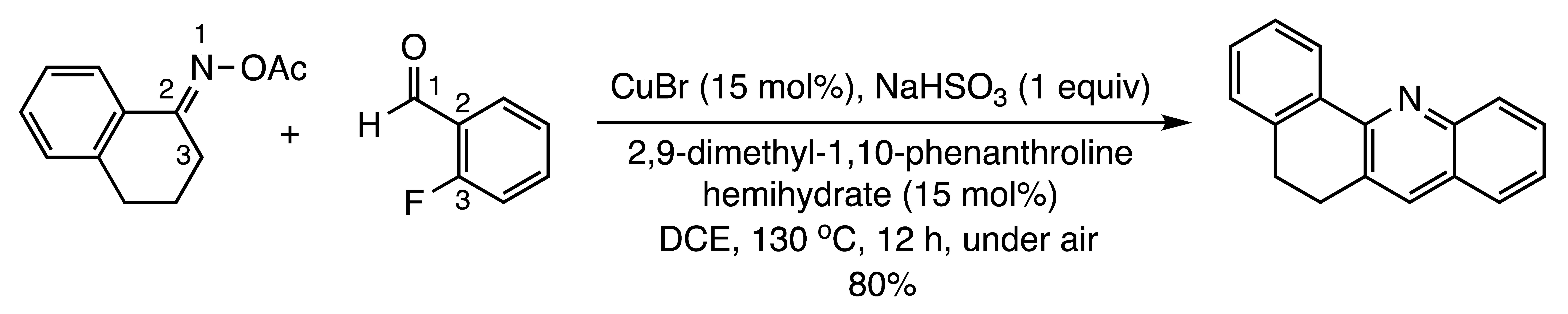 |
| (8) | 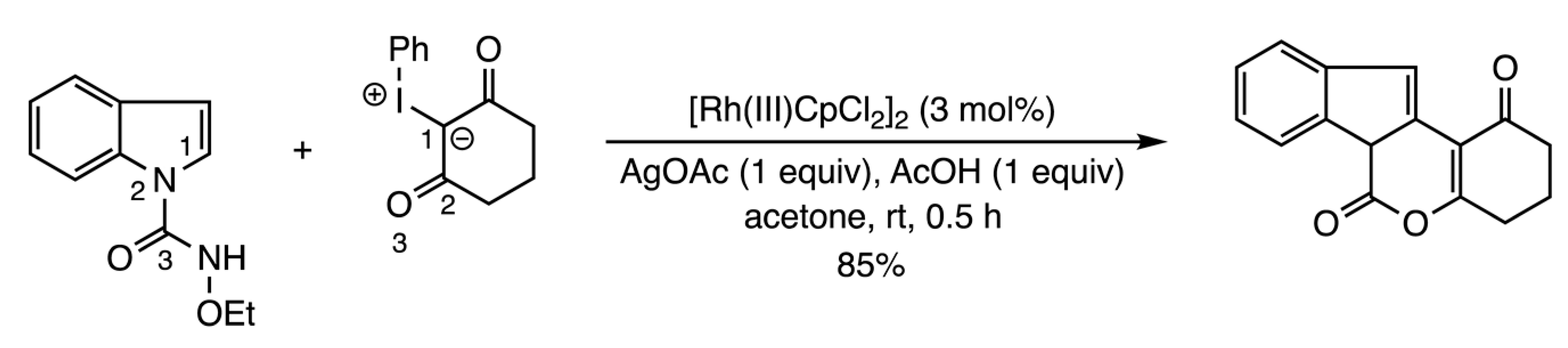 |
| (9) | 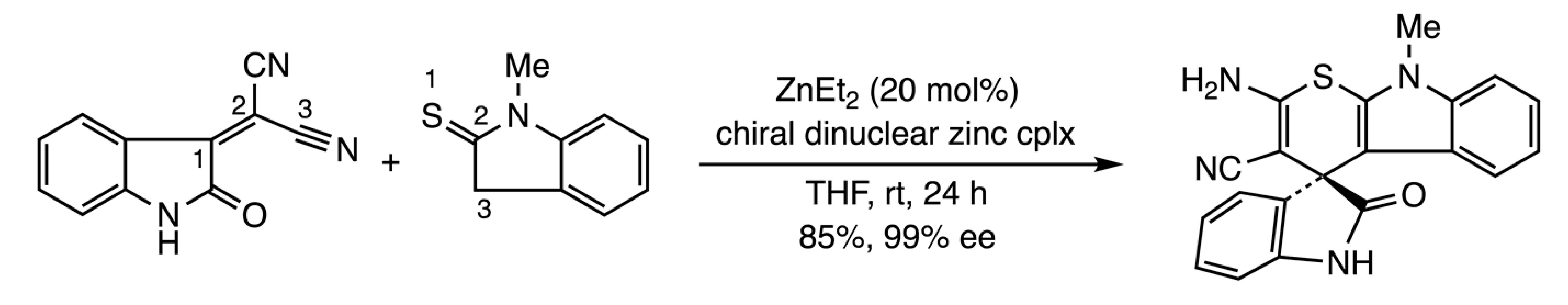 |
| (10) | 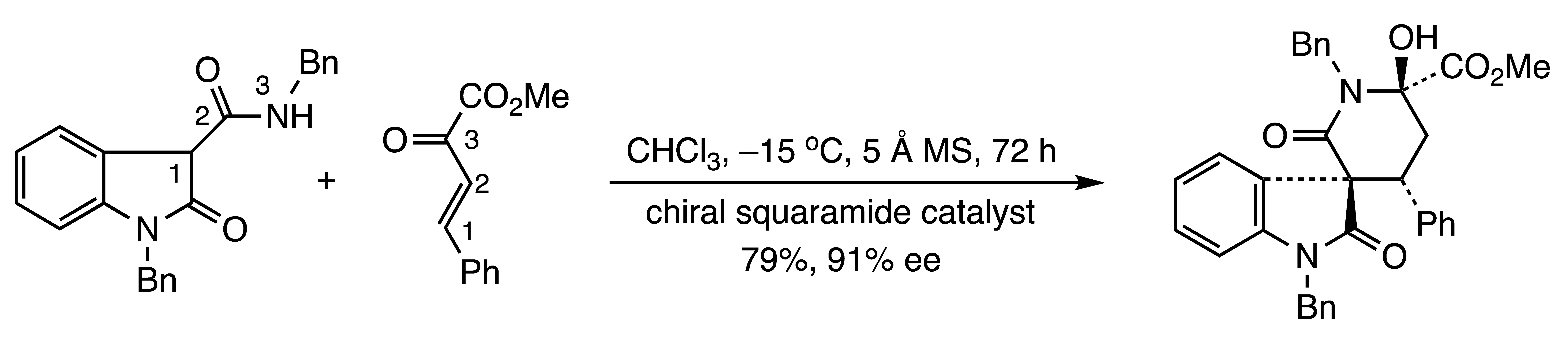 |
The current study reports the use of a domino aldol-SNAr-dehydration reaction to prepare quinolin-2(1H)-ones and 1,8-naphthyridin-2(1H)-ones via the [3+3] annulation (Figure 1) of a double nucleophile (a 2-arylacetamide) with a double electrophile (an SNAr-activated 2-fluorobenzaldehyde or 2-fluoronicotinaldehyde). The aromatic aldehydes are commercially available or easily prepared. The arylacetamides are relatively scarce and expensive to purchase but can be readily synthesized from the corresponding 2-arylacetyl chlorides. The current transformation represents the first use of an activated acetamide to construct potential drug candidates.
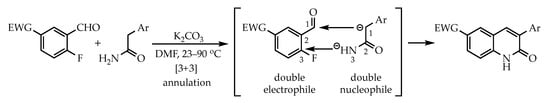
Figure 1.
Generic [3+3] annulation leading to quinolin-2(1H)-ones reported in this work.
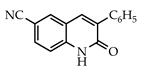
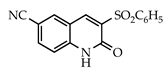
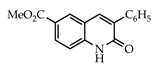
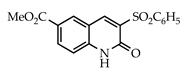
The 3-arylquinolin-2(1H)-one scaffold comprises the core ring system of several important drug candidates (see Figure 2). Compound 1 is a CDK 5 inhibitor and as such may prove useful in the treatment of Alzheimer’s disease and amyotrophic lateral sclerosis [18]. The benzimidazole-linked derivative 2 slowed the growth of NCI-H460 large lung tumor cells in a xenograft mouse model [19]. The quinolone-chalcone hybrid 3 showed significant tubulin polymerization inhibition [20] and thus could find use in curbing the growth of cancer cells by interfering in the mitotic process. Finally, the benzimidazole-substituted quinolin-2(1H)-one 4 demonstrated promising activity against MDA-MB-231 (a model for late-stage breast cancer) and PC-3 (human prostate cancer) cell lines by decreasing CDK-1 and stabilizing the levels of Hsp90 and Hsp70 without initiating a heat shock response [21].
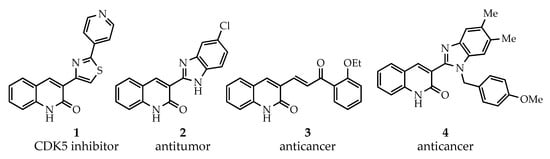
Figure 2.
Drug candidates incorporating the 3-arylquinolin-2(1H)-one core.
2. Results and Discussion
2-Fluoro-5-nitrobenzaldehyde (5) [22] and 5-carbomethoxy-2-fluorobenzaldehyde (8) [5] were prepared as previously described. Other benzaldehydes—5-cyano-2-fluoro-benzaldehyde (6), 2-fluoro-5-(trifluoromethyl)benzaldehyde (7), and 2-fluoronicotinaldehyde (9)—were commercially available. Phenylacetamide (10), 2-(phenylsulfonyl)acetamide (11), ethyl malonate monoamide (12), and 2-(4-methoxyphenyl)acetamide (13) were also acquired from a commercial source, while 2-(2-fluorophenyl)acetamide (14), 2-(4-chlorophenyl)acetamide (15), and 2-(2,5-dimethylphenyl)acetamide (16) were prepared from the corresponding acid chlorides [23].
A listing of the quinolin-2(1H)-ones and 1,8-naphthyridin-2(1H)-ones prepared in this study is given in Table 1. The sequence entails initial aldol addition of the carbanion derived from the 2-arylacetamide to the aldehyde carbon or SNAr addition-elimination of the amide nitrogen at the fluoro-substituted carbon of the activated 2-fluorobenzaldehyde. Following bond rotation of the aldol adduct or equilibration of the amide rotamer to bring the reactive centers into proximity, heterocyclic ring closure would occur to give the ring-fused product. The entire process occurred in a single reaction vessel using K2CO3 as the base with DMF as the solvent. No other catalysts or promoters were added, and this simplified the process optimization. Four activating groups at C5 of the aromatic aldehyde, including NO2, CN, CF3, and CO2Me, have been validated and 2-fluoronicotinaldehyde also gave successful cyclization. Acetamides activated by aromatic or ester groups at C2 were found to be successful, while derivatives incorporating C2 cyano or ketone groups failed to successfully undergo the reaction sequence.

Table 1.
Synthesis of quinolin-2(1H)-one and 1,8-naphthyridin-2(1H)-one derivatives.
As briefly mentioned above, two mechanisms for the formation of the target heterocycles are plausible, differing in the initial bond-forming step (see Scheme 1). Since the pKa values for the pseudo-acidic methylene and amido groups of the 2-arylacetamides are comparable, it might be expected that the anion from either site could initiate the sequence. These pKa’s were estimated from experimentally derived values for PhCH2CO2Et (22.6), PhCONH2 (23.3), CH3CONH2 (25.5) and PhCH2CONH2 (unclearly listed as 24.7) in DMSO [24]. Computer-estimated pKa’s were similar but trended slightly more acidic than the experimental values [25]. Mechanism 1 requires an initial aldolization between the arylacetamide methylene anion and the benzaldehyde to yield aldol conformers A (possibly H-bond stabilized) and B. SNAr ring closure from conformation B would then give the fused-ring β-hydroxyamide, which upon loss of water would afford the quinolin-2(1H)-one. Interestingly, the ring closure in this sequence could occur from either the enolate oxygen or the amide nitrogen. However, as one might expect, cyclization by nitrogen prevails since the quinolin-2(1H)-one product has greater stability than the alternative 2H-chromen-2-imine. For quinolin-2(1H)-ones, it is well known that the amide structure predominates over the quinolinol tautomer [26,27], and with its 10 π electron complement and planarity (all atoms are sp2 hybridized) retains its aromaticity [28]. A similar preference likely applies in the 1,8-naphthyridin-2(1H)-one series. Mechanism 2, involving the addition of the amide nitrogen anion to the aromatic ring, offers an alternative route to the final products. We have shown in the past that primary amides give high yields of SNAr products in DMF [29], but usually require heat for efficient reaction. In this scenario, the less hindered amide rotamer D would be expected to predominate, necessitating a conversion to the more sterically challenged rotamer E before ring formation could occur. To gain insight into the initiating reaction of the sequence, two reactions were run on substrates bearing similar functionality to those used to prepare quinolin-2(1H)-ones. The first, between phenylacetamide and 2-nitrobenzaldehyde in DMF with K2CO3 at room temperature (23 °C), formed two inseparable polar products (by TLC) with loss of the aldehyde carbonyl (IR). The second, employing phenylacetamide with methyl 2-fluoro-5-nitrobenzoate under the same conditions, did not show significant conversion until heat (>50 °C) was applied. While these observations do not have direct relevance in the current application, they strongly suggest that the aldehyde is the most reactive functional group. Since the reaction is stirred at 23 °C for 15–30 min prior to the application of heat, the sequence of events likely begins with aldol formation as in mechanism 1.
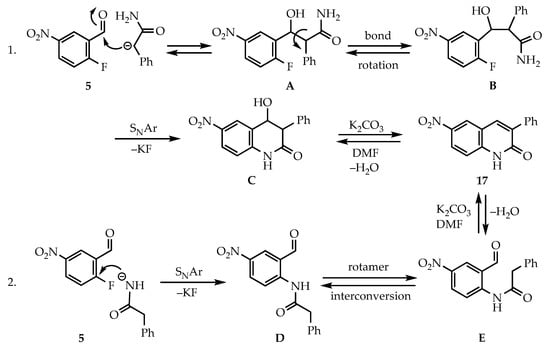
Scheme 1.
Plausible mechanisms for the formation of 17 from 5 and the anion of phenylacetamide (10).
3. Materials and Methods
3.1. General Methods
Unless otherwise indicated, all reactions were performed under dry N2 in oven-dried glassware. All reagents and solvents were used as received. All wash solutions in work-up procedures were aqueous. Reactions were monitored by thin layer chromatography on Analtech No 21521 silica gel GF plates (Newark, DE, USA). Preparative separations were performed by flash chromatography on Davisil®, grade 62, 60–200 mesh silica gel containing 0.5% of UV-05 phosphor (both from Sorbent Technologies, Norcross, GA, USA) slurry packed into quartz columns. Band elution for all chromatographic separations was monitored using a hand-held UV lamp (Fisher Scientific, Pittsburgh, PA, USA). Melting points were obtained using a MEL-TEMP apparatus (Cambridge, MA, USA) and are uncorrected. FT-IR spectra were run as thin films on NaCl disks using a Nicolet iS50 spectrophotometer (Madison WI, USA). 1H- and 13C-NMR spectra were measured using a Bruker Avance 400 system (Billerica, MA, USA) at 400 MHz and 101 MHz, respectively, in the indicated solvents containing 0.05% (CH3)4Si as the internal standard; coupling constants (J) are given in Hz. Low-resolution mass spectra were obtained using a Hewlett-Packard Model 1800A GCD GC-MS system (Palo Alto, CA, USA). Elemental analyses (±0.4%) on all new compounds were determined by Atlantic Microlabs (Norcross, GA, USA). Copies of 1H-NMR and 13C-NMR spectra for all new compounds are given in the Supplementary Materials.
2-Fluoro-5-nitrobenzaldehyde (5) [22] and 5-carbomethoxy-2-fluorobenzaldehyde (8) [5] were prepared as previously described. 5-Cyano-2-fluorobenzaldehyde (6), 2-fluoro-5-(trifluoromethyl)benzaldehyde (7), 2-fluoronicotinaldehyde (9), phenylacetamide (10), 2-(phenylsulfonyl)acetamide (11), ethyl malonate monoamide (12), 2-(4-methoxyphenyl)-acetamide (13), 2-(2-fluorophenyl)acetyl chloride, 2-(4-chlorophenyl)acetyl chloride, and 2-(2,5-dimethylphenyl)acetyl chloride were purchased from Combi Blocks, Inc. (San Diego, CA, USA).
3.2. General Procedure for the Preparation of 2-Arylacetamides
The procedure of Finan and Fothergill was adapted [23]. To a solution of ammonium acetate [ground to a powder and dried under high vacuum (ca. 0.5 mmHg) for 2 h at 23 °C, 1.82 equiv.] in dry acetone (25 mL) was added 1 equiv. of the 2-arylacetyl chloride. The mixture was stirred for 3 h and filtered through Celite® with acetone (50–100 mL). The acetone solution was concentrated, and the resulting solid was triturated with ether in hexane (5:1 v/v). The solid was collected by filtration, recrystallized from water for 14 and 15 (or 19:1 v/v water-ethanol for 16) and dried at 23 °C under a high vacuum to give the yields reported.
3.2.1. 2-(2-Fluorophenyl)acetamide (14)
From 2-(2-fluorophenyl)acetyl chloride, yield 0.89 g (65%) as a white solid, m.p. 156–157 °C (lit. [30] m.p. 157.2–159.2 °C); IR: 3389, 3202, 1645, 1626, 1227 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.34–7.27 (complex, 2H), 7.14 (td, J = 7.6, 1.2 Hz, 1H), 7.09 (ddd, J = 9.6, 8.1, 1.2 Hz, 1H), 5.59 (br s, 1H), 5.51 (br s, 1H), 3.61 (d, J = 1.4 Hz, 2H); 13C NMR (101 MHz, CDCl3): δC 172.2, 160.9 (d, J = 245.8 Hz), 131.6 (d, J = 3.9 Hz), 129.4 (d, J = 8.2 Hz), 124.6 (d, J = 3.6 Hz), 122.0 (d, J = 15.9 Hz), 115.7 (d, J = 21.7 Hz), 36.4 (d, J = 3.0 Hz); MS (m/z) 153 (M+·).
3.2.2. 2-(4-Chlorophenyl)acetamide (15)
From 2-(4-chlorophenyl)acetyl chloride, yield 0.89 g (66%) as a white solid, m.p. 175–176 °C (lit. [31] m.p. 175 °C); IR: 3405, 3204, 1650, 1620 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 7.48 (br s, 1H), 7.36 (d, J = 9.0 Hz, 2H), 7.28 (d, J = 9.0 Hz, 2H), 6.91 (br s, 1H), 3.37 (s, 2H); 13C NMR (101 MHz, DMSO-d6): δC 172.3, 136.0, 131.5, 131.4, 128.5, 41.8; MS (m/z) 168, 170 (M+·, ca 3:1).
3.2.3. 2-(2,5-Dimethylphenyl)acetamide (16)
From 2-(2,5-dimethylphenyl)acetyl chloride, yield 0.91 g (68%) as a white solid, m.p. 152–154 °C (lit. [32] m.p. 154 °C); IR: 3390, 3188, 1645, 1621 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 7.34 (br s, 1H), 7.01 (d, J = 7.6 Hz, 1H), 6.99 (d, J = 1.9 Hz, 1H), 6.92 (dd, J = 7.6, 1.9 Hz, 1H), 6.86 (br s, 1H), 3.35 (s, 2H), 2.23 (s, 3H), 2.19 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δC 172.6, 135.3, 134.8, 133.8, 131.2, 130.1, 127.4, 40.4, 21.0, 19.3; MS (m/z) 163 (M+·).
3.3. Representative Procedure for the Preparation of Quinolin-2(1H)-ones and 1,8-Naphthyridin-2(1H)-ones
A. For amides with a phenyl, phenylsulfonyl, or ester at C2 (substrates 10–12): To a solution of the aldehyde (2.0 mmol, 2.0 equiv.) in DMF (2 mL) was added the amide (1.0 mmol, 1.0 equiv.) and K2CO3 (2.0 equiv.) at 23 °C and the mixture was stirred for 15–30 min. The reaction was then heated for 3–5 h at 90 °C. B. For amides with a substituted aromatic group at C2 (substrates 13–16): To a solution of the aldehyde (1.0 mmol, 1.0 equiv.) in DMF (2 mL) was added the amide (2.0 mmol, 2.0 equiv.) and K2CO3 (2.0 equiv.) using the same time and temperature regime. After TLC (10–20% v/v EtOAc/hexane) indicated the reaction was complete, the crude mixture was cooled to 23 °C, poured into water (30 mL) and the aqueous layer was extracted with EtOAc (3 × 25 mL). The organic layer was washed with 1.0 M HCl (2 × 25 mL) and saturated NaHCO3 (30 mL). The combined organic layers were washed with saturated NaCl (30 mL) and dried (Na2SO4). Removal of the solvent under vacuum gave a crude product, which was further purified by column chromatography and crystallization from ether.
3.3.1. 6-Nitro-3-phenylquinolin-2(1H)-one (17)
Yield: 0.41 g (91%) as a white solid, m.p. 299–300 °C (lit. [33] m.p. 299–302 °C); IR: 1672, 1540, 1336 cm−1; 1H NMR (400 MHz, CDCl3): δH 12.51 (br s, 1H), 8.77 (d, J = 2.2 Hz, 1H), 8.36 (s, 1H), 8.35 (dd, J = 9.1, 2.2 Hz, 1H), 7.76 (d, J = 9.1 Hz, 2H), 7.49–7.39 (complex, 4H); 13C NMR (101 MHz, CDCl3): δC 161.7, 143.1, 142.1, 137.8, 135.8, 133.9, 129.2, 128.9, 128.6, 125.3, 125.0, 119.5, 116.2; MS (m/z): 266 (M+·).
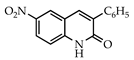
3.3.2. 6-Nitro-3-(phenylsulfonyl)quinolin-2(1H)-one (18)
Yield: 0.35 g (89%) as a white solid, m.p. 329–330 °C; IR: 3160, 1662, 1625, 1338, 1159 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.77 (s, 1H), 9.24 (s, 1H), 9.09 (d, J = 2.2 Hz, 1H), 8.47 (d, J = 9.1 Hz, 1H), 8.02 (d, J = 7.7 Hz, 2H), 7.74 (t, J = 7.4 Hz, 1H), 7.64 (t, J = 7.7 Hz, 2H), 7.49 (d, J = 9.1 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δC 157.0, 145.42, 145.37, 142.6, 139.6, 134.5, 133.1, 129.5, 129.0, 128.4, 127.7, 117.23, 117.16; MS (m/z): 330 (M+·). Anal. Calcd for C15H10N2O5S: C, 54.54; H, 3.05; N, 8.48. Found: C, 54.43; H, 3.01; N, 8.40.
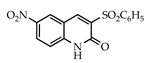
3.3.3. 3-Carbethoxy-6-nitroquinolin-2(1H)-one (19)
Yield: 0.28 g (90%) as a white solid, m.p. 187–188 °C; IR: 3112, 1689, 1619, 1526, 1348 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.58 (s, 1H), 8.57 (d, J = 2.6 Hz, 1H), 8.50 (dd, J = 9.1, 2.6 Hz, 1H), 7.51 (d, J = 9.1 Hz, 1H), 4.45 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3): δC 162.1, 158.4, 154.9, 146.9, 144.3, 128.6, 125.2, 120.6, 118.1, 117.8, 62.6, 14.2; MS (m/z): 262 (M+·). Anal. Calcd for C12H10N2O5: C, 54.97; H, 3.84; N, 10.68. Found: C, 55.01; H, 3.85; N, 10.62.
3.3.4. 3-(4-Methoxyphenyl)-6-nitroquinolin-2(1H)-one (20)
Yield: 0.49 g (90%) as a white solid, m.p. 219–220 °C; IR: 3116, 2846, 1668, 1625, 1525, 1353 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 8.75 (d, J = 2.7 Hz, 1H), 8.40 (dd, J = 9.1, 2.7 Hz, 1H), 8.39 (s, 1H), 7.73 (d, J = 8.8 Hz, 2H), 7.66 (d, J = 9.1 Hz, 1H), 7.07 (d, J = 8.8 Hz, 2H), 3.82 (s, 3H), (NH exchanged); 13C NMR (101 MHz, DMSO-d6): δC 160.5, 159.4, 156.8, 144.1, 138.3, 130.4, 128.6, 126.6, 126.3, 124.6, 120.5, 117.8, 114.3, 55.7; MS (m/z): 296 (M+·). Anal. Calcd for C16H12N2O4: C, 64.86; H, 4.08; N, 9.46. Found: C, 64.79; H, 4.04; N, 9.39.
3.3.5. 3-(2-Fluorophenyl)-6-nitroquinolin-2(1H)-one (21)
Yield: 0.30 g (89%) as a white solid, m.p. 324–325 °C; IR: 3153, 1671, 1929, 1543, 1244, 1344 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.56 (s, 1H), 8.77 (d, J = 2.6 Hz, 1H), 8.38 (dd, J = 9.0, 2.6 Hz, 1H), 8.28 (s, 1H), 7.50 (overlapping t, J = 7.8 Hz, 1H) and d, J = 9.0 Hz, 2H), 7.30 (m, 2H); 13C NMR (101 MHz, DMSO-d6): δC 160.9, 160.1 (d, J = 246.9 Hz), 143.5, 142.2, 140.0, 132.1 (d, J = 3.3 Hz), 131.0 (d, J = 8.4 Hz), 130.2, 125.8, 125.1, 124.7 (d, J = 3.5 Hz), 123.9 (d, J = 14.9 Hz), 119.0, 116.4, 116.1 (d, J = 21.6 Hz); MS (m/z): 284 (M+·). Anal. Calcd for C15H9FN2O3: C, 63.38; H, 3.19; N, 9.86. Found: C, 63.35; H, 3.17; N, 9.81.
3.3.6. 3-(4-Chlorophenyl)-6-nitroquinolin-2(1H)-one (22)
Yield: 0.47 g (88%) as a white solid, m.p. 210–211 °C; IR: 3116, 1662, 1625, 1530, 1340 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 8.76 (d, J = 2.7 Hz, 1H), 8.48 (s, 1H), 8.44 (dd, J = 9.1, 2.7 Hz, 1H), 7.78 (d, J = 8.6 Hz, 2H), 7.68 (d, J = 9.1 Hz, 1H), 7.59 (d, J = 8.6 Hz, 2H), (NH exchanged); 13C NMR (101 MHz, DMSO-d6): δC 159.2, 157.1, 144.1, 140.1, 134.4, 133.3, 130.8, 128.9, 128.0, 126.8, 124.9, 120.2, 118.0; MS (m/z): 299, 301 (M+·, ca 3:1). Anal. Calcd for C15H9ClN2O3: C, 59.92; H, 3.02; N, 9.32. Found: C, 59.88; H, 2.98; N, 9.30.
3.3.7. 3-(2,5-Dimethylphenyl)-6-nitroquinolin-2(1H)-one (23)
Yield: 0.47 g (86%) as a white solid, m.p. 217–218 °C; IR: 3112, 1661, 1623, 1524, 1349 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 8.76 (d, J = 2.8 Hz, 1H), 8.45 (dd, J = 9.1, 2.8 Hz, 1H), 8.20 (s, 1H), 7.69 (d, J = 9.1 Hz, 1H), 7.20 (d, J = 7.8 Hz, 1H), 7.17 (dd, J = 7.8, 1.8 Hz, 1H), 7.12 (d, J = 1.8 Hz, 1H), 2.31 (s, 3H), 2.19 (s, 3H), (NH exchanged); 13C NMR (101 MHz, DMSO-d6): δC 159.1, 157.4, 144.1, 141.7, 135.2, 134.5, 133.7, 130.8, 130.7, 130.5, 129.9, 126.7, 124.8, 120.1, 118.9, 20.9, 19.5; MS (m/z): 294 (M+·). Anal. Calcd for C17H14N2O3: C, 68.91; H, 5.44; N, 9.45. Found: C, 68.86; H, 5.44; N, 9.42.
3.3.8. 6-Cyano-3-phenylquinolin-2(1H)-one (24)
Yield: 0.28 g (85%) as a white solid, m.p. 316–317 °C; IR: 3141, 2227, 1652 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.35 (s, 1H), 8.28 (d, J = 1.9 Hz, 1H), 8.15 (s, 1H), 7.88 dd, J = 8.6, 1.9 Hz, 1H), 7.74 (d, J = 7.1 Hz, 2H), 7.49–7.38 (complex, 4H); 13C NMR (101 MHz, DMSO-d6): δC 161.5, 141.5, 137.1, 136.0, 133.72, 133.66, 133.1, 129.2, 128.8, 128.5, 120.1, 119.4, 116.4, 104.4; MS (m/z): 246 (M+·). Anal. Calcd for C16H10N2O: C, 78.03; H, 4.09; N, 11.38. Found: C, 77.95; H, 4.06; N, 11.25.
3.3.9. 6-Cyano-3-(phenylsulfonyl)quinolin-2(1H)-one (25)
Yield: 0.38 g (91%) as a white solid, m.p. 357–358 °C; IR: 3149, 2230, 1656, 1622, 1322, 1158 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.63 (s, 1H), 9.04 (s, 1H), 8.61 (s, 1H), 8.05–7.95 (complex, 3H), 7.73 (t, J = 7.5 Hz, 1H), 7.63 (t, J = 7.6 Hz, 2H), 7.45 (d, J = 8.7 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δC 156.9, 144.5, 144.0, 139.6, 136.5, 136.1, 134.4, 132.8, 129.5, 129.0, 118.8, 117.7, 117.2, 105.4; MS (m/z): 310 (M+·); Anal. Calcd for C16H10N2O3S: C, 61.93; H, 3.25; N, 9.08. Found: C, 61.90; H, 3.27; N, 9.05.
3.3.10. 6-Carbomethoxy-3-phenylquinolin-2(1H)-one (26)
Yield: 0.29 g (93%) as a white solid, m.p. 254–255 °C; IR: 3155, 1726, 1654, 1621 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.27 (s, 1H), 8.42 (d, J = 2.0 Hz, 1H), 8.27 (s, 1H), 8.05 (dd, J = 8.6, 2.0 Hz, 1H), 7.76 (d, J = 7.7 Hz, 2H), 7.45 (t, J = 7.7 Hz, 2H), 7.40 (d, J = 8.6 Hz, 2H), 3.88 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δC 166.2, 161.7, 142.0, 138.2, 136.3, 132.9, 130.9, 130.7, 129.2, 128.6, 128.5, 123.5, 119.6, 115.5, 52.6; MS (m/z): 279 (M+·); Anal. Calcd for C17H13NO3: C, 73.11; H, 4.69; N, 5.02. Found: C, 73.04; H, 4.65; N, 4.96.
3.3.11. 6-Carbomethoxy-3-(phenylsulfonyl)quinolin-2(1H)-one (27)
Yield: 0.34 g (90%) as a white solid, m.p. 318–319 °C; IR: 3151, 1721, 1662, 1625 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.55 (s, 1H), 9.14 (s, 1H), 8.71 (d, J = 2.0 Hz, 1H), 8.18 (dd, J = 8.7, 2.0 Hz, 1H), 8.00 (d, J = 7.5 Hz, 2H), 7.71 (tt, J = 7.5, 1.2 Hz, 1H), 7.63 (t, J = 8.0 Hz, 2H), 7.42 (d, J = 8.7 Hz, 1H), 3.89 (s, 3H); 13C NMR (101 MHz, DMSO-d6): δC 165.8, 157.0, 145.6, 144.4, 139.9, 134.3, 134.2, 133.3, 132.1, 129.5, 128.9, 124.4, 117.3, 116.4, 52.8; MS (m/z): 343 (M+·); Anal. Calcd for C17H13NO5S: C, 59.47; H, 3.82; N, 4.08. Found: C, 59.49; H, 3.83; N, 4.04.
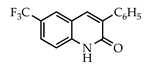
3.3.12. 3-Phenyl-6-(trifluoromethyl)quinolin-2(1H)-one (28)
Yield: 0.27 g (91%) as a white solid, m.p. 234–235 °C; IR: 3152, 1654, 1637, 1331, 1156, 1103 cm−1; 1H NMR (400 MHz, CDCl3): δH 12.20 (s, 1H), 7.96 (s, 1H), 7.90 (t, J = 1.2 Hz, 1H), 7.80 (dt, J = 6.8, 1.7 Hz, 2H), 7.69 (dd, J = 8.7, 2.0 Hz, 1H), 7.54–7.43 (complex, 4H); 13C NMR (101 MHz, DMSO-d6): δC 166.4, 145.9, 142.4, 140.9, 138.2, 133.9, 133.4, 133.3, 131.5 (q, J = 3.5 Hz), 130.9 (q, J = 4.3 Hz), 127.5 (q, J = 32.3 Hz), 124.4, 124.2 (q, J = 272.5 Hz), 120.9; MS (m/z): 289 (M+·). Anal. Calcd for C16H10FNO: C, 66.44; H, 3.48; N, 4.84. Found: C, 66.38; H, 3.45; N, 4.81.
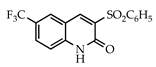
3.3.13. 3-(Phenylsulfonyl)-6-(trifluoromethyl)quinolin-2(1H)-one (29)
Yield: 0.35 g (95%) as a white solid, m.p. 319–320 °C; IR: 3160, 1659, 1634, 1333, 1168, 1133 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.57 (s, 1H), 9.14 (s, 1H), 8.55 (d, J = 2.2 Hz, 1H), 8.05–7.95 (complex, 3H), 7.74 (t, J = 7.5 Hz, 1H), 7.63 (t, J = 7.7 Hz, 2H), 7.51 (d, J = 8.7 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δC 157.0, 145.1, 143.7, 139.7, 134.4, 132.6, 130.2 (q, J = 3.8 Hz), 129.5, 129.0, 124.5 (q, J = 271.9 Hz), 123.7, 123.3, 117.3, 117.1; MS (m/z): 353 (M+·). Anal. Calcd for C16H10F3NO3S: C, 54.39; H, 2.85; N, 3.96. Found: C, 54.33; H, 2.87; N, 3.95.
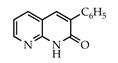
3.3.14. 3-Phenyl-1,8-naphthyridin-2(1H)-one (30)
Yield: 0.32 g (89%) as a white solid, m.p. 242–243 °C; IR: 3164, 1658, 1609 cm−1; 1H NMR (400 MHz, CDCl3): δH 11.99 (s, 1H), 8.77 (dd, J = 4.9, 1.7 Hz, 1H), 7.97 (dd, J = 7.7, 1.7 Hz, 1H), 7.84 (s, 1H), 7.76 (d, J = 7.5 Hz, 2H), 7.50–7.39 (complex, 3H), 7.24 (dd, J = 7.7, 4.9 Hz, 1H); 13C NMR (101 MHz, CDCl3): δC 162.4, 149.9, 149.3, 136.3, 136.0, 135.4, 134.6, 128.9, 128.6, 128.4, 118.7, 115.6; MS (m/z): 222 (M+·). Anal. Calcd for C14H10N2O: C, 75.66; H, 4.54; N, 12.60. Found: C, 75.59; H, 4.51; N, 12.51.
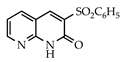
3.3.15. 3-(Phenylsulfonyl)-1,8-naphthyridin-2(1H)-one (31)
Yield: 0.43 g (93%) as a white solid, m.p. 299–300 °C; IR: 3146, 1651, 1608, 1310, 1149 cm−1; 1H NMR (400 MHz, DMSO-d6): δH 12.71 (s, 1H), 9.03 (s, 1H), 8.68 (dd, J = 4.7, 1.8 Hz), 8.49 (dd, J = 7.9, 1.8 Hz, 1H), 8.02 (d, J = 7.5 Hz, 2H), 7.72 (tt, J = 7.5, 1.3 Hz, 1H), 7.62 (t, J = 7.5 Hz, 2H), 7.38 (dd, J = 7.9, 4.7 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δC 156.6, 153.5, 150.6, 143.6, 139.0, 138.7, 133.2, 131.2, 128.4, 127.9, 118.7, 112.0; MS (m/z): 286 (M+·). Anal. Calcd for C14H10N2O3.S: C, 58.73; H, 3.53; N, 9.78. Found: C, 58.68; H, 3.52; N, 9.71.
4. Conclusions
We have successfully developed a domino aldol-SNAr-dehydration [3+3] annulation for the synthesis of 3,6-disubstituted quinolin-2(1H)-ones and 3-substituted 1,8-naphthyridin-2(1H)-ones. The transformation involves an aldol reaction of activated acetamides, substituted at C2 by an aryl, phenylsulfonyl, or ester group with 2-fluorobenzaldehydes substituted with electron-withdrawing substituents at C5 to promote SNAr reaction. The process was also found to be successful for 2-fluoronicotinaldehyde. Both target fused heterocycles were produced in high yields by this process. Since the methylene and amido protons of the amide reacting partner have similar pKa values, two different mechanisms, differing in the initial attacking anion, were possible. Model reactions of phenylacetamide with 2-nitrobenzaldehyde and methyl 2-fluoro-5-nitrobenzoate in DMF with K2CO3 at 23 °C suggested that aldol addition of the phenylacetamide methylene to the aldehyde triggers the cascade of events. The current work represents the first use of a 2-arylacetamide in a potentially valuable synthetic application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155856/s1, Copies of 1H-NMR and 13C-NMR spectra for all new compounds are available online.
Author Contributions
Project conception, project administration, formal analysis and writing the manuscript text, R.A.B.; investigation, methodology, analysis and writing the experimental section, K.F. and E.A. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was obtained from the Oklahoma State University Foundation and the College of Arts and Sciences at Oklahoma State University. The authors are indebted to the OSU College of Arts and Sciences for funds to purchase several departmental instruments including an FT-IR and a 400 MHz NMR unit for the State-wide NMR facility. The NMR facility was initially established with support from the NSF (BIR-9512269), the Oklahoma State Regents for Higher Education, the W. M. Keck Foundation, and Conoco, Inc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
K.F. wishes to thank the OSU Foundation for a K. Darrell Berlin Fellowship in the Summer 2021 and a Smith-Han Fellowship in the Summer 2023; E.A. wishes to thank the OSU Foundation for K. Darrell Berlin Fellowships in Summer 2020 and 2023 as well as a Johnston Chemistry Fellowship in Summer 2021.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Nammalwar, B.; Bunce, R.A.; Nago, T. Recent syntheses of 1,2,3,4-tetrahydroquinolines, 2,3-dihydro-4(1H)-quinolines and 4(1H)-quinolinones. Molecules 2014, 19, 204–232. [Google Scholar] [CrossRef]
- Bunce, R.A.; Schammerhorn, J.E.; Sigle, J. Substituted 4-oxo-1,2,3,4-tetrahydroquinoline-3-carboxylic esters by a tandem imine addition-SNAr reaction. J. Heterocycl. Chem. 2013, 50, 373–380. [Google Scholar] [CrossRef]
- Annor-Gyamfi, J.K.; Meraz, K.; Bunce, R.A. Naphthalenes and quinolines by domino reactions of Morita-Baylis-Hillman acetates. Molecules 2020, 25, 5168. [Google Scholar] [CrossRef] [PubMed]
- Annor-Gyamfi, J.K.; Ametsetor, E.; Meraz, K.; Bunce, R.A. Dihydroquinoline, dihydronaphthyridines and quinolones by domino reactions of Morita-Baylis-Hillman acetates. Molecules 2021, 26, 890. [Google Scholar] [CrossRef] [PubMed]
- Ametsetor, E.; Farthing, S.; Bunce, R.A. Domino aza-Michael-SNAr-heteroaromatization route to C5-substituted N-alkyl-1H-indole-3-carboxylic esters. Molecules 2022, 27, 6998. [Google Scholar] [CrossRef]
- Bunce, R.A.; Rogers, D.; Nago, T.; Bryant, S.A. 4H-1-Benzopyrans by a tandem SN2-SNAr reaction. J. Heterocycl. Chem. 2008, 45, 547–550. [Google Scholar] [CrossRef]
- Gerasyuto, A.I.; Ma, Z.-X.; Buchanan, G.S.; Hsung, R.P. Establishing the concept of aza-[3+3] annulations using enones as a key expansion of this unified strategy in alkaloid synthesis. Beilstein J. Org. Chem. 2013, 9, 1170–1178. [Google Scholar] [CrossRef]
- Li, G.; Carlson, L.J.; Sagamanova, I.K.; Slafer, B.W.; Hsung, R.P.; Gilardi, C.; Sklenicka, H.M.; Sydroenko, N. A Stereodivergent approach for accessing both C2,8a-syn and C2,8a-anti relative stereochemical manifolds in the Lepadin family via a TiCl4-promoted aza-[3+3] annulation. Synthesis 2009, 2009, 2905–2914. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, Y.; Hou, Y.; Tong, X. Access to thiopyrano[2,3-b]indole via tertiary amine-catalyzed formal [3+3] annulations of b′-acetoxy allenoates with indole-2-thiones. Chem. Commun. 2017, 53, 2567–2570. [Google Scholar] [CrossRef]
- Debnath, S.; Kumar, A.S.; Chauhan, S.; Swamy, K.C.K. Lewis-base dependent (3+3) annulations of acetoxy allenoates with iminoindolines: α-Carboline scaffolds with varied substituents. Adv. Synth. Catal. 2022, 364, 4316–4332. [Google Scholar] [CrossRef]
- Yang, W.; Sun, W.; Zhang, C.; Wang, Q.; Guo, Z.; Mao, B.; Liao, J.; Guo, H. Lewis-base-catalyzed asymmetric [3+3] annulation reaction of Morita-Baylis-Hillman carbonates: Enantioselective synthesis of spirocyclohexenes. ACS Catal. 2017, 7, 3142–3146. [Google Scholar] [CrossRef]
- Satham, L.; Namboothiri, I.N.N. (3+3) annulation of nitroallylic acetates with stabilized sulfur ylides for the synthesis of 2-aryl terephthalates. J. Org. Chem. 2018, 83, 9471–9477. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, H.; Deng, G.-J.; Huang, H. Copper-catalyzed formal [3+3] annulations of arylketoximes and o-fluorobenzaldehydes: An entry to quinoline compounds. Org. Lett. 2021, 23, 936–942. [Google Scholar] [CrossRef]
- Nunewar, S.; Kumar, S.; Pandhare, H.; Nanduri, S.; Kanchupalli, V. Rh(III)-catalyzed chemodivergent annulations between indoles and iodonium carbenes: A rapid access to tricyclic and tetracyclic N-heterocycles. Org. Lett. 2021, 23, 4233–4238. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Chen, Y.; Mei, G.-J.; Hua, Y.-Z.; Jia, S.-K.; Wang, M.-C. Zinc-catalyzed enantioselective [3+3] annulation for synthesis of chiral spiro[indolin-3,4′-thiopyrano[2,3-b]indole] derivatives. Molecules 2023, 28, 1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-B.; He, Q.; Wang, T.; Wang, G.; Yang, D.; Han, P.; Jing, L. Organocatalytic asymmetric [3+3] annulations of 3-carboxamide oxindoles with β,γ-unsaturated α-ketoesters: Facile access to chiral spiro-δ-lactam oxindoles. Org. Chem. Front. 2023, 10, 957–962. [Google Scholar] [CrossRef]
- Feng, J.; Liu, B. Formal carbo [3+3] annulation and its application in organic synthesis. Tetrahedron Lett. 2015, 56, 1474–1485. [Google Scholar] [CrossRef][Green Version]
- Zhong, W.; Liu, H.; Kaller, M.R.; Henley, C.; Magal, E.; Nguyen, T.; Osslund, T.D.; Powers, D.; Rzasa, R.M.; Wang, H.-L.; et al. Design and synthesis of quinolin-2(1H)-one derivatives as potent CDK5 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5384–5389. [Google Scholar] [CrossRef]
- Kuang, W.-B.; Huang, R.-Z.; Qin, J.-L.; Lu, X.; Qin, Q.-P.; Zou, B.-Q.; Chen, Z.-F.; Liang, H.; Zhang, Y. Design, synthesis and pharmacological evaluation of new 3-(1H)-benzimidazol-2-yl)quinolin-2(1H)-one derivatives as potential antitumor agents. Eur. J. Med. Chem. 2018, 157, 139–150. [Google Scholar] [CrossRef]
- Mohamed, M.F.A.; Abuo-Rahma, G.E.-D.A. Molecular targets and anticancer activity for quinoline-chalcone hybrids: Literature review. RSC Adv. 2020, 10, 31139–31155. [Google Scholar] [CrossRef]
- Larghi, E.; Bruneau, A.; Sauvage, F.; Alami, M.; Vergnaud-Gauduchon, J. Synthesis and biological activity of 3-(heteroaryl)quinolin-2(1H)-ones bis-heterocycles as potential inhibitors of protein folding machinery. Molecules 2022, 27, 412. [Google Scholar] [CrossRef]
- Gale, D.J.; Wilshire, J.F.K. The preparation of sole polymethine astrazon dyes. Aust. J. Chem. 1970, 23, 1063–1068. [Google Scholar] [CrossRef]
- Finan, P.A.; Fothergill, G.A. The preparation of acid amides from acid chlorides. J. Chem Soc. 1962, 1962, 2824–2825. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Li, R.-Q.; Liu, R.; Guo, Q.-X. First-principle predictions of absolute pKa’s of organic acids in dimethyl sulfoxide solution. J. Am. Chem. Soc. 2004, 126, 814–822. [Google Scholar] [CrossRef]
- Cook, M.J.; Katritzky, A.R.; Linda, P.; Tack, R.D. Aromaticity and tautomerism. Part II. The 4-pyridone, 2-quinolone and 1-isoquinolone series. J. Chem. Soc. Perkin Trans 2 1973, 1973, 1080–1086. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2012; p. 729. ISBN 9780199270293. [Google Scholar]
- Karty, J. Organic Chemistry, 3rd ed.; W.W. Norton: New York. NY, USA, 2022; p. 728. ISBN 9780393544015. [Google Scholar]
- Bunce, R.A.; Smith, C.L.; Knight, C.L. N-(Nitrophenyl)benzamide and benzenesulfonamide derivatives by nucleophilic aromatic substitution. Org. Prep. Proced. Int. 2004, 36, 482–487. [Google Scholar] [CrossRef]
- D’Agostino, V.F.; Dunn, M.J.; Ehrlich, A.E.; Becker, E.I. Absorption spectra of tetracyclones. V. J. Org. Chem. 1958, 23, 1539–1544. [Google Scholar] [CrossRef]
- Rappoport, Z. Handbook of Tables for Organic Compound Identification, 3rd ed.; CRC Press: Cleveland, OH, USA, 1975; ISBN 0-87819-303D. [Google Scholar]
- Skinner, G.S.; Sanderson, T.F.; Bieber, E.R.; Ewadh, H. β-Cyano-α-hydroxycinnamates from the xylenes. J. Org. Chem. 1959, 24, 403–405. [Google Scholar] [CrossRef]
- Wehrmeister, H.L. Synthesis of carbostyril derivatives by reaction of aldehydes with oxazolines. J. Heterocycl. Chem. 1976, 13, 61–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).