Abstract
This paper demonstrates the generation of broadband emission in the visible and infrared ranges induced by a concentrated beam of infrared radiation from CsPbBr3 ceramics doped with Yb3+ ions. The sample was obtained by the conventional solid-state reaction method, and XRD measurements confirmed the phase purity of the material crystallizing in the orthorhombic system. Spectroscopic measurements required further sample preparation in the form of ceramics using a high-pressure press. The research showed that as the excitation power increases, the emission intensity does not increase linearly from the beginning of the experiment. Irradiation of the material results in the accumulation of the delivered energy. Absorption of a sufficient number of photons triggers avalanche emission. It was found that the most intense luminescence is produced in a vacuum. Changes in conductivity were also observed, where the excitation was able to lower the resistivity of the material and it was highly dependent on the excitation power. The mechanism responsible for the generation of the observed phenomenon involving intervalence charge transfer (IVCT) transitions has been postulated.
1. Introduction
Interest in lead halide perovskites has been increasing in recent years due to their unique optical properties, such as precise control of absorption and emission color, either by size or composition. The name “perovskite” was first given by Gustav Rose in 1839 after Russian mineralogist Lev Perovski to the mineral CaTiO3. However, it is now used as a general term for several materials that present the general formula APbX3, where A can be either an organic cation (such as formamidinium or methylammonium) or an inorganic cation (such as Cs+, which is the most common) exhibiting a network of corner-shared PbX6 octahedra, and crystalizing in either cubic, orthorhombic, or tetragonal phases [1], while X can be substituted by oxygen or halide elements, such as Br. The attention to this type of materials has resulted in a number of articles in recent years indicating the application of perovskites, in particular as potential candidates for photovoltaic devices, for example [2,3,4,5,6,7,8,9].
When submitted to infrared laser excitation passing through a focusing lens, a wide variety of materials present a bright broadband emission, commonly called laser-induced emission (LIE). One of the first reports of this phenomenon was made in 2010 by Wang and Tanner, where an intense white light generation was achieved in Ln2O3 (where Ln was Sm3+, Tm3+, and Yb3+) and CeO2 [10]. This emission has a few particular characteristics, such as exclusively appearing with focused excitation, a non-linear dependence of its intensity as a function of excitation power, and pressure surrounding the material, to cite a few. More recently, changes in resistivity were also investigated in some materials and it was found that, upon focused infrared excitation, a decrease in resistivity was observed, coupled with the simultaneous generation of current with LIE, which indicates that the phenomenon is related to promotion of electrons, i.e., it has an electronic nature. It is worth highlighting that this change was strongly dependent on excitation power, meaning that, with higher power, there was a more pronounced decrease in resistivity. This was observed in several materials, such as La1-xNdxAlO3 [11], GaN [12], LiYbP4O12 [13], Sr2CeO4 [14], and graphene [15], to cite a few.
Since its first description, a wide variety of materials were described as capable of producing LIE, such as transition metal- or lanthanide-doped garnets [16,17], transparent ceramics [18,19], a wide variety of inorganic nanocrystals, such as Sr2CeO4 [14,20,21], or LnAlO3 (where Ln can be different lanthanides) [11,22], Sn-based clusters [23,24], YVO4 [25], silica porous materials [26], and even carbon-based materials, such as graphene [27] or diamonds [28]. Despite the fact that many materials have been discovered for LIE, the mechanism is still not agreed upon, and several have been proposed, such as blackbody emission [29,30], oxygen vacancies [25], photon avalanche [31,32], and radiative energy loss (bremsstrahlung emission) [24], to cite a few. However, multiphoton ionization and subsequent intervalence charge transfer (IVCT) have been receiving significant attention. In this mechanism, the absorption of multiple photons leads to the ionization of the material following Keldysh’s theory [33], subsequently creating ionic pairs that undergo an IVCT. To further support the IVCT model, the presence of Yb2+ ions after laser excitation has been observed in Yb3+-doped YAG [34]. Furthermore, the competing nature of LIE and the MLCT (metal-ligand charge transfer) emission band in Sr2CeO4 [20], and, more recently, the threshold dependence on relative permittivity of LIE in alcohols [35], have been reported, which are compelling evidence of the involvement of ionization and a possible IVCT in this complex emission phenomenon. Furthermore, changes in the resistance of the material upon focused laser excitation accompanied by the emission seem to indicate a close relationship between light emission and the ejection of electrons [15,35].
Two main applications were linked to LIE: since it presents a high color rendering index (CRI) close to 100 [36,37,38], it is very favorable for indoor lighting applications. Furthermore, the ejection of electrons was recently linked to the production of H2 [39,40,41], adding great value for research in laser-induced emission since it is a very active field of research for green energy generation. Additionally, LIE is capable of increasing the light power output in tungsten lamps [42], which could significantly increase interest in it.
Although a high number of materials were described to produce LIE, lead bromide perovskites have not yet been explored in this manner. Due to the surging interest in this material, a new possibility for applications might be achieved with LIE. Therefore, here we investigate the emission properties of CsPbBr3:10%Yb3+ under focused infrared excitation. The characteristics of LIE were identified and described, coupled with an IVCT mechanism proposition.
2. Results and Discussion
2.1. Structural and Optical Properties
To confirm the phase purity of the CsPbBr3:10%Yb3+ perovskite powder, XRD measurements were collected and are illustrated in Figure 1a. It can be observed that the obtained result is consistent with the ICSD# 97851 standard, which means that the investigated material crystallizes in an orthorhombic crystallographic system in Pbnm symmetry. Our report shows that using the dry chemistry method in the form of a solid-state reaction leads to obtaining a material that is characterized by micrometric average grain sizes (Figure 1b). Moreover, the resulting crystallites show irregular geometric shapes and their walls are very smooth. Aggregation of CsPbBr3:Yb3+ crystallites was not observed. It is worth emphasizing that, according to the literature reports, dopant ions substitute lead positions due to the similarity of their ionic radii [43,44]. Nevertheless, the introduction into the structure of a dopant with a smaller ionic radius compared to that of the substituted ion results in the formation of defects (probably lead vacancies—VPb [45]) to compensate for the difference in charge.
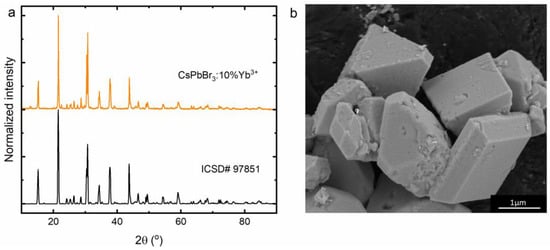
Figure 1.
X-ray diffraction pattern (a) and SEM image (b) of the CsPbBr3:10%Yb3+ perovskite powder.
In order to prove that Yb3+ ions have embedded themselves in the crystal structure of the investigated material, absorption and emission spectra of the CsPbBr3:Yb3+ perovskite powder were recorded upon excitation with a laser diode operating in the 375 nm range measured at 300 K and 190 K, respectively. In Figure 2a, it is clear that from the ultraviolet to the greenish range the intense host band dominates [46]. In addition, a weaker absorption band in the near-infrared range can be found, which is typical of ytterbium ions exhibiting a +3 oxidation state, representing the transition between the 2F7/2 ground state and the 2F5/2 excited state [47]. It was found that excitation of the material in the UV range leads to energy splitting between two emission centers, as presented in Figure 2b. The first is associated with exciton luminescence, while the second is related to the emission of Yb3+ ions, manifested by an intense band with a maximum at 530 nm and 990 nm, respectively [48]. The detection of a strong emission band in the NIR range unequivocally confirms the presence of Yb3+ ions in the studied material. The exclusion of the formation of Yb2+ ions, which theoretically should more easily locate in the positions of Pb2+ ions due to their similar charge, means that before irradiation of the sample with a concentrated beam of a near-infrared laser diode, there are no such ions in the CsPbBr3:Yb3+ structure. The obtained result is extremely important for explaining the mechanism of anti-Stokes luminescence generated from CsPbBr3:Yb3+ ceramics, which is described later in this article.
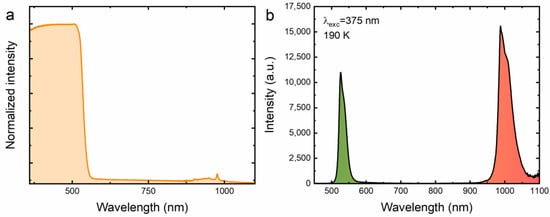
Figure 2.
Absorption (a) and emission (b) spectra of the CsPbBr3:10%Yb3+ perovskite powder recorded at 300 K and 190 K, respectively.
Because LIE generation requires good contact between grains, the irradiation of powder with a concentrated infrared laser beam results in a lack of broadband luminescence. Therefore, ceramics of CsPbBr3:10%Yb3+ with a 0.5 cm diameter were prepared, as shown in Figure 3a. When these ceramics were exposed to a focused infrared laser excitation, a clear bright laser-induced emission was observed (Figure 3b,c). Regardless of whether the excitation wavelength was 808 nm or 975 nm, the characteristics of the emission were very similar, namely a warm light in the orange region, characteristic of several other materials presenting LIE [11,22,36,37]. The precise characterization, i.e., CIE coordinates and CRI, will be explored later.
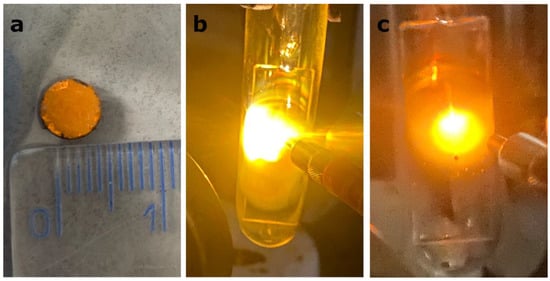
Figure 3.
Digital photograph of the CsPbBr3:10%Yb3+ ceramics in daylight (a), under focused 808 nm excitation (b) and under focused 975 nm excitation (c). The former two are included to showcase the visual characteristics of LIE.
The spectroscopic characterization of the effect observed from the CsPbBr3:10%Yb3+ ceramics was based on the registration of the emission power dependence in two different experiments using two different wavelengths of infrared radiation according to the diagram presented in Figure 4a. It is worth noting that the experiments were performed in a homemade quartz chamber, inside of which a high vacuum (10−5 mbar) was established using a turbomolecular pump. Moreover, to achieve broadband emission, focusing the excitation beam with a lens was essential. The luminescence was observed in both the visible and infrared spectral ranges (Figure 4b), but the need to use two different detectors led to the impression that two bands were generated when, in fact, it was one broad emission band. Figure 4c,d clearly show that for low values of excitation power, the broadband emission was not observed, regardless of the excitation wavelength. A drastic increase in luminescence intensity occurred no sooner than above 0.4 W of laser diode power. This phenomenon is called photon avalanche emission, which empirically can be represented by the power law , where I is the LIE intensity, P stands for the power of the excitation source and the N parameter means the number of absorbed photons involved in the process [26,49]. Such threshold behavior is characteristic of the generation of broadband emission that is also observed in other materials [19,50,51]. Although two different excitation sources were used, the order of magnitude of the received N parameter is similar between them. However, it can be seen that as the excitation energy of the laser diode increases, the N parameter decreases. Reports already published in the literature considering the generation of broadband emission show that this is a common effect [12,21]. Surprisingly, the magnitude of the N parameter varies depending on the measured spectral range. Usually, smaller values are determined for the NIR region, but the trend remains the same as that for the VIS region [12,27]. In order to keep the article clear, and because of the similarity of the obtained spectra, only the emission recorded at 975 nm is presented.
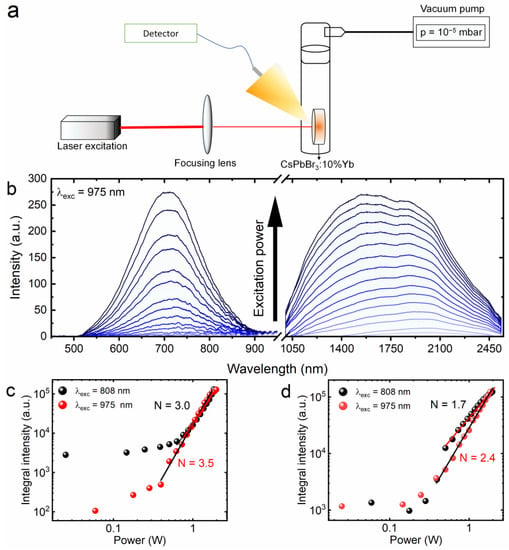
Figure 4.
Schematic representation of the experimental setup used for power dependence measurements (a). Laser-induced emission spectra of CsPbBr3:10%Yb3+ under focused 975 nm excitation (b). Intensity dependence on excitation power under focused 808 nm and 975 nm for the visible range (c) and infrared range (d).
Figure 5 shows the relationship between the pressure surrounding the CsPbBr3:10%Yb3+ ceramics and the intensity of the broadband luminescence upon excitation of the studied material with focused light provided by a near-infrared laser diode. It can be observed that the LIE is most efficient in the pressure range from 10−5 mbar to 0.1 mbar. For higher pressures, the broadband emission intensity degrades quickly and then its quenching occurs. The nature of this phenomenon can be explained by a heat dissipation model described empirically by the following formula:
where p0 is the critical magnitude of pressure of the surrounding atmosphere above which the LIE intensity begins to decrease and I0 is the initial intensity. Briefly, this model assumes that the temperature of the sample is reduced at atmospheric pressure when the investigated material is irradiated with IR laser diode light, either due to the scattering of the excitation beam by air molecules, or thermal diffusion between the surface of the sample and air molecules surrounding the material, where part of the energy is lost to heat instead of emitting light. Consequently, quenching of the luminescence is observed. Similar behavior has been reported in the literature, not only in the air atmosphere but also in the environment of other inert gases [10]. It is worth mentioning that, contrary to other materials, the emission in ambient pressure was not completely quenched, showcasing the possibility of exploring LIE to produce H2, as explored previously in aluminate perovskites and graphene by our group [39,52,53]; however, stability of this material in solvents is a great concern, since water is known to decompose the material, for example.
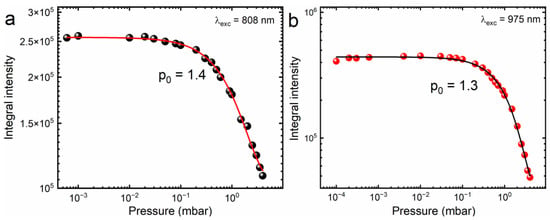
Figure 5.
Integral intensity dependence on ambient pressure for focused 808 nm excitation (a) and 975 nm (b) for CsPbBr3:10%Yb3+ ceramics.
Since the proposed technique for the generation of broadband emission from CsPbBr3:10%Yb3+ ceramics is unusual and pulsed excitation is insufficient to achieve it, conventional luminescence decays could not be recorded. However, in order to obtain deeper insights into the nature of the observed phenomenon, luminescence rise times were recorded upon both excitation lines by measuring the time required to reach the maximum emission intensity from the activation of the excitation source (see Figure 6). It was found that the aforementioned time is relatively long and prolonged as the excitation energy increases from 0.25 s to 0.40 s for 808 nm and 975 nm excitation lines, respectively. This behavior can be attributed to the time required to shift an electron from the valence band to the conduction band. In this case, higher excitation energy accelerates the processes that occur; in other words, the higher the excitation energy, the shorter the mentioned time [11,20].
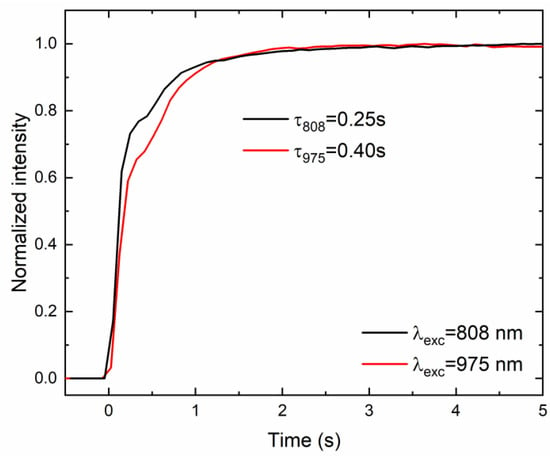
Figure 6.
The rise time of the LIE from CsPbBr3:10%Yb3+ ceramics under 808 nm and 975 nm using fixed 1.0 W excitation power.
In order to precisely characterize the emission observed from CsPbBr3:Yb3+ ceramics presented in Figure 3b,c, its chromatic coordinates were determined based on the photoluminescence spectrum showing the highest emission intensity. The result of the calculation can be found in Figure 7. It was found that the technique proposed for producing broadband emission from the investigated material leads to the acquisition of luminescence exhibiting (x = 0.53908, y = 0.42632), (x = 0.61055, y = 0.38551) coordinates at 808 nm and 975 nm excitations, respectively. As a result, the observed emission is located in the orange-reddish region in the CIE diagram. Further analysis showed that the obtained luminescence reveals a warm color in the range of 2000 K and 1300 K, and has a color rendering index (CRI) equal to 73 and 83, at 808 nm and 975 nm excitations, respectively.
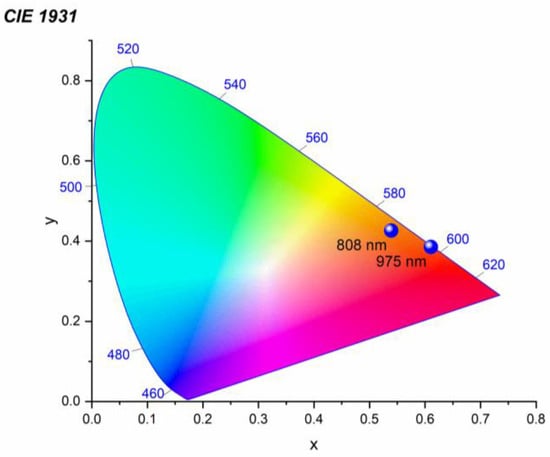
Figure 7.
CIE chromaticity diagram of the LIE produced under focused 808 nm and 975 nm excitations for the CsPbBr3:10%Yb3+ ceramics. The spectra with maximum intensity were chosen to construct the diagram.
2.2. Photoconductivity
Photoconductivity measurements were carried out on a homemade system comprising a quartz cell with electrodes. Gold wires were attached to the surface of the ceramic with silver contacts and connected to the electrodes, and the measurements were made in a vacuum (10−5 mbar). The experimental apparatus is illustrated in Figure 8a,b. Ejection of electrons in LIE was observed in several materials by means of changes in resistance with focused excitation. For CsPbBr3:10%Yb3+ ceramics, a similar behavior was observed, as can be seen in Figure 8c. The experiment was performed with on–off cycles with a 30 s duration, in which the excitation power was increased after every cycle. Two observations can be clearly made: first, when the excitation was turned on, a decrease in resistance occurred, indicating the promotion of electrons to the conduction band. This decrease can also be divided into two different steps, an initial fast one due to the ionization of the sample, and a slower component due to heating [54].
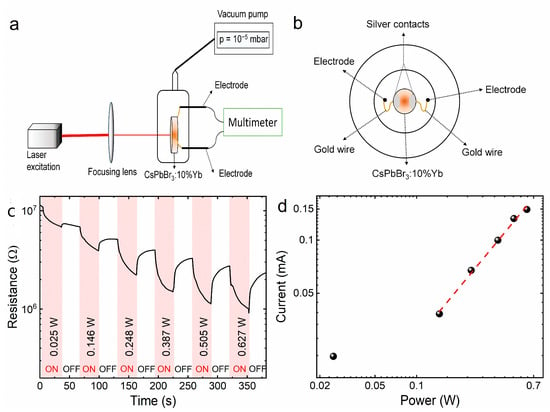
Figure 8.
Schematic representation of the homemade experimental setup for photoconductivity measurements: side view (a) and front view (b). Resistance changes of the CsPbBr3:10%Yb3+ ceramics under on–off cycles with 30 s of focused 975 nm excitation (c). Generated current as a function of excitation power (d).
Furthermore, the magnitude of the decrease is highly dependent on excitation power, indicating that a higher power induces a more pronounced ionization on the material. Similar behavior was observed in LaAlO3:Nd3+ and Y2Si2O7 [11,55], to cite a few. Additionally, a photocurrent was also detected, which presented a non-linear behavior (Figure 8d). Both these facts indicate an ionization process that will be important to the proposed mechanism.
2.3. Energy Transfer Mechanism
Considering the mechanism that may be responsible for the laser-induced emission (LIE) observed from CsPbBr3 ceramics doped with Yb3+ ions, one very important factor must be taken into account, namely the temperature of the sample during exposure to the concentrated infrared beam. Previous literature reports have proven that the temperature of the studied material is relatively low and is usually no higher than 1000 °C [10,56]. This means that the blackbody theory, which explains well the shape of the emission band and the pressure dependence but does not explain the threshold nature of the observed phenomenon, cannot be applied here. In addition, the observation of LIE in a liquid N2 temperature also contradicts the blackbody emission [57]. Additional confirmation of this fact is the lack of change in the position of the luminescence maximum during the measurements of emission intensity as a function of the near-infrared laser diode power density. Moreover, the recent discovery sheds new light on the studied broadband emission. It is worth mentioning that the LIE generated in the presented manner is a surface phenomenon and occurs only from the point of exposure to the laser beam, and not from the entire surface of the material [18]. Furthermore, it was found that the LIE is accompanied by the emission of hot electrons, which is of particular importance for using the studied phenomenon in the photocatalysis process for hydrogen production [53], thereby expanding the possibility for application of this phenomenon. Taking into account all the mechanisms of LIE generation proposed in the literature so far, the most likely seems to be the one associated with the formation of [Yb3+-Yb2+] ion pairs due to the illumination of the sample with focused IR radiation leading to multiphoton absorption and ionization of microcrystalline CsPbBr3:Yb3+ perovskite ceramics. As a result, electron promotion from the valence to the conduction band occurs. The free electrons then combine with Yb3+ to form Yb2+ ions and a charge transfer transition between the mixed valence pairs (IVCT) arises manifested by broadband emission. It is worth noting that this hypothesis was already supported by ab initio calculations [58,59]. The previously mentioned surface defects in the form of lead vacancies may also participate in the proposed mechanism. However, further detailed studies are needed to clarify this.
3. Materials and Methods
CsPb0.9Br3 perovskite powder of micrometric size doped with 10% of Yb3+ ions was prepared by the traditional solid-state reaction method. For this purpose, stoichiometric amounts of starting materials such as CsBr (99.9%, Sigma-Aldrich, Saint Louis, Missouri, USA), PbBr2 (≥98%, Sigma-Aldrich), and YbBr3·xH2O (99.9%, Alfa Aesar, Haverhill, MA, USA) were weighed and ground intensively in an agate mortar in the presence of ethylene alcohol. Such an environment was required to ensure better mixing of the reactants, which was carried out until the alcohol evaporated completely. The powder prepared in this way was placed in a quartz boat and then heated in a tube furnace in an inert atmosphere (nitrogen) under the following regime: 550 °C/3 h, 480 °C/48 h, and 300 °C/24 h. In order to provide better contact between the grains, the resulting orange powder was sintered into ceramics on a press using the low-temperature high-pressure (LTHP) technique at 500 °C under 8 GPa. It is worth emphasizing that the orange color of the sample is a characteristic of the studied material and not the result of doping with lanthanide.
The X-ray diffraction (XRD) pattern was measured with an X’Pert PRO powder diffractometer (Malvern Panalytical, Malvern, UK) equipped with a linear PIXcel detector and using Cu Kα radiation (λ = 1.54056 Å). The chemical composition and general morphology of the samples were checked using a FE-SEM microscope (NanoSEM 230, FEI Nova, Hillsboro, OR, USA). The absorption spectra were measured in the back scattering mode using an Agilent Cary 5000 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). The Stokes emission spectrum was recorded using a Hamamatsu photonic multichannel analyzer PMA-12 equipped with a BT-CCD linear image sensor (Hamamatsu Photonics, Iwata, Japan). A laser diode operating under a 375 nm excitation line was applied as the excitation source. The temperature of the samples during emission measurements was controlled by Linkam THMS600 Heating/Freezing Stage (Linkam Scientific Instruments Ltd., Salfords, United Kingdom). The anti-Stokes emission spectra were measured using focused CW laser diodes (808 and 975 nm) as excitation sources and an AVS-USB2000 Spectrometer (Avantes, Apeldoorn, The Netherlands) as a detector for the visible range and Ocean Optics Nirquest 512-2.5 (Ocean Optics, Inc., Dunedin, FL, USA) as a detector for the near-infrared range. All of the emission spectra were corrected for the detector sensitivity. The rise times of the emission intensity were recorded using a PM101U power meter equipped with a S122C head (Thorlabs, Newton, NJ, USA). Photocurrent measurements were performed using a Keithley 2410 SourceMeter (Keithley Instruments, Cleveland, OH, USA) at room temperature under 975 nm CW laser diode excitation, with 150 V of bias voltage. For electrical measurements, silver electrodes with a gold wire were attached to the ceramic. The sample was illuminated with a focused beam of a 975 nm laser diode between the electrodes. The measurements were performed in 30 s cycles during which the laser diode was turned on and off, varying its power in the range of 0–0.6 W. All measurements were performed at low-pressure conditions using a vacuum cell supplied with a Turbomolecular Drag Pump TMH071 P and a TC 600 electronic drive unit (Pfeiffer, Aßlar, Germany). The focusing lens had a focal length of 40 mm; therefore, the sample was positioned at a distance of 4 cm from it to obtain the smallest excitation area achievable.
4. Conclusions
The conducted research demonstrated that the dry chemistry method, namely the solid-state reaction, can be successfully used to synthesize micrometric inorganic bromide-based perovskites doped with Yb3+ ions in powder form. The obtained material after pressure treatment served as a target illuminated by a concentrated infrared beam, which responded with broadband emission reaching far beyond the visible range. The luminescence exhibited the highest intensity in a vacuum and strong nonlinear power dependence of the NIR excitation laser. It was characterized by a pleasant color with a high CRI. Upon focused laser excitation, a decrease in resistivity, as well as the appearance of current, were observed, indicating both photoionization and heating of the material; the latter was due to the high power density of the excitation achieved with focusing. Interestingly, both the resistivity decrease and the current were strongly dependent on excitation power, suggesting that higher excitation power induces higher degrees of ionization. The mechanism behind the observed phenomenon is probably related to the [Yb2+-Yb3+] ion pairs induced by the laser beam during energy absorption and the following radiative depopulation through intervalence charge transfer transitions. Moreover, Stokes absorption and emission measurements showed the presence of Yb3+ ions and the absence of a trace of Yb2+ in the studied material, indicating that ytterbium(II) ions appear as a result of irradiation of the CsPbBr3:Yb3+ ceramics with a concentrated infrared laser beam. Due to the high CRI and warm light emission, the LIE generated from this material can be used for indoor lighting, and due to the ejected electrons in ambient pressure, it might be even capable of producing H2 in solvents such as alcohols.
Author Contributions
Conceptualization, M.S.; methodology, M.S. and J.M.G.; validation, M.S.; formal analysis, M.S. and J.M.G.; investigation, M.S. and J.M.G.; writing—original draft preparation, M.S. and J.M.G.; writing—review and editing, M.S. and J.M.G.; visualization, M.S. and J.M.G.; supervision, W.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in whole by National Science Center, Poland, grant no. NCN-2021/43/D/ST5/01865. For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Zenodo at DOI: 10.5281/zenodo.8006575.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors upon request.
References
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef]
- Manser, J.S.; Saidaminov, M.I.; Christians, J.A.; Bakr, O.M.; Kamat, P.V. Making and Breaking of Lead Halide Perovskites. Acc. Chem. Res. 2016, 49, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K. Organic-Inorganic Hybrid Lead Halide Perovskites for Optoelectronic and Electronic Applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Rosales, B.A.; Hanrahan, M.P.; Boote, B.W.; Rossini, A.J.; Smith, E.A.; Vela, J. Lead Halide Perovskites: Challenges and Opportunities in Advanced Synthesis and Spectroscopy. ACS Energy Lett. 2017, 2, 906–914. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photonics 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Pan, G.; Chen, X.; Li, D.; Xu, W.; Bai, X.; Song, H. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Downconverter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ding, T.; Liu, X.; Liu, Y.; Wu, K. Quantum-Cutting Luminescent Solar Concentrators Using Ytterbium-Doped Perovskite Nanocrystals. Nano Lett. 2019, 19, 338–341. [Google Scholar] [CrossRef]
- Groenewegen, H. Energy Transfer Processes in Ytterbium Doped Metal Halide Perovskites Probed with Cathodoluminescence. Master Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2021. Available online: https://www.lmpv.nl/wp-content/uploads/2022/01/Heleen-Groenewegen-Master-Thesis-UvA-2021.pdf (accessed on 5 June 2023).
- Wang, J.; Tanner, P.A. Upconversion for White Light Generation by a Single Compound. J. Am. Chem. Soc. 2010, 132, 947–949. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Stefanski, M.; Tomala, R.; Musialek, A.; Strek, W. Laser Induced White Emission Generation from La1−xNdxAlO3 Nanocrystals. Dalt. Trans. 2023, 52, 2073–2079. [Google Scholar] [CrossRef]
- Stefanski, M.; Głuchowski, P.; Strek, W. Laser Induced Emission Spectra of Gallium Nitride Nanoceramics. Ceram. Int. 2020, 46, 29060–29066. [Google Scholar] [CrossRef]
- Strek, W.; Marciniak, L.; Bednarkiewicz, A.; Lukowiak, A.; Wiglusz, R.; Hreniak, D. White Emission of Lithium Ytterbium Tetraphosphate Nanocrystals. Opt. Express 2011, 19, 14083–14092. [Google Scholar] [CrossRef] [PubMed]
- Strek, W.; Tomala, R.; Marciniak, L.; Lukaszewicz, M.; Cichy, B.; Stefanski, M.; Hreniak, D.; Kedziorski, A.; Krosnicki, M.; Seijo, L. Broadband Anti-Stokes White Emission of Sr2CeO4 Nanocrystals Induced by Laser Irradiation. Phys. Chem. Chem. Phys. 2016, 18, 27921–27927. [Google Scholar] [CrossRef] [PubMed]
- Strek, W.; Tomala, R.; Lukaszewicz, M.; Cichy, B.; Gerasymchuk, Y.; Gluchowski, P.; Marciniak, L.; Bednarkiewicz, A.; Hreniak, D. Laser Induced White Lighting of Graphene Foam. Sci. Rep. 2017, 7, 41281. [Google Scholar] [CrossRef] [PubMed]
- Chaika, M.A.; Tomala, R.; Strek, W. Infrared Laser Stimulated Broadband White Emission of Transparent Cr:YAG Ceramics Obtained by Solid State Reaction Sintering. Opt. Mater. 2021, 111, 110673. [Google Scholar] [CrossRef]
- Chaika, M.; Tomala, R.; Strek, W. Surface Related Laser Induced White Emission of Cr:YAG Ceramic. Sci. Rep. 2021, 11, 14063. [Google Scholar] [CrossRef]
- Chaika, M.; Balabanov, S.; Strek, W. Surface Related White Light Emission in Yb2O3 Transparent Nanoceramics. Mater. Res. Bull. 2023, 157, 112011. [Google Scholar] [CrossRef]
- Chaika, M.; Tomala, R.; Oleszko, M.; Strek, W. Surface-Related White Light Emission Phenomenon in Transparent Solids. MRS Adv. 2022, 7, 1095–1098. [Google Scholar] [CrossRef]
- Stefanski, M.; Lukaszewicz, M.; Hreniak, D.; Strek, W. Laser Induced White Emission Generated by Infrared Excitation from Eu3+:Sr2CeO4 Nanocrystals. J. Chem. Phys. 2017, 146, 104705. [Google Scholar] [CrossRef]
- Stefanski, M.; Hreniak, D.; Strek, W. Broadband White Emission from Yb3+ Doped Sr2CeO4 Nanocrystals. Opt. Mater. 2017, 65, 95–98. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Stefanski, M.; Tomala, R.; Strek, W. Bright Warm White Emission of Nd0.9Yb0.1AlO3 Nanocrystals under High Power Density Infrared Excitation. ECS J. Solid State Sci. Technol. 2023, 12, 56002. [Google Scholar] [CrossRef]
- Rojas-León, I.; Christmann, J.; Schwan, S.; Ziese, F.; Sanna, S.; Mollenhauer, D.; Rosemann, N.W.; Dehnen, S. Cluster-Glass for Low-Cost White-Light Emission. Adv. Mater. 2022, 34, 2203351. [Google Scholar] [CrossRef]
- Rosemann, N.W.; Eußner, J.P.; Beyer, A.; Koch, S.W.; Volz, K.; Dehnen, S.; Chatterjee, S. A Highly Efficient Directional Molecular White-Light Emitter Driven by a Continuous-Wave Laser Diode. Science 2016, 352, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, W.; Li, C.; Zhang, H.; Dong, B.; Xu, L.; Xu, S.; Song, H. Broad White Light and Infrared Emission Bands in YVO4:Yb3+,Ln3+ (Ln3+ = Er3+, Tm3+, or Ho3+). Appl. Phys. Express 2012, 5, 092701. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, X.; Wu, J.; Zhang, D.; Zhang, D.; Xu, Z.; Cui, Y.; Qiu, J.; Strek, W. Boosting Continuous-Wave Laser-Driven Nonlinear Photothermal White Light Generation by Nanoscale Porosity. Adv. Mater. 2022, 34, 2106368. [Google Scholar] [CrossRef]
- Strek, W.; Tomala, R. Laser Induced Broadband Emission Spectra of Graphene Foam. Phys. B Condens. Matter 2019, 579, 411840. [Google Scholar] [CrossRef]
- Strek, W.; Oleszko, M.; Wiewiórski, O.; Tomala, R.; Konovalova, A.; Ignatenko, O.; Chaika, M. Laser Induced White Emission of Diamond. J. Chem. Phys. 2022, 157, 134708. [Google Scholar] [CrossRef]
- Debasu, M.L.; Ananias, D.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Rocha, J.; Carlos, L.D. All-in-One Optical Heater-Thermometer Nanoplatform Operative from 300 to 2000 K Based on Er3+ emission and Blackbody Radiation. Adv. Mater. 2013, 25, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, C.I.; Oliveira, A.L.; Pereira, S.C.F.; De Sá, G.F.; Da Luz, L.L.; Alves, S. Bright Thermal (Blackbody) Emission of Visible Light from LnO2 (Ln = Pr, Tb), Photoinduced by a NIR 980 Nm Laser. Dalt. Trans. 2019, 48, 2574–2581. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.F.; Da Silva, R.F.; Santos, E.P.; Maia, L.J.Q.; Moura, A.L. Photon-Avalanche-like Upconversion in NdAl3(BO3)4 nanoparticles Excited at 1064 nm. Appl. Phys. Lett. 2020, 117, 151102. [Google Scholar] [CrossRef]
- Santos, E.P.; Maciel, C.V.T.; da Silva, R.F.; Luz, D.F.; Silva, J.F.; Jacinto, C.; Maia, L.J.Q.; Rego-Filho, F.G.; Moura, A.L. Temperature Triggering a Photon-Avalanche-like Mechanism in NdAl3(BO3)4 Particles under Excitation at 1064 nm. J. Lumin. 2022, 245, 118645. [Google Scholar] [CrossRef]
- Keldysh, L.V. Multiphoton Ionization by a Very Short Pulse. Uspekhi Fiz. Nauk 2017, 60, 1187–1193. [Google Scholar]
- Chaika, M.; Tomala, R.; Strek, W. Laser Induced Broadband Vis and NIR Emission from Yb:YAG Nanopowders. J. Alloys Compd. 2021, 865, 158957. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Stefanski, M.; Tomala, R.; Strek, W. Laser-Induced Broadband White Emission of NdAlO3 Nanocrystals in Alcohols. J. Phys. Chem. C 2023, 127, 10157–10163. [Google Scholar] [CrossRef]
- Bilir, G.; Ozen, G.; Bettinelli, M.; Piccinelli, F.; Cesaria, M.; Di Bartolo, B. Broadband Visible Light Emission From Nominally Undoped and Cr3+ Doped Garnet Nanopowders. IEEE Photonics J. 2014, 6, 2201211. [Google Scholar] [CrossRef]
- Bilir, G.; Bartolo, B. Di Production of Bright, Wideband White Light from Y2O3 Nano-Powders Induced by Laser Diode Emission. Opt. Mater. 2014, 36, 1357–1360. [Google Scholar] [CrossRef]
- Bilir, G.; Ozen, G.; Collins, J.; Cesaria, M.; Bartolo, B. Di Unconventional Production of Bright White Light Emission by Nd-Doped and Nominally Un-Doped Y2O3 Nano-Powders. IEEE Photonics J. 2014, 6, 8200518. [Google Scholar] [CrossRef]
- Strek, W.; Wiewiórski, P.; Mista, W.; Hanulia, T.; Tomala, R. Laser-Induced Hydrogen Generation from Methanol with Graphene Aerogel as the Target. ACS Omega 2021, 6, 3711–3716. [Google Scholar] [CrossRef]
- Strek, W.; Wiewiórski, P.; Miśta, W.; Tomala, R.; Stefanski, M. Laser-Induced Generation of Hydrogen from Methanol Vapor. Int. J. Hydrogen Energy 2022, 47, 27032–27037. [Google Scholar] [CrossRef]
- Strek, W.; Wiewiórski, P.; Miśta, W.; Tomala, R.; Stefanski, M. Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules 2022, 27, 718. [Google Scholar] [CrossRef] [PubMed]
- Hanulia, T.; Oleszko, M.; Tomala, R.; Strek, W. Investigation of Coherence Properties of White Light Emission of Tungsten Lamp Additionally Excited with Laser Radiation. AIP Adv. 2021, 11, 025119. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W.; et al. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.P.; Chen, Y.M.; Zhang, L.M.; Guo, S.Q.; Liu, J.D.; Li, H.; Ye, B.J.; Li, Z.Y.; Zhou, Y.; Zhang, B.B.; et al. Insights into the Local Structure of Dopants, Doping Efficiency, and Luminescence Properties of Lanthanide-Doped CsPbCl3 Perovskite Nanocrystals. J. Mater. Chem. C 2019, 7, 3037–3048. [Google Scholar] [CrossRef]
- Milstein, T.J.; Kroupa, D.M.; Gamelin, D.R. Picosecond Quantum Cutting Generates Photoluminescence Quantum Yields Over 100% in Ytterbium-Doped CsPbCl3 Nanocrystals. Nano Lett. 2018, 18, 3792–3799. [Google Scholar] [CrossRef]
- Li, C.; Zang, Z.; Chen, W.; Hu, Z.; Tang, X.; Hu, W.; Sun, K.; Liu, X.; Chen, W. Highly Pure Green Light Emission of Perovskite CsPbBr3 Quantum Dots and Their Application for Green Light-Emitting Diodes. Opt. Express 2016, 24, 15071. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, T.; He, D.; Guzik, M.; Boulon, G. Influence of Stark Splitting Levels on the Lasing Performance of Yb3+ in Phosphate and Fluorophosphate Glasses. Opt. Express 2015, 23, 1505. [Google Scholar] [CrossRef]
- Lesage, A.; van der Laan, M.; Gomez, L.; Gregorkiewicz, T. Substitutional Doping of Yb3+ in CsPbBrxCl3–x Nanocrystals. J. Phys. Chem. C 2020, 124, 6413–6417. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef]
- Tomala, R.; Hreniak, D.; Strek, W. Influence Concentration of Nd3+ ion on the Laser Induced White Emission of Y2Si2O7:Nd3+. Opt. Mater. 2017, 74, 135–138. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, C.; Li, Z.; Jiang, W.; Wang, Y.; Yin, H.; Wu, L.; Chen, Z.; Zhang, G. High-Efficiency Broadband Anti-Stokes Emission from Yb3+-Doped Bulk Crystals. Opt. Lett. 2016, 41, 2141–2144. [Google Scholar] [CrossRef]
- Strek, W.; Mista, W.; Wiewiorski, P.; Tomala, R. Laser Induced Hydrogen Emission from Ethanol with Dispersed Graphene Particles. Chem. Phys. Lett. 2021, 775, 138649. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Miśta, W.; Wiewiórski, P.; Stefanski, M.; Tomala, R.; Strek, W. Laser Induced Generation of Hydrogen by Using NdAlO3 Nanocrystals as Photocatalysts in Alcohols. Int. J. Hydrogen Energy 2023, 48, 23550–23557. [Google Scholar] [CrossRef]
- Tomala, R.; Strek, W. Emission Properties of Nd3+:Y2Si2O7 Nanocrystals under High Excitation Power Density. Opt. Mater. 2019, 96, 109257. [Google Scholar] [CrossRef]
- Tomala, R.; Hreniak, D.; Strek, W. Laser Induced Broadband White Emission of Y2Si2O7 Nanocrystals. J. Rare Earths 2019, 37, 1196–1199. [Google Scholar] [CrossRef]
- Wu, J.; Xu, C.; Qiu, J.; Liu, X. Conversion of Constant-Wave near-Infrared Laser to Continuum White Light by Yb-Doped Oxides. J. Mater. Chem. C 2018, 6, 7520–7526. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Stefanski, M.; Tomala, R.; Stręk, W. Laser Induced Emission of NdAlO3 Nanocrystals in Vacuum, Air, and Liquid N2. Low Temp. Phys. 2023, 49, 335–337. [Google Scholar] [CrossRef]
- Joos, J.J.; Seijo, L.; Barandiara, Z. Direct Evidence of Intervalence Charge-Transfer States of Eu-Doped Luminescent Materials. J. Phys. Chem. Lett. 2019, 10, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, I.; Joos, J.J.; Barandiarán, Z.; Seijo, L. Mixed-Valence Lanthanide-Activated Phosphors: Invariance of the Intervalence Charge Transfer (IVCT) Absorption Onset across the Series. J. Phys. Chem. C 2020, 124, 2619–2626. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).