Abstract
This study identified phytochemicals in Argemone mexicana (A. mexicana) extracts that are responsible for its medicinal properties, and the best solvent for their extraction. The extracts of the stem, leaves, flowers, and fruits of A. mexicana were prepared at low (corresponding to room temperature) and high temperatures (corresponding to the boiling points) in various solvents, viz., hexane, ethyl acetate, methanol, and H2O. The UV-visible absorption spectra of various phytoconstituents in the isolated extracts were determined through spectrophotometry. Qualitative tests for the screening of phytoconstituents in the extracts were performed to identify various phytochemicals. We identified the presence of terpenoids, alkaloids, cardiac glycosides, and carbohydrates in the plant extracts. The antioxidant and anti-human immunodeficiency virus type 1 reverse transcriptase (anti-HIV-1RT) potential, as well as the antibacterial activity of various A. mexicana extracts were determined. These extracts showed strong antioxidant activities. The extracts exhibited antimicrobial activities against Salmonella typhi, Staphylococcus epidermis, Citrobacter, Neisseria gonorrhoeae, and Shigella flexineri. These extracts significantly inhibited HIV-1 reverse transcriptase activity. The aqueous leaf extract prepared at a temperature equivalent to the boiling point, i.e., 100 °C, was identified to be the most active against pathogenic bacteria and HIV-1 RT.
1. Introduction
Argemone mexicana L. (A. mexicana) (Papaveraceae), also known as the Mexican prickly poppy or Mexican poppy, grows in the tropical and subtropical regions globally. Argemone mexicana L. is mainly found in Mexico but it is now widely distributed across many parts of the world, including India, Bangladesh, the United States and Ethiopia [1,2]. A. mexicana, a globally used medicinal plant, serves as a source of many alternative medicines. The herbs/shrubs and many trees present in the wild state possess a huge number of novel phytochemicals of medicinal significance. Studies on the wild species of certain weeds have been considered of great importance for the treatment of various diseases [3].
The yellow exudate of A. mexicana has been used to treat dropsy, jaundice, scabies, and skin diseases [4,5]. The flowers, leaves, and seeds of this plant have been used to treat diverse diseases including dysentery, ulcers, cough, and hypertension [5,6,7,8,9]. A. mexicana also exhibits hepatoprotective, anticancer, anti-HIV, antiproliferative, anti-inflammatory, antibacterial, antidiabetic, antifertility, antiallergic, nematocidal, and antioxidant activities [10,11]
The leaves and stems of A. mexicana are employed to treat malaria and dropsy. They possess anti-analgesic, antispasmodic, antiparasitic, and narcotic properties with antifungal, hepatoprotective, larvicidal, and chemosterilant activities [12,13]. The leaf extract has been used as a disinfectant, whereas the seed extract has been employed for the treatment of leprosy, warts, skin diseases, and insect bites. These activities are attributed to secondary metabolitesand protein hydrolyzing substances [1,14].
The extracts of different parts of A. mexicana are used in different forms. For example, the root paste is used to treat insect and scorpion bites, etc., in the form of ointment for external use for the treatment of wound healing. For constipation and bloating, the root powder is used orally at a dose of 1–2 g/day. The fruit extracts of A. mexicana have been used as intra-peritoneal injections in anticancer studies in mice [15]. The latex from A. mexicana is useful to treat conjunctivitis, while the oil from the seeds is used to treat asthma, dysentery, and ulcers, etc. [16].
The secondary metabolites of A. mexicana such as flavonoids, polyphenols, phenols, alkaloids, saponins, and tannins, have been shown to be responsible for exhibiting medicinal properties. These secondary metabolites isolated from different parts of A. mexicana contain antimicrobial activities against different species of fungal, bacterial, and viral pathogens [11,17].
The biomedical properties of these phytochemicals isolated in different solvents at high and low temperatures have not been properly studied. In view of this background and the traditional uses of A. mexicana as a medicinal plant, the aim of this study was to optimize the best solvent and temperature for the extraction of secondary metabolites from different parts, namely the leaves, stem, fruits, and flowers of A. mexicana. We have attempted to characterize the chemical ingredients present in the different extracts of the plant through chemical analysis, monitoring their retardation factor (Rf) values employing thin layer chromatography (TLC), and determining the differential absorption of light at varying UV and visible ranges. Further, it has been endeavored to assess their antimicrobial, and antiviral (especially anti-HIV reverse transcriptase) activities.
2. Results
2.1. Spectrophotometric Analysis of Phytochemicals from A. mexicana Extracts at Room Temperature
We noted major absorption peaks at 276, 370, 411, and 669 nm, indicating that flavonoids were the major compounds in the A. mexicana leaf extract prepared at room temperature in hexane, ethyl acetate, methanol and aqueous medium. Pheophytin A was the second most abundant chemical compound present in the leaf extract (peaks observed at 370, 411, and 669 nm). However, these peaks were not noted in the aqueous extract. Unsaturated carbonyl compounds were found in only the aqueous leaf extract at 198 nm and at a lower concentration of the extract (250 µg/mL).
The stem extracts prepared at room temperature in hexane, ethyl acetate, methanol, and aqueous medium revealed major peaks at 332, 340, 410, and 669 nm, indicating the presence of flavonoids. Pheophytin A was present only in a few sections of the extract at 371, 402, and 667 nm. The unsaturated carbonyl compounds were found in only the aqueous stem extract at 340, 415, and 669 nm and at a lower concentration (250 µg/mL). The fruit extract prepared at room temperature in hexane, ethyl acetate, methanol, and aqueous medium revealed peaks at 206, 232, 333, 301, 410, and 667 nm, indicating the occurrence of pheophytin A and flavonoids. Unsaturated carbonyl compounds were detected at 206 and 221 nm. (Supplementary Figures and Tables).
2.2. Spectrophotometric Analysis of Phytochemicals from A. mexicana Extracts at a High Temperature
We noted the peaks in the visible range (190 to 700 nm), which indicated the occurrence of phytochemicals. In the leaf extract produced at a high temperature in hexane, ethyl acetate, methanol, and aqueous medium, the most abundant compounds were flavonoids, with absorption peaks observed at 231, 236, 405, 666, and 668 nm. Pheophytin A (absorbance peaks at 237, 376, and 669 nm) and the unsaturated carbonyl compounds (peak at 205 nm) were also observed.
The stem and fruit prepared at a high temperature in hexane, ethyl acetate, methanol, and aqueous medium extracts contained flavonoids, with absorption peaks noted at 227, 350, 380, 666, and 669 nm. Pheophytin A was the second most abundant phytochemical in the stem extract across all the solvents; the peak was noted at 422 nm. Pheophytin A was not present in the methanolic extract of the fruit, and the extracts of stem and fruit prepared in water. The unsaturated carbonyl compounds were found in the aqueous stem extract at 208 nm only. In the flower extract, flavonoids and pheophytin A were identified (239, 351, and 669 nm) in the methanolic extract, and unsaturated carbonyl compounds at 210 nm at lower solvent concentrations (Supplementary Figures and Tables).
2.3. Phytochemical Analysis of Phytochemicals from A. mexicana Extracts at Room Temperature as Detected by TLC
To confirm the results obtained from spectral analysis, TLC of the fractions which showed the presence of optimal concentrations of phytochemicals was carried out. These results are presented in Figure 1. The ethyl acetate fruit extract of A. mexicana showed the presence of alkaloids, flavonoids, primary and secondary amines. Other phytochemicals included antioxidants, carbohydrates, saponins, glycosides, essential oil, phenols, mycotoxin, and terpenes.
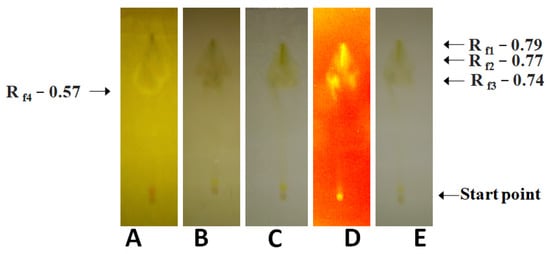
Figure 1.
Phytochemical analysis of the A. mexicana fruits prepared in ethyl acetate at room temperature. (A): Dragendorff’s reagent treatment detecting alkaloids, and primary/secondary amines, (B): anisaldehyde–sulfuric acid reagent treatment detecting various natural products, (C): Mayer’s reagent treatment detecting alkaloids, (D): UV light treatment detecting photoactive compounds, and (E): visible light treatment detecting photoactive compounds.
2.4. Phytochemical Analysis of A. mexicana Extracts Prepared at the Boiling Points of Solvents (High Temperature) Using TLC
The results are shown in Figure 2. The extract of A. mexicana leaves produced in water at 100 °C indicated the occurrence of flavonoids and the primary/secondary amines. Antioxidants, glycosides, steroids, carbohydrates, prostaglandins, saponins, essential oil, phenols, and terpenoids were also detected.
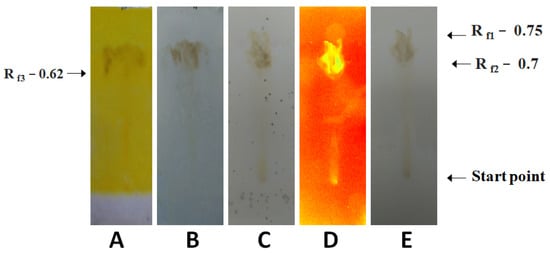
Figure 2.
Analysis of phytochemicals present in the A. mexicana leaf extract prepared in water at a high temperature (100 °C). (A): Dragendorff’s reagent treatment detecting alkaloids, and primary/secondary amines, (B): anisaldehyde–sulfuric acid reagent treatment detecting various natural products, (C): Mayer’s reagent treatment detecting alkaloids, (D): UV light treatment detecting photoactive compounds, and (E): visible light treatment detecting photoactive compounds.
The methanolic fruit extract showed the presence of prostaglandins, antioxidants, carbohydrates, steroids, mycotoxin, phenols, saponins, terpenes, glycosides, and essential oil (Figure 3).
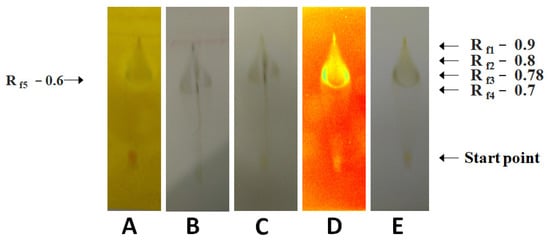
Figure 3.
The analysis of the phytochemicals occurring in the extract of A. mexicana fruits prepared in the methanol at its boiling point (high temperature). (A): Dragendorff’s reagent treatment detecting alkaloids, and primary/secondary amines, (B): anisaldehyde–sulfuric acid reagent treatment detecting various natural products, (C): Mayer’s reagent treatment detecting alkaloids, (D): UV light treatment detecting photoactive compounds, and (E): visible light treatment detecting photoactive compounds.
2.5. Chemical Characterization of A. mexicana Extracts
2.5.1. Qualitative Detection of the Bioactive Compounds Present in the Extracts Produced at Room Temperature
Table 1 lists the various phytochemicals identified in different A. mexicana extracts prepared in diverse solvents at room temperature and high temperature.

Table 1.
Qualitative estimation of the bioactive compounds occurring in the extracts of Argemone mexicana prepared at room and high temperatures.
The leaf extract prepared in these solvents contained terpenoids. The ethyl acetate extract contained cardiac glycosides, phenols, and traces of flavonoids. The aqueous and methanolic leaf extracts contained carbohydrates, phenols, quinones, and traces of flavonoids.
The stem extract of A. mexicana contained only terpenoids, flavonoids, quinones, and small amounts of steroids. Carbohydrates, cardiac glycosides, flavonoids, phenols, quinones, steroids, and terpenoids were detected in the ethyl acetate extract made at a low temperature. The methanolic extract produced at room temperature contained high amounts of alkaloids, carbohydrates, flavonoids, phenols, quinones, saponins, steroids, and terpenoids. The aqueous extract prepared under this condition contained only carbohydrates, phenols, and quinones.
The fruit extract obtained in hexane at a low temperature contained cardiac glycosides, quinones, terpenoids, and phenols. The ethyl acetate extract contained cardiac glycosides, steroids and terpenoids, phenols, and quinones, with traces of carbohydrates. The methanolic fruit extract contained alkaloids, carbohydrates, cardiac glycosides, flavonoids, quinones, steroids, terpenoids, and traces of phenol, whereas the aqueous extract contained carbohydrates, flavonoids, terpenoids, tannins, phenol, and quinones.
The flower extract obtained in various solvents at a low temperature did not have significant phytochemicals (unpublished data).
2.5.2. Qualitative Analysis of the Phytochemicals Present in Different A. mexicana Extracts Prepared at a High Temperature
The leaf extract in hexane obtained at a high temperature contained terpenoids, quinones, and alkaloids, with carbohydrates and cardiac glycosides in small amounts. The ethyl acetate leaf extract contained cardiac glycosides, terpenoids and small amounts of phenols and flavonoids. The leaves extracted in methanol at a high temperature contained high amounts of alkaloids, carbohydrates, phenols, terpenoids, and tannins. Similar phytochemicals were found in the aqueous leaf extract made at 100 °C, except for small amounts of quinones.
The stem extracted in hexane at a high temperature contained carbohydrates, cardiac glycosides, quinones, and terpenoids in significant amounts, followed by phenols and traces of flavonoids. The ethyl acetate extract contained cardiac glycosides, terpenoids, quinones, phenols, and small amounts of flavonoids. Methanol and aqueous stem extracts prepared at a high temperature contained significant amounts of alkaloids, carbohydrates, phenols, terpenoids, and tannins. The aqueous extract also contained quinones.
The fruit extract prepared in hexane contained cardiac glycosides, quinones, steroids, terpenoids, and small amounts of flavonoids and phenols. The ethyl acetate extract contained cardiac glycosides, steroids, and terpenoids, as well as small amounts of carbohydrates, phenols, and quinones. Methanolic and aqueous fruit extracts prepared at a high temperature contained significant amounts of carbohydrates, phenols, quinones, and terpenoids. Steroids were present in methanolic extracts, but absent in aqueous extracts. The aqueous extracts, but not methanolic extracts, contained alkaloids and tannins.
Only the flower extracts prepared using methanol and water had detectable phytochemicals. The methanolic flower extract obtained at a high temperature contained significant amounts of carbohydrates, phenols, quinones, alkaloids, cardiac glycosides, terpenoids, and traces of flavonoids. The aqueous extract contained carbohydrates, phenols, quinones, tannins, and terpenoids, as well as small amounts of alkaloids and flavonoids (Table 1).
2.5.3. Free-Radical Neutralizing Ability of A. mexicana Extracts
The leaf extract revealed the highest free-radical scavenging activity in water, followed by methanol and ethyl acetate at room temperature and the temperature equivalent to their boiling points. The free-radical neutralizing ability of stem extracts in various solvents were as follows: methanol > aqueous > ethyl acetate in extracts prepared at low temperatures. The free-radical quenching activity of the stem extracts obtained at high temperatures was in the following order: ethyl acetate > water > methanol. The fruit extract made at room temperature exhibited similar activity in methanol and water. The fruit extract made at a temperature equivalent to their boiling point revealed similar activity in water and methanol, which was less than that in ethyl acetate. The flower extract displayed free-radical scavenging activity only when made at a temperature equivalent to the boiling points in methanol and water. The IC50 values are listed in Table 2.

Table 2.
Free-radical neutralizing ability of Argemone mexicana extracts prepared at room temperature and high temperature.
2.6. Effect of A. mexicana Extract against the Activity of HIV-1 RT
The leaf extract prepared in distilled water at a high temperature exhibited the maximum inhibitory effect against HIV-1 RT, with an IC50 value of 0.044 mg/mL, followed by ethyl acetate and methanolic extracts. Only the leaf extract prepared in the ethyl acetate at room temperature displayed HIV-1 RT activity (Table 3). Similarly, only the ethyl acetate fruit extract prepared at high and low temperatures exhibited HIV-1 RT activity. No activity of anti-HIV-RT was detected in other extracts.

Table 3.
Effect of Argemone mexicana extracts prepared at a high temperature and room temperature on HIV-1 RT activity.
2.7. Antimicrobial Activity of Various A. mexicana Extracts
The antimicrobial effects of different extracts prepared at room temperature against different bacterial strains are listed in Table 4. Ampicillin (5 mg/mL) was used as a standard. The ethyl acetate and methanolic leaf extracts prepared at a low temperature were effective against S. typhi, Citrobacter, Shigella flexineri, and S. epidermis. Neisseria gonorrhoeae was inhibited only by the ethyl acetate leaf extract synthesized at room temperature. The ethyl acetate and methanolic fruit extracts prepared at room temperature inhibited Salmonela typhi, Neisseria gonorrhoeae, and S. flexineri. S. epidermis was inhibited only by the methanolic fruit extract.

Table 4.
Argemone mexicana extracts prepared at room temperature, showing antibacterial properties against various pathogenic bacterial strains.
Table 5 presents the antimicrobial effects of the plant extracts synthesized at the temperature equivalent to the boiling points of the solvents. Methanol and aqueous leaf extracts prepared at high temperatures inhibited Gonococci and Citrobacter. Salmonella typhi was inhibited by aqueous and ethyl acetate extracts, and Staphylococcus epidermis was inhibited only by the ethyl acetate leaf extract. Methanolic and ethyl acetate fruit extracts prepared at a high temperature inhibited Salmonella typhi and Citrobacter. Only the methanolic fruit extract inhibited Gonococci and S. flexineri, whereas S. epidermis was inhibited only by the ethyl acetate fruit extract.

Table 5.
Effects of the extracts of leaves and fruits of Argemone mexicana prepared at a high temperature on various pathogenic bacterial strains.
3. Discussion
A. mexicana belongs to the Papaveraceae family, which possesses antibacterial [18,19,20], antifungal [21,22], antiviral [23,24], antioxidant [25,26], and cytotoxic/anticancer [27] properties. The emergence of drug-resistant microbial strains [28] has necessitated the search for antimicrobial compounds from natural sources, such as plants and marine microorganisms. Many studies have determined the antimicrobial effects and secondary metabolites of A. mexicana [29,30]. Recently, A study by Orozco-Nunnelly et al., 2021 [28] has shown that the methanolic extract of outer roots and leaves displayed strong antimicrobial activities, especially against Gram-positive bacteria. In this study, different extracts of A. mexicana have been shown tocause an inhibition in the growth of both Gram +ve and Gram −ve bacteria. The results from the present study revealed that the temperatures corresponding to the solvents’ boiling points resulted in a better yield of phytochemicals. Terpenoids were the most abundant, followed by phenols, flavonoids, carbohydrates, and cardiac glycosides, as also reported by [29]. The leaf and the stem extracts exhibited higher free-radical scavenging activity than the fruit and flower extracts, which may be attributed to phenolic compounds [31]. The leaf extract possessed antioxidant activity, as also shown by Perumal et al. (2010) [32].
HIV uses reverse transcriptase (RT) to convert its RNA to cDNA. Ishizuka et al., 2020 [23] have shown that RT exhibits RNA- and DNA-dependent DNA polymerase activities and RNase H activity. In the present study, the aqueous extract of leaves prepared at a high temperature indicated the maximum inhibitory activity against HIV-1 RT, much like any other nonnucleoside reverse transcriptase inhibitor (NNRTI), as demonstrated by Sanna et al., 2018 [33]. The fruit extracts prepared at a high temperature also exhibited anti-HIV-RT activity, which may be attributed to the presence of flavonoids and alkaloids in these extracts [34]. Nuciferine, an alkaloid in the roots of A. mexicana, has been reported to be responsible for its anti-HIV-1RT activity [35]. The methanolic extracts of the entire A. mexicana contains benzo[c]phenanthridine alkaloid and (±)-6-acetonyl dihydrochelerythrine, which exhibited anti-HIV activity [35]. The anti-HIV-RT property was found to be mainly associated with the aqueous extract of the leaves at a high temperature. The results from earlier studies in collaboration with other works have shown anti-HIV-RT activity by some organic compounds present in the leaf extracts, displaying an inhibition of HIV-1 DNA polymerase function [23]. Various plant extracts exhibited antimicrobial activities against different test organisms, namely S. typhi, Gonococci, Citrobacter, S. flexineri, and S. epidermis. Among the extracts prepared at room temperatures, the methanolic extract revealed the best antimicrobial activity. On the other hand, among the extracts prepared at high temperatures, the aqueous extract revealed the maximum antimicrobial activity. The antimicrobial activity may be attributed to phenols, terpenoids, and alkaloids [36].
The methanolic extracts of the outer root of A. mexicana possessed maximum activity against Gram+ve bacteria, and minimum activity against the Gram−ve bacteria and fungi (Orozco-Nunnelly et al., 2021) [28]. The antimicrobial activity of the leaf extract can be attributed to the presence of berberine, an alkaloid [28]. Berberine exerts its effect by damaging the cell membrane and impeding the synthesis of proteins and DNA [37]. Berberin chloride, in combination with various anti-staphylococcal drugs as a reference of CoNS strains, varied greatly across different bacterial stains and drugsused [38]. Rahman et al., 2011 [19] reported that the leaf extract of A. mexicana inhibited Salmonella sp, which was also noted in this study.
4. Materials and Methods
4.1. Reagents and Chemicals
The reagents Luria–Bertani broth (Miller, Appleton, WI, USA) and Mueller–Hinton agar were procured from SRL Pvt. Ltd. (Mumbai, India), Himedia, whereas the ampicillin sodium injection was obtained from Saralife (New Delhi, India). Silica-gel-coated TLC plates, Wagner’s reagent, and Molisch reagent were procured from Merck, Darmstadt, Germany. All other reagents used were of analytical grade.
4.2. Plant Material Collection and Extract Preparation
A. mexicana plants were collected from Prayagraj, India, and nearby areas. The leaves, stems, flowers, and fruits were collected. The collected plant materials were washed under running tap water and then with double-distilled water. The plant parts were then dried in shade and powdered. The powder was dissolved in the ethyl acetate, hexane, water, and methanol at a low temperature (room temperature) and high temperature (temperatures equivalent to the boiling points of the solvents such as 77.1 °C, 68 °C, 100 °C, and 64.7 °C, respectively). The extracts at a high temperature were prepared using the Soxhlet apparatus. These extracts were dried at room temperature and used by dissolving them into the desired volume of the solvents for using them at different concentrations for carrying out different specific experiments, or stored at −20 °C until further use.
4.3. Analysis of A. mexicana Extract Spectrophotometrically
The spectrophotometric analysis of the extracts resulted in different spectral patterns corresponding to the specific absorption profiles under UV and visible wavelengths of various components present in the isolated extracts determined through spectrophotometry. The UV-visible spectra of different concentrations of extracts were placed in a quartz cuvette and recorded from 190 to 750 nm by using the double-beam UV-visible spectrophotometer (model: Scientific Spectroscan UV2700, Thermo Fisher Scientific India Pvt. Ltd., Pune, India) [39,40]. The analysis of the absorption peaks was carried out and compared with the peaks of specific bioactive compounds.
4.4. Thin Layer Chromatography (TLC)
The TLC plates coated with the silica gel (Merck, Darmstadt, Germany) were employed for the analysis of different phytochemicals present in the various extracts of A. mexicana. The fractions of which the UV-visible absorption spectra showed the presence of optimal concentrations of phytochemicals were subject to thin layer chromatography to confirm the results.
4.5. Assay of Phytochemicals
Phytoconstituents including alkaloids, carbohydrates, cardiac glycosides, flavonoids, phenols, steroids, and saponins present in the extracts were qualitatively evaluated using previously described standard procedures [41,42].
4.6. 2,2-Diphenyl-1-picrylhydrazyl Assay
The free radical quenching potential of various extracts was determined using the 2, 2-diphenyl-1-picrylhydrazyl assay (DPPH), as described previously [43], with some modifications. Of the 10 mM DPPH solution, 1 mL was added to different extract concentrations (25, 50, 100, 200, 300, and 500 µg/mL). After 30 min incubation at room temperature, the absorbance was determined at 517 nm. The ascorbic acid (1%) was used as a standard. The free radical scavenging capacity of the extracts was calculated as shown below:
Scavenging activity of DPPH (%) = [(Absorption by control − Absorption by the sample)/(Absorption by control) × 100]
4.7. Examination of Different Plant Extracts for Their Anti-HIV-1RT Activity
The anti-HIV-1RT activity of different plant extracts was determined by employing RT colorimetric assay kits (Roche, NY, USA), in accordance with the manufacturer’s instruction.
4.8. Antimicrobial Activity
4.8.1. Test Microorganisms
Clinically pathogenic bacteria, namely Neisseria gonorrhoeae, Salmonella typhi, Citrobacter, Staphylococcus epidermidis, and Shigella flexineri, were used. These organisms were obtained from the Medical Sciences Institute of BHU, Varanasi, India.
4.8.2. Antibacterial Activity
The agar-well diffusion method [44] was used to examine antibacterial susceptibility. Sterilized discs (6 mm) soaked in different extract concentrations (100, 200, 300, and 500 μg in 5 μL volume each) of extracts were placed on solid media with sterile forceps. Water and ampicillin (5 μg/mL) were employed as the negative and positive controls, respectively. The petri plates were placed for 24 h at 37 °C. The diameter of the zone of inhibition (mm) after 24 h incubation was used as a measure of antimicrobial activity.
4.9. Statistical Analysis
We performed all experiments in triplicate. Data are presented as the mean ± SE of the three replicates. All the data were statistically analyzed using (MS Excel)—Microsoft 365. (Graph pad Prism)—GraphPad Software, Inc., San Diego, CA, USA.
5. Conclusions
In the present study, the leaf extract of A. mexicana prepared in an aqueous medium at high temperatures was found to be the most active against HIV 1-RT and the tested microorganisms. Water at a high temperature was the best solvent to extract the pharmacological activities of bioactive molecules from A. mexicana. The pharmacological properties of A. mexicana may be attributable to various compounds, which may exert their actions through specific and nonspecific mechanisms. Therefore, active chemical components should be identified to elucidate their mechanisms of actions for the scientific utilization of the plant to cure various diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28114428/s1, Figure S1: Spectrophotometric analysis of the phytochemicals present in various parts of A. mexicana, extracts prepared at room temperature (24 ± 2 °C) in various solvents. A- hexane, B-ethyl acetate, C-methanol, and D-water; Figure S2: Spectrophotometric analysis of the phytochemicals present in various parts of A. mexicana, extracts prepared at high temperature in various solvents (corresponding to their boiling points). A-hexane, B-ethyl acetate, C-methanol, and D-water.; Table S1: Spectrophotometric analysis of A. mexicana extractsprepared at room temperature; Table S2: Spectrophotometric analysis of A. mexicana extractsprepared at high temperature [45,46].
Author Contributions
J.J.: Data curation, Formal analysis, Investigation, Methodology and paper writing; N.J.S.: Conceptualization, Resources, Writing—draft of manuscript, Writing—review and editing; S.F.: Resources, Preparing the draft, Formal analysis; M.A.: Funding acquisition, Visualization; S.K.A.: Resources, Writing manuscript; M.G.A.: Resources, Writing draft of the manuscript; M.d.L.P.: Conceptualization, Writing—review and editing; P.S.: Writing draft of the manuscript, Resources; B.S.: organization of the whole manuscript and interpretation of results; Conceptualization, Resources, Supervision, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Researchers Supporting Project (number RSPD2023R656), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (number RSPD2023R656), King Saud University, Riyadh, Saudi Arabia. M.d.L.P. is grateful to the Project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 and LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Alagesaboopathi, C.; Kalaiselvi, N. Antimicrobial activities of the root, stem and leaf extracts of Argemone mexicana L. Int. J. Biosci. 2012, 2, 61–68. [Google Scholar]
- Jaiswal, J.; Sharma, B. A Comparative Study of Antimicrobial and Pharmacological Properties of Argemone mexicana, Solanum xanthocarpum and Thevetia peruviana. Acta Sci. Microbiol. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Alam, A.; Khan, A. Argemone mexicana L.: A weed with versatile medicinal and pharmacological applications. Annu. Phytomed. Int. J. 2020, 9, 218–223. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; NISCOM CSIR: New Delhi, India, 1956; p. 23. [Google Scholar]
- Sharma, J.; Gairola, S.; Gaur, R.D.; Painuli, R.M. The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2012, 143, 262–291. [Google Scholar] [CrossRef]
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants; Agrobios: Jodhpur, India, 2003; pp. 59–60. [Google Scholar]
- Savithramma, N.; Sulochana, C.; Rao, K.N. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh. India J. Ethnopharmacol. 2007, 113, 54–61. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, U.P.; Monteiro, J.M.; Ramos, M.A.; de Amorim, E.L. Medicinal and magic plants from a public market in northeastern Brazil. J. Ethnopharmacol. 2007, 110, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Bieski, I.G.; Rios Santos, F.; de Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; de Oliveira Martins, D.T. Ethnopharmacology of medicinal plants of the pantanal region (Mato Grosso, Brazil). Evid. Based Complement. Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef] [PubMed]
- Nancy, A.; Praveena, A. Argemone mexicana: A Boon to Medicinal and Pharmacological Approaches in Current Scenario. Cardiovasc. Hematol. Agents Med. Chem. 2017, 15, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Favela-Hernandez, J.M.; Guerrero, G.; Garcia-Lujan, C. Phytochemical, Pharmacological and Antimicrobial Properties of the Tissue Extracts of Argemone spp. Acta Sci. Microbiol. 2023, 6, 1–15. [Google Scholar]
- Singh, S.K.; Pandey, V.D.; Singh, A.; Singh, C. Antibacterial activity of seed extracts of Argemone mexicana L. on some pathogenic bacterial strain. Afr. J. Biotechnol. 2009, 8, 7077–7081. [Google Scholar]
- Malik, C.; Mohanty, J.P.; Pradhan, S.; Sharma, C. Phytochemistry and pharmacology of Argemone mexicana Linn-An Indian medicinal plant. Res. J. Pharm. Phytochem. 2023, 15, 27–32. [Google Scholar]
- Panghal, M.; Arya, V.; Yadav, S.; Kumar, S.; Yadav, J.P. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. J. Ethnobiol. Ethnomed. 2010, 6, 4. [Google Scholar] [CrossRef]
- Jain, R.; Pandey, A.; Jain, R.S. Evaluation of Argemone mexicana fruits extract using micronucleus mssay in mouse bone marrow cells. Bull. Pharm. Sci. 2011, 1, 22–24. [Google Scholar]
- Elizondo-Luevano, J.H.; Verde-Star, J.; González-Horta, A.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In Vitro Effect of Methanolic Extract of Argemone mexicana against Trichomonas vaginalis. Korean J. Parasitol. 2020, 58, 135–145. [Google Scholar] [CrossRef]
- Ji, G.; Shukla, S.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive Effect of Argemone mexicana Plant Extract on Acid Corrosion of Mild Steel. Ind. Eng. Chem. Res. 2011, 50, 11954–11959. [Google Scholar] [CrossRef]
- Bhattacharjee, I.; Chatterjee, S.K.; Chatterjee, S.; Chandra, G. Antibacterial potentiality of Argemone mexicana solvent extracts against some pathogenic bacteria. Mem. Inst. Oswaldo Cruz. 2006, 101, 645–648. [Google Scholar] [CrossRef]
- Rahman, M.S.; Salehin, M.F.; Jamal, A.H.M.; Parvin, A.; Alam, M.K. Antibacterial activity of Argemone mexicana L. against water borne microbes. Res. J. Med. Plant. 2011, 5, 621–626. [Google Scholar] [CrossRef]
- Sahu, M.C.; Padhy, R.N. In vitro antibacterial potency of Butea monosperma Lam. against 12 clinically isolated multidrug resistant bacteria. Asian Pac. J. Trop. Dis. 2013, 3, 217–226. [Google Scholar] [CrossRef]
- Kushtwar, R.S.; Tripathy, S. Study on antibacterial activity of aerial part of argemone mexicanalinn. Innov. Int. J. Med. Pharm. Sci. 2017, 6, 1025–1031. [Google Scholar]
- Andleeb, S.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Alkahtani, J.; Bukhari, S.M. In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. King Saud Univ. Sci. 2020, 32, 2053–2058. [Google Scholar] [CrossRef]
- Ishizuka, K.; Tsutsumi, Y.; Baba, M.; Biyani, R.; Meng, C.W.; Biyani, M.; Takagi, M.; Jaiswal, J.; Sharma, B.; Kojima, K.; et al. Inhibition of HIV-1 Reverse Transcriptase Activity by the Extracts of Indian Plants. Int. J. Biol. Macromol. 2020, 20, 17–22. [Google Scholar] [CrossRef]
- Pandeya, K.B.; Ganeshpurkar, A.; Mishra, M.K. Natural RNA dependent RNA polymerase inhibitors: Molecular docking studies of some biologically active alkaloids of Argemone mexicana. Med. Hypotheses 2020, 144, 109905. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Kar, D.; Nayak, S. Antidiabetic activity and modulation of antioxidant status by fractions of Argemone mexicana in alloxan induced diabetic rats. Int. J. Green. Pharm. 2012, 6, 321. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Rajeshkanna, A.; Senthamilselvi, M.M. Antioxidant and Anti-inflammatory activities of the flower extracts of Argemone mexicana L. Int. J. Res. Pharm. Sci. 2020, 11, 323–330. [Google Scholar] [CrossRef]
- More, N.V.; Kharat, A.S. Antifungal and Anticancer Potential of Argemone mexicana L. Medicines 2016, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Nunnelly, D.A.; Pruet, J.; Rios-Ibarra, C.P.; BocangelGamarra, E.L.; Lefeber, T.; Najdeska, T. Characterizing the cytotoxic effects and several antimicrobial phytocompounds of Argemone mexicana. PLoS ONE 2021, 16, e0249704. [Google Scholar] [CrossRef]
- Brahmachari, G.; Gorai, D.; Roy, R. Argemone mexicana: Chemical andpharmacological aspects. Rev. Bras. Farmacogn. 2013, 23, 559–575. [Google Scholar] [CrossRef]
- Khan, A.M.; Bhadauria, S. Analysis of medicinally important phytocompounds from Argemone mexicana. J. King Saud Univ. Eng. Sci. 2018, 31, 1020–1026. [Google Scholar] [CrossRef]
- Sivanandham, V. Free radicals in health and diseases—A mini review. Pharmacologyonline 2011, 1, 1062–1077. [Google Scholar]
- Perumal, P.; Sekar, V.T.; Rajesh, V.; Gandhimathi, S.; Sampathkumar, R.; Nazimudin, K.S. In vitro antioxidant activity of Argemone mexicana roots. Int. J. PharmTech Res. 2010, 2, 1477–1482. [Google Scholar]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Singh, S.K.; Mathur, A.S.; Singh, S.K. In vitro cytotoxicity of Argemone mexicana against Different Human Cancer Cell Lines. Int. J. Chem. Environ. Pharm. Res. 2010, 1, 37–39. [Google Scholar]
- Chang, Y.C.; Hsieh, P.W.; Chang, F.R.; Wu, R.R.; Liaw, C.C.; Lee, K.H.; Wu, Y.C. Two new protopines argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from formosan Argemone mexicana. Planta Med. 2003, 69, 148–152. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Peng, L.; Kang, S.; Yin, Z.; Jia, R.; Song, X.; Li, L.; Li, Z.; Zou, Y.; Liang, X.; Li, L.; et al. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015, 8, 5217–5223. [Google Scholar] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Mularz, T.; Idzik, D. Berberine Enhances the Antibacterial Activity of Selected Antibiotics against Coagulase-Negative Staphylococcus Strains in Vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Soni, A.; Jain, P.; Bhawsar, J. Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTI and GC/MS technique. J. Chem. Pharm. Res. 2016, 8, 1–6. [Google Scholar]
- Jaiswal, J.; Doharey, P.K.; Singh, R.; Tiwari, P.; Singh, N.; Kumar, A.; Gupta, V.K.; Siddiqui, A.J.; Sharma, B. Biochemical Characterization of Different Chemical Components of Parthenium hysterophorus and Their Therapeutic Potential against HIV-1 RT and Microbial Growth. BioMed Res. Int. 2022, 2022, 3892352. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative biochemistry of the flavonoids-VI.: Flavonoid patterns in the bignoniaceae and the gesneriaceae. Phytochemistry 1993, 6, 1–12. [Google Scholar]
- Srivastava, N.; Chauhan, A.S.; Sharma, B. Isolation and characterization of some phytochemicals from Indian traditional plants. Biotechnol. Res. Int. 2012, 2012, 549850. [Google Scholar] [CrossRef]
- Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Parthenium hysterophorus: A probable source of anticancer, antioxidant and anti-HIV agents. BioMed Res. Int. 2013, 2013, 810734. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdianrizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J. Pharm. Sci. 2008, 32, 17–20. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).