Effective Removal of Cd(II) from Aqueous Solutions Using Theobroma cacao Agro-Industrial Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Biosorbent Characterization
2.2. pH Effects
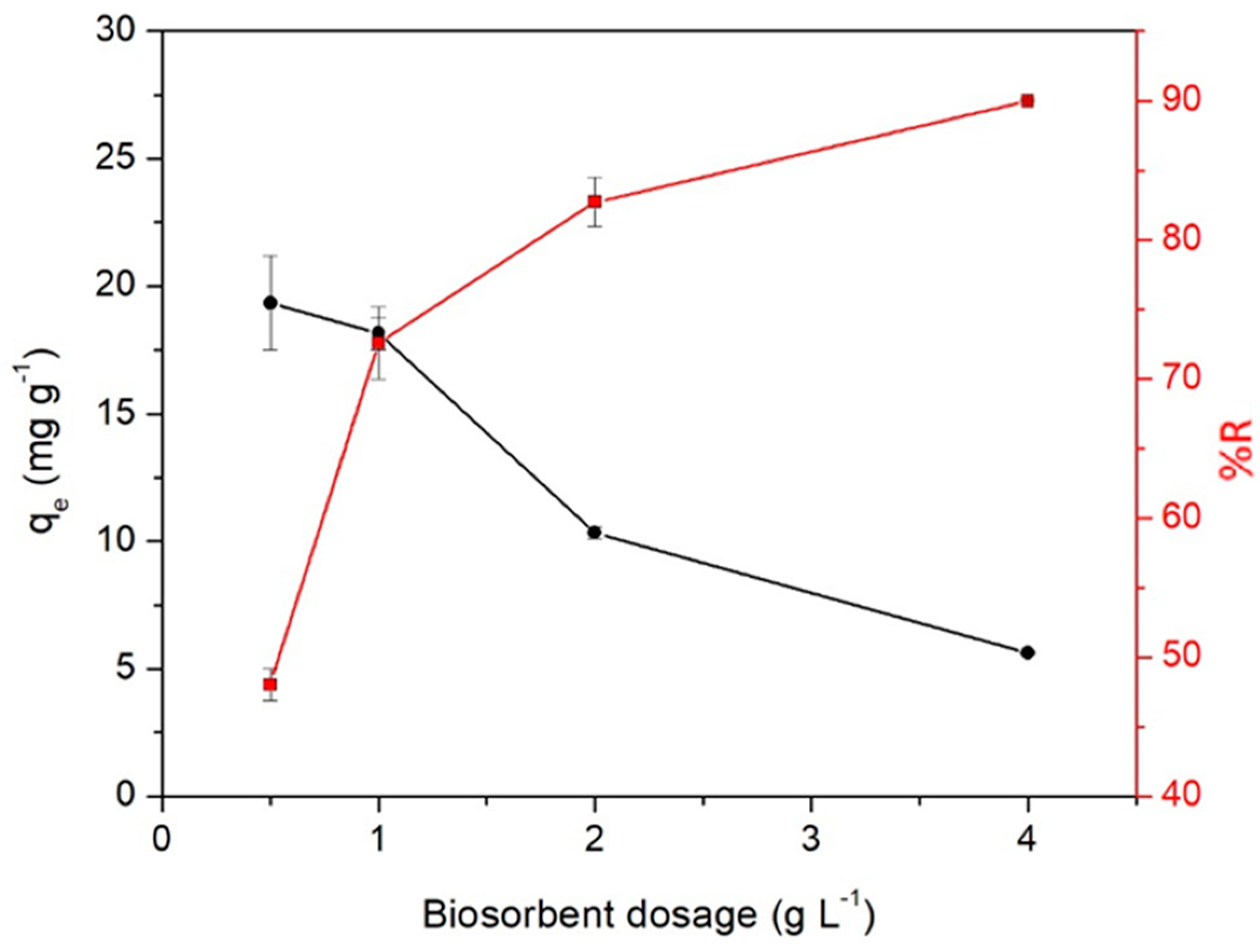
2.3. Biosorbent Dosage Effect
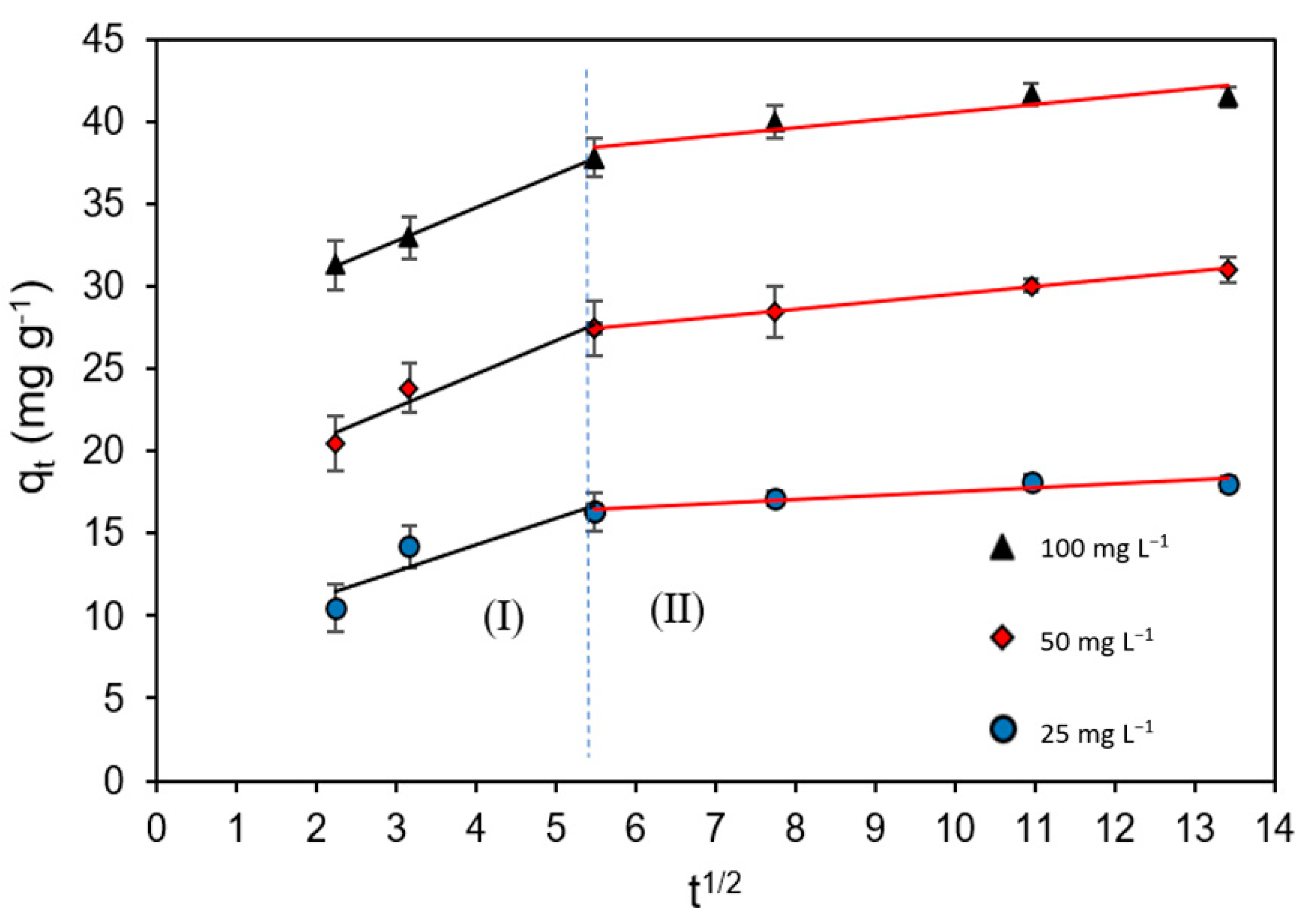
2.4. Kinetic Data
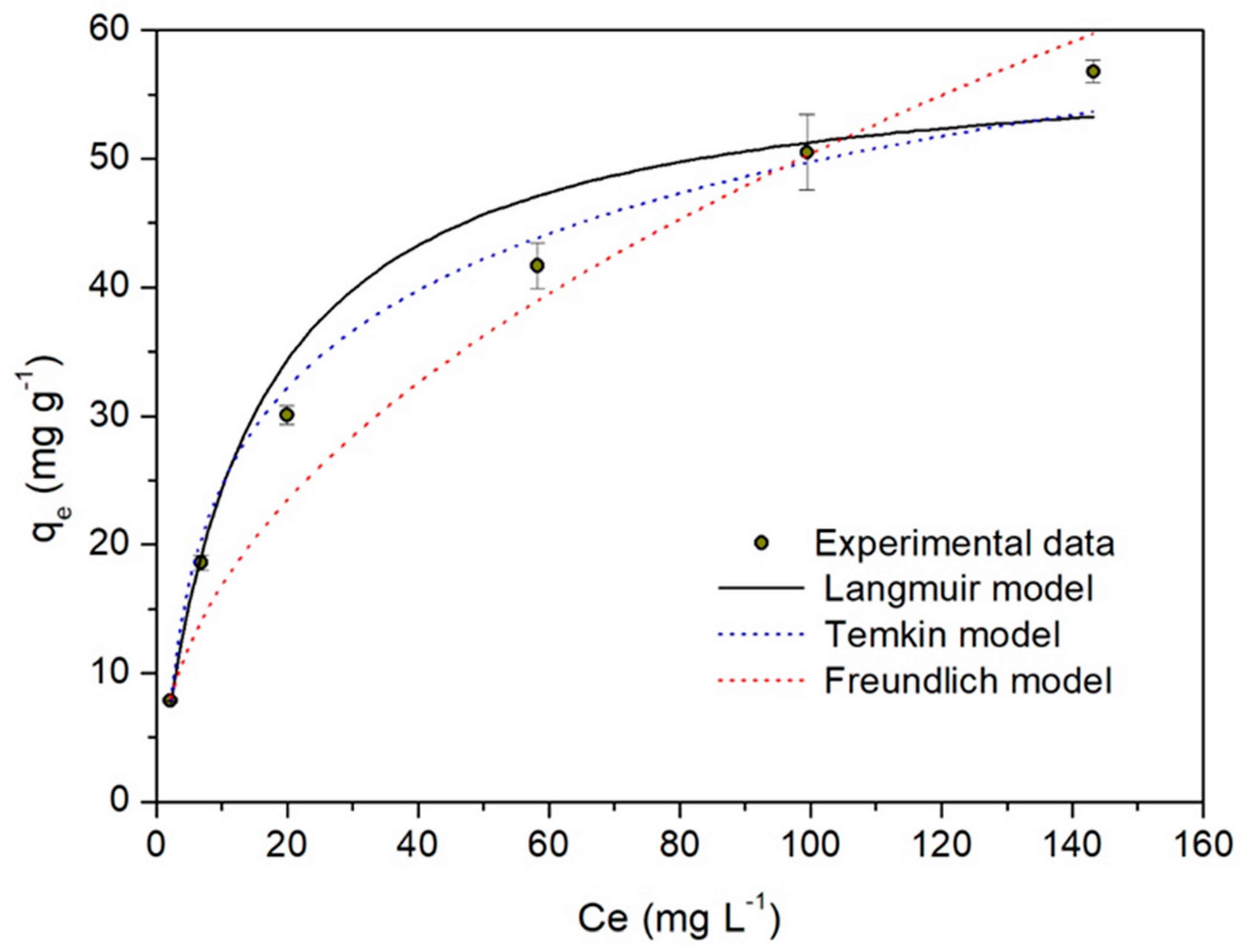
2.5. Biosorption Isotherms
| Adsorbent | dose (g L−1) | qe,max (mg g−1) | References |
|---|---|---|---|
| Foeniculum vulgari biomass | 10 | 26.59 | [41] |
| Cocoa pod waste | 10 | 12.15 | [42] |
| Dried cactus (Opuntia ficus indica) | 0.5 | 30.42 | [43] |
| Cabbage waste Canola biomass | 5 3 | 20.6 25.86 | [44] [45] |
| Barley husk biomass | 3 | 24 | [46] |
| Peanut husk | 3 | 29 | [47] |
| Cassia fistula biomass | 2.5 | 7.24 | [48] |
| Oak waste materials | 5 | 155.9 | [49] |
| Avocado pear exocarp | 0.5 | 13.91 | [14] |
| Spent coffee grounds | 2.5 | 11 | [37] |
| Agave angustifolia biomass | 6.7 | 34.84 | [50] |
| Opuntia fuliginosa biomass | 6.7 | 30.21 | [50] |
| Phyllanthus emblica fruit stone | 1 | 3.15 | [13] |
| Cocoa (Theobroma cacao) pod husk | 20 | 4.42 | [51] |
| Chrysopogon zizanioides root powder | 1 | 26.69 | [30] |
| Theobroma cacao agro-industrial waste | 1 | 58.5 | This work |
2.6. Biosorption Thermodynamics
2.7. Effect of Co-Cations in Binary and Ternary Systems
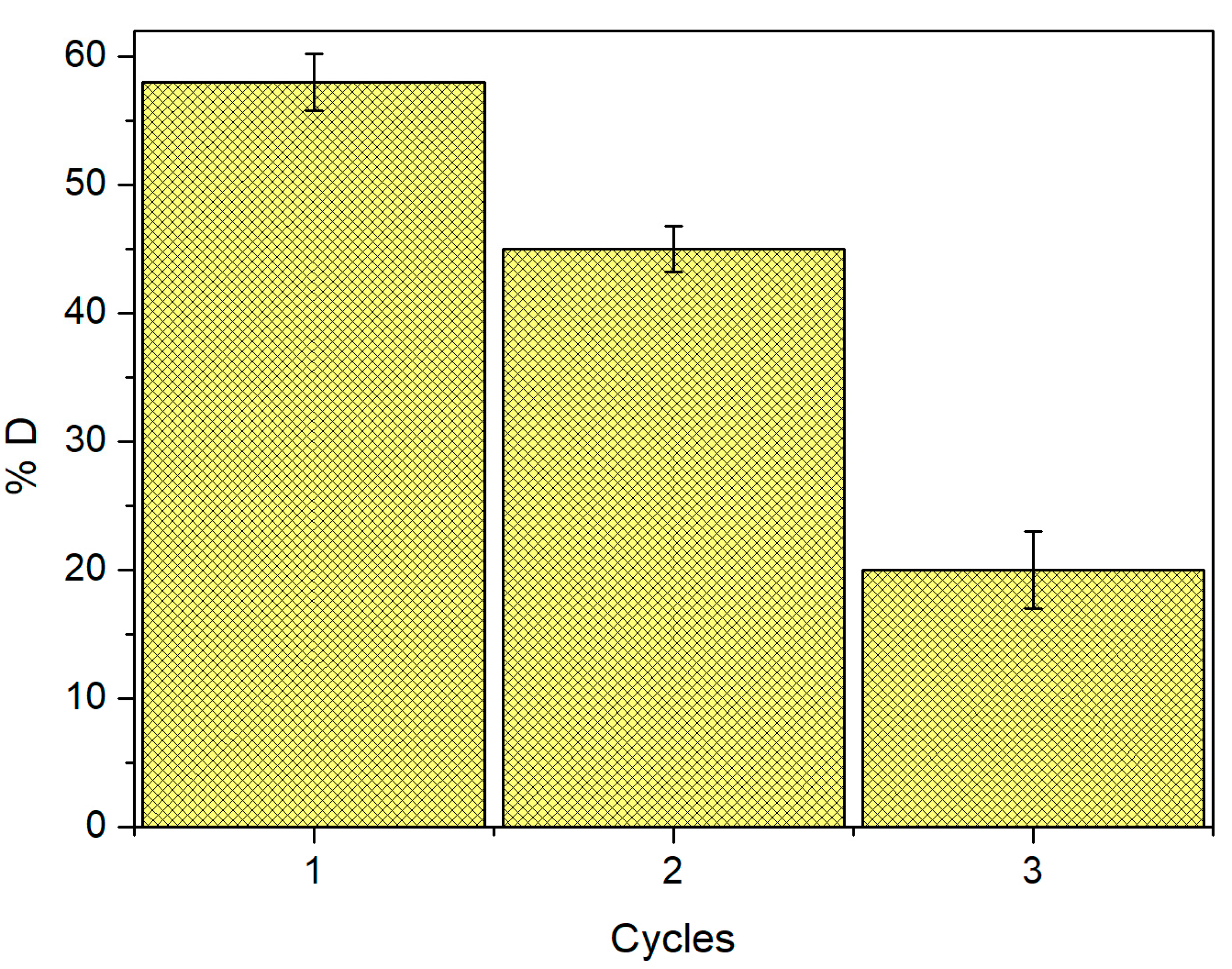
2.8. Desorption
3. Materials and Methods
3.1. Preparation and Characterizations of WTC Biosorbent
3.2. Biosorption Assays
3.3. Competitive Effect of Co-Cations
3.4. Desorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Howard, J.A.; Kuznietsova, H.; Dziubenko, N.; Aigle, A.; Natuzzi, M.; Thomas, E.; Lysenko, V.; David, L.; Brichart, T.; Lux, F.; et al. Combating lead and cadmium exposure with an orally administered chitosan-based chelating polymer. Sci. Rep. 2023, 13, 2215. [Google Scholar] [CrossRef] [PubMed]
- Kavisri, M.; Abraham, M.; Namasivayam, S.K.R.; Aravindkumar, J.; Balaji, D.; Sathishkumar, R.; Sigamani, S.; Srinivasan, R.; Moovendhan, M. Adsorption isotherm, kinetics and response surface methodology optimization of cadmium (Cd) removal from aqueous solution by chitosan biopolymers from cephalopod waste. J. Environ. Manag. 2023, 335, 117484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, S.; Li, Y.; Peng, J.; Yu, J.; Chi, R.; Xiao, C. Efficient bioimmobilization of cadmium contamination in phosphate mining wastelands by the phosphate solubilizing fungus Penicillium oxalicum ZP6. Biochem. Eng. J. 2022, 187, 108667. [Google Scholar] [CrossRef]
- Saeed AA, H.; Harun, N.Y.; Nasef, M.M.; Al-Fakih, A.; Ghaleb, A.A.S.; Afolabi, H.K. Removal of cadmium from aqueous solution by optimized rice husk biochar using response surface methodology. Ain Shams Eng. J. 2022, 13, 101516. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S.; Batool, S.; Yusuff, A.S. An overview of the use of water-stable metal-organic frameworks in the removal of cadmium ion. J. Environ. Chem. Eng. 2023, 11, 109131. [Google Scholar] [CrossRef]
- Weshahy, A.R.; Sakr, A.K.; Gouda, A.A.; Atia, B.M.; Somaily, H.H.; Hanfi, M.Y.; Sayyed, M.I.; El Sheikh, R.; El-Sheikh, E.M.; Radwan, H.A.; et al. Selective Recovery of Cadmium, Cobalt, and Nickel from Spent Ni–Cd Batteries Using Adogen® 464 and Mesoporous Silica Derivatives. Int. J. Mol. Sci. 2022, 23, 8677. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Soliman, N.A.; Ossman, M.E.; Abdel-Fattah, Y.R.; Moawad, M.N. Isotherm and kinetic studies of cadmium biosorption and its adsorption behaviour in multi-metals solution using dead and immobilized archaeal cells. Sci. Rep. 2023, 13, 2550. [Google Scholar] [CrossRef]
- De Freitas, G.R.; da Silva, M.G.C.; Vieira, M.G.A. Biosorption technology for removal of toxic metals: A review of commercial biosorbents and patents. Environ. Sci. Pollut. Res. 2019, 26, 19097–19118. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Biosorption of pollutants by plant leaves: An empirical review. J. Environ. Chem. Eng. 2019, 7, 103100. [Google Scholar] [CrossRef]
- Tee, W.T.; Loh, N.Y.L.; Hiew, B.Y.Z.; Hanson, S.; Thangalazhy-Gopakumar, S.; Gan, S.; Lee, L.Y. Effective remediation of lead(II) wastewater by Parkia speciosa pod biosorption: Box-Behnken design optimisation and adsorption performance evaluation. Biochem. Eng. J. 2022, 187, 108629. [Google Scholar] [CrossRef]
- Kushwaha, S.; Suhas; Chaudhary, M.; Tyagi, I.; Bhutiani, R.; Goscianska, J.; Ahmed, J.; Manila; Chaudhary, S. Utilization of Phyllanthus emblica fruit stone as a Potential Biomaterial for Sustainable Remediation of Lead and Cadmium Ions from Aqueous Solutions. Molecules 2022, 27, 3355. [Google Scholar] [CrossRef] [PubMed]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of Lead and Cadmium from Aqueous Solution in Single and Binary Systems Using Avocado Pear Exocarp: Effects of Competing Ions. Anal. Lett. 2020, 53, 2868–2885. [Google Scholar] [CrossRef]
- Fawzy, M.; Nasr, M.; Abdel-Rahman, A.M.; Hosny, G.; Odhafa, B.R. Techno-economic and environmental approaches of Cd2+ adsorption by olive leaves (Olea europaea L.) waste. Int. J. Phytoremediat. 2019, 21, 1205–1214. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Lee, S.M. Optimization of Cd(II), Cu(II) and Ni(II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar] [CrossRef]
- Eletta, O.A.A.; Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V.; Ayandele, F.O. Valorisation of Cocoa (Theobroma cacao) pod husk as precursors for the production of adsorbents for water treatment. Environ. Technol. Rev. 2020, 9, 20–36. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; De la Cruz-Cerrón, L.; Cisneros-Santos, G.; De la Cruz, A.H.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Arabica-coffee and teobroma-cocoa agro-industrial waste biosorbents, for Pb(II) removal in aqueous solutions. Environ. Sci. Pollut. Res. 2022, 30, 2991–3001. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; De la Cruz-Cerrón, L.; Asencios, Y.J.O.; Marcos, F.C.F.; Dávalos-Prado, J.Z. Alkaline Modification of Arabica-Coffee and Theobroma-Cocoa Agroindustrial Waste for Effective Removal of Pb(II) from Aqueous Solutions. Molecules 2023, 28, 683. [Google Scholar] [CrossRef]
- Pabbenteng; Samawi, F.W.; Maming. The utility of cocoa pods husk M45 (Theobroma cocoa) as adsorbent of heavy metals, iron (Fe) and copper in the laboratory wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 473. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Islam, M.T.; Imam, M.A.; Hyder, A.H.M.G.; Jabbari, V.; Dominguez, N.; Noveron, J.C. Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2018, 6, 6602–6611. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Bonilla-Mancilla, H.; Ortega-Toro, R.; Villabona-Ortíz, A.; Díaz-Illanes, M. The elimination of lead(II) ions in a solution by bio-adsorption: Kinetics, equilibrium, and thermodynamics. J. Water Land Dev. 2022, 53, 118–127. [Google Scholar]
- Jaihan, W.; Mohdee, V.; Sanongraj, S.; Pancharoen, U.; Nootong, K. Biosorption of lead (II) from aqueous solution using Cellulose-based Bio-adsorbents prepared from unripe papaya (Carica papaya) peel waste: Removal Efficiency, Thermodynamics, kinetics and isotherm analysis: Biosorption of lead (II) from aqueous solution. Arab. J. Chem. 2022, 15, 103883. [Google Scholar] [CrossRef]
- Tunali Akar, S.; Arslan, S.; Alp, T.; Arslan, D.; Akar, T. Biosorption potential of the waste biomaterial obtained from Cucumis melo for the removal of Pb 2+ ions from aqueous media: Equilibrium, kinetic, thermodynamic and mechanism analysis. Chem. Eng. J. 2012, 185–186, 82–90. [Google Scholar] [CrossRef]
- Moyo, M.; Pakade, V.E.; Modise, S.J. Biosorption of lead(II) by chemically modified Mangifera indica seed shells: Adsorbent preparation, characterization and performance assessment. Process Saf. Environ. Prot. 2017, 111, 40–51. [Google Scholar] [CrossRef]
- Gök, G.; Kocyigit, H.; Gök, O.; Celebi, H. The use of raw shrimp shells in the adsorption of highly polluted waters with Co2+. Chem. Eng. Res. Des. 2022, 186, 229–240. [Google Scholar] [CrossRef]
- Szostak, K.; Hodacka, G.; Długosz, O.; Pulit-Prociak, J.; Banach, B. Sorption of Mercury in Batch and Fixed-Bed Column System on Hydrochar Obtained from Apple Pomace. Processes 2022, 10, 2114. [Google Scholar] [CrossRef]
- Ali Alhaidrai, S.A.; Al-Hadi, F.A.; Al-Kaf, A.G.; Taj Al- Deen, A. Phytochemical Screening by FTIR Spectroscopic Analysis in the Methanolic Extracts Coffee (C. Arabica. L) to Seeds and Peels (Unroasted and Roasted) Cultivars Grown in Yemen. Bioequiv. Bioavailab. Int. J. 2022, 6, 000179. [Google Scholar]
- Madadgar, S.; Doulati Ardejani, F.; Boroumand, Z.; Sadeghpour, H.; Taherdangkoo, R.; Butscher, C. Biosorption of Aqueous Pb(II), Co(II), Cd(II) and Ni(II) Ions from Sungun Copper Mine Wastewater by Chrysopogon zizanioides Root Powder. Minerals 2023, 13, 106. [Google Scholar] [CrossRef]
- Oliveira, M.R.F.; do Vale Abreu, K.; Romão, A.L.E.; Davi, D.M.B.; de Carvalho Magalhães, C.E.; Carrilho, E.N.V.M.; Alves, C.R. Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium. Environ. Sci. Pollut. Res. 2021, 28, 18941–18952. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Solomon, S.; Daher, M.A.; Walker, G. Efficient removal of anionic and cationic dyes from aqueous systems using spent Yerba Mate “Ilex paraguariensis”. J. Taiwan Inst. Chem. Eng. 2017, 82, 144–155. [Google Scholar] [CrossRef]
- Mangwandi, C.; Kurniawan, T.A.; Albadarin, A.B. Comparative biosorption of chromium (VI) using chemically modified date pits (CM-DP) and olive stone (CM-OS): Kinetics, isotherms and influence of co-existing ions. Chem. Eng. Res. Des. 2020, 156, 251–262. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Tovar, C.; Bonilla-Mancilla, H.; del Pino-Moreyra, J.; Villabona-Ortíz1, A.; Ortega-Toro, R. Efecto de la dosis de adsorbente en la remoción de Pb(II) usando bagazo de caña de azúcar: Cinética e isotermas. Rev. Mex. Ing. Quim. 2022, 19, 1413–1423. [Google Scholar] [CrossRef]
- Chwastowski, J.; Bradło, D.; Żukowski, W. Adsorption of cadmium, manganese and lead ions from aqueous solutions using spent coffee grounds and biochar produced by its pyrolysis in the fluidized bed reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef]
- Akbari, M.; Hallajisani, A.; Keshtkar, A.R.; Shahbeig, H.; Ali Ghorbanian, S. Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)-Co(II) single and binary mixtures on brown algae C. indica. J. Environ. Chem. Eng. 2015, 3, 140–149. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Lange, L.C.; Santos, L.V.S. Simultaneous biosorption of Cd(II), Ni(II) and Pb(II) onto a brown macroalgae Fucus vesiculosus: Mono- and multi-component isotherms, kinetics and thermodynamics. J. Environ. Manag. 2019, 251, 109587. [Google Scholar]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Khan, M.A.; Rehman, F. Utilization of Fennel biomass (Foeniculum vulgari) a medicinal herb for the biosorption of Cd(II) from aqueous phase. Chem. Eng. J. 2010, 156, 106–113. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Oputu, O.U.; Adebowale, K.O.; Ogunsolu, O.; Olujimi, O.O. Biosorption of Cd2+ and Pb2+ ions onto mango stone and cocoa pod waste: Kinetic and equilibrium studies. Sci. Res. Essays. 2012, 7, 1614–1629. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nghiem, L.D.; Hai, F.I.; Vigneswaran, S.; Nguyen, T.V. Competitive adsorption of metals on cabbage waste from multi-metal solutions. Bioresour. Technol. 2014, 160, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Azarpira, H.; Mahdavi, Y.; Balarak, D. Removal of cd(II) by adsorption on agricultural waste biomass. Pharma Chem. 2016, 8, 61–67. [Google Scholar]
- Balarak, D.; Azarpira, H.; Mostafapour, F.K. Thermodynamics of removal of cadmium by adsorption on barley husk biomass. Pharma Chem. 2016, 8, 243–247. [Google Scholar]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Imran, M.; Suddique, M.; Shah, G.M.; Ahmad, I.; Murtaza, B.; Shah, N.S.; Mubeen, M.; Ahmad, S.; Zakir, A.; Schotting, R.J. Kinetic and equilibrium studies for cadmium biosorption from contaminated water using Cassia fistula biomass. Int. J. Environ. Sci. Technol. 2019, 16, 3099–3108. [Google Scholar] [CrossRef]
- Takdastan, A.; Samarbaf, S.; Tahmasebi, Y.; Alavi, N.; Babaei, A.A. Alkali modified oak waste residues as a cost-effective adsorbent for enhanced removal of cadmium from water: Isotherm, kinetic, thermodynamic and artificial neural network modeling. Int. J. Environ. Sci. Technol. 2019, 78, 352–363. [Google Scholar] [CrossRef]
- Flores-Trujillo, A.K.I.; Mussali-Galante, P.; de Hoces, M.C.; Blázquez-García, G.; Saldarriaga-Noreña, H.A.; Rodríguez-Solís, A.; Tovar-Sánchez, E.; Sánchez-Salinas, E.; Ortiz-Hernández, L. Biosorption of heavy metals on Opuntia fuliginosa and Agave angustifolia fibers for their elimination from water. Int. J. Environ. Sci. Technol. 2021, 18, 441–454. [Google Scholar] [CrossRef]
- Obike, A.I.; Igwe, J.C.; Emeruwa, C.N.; Uwakwe, K.J. Equilibrium and kinetic studies of Cu (II), Cd (II), Pb (II) and Fe (II) adsorption from aqueous solution using cocoa (Theobroma cacao) pod husk. J. Appl. Sci. Environ. Manag. 2018, 22, 182. [Google Scholar] [CrossRef]
- Çetintaş, S.; Ergül, H.A.; Öztürk, A.; Bingöl, D. Sorptive performance of marine algae (Ulva lactuca Linnaeus, 1753) with and without ultrasonic-assisted to remove Hg(II) ions from aqueous solutions: Optimisation, equilibrium and kinetic evaluation. Int. J. Environ. Anal. 2022, 102, 1428–1451. [Google Scholar] [CrossRef]
- Tavana, M.; Pahlavanzadeh, H.; Zarei, M.J. The novel usage of dead biomass of green algae of Schizomeris leibleinii for biosorption of copper(II) from aqueous solutions: Equilibrium, kinetics and thermodynamics. J. Environ. Chem. Eng. 2020, 8, 104272. [Google Scholar] [CrossRef]
- Aksu, Z.; Dönmez, G. Binary biosorption of cadmium(II) and nickel(II) onto dried Chlorella vulgaris: Co-ion effect on mono-component isotherm parameters. Process Biochem. 2006, 41, 860–868. [Google Scholar] [CrossRef]
- Sandoval-Flores, G.; Alvarado-Reyna, S.; Elvir-Padilla, L.G.; Mendoza-Castillo, D.I.; Reynel-Avila, H.E.; Bonilla-Petriciolet, A. Kinetics, Thermodynamics, and Competitive Adsorption of Heavy Metals from Water Using Orange Biomass. Water Environ. Res. 2018, 90, 2114–2125. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Ebrahim, S.E.; Mohammed-Ridha, M.J. Equilibrium, kinetic, and thermodynamic biosorption of Pb(II), Cr(III), and Cd(II) ions by dead anaerobic biomass from synthetic wastewater. Environ. Sci. Pollut. Res. 2013, 20, 175–187. [Google Scholar] [CrossRef]
- Carbonel Ramos, D. Cadmium, Copper and Lead Adsorption on Natural and Modified Bentonite, Kaolin and Zeolite: A Review of Process Parameters, Isotherms and Kinetics. Ingeniería 2018, 23, 252–273. [Google Scholar]
- Hossain, A.; Ngo, H.; Guo, W.; Nguyen, V. Biosorption of Cu(II) From Water by Banana Peel Based Biosorbent: Experiments and Models of Adsorption and Desorption. J. Water Sustain. 2012, 2, 87–104. [Google Scholar]
- Do Nascimento, W.J.; Landers, R.; Gurgel Carlos Da Silva, M.; Vieira, M.G.A. Equilibrium and desorption studies of the competitive binary biosorption of silver(I) and copper(II) ions on brown algae waste. J. Environ. Chem. Eng. 2021, 9, 26–32. [Google Scholar] [CrossRef]
- Salazar-Pinto, B.M.; Zea-Linares, V.; Villanueva-Salas, J.A.; Gonzales-Condori, E.G. Cd (II) and Pb (II) biosorption in aqueous solutions using agricultural residues of Phaseolus vulgaris L.: Optimization, kinetics, isotherms and desorption. Rev. Mex. Ing. Quim. 2021, 20, 305–322. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
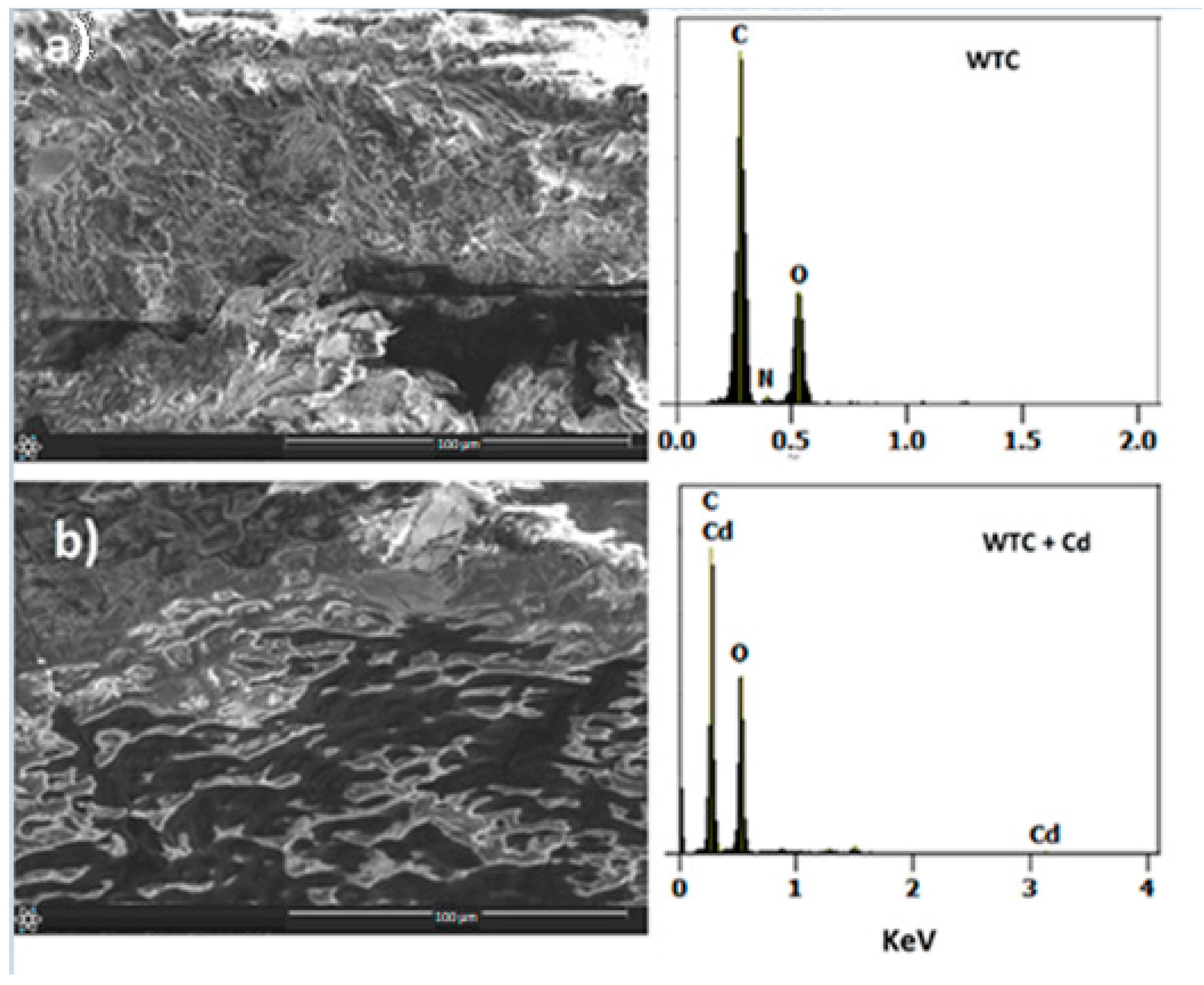
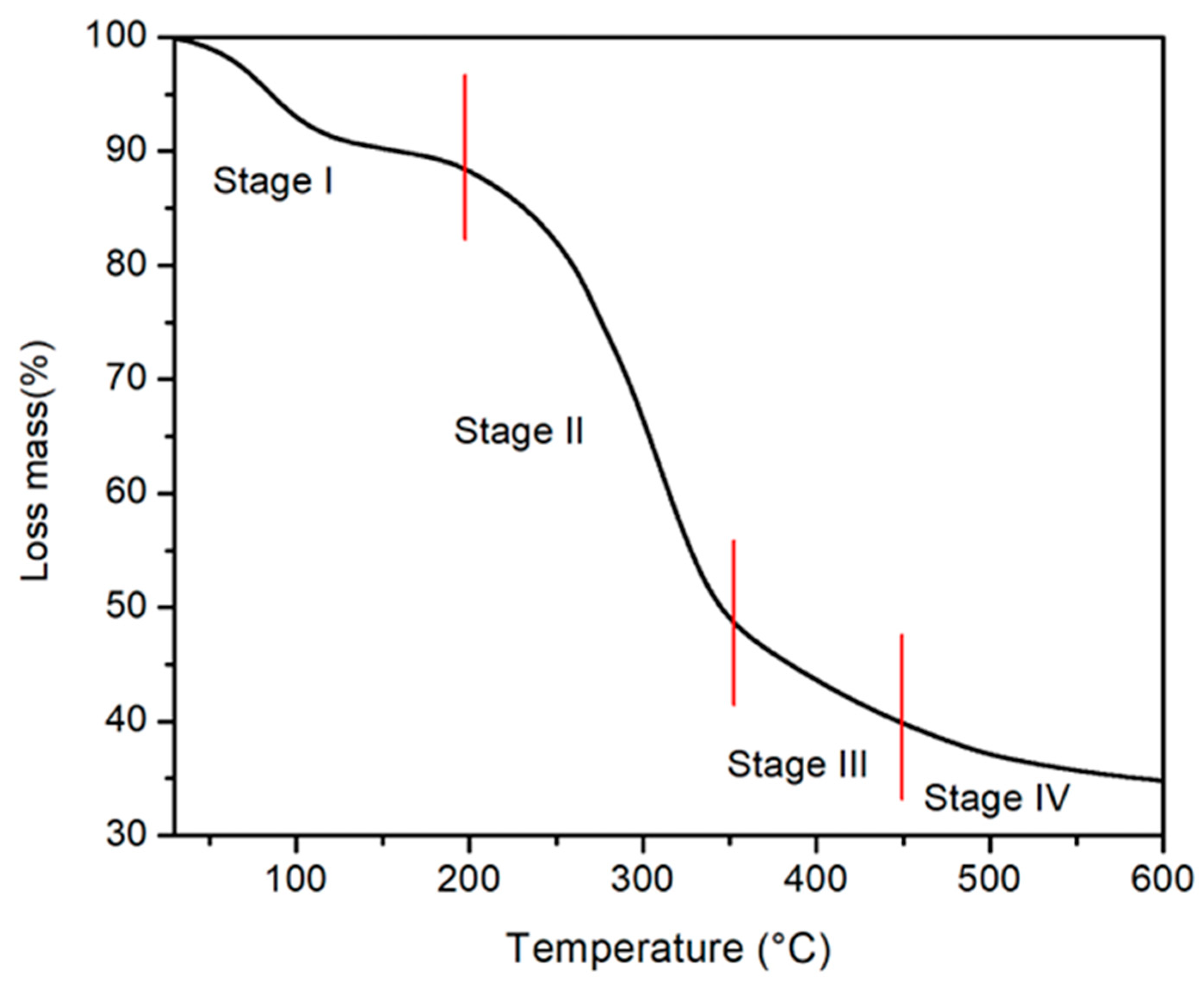
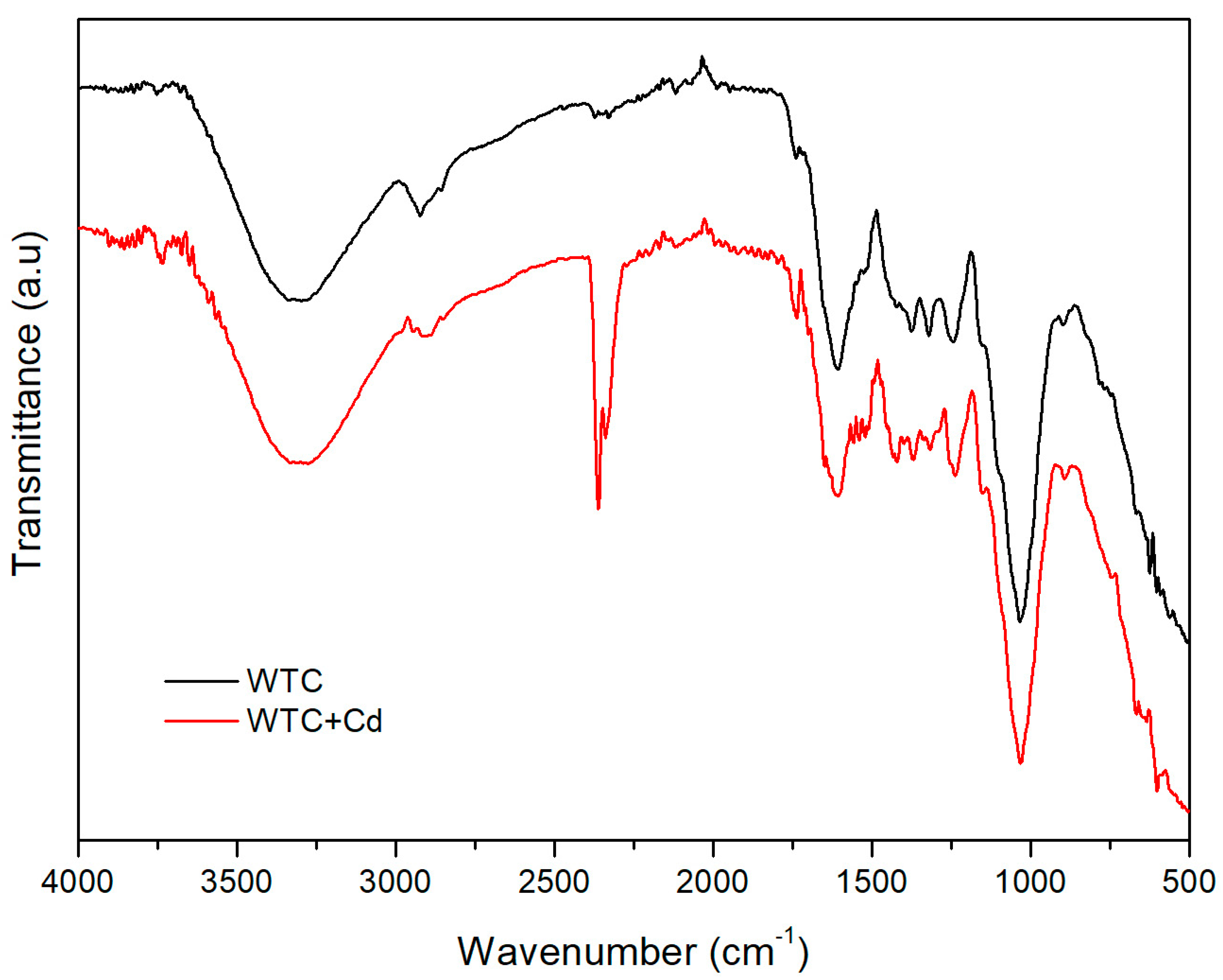




| Model | Parameters | Co = 25 mg L−1 | 50 mg L−1 | 100 mg L−1 |
|---|---|---|---|---|
| Pseudo-first order | qe,cal (mg g−1) | 17.78 ± 0.23 | 30.08 ± 0.43 | 40.82 ± 0.99 |
| k1 (min−1) | 0.17 ± 0.021 | 0.22 ± 0.025 | 0.19 ± 0.033 | |
| R2 χ2 | 0.89 0.16 | 0.90 1.19 | 0.86 8.47 | |
| Pseudo-second-order | qe,cal (mg g−1) | 18.47 ± 0.16 | 30.76 ± 0.23 | 41.99 ± 0.53 |
| k2 (g mg−1·min−1) | 0.015 ± 0.001 | 0.012 ± 0.001 | 0.010 ± 0.001 | |
| h (mg g−1·min−1) | 5.12 | 12.30 | 17.63 | |
| R2 | 0.98 | 0.93 | 0.98 | |
| χ2 | 0.05 | 0.17 | 0.04 | |
| Intra-particle diffusion | kid,I (mg g−1· min−1/2) BI R2 χ2 | 1.62 ± 0.02 7.80 ± 0.52 0.90 0.12 | 2.05 ± 0.03 16.51 ± 0.83 0.95 0.024 | 2.02 ±0.03 26.69 ± 0.92 0.99 0.015 |
| kid,II (mg g−1· min−1/2) BII R2 χ2 | 0.23 ± 0.01 15.27 ± 0.78 0.90 0.10 | 0.45 ± 0.01 25.01 ± 0.81 0.99 0.026 | 0.47 ± 0.02 35.81 ± 0.94 0.85 0.015 |
| Model | Parameters | |
|---|---|---|
| Langmuir | qmax (mg g−1) | 58.5 ± 3.6 |
| KL (L mg−1) | 0.068 ± 0.007 | |
| R2 χ2 | 0.98 2.41 | |
| Freundlich | n | 2.11 ± 0.02 |
| KF (mg0.53 g−1 L0.47) | 5.63 ± 0.44 | |
| R2 χ2 | 0.96 3.42 | |
| Temkin | bT (J mol−1) KT (Lg−1) R2 χ2 | 0.22 ± 0.03 0.95 ± 0.04 0.99 1.85 |
| ΔHo (kJ mol−1) | ΔSo (J K−1 mol−1) | ΔGo (kJ mol−1) | ||
|---|---|---|---|---|
| T = 20 °C | 30 °C | 50 °C | ||
| −8.9 ± 0.9 | −22 ± 2.4 | −2.5 ± 0.7 | −2.3 ± 1.0 | −1.8 ± 0.8 |
| Kinetic Models | Equation | Parameters |
|---|---|---|
| Pseudo-first order | qe (mg g−1): adsorption capacity qt (mg g−1): the amount of Cd (II) retained per unit mass of biosorbent in time t. k1 (min−1): the first-order kinetic rate constant k2 (g mg−1 min−1): rate constant adsorption h: initial sorption rate (mg g−1 min–1) | |
| Pseudo-second order | ||
| Intraparticle diffusion | kid (mg g−1 min−1/2): intraparticle diffusion rate constant C (mg g−1): constant related to the thickness of the adsorbent boundary layer | |
| Isotherm Models | Equation | Parameters |
| Langmuir | Ce (mg L−1): adsorbate concentration in equilibrium qmax (mg g−1): Langmuir constant related to the maximum biosorption capacity KL: Langmuir constant related to the affinity between sorbent and sorbate | |
| Freundlich | KF (L1/n mg(n−1)/n g−1): constant equilibriumn n: constant related to the affinity between sorbent and sorbate. | |
| Temkin | B = RT/bT, R is the gas constant (8.3145 J mol−1 K−1), T absolute temperature bT: Temkin constant related to the heat of adsorption (J mol−1) KT: Temkin isotherm equilibrium binding constant (L g−1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavado-Meza, C.; De la Cruz-Cerrón, L.; Lavado-Puente, C.; Gamarra-Gómez, F.; Sacari-Sacari, E.; Dávalos-Prado, J.Z. Effective Removal of Cd(II) from Aqueous Solutions Using Theobroma cacao Agro-Industrial Waste. Molecules 2023, 28, 5491. https://doi.org/10.3390/molecules28145491
Lavado-Meza C, De la Cruz-Cerrón L, Lavado-Puente C, Gamarra-Gómez F, Sacari-Sacari E, Dávalos-Prado JZ. Effective Removal of Cd(II) from Aqueous Solutions Using Theobroma cacao Agro-Industrial Waste. Molecules. 2023; 28(14):5491. https://doi.org/10.3390/molecules28145491
Chicago/Turabian StyleLavado-Meza, Carmencita, Leonel De la Cruz-Cerrón, Carmen Lavado-Puente, Francisco Gamarra-Gómez, Elisban Sacari-Sacari, and Juan Z. Dávalos-Prado. 2023. "Effective Removal of Cd(II) from Aqueous Solutions Using Theobroma cacao Agro-Industrial Waste" Molecules 28, no. 14: 5491. https://doi.org/10.3390/molecules28145491
APA StyleLavado-Meza, C., De la Cruz-Cerrón, L., Lavado-Puente, C., Gamarra-Gómez, F., Sacari-Sacari, E., & Dávalos-Prado, J. Z. (2023). Effective Removal of Cd(II) from Aqueous Solutions Using Theobroma cacao Agro-Industrial Waste. Molecules, 28(14), 5491. https://doi.org/10.3390/molecules28145491







