A Rational Combination of Cyclocarya paliurus Triterpene Acid Complex (TAC) and Se-Methylselenocysteine (MSC) Improves Glucose and Lipid Metabolism via the PI3K/Akt/GSK3β Pathway
Abstract
1. Introduction
2. Results
2.1. Response Surface Analysis and Optimization
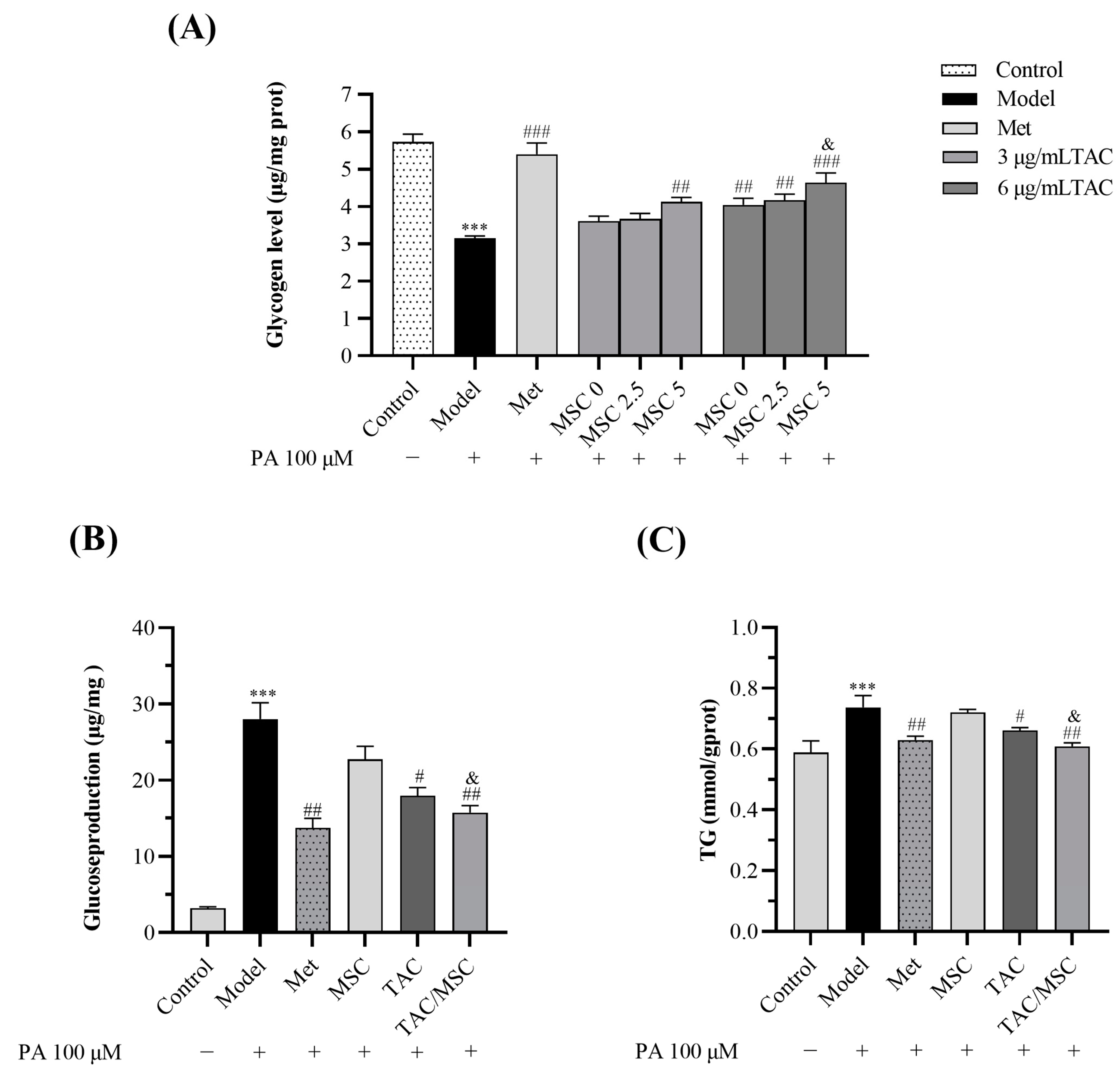
2.2. TAC/MSC-Enhanced Glucose Consumption in HepG2 Cells
2.3. TAC/MSC Can Increase Glycogen Synthesis in Insulin-Resistant HepG2 Cells
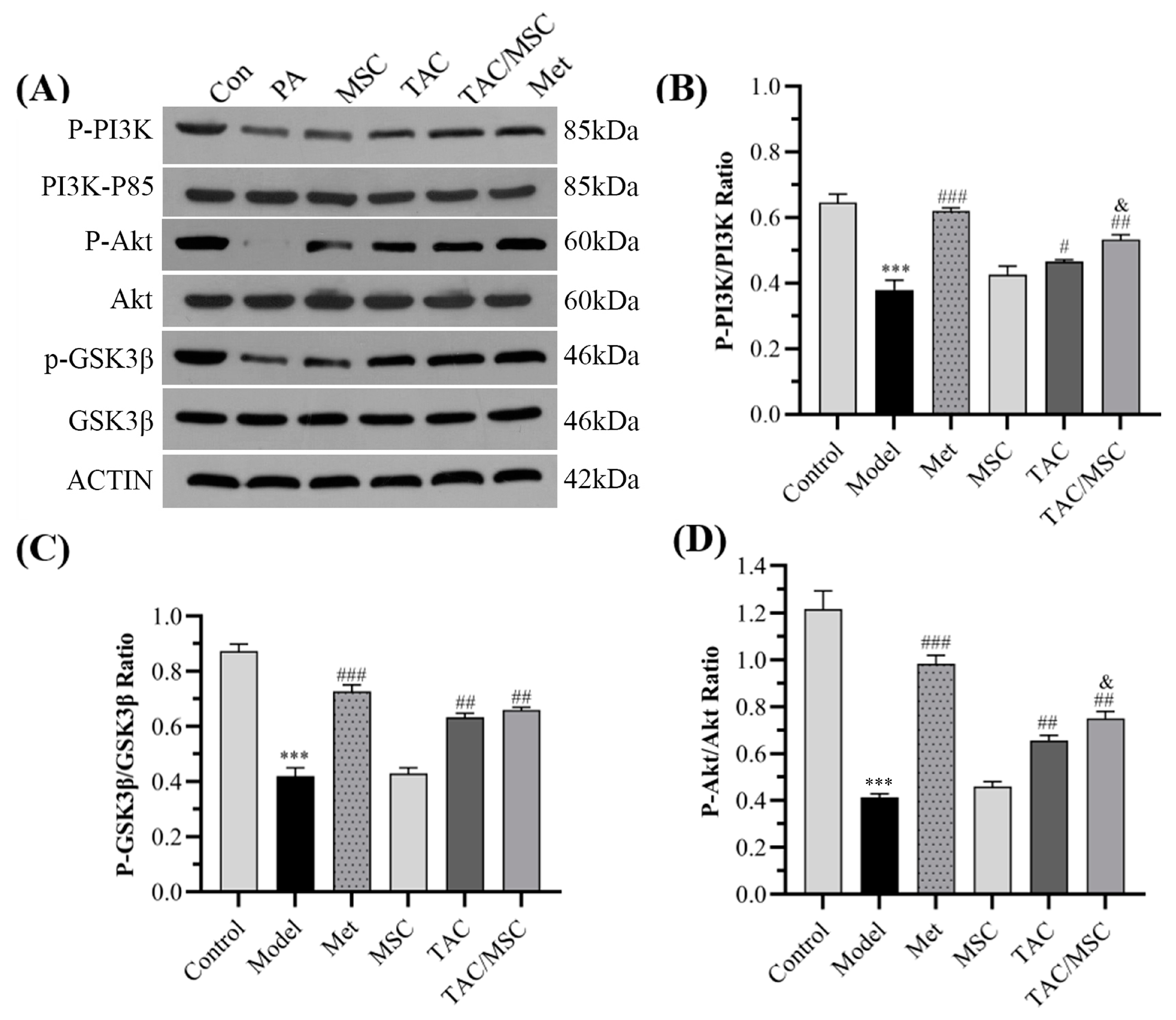
2.4. Effects of TAC/MSC on P-PI3K, PI3K, P-AKT, AKT, P-GSK3β, and GSK3β in HepG2 Cells
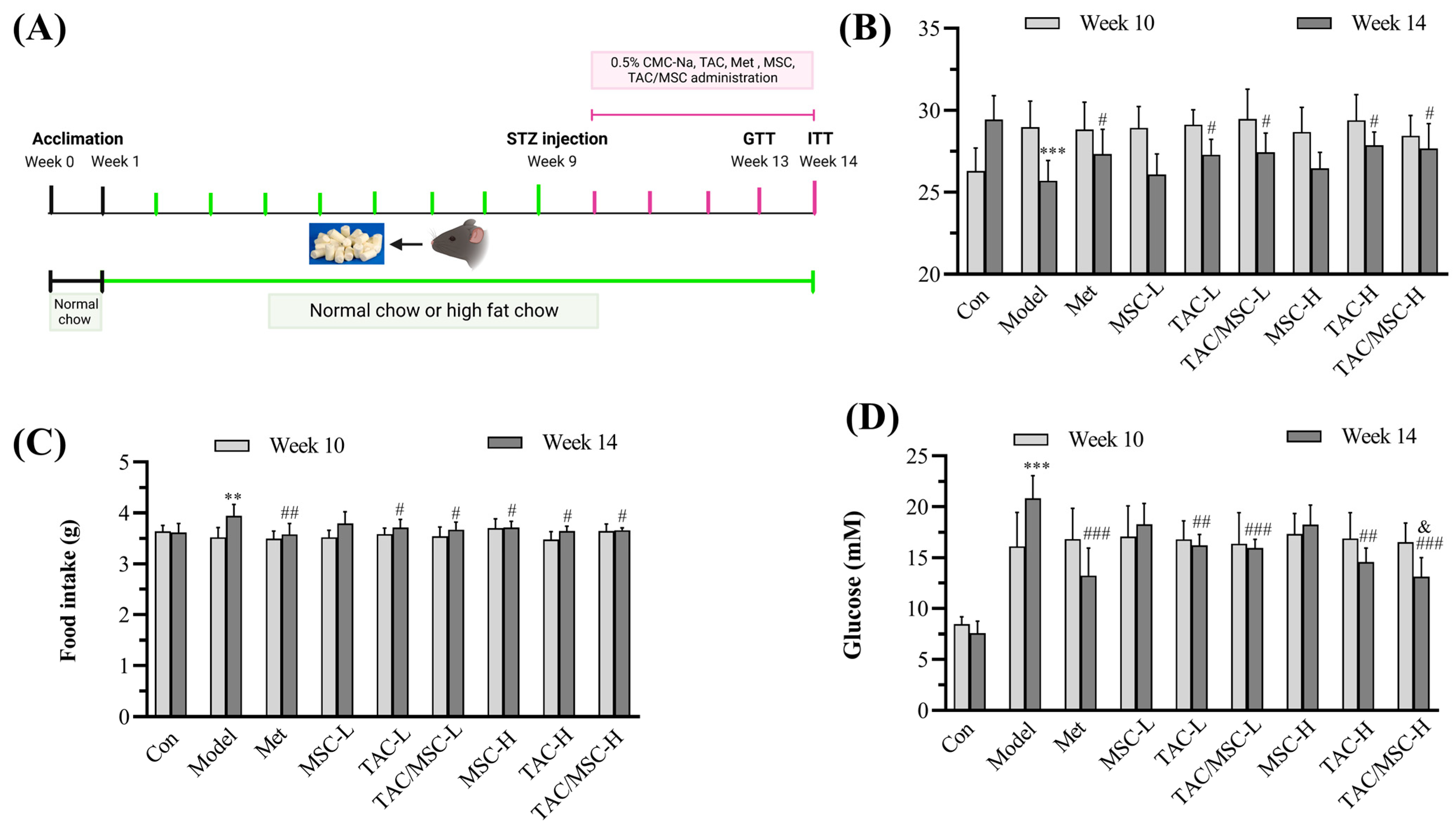
2.5. Effects of TAC/MSC on Body Weight, Food Intake, and Fasting Blood Glucose
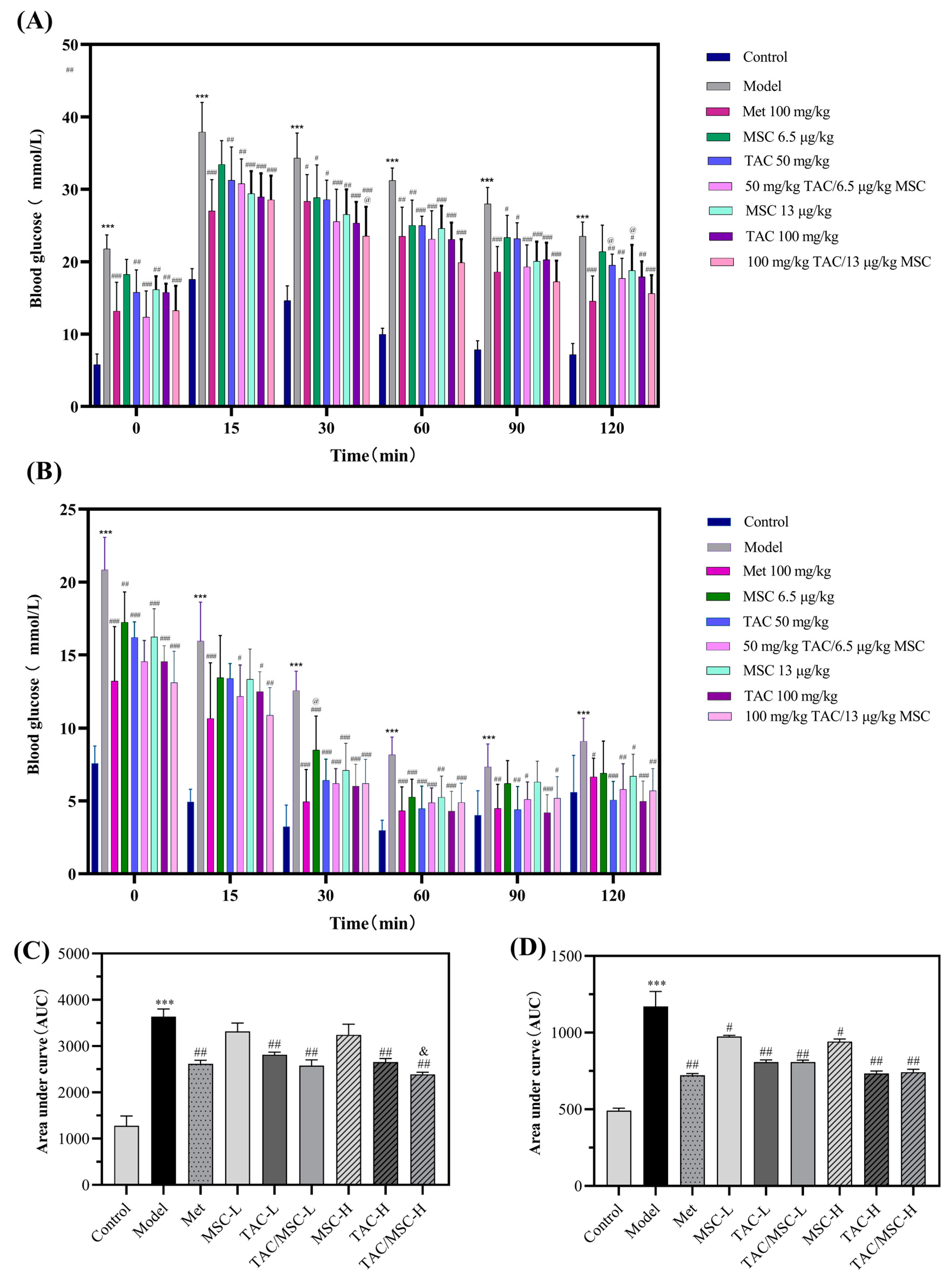
2.6. Effects of TAC/MSC on Glucose Tolerance Tests (GTTs) and Insulin Tolerance Tests (ITTs) of T2DM Mice
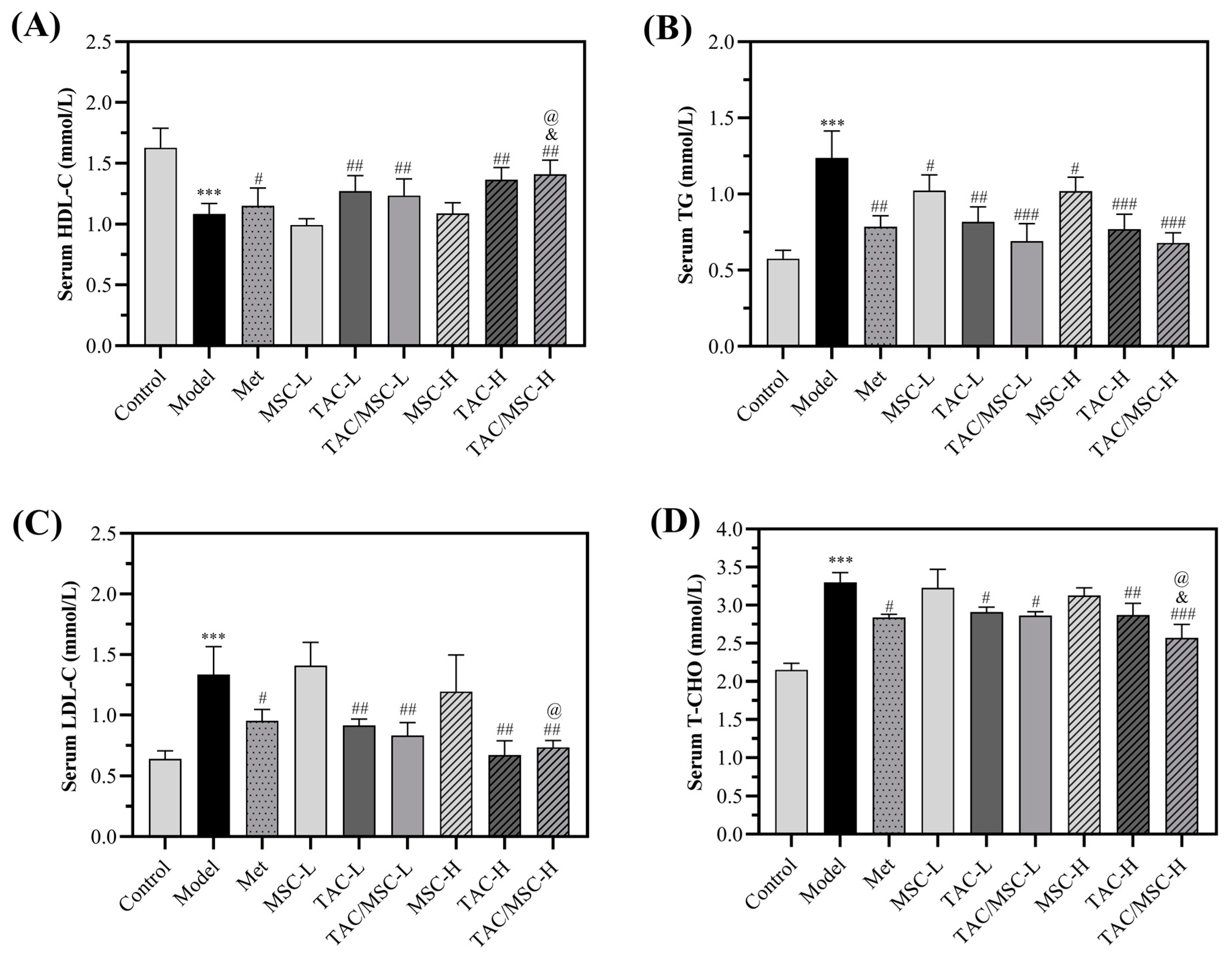
2.7. Effects of TAC/MSC on Serum Lipid Metabolism in T2DM Mice
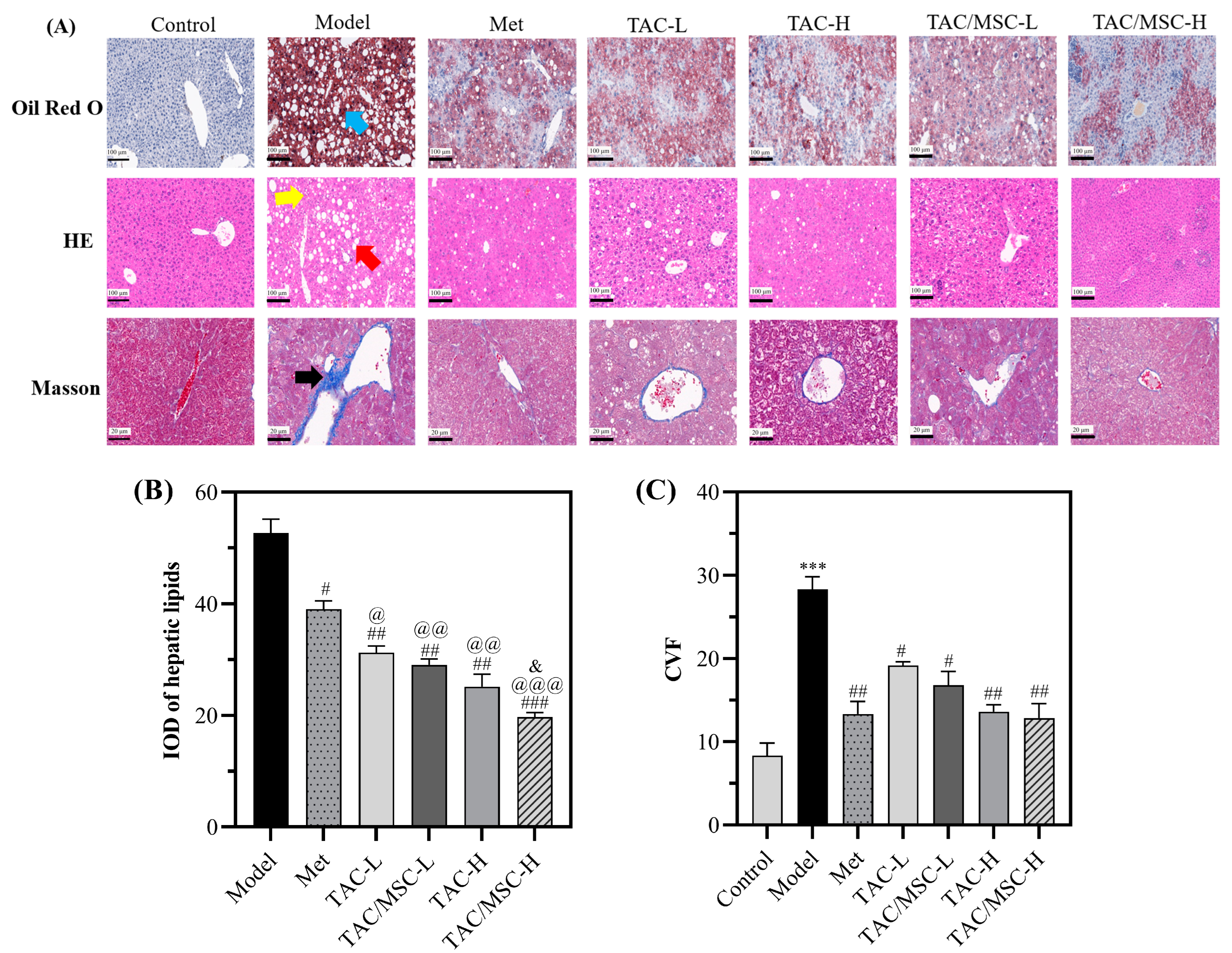
2.8. Effect of TAC/MSC on T2DM-Induced Histopathological Changes in the Liver
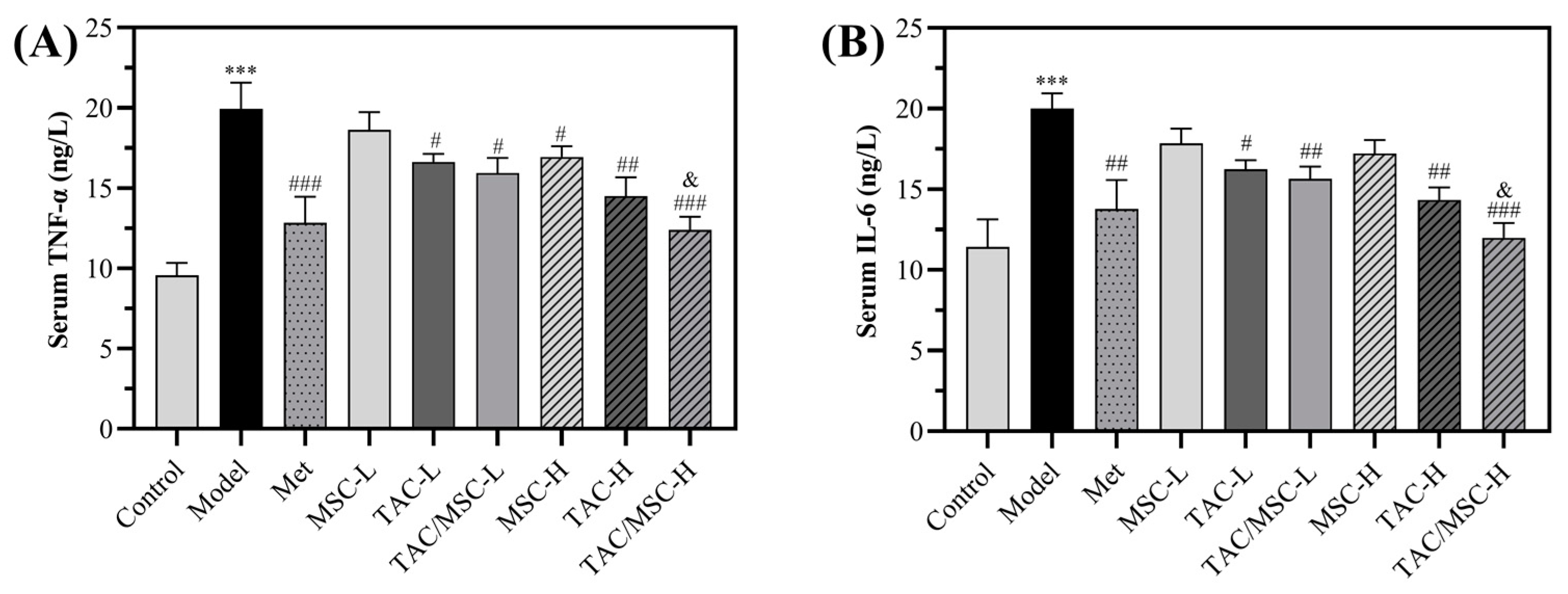
2.9. Effects of TAC/MSC on Proinflammatory Cytokines
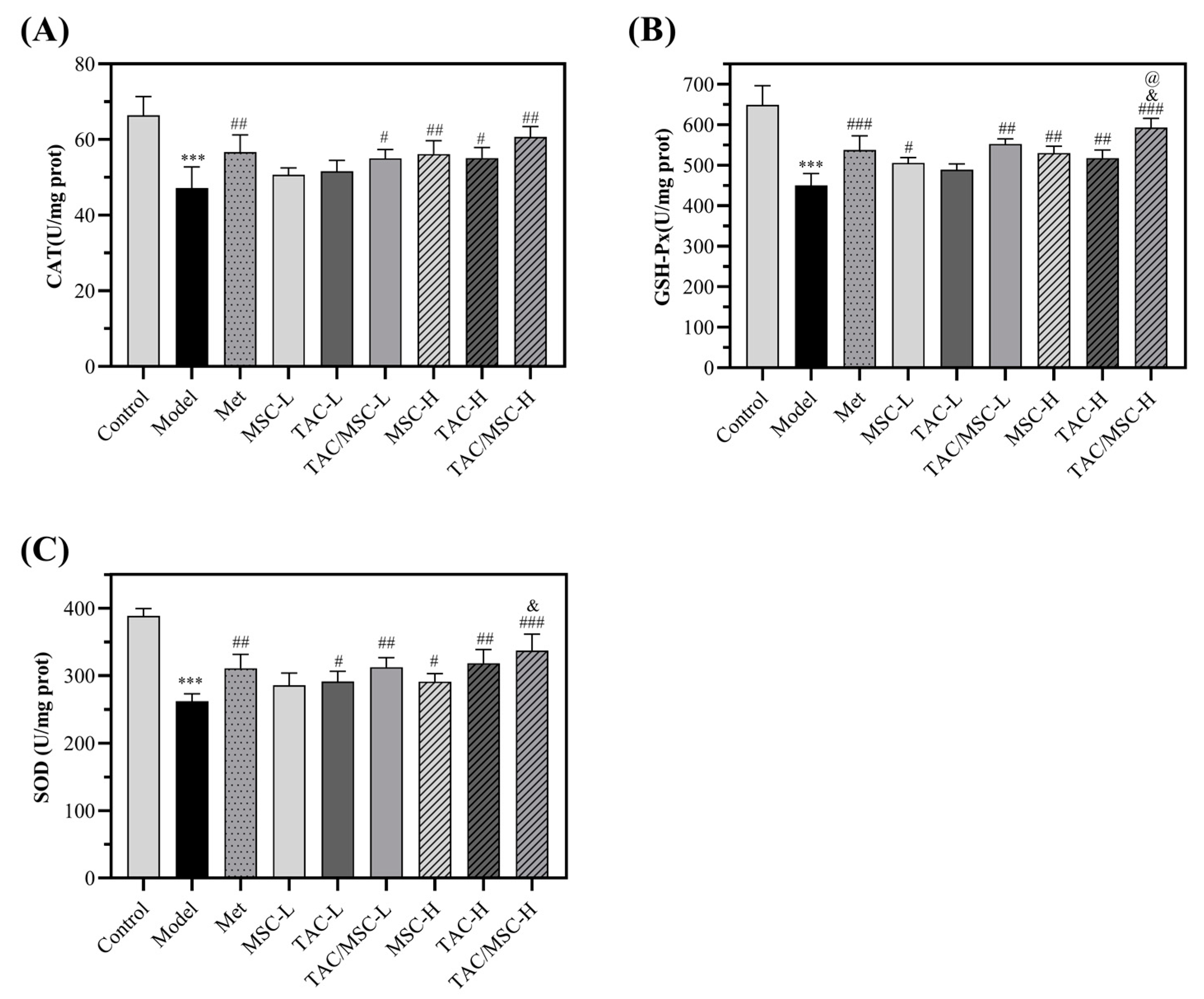
2.10. Effects of TAC/MSC on Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), and Catalase (CAT) in Liver
2.11. Effects of TAC/MSC on ROS in the Liver
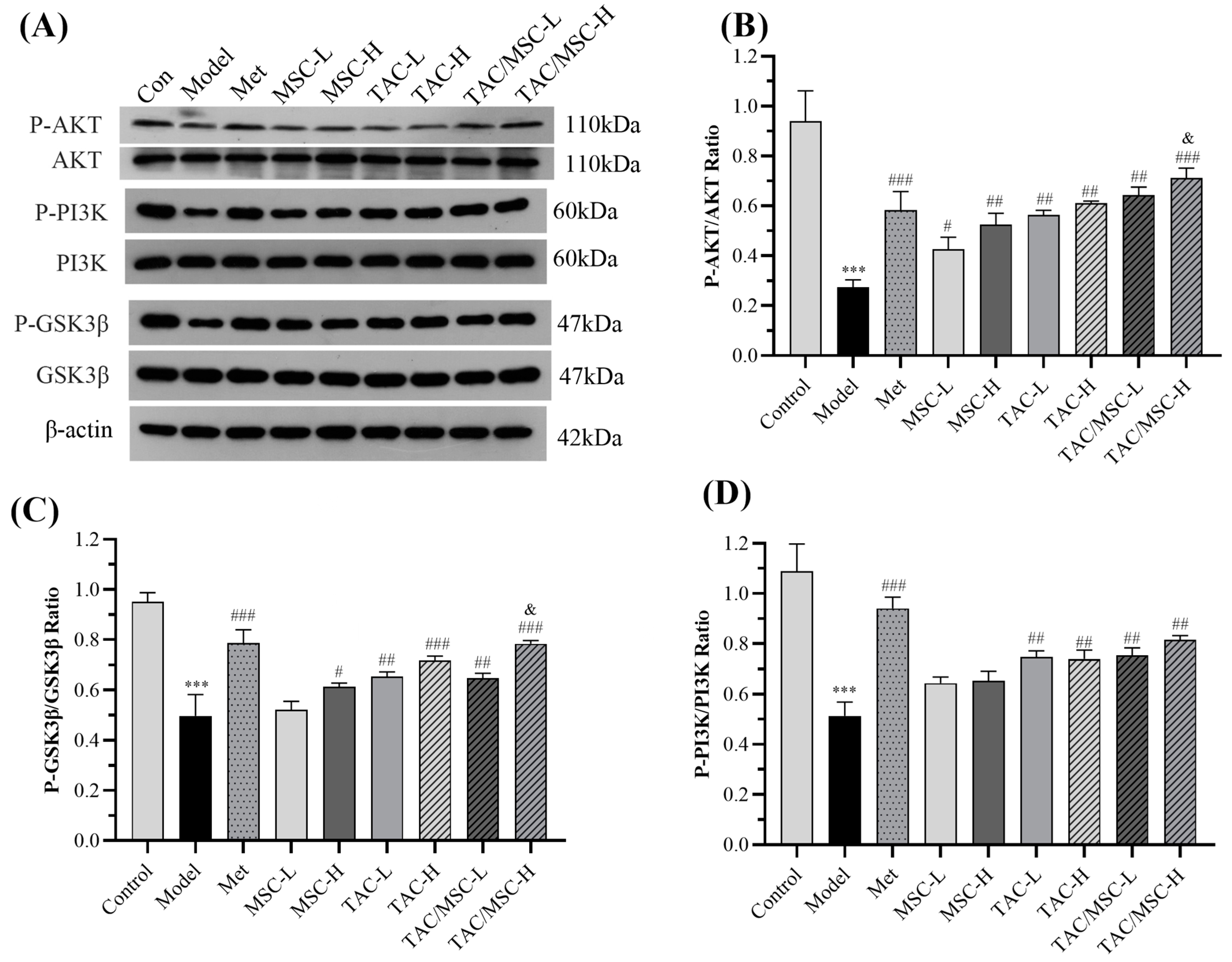
2.12. Effects of TAC/MSC on P-PI3K, PI3K, P-AKT, AKT, P-GSK3β, and GSK3β in the Liver
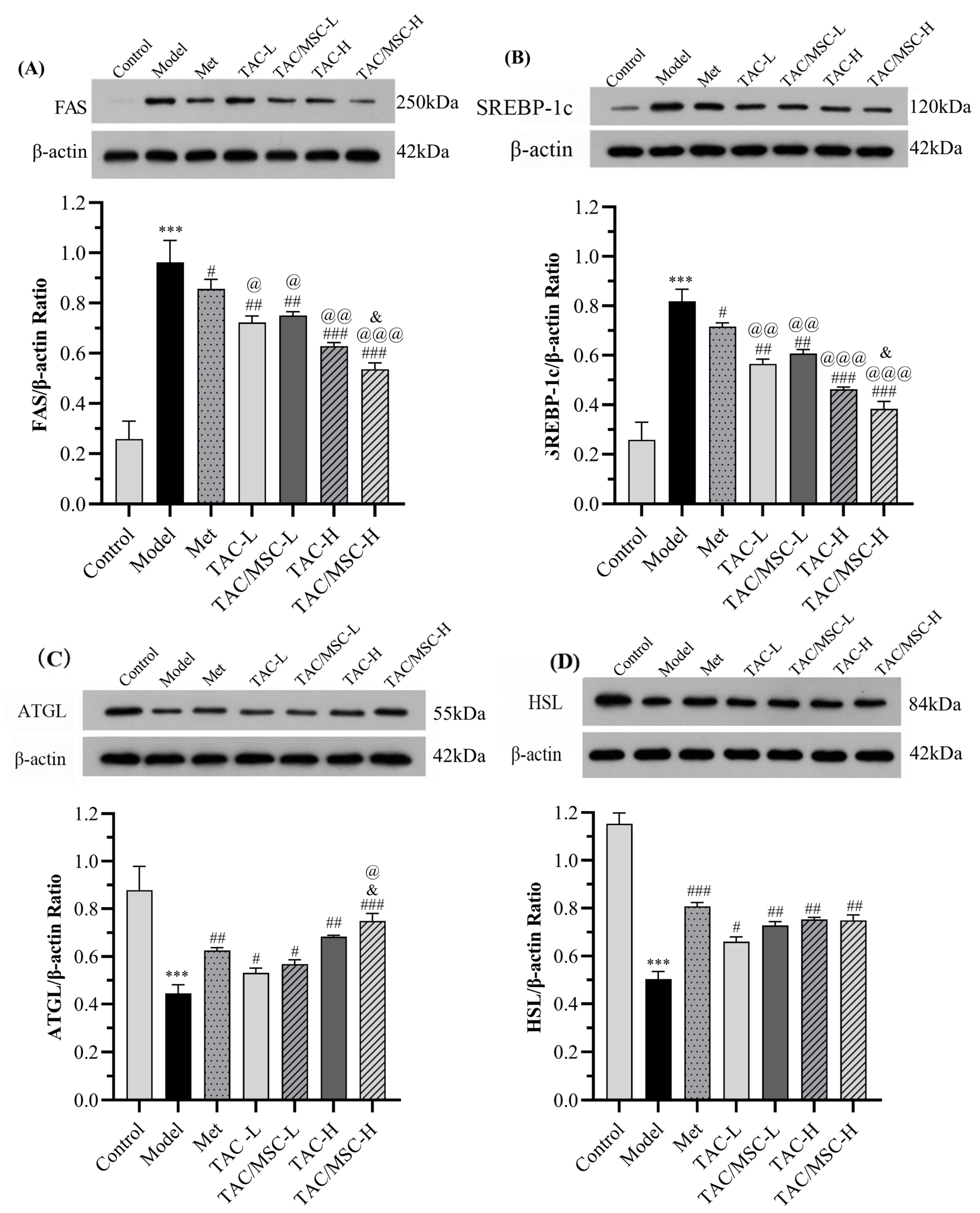
2.13. Effects of TAC/MSC on SREBP-1c, FAS, ATGL, and HSL in the Liver and White Adipose Tissue (WAT)
3. Discussion
4. Materials and Methods
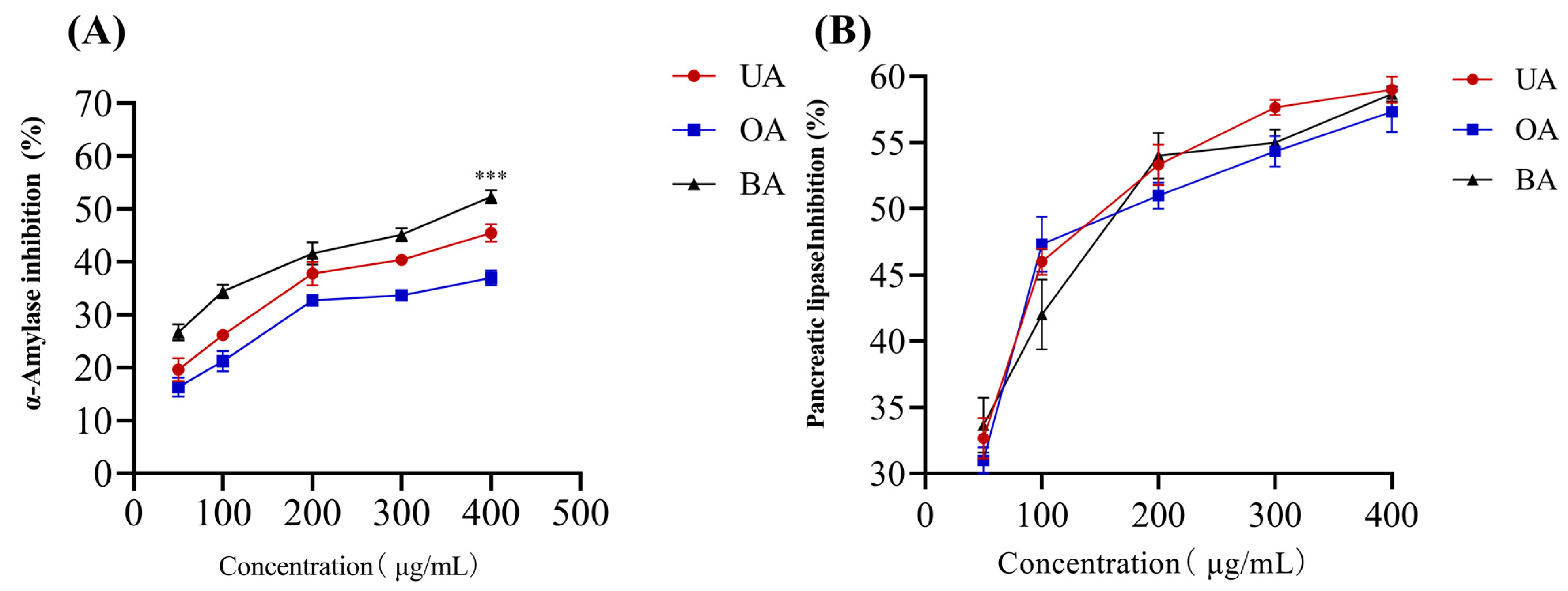
4.1. α-Amylase Inhibition Assay
4.2. Pancreatic Lipase Inhibition Assay
4.3. Process Optimization
4.4. Cell Culture and Treatments
4.5. MTT Assay
4.6. Glucose Consumption Assay
4.7. Glycogen Content Assay
4.8. Glucose Production Assay
4.9. Glucose Production Assay
4.10. In Vivo Experimental Design
4.11. Food Intake, Body Weight, Blood Glucose, and Biochemical Analysis
4.12. In Vivo Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
4.13. Enzyme-Linked Immunosorbent Assay
4.14. Histological Assessment
4.15. Immunofluorescence Analyses
4.16. Western Blot
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Wang, H.; Tang, C.; Gao, Z.Z.; Huang, Y.S.; Zhang, B.X.; Wei, J.H.; Zhao, L.H.; Tong, X.L. Potential role of natural plant medicine Cyclocarya paliurus in the treatment of Type 2 Diabetes mellitus. J. Diabetes Res. 2021, 2021, 1655336. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yan, L.J.; Chen, H.; Wu, N.; Wang, W.B.; Wang, D.S. Cyclocarya paliurus Polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Liu, X.; Yin, J.Y.; Huang, D.F.; Zhang, H.; Xie, M.Y. Simultaneous analysis of 18 mineral elements in Cyclocarya paliurus polysaccharide by ICP-AES. Carbohydr. Polym. 2013, 94, 216–220. [Google Scholar] [CrossRef]
- Yan, H.; Li, X.; Ni, W.; Zhao, Q.; Leng, Y.; Liu, H.Y. Phytochemicals from the leaves of Cyclocarya paliurus and their 11β-HSD1 enzyme inhibitory effects. Chem. Biodivers. 2021, 18, e2000772. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.J.; Sun, H.H.; Chen, Z.H.; Wu, J.P.; Li, J.; Zhu, H.; Huang, L.L.; Chang, X.W.; Ou, S.Y.; Wang, W.X.; et al. Four new prenylflavonol glycosides from the leaves of Cyclocarya paliurus. Nat. Prod. Res. 2022, 36, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Sheng, X.P.; Huang, Y.P.; Wang, Y.T.; Jiang, C.H.; Zhang, J.; Yin, Z.Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates non-alcoholic fatty liver disease via improving oxidative stress and mitochondrial dysfunction. Biomed. Pharmacother. 2018, 104, 229–239. [Google Scholar] [CrossRef]
- Wu, Z.F.; Gao, T.H.; Zhong, R.L.; Lin, Z.; Jiang, C.H.; Ouyang, S.; Zhao, M.; Che, C.T.; Zhang, J.; Yin, Z.Q. Antihyperlipidaemic effect of triterpenic acid-enriched fraction from Cyclocarya paliurus leaves in hyperlipidaemic rats. Pharm. Biol. 2017, 55, 712–721. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.N.; Fang, S.Z.; Wang, T.L.; Yin, Z.Q.; Shang, X.L.; Yang, W.X.; Fu, X.X. Antidiabetic effect of Cyclocarya Paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules 2018, 23, 1042. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, M.G.; Jiang, C.H.; Sheng, X.P.; Yang, H.M.; Liu, Y.; Yao, X.M.; Zhang, J.; Yin, Z.Q. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine 2020, 66, 153130. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, P.Y.; Xin, S.C.; Peng, D.Y.; Chen, J.G.; Yan, J. Stimulation of glucose consumption in 3T3-L1 adipocytes by triterpenoids from Cyclocarya paliurus Leaves. Mod. Food Sci. Technol. 2014, 30, 31–37. [Google Scholar]
- Fang, Z.J.; Shen, S.N.; Wang, J.M.; Wu, Y.J.; Zhou, C.X.; Mo, J.X.; Lin, L.G.; Gan, L.S. Triterpenoids from Cyclocarya paliurus that enhance glucose uptake in 3T3-L1 adipocytes. Molecules 2019, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: Promise or illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Hu, W.L.; Zhao, C.; Hu, H.B.; Yin, S.T. Food sources of selenium and its relationship with chronic diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Lv, M.; Chen, W.H.; Xu, Q.; Tan, K.X.; Xiang, J.Q.; Liu, W. Effect of organic selenium rich fertilizer on selenium, trace elements and functional components in Cyclocarya paliurus leaves. J. Agric. 2016, 6, 39–42. [Google Scholar]
- Zhang, H.; Chen, W.H.; Ma, F.; Wang, S.; Tan, K.X.; Xiang, J.Q.; Liu, W. Effects of selenium rich Cyclocarya paliurus polysaccharide on blood glucose, blood lipid and immunity in diabetes model mice. Food Sci. 2017, 38, 228–232. [Google Scholar]
- Shin, H.S.; Yang, W.J.; Choi, E.M. The Preventive effect of Se-methylselenocysteine on γ-radiation-induced oxidative stress in rat lungs. J. Trace Elem. Med. Biol. 2013, 27, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Du, X.B.; Shi, Q.Q.; Zhao, Y.X.; Xie, Y.L.; Li, X.; Liu, Q.; Iqbal, J.; Zhang, H.; Liu, X.; Shen, L. Se-methylselenocysteine (SMC) improves cognitive deficits by attenuating synaptic and metabolic abnormalities in alzheimer’s mice model: A proteomic study. ACS Chem. Neurosci. 2021, 12, 1112–1132. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane Mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef]
- Kiersztan, A.; Baranska, A.; Hapka, M.; Lebiedzinska, M.; Winiarska, K.; Dudziak, M.; Bryla, J. Differential action of methylselenocysteine in control and alloxan-diabetic rabbits. Chem. Biol. Interact. 2009, 177, 161–171. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-obesity effect of DKB-117 through the inhibition of pancreatic lipase and α-Amylase activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jin, Z.; Jiang, S. Antiproliferative and pro-apoptotic effects of Cyclocarya paliurus polysaccharide and X-Ray irradiation combination on SW480 colorectal cancer cells. Mol. Med. Rep. 2019, 20, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Shen, M.Y.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Li, C.; Huang, D.F.; Wang, Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef]
- Wu, T.; Shen, M.Y.; Guo, X.M.; Huang, L.X.; Yang, J.; Yu, Q.; Chen, Y.; Xie, J.H. Cyclocarya paliurus polysaccharide alleviates liver inflammation in mice via beneficial regulation of gut microbiota and TLR4/MAPK signaling pathways. Int. J. Biol. Macromol. 2020, 160, 164–174. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Shi, M.; Zheng, C.; Liu, Y.; Qin, H.; Zhang, Y.; Li, X. The effect of cyclocarya triterpenoids on autophagy and apoptosis of INS-1 cells injured by streptozotocin. Pharm. Clin. Med. 2017, 33, 89–94. [Google Scholar]
- Liao, E.P. Management of type 2 diabetes: New and future developments in treatment. Am. J. Med. 2012, 125, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-Induced Insulin Resistance. Biochim. Biophys. Acta 2014, 1842, 446–462. [Google Scholar] [CrossRef]
- Ishii, M.; Maeda, A.; Tani, S.; Akagawa, M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch. Biochem. Biophys. 2015, 566, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Belkina, A.C.; Denis, G.V. Obesity genes and insulin resistance. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 472–477. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hamza, A.A. Rosuvastatin ameliorates diabetes-Induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015, 141, 13–19. [Google Scholar] [CrossRef]
- Li, B.B.; Hong, Y.; Gu, Y.; Ye, S.J.; Hu, K.L.; Yao, J.; Ding, K.; Zhao, A.H.; Jia, W.; Li, H.K. Functional metabolomics reveals that astragalus polysaccharides improve lipids metabolism through microbial metabolite 2-Hydroxybutyric acid in obese Mice. Engineering 2022, 9, 111–122. [Google Scholar] [CrossRef]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.B.; Zhao, Y.; Xu, L.J.; Zou, X.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Jiang, C.H.; Yao, N.; Li, Y.; Wang, Q.Q.; Fang, S.Z.; Shang, X.L.; Zhao, M.; Che, C.T.; Ni, Y.C.; et al. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J. Ethnopharmacol. 2015, 166, 286–296. [Google Scholar] [CrossRef]
- Guzik, T.J.; Cosentino, F. Epigenetics and immunometabolism in diabetes and aging. Antioxid. Redox Signal. 2018, 29, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Eom, S.H.; Lee, M.S.; Lee, E.W.; Kim, Y.M.; Kim, T.H. Pancreatic lipase inhibitory activity of phlorotannins isolated from eisenia bicyclis. Phytother. Res. 2013, 27, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, L.S.; Ding, Y.; Cheng, B.C.Y.; Luo, G.; Kong, J.; Liu, T.H.; Zhang, S.F. Edgeworthia Gardneri (Wall.) Meisn. water extract ameliorates palmitate induced insulin resistance by regulating IRS1/GSK3b/FOXO1 signaling pathway in human HepG2 hepatocytes. Front. Pharmacol. 2020, 10, 1666. [Google Scholar] [CrossRef]


| RUN | UA (μg/mL) | BA (μg/mL) | OA (μg/mL) | Pancreatic Lipase Inhibition (%) | α-Amylase Inhibition (%) |
|---|---|---|---|---|---|
| 1 | 100 | 100 | 250 | 45.38 | 34.48 |
| 2 | 400 | 100 | 250 | 43.8 | 43.95 |
| 3 | 100 | 400 | 250 | 42.83 | 48.79 |
| 4 | 400 | 400 | 250 | 56.52 | 59.74 |
| 5 | 100 | 250 | 100 | 60.51 | 46.51 |
| 6 | 400 | 250 | 100 | 61.08 | 54.47 |
| 7 | 100 | 250 | 400 | 62.56 | 53.48 |
| 8 | 400 | 250 | 400 | 63.18 | 59.49 |
| 9 | 250 | 100 | 100 | 58.08 | 44.47 |
| 10 | 250 | 400 | 100 | 60.93 | 59.01 |
| 11 | 250 | 100 | 400 | 58.2 | 46.84 |
| 12 | 250 | 400 | 400 | 61.9 | 62.31 |
| 13 | 250 | 250 | 250 | 62.03 | 58.98 |
| 14 | 250 | 250 | 250 | 64.51 | 52.71 |
| 15 | 250 | 250 | 250 | 62.5 | 55.62 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 781.98 | 9 | 86.89 | 17.77 | 0.0005 | Significant |
| A | 56.6 | 1 | 56.60 | 11.58 | 0.0114 | |
| B | 53.58 | 1 | 53.38 | 10.96 | 0.0129 | |
| C | 10.75 | 1 | 10.75 | 2.20 | 0.1817 | |
| AB | 92.82 | 1 | 92.82 | 18.98 | 0.0033 | |
| AC | 3.88 | 1 | 3.88 | 0.79 | 0.4025 | |
| BC | 0.19 | 1 | 0.19 | 0.039 | 0.8498 | |
| A2 | 204.25 | 1 | 204.25 | 41.77 | 0.0003 | |
| B2 | 270.88 | 1 | 270.88 | 55.4 | 0.0001 | |
| C2 | 92.44 | 1 | 92.44 | 18.91 | 0.0034 | |
| Residual | 34.23 | 7 | 4.89 | |||
| Lack of Fit | 28.08 | 3 | 9.43 | 6.34 | 0.0532 | Not significant |
| Pure Error | 5.95 | 4 | 1.49 | |||
| Cor Total | 816.21 | 16 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 808.70 | 9 | 88.96 | 18.34 | 0.0005 | Significant |
| A | 147.79 | 1 | 147.79 | 30.17 | 0.0009 | |
| B | 451.44 | 1 | 451.44 | 92.14 | <0.0001 | |
| C | 39.08 | 1 | 39.08 | 7.98 | 0.0256 | |
| AB | 0.55 | 1 | 0.55 | 0.11 | 0.7475 | |
| AC | 0.95 | 1 | 0.95 | 0.19 | 0.6722 | |
| BC | 0.23 | 1 | 0.23 | 0.047 | 0.8353 | |
| A2 | 66.16 | 1 | 66.16 | 13.5 | 0.0079 | |
| B2 | 77.82 | 1 | 77.82 | 15.89 | 0.0053 | |
| C2 | 25.18 | 1 | 25.18 | 5.14 | 0.0577 | |
| Residual | 34.29 | 7 | 4.9 | |||
| Lack of Fit | 10.15 | 3 | 3.38 | 0.56 | 0.6668 | Not significant |
| Pure Error | 24.14 | 4 | 6.04 | |||
| Cor Total | 843 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Zhou, H.; Luo, D.; Chen, D.; Fan, J.; Shao, X.; Zhou, J.; Liu, W. A Rational Combination of Cyclocarya paliurus Triterpene Acid Complex (TAC) and Se-Methylselenocysteine (MSC) Improves Glucose and Lipid Metabolism via the PI3K/Akt/GSK3β Pathway. Molecules 2023, 28, 5499. https://doi.org/10.3390/molecules28145499
Bai X, Zhou H, Luo D, Chen D, Fan J, Shao X, Zhou J, Liu W. A Rational Combination of Cyclocarya paliurus Triterpene Acid Complex (TAC) and Se-Methylselenocysteine (MSC) Improves Glucose and Lipid Metabolism via the PI3K/Akt/GSK3β Pathway. Molecules. 2023; 28(14):5499. https://doi.org/10.3390/molecules28145499
Chicago/Turabian StyleBai, Xichen, Hong Zhou, Dan Luo, Dan Chen, Jianyuan Fan, Xiaoting Shao, Jun Zhou, and Wei Liu. 2023. "A Rational Combination of Cyclocarya paliurus Triterpene Acid Complex (TAC) and Se-Methylselenocysteine (MSC) Improves Glucose and Lipid Metabolism via the PI3K/Akt/GSK3β Pathway" Molecules 28, no. 14: 5499. https://doi.org/10.3390/molecules28145499
APA StyleBai, X., Zhou, H., Luo, D., Chen, D., Fan, J., Shao, X., Zhou, J., & Liu, W. (2023). A Rational Combination of Cyclocarya paliurus Triterpene Acid Complex (TAC) and Se-Methylselenocysteine (MSC) Improves Glucose and Lipid Metabolism via the PI3K/Akt/GSK3β Pathway. Molecules, 28(14), 5499. https://doi.org/10.3390/molecules28145499






