A Chiral Metal—Organic Framework Prepared on Large-Scale for Sensitive and Enantioselective Fluorescence Recognition
Abstract
1. Introduction
2. Results and Discussion
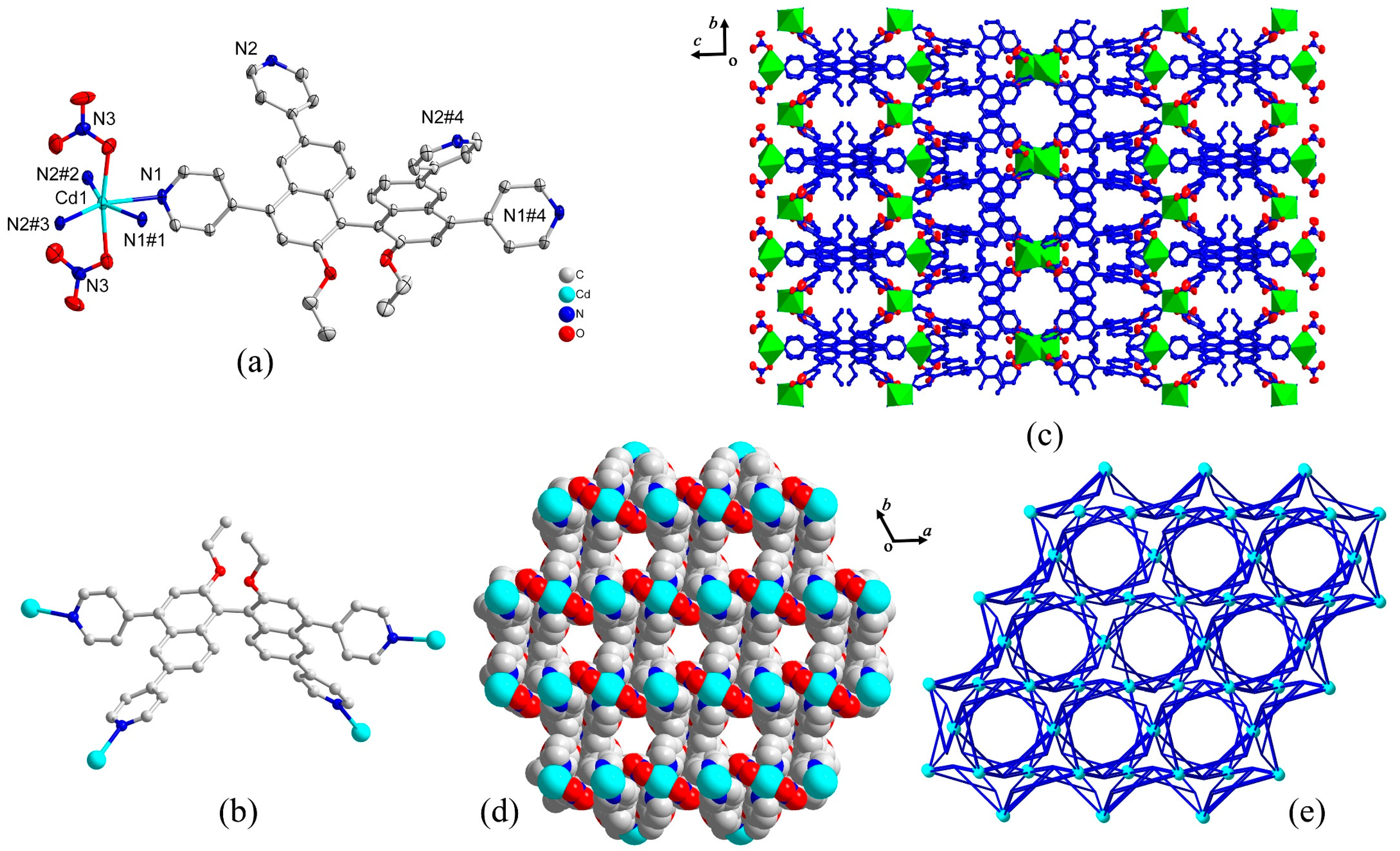
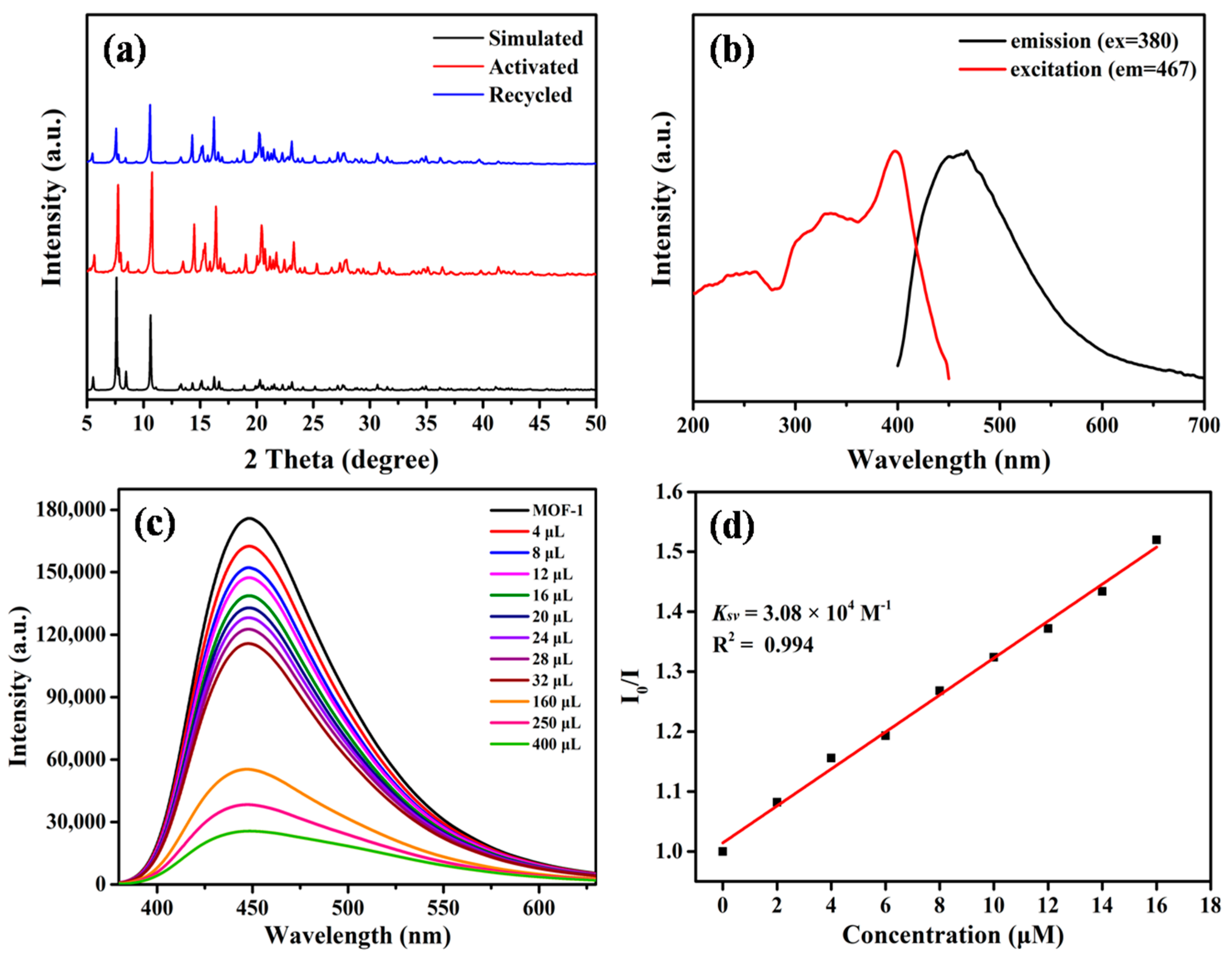
2.1. Synthesis and Characterization
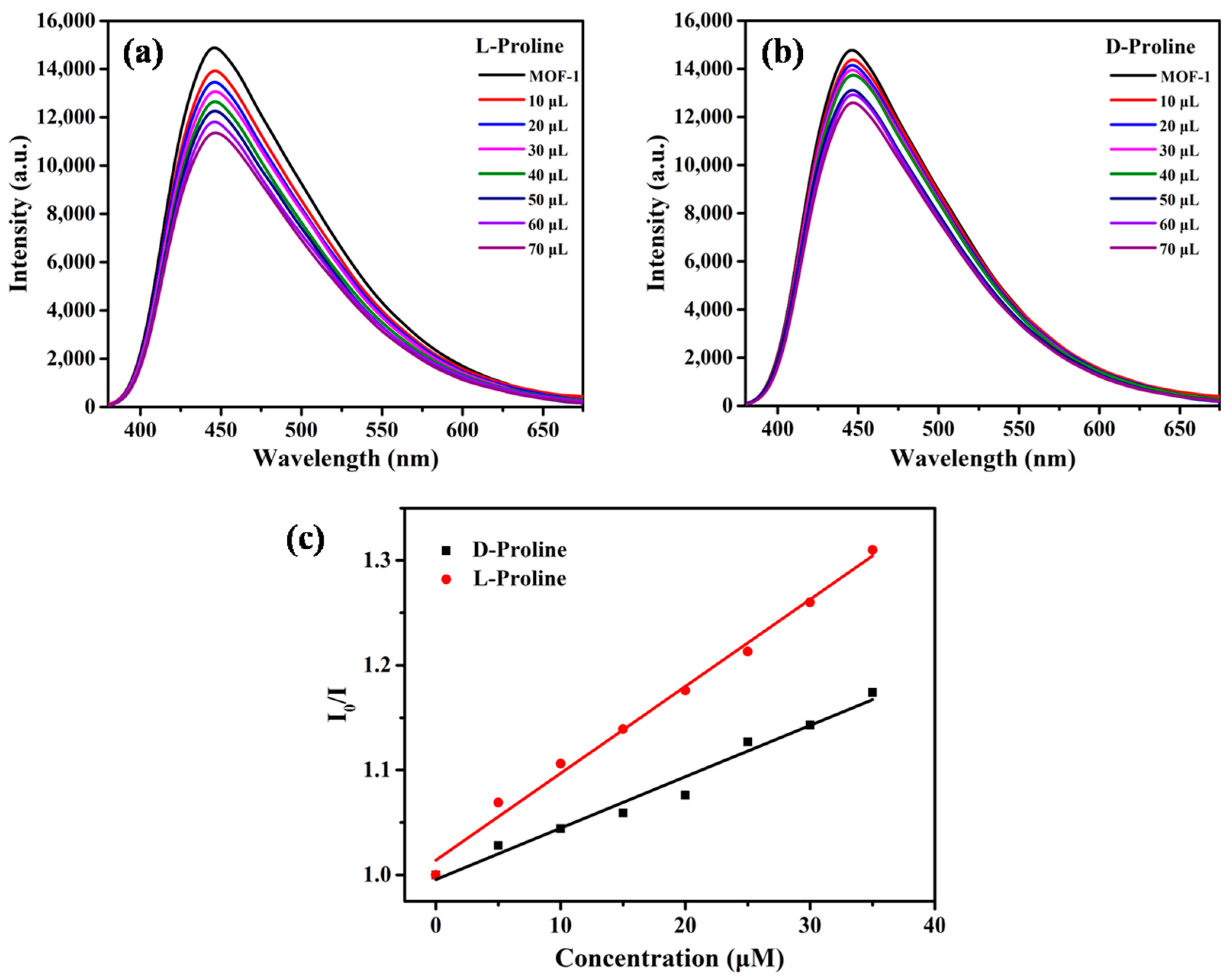
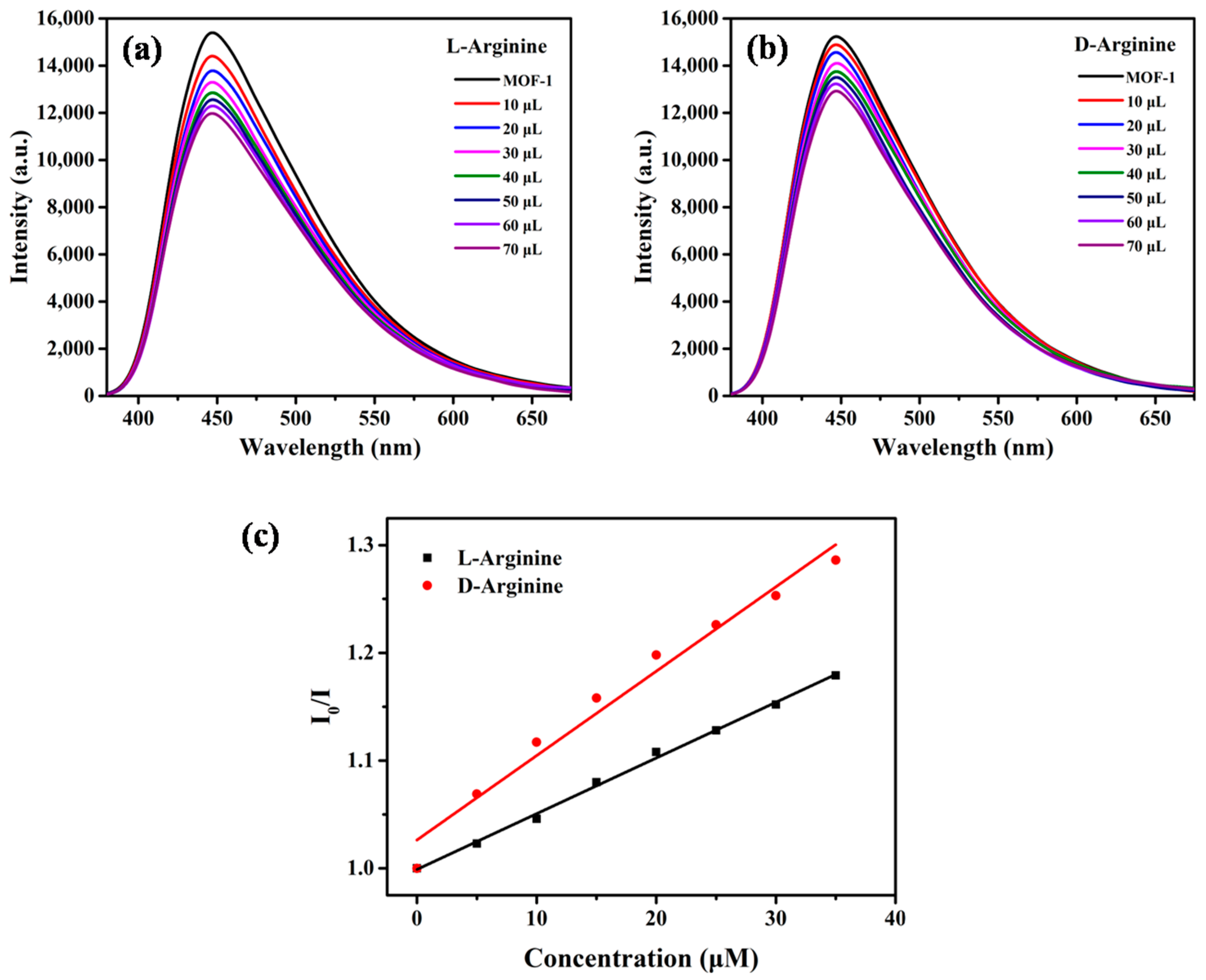
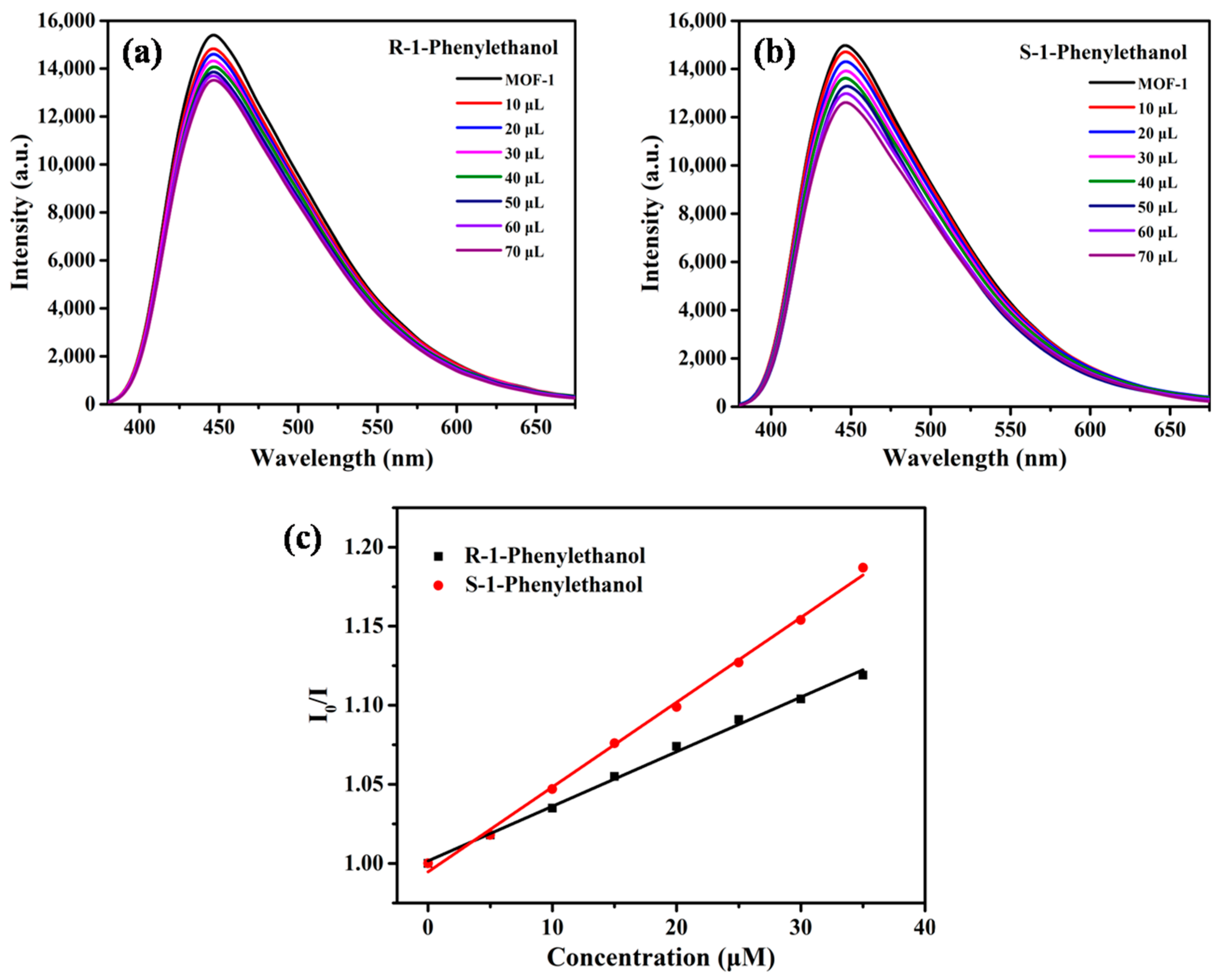
2.2. Enantioselective Fluorescent Sensing
3. Materials and Methods
3.1. General
3.2. Synthesis of [Cd(s-L)](NO3)2(MOF-1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, R.; Kodaimati, M.S.; Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 2021, 50, 5564–5589. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zha, D.; Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Sonwal, S.; Lee, M.J.; Mohana Rani, G.; Lee, E.S.; Jeon, T.J.; Kang, S.M.; Oh, M.H.; Huh, Y.S. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Hou, B.; Cheng, Q.; Zhang, G. A novel magnesium metal-organic framework as a multiresponsive luminescent sensor for Fe(III) ions, pesticides, and antibiotics with high selectivity and sensitivity. Inorg. Chem. 2018, 57, 13330–13340. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Jiang, H.; Wang, X. Recent advances of BINOL-based sensors for enantioselective fluorescence recognition. Analyst 2020, 145, 6769–6812. [Google Scholar] [CrossRef]
- Crassous, J. Chiral transfer in coordination complexes: Towards molecular materials. Chem. Soc. Rev. 2009, 38, 830–845. [Google Scholar] [CrossRef]
- Thoonen, S.; Hua, C. Chiral detection with coordination polymers. Chem.-Asian J. 2021, 16, 890–901. [Google Scholar] [CrossRef]
- Lee, M.; Ok, K.M. Chiral coordination compounds with exceptional enantioselectivity. J. Mater. Chem. C 2022, 10, 17127–17134. [Google Scholar] [CrossRef]
- Alizada, M.; Gul, A.; Oguz, M.; Kursunlu, A.N.; Yilmaz, M. Ion sensing of sister sensors based-on calix[4]arene in aqueous medium and their bioimaging applications. Dyes. Pigment. 2021, 184, 108741. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Sahin, E.; Güler, E. Cu(II) chemo sensor based on a fluorogenic bodipy-salophen combination: Sensitivity and selectivtity studies. J. Fluoresc. 2016, 26, 1997–2004. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.Y. Photoluminescent metal-organic frameworks and their application for sensing biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Raja Lakshmi, P.; Nanjan, P.; Kannan, S.; Shanmugaraju, S. Recent advances in luminescent metal-organic frameworks (LMOFs) based fluorescent sensors for antibiotics. Coord. Chem. Rev. 2021, 435, 213793. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.W.; Lu, W.; Li, D. Metal-organic frameworks as photo luminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.E.; Roncaroli, F. Photofunctional metal-organic framework thin films for sensing, catalysis and device fabrication. Inorg. Chim. Acta 2020, 513, 119926. [Google Scholar] [CrossRef]
- Lin, X.; Hong, Y.; Zhang, C.; Huang, R.; Wang, C.; Lin, W. Pre-Concentration and energy transfer enable the efficient luminescence sensing of transition metal ions by metal-organic frameworks. Chem. Commun. 2015, 51, 16996–16999. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Hu, P.; Kirchon, A.; Zhao, Y.-M.; Pang, J.; Zhang, T.; Zhou, H.-C. Facile fabrication of a multifunctional metal-organic framework-based sensor exhibiting exclusive solvochromic behaviors toward ketone molecules. ACS Appl. Mater. Interfaces 2019, 11, 8227–8233. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical sensors based on metal-organic frameworks. Chempluschem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Huang, F.M.; Wang, M.; Lin, C.; Wang, J.; Wu, P. Luminescent metal-organic frameworks as chemical sensors based on “mechanism-response”: A review. Dalton Trans. 2021, 50, 3429–3449. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; Li, M.; O’Keeffe, M.; Eddaoudi, M. Reticular chemistry 3.2: Typical minimal edge-transitive derived and related nets for the design and synthesis of metal-organic frameworks. Chem. Rev. 2020, 120, 8039–8065. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S.B.; Nayak, S.; Briggs, M.E.; Jansat, S.; Katsoulidis, A.P.; Miller, G.J.; Warren, J.E.; Antypov, D.; Cora, F.; Slater, B.; et al. Chemical and structural stability of zirconium-based metal-organic frameworks with large three-dimensional pores by linker engineering. Angew. Chem. Int. Ed. 2015, 54, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Q.; Zhang, G. A carbazole-functionalized metal-organic framework for efficient detection of antibiotics, pesticides and nitroaromatic compounds. Dalton Trans. 2019, 48, 2683–2691. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Jindal, S.; Savitha, G.; Moorthy, J.N. Temperature-dependent emission and turn-off fluorescence sensing of hazardous “Quat” herbicides in water by a Zn-MOF based on a semi-rigid dibenzochrysenetetra acetic acid linker. Inorg. Chem. 2020, 59, 6202–6213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lei, M.; Zhang, T.; Zhang, Q.; Zhang, R.; Yang, M. A water-stable multi-responsive luminescent Zn-MOF sensor for detecting TNP, NZF and Cr2O72− in aqueous media. Dalton Trans. 2021, 50, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Zhang, L.; Liu, S.; Yuan, G. 8-Hydroxyquinolinate-based metal-organic frameworks: Synthesis, tunable luminescent properties, and highly sensitive detection of small molecules and metal ions. Inorg. Chem. 2019, 58, 2444–2453. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, M.; Kong, F.; Yang, Y. A water-stable homochiral luminescent MOF constructed from an achiral acylamide-containing dicarboxylate ligand for enantioselective sensing of penicillamine. Chem. Commun. 2018, 54, 10901–10904. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Mukhopadhyay, A.; Savitha, G.; Moorthy, J.N. Remarkably selective and enantio differentiating sensing of histidine by a fluorescent homochiral Zn-MOF based on pyrene-tetralactic acid. Chem. Sci. 2016, 7, 3085–3091. [Google Scholar] [CrossRef]
- Jiao, J.; Dong, J.; Li, Y.; Cui, Y. Fine-tuning of chiral microenvironments within triple-stranded helicates for enhanced enantioselectivity. Angew. Chem. Int. Ed. 2021, 60, 16568–16575. [Google Scholar] [CrossRef]
- Thoonen, S.; Tay, H.M.; Hua, C. A chiral binaphthyl-based coordination polymer as an enantioselective fluorescence sensor. Chem.Commun. 2022, 58, 4512–4515. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, D.; Han, S.D.; Pan, J.; Xue, Z.Z.; Li, J.H.; Wang, G.M. A pillared-layer strategy to construct water-stable Zn-organic frameworks for iodine capture and luminescence sensing of Fe3+. Dalton Trans. 2019, 48, 602–608. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Zhang, F.; Cui, Y. A highly fluorescent metallosalalen-based chiral cage for enantioselective recognition and sensing. Chem. Eur. J. 2014, 20, 6455–6461. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tan, C.; Zhang, K.; Liu, Y.; Low, P.J.; Jiang, J.; Cui, Y. Chiral NH-controlled supra molecular metallacycles. J. Am. Chem. Soc. 2017, 139, 1554–1564. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral metal-organic frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhang, M.; Liu, Y.; Chen, Z.; Cui, Y. A chiral quadruple-stranded helicate cage for enantioselective recognition and separation. J. Am. Chem. Soc. 2012, 134, 6904–6907. [Google Scholar] [CrossRef]
- Wanderley, M.M.; Wang, C.; Wu, C.D.; Lin, W. A chiral porous metal-organic framework for highly sensitive and enantioselective fluorescence sensing of amino alcohols. J. Am. Chem. Soc. 2012, 134, 9050–9053. [Google Scholar] [CrossRef]
- Karmakar, M.; Chattopadhyay, S. Synthesis, structure and nitroaromatic sensing ability of a trinuclear zinc complex with a reduced Schiff base ligand: Assessment of the ability of the ligand to sense zinc ion. Polyhedron 2020, 187, 114639. [Google Scholar] [CrossRef]
- Karmakar, M.; Basak, T.; Chattopadhyay, S. penta-nuclear zinc(II) complex with a reduced Schiff base ligand: Assessment of its ability to sense nitroaromatics. New J. Chem. 2019, 43, 4432–4443. [Google Scholar] [CrossRef]
- Dasgupta, S.; Zangrando, E.; Majumder, I. A Comparative Study on “Turn-off” Fluorimetric Nitro Aromatic Detection Using a Class of Dinulear Zinc (II) Schiff Base Complexes. ChemistrySelect 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Xin, X.H.; Lu, W.; Lu, J.; Xu, J.G.; Wang, S.H.; Zheng, F.K.; Guo, G.G. A luminescent barium-based metal-organic framework: Synthesis, structure and efficient detection of 4-nitrobenzoic acid. Inorg. Chem. Commun. 2018, 97, 129–133. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ganguly, S.; Chakraborty, A.; Mandal, A.; Das, D. Green Synthesis of Self Assembled Nanospherical Dysprosium MOFs: Selective and Efficient Detection of Picric Acid in Aqueous and Gas Phase. ACS Sustain. Chem. Eng. 2019, 7, 819–830. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, X.; Zhang, L.; Eubank, J.F.; Liu, X.; Liu, Y. Unique Fluorescence Turn-On and Turn-Off−On Responses to Acids by a Carbazole-Based Metal−Organic Framework and Theoretical Studies. J. Am. Chem. Soc. 2022, 144, 17054–17063. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, N.; Iki, N.; Sugawara, A.; Miyano, S. Selective oxidation of thiacalix[4]arenes to the sulfinyl and sulfonyl counterparts and their complexation abilities toward metal ions as studied by solvent extraction. Tetrahedron 2001, 57, 5557–5563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-M.; Bai, Y.-M.; Ai, L.-L.; Wu, F.-H.; Shan, W.-L.; Kang, Y.-S.; Luo, L.; Chen, K.; Xu, F. A Chiral Metal—Organic Framework Prepared on Large-Scale for Sensitive and Enantioselective Fluorescence Recognition. Molecules 2023, 28, 4593. https://doi.org/10.3390/molecules28124593
Zhang X-M, Bai Y-M, Ai L-L, Wu F-H, Shan W-L, Kang Y-S, Luo L, Chen K, Xu F. A Chiral Metal—Organic Framework Prepared on Large-Scale for Sensitive and Enantioselective Fluorescence Recognition. Molecules. 2023; 28(12):4593. https://doi.org/10.3390/molecules28124593
Chicago/Turabian StyleZhang, Xin-Mei, Yan-Mei Bai, Lu-Lu Ai, Fang-Hui Wu, Wei-Long Shan, Yan-Shang Kang, Li Luo, Kai Chen, and Fan Xu. 2023. "A Chiral Metal—Organic Framework Prepared on Large-Scale for Sensitive and Enantioselective Fluorescence Recognition" Molecules 28, no. 12: 4593. https://doi.org/10.3390/molecules28124593
APA StyleZhang, X.-M., Bai, Y.-M., Ai, L.-L., Wu, F.-H., Shan, W.-L., Kang, Y.-S., Luo, L., Chen, K., & Xu, F. (2023). A Chiral Metal—Organic Framework Prepared on Large-Scale for Sensitive and Enantioselective Fluorescence Recognition. Molecules, 28(12), 4593. https://doi.org/10.3390/molecules28124593



