The Anti-Aggregative Potential of Resolvin E1 on Human Platelets
Abstract
1. Introduction
2. Results
2.1. Characteristics of Participants
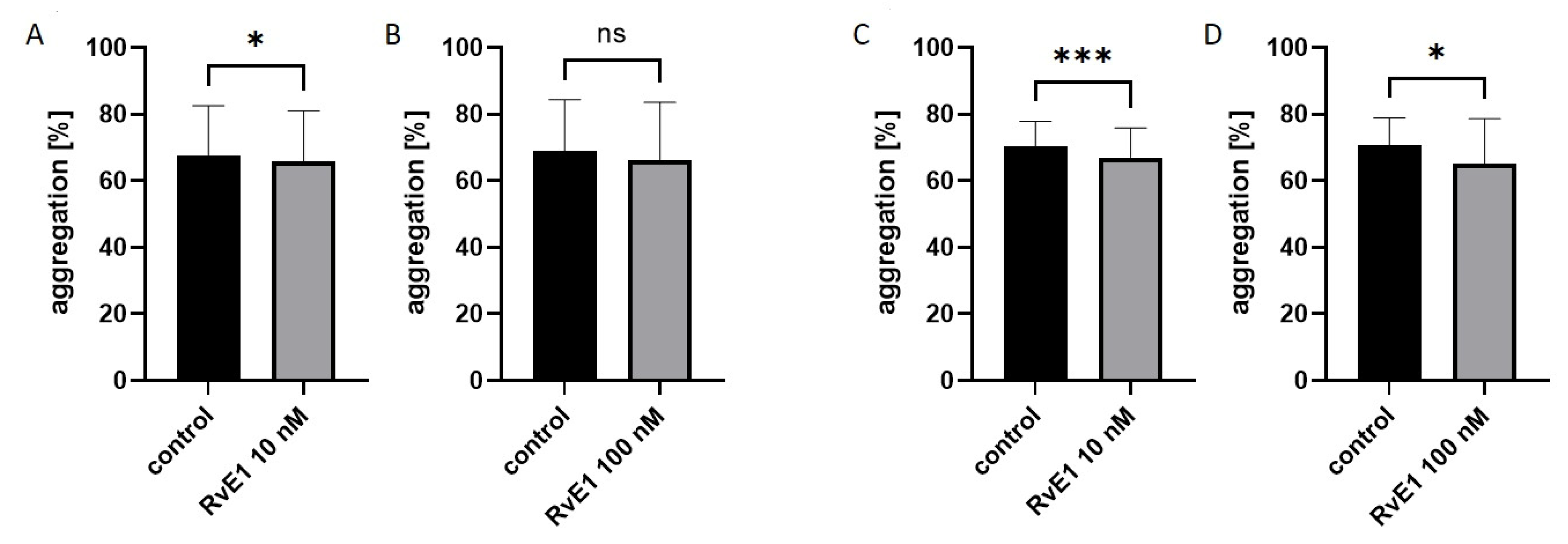
2.2. Effect of Resolvin E1 on Platelet Aggregation in Whole Blood
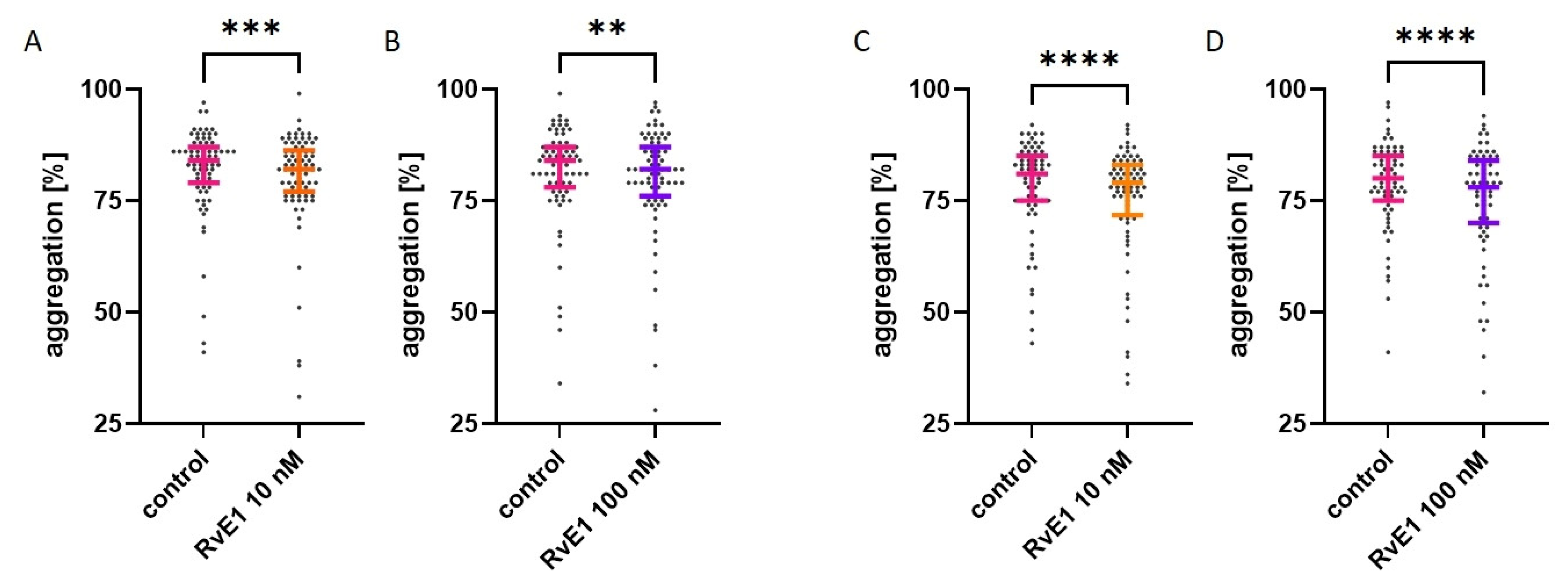
2.3. Effect of Resolvin E1 on Platelet Aggregation in Platelet-Rich Plasma
2.4. Effect of Resolvin E1 on Platelet Aggregation in Isolated Platelets
2.5. Effect of Resolvin E1 on P-Selectin (CD62) Expression in Platelet-Rich Plasma
2.6. Effect of Resolvin E1 on the Fibrinogen-Binding Capacity to Platelets in Platelet-Rich Plasma
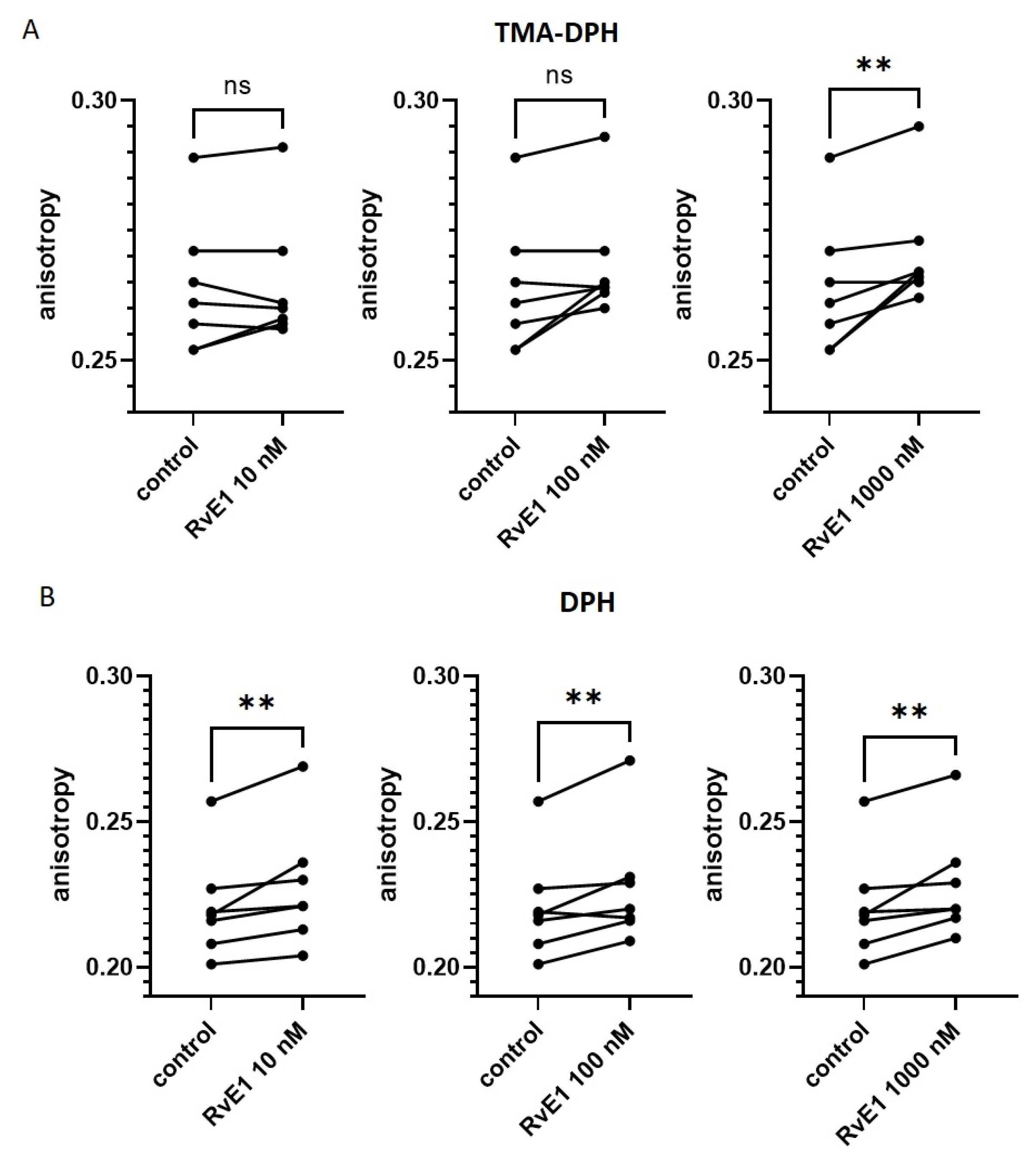
2.7. Effect of Resolvin E1 on Platelet Membrane Fluidity in Isolated Platelets
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Study Population—Recruitment
4.3. Blood Collection and Preparation
4.4. Parameters from Laboratory Results
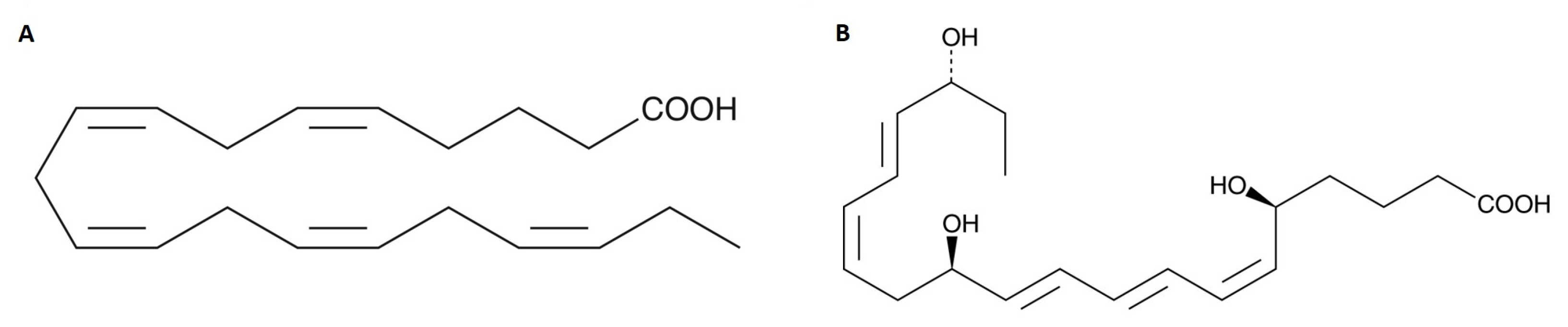
4.5. Chemical Formula of Eicosapentaenoic Acid (EPA) and Its Metabolite (Resolvin E1)
4.6. Platelet Aggregation in Whole Blood
4.7. Platelet Aggregation in Platelet-Rich Plasma
4.8. Platelet Aggregation in Isolated Platelets
4.9. Platelet Activation and Reactivity
4.9.1. P-Selectin Expression
4.9.2. Fibrinogen-Binding
4.10. Platelet Membrane Fluidity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of platelet biomarkers in inflammatory response. Biomark. Res. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Bray, P.F. Platelet hyperreactivity: Predictive and intrinsic properties. Hematol. Oncol. Clin. N. Am. 2007, 21, 633–645. [Google Scholar] [CrossRef]
- Jourdi, G.; Lordkipanidze, M.; Philippe, A.; Bachelot-Loza, C.; Gaussem, P. Current and Novel Antiplatelet Therapies for the Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 13079. [Google Scholar] [CrossRef]
- Schultz-Lebahn, A.; Skipper, M.T.; Hvas, A.M.; Larsen, O.H. Optimized tool for evaluation of platelet function measured by impedance aggregometry. Platelets 2021, 32, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Sorigue, M.; Morales-Indiano, C.; Ruiz-Garcia, L.; Pena, M.; Nieto, J.; Martinez-Iribarren, A.; Feliu, E.; Llopis, M.A.; Orna, E. Adoption of Bio/Data Collagen for the assessment of platelet function with the Multiplate® Analyzer. Thromb. Res. 2018, 161, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Harbi, M.H.; Smith, C.W.; Nicolson, P.L.R.; Watson, S.P.; Thomas, M.R. Novel antiplatelet strategies targeting GPVI, CLEC-2 and tyrosine kinases. Platelets 2021, 32, 29–41. [Google Scholar] [CrossRef]
- Borst, O.; Gawaz, M. Glycoprotein VI-novel target in antiplatelet medication. Pharmacol. Ther. 2021, 217, 107630. [Google Scholar] [CrossRef]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef]
- Contursi, A.; Ballerini, P.; Alberti, S.; Münch, G.; Patrignani, P. The Novel Antiplatelet Agent Revacept in Cardiovascular Medicine: The Promise of Efficacy Without Bleeding. J. Clin. Cardiol. 2022, 3, 12–20. [Google Scholar] [CrossRef]
- Drenjancevic, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids-Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). Int. J. Mol. Sci. 2022, 23, 2104. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Allaire, J.; Mejia, S.B.; Khan, T.A.; Sievenpiper, J.L.; Lamarche, B. Comparing the Effects of Docosahexaenoic and Eicosapentaenoic Acids on Inflammation Markers Using Pairwise and Network Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 128–140. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef]
- Bae, J.H.; Lim, H.; Lim, S. The Potential Cardiometabolic Effects of Long-Chain omega-3 Polyunsaturated Fatty Acids: Recent Updates and Controversies. Adv. Nutr. 2023, 14, 612–628. [Google Scholar] [CrossRef]
- Golanski, J.; Szymanska, P.; Rozalski, M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis-Current Perspectives in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2394. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef] [PubMed]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Stanger, L.; Freedman, C.J.; Standley, M.; Hoang, T.; Adili, R.; Tsai, W.C.; van Hoorebeke, C.; Holman, T.R.; Holinstat, M. DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway. J. Thromb. Haemost. 2021, 19, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Budoff, M.J.; Wani, O.R.; Le, V.; Patel, D.K.; Nelson, A.; Nemiroff, R.L. EPA’s pleiotropic mechanisms of action: A narrative review. Postgrad. Med. 2021, 133, 651–664. [Google Scholar] [CrossRef]
- Welty, F.K.; Schulte, F.; Alfaddagh, A.; Elajami, T.K.; Bistrian, B.R.; Hardt, M. Regression of human coronary artery plaque is associated with a high ratio of (18-hydroxy-eicosapentaenoic acid + resolvin E1) to leukotriene B4. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21448. [Google Scholar] [CrossRef]
- Larson, M.K.; Shearer, G.C.; Ashmore, J.H.; Anderson-Daniels, J.M.; Graslie, E.L.; Tholen, J.T.; Vogelaar, J.L.; Korth, A.J.; Nareddy, V.; Sprehe, M.; et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fatty Acids 2011, 84, 93–98. [Google Scholar] [CrossRef]
- McEwen, B.J.; Morel-Kopp, M.C.; Chen, W.; Tofler, G.H.; Ward, C.M. Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Semin. Thromb. Hemost. 2013, 39, 25–32. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids focusing on eicosapentaenoic acid and docosahexaenoic acid in the prevention of cardiovascular diseases: A review of the state-of-the-art. Expert. Rev. Clin. Pharmacol. 2021, 14, 79–93. [Google Scholar] [CrossRef]
- Dona, M.; Fredman, G.; Schwab, J.M.; Chiang, N.; Arita, M.; Goodarzi, A.; Cheng, G.; von Andrian, U.H.; Serhan, C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008, 112, 848–855. [Google Scholar] [CrossRef]
- Phang, M.; Garg, M.L.; Sinclair, A.J. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-Redefining platelet response to fish oils. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.G.; Cao, J.; Mao, Q.X.; Lu, X.C.; Zhou, X.L.; Fan, L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2013, 226, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Harris, W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids 2002, 37, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Jacob, R.F.; Shrivastava, S.; Sherratt, S.C.R.; Chattopadhyay, A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim. Biophys. Acta 2016, 1858, 3131–3140. [Google Scholar] [CrossRef]
- Vignini, A.; Nanetti, L.; Raffaelli, F.; Sabbatinelli, J.; Salvolini, E.; Quagliarini, V.; Cester, N.; Mazzanti, L. Effect of 1-y oral supplementation with vitaminized olive oil on platelets from healthy postmenopausal women. Nutrition 2017, 42, 92–98. [Google Scholar] [CrossRef]
- Fredman, G.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2005–2013. [Google Scholar] [CrossRef]
- Stupin, M.; Kibel, A.; Stupin, A.; Selthofer-Relatic, K.; Matic, A.; Mihalj, M.; Mihaljevic, Z.; Jukic, I.; Drenjancevic, I. The Physiological Effect of n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) Intake and Exercise on Hemorheology, Microvascular Function, and Physical Performance in Health and Cardiovascular Diseases; Is There an Interaction of Exercise and Dietary n-3 PUFA Intake? Front. Physiol. 2019, 10, 1129. [Google Scholar] [CrossRef]
- Reddoch-Cardenas, K.M.; Sharma, U.; Salgado, C.L.; Cantu, C.; Darlington, D.N.; Pidcoke, H.F.; Bynum, J.A.; Cap, A.P. Use of Specialized Pro-Resolving Mediators to Alleviate Cold Platelet Storage Lesion. Transfusion 2020, 60 (Suppl. S3), S112–S118. [Google Scholar] [CrossRef]
- Lu, L.; Okada, N.; Nakatani, S.; Yoshikawa, K. Eicosapentaenoic acid-induced changes in membrane fluidity and cell adhesion molecules in cultured human keratinocytes. Br. J. Dermatol. 1995, 133, 217–222. [Google Scholar] [CrossRef]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Bhatt, D.L.; Mason, R.P. A biological rationale for the disparate effects of omega-3 fatty acids on cardiovascular disease outcomes. Prostaglandins Leukot. Essent. Fatty Acids 2022, 182, 102450. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Yamasaki, H.; Yazawa, K.; Masumura, S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids 1999, 34, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hiraki, T.; Kawamoto, J.; Kurihara, T.; Nogi, Y.; Kato, C.; Abe, F. Eicosapentaenoic acid plays a role in stabilizing dynamic membrane structure in the deep-sea piezophile Shewanella violacea: A study employing high-pressure time-resolved fluorescence anisotropy measurement. Biochim. Biophys. Acta 2012, 1818, 574–583. [Google Scholar] [CrossRef]
- Bhatt, D.L. My Approach to the patient with CAD and aspirin resistance. Trends Cardiovasc. Med. 2017, 27, 518–519. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: A randomized, controlled trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Bäck, M. Icosapent ethyl in cardiovascular prevention: Resolution of inflammation through the eicosapentaenoic acid-resolvin E1-ChemR23 axis. Pharmacol. Ther. 2023, 247, 108439. [Google Scholar] [CrossRef]
- Đukanović, N.; Obradović, S.; Zdravković, M.; Đurašević, S.; Stojković, M.; Tosti, T.; Jasnić, N.; Đorđević, J.; Todorović, Z. Lipids and Antiplatelet Therapy: Important Considerations and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3180. [Google Scholar] [CrossRef]
- Foster, H.; Wilson, C.; Philippou, H.; Foster, R. Progress toward a Glycoprotein VI Modulator for the Treatment of Thrombosis. J. Med. Chem. 2020, 63, 12213–12242. [Google Scholar] [CrossRef]
- Foster, H.; Wilson, C.; Gauer, J.S.; Xu, R.G.; Howard, M.J.; Manfield, I.W.; Ariens, R.; Naseem, K.; Vidler, L.R.; Philippou, H.; et al. A Comparative Assessment Study of Known Small-molecule GPVI Modulators. ACS Med. Chem. Lett. 2022, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Ntzani, E.E.; Tsapas, A.; Mantzoros, C.S.; Tsiara, S.; Xanthos, T.; Karpettas, N.; Patrikios, I.; Rizos, E.C. Dose-related meta-analysis for Omega-3 fatty acids supplementation on major adverse cardiovascular events. Clin. Nutr. 2022, 41, 923–930. [Google Scholar] [CrossRef]
- Al-Shaer, A.E.; Regan, J.; Buddenbaum, N.; Tharwani, S.; Drawdy, C.; Behee, M.; Sergin, S.; Fenton, J.I.; Maddipati, K.R.; Kane, S.; et al. Enriched Marine Oil Supplement Increases Specific Plasma Specialized Pro-Resolving Mediators in Adults with Obesity. J. Nutr. 2022, 152, 1783–1791. [Google Scholar] [CrossRef]
| Parameter | Healthy Donors (n = 95) |
|---|---|
| PLT [×103/µL] | 253 ± 53 |
| MPV [fL] | 10.3 (9.9–11.0) |
| PCT [%] | 0.25 (0.23–0.29) |
| PDW [fL] | 11.7 (10.9–13.3) |
| RBC [×106/µL] | 4.78 ± 0.48 |
| WBC [×103/µL] | 6.11 ± 1.40 |
| Control | Resolvin E1 10 nM | p Value | Resolvin E1 100 nM | p Value | |
|---|---|---|---|---|---|
| platelet at rest | 59.0 (54.0–68.0) | 60.0 (54.5–69.0) | 0.9999 | 54.0 (51.0–67.5) | 0.1542 |
| platelet after 1 h at RT | 51.5 (43.0–57.8) | 52.0 (41.0–55.8) | 0.6346 | 50.5 (44.3–63.0) | 0.7631 |
| Control | Resolvin E1 10 nM | p Value | Resolvin E1 100 nM | p Value | |
|---|---|---|---|---|---|
| platelets at rest | |||||
| no activation | 1.5 (1.0–2.1) | 1.4 (0.9–2.3) | 0.9999 | 1.3 (1.1–2.3) | 0.9999 |
| collagen activation | 11.9 (8.0–18.7) | 10.5 (5.6–18.2) | 0.0285 | 6.7 (5.1–17.1) | 0.0012 |
| platelets after 1 h at RT | |||||
| no activation | 3.6 (1.5–5.1) | 3.5 (1.8–4.6) | 0.9999 | 3.0 (2.1–5.4) | 0.9999 |
| collagen activation | 16.1 (7.6–26.1) | 12.3 (7.0–24.9) | 0.1324 | 9.5 (7.7–20.0) | 0.4413 |
| Control | Resolvin E1 10 nM | p Value | Resolvin E1 100 nM | p Value | |
|---|---|---|---|---|---|
| platelets at rest | |||||
| no activation | 3.5 ± 1.9 | 3.4 ± 1.8 | 0.8419 | 3.3 ± 1.6 | 0.6961 |
| collagen activation | 45.8 ± 18.5 | 44.1 ± 21.9 | 0.8815 | 43.9 ± 22.9 | 0.8512 |
| platelets after 1 h at RT | |||||
| no activation | 4.0 ± 3.0 | 3.7 ± 2.4 | 0.4364 | 3.3 ± 2.0 | 0.0399 |
| collagen activation | 39.0 ± 18.7 | 33.4 ± 20.2 | 0.1159 | 32.7 ± 20.7 | 0.0714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, P.; Luzak, B.; Miłowska, K.; Golański, J. The Anti-Aggregative Potential of Resolvin E1 on Human Platelets. Molecules 2023, 28, 5323. https://doi.org/10.3390/molecules28145323
Szymańska P, Luzak B, Miłowska K, Golański J. The Anti-Aggregative Potential of Resolvin E1 on Human Platelets. Molecules. 2023; 28(14):5323. https://doi.org/10.3390/molecules28145323
Chicago/Turabian StyleSzymańska, Patrycja, Bogusława Luzak, Katarzyna Miłowska, and Jacek Golański. 2023. "The Anti-Aggregative Potential of Resolvin E1 on Human Platelets" Molecules 28, no. 14: 5323. https://doi.org/10.3390/molecules28145323
APA StyleSzymańska, P., Luzak, B., Miłowska, K., & Golański, J. (2023). The Anti-Aggregative Potential of Resolvin E1 on Human Platelets. Molecules, 28(14), 5323. https://doi.org/10.3390/molecules28145323








