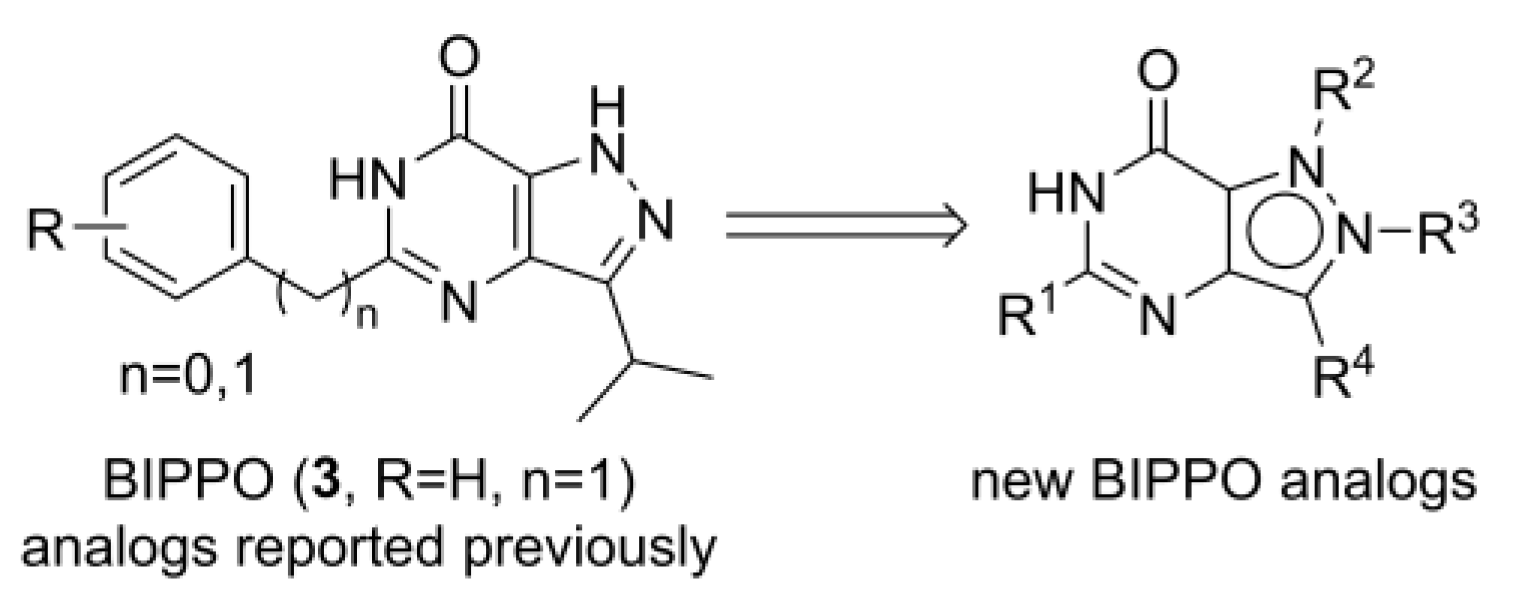
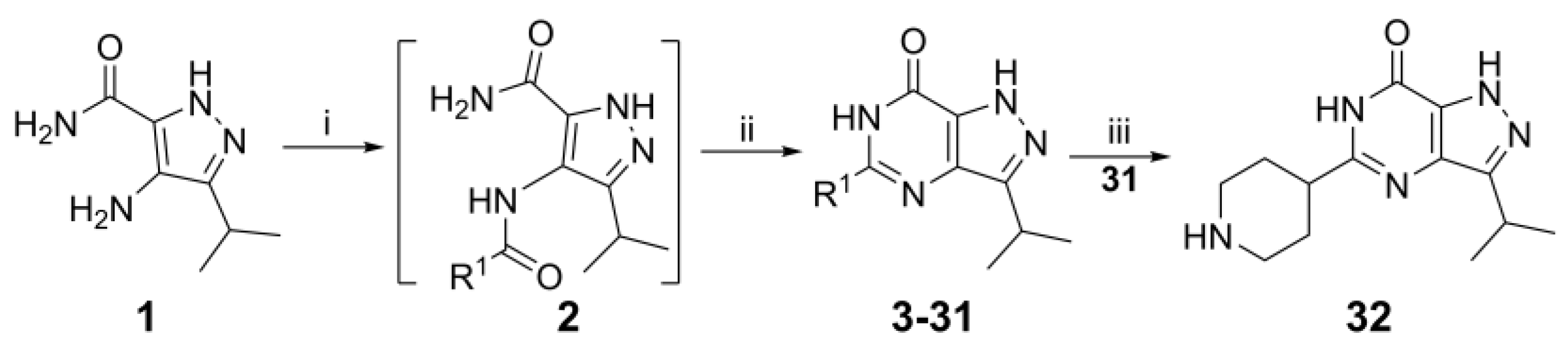
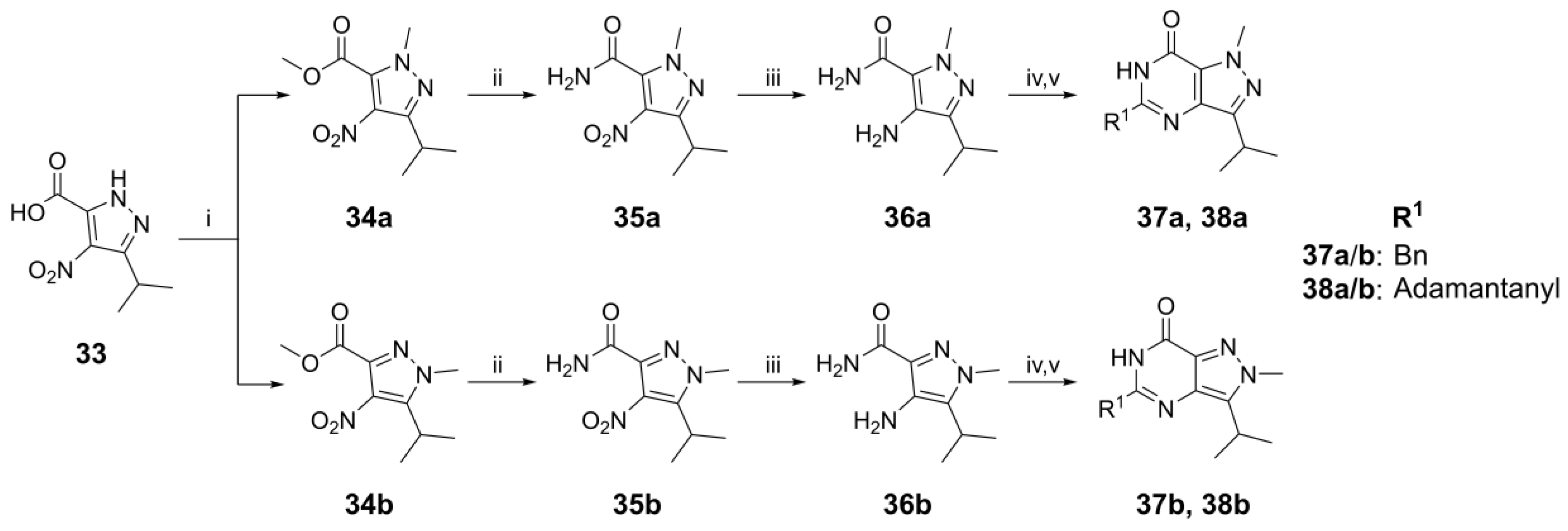
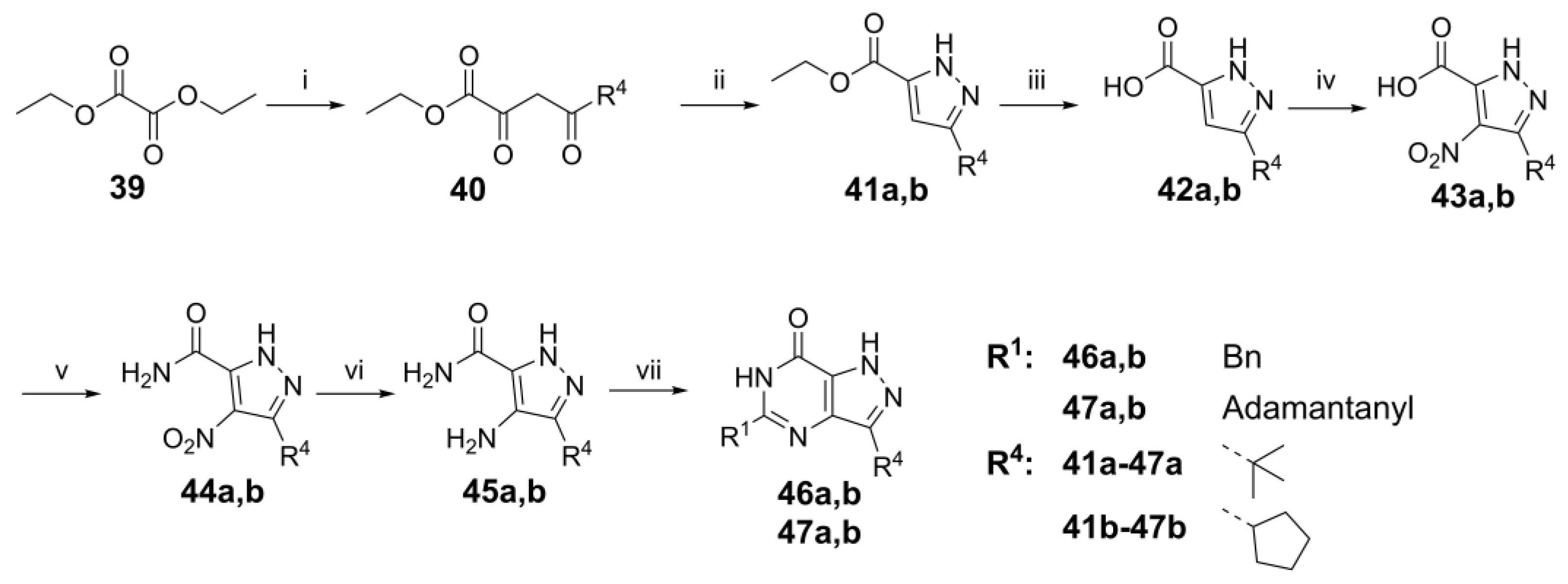
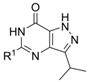
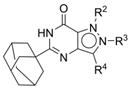
4.1. Chemistry
All starting materials were obtained from commercial suppliers and used without purification. Synthesis of
1,
3,
6,
18,
19,
36b,
37a and
43b was reported previously [
12,
13,
16,
17,
18,
19,
20]. Anhydrous THF, DCM and DMF were obtained by passing through an activated alumina column prior to use. All reactions were carried out under a nitrogen atmosphere unless mentioned otherwise. TLC analyses were performed using Merck F
254 aluminum-backed silica plates and visualized with 254 nm UV light. Flash column chromatography was executed using Biotage Isolera equipment. All HRMS spectra were recorded on a Bruker microTOF mass spectrometer using ESI in positive-ion mode. All NMR spectra were recorded on either a Bruker Avance 300, 500 or 600 spectrometer. The peak multiplicities are defined as follows: s, singlet; d, doublet; t, triplet; q, quartet; p, pentet; dd, doublet of doublets; dt, doublet of triplets; td, triplet of doublets; br, broad; m, multiplet; and app, apparent. The spectra were referenced to the internal solvent peak as follows: CDCl
3 (δ = 7.26 ppm in
1H NMR, δ = 77.16 ppm in
13C NMR) and DMSO-
d6 (δ = 2.50 ppm in
1H NMR, δ = 39.52 ppm in
13C NMR). IUPAC names were adapted from ChemBioDraw Ultra 19.0. Purities were measured with the aid of analytical LC-MS using a Shimadzu LC-20AD liquid chromatography pump system with a Shimadzu SPDM20A diode array detector with the MS detection performed with a Shimadzu LCMS-2010EV mass spectrometer operating in positive ionization mode. The column used was an Xbridge (C18) 5 μm column (100 mm × 4.6 mm). The following solutions were used for the eluents. Acidic mode eluent A: H
2O/HCOOH 999:1, and solvent B: MeCN/HCOOH 999:1. Basic mode eluent A: 0.04% (
w/
v) (NH
4)HCO
3 aqueous solution, and solvent B: 0.04% (NH
4)HCO
3 (
w/
v) in MeCN:H
2O 9:1. The eluent program used is as follows: flow rate: 1.0 mL/min, start with 95% A in a linear gradient to 10% A over 4.5 min, hold 1.5 min at 10% A, in 0.5 min in a linear gradient to 95% A, hold 1.5 min at 95% A, total run time: 8.0 min. Compound purities were calculated as the percentage peak area of the analyzed compound by UV detection at 254 nm. Note: not all
13C signals are visible in spectrum due to tautomerism of non-
N-substituted pyrazoles; 2D NMR (HSQC and HMBC) spectra were measured to assign
13C signals if applicable.
The general method for the synthesis of final compounds: An amine (1.0 eq) and the corresponding acid (1.0 eq), PyBrop (1.1 eq) and TEA (2.0 eq) were combined in DCE and heated using microwave irradiation at 120 °C for 20 min. The reaction mixture was purified using column chromatography to obtain the amide intermediates. Then, the amide intermediate was combined with KOtBu (2.0 eq) in iPrOH and heated using microwave irradiation at 130 °C for 30 min. The reaction mixture was concentrated in vacuo and purified using column chromatography to obtain the final product.
5-Benzyl-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
3 (NPD-0019). Prepared from
1 via the general method to give the title compound as a white solid (87 mg, 68% for two steps).
1H NMR (500 MHz, DMSO-
d6) δ 13.63 (br s, 1H), 12.18 (br s, 1H), 7.37–7.28 (m, 4H), 7.25–7.20 (m, 1H), 3.90 (s, 2H), 3.24 (hept,
J = 6.8 Hz, 1H) and 1.32 (d,
J = 7.0 Hz, 6H).
13C NMR (151 MHz, DMSO-
d6) δ 152.4 (HMBC), 150.3 (HMBC), 137.1, 128.7, 128.4, 126.6, 40.3, 25.8 (HSQC) and 21.8. LC-MS: t
R = 3.66 min, purity: >99%, m/z [M + H]
+: 269; HR-MS: calc. for C
15H
16N
4O [M + H]
+; 269.1397, found 269.1385. Spectral data agree with a previous report [
12].
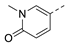
3-Isopropyl-5-(pyridin-4-ylmethyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 4 (NPD-2960). Prepared from 1 via the general method to give the title compound as a white solid (75 mg, 59% for two steps). 1H NMR (500 MHz, DMSO-d6 + 1 drop of D2O) δ 8.48 (d, J = 5.3 Hz, 2H), 7.32 (d, J = 5.6 Hz, 2H), 3.95 (s, 2H), 3.22 (hept, J = 6.9 Hz, 1H) and 1.29 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6 + 1 drop of D2O) δ 150.6 (HMBC), 149.6, 145.9, 124.2, 39.5, 26.1 (HSQC) and 21.8. LC-MS: tR = 2.26 min, purity: 98%, m/z [M + H]+: 270; HR-MS: calc. for C14H15N5O [M + H]+; 270.1349, found 270.1341.
5-(Benzyloxy)-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 5 (NPD-0434). Prepared from 1 via the general method to give the title compound as a white solid (200 mg, 60% for two steps). 1H NMR (600 MHz, DMSO-d6) δ 13.73 (br s, 1H), 12.40 (br s, 1H), 7.33–7.28 (m, 2H), 7.08–7.03 (m, 2H), 6.99–6.95 (m, 1H), 4.95 (s, 2H), 3.26 (app s, 1H) and 1.33 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 157.9, 150.8 (HMBC), 148.9 (HMBC), 142.3 (HMBC), 129.5, 121.2, 114.8, 67.8, 26.2 (HSQC) and 21.8. LC-MS: tR = 3.80 min, purity: >99%, m/z [M + H]+: 285; HR-MS: calc. for C15H16N4O2 [M + H]+; 285.1346, found 285.1341.
3-Isopropyl-5-phenyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
6 (NPD-3200). Prepared from
1 via the general method to give the title compound as a white solid (73 mg, 32% for two steps).
1H NMR (500 MHz, DMSO-
d6 + 1 drop of D
2O) δ 8.04–7.98 (m, 2H), 7.55–7.48 (m, 3H), 3.33 (hept,
J = 7.0 Hz, 1H) and 1.37 (d,
J = 7.0 Hz, 6H).
13C NMR (126 MHz, DMSO-
d6 + 1 drop of D
2O) δ 151.8 (HMBC), 150.4 (HMBC), 143.3 (HMBC), 133.6, 131.3, 129.3, 128.0, 26.6 (HSQC) and 22.4. LC-MS: t
R = 3.78 min, purity: >99%, m/z [M + H]
+: 255; HR-MS: calc. for C
14H
14N
4O [M + Na]
+; 277.1060, found 277.1070. Spectral data agree with a previous report [
12].
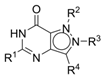
3-Isopropyl-5-(4-(4-methylpiperazin-1-yl)phenyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 7 (NPD-3282). Prepared from 1 via the general method to give the title compound as a white solid (0.11 g, 35% for two steps). 1H NMR (600 MHz, CDCl3) δ 10.78 (br s, 1H), 7.89 (d, J = 7.9 Hz, 2H), 6.87 (app s, 2H), 3.48 (hept, J = 6.6 Hz, 1H), 3.22 (app s, 4H), 2.53 (app s, 4H), 2.33 (s, 3H) and 1.51 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 155.8, 152.9, 152.2 (HMBC), 149.5, 139.0, 128.3, 122.7, 114.8, 54.8, 47.7, 46.2, 26.9 and 22.0. LC-MS: tR = 2.53 min, purity: 98%, m/z [M + H]+: 353; HR-MS: calc. for C19H24N6O [M + H]+; 353.2084, found 353.2078.
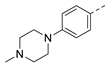
3-Isopropyl-5-(4-(piperidin-1-yl)phenyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 8 (NPD-3283). Prepared from 1 via the general method to give the title compound as a white solid (70 mg, 23% for two steps). 1H NMR (300 MHz, DMSO-d6) δ 13.62 (br s, 1H), 12.03 (br s, 1H), 7.97 (d, J = 8.9 Hz, 2H), 7.00 (d, J = 9.0 Hz, 2H), 3.32–3.26 (m, 5H), 1.60 (app s, 6H) and 1.39 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 152.5, 150.6 (HMBC), 141.8 (HMBC), 128.4, 121.5, 114.1, 48.3, 26.3 (HMBC), 24.9, 24.0 and 21.9. LC-MS: tR = 4.34 min, purity: >99%, m/z [M + H]+: 338; HR-MS: calc. for C19H23N5O [M + H]+; 338.1975, found 338.1964.
3-Isopropyl-5-(thiazol-4-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 9 (NPD-2973). Prepared from 1 via the general method to give the title compound as a white solid (67 mg, 54% for two steps). 1H NMR (500 MHz, DMSO-d6 + 1 drop of D2O) δ 9.26 (s, 1H), 8.50 (s, 1H), 3.33 (app s, 1H) and 1.38 (d, J = 6.8 Hz, 6H). 13C NMR (126 MHz, DMSO-d6 + 1 drop of D2O) δ 155.4 (HSQC), 149.1, 149.0 (HMBC), 144.5, 135.8 (HMBC), 121.9, 25.8 and 22.0. LC-MS: tR = 3.40 min, purity: >99%, m/z [M + H]+: 262; HR-MS: calc. for C11H11N5OS [M + H]+; 262.0757, found 262.0756.
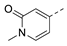
3-Isopropyl-5-(1-methyl-6-oxo-1,6-dihydropyridin-3-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 10 (NPD-2968). Prepared from 1 via the general method to give the title compound as a white solid (46 mg, 34% for two steps). 1H NMR (300 MHz, DMSO-d6) δ 13.73 (br s, 1H), 12.03 (br s, 1H), 8.57 (d, J = 2.6 Hz, 1H), 8.10 (dd, J = 9.6, 2.6 Hz, 1H), 6.51 (d, J = 9.6 Hz, 1H), 3.51 (s, 3H), 3.33 (1H, confirmed by HSQC) and 1.38 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 162.4, 151.5 (HMBC), 142.7 (HMBC), 141.0, 138.9, 119.2, 112.2, 38.1, 26.5 (HSQC) and 22.3. LC-MS: tR = 2.91 min, purity: >99%, m/z [M + H]+: 286; HR-MS: calc. for C14H15N5O2 [M + H]+; 286.1299, found 286.1293.
3-Isopropyl-5-(1-methyl-2-oxo-1,2-dihydropyridin-4-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 11 (NPD-2970). Prepared from 1 via the general method to give the title compound as a white solid (47 mg, 35% for two steps). 1H NMR (500 MHz, DMSO-d6 + 1 drop of D2O) δ 7.78 (d, J = 7.1 Hz, 1H), 7.11 (s, 1H), 6.87 (dd, J = 7.1, 1.7 Hz, 1H), 3.45 (s, 3H), 3.32 (hept, J = 7.0 Hz, 1H) and 1.37 (d, J = 6.9 Hz, 6H). 13C NMR (126 MHz, DMSO-d6 + 1 drop of D2O) δ 162.2, 144.0, 140.2, 117.5, 103.7, 37.2, 26.2 (HSQC) and 22.1. LC-MS: tR = 2.86 min, purity: >99%, m/z [M + H]+: 286; HR-MS: calc. for C14H15N5O2 [M + K]+; 324.0857, found 324.0857.
3-Isopropyl-5-phenethyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
12 (NPD-3281). Prepared from
1 via the general method to give the title compound as a white solid (96 mg, 71% for two steps).
1H NMR (600 MHz, DMSO-
d6) δ 13.52 (br s, 1H), 12.11 (br s, 1H), 7.29–7.25 (m, 4H), 7.20–7.16 (m, 1H), 3.26 (app s, 1H), 3.07–2.97 (m, 2H), 2.88 (app s, 2H) and 1.34 (d,
J = 6.9 Hz, 6H).
13C NMR (151 MHz, DMSO-
d6) δ 154.3 (HMBC), 153.5 (HMBC), 150.8 (HMBC), 141.3, 137.5 (HMBC), 128.9, 128.7, 126.5, 36.2, 33.2, 26.6 (HSQC) and 22.3. LC-MS: t
R = 3.87 min, purity: >99%, m/z [M + H]
+: 283; HR-MS: calc. for C
16H
18N
4O [M + H]
+; 283.1553, found 283.1545. Spectral data agree with a previous report [
12].
(racemic)-3-Isopropyl-5-(1-phenylethyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 13 (NPD-2969). Prepared from 1 via the general method to yield the title compound as a white solid (95 mg, 71% for two steps). 1H NMR (500 MHz, CDCl3) δ 10.48 (br s, 1H), 7.34 (d, J = 7.2 Hz, 2H), 7.24 (app t, J = 7.5 Hz, 2H), 7.18 (t, J = 7.2 Hz, 1H), 4.20 (q, J = 7.0 Hz, 1H), 3.51 (hept, J = 7.0 Hz, 1H), 1.71 (d, J = 7.0 Hz, 3H) and 1.52 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 155.9, 155.3, 151.3, 141.5, 137.6, 129.0, 127.7, 127.6, 127.2, 45.0, 26.8, 21.9, 21.9 and 19.7. Note: one extra carbon signal observed due to hindered rotation of the isopropyl group. LC-MS: tR = 4.41 min, purity: >99%, m/z [M + H]+: 283; HR-MS: calc. for C16H18N4O [M + H]+; 283.1553, found 283.1542.
3-Isopropyl-5-(2-phenylpropan-2-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 14 (NPD-3743). Prepared from 1 via the general method to yield the title compound as a white solid (58 mg, 22% for two steps). 1H NMR (500 MHz, DMSO-d6 + 1 drop of D2O) δ 7.34–7.28 (m, 2H), 7.25–7.17 (m, 3H), 3.31 (hept, J = 7.4 Hz, 1H), 1.68 (s, 6H) and 1.40 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 158.0 (HMBC), 150.7 (HMBC), 146.3, 128.3, 126.4, 126.2, 44.7, 27.8 (HSQC), 26.1 and 21.9. LC-MS: tR = 4.57 min, purity: >99%, m/z [M + H]+: 297; HR-MS: calc. for C17H20N4O [M + H]+; 297.1710, found 297.1706.
(racemic)-3-Isopropyl-5-(methoxy(phenyl)methyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 15 (NPD-3744). Prepared from 1 via the general method to yield the title compound as a white solid (47 mg, 18% for two steps). 1H NMR (500 MHz, DMSO-d6) δ 13.69 (br s, 1H), 12.15 (br s, 1H), 7.53–7.49 (m, 2H), 7.38–7.33 (m, 2H), 7.32–7.27 (m, 1H), 5.25 (s, 1H), 3.35 (s, 3H), 3.29–3.18 (m, 1H), 1.32 (d, J = 2.4 Hz, 3H) and 1.31 (d, J = 2.4 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 152.6 (HMBC), 150.8 (HMBC), 141.9 (HMBC), 138.4, 128.4, 128.3, 127.0, 82.5, 56.9, 26.0 (HSQC), 21.9 and 21.8. Note: one extra carbon signal was observed due to hindered rotation of the isopropyl group. LC-MS: tR = 3.86 min, purity: >99%, m/z [M + H]+: 299; HR-MS: calc. for C17H20N4O [M + H]+; 299.1503, found 299.1499.
3-Isopropyl-5-(1-phenylcyclopropyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 16 (NPD-3746). Prepared from 1 via the general method to yield the title compound as a white solid (74 mg, 28% for two steps). 1H NMR (500 MHz, CDCl3) δ 8.58 (br s, 1H), 7.48–7.36 (m, 5H), 3.39 (hept, J = 7.0 Hz, 1H), 1.83 (app q, J = 3.9 Hz, 2H), 1.44 (d, J = 7.0 Hz, 6H) and 1.37 (app q, J = 3.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 155.5, 154.1, 151.7, 138.6, 138.1, 131.0, 129.8, 128.9, 126.2, 29.4, 26.8, 21.8 and 18.0. LC-MS: tR = 4.21 min, purity: >99%, m/z [M + H]+: 295; HR-MS: calc. for C17H20N4O [M + H]+; 295.1553, found 295.1553.
3-Isopropyl-5-(1-phenylcyclobutyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 17 (NPD-3745). Prepared from 1 via the general method to yield the title compound as a white solid (77 mg, 28% for two steps). 1H NMR (500 MHz, CDCl3) δ 8.65 (s, 1H), 7.40–7.22 (m, 5H), 3.49 (hept, J = 7.0 Hz, 1H), 3.06–2.97 (m, 2H), 2.71–2.62 (m, 2H), 2.22–2.09 (m, 1H), 2.03–1.91 (m, 1H) and 1.52 (d, J = 6.9 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 156.5, 154.9, 152.0, 144.1, 137.5, 129.3, 127.5, 126.6, 126.3, 51.3, 32.7, 27.0, 21.9 and 16.5. LC-MS: tR = 4.67 min, purity: >99%, m/z [M + H]+: 309; HR-MS: calc. for C17H20N4O [M + H]+; 309.1710, found 309.1709.
3-Isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
18 (NPD-3378). Prepared from
1 via the general method to give the title compound as a white solid (75 mg, 32% for two steps).
1H NMR (600 MHz, DMSO-
d6) δ 13.71 (br s, 1H), 12.08 (br s, 1H), 7.80 (s, 1H), 3.26 (hept,
J = 6.9 Hz, 1H) and 1.34 (d,
J = 7.0 Hz, 6H).
13C NMR (151 MHz, DMSO-
d6) δ 153.4 (HMBC), 150.4 (HMBC), 141.8, 136.3 (HMBC), 25.9 (HSQC) and 21.8. LC-MS: t
R = 2.36 min, purity: >99%, m/z [M + H]
+: 179; HR-MS: calc. for C
8H
10N
4O [M + H]
+; 179.0927, found 179.0935. Spectral data are in agreement with a previous report [
17,
18].
3-Isopropyl-5-methyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
19 (NPD-3380). Prepared from
1 via the general method to give the title compound as a white solid (0.16 g, 90% for two steps).
1H NMR (300 MHz, DMSO-
d6) δ 13.52 (br s, 1H), 11.99 (br s, 1H), 3.23 (hept,
J = 6.2 Hz, 1H), 2.31 (s, 3H) and 1.32 (d,
J = 7.0 Hz, 6H).
13C NMR (126 MHz, DMSO-
d6) δ 151.1, 141.1 (HMBC), 26.4 (HSQC), 22.4 and 21.5. LC-MS: t
R = 2.46 min, purity: >99%, m/z [M + H]
+: 193; HR-MS: calc. for C
9H
12N
4O [M + H]
+; 193.1084, found 193.1090. Spectral data agree with a previous report [
19].
3,5-Diisopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 20 (NPD-3379). Prepared from 1 via the general method to give the title compound as a white solid (0.17 g, 87% for two steps). 1H NMR (300 MHz, DMSO-d6) δ 13.54 (br s, 1H), 11.88 (br s, 1H), 3.23 (hept, J = 6.8 Hz, 1H), 2.87 (hept, J = 7.8 Hz, 1H), 1.34 (d, J = 6.9 Hz, 6H) and 1.22 (d, J = 6.8 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 158.1 (HMBC), 150.7 (HMBC), 32.8, 26.3 (HSQC), 21.8 and 20.7. LC-MS: tR = 3.42 min, purity: >99%, m/z [M + H]+: 221; HR-MS: calc. for C11H16N4O [M + H]+; 221.1397, found 221.1405.
5-Butyl-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 21 (NPD-3645). Prepared from 1 via the general method to give the title compound as a white solid (88 mg, 42% for two steps). 1H NMR (500 MHz, DMSO-d6) δ 13.52 (br s, 1H), 12.02 (br s, 1H), 3.29–3.17 (m, 1H), 2.61–2.53 (m, 2H), 1.65 (app p, J = 7.6 Hz, 2H), 1.37–1.28 (m, 8H) and 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 154.5 (HMBC), 150.6 (HMBC), 141.4 (HMBC), 34.2, 29.7, 26.5, 22.3, 22.1 and 14.2. LC-MS: tR = 3.58 min, purity: >99%, m/z [M + H]+: 235; HR-MS: calc. for C12H18N4O [M + H]+; 235.1553, found 235.1562.
5-Cyclopentyl-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 22 (NPD-3373). Prepared from 1 via the general method to give the title compound as a white solid (0.13 g, 60% for two steps). 1H NMR (600 MHz, DMSO-d6) δ 13.51 (br s, 1H), 11.97 (br s, 1H), 3.28–3.19 (m, 1H), 3.07–3.00 (m, 1H), 2.00–1.91 (m, 2H), 1.89–1.81 (m, 2H), 1.77–1.69 (m, 2H), 1.64–1.55 (m, 2H) and 1.34 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 157.4 (HMBC), 150.6 (HMBC), 141.9 (HMBC), 43.3, 31.0, 26.7, 25.2 and 21.8. LC-MS: tR = 3.90 min, purity: >99%, m/z [M + H]+: 247; HR-MS: calc. for C13H18N4O [M + H]+; 247.1553, found 247.1562.
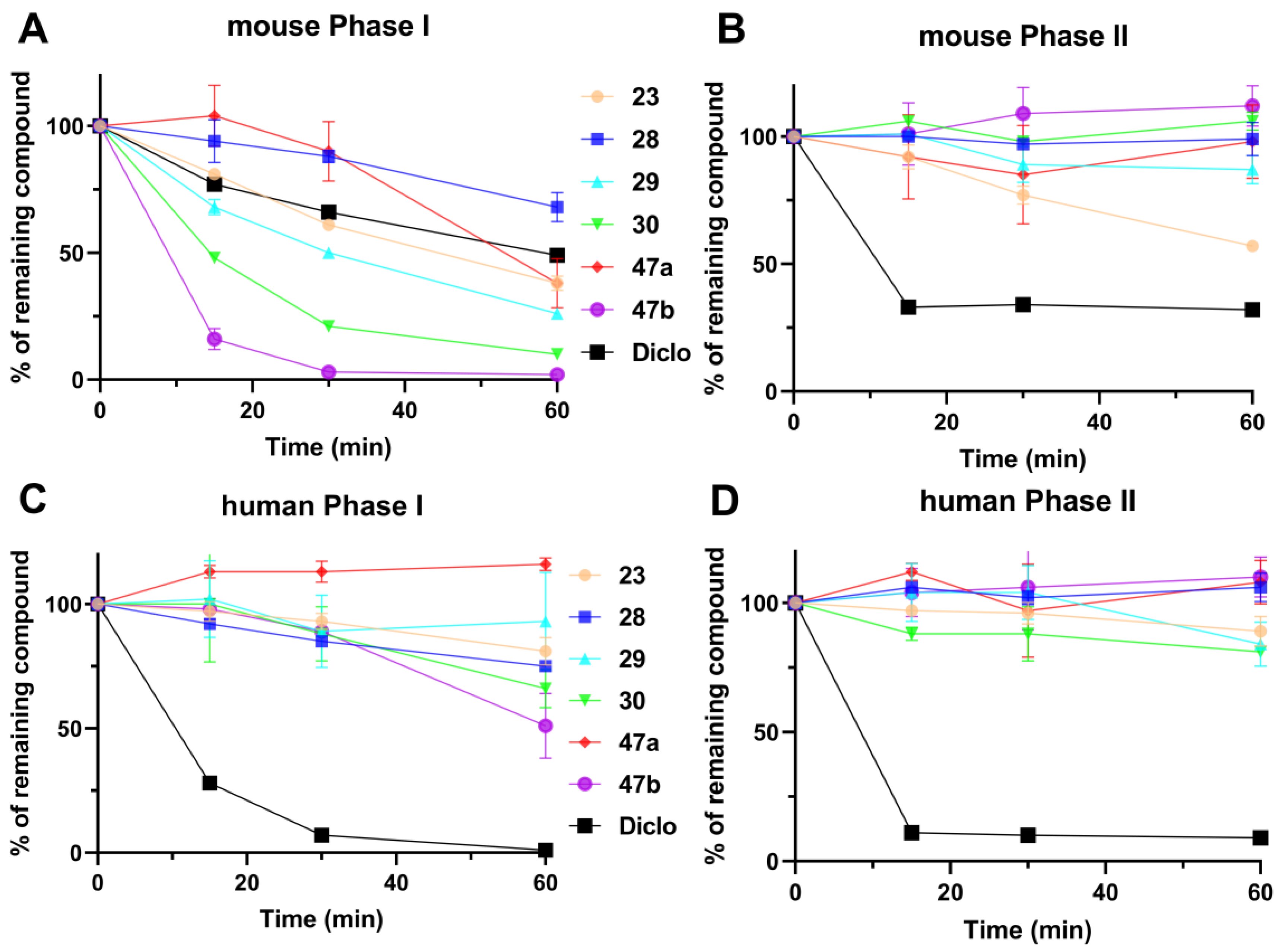
5-Cyclohexyl-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 23 (NPD-3518). Prepared from 1 via the general method to give the title compound as a white solid (98 mg, 42% for two steps). 1H NMR (300 MHz, CD3OD) δ 3.48–3.34 (m, 1H), 2.60 (tt, J = 12.0, 3.5 Hz, 1H), 2.03–1.83 (m, 4H), 1.81–1.57 (m, 3H) and 1.52–1.28 (m, 9H). 13C NMR (151 MHz, CD3OD) δ 180.4 (HMBC), 159.3 (HMBC), 153.1 (HMBC), 144.1 (HMBC), 44.7, 32.1, 27.8 (HSQC), 27.1, 26.9 and 22.2. LC-MS: tR = 4.18 min, purity: >99%, m/z [M + H]+: 261; HR-MS: calc. for C14H20N4O [M + H]+; 261.1710, found 261.1698.
3-Isopropyl-5-(tetrahydro-2H-pyran-4-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 24 (NPD-3542). Prepared from 1 via the general method to give the title compound as a white solid (95 mg, 41% for two steps). 1H NMR (300 MHz, DMSO-d6) δ 13.64 (br s, 1H), 11.70 (br s, 1H), 4.01–3.88 (m, 2H), 3.47–3.17 (m, 3H), 2.92–2.74 (m, 1H), 1.85–1.73 (m, 4H) and 1.34 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 156.1 (HMBC), 150.4 (HMBC), 139.8 (HMBC), 66.6, 39.2, 30.3, 26.1 (HSQC) and 21.8. LC-MS: tR = 3.04 min, purity: >99%, m/z [M + H]+: 263; HR-MS: calc. for C13H18N4O2 [M + H]+; 263.1503, found 263.1497.
3-Isopropyl-5-(1-methylpiperidin-4-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one diformate, 25 (NPD-3374). Prepared from 1 to give the title compound as a white solid (39 mg, 9% for two steps). 1H NMR (600 MHz, DMSO-d6 + 1 drop of D2O) δ 8.31 (s, 2H), 3.36 (app d, J = 12.2 Hz, 2H), 3.24 (hept, J = 7.2 Hz, 1H), 2.88 (app t, J = 11.1 Hz, 2H), 2.80 (app t, J = 11.1 Hz, 1H), 2.66 (s, 3H), 2.06 (app d, J = 12.4 Hz, 2H), 1.93 (app q, J = 11.3 Hz, 2H) and 1.30 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, DMSO-d6 + 1 drop of D2O) δ 167.5, 155.4, 151.4 (HMBC), 53.7, 43.7, 37.9, 27.9, 26.4 and 22.4. LC-MS: tR = 2.14 min, purity: >99%, m/z [M + H]+: 276; HR-MS: calc. for C14H21N5O [M + H]+; 276.1819, found 276.1822.
cis-5-(4-Hydroxycyclohexyl)-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
26 (NPD-3543) and
trans-5-(4-Hydroxycyclohexyl)-3-isopropyl-1,6-dihydro-7H-pyrazolo [4,3-d]pyrimidin-7-one,
27 (NPD-3544). Prepared from
1 with 4-oxocyclohexanecarboxylic acid via the general method to give the title compound
26 as a white solid (79 mg, 24% for two steps) and
27 as a white solid (65 mg, 20% for two steps).
26:
1H NMR (500 MHz, DMSO-
d6) δ 13.53 (br s, 1H), 11.88 (br s, 1H), 4.36 (s, 1H), 3.83 (s, 1H), 3.30–3.20 (m, 1H), 2.62–2.54 (m, 1H), 2.00–1.88 (m, 2H), 1.78–1.67 (m, 2H), 1.65–1.55 (m, 2H), 1.52–1.43 (m, 2H) and 1.34 (d,
J = 6.9 Hz, 6H).
13C NMR (126 MHz, DMSO-
d6) δ 157.4 (HMBC), 150.3 (HMBC), 140.8 (HMBC), 63.8, 41.0, 32.0, 26.1 (HSQC), 24.8 and 21.9. LC-MS: t
R = 2.85 min, purity: >99%, m/z [M + H]
+: 277; HR-MS: calc. for C
14H
20N
4O
2 [M + H]
+; 277.1659, found 277.1659.
27:
1H NMR (500 MHz, DMSO-
d6) δ 13.54 (br s, 1H), 11.90 (br s, 1H), 4.59 (d,
J = 4.4 Hz, 1H), 3.41 (tt,
J = 8.5, 5.2 Hz, 1H), 3.23 (hept,
J = 7.6, 7.1 Hz, 1H), 2.49–2.45 (m, 1H), 1.98–1.81 (m, 4H), 1.66–1.52 (m, 2H), 1.32 (d,
J = 7.0 Hz, 6H) and 1.27–1.14 (m, 2H).
13C NMR (151 MHz, DMSO-
d6) δ 157.1 (HMBC), 150.2 (HMBC), 141.2 (HMBC), 68.3, 41.6, 35.0, 29.0, 26.2 (HSQC) and 21.9. LC-MS: t
R = 2.76 min, purity: >99%, m/z [M + H]
+: 277; HR-MS: calc. for C
14H
20N
4O
2 [M + H]
+; 277.1665, found 277.1653. NMR proof of diastereomers can be found in the
Supporting information (Figure S76).
5-(4,4-Difluorocyclohexyl)-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 28 (NPD-3545). Prepared from 1 via the general method to give the title compound as a white solid (84 mg, 32% for two steps). 1H NMR (500 MHz, DMSO-d6) δ 13.63 (br s, 1H), 12.01 (br s, 1H), 3.24 (hept, J = 7.0 Hz, 1H), 2.79–2.70 (m, 1H), 2.19–2.09 (m, 2H), 2.05–1.96 (m, 2H), 1.95–1.75 (m, 4H) and 1.33 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 155.6, 150.2 (HMBC), 125.7, 123.8, 121.9, 39.0 (HSQC), 32.4 (t, J = 24.3 Hz), 26.8, 26.7, 26.0 (HSQC) and 21.8. LC-MS: tR = 3.95 min, purity: >99%, m/z [M + H]+: 297; HR-MS: calc. for C14H18F2N4O [M + H]+; 297.1521, found 297.1523.
5-(Bicyclo[2.2.2]octan-1-yl)-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 29 (NPD-3546). Prepared from 1 via the general method to give the title compound as a white solid (78 mg, 31% for two steps). 1H NMR (500 MHz, DMSO-d6) δ 3.22 (hept, J = 7.0 Hz, 1H), 1.88–1.81 (m, 6H), 1.67–1.62 (m, 1H), 1.62–1.55 (m, 6H) and 1.34 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 159.4, 150.1 (HMBC), 136.3 (HMBC), 37.0, 28.9, 26.4, 25.8, 24.0 and 22.2. LC-MS: tR = 4.75 min, purity: >99%, m/z [M + H]+: 287; HR-MS: calc. for C16H22N4O [M + H]+; 287.1866, found 287.1873.
5-(Adamantan-1-yl)-3-isopropyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 30 (NPD-3547). Prepared from 1 via the general method to give the title compound as a white solid (85 mg, 31% for two steps). 1H NMR (500 MHz, DMSO-d6) δ 13.56 (br s, 1H), 11.55 (br s, 1H), 3.23 (hept, J = 6.8 Hz, 1H), 2.03 (app s, 3H), 1.99 (app s, 6H), 1.75–1.66 (m, 6H) and 1.35 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 159.7 (HMBC), 150.9 (HMBC), 39.5, 38.9, 36.3, 28.2, 26.5 (HSQC) and 22.3. LC-MS: tR = 5.14 min, purity: >99%, m/z [M + H]+: 313; HR-MS: calc. for C18H24N4O [M + H]+; 313.2023, found 313.2030.
3-Isopropyl-5-(piperidin-4-yl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 32 (NPD-3593). Prepared from 1 (0.20 g, 1.2 mmol), N-BOC-piperidine-4-carboxylic acid (0.27 g, 1.2 mmol) and PyBrop (0.61 g, 1.3 mmol) via the general method to give the intermediate 31 as a white solid (119 mg, 28% for two steps). A solution of 1.0 M HCl was added dropwise to a 1,4-dioxane (50 mL) solution of 31 and stirred for 16 h. The reaction mixture was concentrated in vacuo and purified with a reverse-phase column to give the title compound as a white solid (46 mg, 53%). 1H NMR (500 MHz, CD3OD) δ 3.54 (dt, J = 12.9, 3.7 Hz, 2H), 3.40–3.33 (m, 1H), 3.14 (td, J = 12.6, 3.2 Hz, 2H), 2.96 (tt, J = 9.9, 4.1 Hz, 1H), 2.26–2.20 (m, 2H), 2.17–2.08 (m, 2H) and 1.43 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, CD3OD) δ 155.7 (HMBC), 154.1 (HMBC), 44.5, 38.9, 28.0, 27.7 (HSQC) and 22.2. LC-MS: tR = 2.04 min, purity: >99%, m/z [M + H]+: 262; HR-MS: calc. for C13H19N5O [M + H]+; 262.1662, found 262.1668.
Methyl 3-isopropyl-1-methyl-4-nitro-1H-pyrazole-5-carboxylate,
34a and
methyl 5-isopropyl-1-methyl-4-nitro-1H-pyrazole-3-carboxylate,
34b. To a mixture of K
2CO
3 (13.9 g, 100 mmol), 33 (5.00 g, 25.1 mmol) in DMF (50 mL) was added MeI (3.45 mL, 55.2 mmol); the reaction mixture was heated at 60 °C for 1 h. After that, this mixture was concentrated in vacuo, dissolved in water (50 mL), extracted with EtOAc (3 × 50 mL) and washed with brine. The combined organic layers were concentrated in vacuo, purified using flash column chromatography on silica gel eluting with EtOAc in cyclohexane (10% to 50%) to give the title compounds
34a (1.33 g, 23%) and
34b (1.46 g, 26%) as off-white solids.
34a:
1H NMR (600 MHz, CDCl
3) δ 3.98 (s, 3H), 3.96 (s, 3H), 3.43 (hept,
J = 7.1 Hz, 1H) and 1.30 (d,
J = 6.9 Hz, 6H).
13C NMR (151 MHz, CDCl
3) δ 159.3, 153.3, 132.1, 131.9 (HMBC), 53.7, 39.2, 26.5 and 21.5. LC-MS: t
R = 4.46 min, purity: >99%, m/z [M + H]
+: 228.
34b:
1H NMR (600 MHz, CDCl
3) δ 3.94 (s, 3H), 3.94 (s, 3H), 3.48 (hept,
J = 7.2 Hz, 1H) and 1.40 (d,
J = 7.2 Hz, 6H).
13C NMR (151 MHz, CDCl
3) δ 160.8, 146.3, 137.4, 132.1 (HMBC), 53.1, 39.1, 25.8 and 19.4. LC-MS: t
R = 3.96 min, purity: >99%, m/z [M + H]
+: 228. Regiochemistry confirmed with 1D NOESY spectra (
Supporting information Figure S95).
3-Isopropyl-1-methyl-4-nitro-1H-pyrazole-5-carboxamide, 36a. Ester 34a (1.33 g, 5.84 mmol) was dissolved in 7 M NH3 in MeOH (4.17 mL, 29.2 mmol) and stirred at RT for 16 h. The reaction mixture was then concentrated in vacuo and added to the suspension of 10% palladium on carbon (0.200 g, 1.88 mmol) in EtOH (50 mL) and heated at 75 °C with H2 gas insert for 16 h. After that, the reaction mixture was filtered through celite, concentrated in vacuo and purified using flash column chromatography on silica gel with a gradient elution of MeOH in DCM (0% to 10%) to give the title compound as a pink solid (0.98 g, 92% for two steps). 1H NMR (300 MHz, DMSO-d6) δ 7.51 (br s, 2H), 4.09 (s, 2H), 3.86 (s, 3H), 2.97 (hept, J = 7.0 Hz, 1H) and 1.16 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 162.0, 146.1, 128.0, 124.3, 39.0, 24.3 and 21.8. LC-MS: tR = 2.14 min, purity: 97%, m/z [M + H]+: 183.
4-Amino-5-isopropyl-1-methyl-1H-pyrazole-3-carboxamide,
36b. Ester
34b (1.46 g, 6.88 mmol) was dissolved in 7 M NH
3 in MeOH (4.58 mL, 32.1 mmol) and stirred at RT for 16 h. The reaction mixture was then concentrated in vacuo and added to the suspension of 10% palladium on carbon (0.250 g, 2.35 mmol) in EtOH (50 mL) and heated at 75 °C with H
2 gas insert for 16 h. After that, the reaction mixture was filtered through celite, concentrated in vacuo and purified using flash column chromatography on silica gel with a gradient elution of MeOH in DCM (0% to 10%) to give the title compound as a pink solid (1.20 g, 96% for two steps).
1H NMR (300 MHz, DMSO-
d6) δ 7.07 (s, 1H), 6.94 (s, 1H), 4.42 (s, 2H), 3.71 (s, 3H), 3.06 (hept,
J = 7.0 Hz, 1H) and 1.24 (d,
J = 7.1 Hz, 6H).
13C NMR (151 MHz, DMSO-
d6) δ 166.0, 132.5, 130.4, 129.8, 37.6, 24.3 and 20.0. LC-MS: t
R = 1.78 min, purity: >99%, m/z [M + H]
+: 183. Spectral data are in agreement with a previous report [
16].
5-Benzyl-3-isopropyl-1-methyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
37a (NPD-3647). Prepared from
36a via the general method to yield the title compound
37a as a white solid (0.16 g, 70% for two steps).
1H NMR (500 MHz, CD
3OD) δ 7.34–7.28 (m, 4H), 7.26–7.21 (m, 1H), 4.16 (s, 3H), 3.97 (s, 2H), 3.36 (hept,
J = 7.0 Hz, 1H) and 1.37 (d,
J = 7.0 Hz, 6H).
13C NMR (151 MHz, CD
3OD) δ 156.5, 154.7, 151.8, 138.6, 137.7, 129.7, 129.7, 128.1, 126.0, 41.7, 38.3, 27.4 and 22.4. LC-MS: t
R = 4.16 min, purity: >99%, m/z [M + H]
+: 283; HR-MS: calc. for C
16H
18N
4O [M + H]
+; 283.1553, found 283.1556. Spectral data are in agreement with a previous report [
12].
5-Benzyl-3-isopropyl-2-methyl-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 37b (NPD-3646). Prepared from 36b via the general method to yield the title compound as a white solid (85 mg, 37% for two steps). 1H NMR (600 MHz, CD3OD) δ 7.36–7.28 (m, 4H), 7.25–7.21 (m, 1H), 4.05 (s, 3H), 3.93 (s, 2H), 3.40 (hept, J = 7.0 Hz, 1H) and 1.49 (d, J = 7.0 Hz, 6H). 13C NMR (151 MHz, CD3OD) δ 159.9, 153.4, 143.3, 137.9, 136.7, 134.9, 129.8, 129.6, 128.0, 42.0, 38.8, 27.4 and 21.4. LC-MS: tR = 3.92 min, purity: >99%, m/z [M + H]+: 283; HR-MS: calc. for C16H18N4O [M + H]+; 283.1553, found 283.1554.
5-(Adamantan-1-yl)-3-isopropyl-1-methyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 38a (NPD-3642). Prepared from 36a via the general method to give the title compound as a white solid (123 mg, 46% for two steps). 1H NMR (500 MHz, CDCl3) δ 10.02 (s, 1H), 4.23 (s, 3H), 3.34 (hept, J = 6.9 Hz, 1H), 2.13 (s, 3H), 2.04 (app s, 6H), 1.84–1.74 (m, 6H) and 1.42 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, CDCl3) δ 158.8, 155.4, 151.2, 138.0, 124.7, 40.4, 38.9, 38.2, 36.5, 28.4, 27.0 and 22.0. LC-MS: tR = 5.79 min, purity: 97%, m/z [M + H]+: 327; HR-MS: calc. for C19H26N4O [M + H]+; 327.2179, found 327.2170.
5-(Adamantan-1-yl)-3-isopropyl-2-methyl-2,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 38b (NPD-3641). Prepared from 36b via the general method to give the title compound as a white solid (112 mg, 42% for two steps). 1H NMR (500 MHz, CDCl3) δ 8.72 (s, 1H), 4.04 (s, 3H), 3.30 (hept, J = 7.0 Hz, 1H), 2.13 (app s, 3H), 1.98 (d, J = 2.6 Hz, 6H), 1.77 (app q, J = 12.3 Hz, 6H) and 1.50 (d, J = 7.0 Hz, 6H). 13C NMR (126 MHz, DMSO-d6) δ 157.9, 157.8, 141.7, 135.1, 134.0, 40.4, 38.8, 38.8, 36.5, 28.3, 26.5 and 21.3. LC-MS: tR = 5.31 min, purity: >99%, m/z [M + H]+: 327; HR-MS: calc. for C19H26N4O [M + H]+; 327.2179, found 327.2174.
3-Cyclopentyl-1H-pyrazole-5-carboxylic acid, 42b. NaOEt (3.89 g, 54.9 mmol) was dissolved in EtOH (50 mL) at RT and a solution of diethyl oxalate (7.56 mL, 55.4 mmol) in 1-cyclopentylethanone (5.67 mL, 46.1 mmol) was added dropwise at RT for 30 min. The reaction mixture was diluted with EtOH (50 mL) and heated to 60 °C for 2 h, after which AcOH (8.9 mL, 55 mmol) and 64–65% N2H4 monohydrate (2.20 mL, 46.1 mmol) were added, and the mixture was stirred under reflux for 2 h. The reaction mixture was concentrated under reduced pressure and mixed with aqueous NaOH solution (97 mL, 97 mmol) in 1,4-dioxane (112 mL); the reaction mixture was heated to 50 °C and stirred for 20 h. Then, the reaction was cooled to RT, and 1,4-dioxane was removed under reduced pressure. The residue was washed with diethyl ether (100 mL). The water layer was acidified to pH 1 with concentrated HCl (37%). The white solid was filtered and dried in vacuo to yield the title product 42b as a white solid (5.21 g, 63% for three steps). 1H NMR (600 MHz, DMSO-d6) δ 12.90 (br s, 1H), 6.46 (s, 1H), 3.04 (app p, J = 8.1 Hz, 1H), 2.02–1.94 (m, 2H), 1.73–1.66 (m, 2H) and 1.64–1.53 (m, 4H). 13C NMR (151 MHz, DMSO-d6) δ 104.6, 36.6 (HMBC), 32.7 and 24.6. LC-MS: tR = 3.19 min, purity: >99%, m/z [M − H]−: 179.
3-(tert-Butyl)-4-nitro-1H-pyrazole-5-carboxylic acid, 43a. Ester 41a (25.0 g, 127 mmol) was dissolved in a mixture of THF (100 mL) and water (100 mL), after which NaOH (15.3 g, 382 mmol) was added. The reaction mixture was concentrated under reduced pressure after heating at 60 °C for 4 h, washed with EtOAc (3 × 100 mL), adjusted to pH 1 with concentrated HCl solution, and the off-white solid was filtered as intermediate 42a (16.5 g, 77%), which was used for the next step without further purification. Acid 42a (3.95 g, 23.5 mmol) was added portion-wise to concentrated H2SO4 (19.1 mL, 352 mmol) at RT with stirring. The reaction mixture was then heated to 60 °C, and 65% HNO3 (4.50 mL, 70.4 mmol) was added dropwise, keeping the temperature at 60 °C. The reaction was stirred at 60 °C for 3 h, cooled to RT and poured onto 200 g of ice with stirring. After 15 min, the white precipitate was isolated by filtration, washed with water and dried under reduced pressure to give the title product 43a as a white solid (4.50, 90%). 1H NMR (300 MHz, DMSO-d6) δ 13.82 (s, 1H) and 1.34 (s, 9H). 13C NMR (151 MHz, DMSO-d6) δ 147.3 (HMBC), 32.4 (HSQC) and 28.2. LC-MS: tR = 3.26 min, purity: 96%, m/z [M + H]+: 214.
3-Cyclopentyl-4-nitro-1H-pyrazole-5-carboxylic acid,
43b. Acid
42b (5.21 g, 28.9 mmol) was added portion-wise to concentrated H
2SO
4 (8.91 mL, 159 mmol) at RT with stirring. The reaction mixture was then heated to 60 °C, and 65% HNO
3 (6.95 mL, 101 mmol) was added dropwise, keeping the temperature at 60 °C. The reaction was stirred at 60 °C for 3 h, cooled to RT and poured onto 200 g of ice with stirring. After 15 min, the white precipitate was isolated by filtration, washed with water and dried under reduced pressure to give the title product
43b as a white solid (4.01 g, 61%).
1H NMR (600 MHz, DMSO-
d6 + 1 drop of D
2O) δ 3.47 (p,
J = 8.6 Hz, 1H), 2.09–1.98 (m, 2H), 1.79–1.68 (m, 2H) and 1.68–1.55 (m, 4H).
13C NMR (151 MHz, DMSO-
d6 + 1 drop of D
2O) δ 36.0, 32.0 and 25.5. LC-MS: t
R = 3.26 min, purity: >99%, m/z [M − H]
−: 224. Spectral data are in agreement with a previous report [
17].
4-Amino-3-(tert-butyl)-1H-pyrazole-5-carboxamide, 45a. Oxalyl chloride (6.16 mL, 70.4 mmol) was added dropwise to a suspension of 43a (5.00 g, 23.5 mmol) in DCM (240 mL) containing DMF (0.082 mL, 1.1 mmol) under nitrogen at 0 °C. The reaction mixture was stirred at 0 °C for 1 h, allowed to warm to RT and stirred for a further 2 h. The reaction mixture was concentrated in vacuo and co-evaporated with toluene three times. The residue was dissolved in DCM (100 mL) and added dropwise to 7 M NH3 in MeOH (10.1 mL, 70.4 mmol) at 0 °C. After stirring for 3 h, the reaction mixture was concentrated in vacuo, combined with 10% palladium on carbon (0.85 g, 8.0 mmol) in EtOH (90 mL) and stirred under H2 gas insert at 60 °C for 6 h. The reaction mixture was filtered through celite, and the solid was washed with MeOH (50 mL). The filtrate was concentrated under reduced pressure, and the residue was used for the next step without further purification.
4-Amino-3-cyclopentyl-1H-pyrazole-5-carboxamide, 45b. Oxalyl chloride (1.09 mL, 12.5 mmol) was added dropwise to a suspension of 43b (0.94 g, 4.2 mmol) in DCM (20 mL) containing DMF (0.014 mL, 0.18 mmol) under nitrogen at 0 °C. The reaction was stirred at 0 °C for 1 h, allowed to warm to RT and stirred for a further 2 h. The reaction mixture was concentrated in vacuo, combined with 10% palladium on carbon (0.85 g, 8.0 mmol) in EtOH (90 mL) and stirred under H2 gas insert at 60 °C for 6 h. The reaction mixture was filtered through celite, and the solid was washed with MeOH (50 mL). The filtrate was concentrated under reduced pressure, and the residue was used for the next step without further purification.
5-Benzyl-3-(tert-butyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 46a (NPD-3648). Prepared from 45a via the general method to yield the title compound as a white solid (0.14 g, 46% for four steps). 1H NMR (500 MHz, CD3OD) δ 7.36–7.33 (m, 2H), 7.33–7.27 (m, 2H), 7.25–7.20 (m, 1H), 3.98 (s, 2H) and 1.50 (s, 9H). 13C NMR (126 MHz, CD3OD) δ 155.4 (HMBC), 153.0 (HMBC), 146.6 (HMBC), 138.0, 129.8, 129.6, 128.0, 42.0, 33.8 (HMBC) and 29.9. LC-MS: tR = 4.20 min, purity: >99%, m/z [M + H]+: 283; HR-MS: calc. for C17H18N4O [M + H]+; 283.1553, found 283.1551.
5-Benzyl-3-cyclopentyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 46b (NPD-3604). Prepared from 45b via the general method to give the title compound as a white solid (0.14 g, 14% for four steps). 1H NMR (500 MHz, DMSO-d6) δ 7.34–7.27 (m, 4H), 7.24–7.19 (m, 1H), 3.88 (s, 2H), 3.31 (app p, J = 8.1 Hz, 1H), 2.02–1.94 (m, 2H), 1.84–1.68 (m, 4H) and 1.66–1.56 (m, 2H). 13C NMR (126 MHz, DMSO-d6) δ 153.0, 137.4, 129.1, 129.0, 127.3, 40.6, 36.6, 32.6 and 25.4. LC-MS: tR = 4.05 min, purity: >99%, m/z [M + H]+: 295; HR-MS: calc. for C17H18N4O [M + H]+; 295.1553, found 295.1542.
5-(Adamantan-1-yl)-3-(tert-butyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 47a (NPD-3643). Prepared from 45a via the general method to give the title compound as a white solid (132 mg, 29% for four steps). 1H NMR (500 MHz, CDCl3) δ 9.20 (s, 1H), 2.15 (app s, 3H), 2.02 (app s, 6H), 1.79 (app q, J = 12.3 Hz, 6H) and 1.53 (s, 9H). 13C NMR (126 MHz, DMSO-d6) δ 158.5, 155.1, 154.0, 137.7, 127.2, 40.5, 39.0, 36.5, 33.4, 29.4 and 28.3. LC-MS: tR = 5.55 min, purity: 99%, m/z [M + H]+: 327; HR-MS: calc. for C19H26N4O [M + H]+; 327.2179, found 327.2170.
5-(Adamantan-1-yl)-3-cyclopentyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one, 47b (NPD-3644). Prepared from 45b via the general method to give the title compound as a white solid (97 mg, 11% for four steps). 1H NMR (500 MHz, DMSO-d6 + 1 drop of D2O) δ 3.29 (p, J = 8.2 Hz, 1H), 2.05–1.88 (m, 11H) and 1.88–1.56 (m, 12H). 13C NMR (126 MHz, CD3OD + 1 drop of CDCl3) δ 159.8, 156.8, 148.0 (HMBC), 136.3 (HMBC), 40.3, 39.3, 37.3, 36.7, 32.8, 28.6 and 26.0. LC-MS: tR = 5.52 min, purity: >99%, m/z [M + H]+: 339; HR-MS: calc. for C20H26N4O [M + H]+; 339.2179, found 339.2167.