Abstract
Heterocyclic compounds are significant lead drug candidates based on their various structure–activity relationships (SAR), and their use in pharmaceutics is constantly developing. Benzimidazole (BnZ) is synthesized by a condensation reaction between benzene and imidazole. The BnZ structure consists of two nitrogen atoms embedded in a five-membered imide ring which is fused with a benzene ring. This review examines the conventional and green synthesis of metallic and non-metallic BnZ and their derivatives, which have several potential SARs, along with a wide range of pharmacological properties, including anti-cancer, anti-inflammatory, anti-microbial, anti-tubercular, and anti-protozoal properties. These compounds have been proven by pharmacological investigations to be efficient against different strains of microbes. Therefore, in this review, the structural variations of BnZ are listed along with various applications, predominantly related to their biological activities.
1. Introduction
With regard to BnZ’s limiting efficacy and its extensive advantageous reactive properties for quantitative and qualitative relations, BnZ derivatives have remarkable beneficial components due to their diverse bioactivity and therapeutic uses [1]. Due to the importance of BnZ, it was decided to synthesize a number of novel BnZ-based derivatives with additional heteroatoms and investigate their probable bioactivity. BnZ exhibits a significant electron-rich heterocyclic pharmacophore in its structure which is beneficial for drug design and development [2]. Investigation of the melting point (m.p.) of several BnZ derivatives showed that addition of a substituent to 1-position leads to a reduction in m.p. The presence of two nitrogen atoms in the imide group generally causes polarity, resulting in its solubility in organic solvents and greater solubility in polar solvents. The solubility in non-polar solvents may improve with the addition of non-polar substituents to the BnZ ring in various positions. In contrast, the addition of polar groups to BnZ causes it to become more soluble in polar liquids. Over 300 °C, the BnZ derivatives’ structures are unaltered. BnZ is soluble in dilute acid and has less basicity than imidazole. Additionally, BnZ has sufficient acidity to dissolve in aqueous (aq.) alkali for the synthesis of N-metallic compounds. Similar to imidazole, BnZ’s acidic character shows ion stabilization by resonance. In a weak basic solution such as K2CO3, BnZ is more acidic with improved solubility in an aqueous medium [3]. Thus, the addition of substituents to the BnZ ring at different positions can significantly impact properties such as melting point and solubility in organic and polar solvents. However, BnZ shows significant electronic transition because of its heterocyclic pharmacophore, which contributes to its pharmacological potential. Furthermore, its unique characteristics enable structural modulation of BnZ and its derivatives, which allows for solubility in dilute acid. Therefore, by exploring novel BnZ-based structural variations with additional heteroatoms, further insights into their bioactivity can be gained. These factors highlight the importance of BnZ derivatives and their potential applications.
2. Synthesis of Benzimidazole
BnZ is a bicyclic heterocyclic aromatic compound which is made up of benzene and imidazole rings bonded at 4- and 5-positions. Ortho-phenylene derivatives of BnZ such as methyl-o-phenylenediamine are known as benzoglyoxalines. Recently, several researchers have described various techniques for synthesizing 1- or 1, 2-disubstituted BnZ derivatives by employing various moieties in various reaction environments [4]. Initially, BnZ was synthesized as a 2,5-dimethyl-benzimidazole (III) in 1872 by Hoebrecker [5] through the reduction of 2-nitro-4-methylacetanilide (I) using tin (Sn) and hydrochloric acid (HCl), followed by the dehydration of 2-nitro-4-methylacetanilide(II), as explained in Scheme 1. Details regarding the specific reaction conditions are provided elsewhere [6].
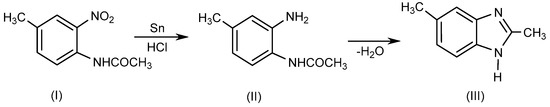
Scheme 1.
Hoebrecker method.
BnZ synthesis proceeds by choosing benzene derivatives that have nitrogen-containing functionalities at ortho positions to each other, e.g., orthophenylenediamine (Figure 1). It is evident that the starting material must have the functionality to enable the preparation of BnZ using a variety of techniques. Ortho-phenylenediamine and its derivatives undergo a condensation process with carboxylic acids or aldehydes. Therefore, several BnZ synthesis processes have been categorized based on the basic nature of o-phenylenediamine [7]. The direct condensation of orthophenylenediamine with nitriles has much use in the synthesis of benzimidazoles as well as in the manufacture of benzimidazole-related agricultural and pharmaceuticals chemicals because nitriles are easily accessible due to their broad supply as commodity chemicals [8].
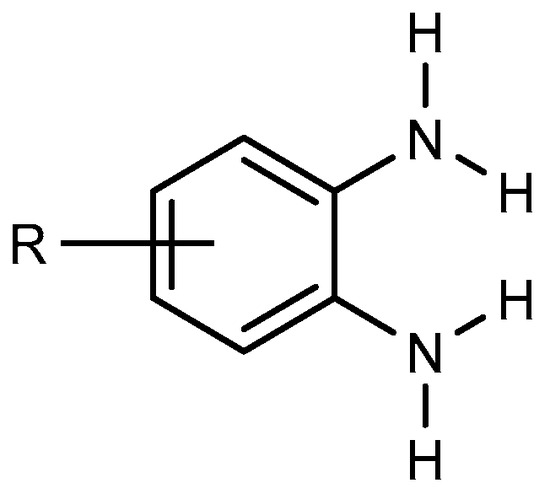
Figure 1.
Orthophenylenediamine compound.
The most typical synthetic approach for producing several BnZ derivatives is known as Phillip’s method, which entails the condensation of o-phenylenediamine (IV) with carboxylic acids (V) itself or its derivatives, and which involves heating the reagents in the presence of concentrated HCl (Scheme 2).
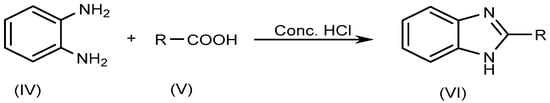
Scheme 2.
Phillip’s method.
A number of new modified BnZ derivatives have been synthesized, having a furan substituent at the second position and an alkyl/aryl substituent at the first position, which further was tested by an in silico study [9].
Several synthetic tactics have been successfully implemented, which are described in Figure 2 for the production of BnZ [A]. A combination of ortho-substituted aniline [B] and bifunctional o-esters produced a BnZ derivative in 2012, according to Bastug et al. [10]. Utilizing heterocyclic aromatic compounds like H2NC6H4NO2 with CH2O2, iron powder, and ammonium chloride, proceeded by the one pot reduction of NO2 followed by imidazole cyclisation [C] into bicyclic 2-H BnZ, was one method used by Hanan et al. (2010) [11]. Yang et al. developed a new method for the one-step synthesis of 2-substituted -N-H, -N-alkyl, and -N-aryl BnZ derivatives in the presence of aldehydes and sodium dithionite through the reduction of o-nitroanilines [D], succeeding in synthesizing BnZ [12]. Another approach for the synthesis of 2-substituted BnZ was published by Cui et al. in 2012 for producing 1,2-phenylenediamines and triacyloxyborane intermediates, which were produced via the interaction between carboxylic acids [E] and borane-THF, to yield 2-substituted BnZ [13]. N-methyl-1,2-phenylenediamine, sodium hydride, and carbonitriles [F] were used as the starting ingredients by Sluiter and Christoffers in 2009 [14]. Wray synthesized [1] 1H-indazoles from common arylamino oximes in 2010 in the presence of several bases, but the synthesis of BnZ was only improved by one base, i.e., triethylamine [G] [15]. Cuprous oxide, potassium carbonate, (CH3NH)2C2H4, and water [H] were used by Peng et al. to synthesize cost-effective and environmentally friendly BnZ [16]. Ortho-bromoaryl compounds are cyclized intramolecularly using cuprous oxide nanoparticles like a catalyst [I]. Saha et al. were able to synthesize substituted BnZ and 2-aminobenzimidazole. The catalyst might also be restored without altering its activity, in addition, with this method [17]. CuI/l-proline was applied by Diao et al. as a catalyst to condense 2-iodoacetanilides and aqueous ammonia for cyclization to create substituted 1H-BnZ by applying heat under acidic circumstances [18]. Kim et al. produced BnZ derivatives by condensation as well as the formation of C-N bonds. Sodium azide, RCHO [J] and 2-haloanilines are active as initial ingredients in one-pot synthesis [19]. Tao Zhang initially presented a model for the reaction of BnZ with [K] (10 mmol) and benzonitrile (NC-R) in a mixture of 20 mL PPA (polyphosphoric acid) and 10 mL H3PO4 under microwave irradiation (MC 275 W, 15 min), with a yield of 92% [20].
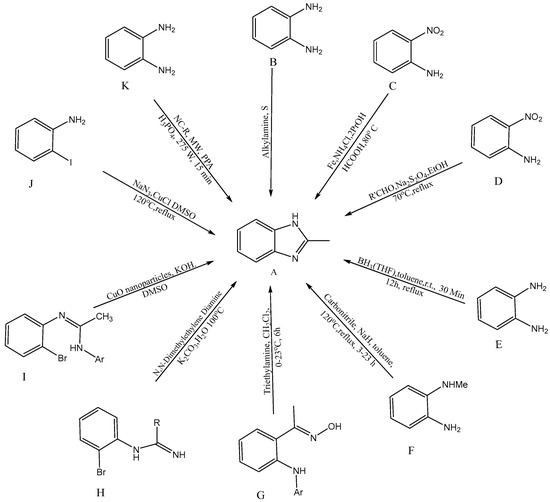
Figure 2.
Synthetic approaches for BnZ.
Several synthetic tactics have been successfully implemented, which are described in Figure 2 for the production of BnZ [A]. A combination of ortho-substituted aniline [B] and bifunctional o-esters produced a BnZ derivative in 2012, according to Bastug et al. [10]. Utilizing heterocyclic aromatic compounds like H2NC6H4NO2 with CH2O2, iron powder, and ammonium chloride, proceeded by the one pot reduction of NO2 followed by imidazole cyclisation [C] into bicyclic 2-H BnZ, was one method used by Hanan et al. (2010) [11]. Yang et al. developed a new method for the one-step synthesis of 2-substituted -N-H, -N-alkyl, and -N-aryl BnZ derivatives in the presence of aldehydes and sodium dithionite through the reduction of o-nitroanilines [D], succeeding in synthesizing BnZ [12]. Another approach for the synthesis of 2-substituted BnZ was published by Cui et al. in 2012 for producing 1,2-phenylenediamines and triacyloxyborane intermediates, which were produced via the interaction between carboxylic acids [E] and borane-THF, to yield 2-substituted BnZ [13]. N-methyl-1,2-phenylenediamine, sodium hydride, and carbonitriles [F] were used as the starting ingredients by Sluiter and Christoffers in 2009 [14]. Wray synthesized [1] 1H-indazoles from common arylamino oximes in 2010 in the presence of several bases, but the synthesis of BnZ was only improved by one base, i.e., triethylamine [G] [15]. Cuprous oxide, potassium carbonate, (CH3NH)2C2H4, and water [H] were used by Peng et al. to synthesize cost-effective and environmentally friendly BnZ [16]. Ortho-bromoaryl compounds are cyclized intramolecularly using cuprous oxide nanoparticles like a catalyst [I]. Saha et al. were able to synthesize substituted BnZ and 2-aminobenzimidazole. The catalyst might also be restored without altering its activity, in addition, with this method [17]. CuI/l-proline was applied by Diao et al. as a catalyst to condense 2-iodoacetanilides and aqueous ammonia for cyclization to create substituted 1H-BnZ by applying heat under acidic circumstances [18]. Kim et al. produced BnZ derivatives by condensation as well as the formation of C-N bonds. Sodium azide, RCHO [J] and 2-haloanilines are active as initial ingredients in one-pot synthesis [19]. Tao Zhang initially presented a model for the reaction of BnZ with [K] (10 mmol) and benzonitrile (NC-R) in a mixture of 20 mL PPA (polyphosphoric acid) and 10 mL H3PO4 under microwave irradiation (MC 275 W, 15 min), with a yield of 92% [20].
3. Metal-Catalyzed Derivatives of Benzimidazole
Metal catalysts have a few features, including the fact that they can be recovered. Catalysts have surface efficiency for catalyzing reactions and further increase overall reaction rate. Indium [III]triflate was used as a reusable catalyst by De et al., in 2006 which described the synthesis of MSBs (IX-a) with high yields. The synthesis of 2-substituted BnZ without solvent occurs by the fusion of o-phenylenediamine (VII) with aldehydes [where R = benzaldehyde (VIII)] utilizing a catalytic quantity of In(OTf)3. VII and VIII were combined in a 1:1.1 molar ratio and heated up to atmospheric temperature for 30 minutes to perform the reactions. They also used various catalysts, such as Copper [II]triflate, Lutetium[III]trifluoromethanesulfonate, Indium[III]trifluoromethanesulfonate and La(OTf)3. Among these, the most recently used catalysts gave the highest output. The process is depicted in Scheme 3 [21].
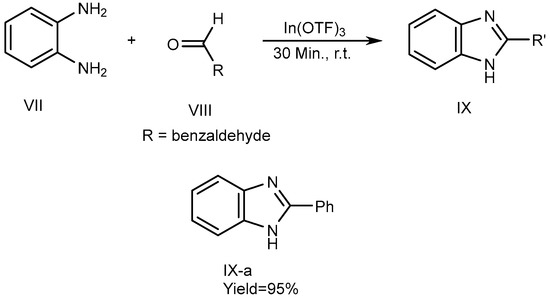
Scheme 3.
Metal-catalyzed reduction of BnZ.
Fu et al., described the method for the synthesis of 2-substituted 1H-BnZ (XIII a-b) in dimethyl sulfoxide using cesium carbonate as a base and 10 mol % Copper[I] bromide as a catalyst. The optimized yield of 2-substituted 1H-BnZ (XIII-c) using this approach was obtained by coupling, hydrolysis, and the intra-molecular cyclization of o-haloacetanilide (X) derivatives with amidines (XI). o-iodoacetanilide is required for the N-arylation of amidines at 60 °C to yield XIII-a, whereas o-bromoacetanilide required a high temperature of 90 °C to yield XIII-b and XIII-c., which is displayed in Scheme 4 [22].
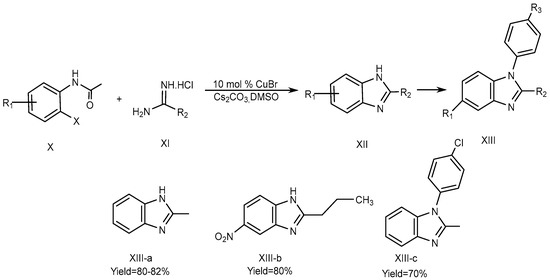
Scheme 4.
CuBr-catalyzed reactions.
In 2013, Nguyen et al. described a process using iron-sulfur as a catalyst to synthesize the BnZ derivative (XVI). For the synthesis of XVI, 4-picoline (XV) and substituted o-nitroanilines (XIV) were used as reactants, and the reaction was carried out at 150 °C under solvent-free conditions using an equimolecular amount of an Fe/S catalyst. Fe/S played a crucial role for promoting the cyclization process. Pyridine and quinoline derivatives possess a methyl group at the 2- or 4-position (XV) and are coupled with XIV to form derivative MSBs (XVI a-c), where all other suitable substrates in the reaction along with o-nitroaniline synthesize the corresponding benzimidazole products XVI-a-b with yields of 83-91%, if o-Nitroanilines have electron-donating and withdrawing substituents on its benzene ring. There are methyl groups of picolines in elemental Chloride (XVI-c), as depicted in Scheme 5 [23].
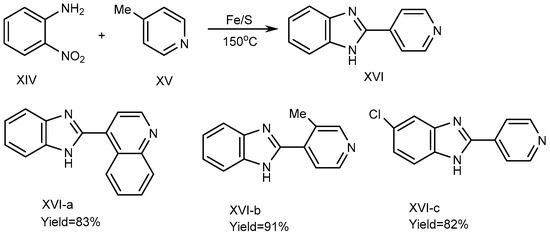
Scheme 5.
Fe/S-catalyzed reaction of BnZ.
Sun et al. (2017) described a procedure for the feasible condensation of phenylhydrosilicon, o-phenylenediamine (XVII) and dimethylformamide (XVIII). According to this scheme, hydrosilicon is utilized as a reaction catalyst, activating the carbonyl group of di-methylformamide to make an Si–O bond at 120 °C for 12 hours. An amine-group in o-phenylenediamine was coupled with activated DMF-hydrosilicon mixture, and further undergoes cyclization, succeeding in the excellent synthesis of BnZ (XIX), as shown in Scheme 6 [24].
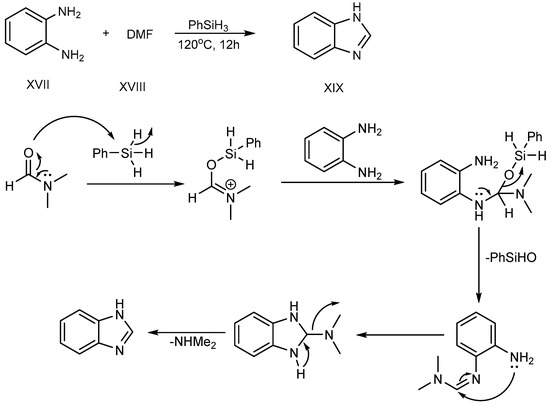
Scheme 6.
Cyclization of o-phenylenediamines with DMF.
A dehydrogenation reaction, using cobalt-pincer complexes (XXII) as a catalyst, was carried out at 150 °C for 24 hours by Milstein et al. (2017). Primary alcohol (XXI) and o-phenylenediamine (XX) were combined to synthesize the 2-MSB derivative (XXIII) (Scheme 7) [25].
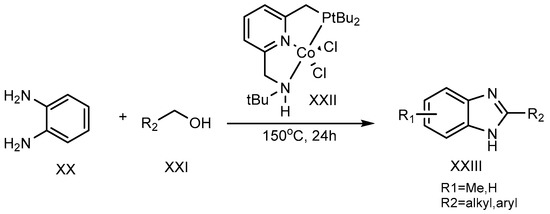
Scheme 7.
Co-complex catalyst-mediated reaction.
4. Metal-Free Catalyzed Derivatives of BnZ
A one-pot synthesis with an exceptional yield was reported by Nguyen et al. in 2012 under dry and metal-free conditions to obtain XXX–XXXIV. In this method, the reaction was completed with trialkyl amine (triethylamine) (XXVIII), benzene-1,2-diamine (XXIX), and derivatives of phenylmethanamines (XXIV–XXVII). Sulphur atoms helped to proceed the reaction for generating C–N bonds, and the researchers succeeded in its cyclization (Scheme 8) [26].
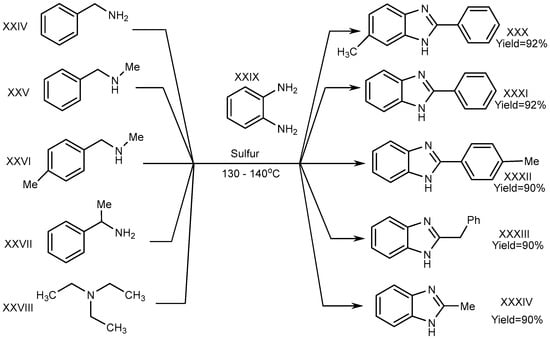
Scheme 8.
Cross–coupling reaction of o–phenylenediamines with aliphatic amines (S).
Shanmugam et al. described the efficient metal-free synthesis of aryl-substituted BnZ (XXXVII-a) through the use of acetic acid as a catalyst under microwave heating conditions. This synthesis involves the reaction of o-phenylenediamine (XXXVI) with a substituted aromatic aldehyde (XXXV). Shown in Scheme 9, the reaction is entirely free from metals, as well as using environmentally friendly green solvent [27].
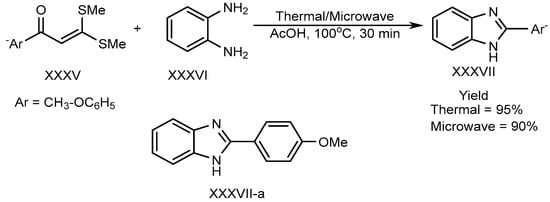
Scheme 9.
Thermal/microwave-assisted BnZ synthesis.
Liu et al. described a metal–free process for the preparation of N-substituted BnZ (XL) under microwave irradiation. The improved yield is produced when fluoro-aryl formamidines (XXXVIII) with primary amines (XXXIX) undergo an SN–Ar reaction [28]. (Scheme 10).
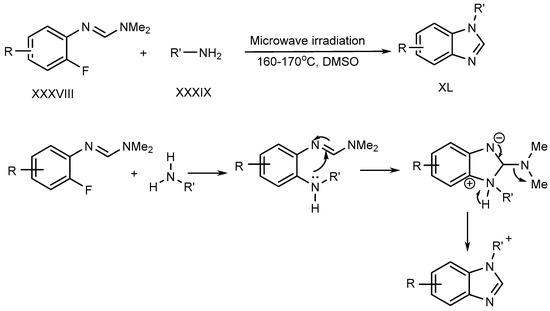
Scheme 10.
Synthesis of BnZ under metal-free conditions with amines.
Mostafavi et al. published a method in 2018 for BnZ biosynthesis. Hexamethyldisilazane regulated the reaction at 120 °C, and transformed o-phenylenediamine (XLI) and N,N-Dimethylformamide into BnZ. There is no need for any acid, transition metal, or fluid for proceeding the reaction to achieve good yields XLIII (Scheme 11) [29].
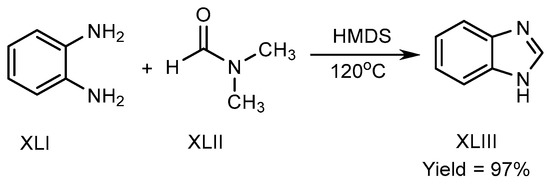
Scheme 11.
Metal-free BnZ biosynthesis.
In the presence of 5ml of dilute hydrochloric acid solution, o-phenylenediamine (XLV) and Ibuprofen (XLIV) are used to produce a BnZ variant XLVI. Further, the mixture was heated in a water bath at 100 °C for two hours with the slow addition of 10% sodium hydroxide, as shown in Scheme 12. The researchers discovered that BnZs have very strong antimicrobial properties [30].
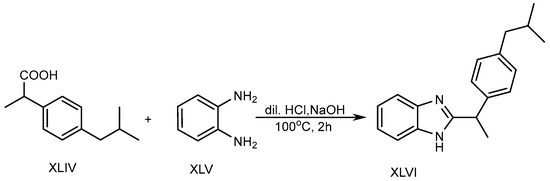
Scheme 12.
Base-catalyzed synthesis of BnZ.
5. Green Synthesis of Benzimidazole
Most chemical organizations or industries, including pharma-enterprises, are now coping with mild ecological issues, like excessive solvent, chemicals waste, and the use of catalysts in the production of BnZ. Green synthesis mediates solvent-free production by using environmentally friendly catalysts like C3H6O3 or B(OH)3 under microwave condition, which is an effective way to get rid of these issues.
XLVII and aldehydes (XLVIII) undergo cyclic condensation in an equal amount of a bioabsorbable solution like ethanol and lactic acid for 4-6 hours at normal temperature. Song et al. (2016) disclosed the one-pot synthesis of disubstituted-benzimidazole (DSBs) (XLIX-a) derivatives. The optimized result is obtained in lactic acid (Scheme 13) [31].
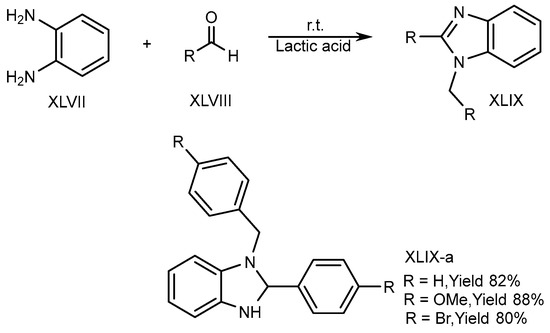
Scheme 13.
Synthesis of XLIX-a in green solvent.
Khunt et al. (2014) described a strategy for the preparation of a BnZ candidate (LII) via the interaction of 1,2-diaminobenzene (L) with aldehydes (LI) by use of an ecofriendly solvent such as polyethylene glycol (PEG)-400. Above 80–85 °C, the process produces the highest yield of PEG-400. The targeted molecule was produced through a new unique synthetic pathway, in which glycerol/water is used (Scheme 14) [32].
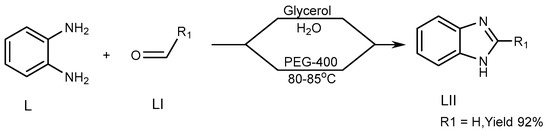
Scheme 14.
Synthesis of BnZ in solution instead of PEG.
Kathirvelan et al. discovered the one-pot synthesis of a monosubstituted benzimidazole (MSB) (LV a-b) scaffold in 2013 (Scheme 15) by the coupling of benzene-1,2-diamine (LIII) and aldehydes (LIV) using an ecofriendly feasible catalyst, i.e., ammonium chloride in ethanol at 80–90 °C [33].
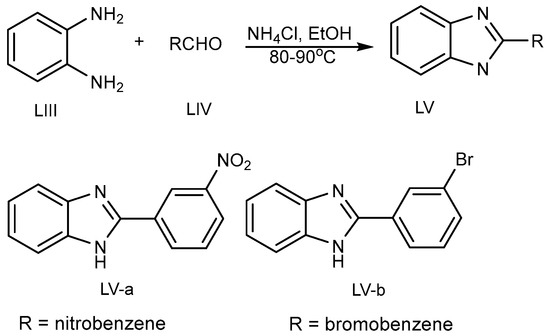
Scheme 15.
BnZ synthesis using ammonium chloride.
Kidwai et al. (2010) described a unique approach using ceric ammonium nitrate (CAN) catalyst for the synthesis of BnZ scaffold (LVIII) in a polyethylene glycol at 50 °C for 2 h. Benzene-1,2-diamine (LVI) combined with aldehyde (LVII) in CAN catalyst tends to lead to exceptional PEG yields (LVIII-a) (Scheme 16) [34].
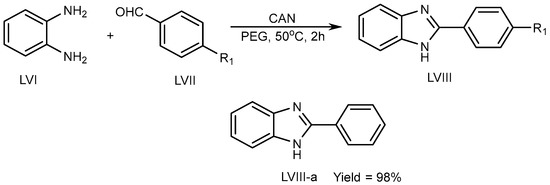
Scheme 16.
CAN-catalyzed polyethylene glycol–MSB reaction.
Azarifar et al. (2010) suggested an eco-friendly microwave-assisted reflux reaction of o-phenylenediamine (LIX) with an RCHO derivative (LX) at 80 °C for the synthesis of MSBs and DSBs (LXI, LXII) (Scheme 17) [35].
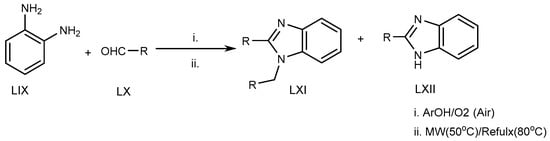
Scheme 17.
Microwave-irradiation-mediated BnZ synthesis.
6. Multifaceted Efficacy of Benzimidazole
The significance of chiral BnZs has led to a relatively new sector of drug discovery for various biological applications; therefore, its synthesis has received special attention [36]. Additionally, chiral BnZs have been utilized as organocatalysts for the enantioselective chlorination, Diels–Alder reactions, asymmetric Michael additions, and asymmetric aldol-type reactions. However, Rh- and Pd-based BnZ complexes are synthesized using a Mizoroki–Heck reaction [37]. The reduction processes is performed using Suzuki–Miyaura coupling reactions [38]. Earlier surveys demonstrate that the BnZ scaffold is crucial for its therapeutic usage, in addition to as optical sensors for use in nanomaterial properties [39]. These properties can apparently benefit the numerous sensing devices, as well as its cost effectiveness and operational flexibility; therefore, it seems to have specific usage in medicine, geology, and industrial innovation (Figure 3). Another use of BnZ and its derivatives is in supra-molecular assemblies with intriguing features for various uses, such as thermostable polymers, adsorbent materials, nano-containers, and liquid crystals for electronic conduction. For the last few years, it has been seen in a lot of research in the area of polybenzimidazole (PBI) derivatives, such as for solid electrolytes of fuel cells [40,41,42,43,44], fiber [45], thin coatings [46], advanced protective coverings [47], or to remove palladium, Th, and U from a watery medium [48]. PBI-based technologies exist in Charlotte, North Carolina, used in the pioneering industrial applications for firefighter safety in Europe, United States, and Middle East, with 32 years of expertise. PBI materials are famous for their tested protection against heat and flames, and shield the firemen in a various fire services [49].
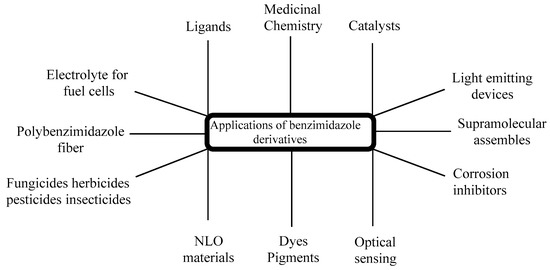
Figure 3.
Applications of BnZ derivatives.
Another use of poly-Benzimidazole, as a combined matrix, shows extraordinarily strong evaporation property due to its high porosity and selectivity [50]. Additionally, this is the most recommended material for another photonic technology in organic frames. This has led to the study of a number of small molecules of BnZ derivatives like 2-mercaptobenzimidazole, 2-phenylbenzimidazole, and 2-hydroxybenzimidazole, with excellent non-linear optical (NLO) properties [51] and highly intricate structures [52]. Benlate and Carbendazim both used BnZ as fungicides with minimal cytotoxicity at lower concentrations and no risk for carcinogenic or genetic effects [53]. There is documentation regarding the utilization of BnZ as fertilizers and insecticides, which also specifies their uses [54]. BnZ is an organic molecule, recently highlighted as a corrosion inhibitor for copper, iron, or zinc in acidic circumstances [55,56] because of its properties of BnZ-bonded electron donating and accepting substituents. Scientists have demonstrated that BnZ is a flexible and necessary chromophore for natural dyes, with excellent photophysical, electrochemical, and photovoltaic characteristics [57]. Benzimidazol-2-one is highly considerable because of its durability and light-resistance properties. It has been used for the last three decades to produce a wide variety of subtleties in water for color painting and electro-photographic toner [58]. BnZ has proven to be a vital molecule in organic light-emitting devices (OLEDs), with excellent phosphorescence, thermal features, and morphological stabilities [59]. For innovative research with a new mode of action, heterocyclic compounds are frequently used. Aryl aziridines is a heterocyclic compound [60] and BnZ derivatives play a significant role because of the vast range of biological actions they exhibit. According to a literature survey, BnZ and its derivatives have physiological and pharmacological activities. These are able to treat a broad range of disorders, particularly epilepsy, diabetes, and pregnancy. Such derivatives exhibit a variety of bioactivities like antiallergic [61], antihistamine [62], anti-ulcerative, antiproliferative [63], antioxidant [64,65], antifungal, antibacterial, antitubercular [66], antihypertensive [67], anti-inflammatory, analgesic, antiamoebic [68], antitumor [69,70,71], antimalarial [72], and anti-kinase activity [73]. Several BnZ derivatives are also being investigated as cholinesterase inhibitors and anti-parasitic drugs [74,75,76,77].
7. Benzimidazole-Derivative-Based Biological Activities
Numerous derivatives have been explored in individual investigations, for various therapeutic treatments. These include the antiemetic, KB-R-6933; the anti-cancer, Bendastumide; the antiviral, l Hoechst 33342; the antihistamine, Clemizole; the anti-ulcerative, Omeprazole; and the anti-hypertensive, Telmisartan. Additionally, Thiabendazol, an anti-fungal agent, has been employed to examine its effects (Figure 4) [78].
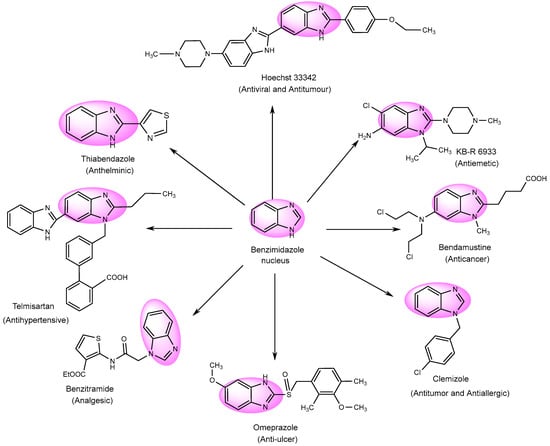
Figure 4.
Multifunctional examples of BnZ derivatives.
8. Anti-Cancer Activity
Cancer is responsible for the second-largest number of disease-related deaths in the world. Anticancer drugs must harm cancer-affected cells but not ordinary cells, and act effectively against cancer. For the last few years, anti-cancer medicines have shown relatively significant damage to both normal and tumor cells. Cancer patients are discontinuing chemotherapy because of toxicities and the side effects of anticancer medicines. It should be observed that the investigation of novel anticancer medicines with a high efficacy and minimal side effects are both an urgent necessity and a challenging problem.
In Figure 5, Zhe Wang et al. evaluated the anti-cancer activities of several chrysin-based BnZ derivatives. Entity 1 (7-(4-(2-methyl-1H-benzo[d]imidazol-1-yl)butoxy)-4-methylene-2-phenyl-4H-chromen-5-ol) was synthesized and exhibited the most anti-proliferative effects on MCF cell lines, having an IC50 of 25.72 ± 3.95 μM. Flow cytometry results showed that drug 1 accelerates MCF cell-line apoptosis in a daily dosage manner. When the anti-cancer activity of compound 1 was examined in tumor-suffering mice, the resultant tumor growth was shown to be suppressed [79]. Goreti Ribeiro Morais et al. [80] freshly developed BnZ variants with Na3AlF6 and R-OH substituents, which has been further tested for anti-cancer activity. “Drug 2” (4-(1H-benzo[d]imidazol-2-yl)-N-(2-fluoroethyl)-N-methylaniline) was demonstrated to be the most significant anticancer drug. The BnZ nucleus was substituted by a 2-fluoroethyl string at the aniline nitrogen, which demonstrated acceptable cytotoxicity against U87 glioblastoma cell lines (IC50 = 45.2 ± 13.0 μM) compared to DOX (IC50 = 16.6 ± 2.5 μM), i.e., a generic anticancer medicine [81].
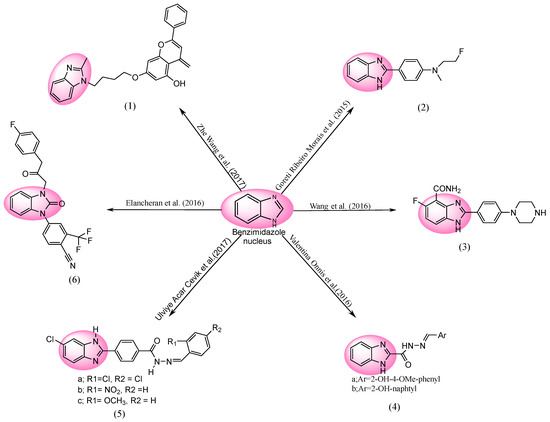
Figure 5.
BnZ-based anti-cancer drugs [79,80,81,82,83,84,85].
Unique 5-fluoro-benzimidazole-4-carboxamide analogues were synthesized by Wang et al. One highly effective anti-cancerous drug, 3 (5-fluoro-2-(4-(piperazin-1-yl)phenyl)-1H-benzo[d]imidazole-4-carboxamide), was identified to have an IC50 of 7.4 μM, with good cell inhibition against HCT116 cell lines [82]. The antiproliferative effect of BnZ derivatives was investigated by Valentina Onnis et al. All investigated cell lines were precisely suppressed in terms of tumor growth by the hydrazones 4a ((E)-N′-(2-hydroxy-4-methoxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide) and 4b ((E)-N1-((2-Hydroxynaphthalen-1-yl)methylene)-1H-benzo[d]imidazole-2-carbohydrazide). A growth inhibitor was tested against cell lines of human T-lymphoblastic leukemia for entity 4a and 4b, with an IC50 of 0.98 ± 0.02 and 1.0 ± 0.01 μM, and human pancreatic carcinoma, with an IC50 of 6.3 ± 3.2 and 7.9 ± 0.3 (μM), and murine leukemia, with an IC50 of 1.6 ± 0.9 and 2.9 ± 1.3 μM, respectively. They noticed that mixing BnZ with hydrazone enhanced its pharmacological activities, producing a new featured chemical compound that has strong antiproliferative properties [83]. Recently, the anti-cancer effect of BnZ derivatives was investigated by Ulviye Acar Cevik et al. Besides these examined chemicals, they also noticed that compounds 5a (4-(6-chloro-1H-benzo[d]imidazol-2-yl)-N′-(2,4-dichlorobenzylidene)benzohydrazide) and 5b (4-(6-chloro-1H-benzo[d]imidazol-2-yl)-N′-(4-nitrobenzylidene)benzohydrazide) had noticeable cytotoxicity. Compounds 5a and 5b, when applied to human-lung-cell-line adenocarcinoma, have an IC50 of 0.0316 μM. Compared with the classical anticancer medicine cisplatin (IC50 0.045 and 0.052 μM), 5c (4-(6-chloro-1H-benzo[d]imidazol-2-yl)-N′-(2-methoxybenzylidene)benzohydrazide) showed significant cytotoxicity, with an IC50 of around 0.06 μM [84]. Elancheran et al. synthesized and examined a number of oxo-bBnZ derivatives for their ability to inhibit androgen receptor functions. They had effective actions against PC-3, with an IC50 of 12.19 ± 0.25 μM, and on LNCaP, with an IC50 of 11.2 ± 0.13 μM. Also, cytotoxicity effects were actively expressed by compound 6 (4-(3-(3-(4-fluorophenyl)-2-oxopropyl)-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-2-(trifluoromethyl)benzonitrile) [85].
9. Anti-Inflammatory and Analgesic Activity
Inflammation is an important characteristic of the body’s immunological system. The common early symptoms and indications are heat, redness, pain, swelling, and the lack of ability of the immune system. Since non-steroidal anti-inflammatory drugs (NSAIDs) naturally have the capacity to block cyclooxygenases (COXs), they are frequently employed to reduce inflammation. The synthesis of prostaglandins from arachidonic acid is mediated by cyclooxygenase. Medical scientists have investigated a number of analgesic and anti-inflammatory drugs using BnZ bases [86,87,88], as shown in Figure 6.
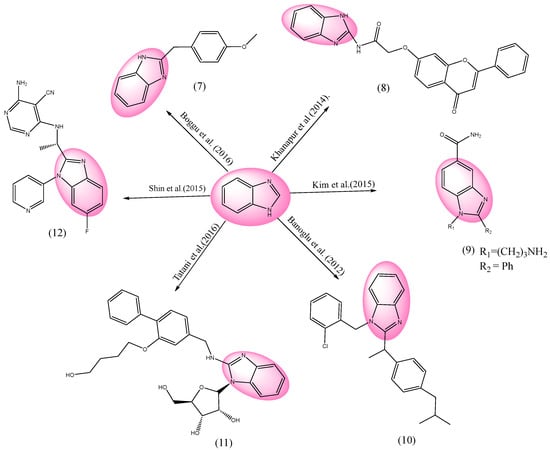
Figure 6.
BnZ-based anti-inflammatory drugs [89,90,91,92,93,94].
One of the BnZ-derivative compounds 7 (2-(4-methoxybenzyl)-1H-benzo[d]imidazole) was investigated by Boggu et al., and had the greatest nuclear factor (κB) at its optimized concentration, with an IC50 of 1.7 μM. An additional synthesis for the possible suppression of activity was examined in unsubstituted BnZ, which had only one carbon spacer and a methoxy group towards the phenyl ring. By refluxing 2-aminobenzenethiol using xylene in the presences of 4-methoxyphenylacetic acid, white solid benzothiazole with a yield of 48% and a melting point 60–62 °C was produced [89]. Khanapur et al. synthesized BnZ derivatives, and examined their effect. Remarkable anti-inflammatory effect was shown by compound 8 (N-(1H-benzo[d]imidazol-2-yl)-2-((4-oxo-2-phenyl-4H-chromen-7-yl)oxy)acetamide). Several heterocyclic moieties were bonded with an acetamide that enhanced their activities, with substituted ester at the 7th position. It was revealed that bicyclic moieties are more effective with substituents at the five and six position throughout heterocyclic molecules. Cyclooxygenase (COX) inhibition at 10 μM for COX 2 was 85.91 ± 0.23%, with an IC50 of 3.11 ± 0.41 μM, and for COX 1 was 25.91 ± 0.77%, with an IC50 of 57.89 ± 0.19 μM. Using column chromatography (petroleum ether/EtOAc), the residue was purified as a colorless solid with a yield of 87% [90]. Only mild inhibitory action was demonstrated by the BnZ derivative 9 (2-(2-aminoethyl)-1-(3-aminopropyl)-1H-benzo[d]imidazole-5-carboxamide), which was synthesized by Kim et al., who chose a 3-aminopropyl group as the substituent at the N1 antagonist to Janus kinase 1 (JAK1), with an IC50 of 6.1 and 11.3 μM, and Janus kinase 3 (JAK3), with an IC50 of 1.5 and 1.8 μM [91]. Entity 10 (1-(2-chlorobenzyl)-2-(1-(4-isobutylphenyl)ethyl)-1H-benzo[d]imidazole) is a collection of BnZ derivatives synthesized by Banoglu et al., with an isobutyl-phenylethyl profile of ibuprofen, which noticeably reduced cellular 5-Lipoxygenase (5-LO), with an IC50 value of 0.31 μM, but was considerably less effective on free-cell 5-LO, which was hardly inhibited by 23% at 10 μM [92]. However, its inhibitory effects towards human concentrative nucleoside transporter 2 (hCNT2) and rat concentrative nucleoside transporter 2 (rCNT2) was greatly improved after substituting adenine, upon which the resultant BnZ product 11 ((2S,3R,4S,5R)-2-(2-(((2-(4-hydroxybutoxy)-[1,1′-biphenyl]-4-yl)methyl)amino)-1H-benzo[d]imidazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol) was formed. So, the most effective hCNT2 inhibitor examined was the semi-synthetic derivative of BnZ 11, with an IC50 value of 0.062 μM. This was also effective as an rCNT2 inhibitor, with an IC50 of 1.5 μM. Similarly, compound 11 had a substantially greater solubility of 0.120 mg/mL than others compound in Japanese Pharmacopoeia second fluid (JP2) at 37 °C. Compound 11, in combination with purine, greatly reduced the exaggerated uric acid in plasma at a lower dose of 1.0 mg/kg, which likely seemed to be an in vitro improvement. This compound, at a greater dose (10 mg/kg), did not significantly improve the suppressive effect [93]. Shin et al. assessed the PI3Kd-specific inhibition activity of BnZ derivatives. Substance 12((S)-4-amino-6-((1-(6-fluoro-1-(pyridin-3-yl)-1H-benzo[d]imidazol-2-yl)ethyl)amino)pyrimidine-5-carbonitrile) showed strong PI3Kd activity. At day 10, plasma was collected after examining exposures of different doses of compound 12. Unbound substances were determined and shown via LC-MS/MS with respect to human blood. Unbound phospho-AKT (pAKT) had an IC50 of 1.0 μM, phosphoinositide 3-kinase delta (PI3Kδ) had an IC50 of 3.1 μM in an in vitro study in mice, and PI3Kβ showed an IC50 of 650 μM in an in vitro study in humans. A pyridine ring was substituted by the phenyl moiety, which produced high oral bioavailability [94].
10. Anti-Microbial Activity
Since they are frequently exploited against microbial infections, certain bacteria have developed resistance to conventional antibiotics. A variety of antibacterial compounds are synthesized to treat and cure microbial illnesses [95]. The flexible and most primary compound is known as a BnZ, which has a wide spectrum of biological actions, most especially for anti-bacterial [96] and antifungal [97,98] properties. Figure 7 depicts BnZ-containing anti-microbial compounds.
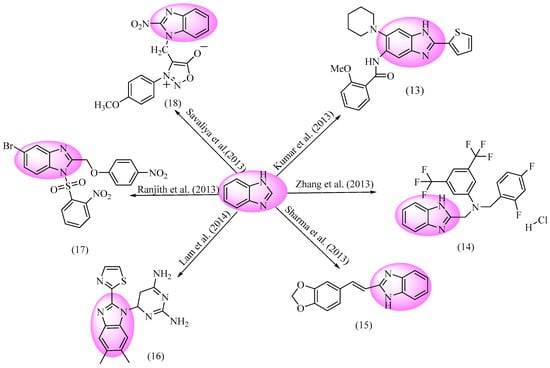
Figure 7.
BnZ-based anti-Microbial [99,100,101,102,103,104].
Several BnZ-based variants were developed by Kumar et al., with potential efficiency in antibacterial properties. Entity 13 (2-methoxy-N-(6-(piperidin-1-yl)-2-(thiophen-2-yl)-1H-benzo[d]imidazol-5-yl)benzamide) was evaluated and shown to provide excellent anti-microbial activity. Select compounds have also been assessed for their ex vivo high potency against F. tularensis Live vaccine strain (LVS). An ex vivo study of the leading drug 13 against the F. tularensis LVS of RAW macrophages was performed. In addition to 13, the dose-dependent leading compound significantly decreased the extent of bacterial action at concentrations of 10 and 50 g/mL. Its MIC90 (μg/mL) against F. tularensis LVS was 1.6 and its cytotoxicity (μg/mL) on Vero cells was >200 µg/mL with compound 13. Entity 13 is a white solid with a melting point 165–167 °C [99]. Zhang et al. produced variants of BnZ and examined its capability to fight against bacteria. Further, the most effective component was discovered to be 14 (N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(2,4-difluorobenzyl)-3,5-bis(trifluoromethyl)aniline). This had the strongest antibacterial effect against the relevant microorganisms at a dose range from 8–32 mg/mL. Particularly, it had an MIC of 8 mg/mL against the S. aureus, B. subtilis, and M. luteus species, as well as 32 mg/mL against E. coli. Component 14 has a yellow solid color with a melting point of 137–138 °C [100]. Sharma et al. developed a number of BnZ and benzothiazole equivalents and tested their effectiveness against microorganisms. Resultantly, compound 15 ((E)-2-(2-(benzo[d][1,3]dioxol-5-yl)vinyl)-1H-benzo[d]imidazole) demonstrated excellent efficacy against. P. aeruginosa, having corresponding MIC values of 0.01 mM and 3.78 mM. In order to produce novel Schiff bases with heterogeneous complexes using microwaves, methylenedioxy cinnamaldehyde was used as a raw material. Structure activity relationship (SAR) investigations revealed that it increased a broad range of activities compared to the species under study [101]. Lam et al. synthesized several 7-(benzimidazol-1-yl)-2,4-diamino-quinazoline derivatives, and their capability for antibacterial action was assessed. Further, a new investigation of drug 16 (6-(5,6-dimethyl-2-(thiazol-2-yl)-1H-benzo[d]imidazol-1-yl)-5,6-dihydropyrimidine-2,4-diamine) showed it to be the most effective against S. aureus, with an MIC of 0.015 mg/mL. A discovered BnZ variant with a thiazol-2-yl group at the 2-position proved to be an incredibly efficient and specific drug. Great potency and selectivity were both exhibited by a thiazol-2-yl group in the BnZ against S. aureus dihydrofolate reductase DHFR Ki = 0.002 μM. Compound 16 is white solid in its physical state [102]. Compound 17 (5-bromo-2-((4-nitrophenoxy)methyl)-1-((2-nitrophenyl)sulfonyl)-1H-benzo[d]imidazole) had quite good efficacy against the tested fungi and bacteria. This included notable efficacy against the M. tuberculosis H37Rv strain. Additionally, substance 17 was found to be effective against the M. tuberculosis H37Rv strain at around 0.625 μg/mL, but was comparably less efficient against M. smegmatis, requiring a concentration of 1.25 μg/mL (ATCC 19420). It is notable that the drug had either an improved or consistent action against M. fortuitum with reference to the drug isoniazid (ATCC 19542) at 6.25 μg/mL. It has also shown promising efficacy against the MDR-TB strain. Inhibitory action of Drug 17 against S. aureus and K. pneumonia was demonstrated at an MIC ratio of 4 μg/mL. However, its antibacterial potential has already been shown, so these are only the preliminary screening data. More research has been performed to determine the action mechanism [103]. Compound 18 (3-(4-methoxyphenyl)-4-((2-nitro-1H-benzo[d]imidazol-1-yl)methyl)-1,2,3-oxadiazol-3-ium-5-olate) is a different class of BnZ derivatives and was synthesized by Savaliya et al. They discovered its exceptional effectiveness of inhibitory action against Gram-positive bacteria S. aureus, with an MIC of 40 μg/mL and inhibition zone (IZ) of 21 μg/mL. Furthermore, its inhibitory action against B. subtilis, with an MIC value of 300 μg/mL and an IZ of 12 μg/mL, was noted. It also demonstrated inhibitory action against Gram-negative bacteria E. coli, with an MIC value 200 μg/mL and IZ of 14 μg/mL, and against P. aeruginosa, with an MIC value 500 μg/mL and an IZ of 10 μg/mL. According to the SAR study for all compounds, sydnone with 4-nitro benzothiazole had good efficacy against all bacterial and fungal strains [104].
11. Anti-Tubercular Activity
The 2nd second-leading reason for mortality globally is tuberculosis (TB), a bacterial infection, and illness due to mycobacterium tuberculosis (M. tuberculosis) [105]. The present scenario to treat TB is DOTS (directly observed therapy short-course), which is the prescription of a mixture of three to four medicines for between six and twelve months [106,107]. These contain isoniazid, and ethambutol, etc. Figure 8 depicts a variety of anti-tubercular drugs with BnZ nuclei. A number of compounds of phenoxy alkyl-BnZ were discovered by Chandrasekera et al. A minimal inhibitory concentration (MIC)99 is needed to totally suppress M. tuberculosis formations in aqueous culture. Entity 19 (4-bromo-N-(3-(2-ethyl-1H-benzo[d]imidazol-1-yl)propyl)-3-methylaniline) was discovered to be the most effective compound, having an MIC of 52 μM [108]. Yoon et al. discovered a number of BnZ-based derivatives, including compounds examined in in vitro assay, that were modified from the microdilution of AlamarBlue (AB) complex 20 (ethyl-1-(3-(2-oxopyrrolidin-1-yl)propyl)-2-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole-5-carboxylate) (m.p. is 99–100 °C). It was discovered to be a highly effective anti-mycobacterial agent against Mycobacterium tuberculosis H37Rv, with an IC50 value of 11.52 μM. It was discovered that an electron-withdrawing group at the 4th position of the phenyl ring is significant for the activity of this compound [109].
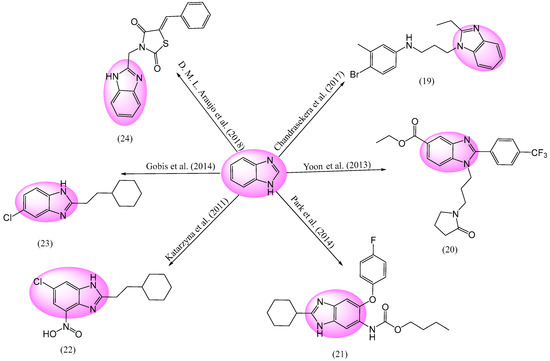
Figure 8.
BnZ-based anti-tubercular activity [108,109,110,111,112,113,114,115,116,117].
Park et al. developed a method and synthesized a collection of 6, 5, or 2-tri-substituted BnZ variants, which later were evaluated for their effectiveness at treating TB. The efficacy of BnZ derivative 21 (butyl(2-cyclohexyl-5-(4-fluorophenoxy)-1H-benzo[d]imidazol-6-yl)carbamate) at preventing Mycobacterium tuberculosis filamentous temperature-sensitive mutant Z (Mtb-FtsZ) polymerization was tested [110]. The investigation of the chemically affected suppression of FtsZ polymerization was conducted using a light scattering test [111,112]. Entity 21 was discovered as a highly effective drug among all the produced compounds. Mtb Hinshaw 37, and Rockefeller university variant (H37Rv) strain were targeted by a variety of drugs, demonstrating effective MIC values in the range of 0.63–12.5 μg/mL [113]. Based on the procedure outlined by Katarzyna Gobis et al., complex 22 ((6-chloro-2-(2-cyclohexylethyl)-1H-benzo[d]imidazol-4-yl)(oxo)-l4-azanol) was produced via the heating of 3-cyclohexylpropanoic acid with 5-chloro-3-nitrobenzene-1,2-diamine, resulting in a yield of light yellow crystals. Compound 22 showed considerable anti-tuberculostatic action against M. tuberculosis, ranging between 1.5 and 12.5 mg/mL and having an m.p. of 190–192 °C [114]. In the study of Gobis et al, compound 23 (5-chloro-2-(2-cyclohexylethyl)-1H-benzo[d]imidazole) was produced by the heating of 3-cyclo-hexylpropanoic acid with the required di-amines. Again, component 23 was identified as the most effective antituberculosis molecule against Mycobacterium tuberculosis (M. TB) and Mycobacterium bovis (M. bovis), with an assessed MIC range from 0.75 to 1.5 mg/mL. Molecules having cyclohexyl ethyl substituents at the C-2 position are highly active compared to compounds with a longer chain [115]. Mycobacterium tuberculosis H37Rv strain was used to test the in vitro antitubercular activity of drug 24 ((Z)-3-((1H-benzo[d]imidazol-2-yl)methyl)-5-benzylidenethiazolidine-2,4-dione), which displayed a potent Mtb inhibitory effect, with an MIC of 6.25 g/mL. Compound 24 was believed to be the most potent analogue against mycobacterium, with equal efficiency against TB as the common antibacterial combination of streptomycin 2-(Chloromethyl)-1H-benzo[d]imidazole and thiazolidine-2,4-dione. This dual combination tends to give a greenish yellow color after 71 h. of reaction in acetonitrile, with a yield of 82% and an m.p. of 210 °C. TLC was used to observe the response of dry liquid, followed by the dilution of the residue with water, drying, and treatment with diethyl ether, to obtain the required components [116,117].
12. Anti-Protozoal Activity
Parasitic diseases are a significant threat to global health. Diseases that cause diarrhea, which are classified as one of the top five killers globally, include several gastrointestinal infections. Each of the global population suffers by one or more unnoticed tropical illnesses yearly, with around 1.6 million persons per day [118,119,120]. Figure 9 depicts some anti-protozoal medicines with BnZ-based moieties. The compound 2-anilinoBnZ was synthesized by Karaaslan et al, and the anti-protozoal potency of this drug was assessed. Compound 25 (2-((3,4-dichlorophenyl)amino)-1H-benzo[d]imidazole-5-carboximidamide) was examined in vitro for its anti-parasitic effect against T. b. rhodesiense, i.e., the bloodstream forms of trypomastigote and P. falciparum at erythrocytic stages [121]. Compound 25 displayed an IC50 value of 0.138 ± 0.017 μM against plasmodium falciparum, indicating a strong anti-malarial activity. In contrast, it also exhibited an IC50 value of 21.80 ± 8.01 μM against Trypanosoma brucei rhodesiense, indicating moderate activity against this parasite. Additionally, a cytotoxicity of compound 25, with an IC50 value of 39.06 ± 0.47 μM, was demonstrated against L6 cells (rat skeletal muscle cells), which indicates that this compound exhibited some toxicity toward these cells at higher concentrations. Drug 26 (N-(3-(4-methyl-1H-benzo[d]imidazol-2-yl)phenyl)thiophene-2-carboxamide), i.e., 2-arylBnZ, was synthesized by Keurulainen et al. It was effective against Tamm–Horsfall protein 1 (THP-1) cells infected with Leishmania donovani (L. donovani), displaying a 46% parasite reduction at 5 μM. The compounds 4- and 5-methylbenzimidazole variants displayed intriguing SARs. They showed comparable IC50 values and suppressed axenic amastigotes at all doses, but their effects on THP-1 cells were quite different. While 4-methyl derivative showed considerable action, the 5-methyl variant essentially demonstrated little effect [122].
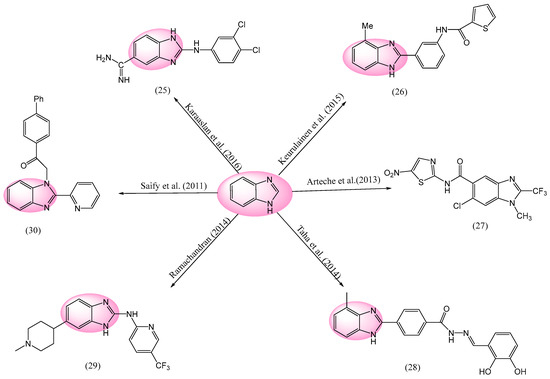
Figure 9.
BnZ-based anti-protozoal activity [121,122,123,124,125,126].
Numerous BnZ-based compounds were discovered by Arteche et al., which were further tested for their antiprotozoal effects. Compound 27 (6-chloro-1-methyl-N-(5-nitrothiazol-2-yl)-2-(trifluoromethyl)-1H-benzo[d]imidazole-5-carboxamide) induced good antiprotozoal action against T. vaginalis, E. histolytica, and G. intestinalis, exhibiting IC50 values of 0.041 ± 0.005, 0.006 ± 0.002, and 0.021± 0.005 μM, respectively. This activity was increased by the addition of a methyl or a trifluoromethyl group at the 2 position of the BnZ nucleus [123]. The highly effective anti-leishmanial compound 28 ((E)-N′-(2,3-dihydroxybenzylidene)-4-(4-methyl-1H-benzo[d]imidazol-2-yl)benzohydrazide) was synthesized by Taha et al., which had an IC50 value of 37.80 ± 2.5 μM. The activity of the SAR is mostly influenced by the number and location of hydroxyl groups. The di-hydroxy substituents in drug 28 had outstanding activity, with variations in their action. The least active molecule among the di-hydroxy analogues is compound 28 (IC50 = 220.15 ± 1.95 μM) because it has high intra-molecular H-bonding, which reduces the activity of the active component methylglyoxal. Compound 28 was tested in vitro for its efficacy to scavenge DPPH radicals. It displayed a variable DPPH radical-scavenging activity of 12.05 ± 0.45, respectively [124]. Ramachandran et al. designed and evaluated a novel family of naryl-2-amino-benzoimidazol 29 named (6-(1-methylpiperidin-4-yl)-N-(5-(trifluoromethyl)pyridin-2-yl)-1H-benzo[d]imidazol-2-amine) for its anti-malarial activity against P. falciparum. Compound 29 was demonstrated to being highly effective, with an IC50 value of 36 μM. It was also easily available and efficient in a rat version of malaria. Furthermore, this series of drug exhibited a highly efficient death rate comparable to chloroquine in a unique in vitro study of its parasite-reduction ratio. More new clinical candidates from this chemical family should be ascertained, possibly by additional lead optimization attempts in combination with in vivo unsafe analysis [125]. The in vitro anti-plasmodial effects of a variety of BnZ derivatives, synthesized by Saify et al., were assessed against human Cytidine triphosphate D (CTP D) and P. falciparum plasmepsin II. Compound 30 (1-([1,1′-biphenyl]-4-yl)-2-(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)ethan-1-one) demonstrated substantial anti-plasmodial action, with an IC50 value of 160 μM. Cathepsin D is inhibited by compound 30, with an inhibitory potency and selectivity comparable to that of plasmepsin II (IC50 = 2.0 μM vs. 14.7 μM). According to SAR studies, the additional phenyl group at the p-position of the acetophenone cause the moiety mosque’s enhanced anti-plasmodial action [126].
13. Conclusions
In conclusion, BnZ and its derivatives have been the focus of extensive investigations of their biological applications in several research areas, including their potential to treat cancer, reduce inflammation, fight against bacteria, treat tuberculosis, and inhibit protozoal growth. Green, non-metallic, and metallic methods have been used to synthesize BnZ and its derivatives. The desired product, as well as economic and environmental factors, all have an important role in the selection of the synthesis route. Recent research has placed a lot of emphasis on the creation of BnZ derivatives with enhanced biological activity, highlighting the potential of these substances as prospective medicines for treating different disorders. So, additional research is required to understand the mechanisms of action of these substances to enhance their biological activities.
Author Contributions
Conceptualization, M.P. and G.A.; methodology, G.A.; validation, G.A. and B.-H.J.; formal analysis, A.G. and H.-K.P.; writing—original draft preparation, M.P. and G.A.; writing—review and editing, G.A., A.G., M.S.A., H.-K.P. and B.-H.J.; supervision, G.A.; funding acquisition, M.S.A. and B.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University (KKU) for funding this research through the Research Group Program under the grant number (R.G.P.2/517/44). This work was supported by the Mid-Career Researcher Program (grant no. 2020R1A2C3004237) through the National Research Foundation of the Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Rao, G.E.; Babu, P.S.; Koushik, O.S.; Sharmila, R.; Vijayabharathi, M.; Maruthikumar, S.; Prathyusha, R.; Pavankumar, P. A Review on Chemistry of Benzimidazole Nucleus and its Biological Significance. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 227–232. [Google Scholar]
- Manna, K.; Agrawal, Y.K. Microwave Assisted Synthesis of New Indophenazine 1,3,5-Trisubstruted Pyrazoline Derivatives of Benzofuran and Their Antimicrobial Activity. Bioorg. Med. Chem. Lett. 2009, 19, 2688–2692. [Google Scholar] [CrossRef]
- Kalidhar, U.; Kaur, A. An Overview on Some Benzimidazole and Sulfonamide Derivatives with Anti-Microbial Activity. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 1116–1135. [Google Scholar]
- wright, J.B. The Chemistry of the Benzimidazoles. Chem. Rev. 1951, 48, 397–541. [Google Scholar] [CrossRef] [PubMed]
- Hoebrecker, F. Benzimidazole. Ber 1872, 5, 920–926. [Google Scholar]
- Fei, F.; Zhou, Z. New Substituted Benzimidazole Derivatives: A Patent Review (2010–2012). Expert Opin. Ther. Pat. 2013, 23, 1157–1179. [Google Scholar] [CrossRef]
- Azeez, S.; Sureshbabu, P.; Chaudhary, P.; Sabiah, S.; Kandasamy, J. Tert-Butyl Nitrite Catalyzed Synthesis of Benzimidazoles from o-Phenylenediamine and Aldehydes at Room Temperature. Tetrahedron Lett. 2020, 61, 151735. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, R.; Chen, J.; Li, T.; Xu, Y. Hydrogenative Coupling of Nitriles with Diamines to Benzimidazoles Using Lignin-Derived Rh2P Catalyst. iScience 2021, 24, 103045. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, P.B.; Haridasan, A.P. Pharmacognostic and Preliminary Phytochemical Evaluation of Nelamuchchala (Gymnostachyum Febrifugum Benth.). Int. J. Res. Ayush Pharm. Sci. 2017, 1, 1–6. [Google Scholar]
- Bastug, G.; Eviolitte, C.; Markó, I.E. Functionalized Orthoesters as Powerful Building Blocks for the Efficient Preparation of Heteroaromatic Bicycles. Org. Lett. 2012, 14, 3502–3505. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.J.; Chan, B.K.; Estrada, A.A.; Shore, D.G.; Lyssikatos, J.P. Mild and General One-Pot Reduction and Cyclization of Aromatic and Heteroaromatic 2-Nitroamines to Bicyclic 2 H-Imidazoles. Synlett 2010, 18, 2759–2764. [Google Scholar] [CrossRef]
- Yang, D.; Fokas, D.; Li, J.; Yu, L.; Baldino, C.M. A Versatile Method for the Synthesis of Benzimidazoles from O-Nitroanilines and Aldehydes in One Step via a Reductive Cyclization. Synthesis 2005, 1, 47–56. [Google Scholar] [CrossRef]
- Cui, W.; Kargbo, R.B.; Sajjadi-Hashemi, Z.; Ahmed, F.; Gauuan, J.F. Efficient One-Pot Synthesis of 2-Substituted Benzimidazoles from Triacyloxyborane Intermediates. Synlett 2012, 2, 247–250. [Google Scholar] [CrossRef]
- Sluiter, J.; Christoffers, J. Synthesis of 1-Methylbenzimidazoles from Carbonitriles. Synlett 2009, 1, 63–66. [Google Scholar] [CrossRef]
- Wray, B.C.; Stambuli, J.P. Synthesis of N-Arylindazoles and Benzimidazoles from a Common Intermediate. Org. Lett. 2010, 12, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-Catalyzed Intramolecular C-N Bond Formation: A Straightforward Synthesis of Benzimidazole Derivatives in Water. J. Org. Chem. 2011, 76, 716–719. [Google Scholar] [CrossRef]
- Saha, P.; Ramana, T.; Purkait, N.; Ali, M.A.; Paul, R.; Punniyamurthy, T. Ligand-Free Copper-Catalyzed Synthesis of Substituted Benzimidazoles, 2-Aminobenzimidazoles, 2-Aminobenzothiazoles, and Benzoxazoles. J. Org. Chem. 2009, 74, 8719–8725. [Google Scholar] [CrossRef]
- Diao, X.; Wang, Y.; Jiang, Y.; Ma, D. Assembly of Substituted 1H-Benzimidazoles and 1,3-Dihydrobenzimidazol-2- Ones via CuI/L-Proline Catalyzed Coupling of Aqueous Ammonia with 2-Iodoacetanilides and 2-Iodophenylcarbamates. J. Org. Chem. 2009, 74, 7974–7977. [Google Scholar] [CrossRef]
- Kim, Y.; Kumar, M.R.; Park, N.; Heo, Y.; Lee, S. Copper-Catalyzed, One-Pot, Three-Component Synthesis of Benzimidazoles by Condensation and C-N Bond Formation. J. Org. Chem. 2011, 76, 9577–9583. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, L.-Z.; Wu, J.; Lu, D.; Ma, B.-L.; Du, Z.-T. Microwave-Assisted Syntehsis of 2-Substituted 1H-Benzo[d]imidazoles and Their Antifungal Activities in vitro. Heterocycles 2013, 87, 1545–1552. [Google Scholar] [CrossRef]
- Trivedi, R.; De, S.K.; Gibbs, R.A. A Convenient One-Pot Synthesis of 2-Substituted Benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11. [Google Scholar] [CrossRef]
- Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Copper-Catalyzed Synthesis of Benzimidazoles via Cascade Reactions of o-Haloacetanilide Derivatives with Amidine Hydrochlorides. J. Org. Chem. 2008, 73, 7841–7844. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-mourabit, A. Iron Sulfide Catalyzed Redox/Condensation Cascade Reaction between 2-Amino/Hydroxy Nitrobenzenes and Activated Methyl Groups: A Straightforward Atom Economical Approach to 2-Hetaryl-Benzimidazoles and -Benzoxazoles. J. Am. Chem. Soc. 2013, 135, 118–121. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Miao, C.; Liu, W.; Sun, W. Synthesis of Benzimidazoles from O-Phenylenediamines and DMF Derivatives in the Presence of PhSiH3. Tetrahedron 2017, 73, 3458–3462. [Google Scholar] [CrossRef]
- Daw, P.; Ben-David, Y.; Milstein, D. Direct Synthesis of Benzimidazoles by Dehydrogenative Coupling of Aromatic Diamines and Alcohols Catalyzed by Cobalt. ACS Catal. 2017, 7, 7456–7460. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Dean, W.A.; Al-Mourabit, A. Benzazoles from Aliphatic Amines and o-Amino/Mercaptan/Hydroxyanilines: Elemental Sulfur as a Highly Efficient and Traceless Oxidizing Agent. Org. Lett. 2012, 14, 5948–5951. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, P.; Thimmarayaperumal, S.; Shanmugam, S. Metal Catalyst Free One-Pot Synthesis of 2-Arylbenzimidazoles from α-Aroylketene Dithioacetals. RSC Adv. 2014, 4, 12028–12036. [Google Scholar] [CrossRef]
- Liu, X.; Cao, H.; Bie, F.; Yan, P.; Han, Y. C A N Bond Formation and Cyclization: A Straightforward and Metal-Free Synthesis of N -1-Alkyl-2-Unsubstituted Benzimidazoles. Tetrahedron Lett. 2019, 60, 1057–1059. [Google Scholar] [CrossRef]
- Mostafavi, H.; Islami, M.R.; Ghonchepour, E.; Tikdari, A.M. Synthesis of 1H-1,3-Benzimidazoles, Benzothiazoles and 3H-Imidazo[4,5-c]Pyridine Using DMF in the Presence of HMDS as a Reagent under the Transition-Metal-Free Condition. Chem. Pap. 2018, 72, 2973–2978. [Google Scholar] [CrossRef]
- Jain, P.; Tiwari, M. Synthesis and Antimicrobial Activity of Some Benzimidazole and 2-Methylbenzimidazole Derivatives. Asian J. Chem. 2017, 29, 838–842. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Zhou, J.; Fang, Q.S.; Chen, L.; Song, Z.B. Chemoselective Synthesis of 1,2-Disubstituted Benzimidazoles in Lactic Acid without Additive. Chem. Pap. 2016, 70, 1293–1298. [Google Scholar] [CrossRef]
- Khunt, M.D.; Kotadiya, V.C.; Viradiya, D.J.; Baria, B.H. Easy, Simplistic and Green Synthesis of Various Benzimidazole and Benzoxazole Derivatives Using PEG 400 as a Green Solvent. Int. Lett. Chem. Phys. Astron. 2014, 25, 61–68. [Google Scholar] [CrossRef]
- Kathirvelan, D.; Yuvaraj, P.; Babu, K.; Nagarajan, A.S.; Reddy, B.S.R. A Green Synthesis of Benzimidazoles Benzimidazole Moieties Are a Very Important Class of Heterocyclic Compounds That Have Many Applications in Pharmaceutical Chemistry. Indian J. Chem. 2013, 52, 1152–1156. [Google Scholar] [CrossRef]
- Kidwai, M.; Jahan, A.; Bhatnagar, D. Polyethylene Glycol: A Recyclable Solvent System for the Synthesis of Benzimidazole Derivatives Using CAN as Catalyst. J. Chem. Sci. 2010, 122, 607–612. [Google Scholar] [CrossRef]
- Azarifar, D.; Pirhayati, M.; Maleki, B.; Sanginabadi, M.; Yami, R.N. Acetic Acid-Promoted Condensation of o-Phenylenediamine with Aldehydes into 2-Aryl-1-(Arylmethyl)-1H-Benzimidazoles under Microwave Irradiation. J. Serbian Chem. Soc. 2010, 75, 1181–1189. [Google Scholar] [CrossRef]
- Khose, V.N.; John, M.E.; Pandey, A.D.; Karnik, A.V. Chiral Benzimidazoles and Their Applications in Stereodiscrimination Processes. Tetrahedron Asymmetry 2017, 28, 1233–1289. [Google Scholar] [CrossRef]
- Said, N.R.; Mustakim, M.A.; Sani, N.N.M.; Baharin, S.N.A. Heck Reaction Using Palladium-Benzimidazole Catalyst: Synthesis, Characterisation and Catalytic Activity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012019. [Google Scholar] [CrossRef]
- Günnaz, S.; Gökçe, A.G.; Türkmen, H. Synthesis of Bimetallic Complexes Bridged by 2,6-Bis(Benzimidazol-2-Yl) Pyridine Derivatives and Their Catalytic Properties in Transfer Hydrogenation. Dalt. Trans. 2018, 47, 17317–17328. [Google Scholar] [CrossRef]
- Horak, E.; Kassal, P.; Murković Steinberg, I. Benzimidazole as a Structural Unit in Fluorescent Chemical Sensors: The Hidden Properties of a Multifunctional Heterocyclic Scaffold. Supramol. Chem. 2018, 30, 838–857. [Google Scholar] [CrossRef]
- Agarwal, R.A.; Aijaz, A.; Ahmad, M.; Sañudo, E.C.; Xu, Q.; Bharadwaj, P.K. Two New Coordination Polymers with Co(II) and Mn(II): Selective Gas Adsorption and Magnetic Studies. Cryst. Growth Des. 2012, 12, 2999–3005. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal Organic Frameworks Mimicking Natural Enzymes: A Structural and Functional Analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wei, B.; Liang, T.; Yang, X.; Wu, Y. Anhydrous Proton Conduction in Liquid Crystals Containing Benzimidazole Moieties. RSC Adv. 2016, 6, 34038–34042. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(Imide Benzimidazole)s for High Temperature Polymer Electrolyte Membrane Fuel Cells. J. Membr. Sci. 2014, 454, 351–358. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Pan, M.; Liu, J.-M.; Wang, W.; Su, C.-Y. Assembly of Robust and Porous Hydrogen-Bonded Coordination Frameworks: Isomorphism, Polymorphism, and Selective Adsorption. Inorg. Chem. 2010, 49, 10166–10173. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Dong, J.; Zhang, Z.; Zhang, Q.; Lin, J. Structure and Properties of Polyimide Fibers Containing Benzimidazole and Amide Units. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 183–191. [Google Scholar] [CrossRef]
- Nabavian, S.; Naderi, R.; Asadi, N. Determination of Optimum Concentration of Benzimidazole Improving the Cathodic Disbonding Resistance of Epoxy Coating. Coatings 2018, 8, 471. [Google Scholar] [CrossRef]
- Iqbal, H.M.S.; Bhowmik, S.; Benedictus, R. Performance Evaluation of Polybenzimidazole Coating for Aerospace Application. Prog. Org. Coat. 2017, 105, 190–199. [Google Scholar] [CrossRef]
- VijayaKumar, V.; Ramesh Kumar, C.; Suresh, A.; Jayalakshmi, S.; Kamachi Mudali, U.; Sivaraman, N. Evaluation of Polybenzimidazole-Based Polymers for the Removal of Uranium, Thorium and Palladium from Aqueous Medium. R. Soc. Open Sci. 2018, 5, 171701. [Google Scholar] [CrossRef]
- Mandal, S.; Song, G. Characterizing Thermal Protective Fabrics of Firefighters’ Clothing in Hot Surface Contact. J. Ind. Text. 2018, 47, 622–639. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Villalobos, L.F.; Vovusha, H.; Shevate, R.; Schwingenschlögl, U.; Peinemann, K.V. Polybenzimidazole-Based Mixed Membranes with Exceptionally High Water Vapor Permeability and Selectivity. J. Mater. Chem. A 2017, 5, 21807–21819. [Google Scholar] [CrossRef]
- Muthuraja, A.; Kalainathan, S. A Study on Growth, Optical, Mechanical, and NLO Properties of 2-Mercaptobenzimidazole, 2-Phenylbenzimidazole and 2-Hydroxy Benzimidazole Single Crystals: A Comparative Investigation. Mater. Technol. 2017, 32, 335–348. [Google Scholar] [CrossRef]
- Tayade, R.P.; Sekar, N. Benzimidazole-Thiazole Based NLOphoric Styryl Dyes with Solid State Emission—Synthesis, Photophysical, Hyperpolarizability and TD-DFT Studies. Dye. Pigment. 2016, 128, 111–123. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of Fungicides, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Ermler, S.; Scholze, M.; Kortenkamp, A. Seven Benzimidazole Pesticides Combined at Sub-Threshold Levels Induce Micronuclei in Vitro. Mutagenesis 2013, 28, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Eldebss, T.M.A.; Farag, A.M.; Shamy, A.Y.M. Synthesis of Some Benzimidazole-Based Heterocycles and Their Application as Copper Corrosion Inhibitors. J. Heterocycl. Chem. 2019, 56, 371–390. [Google Scholar] [CrossRef]
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric Schistosomiasis Parasite Found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Saltan, G.M.; Dinçalp, H.; Kiran, M.; Zafer, C.; Erbaş, S.Ç. Novel Organic Dyes Based on Phenyl-Substituted Benzimidazole for Dye Sensitized Solar Cells. Mater. Chem. Phys. 2015, 163, 387–393. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Khafizova, E.A.; Syakaev, V.V.; Gubaidullin, A.T.; Samigullina, A.I.; Algaeva, N.E.; Latypov, S.K. The Rearrangement of 1H,1′H-Spiro[Quinoline-4,2′-Quinoxaline]-2,3′ (3H,4′H)-Diones—A New and Efficient Method for the Synthesis of 4-(Benzimidazol-2-Yl)Quinolin-2(1H)-Ones. Tetrahedron 2018, 74, 6544–6557. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Qiu, P.; Li, X.; Wang, Q.; Chen, J.; Ma, D. New Benzimidazole-Based Bipolar Hosts: Highly Efficient Phosphorescent and Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes Employing the Same Device Structure. ACS Appl. Mater. Interfaces 2016, 8, 2635–2643. [Google Scholar] [CrossRef]
- Giovine, A.; Muraglia, M.; Florio, M.A.; Rosato, A.; Corbo, F.; Franchini, C.; Musio, B.; Degennaro, L.; Luisi, R. Synthesis of Functionalized Arylaziridines as Potential Antimicrobial Agents. Molecules 2014, 19, 11505–11519. [Google Scholar] [CrossRef]
- Šindelář, Z.; Kopel, P. Bis(Benzimidazole) Complexes, Synthesis and Their Biological Properties: A Perspective. Inorganics 2023, 11, 113. [Google Scholar] [CrossRef]
- Mirzaeian, F.; Eshghi, H. Synthesis of Benzimidazoles by Ni-Catalyzed Hydrogen Transfer Reduction of Nitroarenes with Alcohols. In Proceedings of the 21st ICS International Chemistry Congres, Mashhad, Iran, 26–28 July 2022; Available online: https://profdoc.um.ac.ir/paper-abstract-1091156.html (accessed on 1 May 2023).
- Haddadi, M.H. Benzimidazole Derivatives: A Versatile Scaffold for Drug Development against Helicobacter Pylori-Related Diseases. Fundam. Clin. Pharmacol. 2022, 36, 930–943. [Google Scholar] [CrossRef]
- Haque, R.A.; Iqbal, M.A.; Asekunowo, P.; Majid, A.M.S.A.; Khadeer Ahamed, M.B.; Umar, M.I.; Al-Rawi, S.S.; Al-Suede, F.S.R. Synthesis, Structure, Anticancer, and Antioxidant Activity of Para-Xylyl Linked Bis-Benzimidazolium Salts and Respective Dinuclear Ag(I) N-Heterocyclic Carbene Complexes (Part-II). Med. Chem. Res. 2013, 22, 4663–4676. [Google Scholar] [CrossRef]
- Nile, S.H.; Kumar, B.; Park, S.W. In Vitro Evaluation of Selected Benzimidazole Derivatives as an Antioxidant and Xanthine Oxidase Inhibitors. Chem. Biol. Drug Des. 2013, 82, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Khuntia, A.; Yellasubbaiah, N.; Ayyanna, C.; Naga Sudha, B.; Harika, M.S. Design, Synthesis of Novel Azo Derivatives of Benzimidazole as Potent Antibacterial and Anti Tubercular Agents. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 646–651. [Google Scholar] [CrossRef]
- Iqbal, H.; Verma, A.K.; Yadav, P.; Alam, S.; Mishra, D.; Khan, F.; Hanif, K.; Negi, A.S.; Chanda, D. Antihypertensive Effect of a Novel Angiotensin II Receptor Blocker Fluorophenyl Benzimidazole: Contribution of CGMP, Voltage-Dependent Calcium Channels, and BK Ca Channels to Vasorelaxant Mechanisms. Front. Pharmacol. 2021, 12, 611109. [Google Scholar] [CrossRef]
- Bhagat, P.K.; Raj, R.; Thakur, D.N. Preparation and Characterization of Some Bivalent Metal Complexes with Bis [2-(2ethyledenebenzimidazolyl) Thiocarbohydrazide] and Studies of Their Antiamoebic and Antihelminthic Activities. Int. J. Chem. Environ. Sci. 2023, 4, 49–53. [Google Scholar] [CrossRef]
- Hernández-Romero, D.; Rosete-Luna, S.; López-Monteon, A.; Chávez-Piña, A.; Pérez-Hernández, N.; Marroquín-Flores, J.; Cruz-Navarro, A.; Pesado-Gómez, G.; Morales-Morales, D.; Colorado-Peralta, R. First-Row Transition Metal Compounds Containing Benzimidazole Ligands: An Overview of Their Anticancer and Antitumor Activity. Coord. Chem. Rev. 2021, 439, 213930. [Google Scholar] [CrossRef]
- Hu, J.; Cao, T.; Yuan, B.; Guo, Y.; Zhang, J.; Zhao, X.; Hou, H. Benzimidazole-Quinoline-Based Copper Complexes: Exploration for Their Possible Antitumor Mechanism. Polyhedron 2022, 211, 115563. [Google Scholar] [CrossRef]
- Son, D.-S.; Lee, E.-S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. Available online: https://immunenetwork.org/DOIx.php?id=10.4110/in.2020.20.e29 (accessed on 1 May 2023). [CrossRef]
- Dziwornu, G.A.; Coertzen, D.; Leshabane, M.; Korkor, C.M.; Cloete, C.K.; Njoroge, M.; Gibhard, L.; Lawrence, N.; Reader, J.; van der Watt, M. Antimalarial Benzimidazole Derivatives Incorporating Phenolic Mannich Base Side Chains Inhibit Microtubule and Hemozoin Formation: Structure–Activity Relationship and in Vivo Oral Efficacy Studies. J. Med. Chem. 2021, 64, 5198–5215. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sinha, D.; Sanjay, K.; Singh, V.K. Novel Benzimidazole Analogs as Inhibitors of EGFR Tyrosine Kinase. Chem. Biol. Drug Des. 2012, 80, 625–630. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Ali, M.A.; Wei, A.C.; Choon, T.S.; Khaw, K.Y.; Murugaiyah, V.; Osman, H.; Masand, V.H. Synthesis, Characterization, and Molecular Docking Analysis of Novel Benzimidazole Derivatives as Cholinesterase Inhibitors. Bioorg. Chem. 2013, 49, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, C.F.; Li, X.; Wu, G.S.; Xie, S.; Hu, Q.N.; Deng, Z.; Zhu, M.X.; Luo, H.R.; Hong, X. Synthesis, Biological Evaluation and Molecular Modeling of Substituted 2-Aminobenzimidazoles as Novel Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Bioorg. Med. Chem. 2013, 21, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, L.; Virkel, G.; Elissondo, C.; Canton, C.; Canevari, J.; Murno, G.; Denegri, G.; Lanusse, C.; Alvarez, L. A Pharmacology-Based Comparison of the Activity of Albendazole and Flubendazole against Echinococcus Granulosus Metacestode in Sheep. Acta Trop. 2013, 127, 216–225. [Google Scholar] [CrossRef]
- Pérez-Villanueva, J.; Hernández-Campos, A.; Yépez-Mulia, L.; Méndez-Cuesta, C.; Méndez-Lucio, O.; Hernández-Luis, F.; Castillo, R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 4221–4224. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Sharma, D.; Kumar, P. Benzimidazole: A Medicinally Important Heterocyclic Moiety. Med. Chem. Res. 2012, 21, 269–283. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Xiong, S.; Xiong, R.; Liu, J.; Zou, L.; Lei, X.; Cao, X.; Xie, Z.; Chen, Y.; et al. Design, Synthesis and Biological Evaluation of Chrysin Benzimidazole Derivatives as Potential Anticancer Agents. Nat. Prod. Res. 2018, 32, 2900–2909. [Google Scholar] [CrossRef]
- Morais, G.R.; Palma, E.; Marques, F.; Gano, L.; Oliveira, M.C.; Abrunhosa, A.; Miranda, H.V.; Outeiro, T.F.; Santos, I.; Paulo, A. Synthesis and Biological Evaluation of Novel 2-Aryl Benzimidazoles as Chemotherapeutic Agents. J. Heterocycl. Chem. 2017, 54, 255–267. [Google Scholar] [CrossRef]
- Klenc, J.; Raux, E.; Barnes, S.; Sullivan, S.; Duszynska, B.; Bojarski, A.J.; Strekowski, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino) Pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, H.; Ji, D.; Li, Y.; Xu, Y.; Zhu, Q. Design, Synthesis and Biological Evaluation of Novel 5-Fluoro-1H-Benzimidazole-4-Carboxamide Derivatives as Potent PARP-1 Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4127–4132. [Google Scholar] [CrossRef]
- Onnis, V.; Demurtas, M.; Deplano, A.; Balboni, G.; Baldisserotto, A.; Manfredini, S.; Pacifico, S.; Liekens, S.; Balzarini, J. Design, Synthesis and Evaluation of Antiproliferative Activity of New Benzimidazolehydrazones. Molecules 2016, 21, 4–12. [Google Scholar] [CrossRef]
- Acar Çevik, U.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S. Antiproliferative, Cytotoxic, and Apoptotic Effects of New Benzimidazole Derivatives Bearing Hydrazone Moiety. J. Heterocycl. Chem. 2018, 55, 138–148. [Google Scholar] [CrossRef]
- Elancheran, R.; Saravanan, K.; Choudhury, B.; Divakar, S.; Kabilan, S.; Ramanathan, M.; Das, B.; Devi, R.; Kotoky, J. Design and Development of Oxobenzimidazoles as Novel Androgen Receptor Antagonists. Med. Chem. Res. 2016, 25, 539–552. [Google Scholar] [CrossRef]
- Renard, J.F.; Lecomte, F.; Hubert, P.; De Leval, X.; Pirotte, B. N-(3-Arylaminopyridin-4-Yl)Alkanesulfonamides as Pyridine Analogs of Nimesulide: Cyclooxygenases Inhibition, Anti-Inflammatory Studies and Insight on Metabolism. Eur. J. Med. Chem. 2014, 74, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective Inhibition of Inducible Cyclooxygenase 2 in Vivo Is Antiinflammatory and Nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.G.; Das, N.; Shengule, S.A.; Srivastava, R.S.; Shrivastava, S.K. Synthesis, Characterization, Evaluation and Molecular Dynamics Studies of 5, 6-Diphenyl-1,2,4-triazin-3(2 H)-one Derivatives Bearing 5-Substituted 1,3,4-Oxadiazole as Potential Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2015, 101, 81–95. [Google Scholar] [CrossRef]
- Boggu, P.R.; Venkateswararao, E.; Manickam, M.; Kwak, D.; Kim, Y.; Jung, S.H. Exploration of 2-Benzylbenzimidazole Scaffold as Novel Inhibitor of NF-ΚB. Bioorg. Med. Chem. 2016, 24, 1872–1878. [Google Scholar] [CrossRef]
- Khanapur, M.; Pinna, N.K.; Badiger, J. Synthesis and Anti-Inflammatory in Vitro, in Silico, and in Vivo Studies of Flavone Analogues. Med. Chem. Res. 2015, 24, 2656–2669. [Google Scholar] [CrossRef]
- Kim, M.K.; Shin, H.; Park, K.S.; Kim, H.; Park, J.; Kim, K.; Nam, J.; Choo, H.; Chong, Y. Benzimidazole Derivatives as Potent JAK1-Selective Inhibitors. J. Med. Chem. 2015, 58, 7596–7602. [Google Scholar] [CrossRef]
- Banoglu, E.; Çalişkan, B.; Luderer, S.; Eren, G.; Özkan, Y.; Altenhofen, W.; Weinigel, C.; Barz, D.; Gerstmeier, J.; Pergola, C.; et al. Identification of Novel Benzimidazole Derivatives as Inhibitors of Leukotriene Biosynthesis by Virtual Screening Targeting 5-Lipoxygenase- Activating Protein (FLAP). Bioorg. Med. Chem. 2012, 20, 3728–3741. [Google Scholar] [CrossRef]
- Tatani, K.; Hiratochi, M.; Kikuchi, N.; Kuramochi, Y.; Watanabe, S.; Yamauchi, Y.; Itoh, F.; Isaji, M.; Shuto, S. Identification of Adenine and Benzimidazole Nucleosides as Potent Human Concentrative Nucleoside Transporter 2 Inhibitors: Potential Treatment for Hyperuricemia and Gout. J. Med. Chem. 2016, 59, 3719–3731. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Suchomel, J.; Cardozo, M.; Duquette, J.; He, X.; Henne, K.; Hu, Y.L.; Kelly, R.C.; McCarter, J.; McGee, L.R.; et al. Discovery, Optimization, and in Vivo Evaluation of Benzimidazole Derivatives AM-8508 and AM-9635 as Potent and Selective PI3Kδ Inhibitors. J. Med. Chem. 2016, 59, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Synthesis of Antimicrobial Benzimidazole–Pyrazole Compounds and Their Biological Activities. Antibiotics 2021, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent Advance in Oxazole-Based Medicinal Chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Deepthi, S.B.; Ramesh, P.; Trivedi, R.; Buddana, S.K.; Prakasham, R.S. Carbohydrate Triazole Tethered 2-Pyridyl-Benzimidazole Ligands: Synthesis of Their Palladium (II) Complexes and Antimicrobial Activities. Inorganica Chim. Acta 2015, 435, 200–205. [Google Scholar] [CrossRef]
- Siddiqi, Z.A.; Shahid, A.M.; Khalid, M.; Sharma, P.K.; Siddique, A. Spectroscopic, Luminescence, Electrochemical and Antimicrobial Studies of Lanthanide Complexes of Bis-Benzimidazole Derived Ligands. J. Mol. Struct. 2013, 1037, 402–411. [Google Scholar] [CrossRef]
- Kumar, K.; Awasthi, D.; Lee, S.Y.; Cummings, J.E.; Knudson, S.E.; Slayden, R.A.; Ojima, I. Benzimidazole-Based Antibacterial Agents against Francisella Tularensis. Bioorg. Med. Chem. 2013, 21, 3318–3326. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Damu, G.L.V.; Cai, G.X.; Zhou, C.H. Design, Synthesis and Antimicrobial Evaluation of Novel Benzimidazole Type of Fluconazole Analogues and Their Synergistic Effects with Chloromycin, Norfloxacin and Fluconazole. Eur. J. Med. Chem. 2013, 64, 329–344. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sood, S.; Sharma, N.; Rahi, P.; Kumar, R.; Sinha, A.K.; Gulati, A. Synthesis and SAR Investigation of Natural Phenylpropene-Derived Methoxylated Cinnamaldehydes and Their Novel Schiff Bases as Potent Antimicrobial and Antioxidant Agents. Med. Chem. Res. 2013, 22, 5129–5140. [Google Scholar] [CrossRef]
- Lam, T.; Hilgers, M.T.; Cunningham, M.L.; Kwan, B.P.; Nelson, K.J.; Brown-Driver, V.; Ong, V.; Trzoss, M.; Hough, G.; Shaw, K.J.; et al. Structure-Based Design of New Dihydrofolate Reductase Antibacterial Agents: 7-(Benzimidazol-1-Yl)-2,4-Diaminoquinazolines. J. Med. Chem. 2014, 57, 651–668. [Google Scholar] [CrossRef]
- Ranjith, P.K.; Rajeesh, P.; Haridas, K.R.; Susanta, N.K.; Guru Row, T.N.; Rishikesan, R.; Suchetha Kumari, N. Design and Synthesis of Positional Isomers of 5 and 6-Bromo-1-[(Phenyl) Sulfonyl]-2-[(4-Nitrophenoxy)Methyl]-1H-Benzimidazoles as Possible Antimicrobial and Antitubercular Agents. Bioorg. Med. Chem. Lett. 2013, 23, 5228–5234. [Google Scholar] [CrossRef]
- Savaliya, P.P.; Akbari, V.K.; Modi, J.A.; Patel, K.C. Synthesis, Characterization, and Antimicrobial Screening of Some Mannich Base Sydnone Derivatives. Med. Chem. Res. 2013, 22, 5789–5797. [Google Scholar] [CrossRef]
- Raviglione, M.; Marais, B.; Floyd, K.; Lönnroth, K.; Getahun, H.; Migliori, G.B.; Harries, A.D.; Nunn, P.; Lienhardt, C.; Graham, S.; et al. Scaling up Interventions to Achieve Global Tuberculosis Control: Progress and New Developments. Lancet 2012, 379, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Brossier, F.; Boudinet, M.; Jarlier, V.; Petrella, S.; Sougakoff, W. Comparative Study of Enzymatic Activities of New KatG Mutants from Low-and High-Level Isoniazid-Resistant Clinical Isolates of Mycobacterium Tuberculosis. Tuberculosis 2016, 100, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Surana, S.; Jain, P.; Patel, H.M. Mycobacterium Tuberculosis (MTB) GyrB Inhibitors: An Attractive Approach for Developing Novel Drugs against TB. Eur. J. Med. Chem. 2016, 124, 160–185. [Google Scholar] [CrossRef]
- Chandrasekera, N.S.; Berube, B.J.; Shetye, G.; Chettiar, S.; O’Malley, T.; Manning, A.; Flint, L.; Awasthi, D.; Ioerger, T.R.; Sacchettini, J.; et al. Improved Phenoxyalkylbenzimidazoles with Activity against Mycobacterium Tuberculosis Appear to Target QcrB. ACS Infect. Dis. 2017, 3, 898–916. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Ali, M.A.; Wei, A.C.; Choon, T.S.; Ismail, R. Synthesis and Evaluation of Antimycobacterial Activity of New Benzimidazole Aminoesters. Eur. J. Med. Chem. 2015, 93, 614–624. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lutkenhaus, J. Analysis of FtsZ Assembly by Light Scattering and Determination of the Role of Divalent Metal Cations. J. Bacteriol. 1999, 181, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, D.; Kumar, K.; Knudson, S.E.; Slayden, R.A.; Ojima, I. SAR Studies on Trisubstituted Benzimidazoles as Inhibitors of Mtb FtsZ for the Development of Novel Antitubercular Agents. J. Med. Chem. 2013, 56, 9756–9770. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Awasthi, D.; Lee, S.Y.; Zanardi, I.; Ruzsicska, B.; Knudson, S.; Tonge, P.J.; Slayden, R.A.; Ojima, I. Novel Trisubstituted Benzimidazoles, Targeting Mtb FtsZ, as a New Class of Antitubercular Agents. J. Med. Chem. 2011, 54, 374–381. [Google Scholar] [CrossRef]
- Park, B.; Awasthi, D.; Chowdhury, S.R.; Melief, E.H.; Kumar, K.; Knudson, S.E.; Slayden, R.A.; Ojima, I. Design, Synthesis and Evaluation of Novel 2,5,6-Trisubstituted Benzimidazoles Targeting FtsZ as Antitubercular Agents. Bioorg. Med. Chem. 2014, 22, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Gobis, K.; Foks, H.; Bojanowski, K.; Augustynowicz-Kopeć, E.; Napiórkowska, A. Synthesis of Novel 3-Cyclohexylpropanoic Acid-Derived Nitrogen Heterocyclic Compounds and Their Evaluation for Tuberculostatic Activity. Bioorg. Med. Chem. 2012, 20, 137–144. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Serocki, M.; Augustynowicz-Kopeć, E.; Napiórkowska, A. Synthesis and Evaluation of in Vitro Antimycobacterial Activity of Novel 1H-Benzo[d]Imidazole Derivatives and Analogues. Eur. J. Med. Chem. 2015, 89, 13–20. [Google Scholar] [CrossRef]
- Vinet, L.; Zhedanov, A. A “missing” Family of Classical Orthogonal Polynomials. J. Phys. A Math. Theor. 2011, 44, 1–3. [Google Scholar] [CrossRef]
- Araujo, D.M.L.; Maste, M.M.; Alegaon, S.; Saxena, A. Synthesis, Antitubercular Evaluation and Docking Studies Of Novel Benzimidazole Analogues. Int. J. Pharm. Sci. Res. 2018, 12, 3696–3704. [Google Scholar]
- Hata, Y.; De Mieri, M.; Ebrahimi, S.N.; Mokoka, T.; Fouche, G.; Kaiser, M.; Brun, R.; Potterat, O.; Hamburger, M. Identification of Two New Phenathrenones and a Saponin as Antiprotozoal Constituents of Drypetes Gerrardii. Phytochem. Lett. 2014, 10, cxxxiii–cxl. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Rzeppa, S.; Kaiser, M.; Brun, R. Larrea Tridentata—Absolute Configuration of Its Epoxylignans and Investigations on Its Antiprotozoal Activity. Phytochem. Lett. 2012, 5, 632–638. [Google Scholar] [CrossRef]
- Molyneux, D.H. Neglected Tropical Diseases: Now More than Just ’Other Diseases’- the Post-2015 Agenda. Int. Health 2014, 6, 172–180. [Google Scholar] [CrossRef]
- Karaaslan, C.; Kaiser, M.; Brun, R.; Göker, H. Synthesis and Potent Antiprotozoal Activity of Mono/Di Amidino 2-Anilinobenzimidazoles versus Plasmodium Falciparum and Trypanosoma Brucei Rhodesiense. Bioorg. Med. Chem. 2016, 24, 4038–4044. [Google Scholar] [CrossRef]
- Keurulainen, L.; Siiskonen, A.; Nasereddin, A.; Kopelyanskiy, D.; Sacerdoti-Sierra, N.; Leino, T.O.; Tammela, P.; Yli-Kauhaluoma, J.; Jaffe, C.L.; Kiuru, P. Synthesis and Biological Evaluation of 2-Arylbenzimidazoles Targeting Leishmania Donovani. Bioorg. Med. Chem. Lett. 2015, 25, 1933–1937. [Google Scholar] [CrossRef]
- Soria-Arteche, O.; Hernández-Campos, A.; Yépez-Mulia, L.; Trejo-Soto, P.J.; Hernández-Luis, F.; Gres-Molina, J.; Maldonado, L.A.; Castillo, R. Synthesis and Antiprotozoal Activity of Nitazoxanide-N-Methylbenzimidazole Hybrids. Bioorg. Med. Chem. Lett. 2013, 23, 6838–6841. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Jamil, W.; Rashwan, H.; Kashif, S.M.; Sain, A.A.; Adenan, M.I.; Anouar, E.H.; Ali, M.; Rahim, F.; et al. Synthesis of Novel Derivatives of 4-Methylbenzimidazole and Evaluation of Their Biological Activities. Eur. J. Med. Chem. 2014, 84, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Hameed, P.S.; Srivastava, A.; Shanbhag, G.; Morayya, S.; Rautela, N.; Awasthy, D.; Kavanagh, S.; Bharath, S.; Reddy, J. N-Aryl-2-Aminobenzimidazoles: Novel, Efficacious, Antimalarial Lead Compounds. J. Med. Chem. 2014, 57, 6642–6652. [Google Scholar] [CrossRef] [PubMed]
- Saify, Z.S.; Azim, M.K.; Ahmad, W.; Nisa, M.; Goldberg, D.E.; Hussain, S.A.; Akhtar, S.; Akram, A.; Arayne, A.; Oksman, A.; et al. New Benzimidazole Derivatives as Antiplasmodial Agents and Plasmepsin Inhibitors: Synthesis and Analysis of Structure-Activity Relationships. Bioorg. Med. Chem. Lett. 2012, 22, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).