Abstract
In this study, we explorethe synthesis of binaphthyl-based chiral macrocyclic hosts for the first time. They exhibited the selective recognition abilities of iodide anions which can be favored over those of other anions (AcO−, NO3−, ClO4−, HSO4−, Br−, PF6−, H2PO4−, BF4−, and CO3F3S−), as confirmed by UV-vis, HRMS, and 1H NMR spectroscopy experiments, as well as DFT calculations. Neutral aryl C–H···anion interactions play an important role in the formation complexes. The recognition process can be observed by the naked eye.
1. Introduction
Anions are ubiquitous and play an important role in our body. For example, iodine [1,2,3] is araw material used for the synthesis of thyroid hormones, which can promote the metabolism of substances; regulate the metabolism of proteins, fats, and sugars; and help regulate the metabolism of water and salt. However, iodine deficiency can cause diseases such as goiter or hypothyroidism. However, the most common effects of hyperiodine on thyroid function are iodine-induced goiter (IH) and hyperiodine hyperthyroidism. Moreover, the radioisotopes of 129I− and 130I− are considered harmful to the environment [4]. Therefore, the development of iodide anion receptors is of great value and has attracted considerable interest.
The continuous synthesis of novel macrocyclic host molecules and their unique molecular recognition properties have driven the development of supramolecular chemistry [5,6,7,8,9,10,11]. During the past several decades, various macrocyclic hosts have been developed and show excellent recognition properties for anions (such as fluoride, nitrate, oxyacid, and other anions). Outstanding examples include Sessler’s calixpyrrole [12,13,14], Farnham’s fluorinated macrocyclic ethers [15], Flood’s triazolophane [16,17,18], Sindelar’sbambusuril macrocycle [19,20,21], Beer’s rotaxanes and catenanes [22,23,24,25], and so on [26,27,28,29,30]. Most strategies involve the modification of cavities by employing hydrogen bonds offered by specific bindingsites to bind anions with size and shape selectivity in various media. However, the purpose of constructing iodide anion receptor macrocyclic [31,32], due the large diameter and low electron density [33], rarely makes it difficult to form hydrogen bonds and iodide anion–π interactions. After neutral C–H···anion interactions were elucidated by chemists in 2008 [34,35,36,37], this kind of interaction has attracted significant interest and has developed rapidly. Flood and co-works successfully prepared triazolophane and cages and found that these triazolophane and cages display strong affinity for anions through neutral C–H···anion interactions [16,17,18]. Li’s group reported anionsin water recognized by cages through neutral C–H···anion interactions [38,39,40]. Huang’s group demonstrated that a preorganized rigid macrocycle cyclo[4]carbazole can act as an iodide anion receptor through neutral C–H···anion interactions [41]. Despite these seminal reports, we still need to explorethe application of neutral C–H···anion interactions, especially for the selective recognition of iodide anions.
Since the aryl C–H groups form stronger hydrogen bonds with anions than alkyl C–H group [42], we explored the possibility of using binaphthyl units containing a large number of aryl C–H groups as building blocks to construct macrocyclic arene that can selectively recognize iodide anionsthrough neutral C–H···anion interactions. Recently, Wang and co-works reported the easy preparation of a series of triazine- and binaphthol-based chiral macrocycles and cages [43]. Inspired by their work, in this study, we prepared enantiopure macrocyclic arene composed of chiral enantiomeric binaphthyl units, named RR-1 and SS-1, respectively (Figure 1). It was found that RR-1 and SS-1 have a proper cavity size (approximately 10.100 Å × 9.000 Å). 1H NMR and UV-vis experiments demonstrated that RR-1 and SS-1 could be used as exclusive selectivity sites for iodide anion receptors relative to other anions (such as AcO−, NO3−, ClO4−, HSO4−, Br−, PF6−, H2PO4−, BF4−, and CO3F3S−). Neutral C–H···anion interactions play an important role in the formation of RR-1/iodineand SS-1/iodide anion complexes.
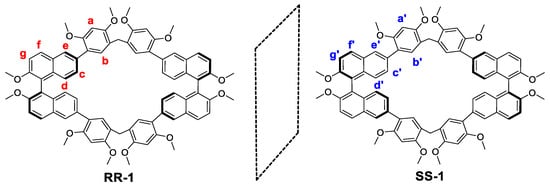
Figure 1.
Structural formula and proton designations of RR-1 and SS-1.
2. Results and Discussion
The synthesis of RR-1 and SS-1 isoutlined in Scheme 1. R-3 and S-3 were prepared according to the literature [44]. We then obtained R-2 and S-2 viathe Suzuki coupling reaction of 2,4-dimethoxybenzeneboronic acid with R-3 or S-3 inthe presence of Pd(PPh3)4 as the catalyst in a 68–70% yield. Finally, RR-1 and SS-1 weresynthesized in moderate yield following thetreatment of R-2 or S-2 with paraformaldehyde and boron trifluoride diethyl etherate in dichloromethane at room temperature. The structuresof RR-1 and SS-1 were confirmed by 1H NMR, 13C NMR, as well as HRMS spectra. The CD spectra of RR-1 (black line) and SS-1 (red line) showed mirror images (Figure 2c), providing strong evidence for the handedness of enantiopure macrocycles.
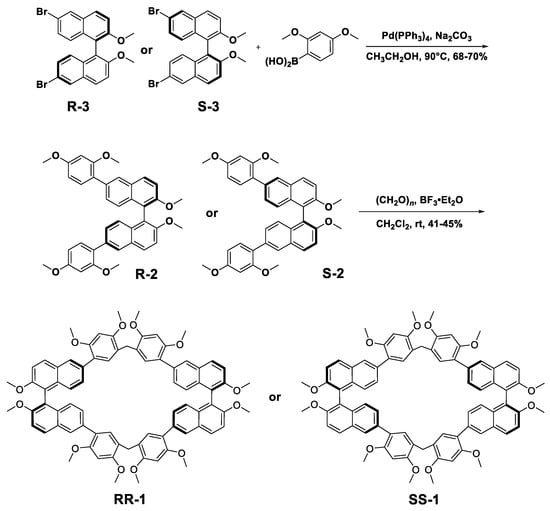
Scheme 1.
The synthesis of RR-1 and SS-1.
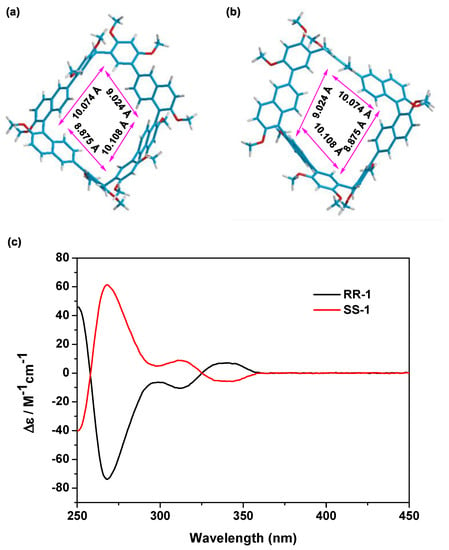
Figure 2.
Theenergy-minimized structures of RR–1 (a) and SS–1 (b) simulated by Gaussian computer program; (c) CD spectra of RR–1 (black line) and SS–1 (red line).
The attempts to obtain the single crystals of RR-1 and SS-1 that are suitable for X-ray analysis ended in failure. Thus, density functional theory (DFT) methods were used to gain further insightinto the structures of RR-1 and SS-1 by usingGaussian 09 software and by choosing 6-311G as the basis sets. As shown in Figure 2, both RR-1 and SS-1 hadbox-like structures with a cavity size of approximately 10.100 Å × 9.000 Å. Initially, we hypothesized that RR-1 and SS-1 contain electron-rich cavities that could be used for complexes with cationic guest molecules. Unfortunately, when RR-1 (4.0 mM) and 1.0 equiv. tetramethylammonium hexafluorophosphate were mixed in CDCl3/CD3CN (v/v = 1/1), the proton signals of both RR-1 and tetramethylammonium hexafluorophosphate were not shifted, suggesting that no complexation occurred between RR-1 and tetramethylammonium hexafluorophosphate (Figure S10). Since RR-1 and SS-1 contain a large number of neutral aryl C–H bonds, we questioned whether RR-1 and SS-1 can be used as receptorsfor anions.
Consequently, UV-vis experiments were carried out to verify our hypothesis and commercially available tetrabutylammonium salts (TBAX) were used as anion sourcesdue totheir simple composition and good solubility in chloroform. As shown in Figure 3a, after the addition of 5.0 equiv. tetrabutylammonium salts (TBAX, X = AcO−, NO3−, ClO4−, HSO4−, Br−, I−, PF6−, H2PO4−, BF4−, and CO3F3S−) to the solution of RR-1 in chloroform, the color of the solution containing RR-1 and TBAI changed from colorless to light orange, whereas the others remained colorless. This obvious color change suggests that interactions between RR-1 and TBAI may have occurred. UV-vis experiments further reveal the interactions behavior between RR-1 and TBAX. Upon the addition of 5.0 equiv TBAI, the absorption at 300 nm and 350 nm was enhanced significantly, and new absorption bands appeared at 375 nm, indicating the formation of RR-1/iodide complexes in the solution. On the other hand, no absorption spectral changes were observed after the addition of other TBAX salts mentioned above. All the above results indicate that RR-1 has the ability toselectively recognize iodide anionsover other tested anions. Similar to RR-1, SS-1 also showedthe selective recognition of iodide anionsover other tested anions (Figure 3b).
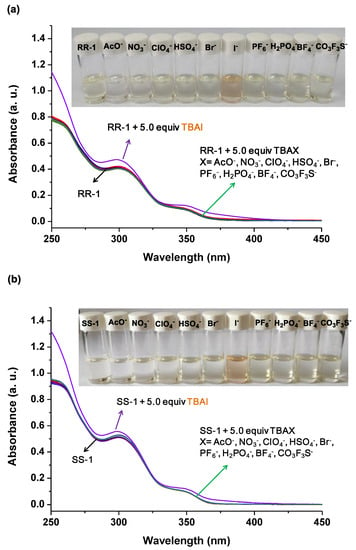
Figure 3.
(a) UV–vis spectra of RR–1 (1.00 × 10−2 mM) in CHCl3 and mixed solutions of RR–1 and TBAX (X = AcO−, NO3−, ClO4−, HSO4−, Br−, I−, PF6−, H2PO4−, BF4−, and CO3F3S−) (5.0 equiv); (b) UV–vis spectra of SS–1 (1.00 × 10−2 mM) in CHCl3 and mixed solutions of SS-1 and TBAX (X = AcO−, NO3−, ClO4−, HSO4−, Br−, I−, PF6−, H2PO4−, BF4−, and CO3F3S−) (5.0 equiv). Inset: photograph showing the colors of these mixed solutions.
1H NMR experiments further provided the evidence for the formation of RR-1/iodide complexes in the solution. After RR-1 and TBAI with a 1:1 molar ratio were mixed in CDCl3, a new set of proton signals that differedfrom RR-1 and TBAI were observed on the1H NMR spectrum, indicating that a new complex RR-1/iodide was formed (Figure 4). The protons b and e corresponding to the RR-1 shift up-field by 0.006 and 0.004 ppm, respectively, which could be attributed to the formation of neutral C–H···anion interactions between RR-1 and TBAI. Only the protons b and e corresponding to RR-1 wereshifted, leading us to doubt that the recognition of iodide using RR-1 may occur on the outside of the cavity. Moreover, unlike the recognition of iodide usingcyclo[4]carbazole [41], which is a slow process and occurs inside the cavity, the complex and decomplex between RR-1 and TBAI is a fast exchange process on the NMR time scale at room temperature. This differencemay be due to the iodide being recognized by RR-1 through neutral C–H···anion interactions that are outside of the cavity. To further obtaininsights into the complexation process between RR-1 and TBAI, 1H NMR spectroscopic titrations experiments were then carried out. By monitoring the change inproton b corresponding to RR-1 followingthe addition of TBAI, a1:1 complex between RR-1 and TBAI was formed by the mole ratio plot. The binding constant Ka of the complex RR-1/iodide was determined to be 132.8 ± 33.8 M−1 using BindFit software (http://supramolecular.org). Consequently, the 1:1 complex of SS-1/iodide was also formed and the binding constant was calculated to be Ka = 119.1 ± 32.6 M−1 (Figure S9).
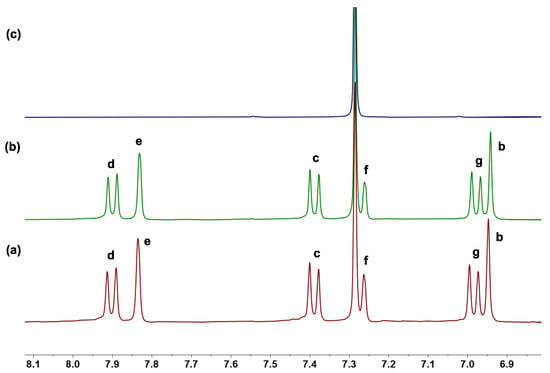
Figure 4.
Partial 1H NMR spectra (400 MHz, CDCl3, 298 K) of (a) free RR-1, (b) RR-1 and 1.0 equiv. of TBAI, and (c) free TBAI. [RR-1]0 = 4.0 mM.
The energy-minimized optimized structure of RR-1/iodide and SS-1/iodide further supported the formation of neutral C–H···anion interactions. As shown in Figure 5a, the iodide anion waslocated outside the cavity of RR-1 through C–H···anion interactions with distances of 3.307 and 3.123 Å, respectively. Only protons b and e corresponding to RR-1 participated in the formation of hydrogen bonds with iodide anions, which is consistent with the results in 1H NMR experiments indicating that only the protons b and e shifted up-field. In the structure of the complex SS-1/iodide, an iodide anionwasalso located outside the cavity of SS-1 through C–H···anion interactions with distances of 3.307 and 3.123 Å, respectively (Figure 5b).
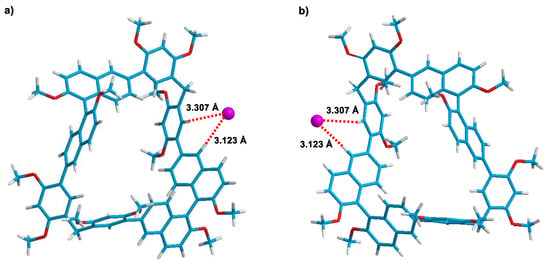
Figure 5.
The energy-minimized structures of RR-1/iodide (a,b) SS-1/iodide simulated by the Gaussian computer program.
3. Materials and Methods
3.1. General Considerations
Unless otherwise noted, all reagents were obtained from commercial suppliers and used without further purification. 1H NMR and13C NMR spectra were recorded witha Bruker DMX400 NMR spectrometer. Electrospray ionization mass spectra (ESI-MS) were recorded on the Thermo Fisher® Exactive LC-MS spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
3.2. Typical Procedure for the Synthesis of RR-1
To a mixture of R-2 (1.17 g, 2.0 mmol) and paraformaldehyde (180 mg, 6.0 mmol) in dichloromethane (150 mL),boron trifluoride diethyl etherate (0.3 mL, 2.4 mmol) was added. The mixture was stirred at room temperature for 0.5 h. Then, the reaction was quenched by the addition of 150 mL of water. The organic layer was separated and dried with anhydrous MgSO4. The solvent was removed in vacuo and the residue was separated viacolumn chromatography on silica gel (eluent: 2:1 DCM/petroleum ether) to give RR-1 (538 mg, 45%) as a yellow solid. 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 9.0 Hz, 4H), 7.84 (s, 4H), 7.39 (d, J = 9.0 Hz, 4H), 7.26 (s, 4H), 7.01–6.93 (m, 8H), 6.60 (s, 4H), 3.97 (s, 4H), 3.93 (s, 12H), 3.81 (s, 12H), 3.76 (s, 12H). 13C NMR (101 MHz, CDCl3) δ 157.6, 155.8, 154.8, 133.7, 132.7, 132.1, 129.3, 129.2, 128.7, 127.5, 124.6, 122.7, 121.4, 119.6, 114.1, 96.0, 57.0, 56.0, 55.9, 27.8. HRMS (APCI) m/z: [M+Na]+ calculatedfor C78H68O12Na, 1219.4608; found, 1219.4559.
4. Conclusions
In summary, we successfully designed and synthesized enantiopure macrocyclic arene RR-1 and SS-1 composed of chiral enantiomeric binaphthyl units. The anion receptor ability of RR-1 and SS-1 was investigated using UV-vis and 1H NMR experiments. RR-1 and SS-1 were found to be able to selectivity bind the iodide anion in a 1:1 manner among the ten tested anions, and neutral C–H···anion interactions were found to play an important role in the formation of the RR-1/iodideand SS-1/iodide anion complex. The complexation between the iodide with RR-1 or SS-1 can be observed by the naked eye, and the color of solution changes from colorless to light orange. We believe that this new kind of iodide receptor will pavethe way to the design of new anion receptors via neutral C–H···anion interactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28124784/s1, indicating the 1H and 13C spectra of thenew compounds, as well asthe determination of the association constants of complexes.
Author Contributions
Z.-C.W. and Y.-Z.T. performed the synthesis. L.-L.T. performed the 1H NMR spectroscopy experiments and F.Z. conceived and supervised the project and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21602055); the Natural Science Foundation of Hunan Province (2017JJ3094); the Construct Program of Applied Characteristic Discipline in Hunan Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available upon request from the authors.
References
- Ahad, F.; Ganie, S.A. Iodine, iodine metabolism and iodine deficiency disorders revisited. Indian J. Endocrinol. Metab. 2010, 14, 13–17. [Google Scholar] [PubMed]
- Chung, H.R. Iodine and thyroid function. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 8–12. [Google Scholar] [CrossRef]
- Dai, G.; Levy, O.; Carrasco, N. Cloning and characterization of the thyroid iodide transporter. Nature 1996, 379, 458–460. [Google Scholar] [CrossRef]
- Di Lemma, F.G.; Colle, J.Y.; Beneš, O.; Konings, R.J.M. A separate effect study of the influence of metallic fission products on CsI radioactive release from nuclear fuel. J. Nucl. Mater. 2015, 465, 499–508. [Google Scholar] [CrossRef]
- Gloe, K. Macrocyclic Chemistry: Current Trends and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Han, Y.; Meng, Z.; Ma, Y.-X.; Chen, C.-F. Iptycene-derived crown ether hosts for molecular recognition and self-assembly. Acc. Chem. Res. 2014, 47, 2026–2040. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Li, C. Biphen[n]arenes: Modular Synthesis, Customizable Cavity Sizes, and Diverse Skeletons. Acc. Chem. Res. 2022, 55, 916–929. [Google Scholar] [CrossRef]
- Zeng, F.; Cheng, L.; Ou, G.-C.; Tang, L.-L.; Ding, M.-H. Pyromellitic Diimide-Extended Pillar[6]arene: Synthesis, Sturcture, and Its Complexation with Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2022, 87, 3863–3867. [Google Scholar] [CrossRef]
- Ding, M.-H.; Liao, J.; Tang, L.-L.; Ou, G.-C.; Zeng, F. High-yield synthesis of a novel water-soluble macrocycle for selective recognition of naphthalene. Chin. Chem. Lett. 2021, 32, 1665–1668. [Google Scholar] [CrossRef]
- Zeng, F.; Cheng, L.; Zhang, W.-J.; Tang, L.-L.; Wang, X.-F. Phenanthrene[2]arene: Synthesis and application as nonporous adaptive crystals in the separation of benzene from cyclohexane. Org. Chem. Front. 2022, 9, 3307–3311. [Google Scholar] [CrossRef]
- Zeng, F.; Xiao, X.-S.; Gong, S.-F.; Yuan, L.; Tang, L.-L. An electron-deficient supramolecular macrocyclic host for the selective separation of aromatics and cyclic aliphatics. Org. Chem. Front. 2022, 9, 4829–4833. [Google Scholar] [CrossRef]
- Gale, P.A.; Sessler, J.L.; Král, V.; Lynch, V. Calix[4]pyrroles: Old Yet New Anion-Bing Agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [Google Scholar] [CrossRef]
- Kim, S.K.; Sessler, J.L. Calix[4]pyrrole-Based Ion Pair Receptors. Acc. Chem. Res. 2014, 47, 2525–2536. [Google Scholar] [CrossRef]
- Kim, D.S.; Sessler, J.L. Calix[4]pyrroles: Versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015, 44, 532–546. [Google Scholar] [CrossRef]
- Farnham, W.B.; Roe, D.C.; Dixon, D.A.; Calabrese, J.C.; Harlow, R.L. Fluorinated macrocyclic ethers as fluoride ion hosts. Novel structures and dynamic properties. J. Am. Chem. Soc. 1990, 112, 7707–7718. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Chen, C.-H.; Flood, A.H. Chloride capture using a C-H hydrogen-bonding cage. Science 2019, 365, 159–161. [Google Scholar]
- Hua, Y.; Flood, A.H. Click chemistry generates privileged CH hydrogen-bonding triazoles: The latest addition to anion supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 1262–1271. [Google Scholar] [CrossRef]
- McDonald, K.P.; Hua, Y.; Lee, S.; Flood, A.H. Shape persistence delivers lock-and-key chloride binding in triazolophanes. Chem. Commun. 2012, 48, 5065–5075. [Google Scholar] [CrossRef]
- Yawer, M.A.; Havel, V.; Sindelar, V.A. Bambusuril Macrocycle that Binds Anions in Water with High Affinity and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Svec, J.; Necas, M.; Sindelar, V. Bambus[6]uril. Angew. Chem. Int. Ed. 2010, 49, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Havel, V.; Sindelar, V.; Necas, M.; Kaifer, A.E. Water-mediated inclusion of benzoates and tosylates inside the bambusuril macrocycle. Chem. Commun. 2014, 50, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, M.; Langton, M.J.; Beer, P.D. Anion recognition in water by a rotaxane containing a secondary rim functionalised cyclodextrin stoppered axle. Chem. Commun. 2015, 51, 4499–4502. [Google Scholar] [CrossRef]
- Langton, M.J.; Duckworth, L.C.; Beer, P.D. Nitrate anion templated assembly of a [2]rotaxane for selective nitrate recognition in aqueous solvent mixtures. Chem. Commun. 2013, 49, 8608–8610. [Google Scholar] [CrossRef]
- Mullen, K.M.; Beer, P.D. Sulfate anion templation of macrocycles, capsules, interpenetrated and interlocked structures. Chem. Soc. Rev. 2009, 38, 1701–1713. [Google Scholar] [CrossRef]
- Barendt, T.A.; Robinson, S.W.; Beer, P.D. Superior anion induced shuttling behaviour exhibited by a halogen bonding two station rotaxane. Chem. Sci. 2016, 7, 5171–5180. [Google Scholar] [CrossRef]
- Tuo, D.-H.; Ao, Y.-F.; Wang, Q.-Q.; Wang, D.-X. Naphthalene-pillared benzene triimide cage: An efficient receptor for polyhedral anions and a general tool for probing theoretically existing anion-πbinding motifs. CCS Chem. 2022, 4, 2806–2815. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, M.X. Exploring anion–π interactions and their applications in supramolecular chemistry. Acc. Chem. Res. 2020, 53, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Tuo, D.-H.; Ao, Y.-F.; Wang, Q.-Q.; Wang, D.-X. Benzene triimide cage as a selective container of azide. Org. Lett. 2019, 21, 7158–7162. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, F.; Zhao, T.; Li, A.; Xu, G.; Sessler, J.L.; He, Q. Selective Inclusion of Fluoride within the Cavity of a Two-Wall Biscalix[4]pyrrole. Org. Lett. 2020, 22, 4451–4455. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.; Lee, A.; Lynch, V.M.; He, Q.; Sessler, J.L.; Kim, S.K. Tetraamidoindoly calix[4]arene as a selective ion pair receptor. Org. Chem. Front. 2022, 9, 6888–6893. [Google Scholar] [CrossRef]
- Li, Y.; Pink, M.; Karty, J.A.; Flood, A.H. Dipole-Promoted and Size-Dependent Cooperativity between Pyridyl-Containing Triazolophanes and Halides Leads to Persistent Sandwich Complexes with Iodide. J. Am. Chem. Soc. 2008, 130, 17293–17295. [Google Scholar] [CrossRef]
- Mendy, J.S.; Saeed, M.A.; Fronczek, F.R.; Powell, D.R.; Hossain, M.A. Anion Recognition and Sensing by a New Macrocyclic Dinuclear Copper(II) Complex: A Selective Receptor for Iodide. Inorg. Chem. 2010, 49, 7223–7225. [Google Scholar] [CrossRef]
- Lee, D.Y.; Singh, N.; Kim, M.J.; Jang, D.O. Chromogenic and Fluorescent Recognition of Iodide with a Benzimidazole-Based Tripodal Receptor. Org. Lett. 2011, 13, 3024–3027. [Google Scholar] [CrossRef]
- Zhu, S.S.; Staats, H.; Brandhorst, K.; Grunenberg, J.; Gruppi, F.; Dalcanale, E.; Luetzen, A.; Rissanen, K.; Schalley, C.A. Anion Binding to Resorcinarene-Based Cacitands: The Importance of C-H… Anion Interactions. Angew. Chem. Int. Ed. 2008, 47, 788–792. [Google Scholar] [CrossRef]
- Li, Y.; Flood, A.H. Pure C-H Hydrogen Binding of Chloride Ions: A Preorganized and Rigid Macrocyclic Receptor. Angew. Chem. Int. Ed. 2008, 47, 2649–2652. [Google Scholar] [CrossRef]
- Juwarker, H.; Lenhardt, J.M.; Pham, D.M.; Craig, S.L. 1,2,3-Triazole CH…Cl− Contacts Guide Anion Binding and Concomitant Folding in 1,4-Diaryl Triazole Oligomers. Angew. Chem. Int. Ed. 2008, 47, 3740–3743. [Google Scholar] [CrossRef]
- Berryman, O.B.; Sather, A.C.; Hay, B.P.; Meisner, J.S.; Johnson, D.W. Solution Phase Measurement of Both Weak σ and C−H···X− Hydrogen Bonding Interactions in Synthetic Anion Receptors. J. Am. Chem. Soc. 2008, 130, 10895–10897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, G.; Chen, L.; Tong, L.; Lei, Y.; Shen, L.; Jiao, T.; Li, H. Selective Recognition of Chloride Anion in Water. Org. Lett. 2020, 22, 4878–4882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, S.; Wu, G.; Lei, Y.; Chen, Q.; Wang, H.; Wu, Y.; Lin, C.; Hong, X.; Kim, S.K.; et al. Constraining Homo- and Heteroanion Dimers in Ultraclos Proximity within a Self-Assembled Hexacationic Cage. J. Am. Chem. Soc. 2020, 142, 20182–20190. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Fang, S.; Zhu, D.; Chen, Y.; Ge, C.; Tang, H.; Li, H. A Self-Assembled Cage Binding Iodide Anions over Other Halid Ions in Water. Angew. Chem. Int. Ed. 2022, 61, e202209078. [Google Scholar]
- Zhu, H.; Shi, B.; Chen, K.; Wei, P.; Xia, D.; Mondal, J.H.; Huang, F. Cyclo[4]carbazole, an Iodide Anion Macrocyclic Receptor. Org. Lett. 2016, 18, 5054–5057. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Hay, B.P. Are C−H Groups Significant Hydrogen Bonding Sites in Anion Receptors? Benzene Complexes with Cl−, NO3−, and ClO4−. J. Am. Chem. Soc. 2005, 127, 8282–8283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ao, Y.-F.; Wang, D.-X.; Wang, Q.Q. Triazine- and Binaphthol-Based Chiral Macrocycles and Cages: Synthesis, Structure, and Solid-State Assembly. J. Org. Chem. 2022, 87, 3491–3497. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liang, F.; Wang, Y.; Xu, M.; Wang, X. A highly sensitive water-soluble system to sense glucose in aqueous solution. Org. Biomol. Chem. 2011, 9, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).