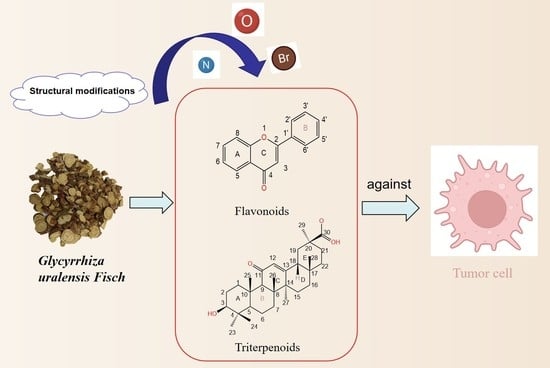
Research Progress on Structural Modification of Effective Antitumor Active Ingredients in Licorice
Abstract
1. Introduction
2. Ingredient Research
2.1. Flavonoids
2.1.1. Flavonoids
2.1.2. Flavonols
2.1.3. Isoflavones
2.1.4. Chalcones
2.1.5. Dihydroflavonoids
2.2. Triterpenoids
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Committee, N.P. Pharmacopoeia of the People’s Republic of China: Part I; China Medical Science and Technology Press: Beijing, China, 2020; pp. 88–89. [Google Scholar]
- Shi, L.; Guo, Y.; Cao, S.; Pei, L.; Li, Y. Tracing to the Origin of Glycyrrhiza uralensis Fisc. Res. Pract. Chin. Med. 2020, 34, 82–86. [Google Scholar]
- Zhao, J.; Wang, Y.; Wong, Q.; Jin, Y.; Zhang, W.; Peng, H.; Cai, Q.; Li, B.; Yang, H.; Zhang, H.; et al. Herbal Textual Research on Glycyrrhizae Radix et Rhizoma in Chinese Classical Prescriptions. Mod. Chin. Med. 2020, 22, 1162–1174. [Google Scholar]
- Huang, S.; Li, C.; Huang, J.; Mo, D.; Luo, P.; Wang, H. Effects of glabridin on proliferation, invasion and apoptosis of Hela cells from cervical cancer and its mechanisms. J. Mod. Oncol. 2020, 28, 3289–3293. [Google Scholar]
- Yuan, Y.; Zhang, X.; Zhu, X.; Wang, W.; Zeng, H.; Le, J. Effect of Licochalcone A on Apoptosis in Human Breast Cancer MDA-MB-231 Cells. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 95–100. [Google Scholar]
- Xin, H.; Xu, W. Effect of licochalcone A on autophagy in renal cell carcinoma via PI3K/Akt/mTOR signaling pathway. China J. Chin. Mater. Medica 2018, 43, 3545–3552. [Google Scholar]
- Wang, J.; Luo, P.; Fei, Z. Anti–tumor effects and cellular mechanisms of Licochalcone A. J. Mod. Oncol. 2016, 24, 3870–3874. [Google Scholar]
- Zhang, N.; Zhang, X. Effect of Glycyrrhizin on Immune Function and Apoptosis of U14 Cervical Cancer Mice. Sci. Technol. Food Ind. 2021, 42, 370–376. [Google Scholar]
- Luo, Z.; Li, Q.; Zhang, J.; Yang, M. Advances in studies on Glycyrrhiza glabra. Chin. Tradit. Herb. Drugs 2011, 42, 2154–2158. [Google Scholar]
- Li, N.; Zhang, C.; Zhang, G.; Liu, H.; Chen, S.; Chen, F.; Li, M.; Liao, W.; Ren, Y. Research progress on chemical constituents and pharmacological effects of different varieties of Glycyrrhizae Radix et Rhizoma and predictive analysis of quality markers. Chin. Tradit. Herb. Drugs 2021, 52, 7680–7692. [Google Scholar]
- Yahara, S.; Nishioka, I. Flavonoid glucosides from licorice. Phytochemistry 1984, 23, 2108–2109. [Google Scholar] [CrossRef]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Mei, L.; Zhi, Y.H.; Zhi, Y.H. In Vitro Evaluation of The Inhibitory Effect of Commonly Used Pharmaceutical Excipients on the Six CYP Isoforms. In Proceedings of the 4th Asian Pacific Regional International Society for the Study of Xenobiotics Meeting, Taiwan, China, 22–25 April 2011. [Google Scholar]
- Leng, J.; Zhu, Y.; Zhu, L.; Wang, S. Two new triterpenoid saponins from roots and rhizomes of Glycyrrhiza uralensis. Chin. Tradit. Herb. Drugs 2015, 46, 1576–1582. [Google Scholar]
- Wang, Q.; Miao, W.; Xiang, C.; Guo, D.; Ye, M. Chemical constituents from Glycyrrhiza uralensis. Chin. Tradit. Herb. Drugs 2012, 43, 1886–1890. [Google Scholar]
- Jia, W.; Liu, D.; Zheng, X.; Zhang, Y.; Zhang, Y. Two new isoprenyl flavonoids from the leaves of glycyrrhiza uralensis fisch. Acta Pharm. Sin. 1993, 1993, 28–31. [Google Scholar]
- Xiang, H.; Geng, Y.; Wu, Z.; Zhao, X.; Jian, J. Synthesis and Anti-hepatoma Activities of Apigenin etherified and brominated derivatives. J. Hunan Norm. Univ. 2009, 6, 26–29. [Google Scholar]
- Cárdenas, M.; Marder, M.; Blank, V.C.; Roguin, L.P. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg. Med. Chem. 2006, 14, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, T.; Ohmoto, T.; Kinoshita, T.; Sankawa, U.; Monache, F.D.; Botta, B.; Tomimori, T.; Miyaichi, Y.; Shirataki, Y.; Yokoe, I.; et al. Inhibition of adenosine 3′,5′-cyclic monophosphate phosphodiesterase by flavonoids III. Chem. Pharm. Bull. 1989, 37, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Daskiewicz, J.B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J.M.; Johnson, I.T.; Ndjoko, K.; Hostettmann, K. Effects of flavonoids on cell proliferation and caspase activation in a human colonic cell line HT29: An SAR study. J. Med. Chem. 2005, 48, 2790–2804. [Google Scholar] [PubMed]
- Colvin, O.M. An overview of cyclophosphamide development and clinical applications. Curr. Pharm. Des. 1999, 5, 555–560. [Google Scholar] [CrossRef]
- Han, T. Design and Synthesis of Six Kinds of Flavone-7-Phosphoramidate Derivatives. Master’s Thesis, Dalian University of Technology, Dalian, China, 2012. [Google Scholar]
- Yang, Y.; Tao, L.; Zhang, Y.; Sun, S.; Guo, H. Structure and Biological Activity of the La(III) Complex with Apigenin. Nat. Prod. Res. Dev. 2014, 26, 1402–1406. [Google Scholar]
- Patil, S.M.; Patil, M.B.; Sapkale, G.N. antimicrobial activity of glycyrrhiza glabra linn. Roots. Int. J. Chem. Sci. 2009, 7, 585–591. [Google Scholar]
- Zhou, B.; Wan, C. Chemical constituents from aerial parts of Glycyrrhiza uralensis. Chin. Tradit. Herb. Drugs 2016, 47, 21–25. [Google Scholar]
- Li, W.; Song, X.; Zhang, L.; Yu, H.; An, J. Advances in Research on Chemical Constituents of Radix Glycyrrhiza. J. Liaoning Univ. Tradit. Chin. Med. 2012, 14, 40–44. [Google Scholar]
- Fan, Y. Isolation, Identification, Quantification and Bioactivity Assessment of the Chemical Constituents in Glycyrrhiza Uralensis Leaves. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2018. [Google Scholar]
- Xiao, Y.; Zhu, J.; Liu, X.; Jin, J.; Li, Y.; Ge, Z.; Ma, J.; Zhang, C. Research progress on chemical constituents, pharmacological effects and clinical applications of Shaoyao Gancao Decoction and predictive analysis of its Q-Marker. Chin. Tradit. Herb. Drugs 2022, 53, 7960–7969. [Google Scholar]
- Luo, Y.; You, P.; Yang, M.; Liu, Y.; Liu, D. Synthesis and Antitumor Activities of Nitrogen-Containing Galangin Derivatives. Nat. Prod. Res. Dev. 2017, 29, 575–578. [Google Scholar]
- Zhao, L.; Wu, F. Structural modification of galangin and the study of inhibition activity on K562 cells. J. Wuhan Polytech. Univ. 2016, 35, 31–36. [Google Scholar]
- Gf, A.; Fas, B.; Sv, C.; Aa, D.; Hs, E.; Ak, F.; Mt, G.; Ab, G. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wen, Q.; Zhao, X. Quercetin attenuates the proliferation of arsenic-related lung cancer cells via a caspase-dependent DNA damage signaling. Mol. Carcinog. 2022, 61, 655–663. [Google Scholar] [CrossRef]
- Soofiyani, S.R.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Tarhriz, V.; Asgharian, P.; Reiner, Ž.; Sharifi-Rad, J.; Cho, W.C. Quercetin as a Novel Therapeutic Approach for Lymphoma. Oxidative Med. Cell. Longev. 2021, 2021, 3157867. [Google Scholar] [CrossRef]
- Parvaresh, A.; Razavi, R.; Rafie, N.; Ghiasvand, R.; Miraghajani, M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 34. [Google Scholar]
- Sun, T.; Sun, C.; Dai, G.; Zhang, H. Syntheses of derivatives of 4′-alkylaminoalkylquercetin. Chin. J. Med. Chem. 2003, 13, 41–44. [Google Scholar]
- Mulholland, P.J.; Ferry, D.R.; Anderson, D.; Hussain, S.A.; Young, A.M.; Cook, J.E.; Hodgkin, E.; Seymour, L.W.; Kerr, D.J. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Golding, B.T.; Griffin, R.J.; Quarterman, C.P.; Slack, J.A.; Williams, J.G. Analogues or Derivatives of Quercetin (Prodrugs). U.S. Patent 6,258,840, 31 December 1997. [Google Scholar]
- Liu, Q.; Feng, Y.; Qu, W.; Zhai, G. Synthesis and Biological Activities of Quercetin Amide Derivatives. Chin. Pharm. J. 2019, 54, 1565–1574. [Google Scholar]
- Durgo, K.; Halec, I.; Šola, I.; Franekić, J. Cytotoxic and Genotoxic Effects of the Quercetin/Lanthanum Complex on Human Cervical Carcinoma Cells In Vitro. Arch. Ind. Hyg. Toxicol. 2011, 62, 221–227. [Google Scholar]
- Du, L.; Chang, B.; Zhang, Q.; Yang, F.; Du, W. Chemical constituents of flavonoids from roots of Glycyrrhiza glabra. Chin. Tradit. Herb. Drugs 2018, 49, 4780–4784. [Google Scholar]
- Zhang, Y.; Xu, X.; Hu, B.; Liu, Q.; Hou, C.; Liu, Y.; Yang, J. Isoflavones from glycyrrhiza eurycarpa. Acta Pharm. Sin. 1997, 32, 62–65. [Google Scholar]
- Wang, Z.; Han, L.; Liu, X.; Yuan, Y.; Liu, K. Preparation and separation of onon in from glycyrrhiza uralensisi fish with high-speed counter current chromtography. Shandong Sci. 2010, 23, 18–21. [Google Scholar]
- Liu, Y. Study of licorice composition: Structure of glycyrrhizone. J. Int. Pharm. Res. 1975, 01, 54–55. [Google Scholar]
- Liu, Q.; Liu, Y. An overview of research on flavonoids in licorice. Chin. Pharm. J. 1989, 12, 705–709. [Google Scholar]
- Asada, Y.; Li, W.; Yoshikawa, T. The first prenylated biaurone, licoagrone from hairy root cultures of Glycyrrhiza glabra. Phytochemistry 1999, 50, 1015–1019. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The Potential Therapeutic Properties of Prunetin against Human Health Complications: A Review of Medicinal Importance and Pharmacological Activities. Drug Metab. Bioanal. Lett. 2022, 15, 166–177. [Google Scholar]
- Hayashi, H.; Yasuma, M.; Hiraoka, N.; Ikeshiro, Y.; Yamamoto, H.; Ye?Ilada, E.; Sezik, E.; Honda, G.; Tabata, M. Flavonoid variation in the leaves of Glycyrrhiza glabra. Phytochemistry 1996, 42, 701–704. [Google Scholar] [CrossRef]
- Li, W.; Asada, Y.; Yoshikawa, T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry 2000, 55, 447–456. [Google Scholar] [CrossRef]
- Zhang, D. Talking about the ingredients of licorice and their effects. Chin. Med. Mod. Distance Educ. China 2012, 10, 128–129. [Google Scholar]
- Kai, L.; Ji, S.; Wei, S.; Kuang, Y.; Ye, M. Glycybridins A-K, Bioactive Phenolic Compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar]
- Liu, Y.; Chen, Y.; Wang, D.; Gao, X.; Guo, H.; Fu, X.; Wang, W. Studies on chemical constituents on roots of Glycyrrhiza uralensis. Chin. J. Pharm. Anal. 2011, 31, 1251–1255. [Google Scholar]
- Rui, C. Domestic research progress on the chemical composition of licorice. Chin. J. Sch. Dr. 2006, 1, 105–106. [Google Scholar]
- Liu, J.; Yang, S.; Fu, Y.; Xu, D.; Jiang, C.; Hou, F. Study on the flavonoids of licorice. Chin. Tradit. Herb. Drugs 1992, 23, 349–350. [Google Scholar]
- Xing, G.; Li, N.; Wang, T.; Yao, M. Advances in studies on flavonoids of licorice. China J. Chin. Mater. Med. 2003, 28, 593–597. [Google Scholar]
- Huang, Y.; Chi, Z.; Wang, S.; Wu, H.; Qin, K.; Li, W. Research progress on Licorice flavonoids and their antitumor activities. Chin. J. New Drugs 2017, 26, 1532–1537. [Google Scholar]
- Yuldashev, M.P.; Batirov, E.K.; Vdovin, A.D.; Abdullaev, N.D. Structural study of glabrisoflavone, a novel isoflavone fromGlycyrrhiza glabraL. Russ. J. Bioorg. Chem. 2000, 26, 784–786. [Google Scholar] [CrossRef]
- Fukai, T.; Tantai, L.; Nomura, T. Isoprenoid-substituted flavonoids from Glycyrrhiza glabra. Phytochemistry 1996, 43, 531–532. [Google Scholar] [CrossRef]
- Bhardwaj, D.K.; Seshadri, T.R.; Singh, R. Glyzarin, a new isoflavone from Glycyrrhiza glabra. Phytochemistry 1977, 16, 402–403. [Google Scholar] [CrossRef]
- Hong, B.; Wang, Y.; Zhan, X.; Dou, D.; Pei, Y.; Chen, Y.; Li, W.; Koike, K.; Nikaido, T. Study on the chemical constituents of aerial parts of cultivated licorice. Northwest Pharm. J. 2005, 2, 59–61. [Google Scholar]
- Zhuang, Y.; Zhou, C.; Wang, Y.; Li, D. Research progress of nitrogen mustard antitumor drugs. Chin. Pharm. J. 2008, 17, 1281–1287. [Google Scholar]
- Hu, K.; Xu, H.; Xin, W.; Chen, X.; Ren, J. Synthesis and antitumor activity of nitrogen mustard derivatives of formononetin. J. China Pharm. Univ. 2012, 43, 113–119. [Google Scholar]
- Xin, F.; Yang, X.; Huang, L.; Lin, J.; Wei, J. Synthesis and characterization of anthocyanin derivatives with NO release. Chem. Reag. 2011, 33, 958–960. [Google Scholar]
- Polkowski, K.; Popiołkiewicz, J.; Krzeczyński, P.; Ramza, J.; Pucko, W.; Zegrocka-Stendel, O.; Boryski, J.; Skierski, J.S.; Mazurek, A.P.; Grynkiewicz, G. Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines. Cancer Lett. 2004, 203, 59–69. [Google Scholar] [CrossRef]
- Popio?Kiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs-genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, C.; Wu, Q.; Yao, B.; Dai, Y.; Zhang, L.; Shen, X. Design and synthesis of genistein derivatives 5-hydroxy-4′-nitro-7-substituted-acyloxo-isoflavone and their antitumor effects. Acad. J. Nav. Med. Univ. 2005, 26, 182–185. [Google Scholar]
- Li, H.Q.; Ge, H.M.; Chen, Y.X.; Xu, C.; Shi, L.; Ding, H.; Zhu, H.L.; Tan, R.X. Synthesis and Cytotoxic Evaluation of a Series of Genistein Derivatives. Chem. Biodivers. 2006, 3, 463–472. [Google Scholar] [CrossRef]
- Zhang, L.N.; Xiao, Z.P.; Ding, H.; Ge, H.M.; Tan, R.X. Synthesis and Cytotoxic Evaluation of Novel 7-O-Modified Genistein Derivatives. Chem. Biodivers. 2010, 4, 248–255. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhiza glabra roots and their PPAR-γ ligand-binding activity. Bioorg. Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Li, Y.; Chen, X.; Guo, D.A. An integrated exact mass spectrometric strategy for comprehensive and rapid characterization of phenolic compounds in licorice. Rapid Commun. Mass. Spectrom. 2013, 27, 2297–2309. [Google Scholar] [CrossRef]
- Kinoshita, T.; Kajiyama, K.; Hiraga, Y.; Takahashi, K.; Tamura, Y.; Mizutani, K. The Isolation of New Pyrano-2-arylbenzofuran Derivatives from the Root of Glycyrrhiza glabra. Chem. Pharm. Bull. 1996, 44, 1218–1221. [Google Scholar] [CrossRef][Green Version]
- Asada, Y.; Wei, L.; Yoshikawa, T. Isoprenylated flavonoids from hairy root cultures of Glycyrrhiza glabra. Phytochemistry 1998, 47, 389–392. [Google Scholar] [CrossRef]
- Liu, T.; Hu, Y. Mannich Reaction of 2′-Hydroxychalcone and Biological Activity of Its Mannich Base. Chin. J. Org. Chem. 2006, 26, 983–987. [Google Scholar]
- Kong, Y.; Wang, K.; Edler, M.C.; Hamel, E.; Mooberry, S.L.; Paige, M.A.; Brown, M.L. A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg. Med. Chem. 2010, 18, 971–977. [Google Scholar] [CrossRef]
- Ma, Y. Synthesis and Antitumor Activity of Isoglycyrrhizin and Its Derivatives. Master’s thesis, Northeast Forestry University, Harbin, China, 2009. [Google Scholar]
- Yang, X.; Wang, Y.; Muhebuli, A.; Xilizhati, A.; Ren, B. Synthesis of Chalcone Isoliquiritigenin Compounds and Its Anti-cervical Cancer Activity. Chin. J. Mod. Appl. Pharm. 2016, 33, 1–6. [Google Scholar]
- Xu, F.; Mourboul, A.; Gao, M.; Sheng, L.; Wang, Y.; Abulizi, A. Anticaner activity of licorice chalcone derivatives in human Bel-7402 hepatocarinoman cell lines. J. Xinjiang Med. Univ. 2013, 36, 143–149. [Google Scholar]
- Somjen, D.; Katzburg, S.; Vaya, J.; Kaye, A.M.; Hendel, D.; Posner, G.H.; Tamir, S. Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. J. Steroid Biochem. Mol. Biol. 2004, 91, 241–246. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef]
- Han, J.B.; Wu, Y.; Wang, S.; Yi, L.; Hu, Y.L. Chemical constituents and chemotaxonomic study of Glycyrrhiza glabra L. Biochem. Syst. Ecol. 2020, 92, 104130. [Google Scholar] [CrossRef]
- Litvinenko, V.I.; Obolentseva, G.V. Chemical and pharmaceutical research on flavanoids of glycyrrhiza glabra l. and glycyrrhiza uralensis fisch. Med. Prom. Sssr 1964, 19, 20–23. [Google Scholar]
- Fukui, H.; Goto, K.; Tabata, M. Two antimicrobial flavanones from the leaves of Glycyrrhiza glabra. Chem. Pharm. Bull. 1988, 36, 4174–4176. [Google Scholar] [CrossRef]
- Ren, J.; Pan, S.; Hu, K. Synthesis and anticancer activity of liquiritigenin thiosemicarbazone derivatives. J. Shenyang Pharm. Univ. 2011, 28, 192–197. [Google Scholar]
- Ma, J.; Zhang, J.; Yao, J.; Zheng, S. GC-MS analysis of volatile constituents from the leaves of Glycyrrhiza glabra Linn. Northwest Pharm. J. 2006, 4, 153–155. [Google Scholar]
- Wei, J. Study on the Chemical Composition and Activity of Triterpene Saponins of Licorice; Nanjing University of Chinese Medicine: Nanjing, China, 2015. [Google Scholar]
- Zheng, Y.; Sun, J.; Duan, W.; Li, Y.; Chen, L.; Lu, T.; Li, C.; Peng, G. Chemical constituents of triterpenoid saponins from Glycyrrhiza glabra. Acta Pharm. Sin. 2021, 56, 289–295. [Google Scholar]
- Shi, R.; Kan, Y.; Li, X. Study on chemical composition of dichloromethane extraction site of prickly fruit licorice. J. Chin. Med. Mater. 2001, 1, 38–39. [Google Scholar]
- Zhang, N.; Cui, X.; Zhao, X.; Li, D.; Dai, L.; Tao, Z. Synthesis and Antitumor Activities of 18α-Glycyrrhetinic Acid Derivatives. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 37–41. [Google Scholar]
- Csuk, R.; Schwarz, S.; Kluge, R.; Ströhl, D. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur. J. Med. Chem. 2010, 45, 5718–5723. [Google Scholar] [CrossRef]
- Fiore, C.; Salvi, M.; Palermo, M.; Sinigaglia, G.; Armanini, D.; Toninello, A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta 2004, 1658, 195–201. [Google Scholar] [CrossRef]
- Mauro, S.; Cristina, F.; Valentina, B.; Mario, P.; Decio, A.; Antonio, T. Carbenoxolone induces oxidative stress in liver mitochondria, which is responsible for transition pore opening. Endocrinology 2005, 146, 2306–2312. [Google Scholar]
- Liu, Y.; Qian, K.; Wang, C.Y.; Chen, C.H.; Yang, X.; Lee, K.H. Synthesis and biological evaluation of novel spin labeled 18β-glycyrrhetinic acid derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7530–7533. [Google Scholar] [CrossRef]
- Shen, L.H.; Lai, Y.S.; Zhang, Y.H.; Luo, X.X.; Zhang, L.Y. Synthesis and anti-tumor activities of nitrate derivatives of glycyrrhetinic acid. J. China Pharm. Univ. 2008, 39, 103–107. [Google Scholar]

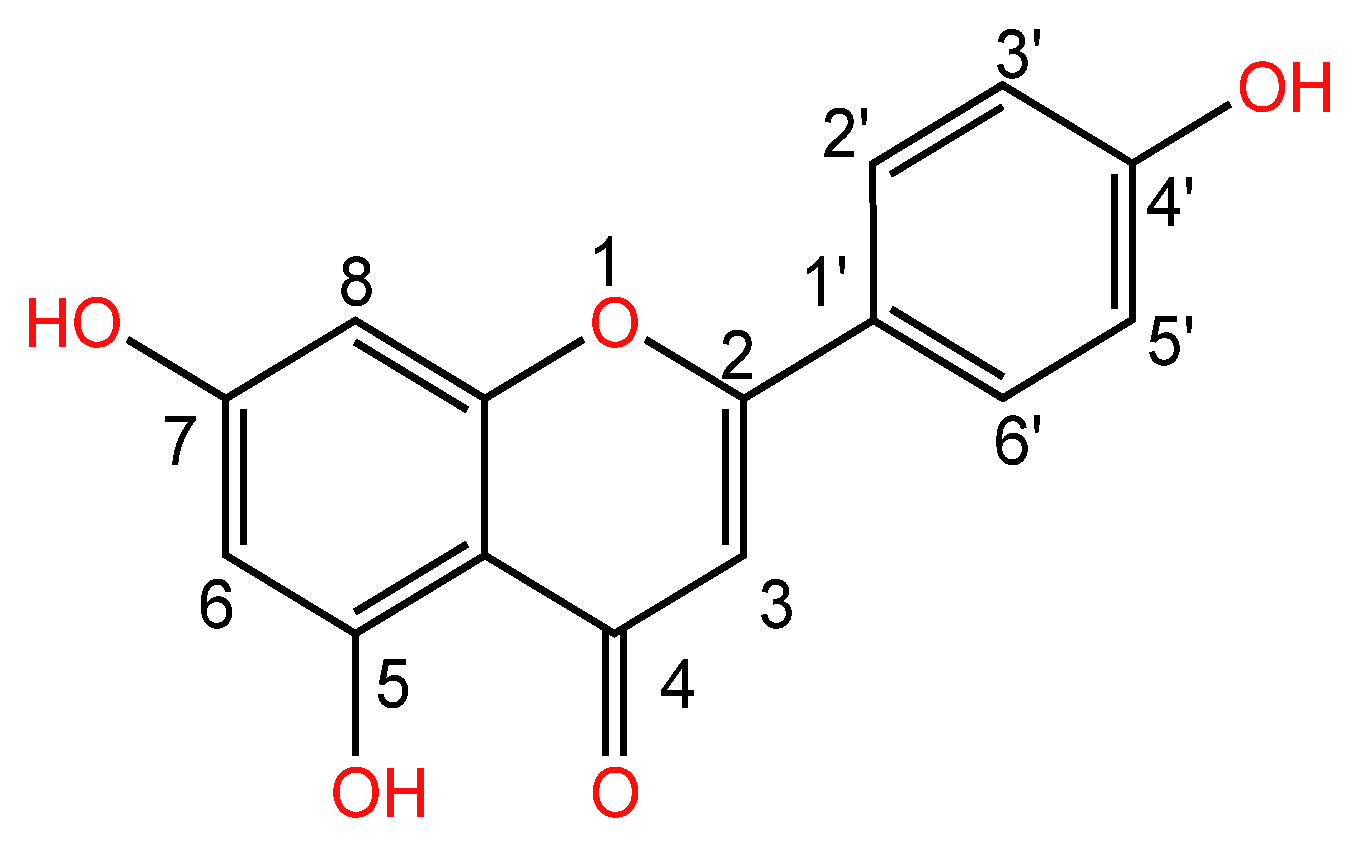

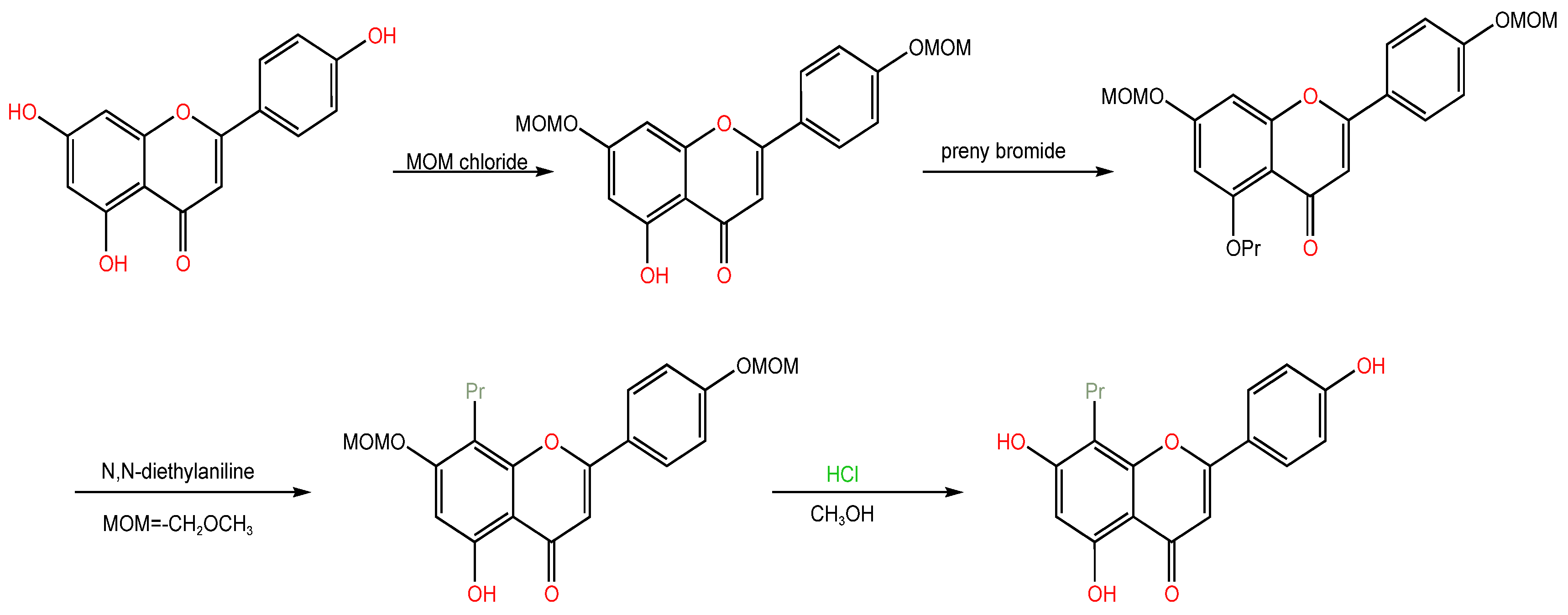


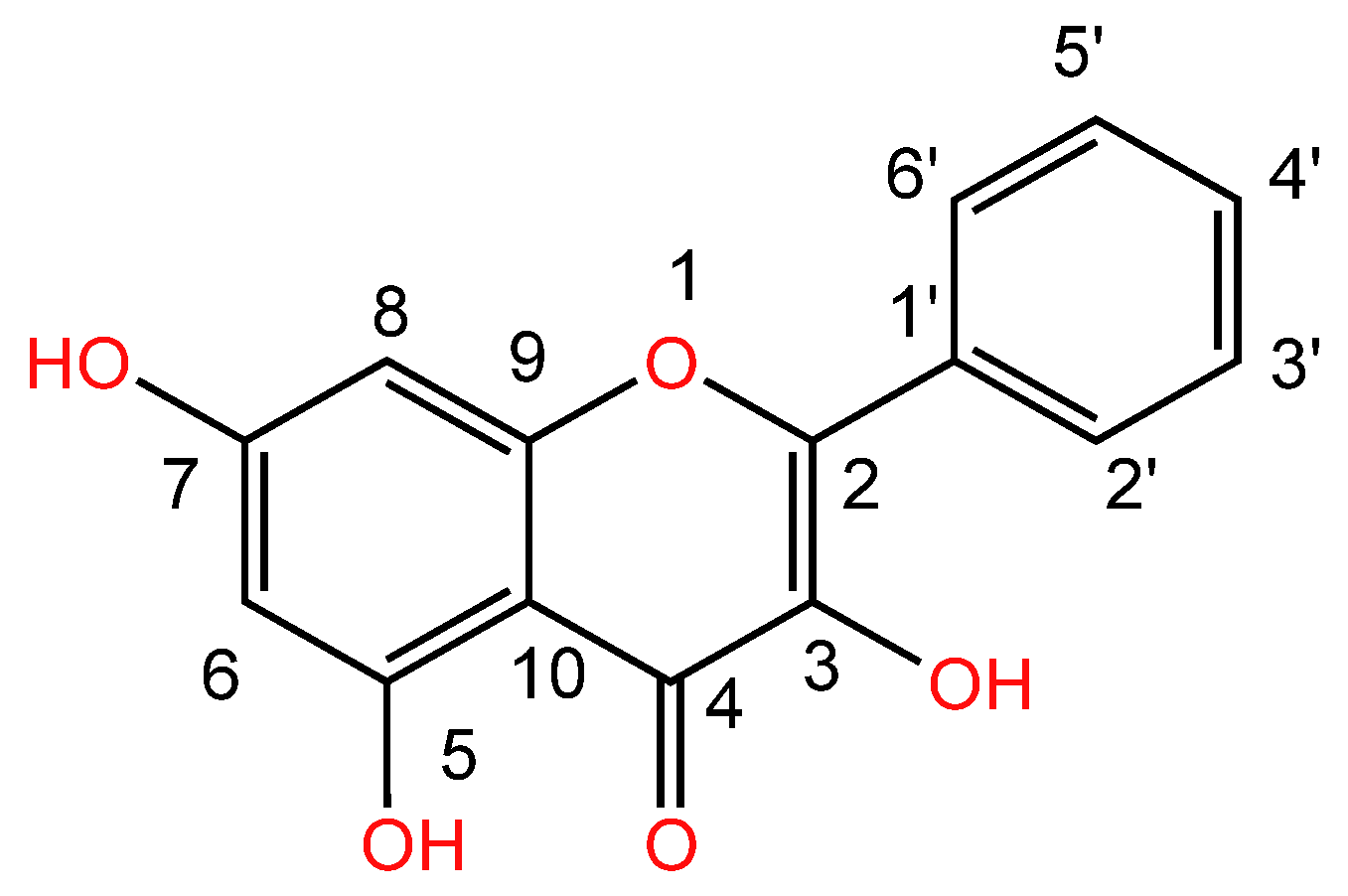




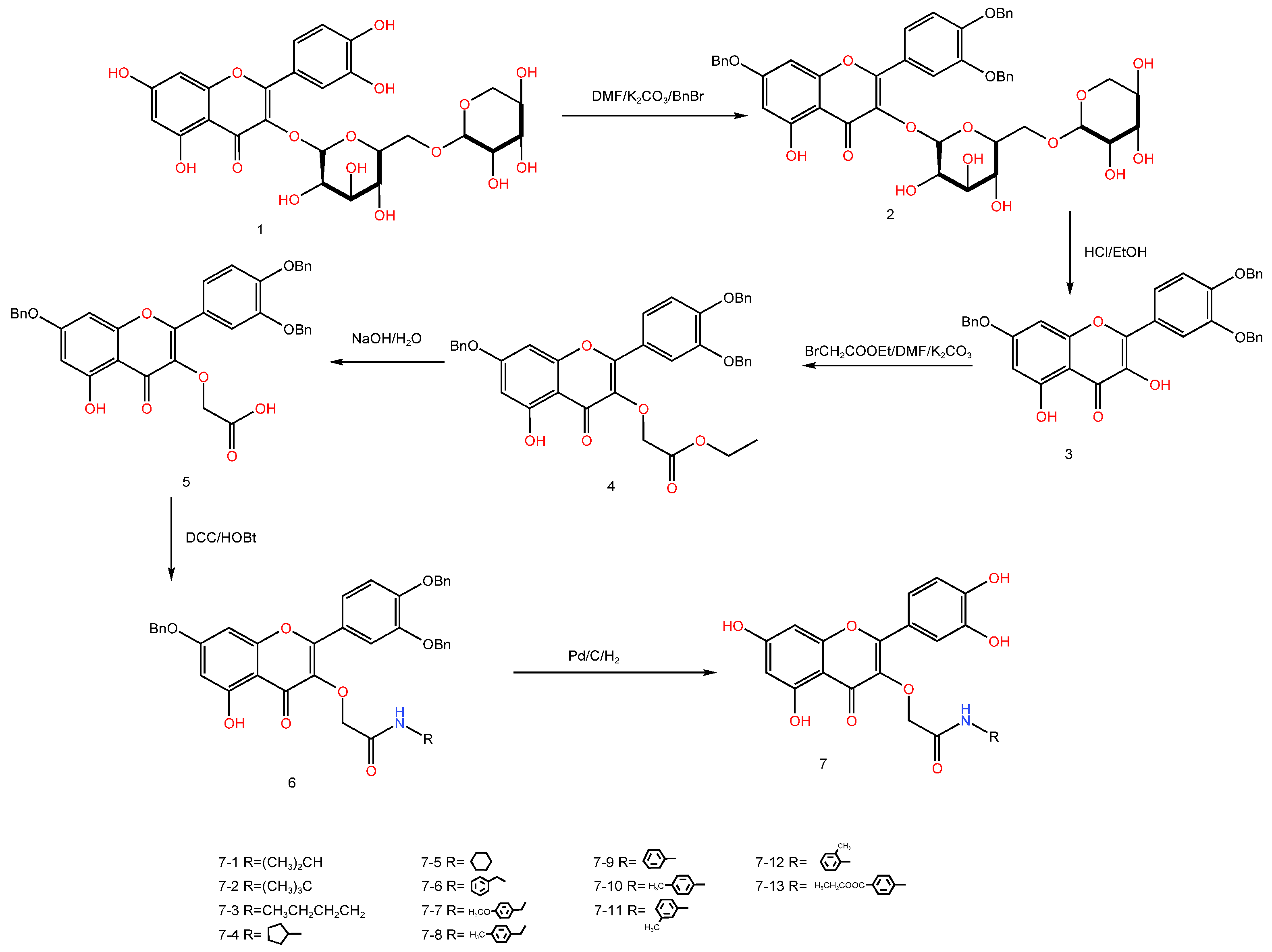
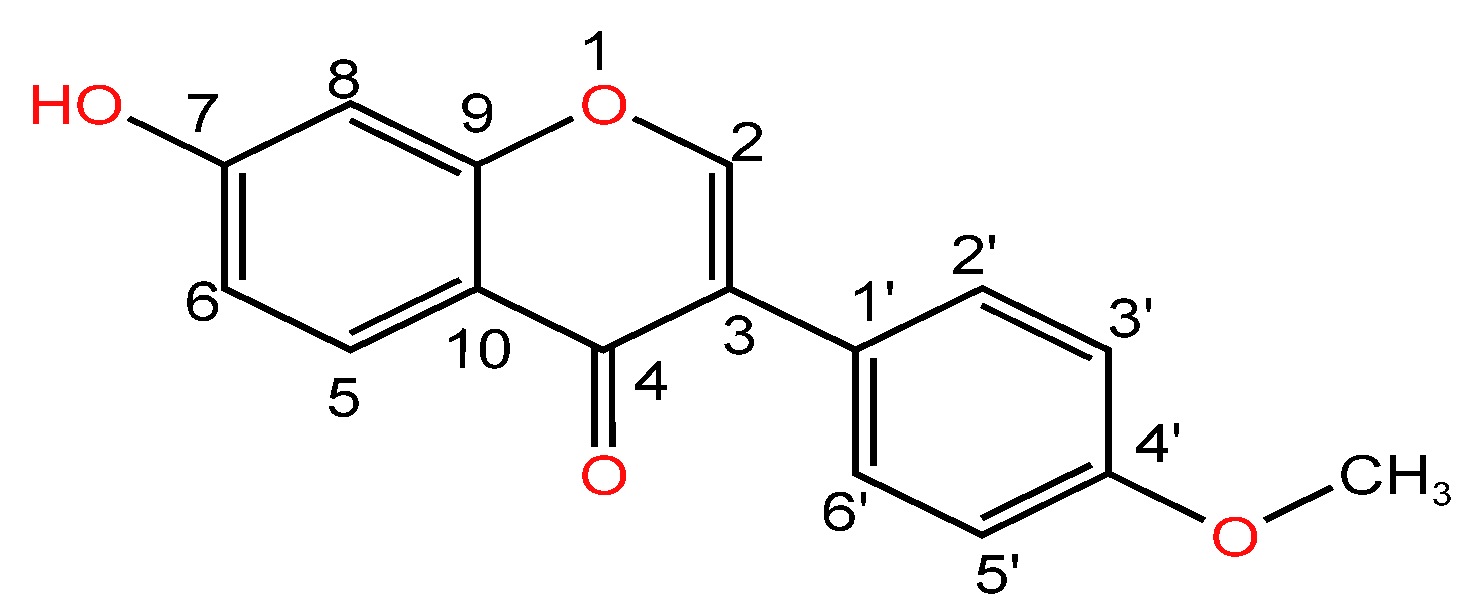
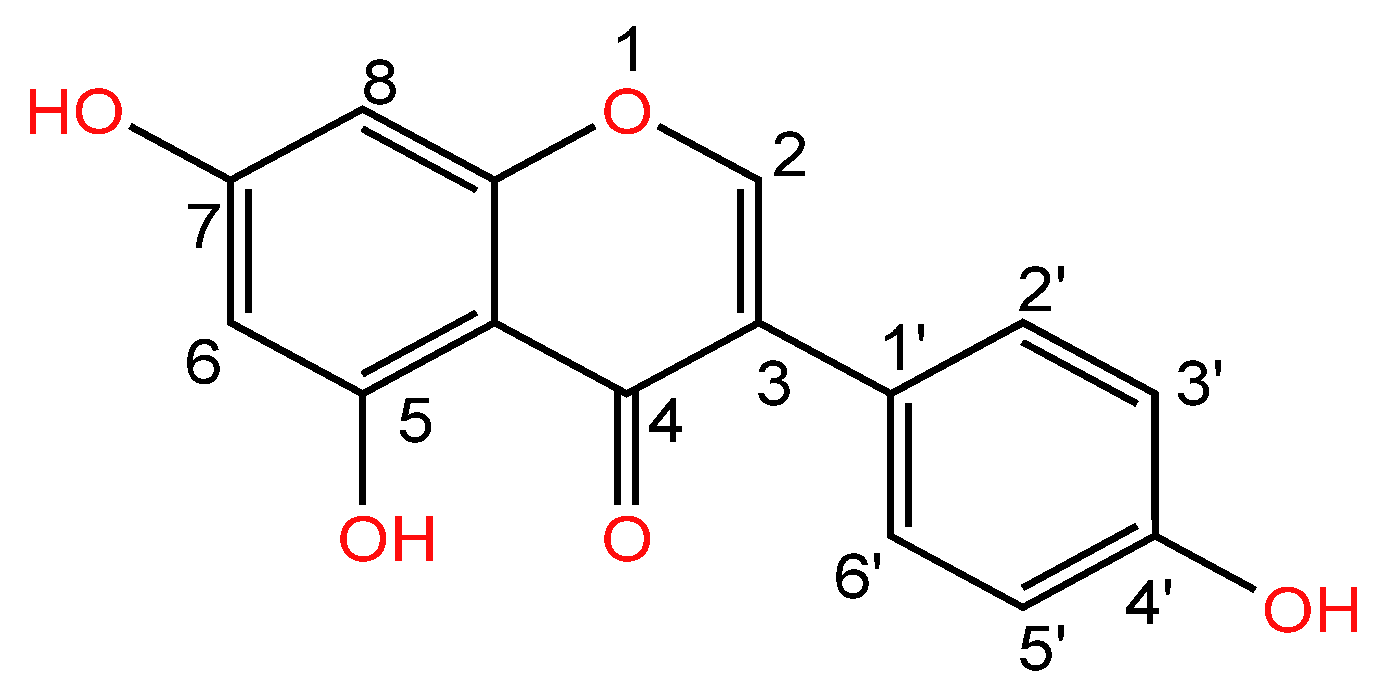
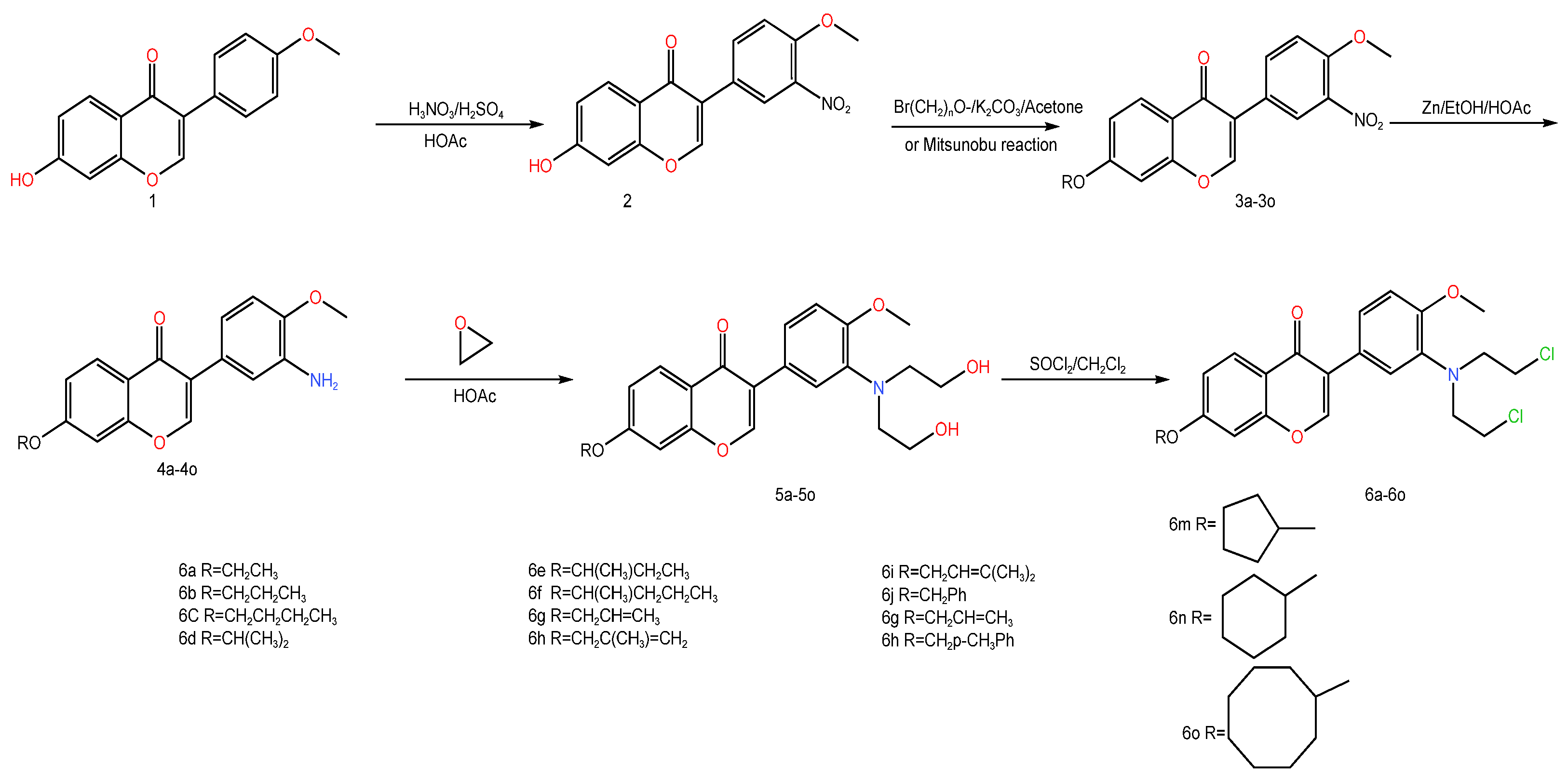

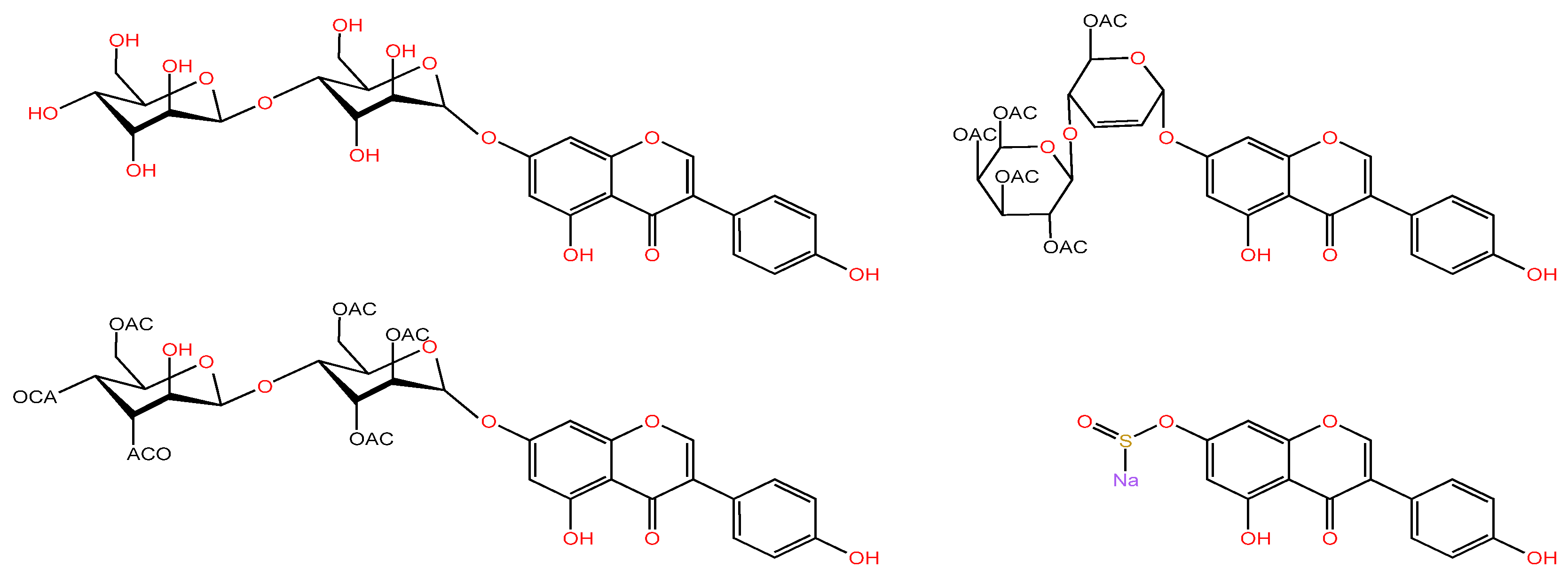
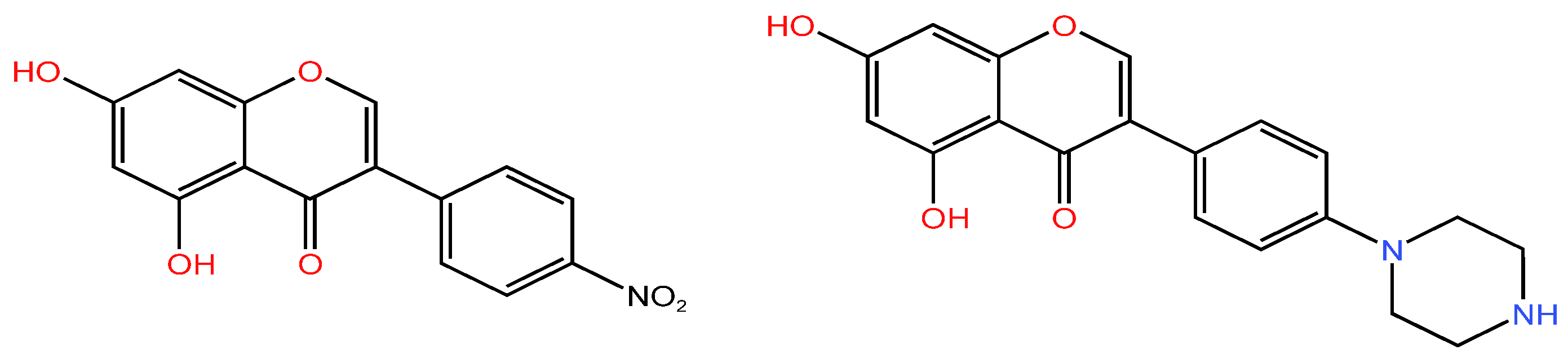

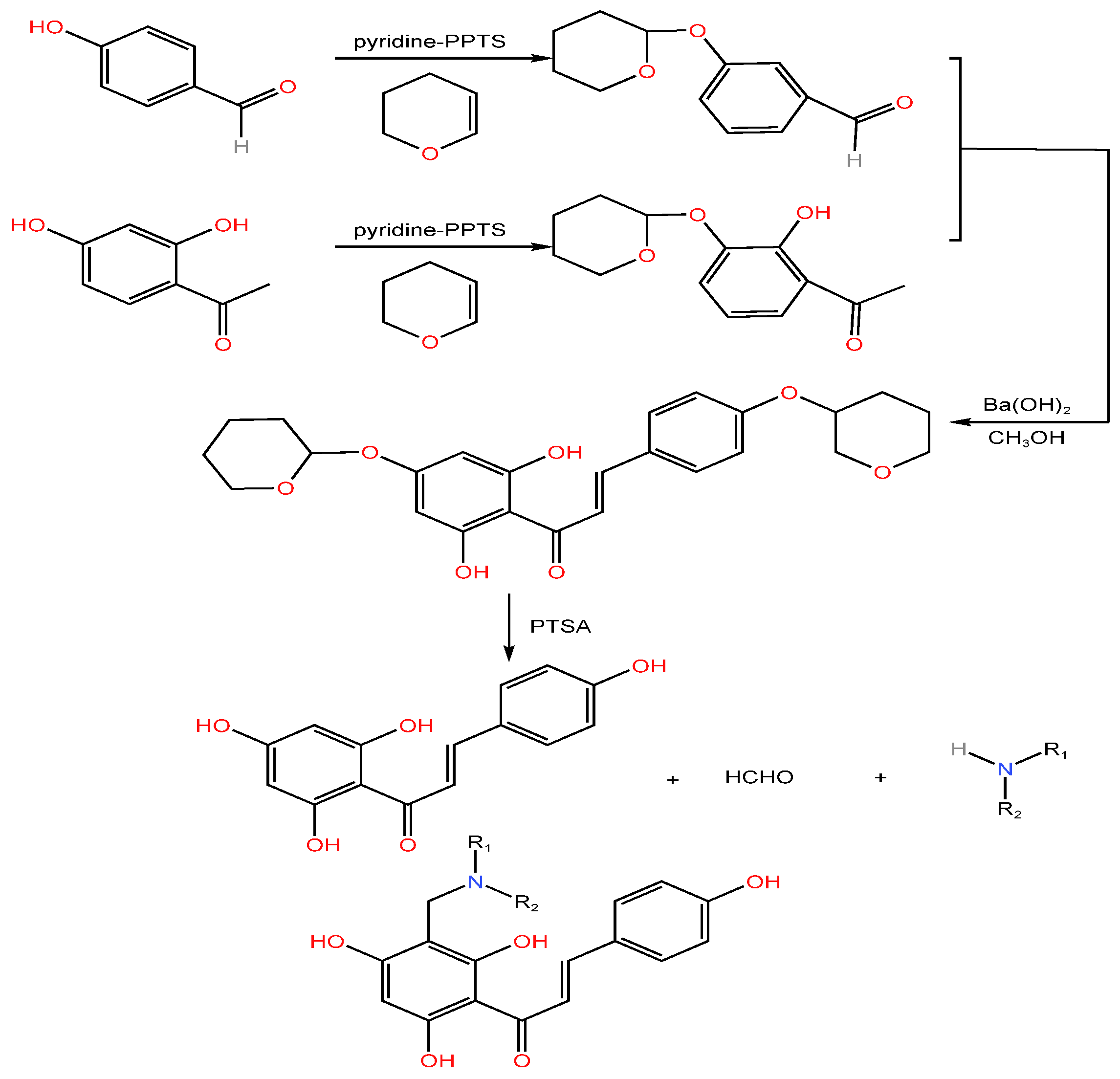
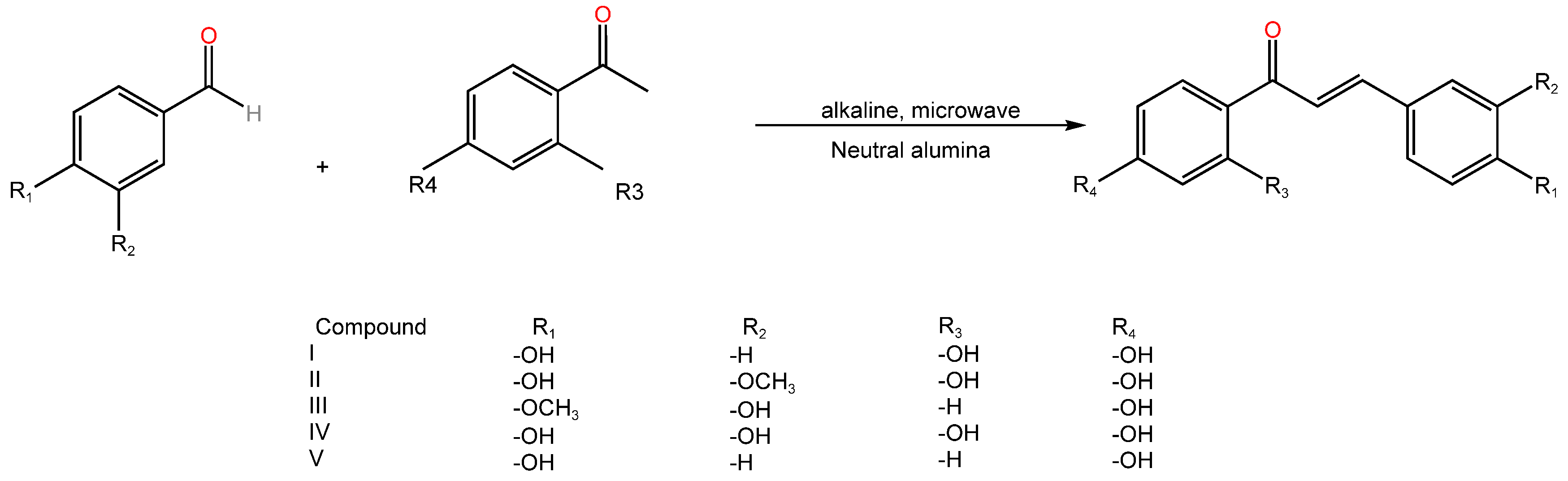
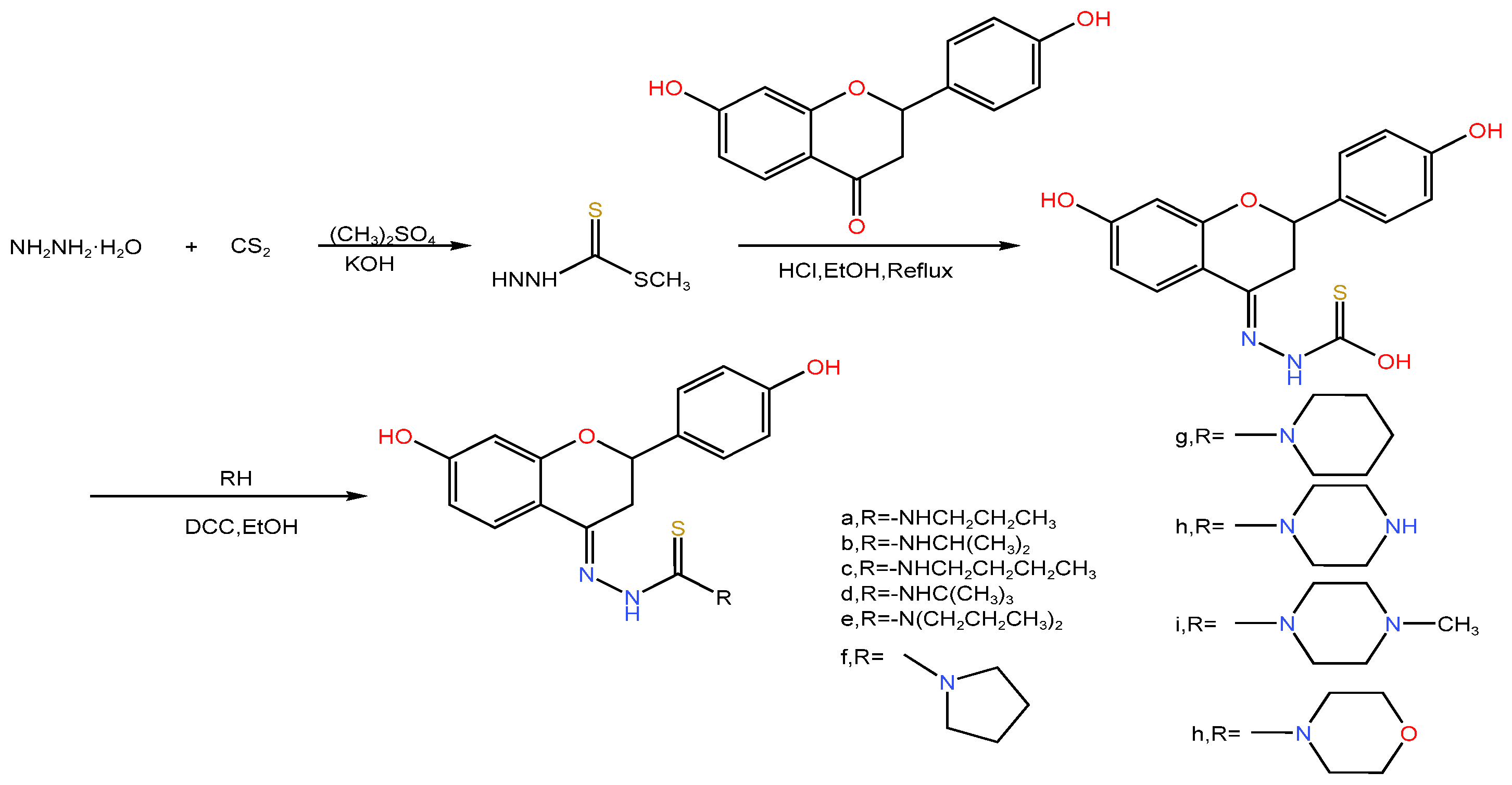
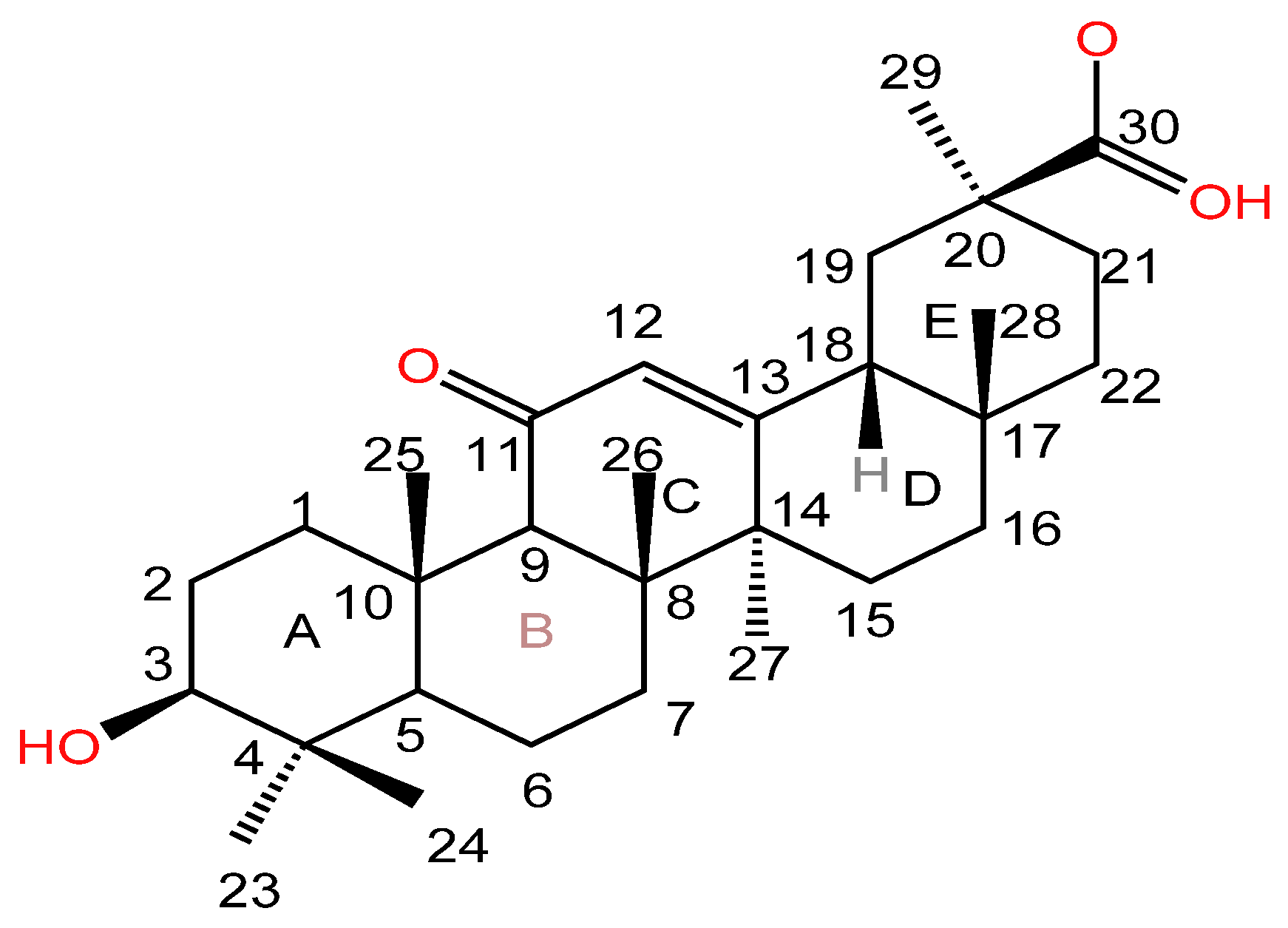

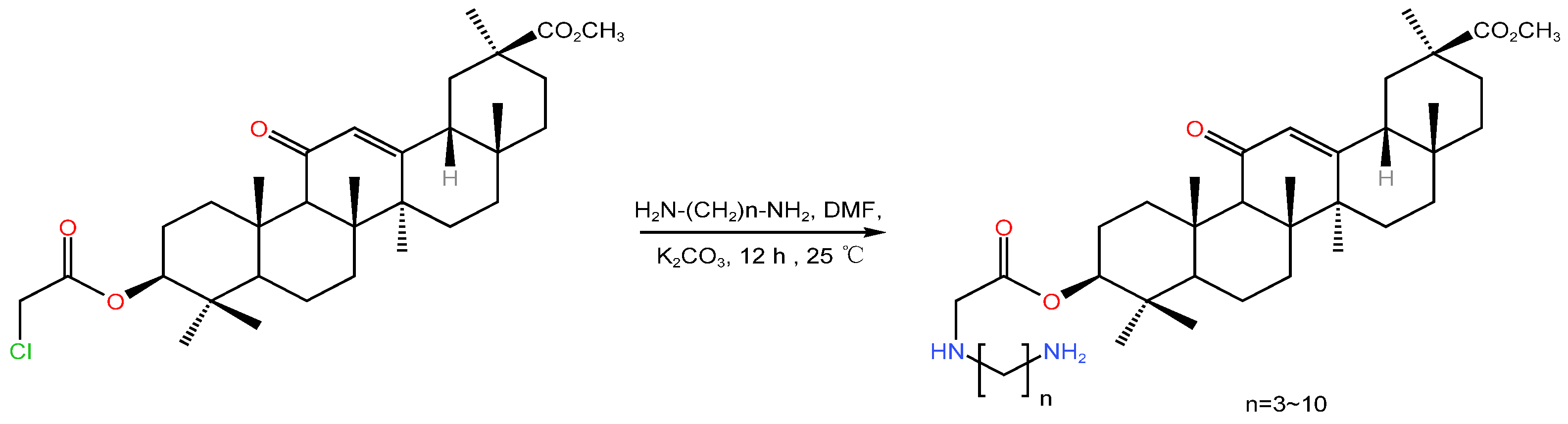
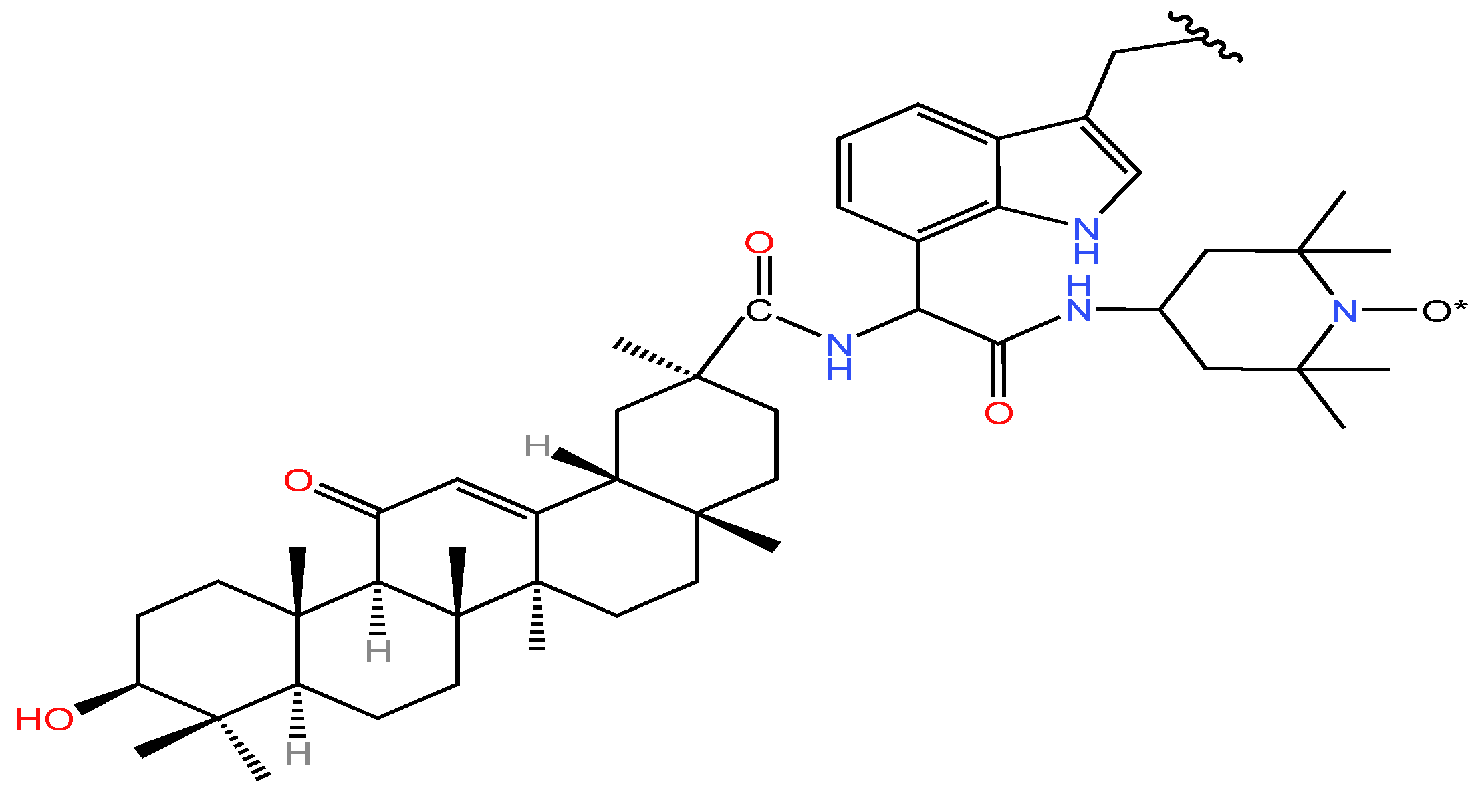
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | schaftoside | C26H28O14 | [9] |
| 2 | vicenin-2 | C27H30O15 | [9] |
| 3 | isoshaftoside | C26H28O14 | [9] |
| 4 | shaftoside | C26H28O14 | [9] |
| 5 | isoviolanthin | C27H30O14 | [9] |
| 6 | isoschaftoside | C26H28O14 | [10] |
| 7 | violanthin | C27H30O14 | [10] |
| 8 | apigenin | C15H10O5 | [11] |
| 9 | licoflavon | C20H18O4 | [12] |
| 10 | kumatakenin | C17H14O6 | [13] |
| 11 | naringin | C27H32O14 | [14] |
| 12 | isoangustone A | C25H26O6 | [15] |
| 13 | genkwanin | C16H12O5 | [16] |
| 14 | uralenol | C21H18O7 | [15] |
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | licoflavonol | C20H18O6 | [24] |
| 2 | galangin | C15H10O5 | [25] |
| 3 | kaempferol | C15H10O6 | [16] |
| 4 | isoquercetin | C21H20O12 | [16] |
| 5 | astragalin | C21H20O11 | [16] |
| 6 | narcissin | C28H32O16 | [26] |
| 7 | isoquercitrin | C21H20O12 | [27] |
| 8 | quercetin | C15H10O7 | [28] |
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | licoisoflavone A | C20H18O6 | [41] |
| 2 | eurycarpin A | C20H18O5 | [42] |
| 3 | formononetin | C16H12O4 | [43] |
| 4 | eurycarpin B | C20H16O5 | [42] |
| 5 | gancaonin G | C21H20O5 | [36] |
| 6 | licoricone | C22H22O6 | [44] |
| 7 | afromosin | C17H14O5 | [45] |
| 8 | odoratin | C17H14O6 | [46] |
| 9 | prunetin | C16H12O5 | [47] |
| 10 | genistein | C15H10O5 | [48] |
| 11 | ononin | C22H22O9 | [42] |
| 12 | licoagroside A | C23H25O12 | [49] |
| 13 | lupiwighteone | C20H18O5 | [50] |
| 14 | 2,3-dehydrokievitone | C20H18O6 | [51] |
| 15 | gancaonin H | C25H24O6 | [52] |
| 16 | glycyroside | C27H30O13 | [53] |
| 17 | calycosin | C16H12O5 | [54] |
| 18 | glabrene | C20H18O4 | [55] |
| 19 | 2′-hydroxyisolupalbigenin | C25H26O6 | [56] |
| 20 | licoricone | C22H22O6 | [55] |
| 21 | glicoricone | C21H20O6 | [55] |
| 22 | 3′,6′-diethoxyfluorescein | C24H20O5 | [55] |
| 23 | 3,7-dimethyl licoflavonol | C22H24O6 | [15] |
| 24 | glabrizoflavone | C20H18O6 | [57] |
| 25 | 6,8-di-(dimethylallyl)-genistein | C25H26O5 | [58] |
| 26 | glyzarin | C18H14O4 | [59] |
| 27 | isoglabrone | C20H16O5 | [60] |
| 28 | parvisoflavone B | C20H16O6 | [56] |
| 29 | 3′,4′-dihydroxy-7-methoxyisoflavone | C16H12O5 | [10] |
| 30 | derrone | C20H16O5 | [51] |
| 31 | parvisoflavones A | C20H16O6 | [51] |
| 32 | isoglabrone | C20H16O5 | [51] |
| 33 | glycybridin D | C25H26O4 | [55] |
| 34 | glycybridin E | C25H24O4 | [55] |
| 35 | glycybridin J | C21H22O6 | [55] |
| 36 | semilicoisoflavone B | C20H16O6 | [42] |
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | isoliquiritigenin | C15H12O4 | [55] |
| 2 | isoliquiritin | C21H22O9 | [12] |
| 3 | licuraside | C26H30O13 | [12] |
| 4 | licochalcone A | C21H22O4 | [12] |
| 5 | licochalcone B | C16H14O5 | [12] |
| 6 | neoisoliquiritin | C21H22O9 | [12] |
| 7 | echinatin | C16H14O4 | [69] |
| 8 | glycybridin A | C20H20O5 | [51] |
| 9 | glycybridin B | C20H22O5 | [51] |
| 10 | glycybridin C | C25H30O5 | [51] |
| 11 | kanzonol Y | C25H30O5 | [51] |
| 12 | kanzonol C | C25H28O4 | [51] |
| 13 | kanzonol B | C20H18O4 | [51] |
| 14 | licoagrochalcone A | C20H20O4 | [51] |
| 15 | paratocarpins B | C25H26O4 | [51] |
| 16 | isobavachalcone | C20H20O4 | [51] |
| 17 | chalconaringenin4-O-glucoside | C21H22O10 | [70] |
| 18 | 3-hydroxylicochalcone E | C21H22O5 | [70] |
| 19 | licoagrochalcone D | C21H22O5 | [70] |
| 20 | [6″,6″-dimethylpyran-(2″,3″4,5)]-3′-γ,γ-dimethylally-2′,3,4′-thrihydroxychalcone | C25H26O5 | [71] |
| 21 | licoagrochalcone C | C21H22O5 | [60] |
| 22 | kanzonol C | C25H28O4 | [60] |
| 23 | 4-hydroxylonchocarpin | C20H18O4 | [72] |
| 24 | licoagrochalcone B | C21H20O4 | [49] |
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | glabrol | C25H28O4 | [78] |
| 2 | glabranin | C20H20O4 | [55] |
| 3 | liquiritigenin | C15H12O4 | [55] |
| 4 | glabranin isomer | C20H20O4 | [79] |
| 5 | liquiritin | C21H22O9 | [12] |
| 6 | liquiritin apioside | C26H30O13 | [12] |
| 7 | pinocembrin | C15H12O4 | [80] |
| 8 | licoflavanone | C20H18O5 | [80] |
| 9 | xambioona | C25H24O4 | [51] |
| 10 | shinflavanone | C25H26O4 | [51] |
| 11 | (2S)-abyssinone I | C20H18O4 | [51] |
| 12 | euchrenone a5 | C25H26O4 | [51] |
| 13 | naringenin | C15H12O5 | [51] |
| 14 | abyssinone II | C20H20O4 | [51] |
| 15 | naringin | C27H32O14 | [51] |
| 16 | pinocembroside | C21H22O9 | [9] |
| 17 | shinflavone | C25H26O4 | [55] |
| 18 | licoagrodin | C45H44O9 | [81] |
| 19 | neoliquiritin | C21H22O9 | [81] |
| 20 | 3′-prenylnaringenin | C21H22O9 | [82] |
| 21 | cyclolicoflavanone | C20H20O5 | [82] |
| 22 | glabranine | C20H20O4 | [82] |
| Serial Number | Name of the Compound | Molecular Formula | Reference |
|---|---|---|---|
| 1 | glycyrrhizic acid | C42H62O16 | [84] |
| 2 | glycyrrhetinic acid | C30H46O4 | [84] |
| 3 | glycyrrhizin | C42H62O16 | [84] |
| 4 | glycyrrhetol | C30H48O3 | [12] |
| 5 | glabric acid | C30H46O5 | [12] |
| 6 | liquoric acid | C30H44O5 | [12] |
| 7 | glabrolide | C30H44O4 | [12] |
| 8 | isoglabrolide | C30H44O4 | [12] |
| 9 | ursolic acid | C30H48O3 | [80] |
| 10 | betulin | C30H50O2 | [80] |
| 11 | lupeol | C30H50O | [80] |
| 12 | licorice-saponin M3 | C48H74O19 | [85] |
| 13 | licorice-saponin N4 | C54H84O23 | [85] |
| 14 | licorice-saponin O4 | C54H84O24 | [85] |
| 15 | licoricesaponin G2 | C42H62O17 | [85] |
| 16 | 18α-licorice-saponin G2 | C42H62O17 | [85] |
| 17 | macedonoside A | C42H62O17 | [85] |
| 18 | 29-hydroxy-glycyrrhizin | C42H62O16 | [85] |
| 19 | licorice-saponin A3 | C48H72O21 | [85] |
| 20 | 24-hydroxy-licorice-saponin A3 | C48H72O22 | [85] |
| 21 | 22β-acetoxyl-glycyrrhizin | C44H64O18 | [85] |
| 22 | licorice-saponin H2 | C42H62O16 | [12] |
| 23 | licorice-saponin R3 | C48H74O20 | [86] |
| 24 | licoricesaponin S3 | C48H75O20 | [86] |
| 25 | uralsaponin C | C42H64O16 | [86] |
| 26 | 18α-glycyrrhizic acid | C42H62O16 | [10] |
| 27 | 18β-glycyrrhizic acid | C42H62O16 | [10] |
| 28 | licorice-saponin P2 | C42H62O17 | [10] |
| 29 | betulic acid | C30H48O3 | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ma, Q.; Gong, G.; Su, F. Research Progress on Structural Modification of Effective Antitumor Active Ingredients in Licorice. Molecules 2023, 28, 5855. https://doi.org/10.3390/molecules28155855
Liu C, Ma Q, Gong G, Su F. Research Progress on Structural Modification of Effective Antitumor Active Ingredients in Licorice. Molecules. 2023; 28(15):5855. https://doi.org/10.3390/molecules28155855
Chicago/Turabian StyleLiu, Chi, Qing Ma, Guangfei Gong, and Fengyan Su. 2023. "Research Progress on Structural Modification of Effective Antitumor Active Ingredients in Licorice" Molecules 28, no. 15: 5855. https://doi.org/10.3390/molecules28155855
APA StyleLiu, C., Ma, Q., Gong, G., & Su, F. (2023). Research Progress on Structural Modification of Effective Antitumor Active Ingredients in Licorice. Molecules, 28(15), 5855. https://doi.org/10.3390/molecules28155855