Abstract
A potassium carbonate promoted tandem oxy-Michael addition/cyclization of α,β-unsaturated carbonyl compounds with naphthol derivatives for the synthesis of 2-substituted naphthopyrans was developed. Using the readily available, inexpensive potassium carbonate as the promoter, a range of different substituted naphthopyrans were prepared.
1. Introduction
Naphthopyran derivatives are widely used in medicine and industry. Many naphthopyran derivatives have important biological and pharmacological activities. Natural products with a naphthopyran core, busseihydroquinones C–D, have been isolated from the roots of Pentas bussei, a plant found in Kenya, and a decoction of the roots is used as a remedy for gonorrhea, syphilis, and dysentery [1,2]. Compound A has shown anti-bacterial activity against some gram-positive and gram-negative bacteria [3,4]. Compound B showed low cytotoxicity toward KB cells in vitro and was inactive against bacteria and fungi [5,6]. In addition, naphthopyran analogs of LY290181 can act as tumor vascular disrupting agents [7]. Compound C with a naphthopyran core exhibits photochromic properties, which are useful for many applications, e.g., in the manufacture of ophthalmic lenses, contact lenses, solar protection glasses, filters, camera optics, transmission devices, agrochemical films, glazes, decorative objects, and in information storage using optical inscription (Figure 1) [5,6,8,9,10,11].
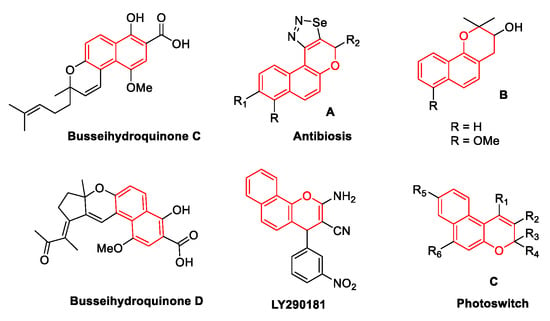
Figure 1.
Representative naphthopyrans used in medicine and industry.
Therefore, efficient methods toward the synthesis of the naphthopyran skeleton have long been valued by synthetic chemists, and many synthetic strategies have been reported. The main route involves the reaction of naphthols and olefins catalyzed by a Lewis acid, including the reaction of naphthols with conjugated dienes [4,12,13,14,15] and the reaction of naphthols with analogs, such as allyl alcohol [5,9,16,17,18,19,20,21,22,23,24]. In these strategies, precious-metal catalysts or a large amount of acid catalysts are used. More importantly, this type of reaction is not suitable for the synthesis of acid-sensitive natural products. Therefore, it is desirable to develop a method to obtain naphthopyran compounds using alkali base (Scheme 1a).
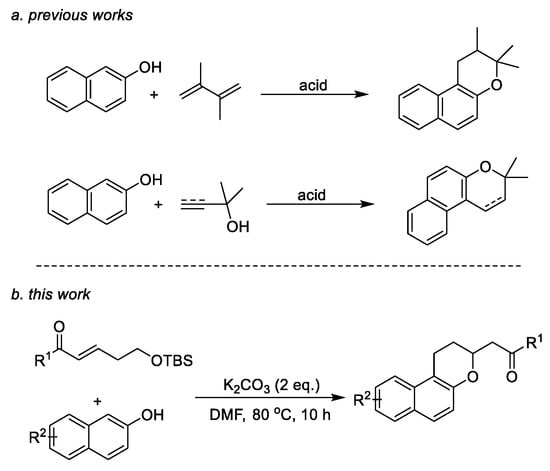
Scheme 1.
Approaches to naphthopyrans.
We speculated that the reaction sites of phenolic hydroxyl groups and unsaturated ketones can be subjected to a base [25] to obtain naphthopyran products, while the remaining carbonyl group in the product would allow for further structural modification, enabling the synthesis of various pharmacological compounds (Scheme 1b).
2. Results and Discussion
Here, we investigated the activity of readily available, inexpensive potassium carbonate as a promoter for the synthesis of naphthopyran compounds by the reaction of naphthol with unsaturated ketones. The results indicated that this method may be an economic, efficient, and practical synthesis for naphthopyran natural products.
In the first set of experiments, we used tert-butyldimethylsilyl (TBS) as the protecting group for the screening of subsequent reaction conditions. Different solvents, THF, DMSO, MeOH, 1,4-dioxane, DCE, and EtOAc, were then examined; only low yields were obtained and most of the raw materials did not react (Table 1, entries 1–7). The reaction proceeded efficiently in the strong polar solvent DMF and provided the product 4aa in 41% yield (Table 1, entry 8). We then examined different bases for the reaction in DMF. However, no desired product 4aa was obtained when t-BuOK or pyridine were used (Table 1, entries 9–10). When CsF or K3PO4 were used, only a trace amount of product was obtained (Table 1, entries 11–12). K2CO3 was found to be the optimal base and 4aa was obtained in an excellent yield of 91% and was successfully isolated in 88% yield (Table 1, entry 13). Raising the temperature to 80 °C increased the reaction rate.

Table 1.
Optimization of the reaction conditions (a).
Having established the optimized reaction conditions, we investigated the substrate scope of the reaction with various α,β-unsaturated carbonyls and naphthalens. As summarized in Scheme 2, we first investigated the reaction of naphthalen-2-ol 2a with a variety of α,β-unsaturated carbonyls 1 under the optimal conditions. Substituted α,β-unsaturated carbonyls, with both electron-donating (4ad–4ag) and electron-withdrawing (4ah and 4ai) groups at different positions on the benzene ring, were suitable substrates and gave the desired 2-substituted naphthopyran products. The structure of 4ag was unambiguously determined by single-crystal X-ray diffraction analysis (CCDC 2171562 (4ag) contains the supplementary crystallographic data for this paper. For details, see the supporting information). A cyclopropane-substituted, α,β-unsaturated carbonyl compound was also tolerated, giving the corresponding product in 70% yield (4ab). In addition, esters could also react with naphthol and gave the naphthopyran products (4aj–4ak) in good yields.
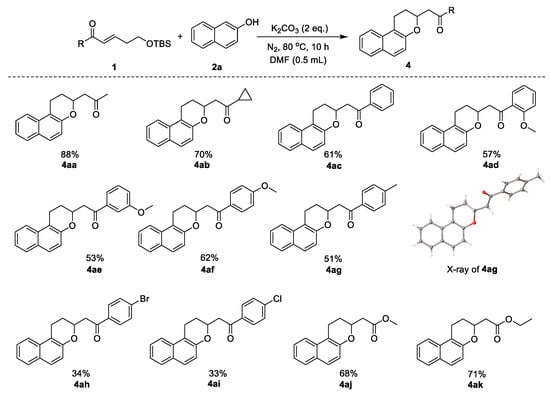
Scheme 2.
Substrate scope of α,β-unsaturated carbonyls in the synthesis of 2-substituted naphthopyrans. Reaction conditions: naphthalen-2-ol (1.5 equiv), K2CO3 (2 equiv), DMF (0.5 mL), 80 °C, 10 h. Isolated yields.
We further investigated the scope of the naphthalens for this reaction. As illustrated in Scheme 3, a series of different naphthalens 2 were reacted with α,β-unsaturated carbonyl 1a under the optimized conditions. This transformation showed good generality and reactivity; all the tested naphthalens were tolerated and produced the corresponding 2-substituted naphthopyran 4. Starting materials bearing electron-withdrawing and electron-donating groups afforded the corresponding products (Scheme 3, 4ba–4ga). Moreover, 4-hydroxycoumarin and 1,3-cyclohexanedione were also tolerated and provided the desired products in 48% and 79% yield, respectively (Scheme 4, 5aa and 5ab).
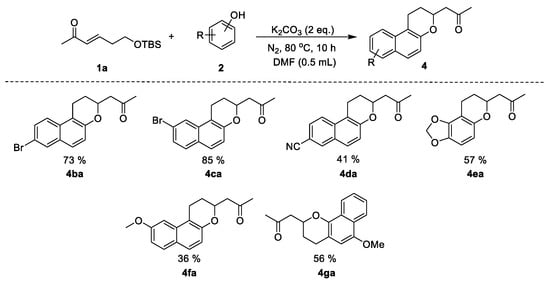
Scheme 3.
Substrate scope of naphthalens in the synthesis of 2-substituted naphthopyrans. Reaction conditions: naphthalen-2-ol (1.5 equiv), K2CO3 (2 equiv), DMF (0.5 mL), 80 °C, 10 h. Isolated yields.
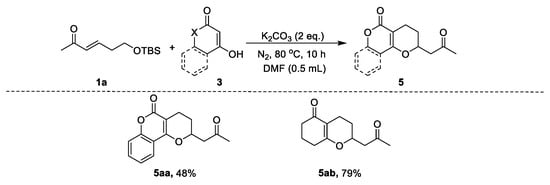
Scheme 4.
Substrate scope of 1,3-dicarbonyl compounds in the synthesis of pyrans. Reaction conditions: naphthalen-2-ol (1.5 equiv), K2CO3 (2 equiv), DMF (0.5 mL), 80 °C, 10 h. Isolated yields.
Subsequently, we explored a gram-scale reaction. α,β-Unsaturated carbonyl 1a was treated with naphthalen-2-ol 2a under the standard conditions (Scheme 5). The corresponding naphthopyran product 4aa was obtained in 81% yield, slightly lower than the small-scale reaction yield.
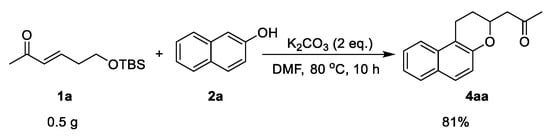
Scheme 5.
The gram-scale reaction.
The reaction of α,β-Unsaturated carbonyl 1a and 1-naphthol was checked as well, which produced the desired product in 31% yield (See NMR spectra in Supplementary Materials). Herein, we proposed an immature process. Initially, deprotonation of the OH group of 2-naphthol 2a with K2CO3 generated 2-naphtholanion I, which undergoes an oxy-Michael addition reaction to the α,β-unsaturated carbonyl compound 1 to afford the alkylated naphthol intermediate III. This intermediate then might undergo an intramolecular SN2-type cyclization process to form the 2-substituted naphthopyran product 4aa along with the loss of TBSOH (Scheme 6). However, we have to realize that the mechanism of the reaction is unclear at this stage.
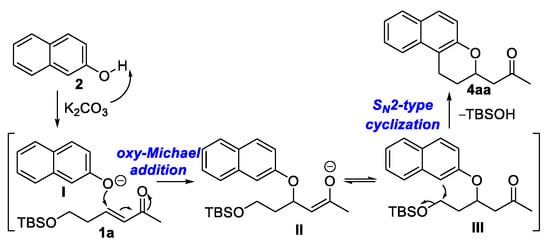
Scheme 6.
Possible mechanism for the formation of 4aa.
In summary, we have developed an approach to access 2-substituted naphthopyrans using α,β-unsaturated carbonyl compounds and naphthol derivatives in DMF at 80 °C with K2CO3 as the promoter. The reaction is proposed to undergo a tandem oxy-Michael addition/cyclization process, and various substitutions on both reaction partners were tolerated. This reaction is the first method for synthesizing naphthopyran compounds using basic medium.
3. Materials and Methods
3.1. General Information
Unless otherwise noted, all reactions were carried out under air atmosphere. 1,2-dichloroethane, N,N-Dimethylformamide, naphthols, ferric chloride, and potassium carbonate were from commercial sources and used as received without further purification. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.20 mm silica gel plates using UV light as the visualizing agent, and iodine and an acidic solution of phosphomolybdic acid (PMA) with heat as the stains. Column chromatography was performed on silica gel (200–300 meshes) using petroleum ether and ethyl acetate as eluent. All new compounds were characterized by means of 1H NMR, 13C NMR and HRMS. NMR spectra were recorded using a Bruker AVANCE NEO 500 MHz NMR spectrometer and can be found at the end of the paper. All 1HNMR data are reported in δ units, parts per million (ppm), and were calibrated relative to the signals for residual chloroform (7.26 ppm) in deuterochloroform (CDCl3). All 13C NMR data are reported in ppm relative to CDCl3 (77.16 ppm) and were obtained with 1H decoupling. The following abbreviations or combinations, thereof, were used to explain the multiplicities: s = singlet, bs = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Single crystal X-ray diffraction (SCXRD) of 4ag was carried out at 100(2) K on a Bruker D8 VENTURE diffractometer using Mo-Kα radiation (λ = 0.71073 Å). Integration and scaling of intensity data was performed using the SAINT program. Data were corrected for the effects of absorption using SADABS. The structures were solved by direct method and refined with the full-matrix least-squares technique using SHELX-2014 software. Non-hydrogen atoms were refined with anisotropic displacement parameters, and hydrogen atoms were placed in calculated positions and refined with a riding model.
3.2. General Information
α,β-Unsaturated carbonyl compound 1 (0.2 mmol, 1.0 equiv), naphthol 2 (0.3 mmol, 1.5 equiv), and K2CO3 (0.4 mmol, 2.0 equiv) were added into a 15 mL tube under N2 atmosphere. DMF (1.5 mL) was added to the reaction tube. Then, the reaction mixture was heated to 80 °C. After a time period of 10 h, the solution was diluted with EtOAc (2 mL × 3), washed with brine (1 mL × 1), and concentrated in vacuo. The crude product was purified by a flash chromatography on silica gel to afford the corresponding product 4.
3.3. Characterization of Products in Details
- 1-(2,3-dihydro-1H-benzo[f]chromen-3-yl)propan-2-one (4aa):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.09–8.05 (m, 1H), 7.75–7.72 (m, 1H), 7.44–7.40 (m, 2H), 7.34 (d, J = 8.3 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 4.69–4.62 (m, 1H), 3.13–2.97 (m, 2H), 2.88–2.83 (m, 1H), 2.77 (dd, J = 15.8, 5.3 Hz, 1H), 2.34 (s, 3H), 2.22–2.15 (m, 1H), 1.92–1.82 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.7, 149.0, 133.1, 127.6, 127.4, 125.6, 125.2, 125.1, 121.2, 119.8, 115.3, 72.5, 49.1, 31.1, 27.4, 24.6. HRMS (ESI) m/z 263.1053 [(M+Na)+; calcd for C16H16O2Na: 263.1048].
- 1-cyclopropyl-2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)ethan-1-one (4ab):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-cyclopropylpent-2-en-1-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a white solid. 1H NMR (500 MHz, CDCl3) δ 7.82 (m, 2H), 7.65 (d, J = 8.9 Hz, 1H), 7.53 (m, 1H), 7.39 (m, 1H), 7.07 (d, J = 8.9 Hz, 1H), 4.64 (m, 1H), 3.20–3.05 (m, 3H), 2.88 (dd, J = 16.0, 6.2 Hz, 1H), 2.30 (m, 1H), 2.07 (m, 1H), 1.92 (m, 1H), 1.25–1.10 (m, 2H), 1.05–0.90 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 208.4, 152.1, 133.1, 129.0, 128.4, 127.7, 126.4, 123.3, 121.9, 119.1, 113.5, 71.8, 48.5, 27.2, 21.3, 21.0, 11.1, 11.0. HRMS (ESI) m/z 289.1263 [(M+Na)+; calcd for C18H18O2Na: 289.1199].
- 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)-1-phenylethan-1-one (4ac):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-phenylpent-2-en-1-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 8.3 Hz, 2H), 7.84 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 9.8 Hz, 1H), 7.65–7.59 (m, 2H), 7.52 (t, J = 8.1 Hz, 3H), 7.40–7.35 (m, 1H), 7.03 (d, J = 8.9 Hz, 1H), 4.80 (m, 1H), 3.66 (dd, J = 16.4, 6.0 Hz, 1H), 3.27 (dd, J = 16.4, 6.7 Hz, 1H), 3.17 (m, 2H), 2.42 (m, 1H), 1.99 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 197.7, 152.1, 137.1, 133.4, 133.0, 129.0, 128.7, 128.4, 128.3, 127.7, 126.4, 123.3, 121.9, 119.1, 113.5, 72.1, 43.9, 27.3, 21.0. HRMS (ESI) m/z 303.1389 [(M+H)+; calcd for C21H19O2: 303.1385].
- 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)-1-(2-methoxyphenyl)ethan-1-one (4ad):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-(2-metho-xyphenyl)pent-2-en-1-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 9.5 Hz, 1H), 7.79 (m, 2H), 7.62 (d, J = 8.8 Hz, 1H), 7.51 (m, 2H), 7.37 (t, J = 7.5 Hz, 1H), 7.10–6.97 (m, 3H), 4.76 (m, 1H), 3.93 (s, 3H), 3.64 (dd, J = 16.8, 6.2 Hz, 1H), 3.37 (dd, J = 16.8, 6.8 Hz, 1H), 3.19–3.07 (m, 2H), 2.38 (m, 1H), 1.96 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 199.7, 158.7, 152.3, 133.7, 133.1, 130.4, 128.9, 128.4, 128.3, 127.6, 126.3, 123.2, 121.9, 120.8, 119.2, 113.6, 111.6, 72.2, 55.6, 49.1, 27.2, 21.1. HRMS (ESI) m/z 333.1492 [(M+H)+; calcd for C22H21O3: 333.1491].
- 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)-1-(3-methoxyphenyl)ethan-1-one (4ae):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-(3-methox-yphenyl)pent-2-en-1-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 7.3 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.63 (d, J = 9.3 Hz, 2H), 7.58 (s, 1H), 7.52 (m, 1H), 7.45–7.35 (m, 2H), 7.17 (dd, J = 8.2, 2.7 Hz, 1H), 7.03 (d, J = 8.9 Hz, 1H), 4.80 (m, 1H), 3.90 (s, 3H), 3.64 (dd, J = 16.5, 6.1 Hz, 1H), 3.26 (dd, J = 16.4, 6.7 Hz, 1H), 3.22–3.08 (m, 2H), 2.41 (m, 1H), 1.99 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 197.5, 159.9, 152.1, 138.5, 133.0, 129.7, 129.0, 128.4, 127.7, 126.4, 123.3, 121.9, 121.0, 119.9, 119.1, 113.5, 112.4, 72.1, 55.5, 44.0, 27.3, 21.0. HRMS (ESI) m/z 333.1494 [(M+H)+; calcd for C22H21O3: 333.1491].
- 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)-1-(4-methoxyphenyl)ethan-1-one (4af):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-(4-methoxy-phenyl)pent-2-en-1-one(0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.9 Hz, 2H), 7.84 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 8.9 Hz, 1H), 7.51 (t, J = 8.3 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.03 (d, J = 8.9 Hz, 1H), 6.98 (d, J = 8.9 Hz, 2H), 4.83–4.73 (m, 1H), 3.91 (s, 3H), 3.61 (dd, J = 16.2, 5.9 Hz, 1H), 3.21 (dd, J = 16.2, 6.8 Hz, 1H), 3.18–3.07 (m, 2H), 2.41 (m, 1H), 1.98 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 196.23, 163.72, 152.13, 133.05, 130.63, 130.25, 128.96, 128.41, 127.66, 126.33, 123.26, 121.95, 119.13, 113.81, 113.56, 72.34, 55.53, 43.52, 27.28, 21.02. HRMS (ESI) m/z 355.1308 [(M+Na)+; calcd for C22H20O3Na: 355.1310].
- 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)-1-(p-tolyl)ethan-1-one (4ag):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-(p-tolyl)pent-2-en-1-one(0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 2H), 7.84 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 8.9 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 8.9 Hz, 1H), 4.80 (m, 1H), 3.63 (dd, J = 16.3, 5.9 Hz, 1H), 3.24 (dd, J = 16.3, 6.8 Hz, 1H), 3.16 (m, 2H), 2.52–2.37 (m, 4H), 1.98 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 197.3, 152.1, 144.2, 134.7, 133.1, 129.4, 129.0, 128.4, 128.4, 127.7, 126.3, 123.3, 122.0, 119.1, 113.5, 72.2, 43.8, 27.3, 21.7, 21.0. HRMS (ESI) m/z 317.1545 [(M+H)+; calcd for C22H21O2: 317.1542].
- 1-(4-bromophenyl)-2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)ethan-1-one (4ah):
The title compound was prepared from (E)-1-(4-bromophenyl)-5-((tert-butyldimethylsilyl)oxy)-pent-2-en-1-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.91 (d, J = 8.6 Hz, 2H), 7.84 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.68–7.60 (m, 3H), 7.54–7.49 (m, 1H), 7.40–7.33 (m, 1H), 7.00 (d, J = 8.9 Hz, 1H), 4.77 (m, 1H), 3.61 (dd, J = 16.3, 6.3 Hz, 1H), 3.24–3.11 (m, 3H), 2.40 (m, 1H), 1.99 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 196.83, 151.94, 135.78, 132.98, 132.01, 129.87, 128.98, 128.64, 128.44, 127.75, 126.41, 123.37, 121.94, 119.02, 113.47, 72.06, 43.80, 27.25, 20.99. HRMS (ESI) m/z 403.0312 [(M+Na)+; calcd for C21H17O2NaBr: 403.0310].
- 1-(4-chlorophenyl)-2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)ethan-1-one (4ai):
The title compound was prepared from (E)-5-((tert-butyldimethylsilyl)oxy)-1-(4-chlorophenyl)-pent-2-en-1-one(0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.99 (d, J = 8.6 Hz, 2H), 7.84 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.62 (d, J = 8.9 Hz, 1H), 7.54–7.46 (m, 3H), 7.38 (t, J = 7.5 Hz, 1H), 7.00 (d, J = 8.9 Hz, 1H), 4.78 (m, 1H), 3.62 (dd, J = 16.3, 6.3 Hz, 1H), 3.21 (dd, J = 16.4, 6.3 Hz, 1H), 3.18–3.07 (m, 2H), 2.44–2.36 (m, 1H), 1.99 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 196.62, 151.95, 139.87, 135.39, 132.98, 129.76, 129.01, 128.98, 128.44, 127.75, 126.40, 123.36, 121.93, 119.03, 113.47, 72.07, 43.82, 27.26, 21.00. HRMS (ESI) m/z 359.0807 [(M+Na)+; calcd for C21H17O2NaCl: 359.0815].
- methyl 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)acetate (4aj):
The title compound was prepared from methyl (E)-5-((tert-butyldimethylsilyl)oxy)pent-2-enoate (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, Chloroform-d) δ 7.83–7.74 (m, 2H), 7.62 (d, J = 8.8 Hz, 1H), 7.49 (m, 1H), 7.35 (m, 1H), 7.04 (d, J = 8.9 Hz, 1H), 4.59–4.53 (m, 1H), 3.77 (d, J = 1.8 Hz, 3H), 3.17–3.03 (m, 2H), 2.88 (dd, J = 15.5, 7.3 Hz, 1H), 2.69 (dd, J = 15.5, 6.0 Hz, 1H), 2.32–2.24 (m, 1H), 1.98–1.89 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 171.22, 151.87, 132.90, 128.94, 128.37, 127.68, 126.30, 123.27, 121.84, 119.05, 113.25, 71.94, 51.83, 40.09, 26.92, 20.93. HRMS (ESI) m/z 279.0999 [(M+Na)+; calcd for C16H16O3Na: 279.0997].
- methyl 2-(2,3-dihydro-1H-benzo[f]chromen-3-yl)acetate (4ak):
The title compound was prepared from ethyl (E)-5-((tert-butyldimethylsilyl)oxy)pent-2-enoate (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a yellow solid. 1H NMR (500 MHz, Chloroform-d) δ 7.81 (dd, J = 11.7, 8.3 Hz, 2H), 7.64 (d, J = 8.9 Hz, 1H), 7.51 (t, J = 7.3 Hz, 1H), 7.37 (t, J = 7.4 Hz, 1H), 7.06 (d, J = 8.9 Hz, 1H), 4.59–4.57 (m, 1H), 4.25 (q, J = 7.1 Hz, 2H), 3.22–3.03 (m, 2H), 2.89 (dd, J = 15.3, 7.3 Hz, 1H), 2.69 (dd, J = 15.3, 6.0 Hz, 1H), 2.33–2.29 (m, 1H), 1.98–1.94 (m, 1H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.84, 151.98, 132.97, 128.99, 128.43, 127.72, 126.35, 123.31, 121.90, 119.12, 113.33, 72.09, 60.73, 40.41, 26.99, 21.00, 14.28. HRMS (ESI) m/z 293.1153 [(M+Na)+; calcd for C17H18O3Na: 293.1154].
- 1-(8-bromo-2,3-dihydro-1H-benzo[f]chromen-3-yl)propan-2-one (4ba):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 6-bromonaphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a white solid. 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J = 2.1 Hz, 1H), 7.65 (d, J = 8.9 Hz, 1H), 7.55 (dd, J = 9.0, 2.1 Hz, 1H), 7.51 (d, J = 8.9 Hz, 1H), 7.04 (d, J = 9.0 Hz, 1H), 4.61–4.51 (m, 1H), 3.13–2.97 (m, 3H), 2.73 (dd, J = 16.3, 5.5 Hz, 1H), 2.33–2.22 (m, 4H), 1.87 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.3, 152.3, 131.6, 130.3, 130.2, 129.5, 126.8, 123.8, 120.2, 117.0, 113.7, 71.8, 48.8, 31.0, 27.0, 21.0. HRMS (ESI) m/z 319.0337 [(M+H)+; calcd for C16H16BrO2: 319.0334].
- 1-(9-bromo-2,3-dihydro-1H-benzo[f]chromen-3-yl)propan-2-one (4ca):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 7-bromonaphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a white solid. 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 1.9 Hz, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.57 (d, J = 8.8 Hz, 1H), 7.43 (dd, J = 8.6, 1.9 Hz, 1H), 7.02 (d, J = 8.9 Hz, 1H), 4.62–4.48 (m, 1H), 3.09–2.95 (m, 3H), 2.73 (dd, J = 16.3, 5.4 Hz, 1H), 2.33–2.21 (m, 4H), 1.87 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.3, 152.8, 134.4, 130.0, 127.6, 127.4, 126.6, 124.4, 120.9, 119.5, 112.8, 71.9, 48.8, 31.0, 26.9, 21.0. HRMS (ESI) m/z 319.0336 [(M+H)+; calcd for C16H16BrO2: 319.0334].
- 3-(2-oxopropyl)-2,3-dihydro-1H-benzo[f]chromene-8-carbonitrile (4da):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 6-hydroxy-2-naphthonitrile (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 1.7 Hz, 1H), 7.84 (d, J = 8.8 Hz, 1H), 7.65–7.56 (m, 2H), 7.11 (d, J = 9.0 Hz, 1H), 4.63–4.57 (m, 1H), 3.10–3.00 (m, 3H), 2.76 (dd, J = 16.5, 5.5 Hz, 1H), 2.29 (s, 4H), 1.88 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.0, 154.6, 134.8, 134.3, 128.2, 127.9, 127.1, 123.1, 121.0, 119.6, 113.9, 106.4, 72.1, 48.6, 31.0, 26.7, 21.0. HRMS (ESI) m/z 266.1177 [(M+H)+; calcd for C17H16NO2: 266.1181].
- 1-(8,9-dihydro-7H-[1,3]dioxolo[4,5-f]chromen-7-yl)propan-2-one (4ea):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), benzo[d][1,3]dioxol-5-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 6.50 (s, 1H), 6.33 (s, 1H), 5.87 (q, J = 1.4 Hz, 2H), 4.47–4.34 (m, 1H), 2.92 (dd, J = 16.1, 7.3 Hz, 1H), 2.81 (m, 1H), 1.78–1.66 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.5, 148.9, 146.4, 141.4, 113.0, 108.2, 100.8, 98.6, 72.0, 49.0, 31.0, 27.4, 24.5. HRMS (ESI) m/z 235.0972 [(M+H)+; calcd for C13H15O4: 235.0970].
- 1-(9-methoxy-2,3-dihydro-1H-benzo[f]chromen-3-yl)propan-2-one (4fa):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 7-methoxynaphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.8 Hz, 1H), 7.56 (d, J = 8.8 Hz, 1H), 7.08 (d, J = 2.5 Hz, 1H), 7.04 (dd, J = 8.8, 2.5 Hz, 1H), 6.90 (d, J = 8.8 Hz, 1H), 4.61–4.53 (m, 1H), 3.95 (s, 3H), 3.07–2.99 (m, 3H), 2.74 (dd, J = 16.1, 5.6 Hz, 1H), 2.32–2.24 (m, 4H), 1.96–1.85 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.6, 158.3, 152.6, 134.3, 130.0, 127.5, 124.2, 116.5, 115.1, 112.5, 101.4, 71.7, 55.3, 48.8, 31.1, 27.2, 21.2. HRMS (ESI) m/z 271.1339 [(M+H)+; calcd for C17H19O3: 271.1334].
- 1-(6-methoxy-3,4-dihydro-2H-benzo[h]chromen-2-yl)propan-2-one (4ga):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 4-methoxynaphthalen-1-ol (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 8.24–8.13 (m, 1H), 8.13–8.00 (m, 1H), 7.48 (m, 2H), 6.49 (s, 1H), 4.60 (m, 1H), 3.96 (s, 3H), 3.19–2.90 (m, 2H), 2.90–2.67 (m, 2H), 2.34 (s, 3H), 2.17 (m, 1H), 1.94–1.79 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 206.9, 149.1, 143.0, 126.0, 125.9, 125.1, 125.1, 121.7, 121.0, 114.6, 105.1, 72.3, 55.7, 49.2, 31.1, 27.7, 25.0. HRMS (ESI) m/z 293.1149 [(M+Na)+; calcd for C17H18O3Na: 293.1154].
- 2-(2-oxopropyl)-3,4-dihydro-2H,5H-pyrano[3,2-c]chromen-5-one (5aa):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 4-hydroxy-2H-chromen-2-one (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.68 (dd, J = 7.9, 1.6 Hz, 1H), 7.51 (ddd, J = 8.6, 7.3, 1.6 Hz, 1H), 7.32 (dd, J = 8.4, 1.1 Hz, 1H), 7.26 (ddd, J = 8.2, 7.4, 1.1 Hz, 1H), 4.75 (m, 1H), 3.10 (dd, J = 16.9, 7.4 Hz, 1H), 2.81 (dd, J = 16.9, 5.3 Hz, 1H), 2.70 (m, 1H), 2.60 (m, 1H), 2.32 (s, 3H), 2.22 (m, 1H), 1.80 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 204.9, 163.0, 159.5, 152.4, 131.5, 123.8, 122.2, 116.6, 115.6, 101.1, 73.7, 48.2, 31.0, 26.1, 18.9. HRMS (ESI) m/z 281.0795 [(M+Na)+; calcd for C15H14O4Na: 281.0790].
- 1-(2-oxopropyl)-2,3,4,6,7,8-hexahydro-5H-chromen-5-one (5ab):
The title compound was prepared from (E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), 3-hydroxycyclohex-2-en-1-one (0.3 mmol), and potassium carbonate (0.4 mmol). The product was a white solid. 1H NMR (500 MHz, CDCl3) δ 4.45–4.38 (m, 1H), 2.86 (dd, J = 16.7, 7.6 Hz, 1H), 2.60 (dd, J = 16.7, 5.1 Hz, 1H), 2.40–2.30 (m, 5H), 2.21 (s, 3H), 2.19–2.10 (m, 1H), 2.00–1.86 (m, 3H), 1.58–1.49 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 205.5, 198.2, 170.9, 111.3, 73.1, 48.3, 36.6, 30.8, 28.5, 26.5, 20.8, 17.3. HRMS (ESI) m/z 231.0995 [(M+Na)+; calcd for C12H16O3Na: 231.0997].
- The gram-scale reaction:
(E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one (0.2 mmol), naphthalen-2-ol (0.3 mmol), and potassium carbonate (0.4 mmol) were added into a round bottom flask under N2 atmosphere. N,N-Dimethylformamide was added to the reaction tube. Then, the reaction mixture was heated to 80 °C. After a time period of 10 h, the solution was diluted with ethyl acetate, washed with brine, and concentrated in vacuo. The crude product was purified by column chromatography on silica gel, eluting with EtOAc/petroleum ether (1/20, v/v) to afford the corresponding product.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28145502/s1, Table S1: Optimization of Protecting groups; Table S2: Optimization of Solvents; Table S3: Optimization of Bases; X-ray data for products 4ag; copies of 1H, 13C spectra. References [26,27,28,29] are cited in the Supplementary Materials.
Author Contributions
S.-S.L., L.-L.Z., M.P. and A.-J.M. performed the project; N.F., J.-B.P. and A.-J.M. provided the fund and data analysis; S.-S.L., L.-L.Z. and A.-J.M. prepared and checked the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Guangdong Province Rural Revitalization Strategy Special Fund (No. Jiangke (2021) 183), the Department of Education of Guangdong Province (2021KQNCX101), the Hong Kong–Macao Joint Research and Development Fund of Wuyi University (2019WGALH12 and 2021WGALH12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the synthesized compounds presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Bukuru, J.F.; Van, T.N.; Puyvelde, L.V.; Mathenge, S.G.; Mudida, F.P.; Kimpe, N.D. A Benzochromene from the Roots of Pentas bussei. J. Nat. Prod. 2002, 65, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Ekberg, A.; Akala, H.M.; Alao, J.P.; Sunnerhagen, P.; Yenesew, A.; Erdélyi, M. Busseihydroquinones A−D from the Roots of Pentas bussei. J. Nat. Prod. 2012, 75, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Karnik, A.V.; Kulkarni, A.M.; Malviya, N.J.; Mourya, B.R.; Jadhav, B.L. Synthesis and in vitro anti-bacterial evaluation of tetracyclic-ortho-fused 4H-naphtho[1′,2′–5,6]pyrano[3,4-d](1,2,3)selenadiazole and its derivatives. Eur. J. Med. Chem. 2008, 43, 2615–2617. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.F.; Abd, E.-W. Synthesis, Reactions and Evaluation of the Antimicrobial Activity of Some 4-(p-Halophenyl)-4H-naphthopyran, Pyranopyrimidine and Pyranotriazolopyrimidine Derivatives. Pharmaceuticals 2012, 5, 745–757. [Google Scholar]
- Schmitta, F.; Golda, M.; Rothemund, M.; Andronache, I.C.; Biwesack, B.; Schobert, R.; Muellet, T. New naphthopyran analogues of LY290181 as potential tumor vascular-disrupting agents. Eur. J. Med. Chem. 2019, 163, 160–168. [Google Scholar] [CrossRef]
- Schmitta, F.; Kasparkovab, J.; Brabecc, V.; Begemann, G.; Schobert, R.; Biwesack, B. New (arene)ruthenium(II) complexes of 4-aryl-4H-naphthopyrans with anticancer and anti-vascular activities. J. Inorg. Biochem. 2018, 184, 69–78. [Google Scholar] [CrossRef]
- Ferrart, F.; Monache, G.D.; de Lima, R. Two naphthopyran derivatives from Faramea cyanea. Phytochemistry 1985, 24, 2753–2755. [Google Scholar] [CrossRef]
- Gemert, B.V. Benzo and Naphthopyrans (Chromenes). In Organic Photochromic and Thermochromic Compounds, 1st ed.; Crano, J.C., Robert, G., Eds.; Plenum Press: New York, NY, USA, 1999; Volume 1, pp. 111–138. [Google Scholar]
- Pan, G.-L.; Wei, J.-Q.; Zhu, A.-P.; Ming, Y.-F.; Fan, M.-G.; Yao, S.-D. Photochromic properties and reaction mechanism of naphthopyran. Sci. China Ser. B Chem. 2001, 44, 276–282. [Google Scholar] [CrossRef]
- Kumar, S.; Hernandez, D.; Hoa, B.; Lee, Y.; Yang, J.S.; McCurdy, A. Synthesis, Photochromic Properties, and Light-Controlled Metal Complexation of a Naphthopyran Derivative. Org. Lett. 2008, 10, 3761–3764. [Google Scholar] [CrossRef]
- Blackwell, C.J.; Gabbutt, C.D.; Guthrie, J.T.; Heron, B.M. The synthesis and properties of vinyl substituted naphthopyrans and their styrene copolymers. Dye. Pigment. 2012, 95, 408–420. [Google Scholar] [CrossRef]
- Wang, J.; Dong, W.; Gao, B.; Liu, D.; Duan, Q. Syntheses and characterizations of Zn(II) Phthalocyanines & Naphthopyrans based polymers for improved nonlinear optical properties. Dye. Pigment. 2020, 182, 108662. [Google Scholar]
- Rawat, M.; Prutyanov, V.; Wulff, W.D. Chromene Chromium Carbene Complexes in the Syntheses of Naphthopyran and Naphthopyrandione Units Present in Photochromic Materials and Biologically Active Natural Products. J. Am. Chem. Soc. 2006, 128, 11044–11053. [Google Scholar] [CrossRef]
- Youn, S.W.; Eom, J.I. Ag(I)-catalyzed sequential C-C and C-O bond formations between phenols and dienes with atom economy. J. Org. Chem. 2006, 71, 6705–6707. [Google Scholar] [CrossRef] [PubMed]
- Adrio, L.A.; Hii, K.K. A recyclable copper(II) catalyst for the annulation of phenols with 1,3-dienes. Chem. Commun. 2008, 20, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W. Reusable Scandium/Ionic Liquid Catalyst System for Sequential C-C and C-O Bond Formations between Phenols and Dienes with Atom Economy. Synlett 2007, 19, 3050–3054. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Maati, H.; Abdelouahdi, K.; Fihri, A.; Zahouily, M.; Varma, R.S.; Aolhy, A. Highly Efficient One-Pot Three-Component Synthesis of Naphthopyran Derivatives in Water Catalyzed by Phosphates. ACS Sustain. Chem. Eng. 2013, 1, 1154–1159. [Google Scholar] [CrossRef]
- Madabhushi, S.; Jillella, R.; Godala, K.R.; Mallu, K.K.R.; Beeram, C.R.; Chinthala, N. An efficient and simple method for synthesis of 2,2-disubstituted-2H-chromenes by condensation of a phenol with a 1,1-disubstituted propargyl alcohol using BF3·Et2O as the catalyst. Tetrahedron Lett. 2012, 53, 5275–5279. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Itonaga, K. Synthesis of Chromans via [3 + 3] Cyclocoupling of Phenols with Allylic Alcohols Using a Mo/o-Chloranil Catalyst System. Org. Lett. 2009, 11, 717–720. [Google Scholar] [CrossRef]
- Xu, X.-B.; Liu, J.; Liang, L.-F.; Li, H.; Li, Y. Iron-Catalyzed Regioselective Hydroaryloxylation of CC Triple Bonds: An Efficient Synthesis of 2H-1-Benzopyran Derivatives. Adv. Synth. Catal. 2009, 351, 2599–2604. [Google Scholar] [CrossRef]
- Zhu, W.-R.; Su, Q.; Diao, H.-J.; Wang, E.-X.; Wu, F.; Zhao, Y.-L.; Weng, J.; Lu, G. Enantioselective Dehydrative γ-Arylation of α-Indolyl Propargylic Alcohols with Phenols: Access to Chiral Tetrasubstituted Allenes and Naphthopyrans. Org. Lett. 2020, 22, 6873–6878. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Xu, F. Synthesis of Chromans via Domino Reaction of Phenols with Prenyl Bromide Catalyzed by Zinc Chloride. Chin. J. Org. Chem. 2016, 36, 2499–2503. [Google Scholar] [CrossRef]
- Schreiber, S.L. Fragmentation reactions of α-alkoxy hydroperoxides and application to the synthesis of the macrolide (+)-recifeiolide. J. Am. Chem. Soc. 1980, 102, 6163–6165. [Google Scholar] [CrossRef]
- Dong, Y.-W.; Wang, G.-W.; Wang, L. Solvent-free synthesis of naphthopyrans under ball-milling conditions. Tetrahedron 2008, 64, 10148–10154. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.-W.; Wang, G.-W.; Komatsu, K. Highly Efficient Mechanochemical Reactions of 1,3-Dicarbonyl Compounds with Chalcones and Azachalcones Catalyzed by Potassium Carbonate. Synlett 2004, 1, 61–64. [Google Scholar] [CrossRef]
- Khrizman, A.; Cheng, H.Y.; Moyna, G. Synthesis of sequentially deuterated 1-n-butyl-3-methylimidazolium ionic liquids. J. Label. Compd. Rad. 2011, 54, 401–407. [Google Scholar] [CrossRef]
- Balasubramanyam, P.; Reddy, G.C.; Salvanna, N.; Das, B. Efficient stereoselective total synthesis of (+)-cryptofolione and the first synthesis of (-)-cryptocaryalactone. Synthesis 2011, 22, 3706–3710. [Google Scholar]
- Lin, L.; Romano, C.; Mazet, C. Palladium-Catalyzed Long-Range Deconjugative Isomerization of Highly Substituted α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2016, 138, 10344–10350. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Li, D.; Liu, X.; Wang, P.; Wang, K.; Zhu, H.; Bai, L.; Wang, R. The Important Role of the Byproduct Triphenylphosphine Oxide in the Magnesium(II)-Catalyzed Enantioselective Reaction of Hemiacetals and Phosphorus Ylides. Angew. Chem. Int. Ed. 2018, 57, 9088–9092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).