Abstract
The aim of this essay is to disclose the similarity of a great variety of reactions that proceed between nucleophiles and π-electrophiles—both aromatic and aliphatic. These reactions proceed via initial reversible addition, followed by a variety of transformations that are common for the adducts of both aliphatic and aromatic electrophiles. We hope that understanding of this analogy should help to expand the scope of the known reactions and inspire the search for new reactions that were overlooked.
1. Introduction
This essay aims to disclose the similarity of a great variety of reactions that proceed between nucleophiles and aliphatic and aromatic π-electrophiles. There is a common belief that these reactions are mechanistically different, which we believe to be incorrect. Therefore, we hope that understanding and acceptance of this analogy should help to expand the scope of the known reactions and inspire the search for new reactions that were so far overlooked.
Although there is a great variety of aliphatic π-electrophiles (carbonyl compounds, imines, iminium ions, nitriles, Michael acceptors, etc.) and aromatic π-electrophiles with electrophilic centers in the ring: nitroarenes, azines, etc., as well as a great, practically unlimited variety of nucleophiles; therefore, numerous reactions that proceed between them—there are just a few fundamental initial processes. All these reactions proceed via the initial reversible addition of nucleophiles to the electrophilic center of an electrophilic partner to form σ adducts that are subsequently converted into the final products in a variety of ways. The addition to aliphatic electrophiles results in the formation of a new σ bond and conversion of the π bond into a σ bond, which as a rule is energetically favorable, with the formation of adducts that are energetically stable intermediates. Due to stability, upon protonation, they can form final products, or they can react further in a great variety of ways. On the other hand, the addition of nucleophiles to electron-deficient arenes results in dearomatization, thus it is energetically unfavorable so, as a rule, the produced σ adducts are unstable, short-lived intermediates. These σ adducts tend to recover aromaticity via dissociation or fast further transformations, thus the number of different final reactions appears to be limited. However, recent literature reports indicate that adducts of nucleophiles to aromatic electrophiles can enter a variety of reactions, and thus they display reactivity identical to their aliphatic counterparts [1,2,3].
The second difference between aliphatic and aromatic π-electrophiles is that aromatic π-electrophiles usually contain more than one electrophilic center that is capable of adding nucleophilic agents, and the addition can result in the formation of isomeric adducts. In turn, aliphatic π-electrophiles usually react selectively, giving single, predictable intermediates. However, the difference also seems to be apparent, as it arises from the structure of individual substrates, and varies depending on substitution or length of the conjugation pathway.
These observations should inspire the search for new processes based on the analogies between aromatic and aliphatic π-electrophiles. In this essay, the similarity of reactions of nucleophiles with aromatic and aliphatic π-electrophiles will be presented consecutively, and for the sake of clarity, electrophiles and nucleophiles with or without leaving groups will be discussed separately. We believe that the clear-cut analogy between aromatic and aliphatic π-electrophiles should inspire the search for new synthetic transformations, which were overlooked, due to the simplified picture of their reactivity, presented in chemical textbooks.
2. Addition of Nucleophiles to π-Electrophiles Containing Leaving Groups at Electrophilic Center
2.1. Addition of Nucleophiles without Leaving Groups
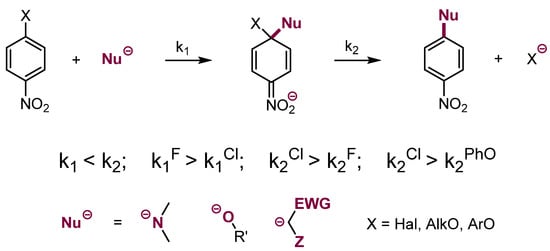
The most common and studied reaction between nucleophiles and aromatic electrophiles is substitution of halogens and other leaving groups in nitroarenes. This reaction, known for more than 150 years, proceeds via the addition of nucleophiles at positions ortho or para to the nitro group occupied by a leaving group X to form σX adducts. As was mentioned earlier, the addition is connected with dearomatization, thus the adducts undergo fast rearomatization via the departure of X− to form products of substitution, SNAr. As a consequence, in this two-step process, the addition is the slower, rate-limiting step, therefore usually the substitution proceeds faster when X = F than X = Cl [4].
In some cases, when X = Cl, the departure of Cl− is so fast that the process has a synchronous character [5,6]. It is therefore a peculiar situation that the two-step substitution of fluorine proceeds faster than the synchronous substitution of chlorine.
Substitution of leaving groups in electron-deficient arenes (nitroarenes, azines, etc.) proceeds with various C, N, O, S, etc. nucleophiles. The most common leaving groups are halogens, but alkoxy and aryloxy groups can also behave as leaving groups (Scheme 1). The substitution of fluoride in 2-fluoropyridine is a versatile way of preparing pyridine derivatives [7].
Scheme 1.
SNAr in nitroarenes.
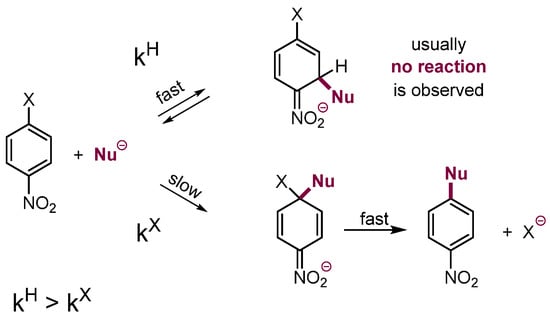
As previously mentioned, in p- and o-halonitrobenzenes and analogues there are also positions occupied by hydrogen available for the addition of nucleophiles. It should be stressed that addition of nucleophiles at these positions is, as a rule, faster than at positions occupied by halogen, but the σH adducts have no direct way for further conversion; hence, they usually dissociated. Nevertheless, when such a possibility exists, further conversion of the σH adducts results in nucleophilic substitution of hydrogen. The general picture is presented in Scheme 2 [4,8].
Scheme 2.
Addition of nucleophiles to nitroarenes.
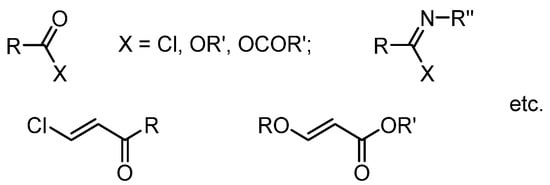
There is a great variety of aliphatic π-electrophiles containing leaving groups at the electrophilic centers. The simplest examples of such electrophiles are acyl chlorides (halides), esters of carboxylic acids, imidoyl chlorides, etc., as well as electron-deficient alkenes containing leaving groups: β-halo or β-alkoxyvinyl ketones, nitriles, esters, etc. (Scheme 3).
Scheme 3.
Aliphatic π-electrophiles.
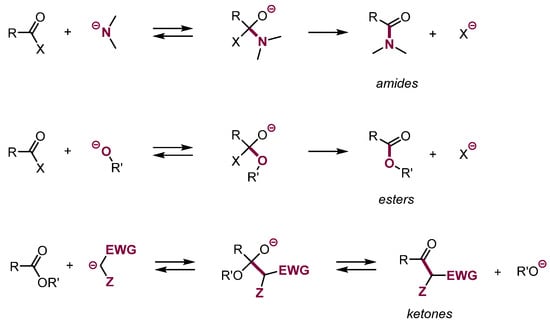
Numerous common reactions such as acylation of carbanions by acyl chlorides and esters, synthesis of esters via acylation of alcohols and transesterification, synthesis of amides, hydrazides, hydroxamic acids, etc. proceed via the addition of nucleophiles to carbonyl and amino groups substituted with a leaving group, followed by elimination of the leaving group (Scheme 4). Many names and common reactions, e.g., Claisen condensation, transesterification, etc., belong to this category. It appears difficult to see the analogy between the Claisen condensation and SNAr reaction, but indeed these reactions proceed similarly as follows: addition of nucleophile followed by elimination of a leaving group from the addition center.
Scheme 4.
Nucleophilic substitution at the carbonyl group.
The reactions of nucleophiles with π-electrophiles containing leaving groups at the electrophilic centers of electron deficient alkenes are less common. Nevertheless, there are many examples of substitution of β-halogens or β-alkoxy groups in vinyl nitriles, ketones, or sulfones that proceed via an addition–elimination mechanism analogous to SNAr [9,10]. Similar to the SNAr, addition is usually the rate-determining step, hence for instance substitution of fluorine is faster than chlorine (Scheme 5). Selected synthetic examples of nucleophilic substitution in aliphatic systems are presented in Scheme 6 [11,12,13].
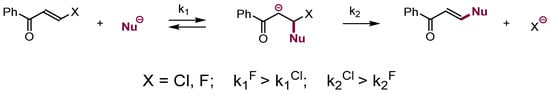
Scheme 5.
Nucleophilic substitution of leaving groups in electrophilic alkenes.
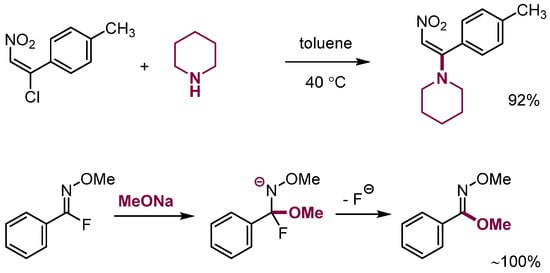
Scheme 6.
Examples of nucleophilic substitution of leaving groups in aliphatic systems.
Similar to the case of aromatic electrophiles there are two main mechanistic variants of substituting chlorine in β-chlorovinyl ketones through two-step processes in which the adducts are intermediates or synchronous reaction when the dissociation is faster than addition and the adducts are transition states [9,10]. Occasionally, reactions other than elimination may be observed following the addition of nitrogen [14,15,16], oxygen [17,18], carbon [19,20], or fluoride [21] nucleophiles to electron-deficient fluoroalkenes, including SN2’ reactions [22].
2.2. Addition of Nucleophiles Containing Leaving Groups
There are few examples of substitution of halogens in nitroarenes with nucleophiles containing leaving groups, e.g., α-chlorocarbanions, mostly because nucleophilic addition to such arenes proceeds faster from the position occupied by hydrogen. The formed σH adducts of α-chlorocarbanions usually undergo β-elimination to form products of vicarious nucleophilic substitution (VNS) [23] faster than dissociation and addition at positions occupied by a leaving group (see below, Section 3.2). Nevertheless, there are examples of substitution of halogens, particularly fluorine, in fluoronitrobenzenes by carbanions of α-chloroalkyl sulfones [24,25], alkoxynitriles, sulfenamides, and anion of t-butylhydroperoxide (Scheme 7) [26]. These reactions have rather limited practical application.
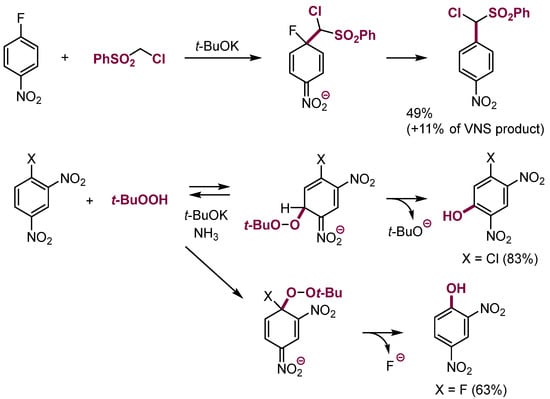
Scheme 7.
SNAr in nitroarenes with nucleophiles containing leaving groups.
There are also not frequent reports of reactions of such nucleophiles with acyl chlorides or esters of carboxylic acids [27]. Nevertheless, adding anions of hydroperoxides to acyl chlorides is a way of synthesis of acyl peroxides in moderate to good yields (Scheme 8) [28].
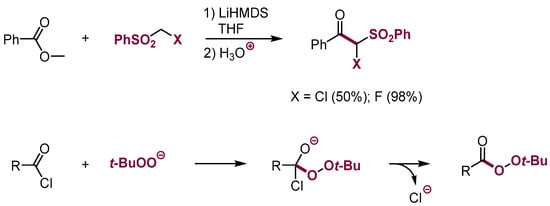
Scheme 8.
Nucleophilic substitution in the carbonyl group with nucleophiles containing leaving groups.
3. Addition of Nucleophiles to π-Electrophiles without Leaving Groups at the Electrophilic Center
3.1. Addition of Nucleophiles without Leaving Groups
For many years, the known reactions of nucleophiles with electron-deficient arenes were limited to those proceeding via addition at positions occupied by halogens or other leaving groups. Only recently was it established that adding nucleophiles to nitroaromatic rings also proceeds to rings that do not contain leaving groups. Moreover, addition to the rings containing halogens proceeds faster at positions occupied by hydrogen than halogen [1,2,3,8]. This process was overlooked because the initial fast addition is a reversible process and the initially formed σH adducts dissociate and slower addition at positions occupied by halogens leading to SNAr taking place (see Scheme 1). It should be mentioned that σH adducts of C, N, O, etc. nucleophiles to highly electrophilic arenes, such as trinitrobenzene, are stable and upon protonation, trinitrocyclohexadiene-type products known as “Meisenheimer complexes”, that are in fact trinitrocyclohexadiene derivatives, are formed (Scheme 9) [29].
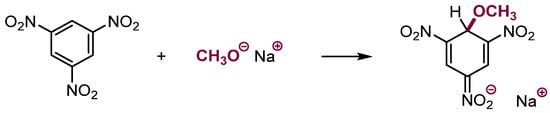
Scheme 9.
Formation of stable adducts of nucleophiles to trinitrobenzene.
Carbanions generated from 1,3-dicarbonyl compounds add to such nitroarenes to form upon protonation peculiar bicyclic products in moderate yields (Scheme 10) [30]. These important and interesting observations have limited application in organic synthesis.
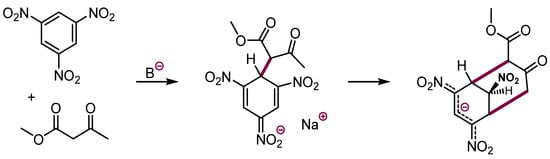
Scheme 10.
Formation of bicyclic structures from trinitrobenzene and carbanions of 1,3-dicarbonyl compounds.
Anionic σH adducts of nucleophiles to mononitroarenes are short-lived species; nevertheless, protonation or silylation of such σH adducts of some carbanions results in the elimination of water to form nitrosoarenes that can be isolated in the form of quinoneoximes [31] or further converted to anthranils (Scheme 11) [32].
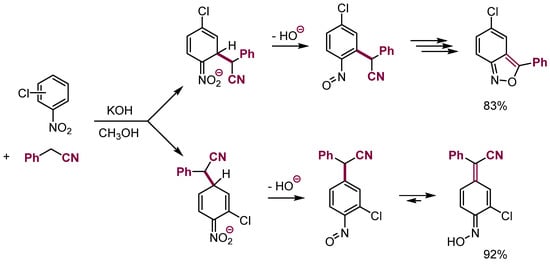
Scheme 11.
Addition of nucleophiles to nitroarenes followed by elimination.
Silylation of σH adducts of methinic carbanions followed by elimination of silanol gave substituted nitrosobenzenes (Scheme 12) [33].
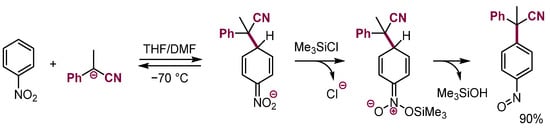
Scheme 12.
Formation of nitrosoarenes via silylation of σH adducts and elimination of silanol.
The scope of these interesting and valuable synthesis processes is rather limited. Short-lived σH adducts of anilines to mononitroarenes upon protonation also undergo the elimination of water to form nitrosodiphenyl amines (Scheme 13) [34,35].
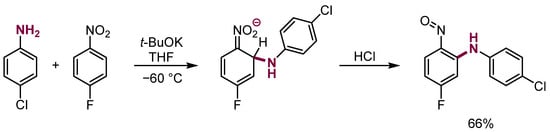
Scheme 13.
Synthesis of nitrosoarylanilines.
Another important way of further transformation of σH adducts of C, N, O, and even P nucleophiles to nitroarenes is oxidation by external oxidants to form products of oxidative nucleophilic substitution of hydrogen (ONSH). It was recently shown that this process is of the general character of wide application in organic synthesis. Examples of ONSH in nitroarenes are presented in Scheme 14 [33,36,37].
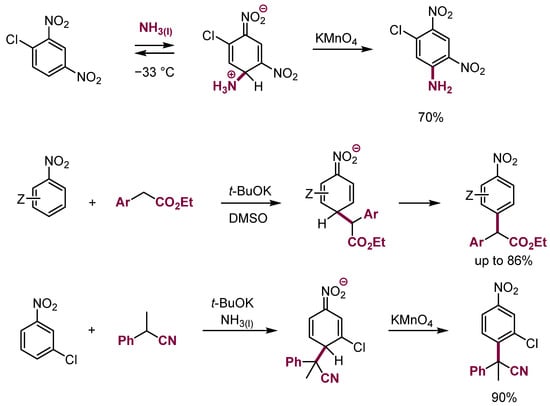
Scheme 14.
Examples of ONSH in nitroarenes.
Further, adding nucleophiles to aliphatic π-electrophiles containing carbon-heteroatom double bonds that do not contain leaving groups is energetically favorable; hence, the usual protonation of the adducts provides stable products. This can be exemplified by the addition of cyanide anion to aldehydes and ketones that upon protonation form cyanohydrines or silylation O-silylated cyanohydrines (Scheme 15) [38,39]. Similarly, the addition of carbanions to aldehydes and ketones followed by protonation gives aldol-type products.
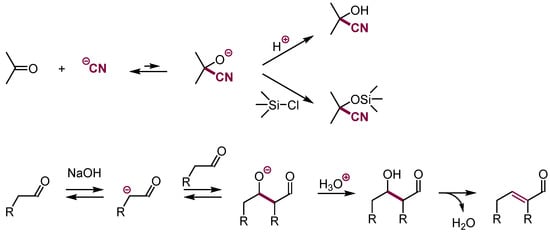
Scheme 15.
Addition of carbon nucleophiles to carbonyl groups.
Usually, the protonated adducts—aldols—enter fast further reactions, most often elimination of water to form alkenes (Knoevenagel reaction) [40]. Additionally, adding ammonia and a variety of amines to aldehydes and ketones results in the formation of aminals and subsequently, upon elimination of water, aldimines or enamines (Scheme 16).
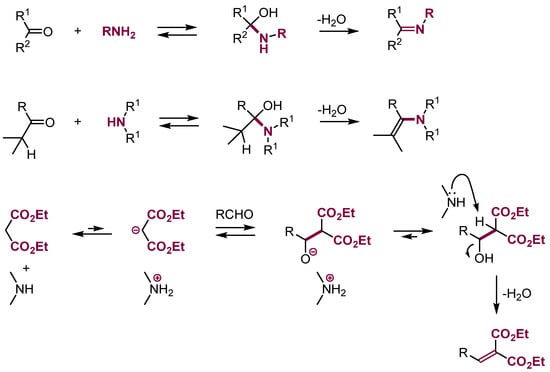
Scheme 16.
Addition of nucleophiles to carbonyl groups followed by elimination.
We would like to stress the similarity of the conversion of the σH adducts to nitroarenes and to aldehydes and ketones via protonation and the elimination of water. The addition of nucleophiles to highly electron-deficient nitroarenes upon protonation results in formation of relatively stable adducts, similarly to formation of aldols, whereas addition to mononitroarenes followed by protonation and elimination of water gives nitrosoarenes, isolated usually as methylenequinone oximes (Scheme 11, Scheme 12 and Scheme 13) in a process analogous to the Knoevenagel reaction.
Oxidation analogous to that of σH adducts of nucleophiles to nitroarenes, ONSH, also proceeds with the adducts of nucleophiles to aliphatic π-electrophiles–aldehydes. For instance, aromatic aldehydes treated with potassium permanganate in liquid ammonia form amides in moderate yields (Scheme 17) [41].
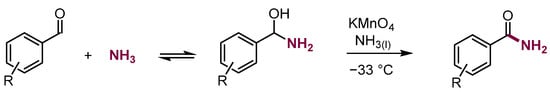
Scheme 17.
Synthesis of amides from aldehyde and ammonia.
Formally, the oxidation of protonated adducts of RLi and RMgX to aldehydes, that is alcohols, may be viewed as another example belonging to this category. ONSH reaction also proceeds with electron-deficient alkenes, for example, in quinone or maleimide derivatives [42,43,44,45,46,47] or even nitroalkenes [48] (Scheme 18).
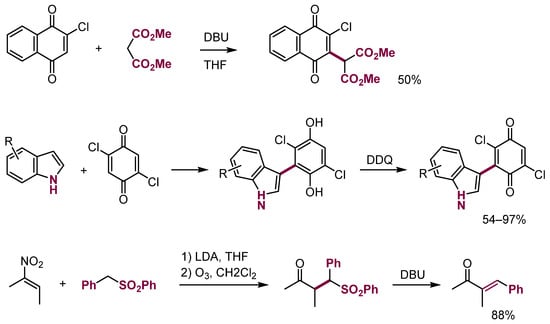
Scheme 18.
ONSH in aliphatic π-electrophiles.
ONSH is also a well-established process for the functionalization of two structurally similar classes of π-electrophiles–aromatic N-oxides [49,50,51] and aliphatic nitrones (Scheme 19) [52,53].
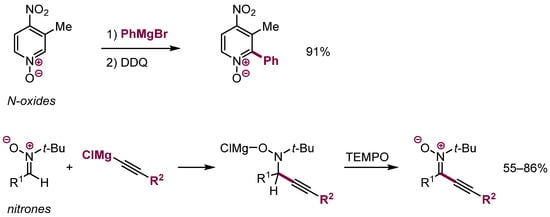
Scheme 19.
ONSH in N-oxides and nitrones.
3.2. Addition of Nucleophiles Containing Leaving Groups
Of particular interest and value are reactions of aromatic and aliphatic π-electrophiles with nucleophiles that contain leaving groups at the nucleophilic center. Such nucleophiles are exemplified by α-halocarbanions, sulfonium and phosphonium ylides, t-butylhydroperoxide anion, substituted amines, etc.
The addition of α-halocarbanions to nitroarenes results in the initial formation of the σH adducts that subsequently enter base-induced β-elimination of hydrogen halide and protonation to give products of vicarious nucleophilic substitution (VNS). This reaction can proceed provided the σH adducts exist for a sufficiently long time and the base is present in excess (Scheme 20) [23,54].
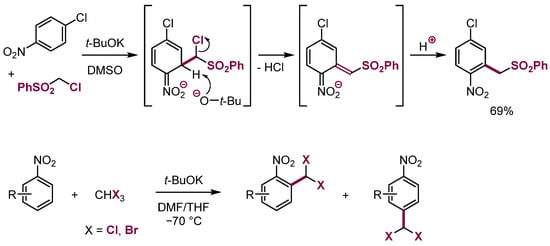
Scheme 20.
Examples of the VNS reaction.
Similar reaction proceeds with carbanions containing other leaving groups able to be eliminated in the base-induced E2 process. For example, VNS cyanomethylation of nitroarenes in the reaction with aryloxyacetonitriles is widely used to synthesize indoles (Scheme 21) [55].
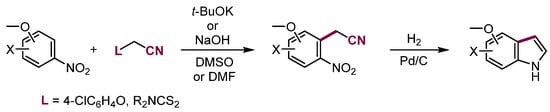
Scheme 21.
Synthesis of indoles from nitroaryl acetonitriles obtained in VNS with aryloxyacetonitriles.
There are a few examples of VNS with sulfonium [56,57,58] and phosphonium ylides (Scheme 22) [59,60].
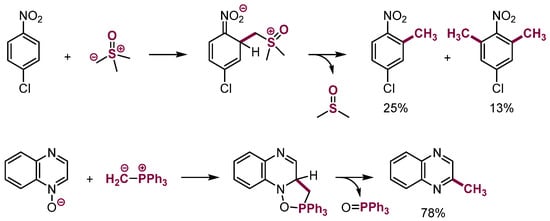
Scheme 22.
Substitution of hydrogen in electron-deficient aromatic systems with ylides.
It should be stressed that an identical process of addition—β-elimination proceeds with an anion of t-butylhydroperoxide to produce nitrophenols and N-anions, generated from some sulfenamides, trimethylhydrazonium iodide, hydroxylamine derivatives, etc. to give nitroanilines (Scheme 23) [26,61,62,63].
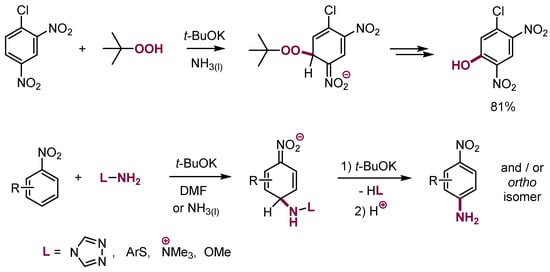
Scheme 23.
VNS in nitroarenes with oxygen and nitrogen nucleophiles.
Thus, VNS is a general process for introducing carbon, nitrogen, and oxygen substituents into aromatic rings.
Interestingly, when delocalization of the negative charge in the σH adduct of α-halocarbanions or sulfonium ylides to electron-deficient arenes is inefficient, they react further not via β-elimination but via intramolecular substitution to form aziridines or cyclopropanes (Scheme 24) [64,65,66,67].
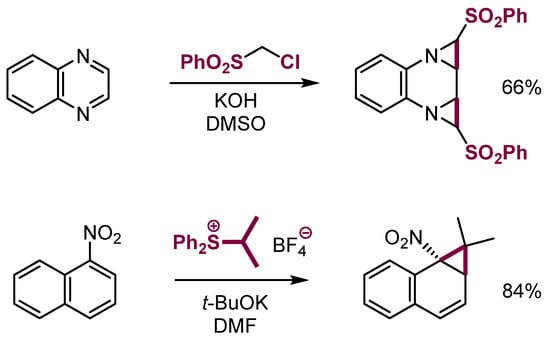
Scheme 24.
Aziridination and cyclopropanation of aromatic systems.
On the other hand, adducts of α-chlorocarbanions and sulfonium ylides to aliphatic electrophiles containing carbonyl groups result in formation of anionic σ adducts that undergo intramolecular 1,3-substitution to form oxiranes (Darzens [68,69] or Corey-Chaykovsky [70,71,72,73] reactions). The reaction of such nucleophiles with imines to form aziridines proceeds similarly (Scheme 25) [74,75].
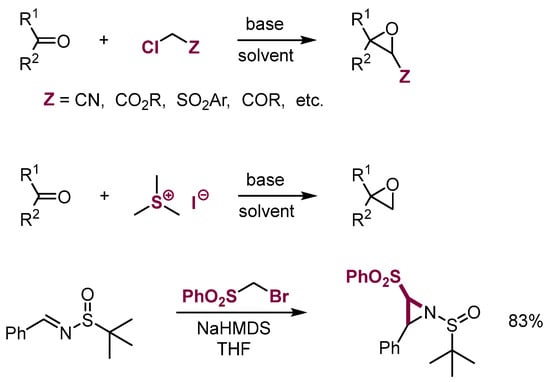
Scheme 25.
Formation of three-membered rings in the reactions of aliphatic π-electrophiles with nucleophiles containing leaving groups.
The high affinity of phosphorus to oxygen causes a different behavior of phosphonium ylides. Their anionic adducts to aldehydes and ketones react further via a combination of the O-anion with a positively charged phosphorous atom giving oxaphosphetanes, followed by the elimination of phosphine oxide to produce alkenes (Wittig reaction; Scheme 26). A similar reaction course leading to alkenes has been reported for adding phosphonium ylides to N-sulfonylimines [76,77], whereas simple N-arylimines give allenes [78].
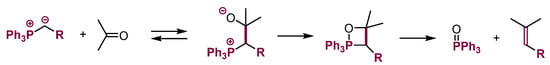
Scheme 26.
The Wittig reaction.
The addition of the nucleophiles containing leaving groups to electron-deficient alkenes results in the formation of anionic adducts that are γ-halocarbanions and related intermediates and is followed by intramolecular 1,3-substitution to give cyclopropanes. This is one of the major ways to synthesize substituted cyclopropanes (Scheme 27) [71,79,80]. A similar process—addition followed by 1,3-intramolecular substitution reaction—proceeds between the anion of t-butylhydroperoxide and some electron-deficient alkenes to give oxiranes, although protonation of the intermediate adducts to form Michael-type products dominates [81]. Similarly, the addition of N-nucleophiles containing leaving groups followed by 1,3-intramolecular substitution is an efficient way of synthesis of aziridines [75].
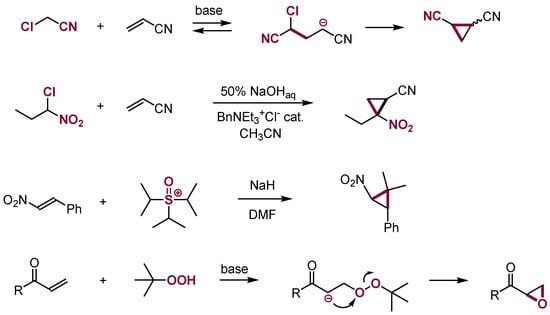
Scheme 27.
Formation of cyclopropanes and oxiranes from Michael acceptors.
Surprisingly, there are no reports of the possibility of converting adducts of α-chlorocarbanions to aliphatic π-electrophiles containing a carbon-heteroatom double bond via base-induced β-elimination, although such attempts were disclosed in the literature [82]. Motivated by the similarity of aromatic and aliphatic π-nucleophiles reactivity, we have attempted such reactions. β-Elimination in the adducts of α-chlorocarbanions to benzaldehyde does not proceed as it is hampered by the vicinity of the negatively charged oxygen which engages in facile intramolecular substitution to form oxiranes. On the other hand, β-elimination in the adducts of α-chlorocarbanions to electron-deficient imines proceeded satisfactorily (Scheme 28) [83].
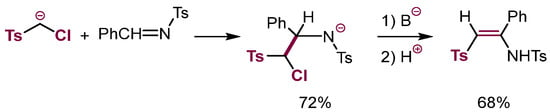
Scheme 28.
VNS in imines.
No examples of β-elimination of HCl from the adducts of α-chlorocarbanions to electron-deficient alkenes were known for a long time. We have shown that such processes, identical to VNS in nitroarenes, proceed under appropriate conditions with electron-deficient alkenes and carbanions containing an aryloxy leaving group or even with α-chlorocarbanions (Scheme 29) [42,84,85].
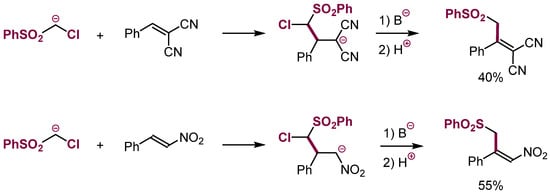
Scheme 29.
VNS in malononitrile derivatives and in nitroalkenes.
More examples of this kind appeared in recent years, including a reaction of nitroalkanes with NO2 acting as a carbanion-stabilizing and leaving group (Scheme 30) [86,87]. A reaction of a nitrogen nucleophile bearing a Ph2S leaving the group with maleimides and naphthoquinone in good yields has also been reported (Scheme 31) [88,89,90].
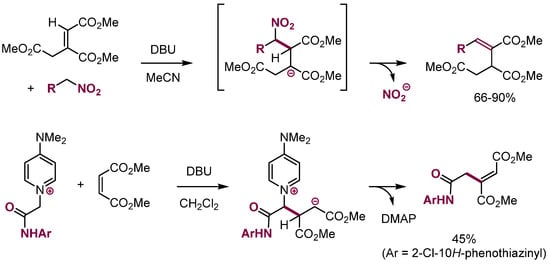
Scheme 30.
VNS in aliphatic systems.
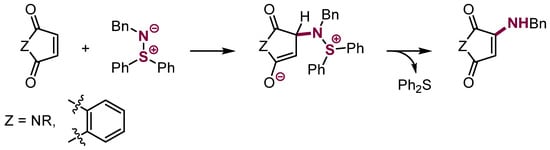
Scheme 31.
VNS with a nitrogen nucleophile in aliphatic systems.
4. Reactions of Specific Nucleophiles
Several kinds of specific nucleophiles also enter analogous reactions with aromatic and aliphatic π-electrophiles. To this category belong 1,3-dipoles that enter 1,3-dipolar cycloaddition both to electron-deficient arenes [91,92] and alkenes [93,94] (Scheme 32).
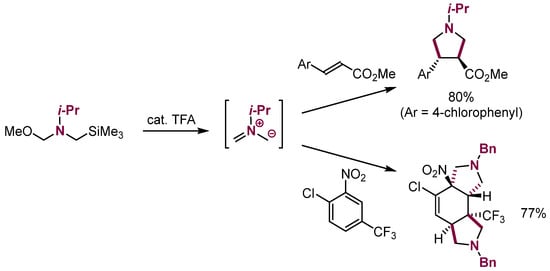
Scheme 32.
1,3-Dipolar cycloaddition of azomethine ylides to aliphatic and aromatic systems.
Of substantial interest are masked acyl carbanions, key intermediates in the Stetter reaction [95]. This reaction proceeds via addition of cyanide anion or nucleophilic carbenes to aldehydes and subsequent intramolecular migration of proton to generate masked acyl carbanions that add to active electrophiles, mainly Michael acceptors. Subsequent dissociation gives Michael adducts of acyl carbanions (Scheme 33).
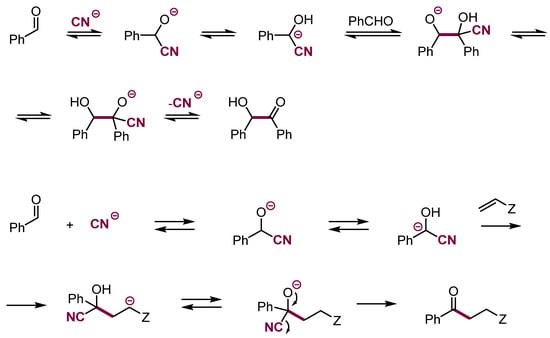
Scheme 33.
Benzoin condensation and Michael addition of masked acyl carbanions.
Examples of the Stetter reaction with nitroarenes that gave as the final products nitrobenzophenones were reported (Scheme 34) [96,97].
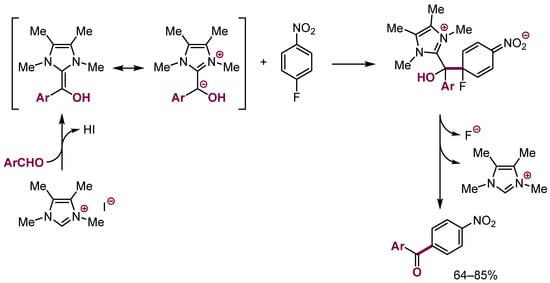
Scheme 34.
Stetter reaction with SNAr in nitroarenes with a NHC as catalyst.
It should be mentioned that the Stetter reaction follows the concept of Umpolung introduced by Seebach to invert the polarity of the carbonyl group in aldehydes [98]. Conversion of aldehydes into dithioacetals followed by deprotonation produced masked acyl carbanions. Subsequent reactions with a variety of electrophiles, followed by deacetalization, give products of reactions of acyl carbanions. Reactions of such carbanions with nitroarenes were also reported. A more convenient approach is generating masked acyl carbanions via the conversion of aldehydes into cyanohydrines, followed by protection in the form of acetals via reactions with vinyl ethers (Scheme 35). Deprotonation of such protected cyanohydrins gave stable equivalents of acyl carbanions [99,100,101]. Thanks to the stability of all intermediates involved, this way of generating masked acyl carbanions appears to be more versatile than the original Stetter reaction.
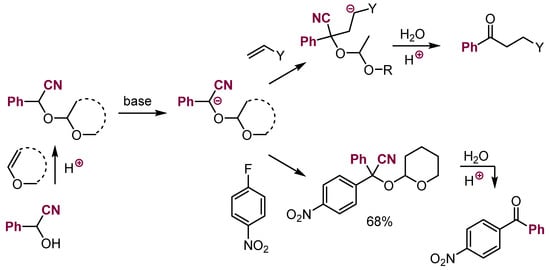
Scheme 35.
Reactions with deprotonated cyanohydrine ethers as masked acyl carbanions.
5. General Comments
Finally, an interesting general question should be addressed. As it was mentioned earlier, nucleophilic addition to ortho and para halonitrobenzenes proceeds faster at positions occupied by hydrogen than halogens, therefore nucleophilic substitution of hydrogen: VNS, ONSH, etc. proceeds as a rule faster than conventional SNAr of halogens. Based on these observations, it was often considered that halogens in electron-deficient arenes partially protect their positions against nucleophilic addition. It was interesting to see that the same observation was made in rather few experiments with aliphatic π-electrophiles. Thus in 2-chloronaphthoquinone VNS reaction with α-chlorocarbanions proceeds exclusively (Scheme 36) [42]. Mayr has shown that nucleophilic addition to chlorobenzoquinones also proceeds faster at positions occupied by hydrogen [102].
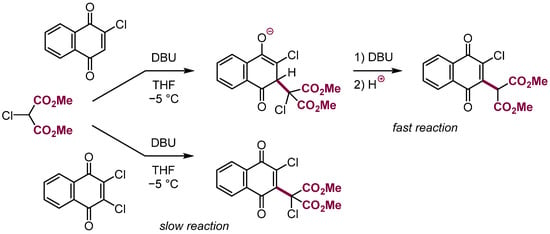
Scheme 36.
VNS and SNAr in chloronaphthoquinones.
We have also found that carbanions add faster at carbon occupied by hydrogen (in position 2) of 1-chlorofumarate and maleate [103]. However, the statement that chlorine decelerates nucleophilic addition at positions it occupies, although supported by many observations, is unjustified simply because competition between addition of nucleophiles at a position occupied by hydrogen and chlorine in para or ortho chloronitrobenzenes is unfair. Positions occupied by hydrogen are additionally activated by electron withdrawing chlorine substituent in the ring or at the π bond, whereas those occupied by chlorine are not additionally activated. This interesting and important problem should be solved using unbiased models. For instance, we have observed that in competition between nitrobenzene and p-chloronitrobenzene for the reaction with a bulky methinic carbanion which does not react in the ortho position, the addition of this carbanion in position para of nitrobenzene proceeds faster. Similarly, the VNS reaction of chloromalonates with 2-chloronaphthoquinones proceeds faster than with 2,3-dichloronaphthoquinones, also 1,2-dichloromaleate reacts slower than monochloro with carbanion of chloromethyl phenyl sulfone (Scheme 36).
These observations support the hypothesis that chlorine substituents indeed decelerate nucleophilic addition at positions they occupy in aromatic and aliphatic π-electrophiles. These interesting observations and hypotheses need further studies.
For instance, there remains an important matter of the relative activity of aldehydes and the corresponding acyl chlorides. We hypothesize that the electrophilic activity of aldehydes is higher, but we are not aware of any experimental evidence. The formation of benzoate of benzaldehyde cyanohydrine when KCN or LiCN is added to a mixture of benzaldehyde and benzoyl chloride is not sufficient [38,104].
6. Kinetic vs. Thermodynamic Control
In the electron-deficient aromatic rings, e.g., nitroarenes, there are usually two or even three electrophilic sites able to add nucleophiles, thus the question of kinetic and thermodynamic control is of crucial importance. According to the recently formulated general mechanism of nucleophilic aromatic substitution in nitroarenes, the addition of nucleophiles to nitroarenes proceeds rapidly at positions ortho or para to the nitro group to form σH adducts [1,8,105]. The adducts are short-lived species and usually dissociate and slower addition at positions occupied by halogens X followed by the fast departure of halide anion results in the substitution of halogens, thus this process can be considered thermodynamically controlled. Furthermore, when, with proper structure of nucleophiles and conditions, the initially formed σH adducts are converted into products of substitution of hydrogen, the process can be considered kinetically controlled. Additionally, the orientation of nucleophilic substitution of hydrogen can be kinetically and thermodynamically controlled. For instance, VNS in nitrobenzene with methylenic carbanions under kinetic control—excess of base, low temperature—proceeds mostly in the ortho position, whereas under thermodynamic control (r.t., slow addition of the carbanion solution to a solution of nitroarene) para substitution dominates. Exemplification of the kinetic vs. thermodynamic control in the reaction of an α-chlorocarbanion with nitroarenes is shown in Scheme 37.
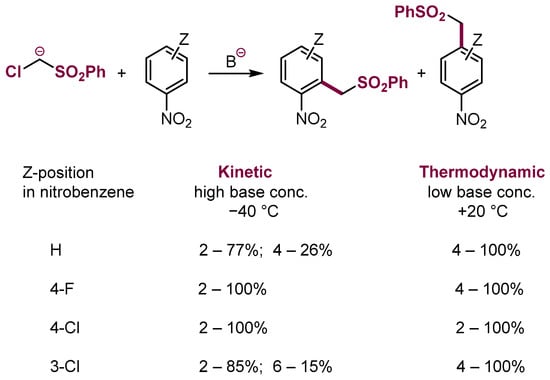
Scheme 37.
Kinetic and thermodynamic control in the VNS reaction. Distributions of 2- and 4-substitution products are given.
These experimental observations are confirmed by calculations of free energies of transition states of the addition and σ adducts [105].
In most aliphatic π-electrophiles, there is one electrophilic center to which the addition of nucleophiles occurs. Nevertheless, there are numerous electrophiles with two such centers, e.g., acrolein, vinyl ketones, acrylates, etc., reactions of which nucleophiles can proceed under kinetic or thermodynamic control (1,2- vs. 1,4-addition) [70].
7. Conclusions
We hope that the general concept of this paper—the analogy between reactions of nucleophiles with aliphatic and aromatic π-electrophiles—is convincingly supported by the presented examples and their interpretation. The mechanism of reactions of nucleophiles with both kinds of π-electrophiles are in fact identical; they are all initiated by a nucleophilic addition to the π system, at the position occupied by a leaving group or by hydrogen, with the latter process usually faster. There are several ways of further conversion of the adducts which are common for both aromatic and aliphatic systems. If the π system of the electrophilic partner contains a heteroatom, Knoevenagel-type elimination may also be possible. Importantly, examples of each type of reaction course can be given for both aliphatic and aromatic electrophiles.
Two important conclusions can be drawn from our analysis. First, some types of reactions are underrepresented within either the aliphatic or aromatic electrophiles group, which should inspire the search for new reactions that remain to be discovered. Second, this essay should change the general opinion that reactions of aliphatic electrophiles are more diversified than those of aromatic ones. It is just the opposite, as exemplified by benzoyl chloride, which is capable only of chloride substitution, and chloronitrobenzene which, upon nucleophilic addition, undergoes SNAr, VNS, or two variants of the ONSH reaction [106].
Author Contributions
Conceptualization, M.M.; writing—original draft preparation, M.B., M.F., R.L. and M.M.; writing—review and editing, M.B., M.F., R.L. and M.M.; visualization, M.B., M.F. and R.L.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Loska, R.; Mąkosza, M. Introduction of Carbon Substituents into Nitroarenes via Nucleophilic Substitution of Hydrogen: New Developments. Synthesis 2020, 52, 3095–3110. [Google Scholar] [CrossRef]
- Mąkosza, M. Reaction of Nucleophiles with Nitroarenes—Multifacial and Versatile Electrophile. Chem. Eur. J. 2014, 20, 5536–5545. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M. Nucleophilic Substitution of Hydrogen in Electron-Deficient Arenes, a General Process of Great Practical Value. Chem. Soc. Rev. 2010, 39, 2855–2868. [Google Scholar] [CrossRef]
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Rohrbach, S.; Smith, A.J.; Pang, J.H.; Poole, D.L.; Tuttle, T.; Chiba, S.; Murphy, J.A. Concerted Nucleophilic Aromatic Substitution Reactions. Angew. Chem. Int. Ed. 2019, 58, 16368–16388. [Google Scholar] [CrossRef] [PubMed]
- Kwan, E.E.; Zeng, Y.; Besser, H.A.; Jacobsen, E.N. Concerted nucleophilic aromatic substitutions. Nature Chem. 2018, 10, 917–923. [Google Scholar] [CrossRef]
- Fier, P.S.; Hartwig, J.F. Synthesis and Late-Stage Functionalization of Complex Molecules through C–H Fluorination and Nucleophilic Aromatic Substitution. J. Am. Chem. Soc. 2014, 136, 10139–10147. [Google Scholar] [CrossRef]
- Mąkosza, M. How does Nucleophilic Aromatic Substitution Really Proceed: General Mechanism. Synthesis 2017, 49, 3247–3254. [Google Scholar] [CrossRef]
- Rappoport, Z. Nucleophilic vinylic substitution. A single- or a multi-step process? Acc. Chem. Res. 1981, 14, 7–15. [Google Scholar] [CrossRef]
- Rappoport, Z. The rapid steps in nucleophilic vinylic addition-elimination substitution. Recent developments. Acc. Chem. Res. 1992, 25, 474–479. [Google Scholar] [CrossRef]
- Gao, M.; Xu, B. Copper Nitrate Mediated Regio- and Stereoselective Difunctionalization of Alkynes: A Direct Approach to α-Chloro-β-nitroolefins. Org. Lett. 2016, 18, 4746–4749. [Google Scholar] [CrossRef]
- Johnson, J.E.; Dolliver, D.D.; Yu, L.; Canseco, D.C.; McAllister, M.A.; Rowe, J.E. Mechanism of Methoxide Ion Substitution in the Z and E Isomers of O-Methylbenzohydroximoyl Halides. J. Org. Chem. 2004, 69, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Dolliver, D.D.; Delatte, D.B.; Linder, D.B.; Johnson, J.E.; Canesco, D.C.; Rowe, J.E. Nucleophilic substitution reactions of N-alkoxyimidoyl fluorides by carbon nucleophiles. Can. J. Chem. 2007, 85, 913–922. [Google Scholar] [CrossRef]
- Petko, K.I.; Filatov, A.A. Addition reaction of various azoles to perfluoromethyl vinyl ether. Chem. Het. Comp. 2021, 57, 666–671. [Google Scholar] [CrossRef]
- Ishida, N.; Adachi, T.; Iwamoto, H.; Ohashi, M.; Ogoshi, S. Copper(I)-mediated C–N/C–C Bond-forming Reaction with Tetrafluoroethylene for the Synthesis of N-Fluoroalkyl Heteroarenes via an Azacupration/Coupling Mechanism. Chem. Lett. 2021, 50, 442–444. [Google Scholar] [CrossRef]
- Cox, D.G.; Sprague, L.G.; Burton, D.J. The facile preparation of HF free polyfluorinated acyl fluorides. J. Fluorine Chem. 1983, 23, 383–388. [Google Scholar] [CrossRef]
- Yang, E.; Reese, M.R.; Humphrey, J.M. Synthesis of α,α-Difluoroethyl Aryl and Heteroaryl Ethers. Org. Lett. 2012, 14, 3944–3947. [Google Scholar] [CrossRef] [PubMed]
- Fuss, A.; Koch, V. Chemistry of 3-Hydroxypyridine Part 3: Synthesis of Substituted 3-[Fluoro(chloro)alkoxy]pyridines from Halo- or Amino-3-hydroxypyridines. Synthesis 1990, 1990, 604–608. [Google Scholar] [CrossRef]
- Krespan, C.G. Derivatives of functionalized fluoro esters and fluoro ketones. New fluoromonomer syntheses. J. Org. Chem. 1986, 51, 326–332. [Google Scholar] [CrossRef]
- Saito, A.; Okada, M.; Nakamura, Y.; Kitagawa, O.; Horikawa, H.; Taguchia, T. Carbocyclization reactions of terminally difluorinated alkenyl active methine compounds mediated by SnCl4 and amine. J. Fluorine Chem. 2003, 123, 75–80. [Google Scholar] [CrossRef]
- Loska, R.; Mąkosza, M. Synthesis of Perfluoroalkyl-Substituted Azines via Nucleophilic Substitution of Hydrogen with Perfluoroisopropyl Carbanions. J. Org. Chem. 2007, 72, 1354–1365. [Google Scholar] [CrossRef]
- Xu, B.; Hammond, G.B. Difluoroallenyl Bromide as a Wide-Ranging Difluoromethylene Cation Equivalent: SN2 Substitution of Difluoropropargyl Bromide through Sequential SE2′ and SN2′ Reactions. Angew. Chem. Int. Ed. 2005, 44, 7404–7407. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M.; Winiarski, J. Vicarious nucleophilic substitution of hydrogen. Acc. Chem. Res. 1987, 20, 282–289. [Google Scholar] [CrossRef]
- Mąkosza, M.; Lemek, T.; Kwast, A.; Terrier, F. Elucidation of the Vicarious Nucleophilic Substitution of Hydrogen Mechanisms via Studies of Competition between Substitution of Hydrogen, Deuterium, and Fluorine. J. Org. Chem. 2002, 67, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Glinka, T.; Mąkosza, M. On the Mechanism of the Vicarious Nucleophilic Substitution of Hydrogen. J. Org. Chem. 1983, 48, 3860–3861. [Google Scholar]
- Mąkosza, M.; Sienkiewicz, K. Hydroxylation of Nitroarenes with Alkylhydroperoxide Anions via Vicarious Nucleophilic Substitution of Hydrogen. J. Org. Chem. 1998, 63, 4199–4208. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, L.; Hu, J. Synthesis of Fluorinated β-Ketosulfones and gem-Disulfones by Nucleophilic Fluoroalkylation of Esters and Sulfinates with Di- and Monofluoromethyl Sulfones. J. Org. Chem. 2009, 74, 3767–3771. [Google Scholar] [CrossRef]
- Milas, N.A.; Surgenor, D.M. Studies in Organic Peroxides. IX. t-Butyl Peresters. J. Am. Chem. Soc. 1946, 68, 642–643. [Google Scholar] [CrossRef]
- Cox, J.P.L.; Crampton, M.R.; Wight, P. Comparison of the intrinsic reactivities of carbon and oxygen nucleophiles at the 1,3,5-trinitro-substituted aromatic ring. J. Chem. Soc. Perkin Trans. II 1988, 2, 25–29. [Google Scholar] [CrossRef]
- Strauss, M.J.; Jensen, T.C.; Schran, H.; O’Conner, K. Condensation-cyclization of diketones and keto esters with electron-deficient aromatics. I. Formation and structure of some stable delocalized anions containing the bicyclo[3.3.1]nonane skeleton. J. Org. Chem. 1970, 35, 383–388. [Google Scholar] [CrossRef]
- Rad, N.I.; Teslenko, Y.O.; Obushak, M.D.; Matiychuk, V.S.; Lytvyn, R.Z.J. Oximes as products in the reactions of 5-substituted 2-nitrothiophenes with arylacetonitriles. Heterocycl. Chem. 2011, 48, 1371–1374. [Google Scholar] [CrossRef]
- Davis, R.B.; Pizzini, L.C.; Benigni, J.D. The Condensation of Aromatic Nitro Compounds with Arylacetonitriles. I. Nitrobenzene. J. Am. Chem. Soc. 1960, 82, 2913–2915. [Google Scholar] [CrossRef]
- Mąkosza, M.; Staliński, K. Oxidative Nucleophilic Substitution of Hydrogen in Nitroarenes. Chem. Eur. J. 1997, 3, 2025–2031. [Google Scholar] [CrossRef]
- Więcław, M.; Bobin, M.; Kwast, A.; Bujok, R.; Wróbel, Z.; Wojciechowski, K. General synthesis of 2, 1-benzisoxazoles (anthranils) from nitroarenes and benzylic C–H acids in aprotic media promoted by combination of strong bases and silylating agents. Mol. Divers. 2015, 19, 807–816. [Google Scholar] [PubMed]
- Wróbel, Z.; Kwast, A. Simple Synthesis of N-aryl-2-nitrosoanilines in the Reaction of Nitroarenes with Aniline Anion Derivatives. Synthesis 2010, 22, 3865–3872. [Google Scholar] [CrossRef]
- Szpakiewicz, B.; Grzegozek, M. Amination of Some 1,3-Dinitrobenzenes with Liquid Ammonia–Potassium Permanganate. Russ. J. Org. Chem. 2004, 40, 829–833. [Google Scholar] [CrossRef]
- Lovato, K.; Guo, L.; Xu, Q.-L.; Liu, F.; Yousufuddin, M.; Ess, D.H.; Kürti, L.; Gao, H. Transition metal-free direct dehydrogenative arylation of activated C(sp3)–H bonds: Synthetic ambit and DFT reactivity predictions. Chem. Sci. 2018, 9, 7992–7999. [Google Scholar] [CrossRef]
- Yoneda, R.; Santo, K.; Harusawa, S.; Kurihara, T. A Simple One-Pot Synthesis of Silylated and Acylated Cyanohydrins. Synthesis 1986, 12, 1054–1055. [Google Scholar] [CrossRef]
- Kazuaki, S. A Convenient One-Pot Cyanosilylation of Aldehydes and Ketones Using Potassium or Sodium Cyanide Impregnated on Amberlite XAD Resin and Trimethylsilyl Chloride. Bull. Chem. Soc. Jpn. 1987, 60, 3820–3822. [Google Scholar]
- Jones, G. The Knoevenagel Condensation. Org. React. 1967, 15, 204–599. [Google Scholar]
- Antoniak, D.; Sakowicz, A.; Loska, R.; Mąkosza, M. Direct Conversion of Aromatic Aldehydes into Benzamides via Oxidation with Potassium Permanganate in Liquid Ammonia. Synlett 2015, 26, 84–86. [Google Scholar] [CrossRef]
- Mąkosza, M.; Nizamov, S. Vicarious nucleophilic substitution of hydrogen (VNS) in 1,4-naphthoquinone derivatives—Competition between VNS and vinylic nucleophilic substitution (SNV). Tetrahedron 2001, 57, 9615–9621. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Park, K.; Li, Z. Synthesis of 3-Indolyl-2,5-dihydroxybenzoquinones. Org. Lett. 2001, 3, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Li, H.-H.; Zhang, X.; Xiang, S.-H.; Li, S.; Tan, B. Organocatalytic Enantioselective Synthesis of Atropisomeric Aryl-p-Quinones: Platform Molecules for Diversity-Oriented Synthesis of Biaryldiols. Angew. Chem. Int. Ed. 2020, 59, 11374–11378. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ye, J.-X.; Luo, Q.-Q.; Mei, T.; Shen, A.; Huang, P.; Chen, J.; Zhang, X.; Xie, C.; Shi, Z.-C. (NH4)2S2O8-Promoted Direct C–C Coupling of Indoles with Quinones/Hydroquinones without Catalyst. Synlett 2021, 32, 1772–1776. [Google Scholar] [CrossRef]
- Yang, Z.-H.; An, Y.-L.; Chen, Y.; Shao, Z.-Y.; Zhao, S.-Y. Copper(I) Iodide-Catalyzed Sulfenylation of Maleimides and Related 3-Indolylmaleimides with Thiols. Adv. Synth. Catal. 2016, 358, 3869–3875. [Google Scholar] [CrossRef]
- McInnis, E.L.; Grant, B.; Arcelo, E. A Reexamination of the Acid Catalyzed Addition of Ethanethiol to Naphthoquinone. Tetrahedron Lett. 1981, 22, 3807–3810. [Google Scholar] [CrossRef]
- Awen, B.Z.; Miyashita, M.; Shiratani, T.; Yoshikoshi, A.; Irie, H. An Expedient Synthesis of α,β-Unsaturated Ketones Using Nitroalkenes and Sulfones. Chem. Lett. 1992, 21, 767–768. [Google Scholar] [CrossRef]
- Hamana, M.; Iwasaki, G.; Saeki, S. Nucleophilic Substitution of 4-Chloroquinoline 1-Oxide and Related Compounds by Means of Hydride Elimination. Heterocycles 1982, 17, 177–181. [Google Scholar] [CrossRef]
- Tagawa, Y.; Nomura, M.; Yamashita, H.; Goto, Y.; Hamana, M. Reaction of Quinoline N-Oxides with Alkyl- and Aryllithiums in the Presence of Oxidant. Heterocycles 1999, 51, 2385–2397. [Google Scholar]
- Zhang, F.; Duan, X.-F. Facile One-Pot Direct Arylation and Alkylation of Nitropyridine N-Oxides with Grignard Reagents. Org. Lett. 2011, 13, 6102–6105. [Google Scholar] [CrossRef]
- Murarka, S.; Studer, A. Transition Metal-Free TEMPO-Catalyzed Oxidative Cross-Coupling of Nitrones with Alkynyl-Grignard Reagents. Adv. Synth. Catal. 2011, 353, 2708–2714. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Li, Y.; Zhou, B. A [4+1] Cyclative Capture Approach to 3H-Indole-N-oxides at Room Temperature by Rhodium(III)-Catalyzed C–H Activation. Angew. Chem. Int. Ed. 2015, 54, 15400–15404. [Google Scholar] [CrossRef]
- Mąkosza, M.; Owczarczyk, Z. Dihalomethylation of Nitroarenes via Vicarious Nucleophilic Substitution of Hydrogen with Trihalomethyl Carbanions. J. Org. Chem. 1989, 54, 5094–5100. [Google Scholar] [CrossRef]
- Mąkosza, M.; Danikiewicz, W.; Wojciechowski, K. Simple and General Synthesis of Hydroxy- and Methoxyindoles via Vicarious Nucleophilic Substitution of Hydrogen. Liebigs Ann. Chem. 1988, 1988, 203–208. [Google Scholar] [CrossRef]
- Metzger, H.; König, H.; Seelert, K. Methylierung mit dimethyl-oxo-sulfoniummethylid. Tetrahedron Lett. 1964, 5, 867–868. [Google Scholar] [CrossRef]
- Traynelis, V.J.; McSweeney, J.V. Ylide Methylation of Aromatic Nitro Compounds. J. Org. Chem. 1966, 31, 243–247. [Google Scholar] [CrossRef]
- An, W.; Choi, S.B.; Kim, N.; Kwon, N.Y.; Ghosh, P.; Han, S.H.; Mishra, N.K.; Han, S.; Hong, S.; Kim, I.S. C2-Selective C–H Methylation of Heterocyclic N-Oxides with Sulfonium Ylides. Org. Lett. 2020, 22, 9004–9009. [Google Scholar] [CrossRef]
- Ghosh, P.; Kwon, N.Y.; Han, S.; Kim, S.; Han, S.H.; Mishra, N.K.; Jung, Y.H.; Chung, S.J.; Kim, I.S. Site-Selective C–H Alkylation of Diazine N-Oxides Enabled by Phosphonium Ylides. Org. Lett. 2019, 21, 6488–6493. [Google Scholar] [CrossRef]
- Han, S.; Chakrasali, P.; Park, J.; Oh, H.; Kim, S.; Kim, K.; Pandey, A.K.; Han, S.H.; Han, S.B.; Kim, I.S. Reductive C2-Alkylation of Pyridine and Quinoline N-Oxides Using Wittig Reagents. Angew. Chem. Int. Ed. 2018, 57, 12737–12740. [Google Scholar] [CrossRef]
- Mąkosza, M.; Białecki, M. Nitroarylamines via Vicarious Nucleophilic Substitution of Hydrogen: Amination, Alkylamination and Arylamination of Nitroarenes with Sulfenamides. J. Org. Chem. 1998, 63, 4878–4888. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Laurenzo, K.S. Direct amination of nitrobenzenes by vicarious nucleophilic substitution. J. Org. Chem. 1986, 51, 5039–5040. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Mitchell, A.R.; Schmidt, R.D. 1,1,1-Trimethylhydrazinium Iodide: A Novel, Highly Reactive Reagent for Aromatic Amination via Vicarious Nucleophilic Substitution of Hydrogen. J. Org. Chem. 1996, 61, 2934–2935. [Google Scholar] [CrossRef] [PubMed]
- Goliński, J.; Mąkosza, M.; Rykowski, A. Formation of aziridine and cyclopropane rings in reaction of quinozalines and naphthyridines with α-halocarbanions. Tetrahedron Lett. 1983, 24, 3279–3280. [Google Scholar] [CrossRef]
- Mąkosza, M.; Glinka, T.; Ostrowski, S.; Rykowski, A. Vicarious Nucleophilic Substitution of Hydrogen versus Bis-Annulation in the Reaction of Chloromethyl Aryl Sulfone Carbanion with Electrophilic Arenes. Chem. Lett. 1987, 16, 61–64. [Google Scholar] [CrossRef]
- Mąkosza, M.; Goliński, J.; Ostrowski, S.; Sahasrabudhe, A.B.; Rykowski, A. Vicarious Nucleophilic Substitution of Hydrogen, Bisannulation and Competitive Reactions of α-Haloalkyl Carbanions with Bicyclic Azaaromatic Compounds. Chem. Ber. 1991, 124, 577–585. [Google Scholar] [CrossRef]
- Antoniak, D.; Barbasiewicz, M. Corey–Chaykovsky Cyclopropanation of Nitronaphthalenes: Access to Benzonorcaradienes and Related Systems. Org. Lett. 2019, 21, 9320–9325. [Google Scholar] [CrossRef]
- Ballester, M. Mechanisms of The Darzens and Related Condensations. Chem. Rev. 1955, 55, 283–300. [Google Scholar] [CrossRef]
- Sweeney, J. Aziridine Synthesis via Nucleophilic Attack of Carbene Equivalents on Imines: The Aza-Darzens Reaction. Eur. J. Org. Chem. 2009, 29, 4911–4919. [Google Scholar] [CrossRef]
- Trost, B.M.; Melvin, L.S., Jr. Sulfur Ylides. Emerging Synthetic Intermediates (Organic Chemistry; A Series of Monographs, Vol. 31); Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1975. [Google Scholar]
- Sun, X.-L.; Tang, Y. Ylide-Initiated Michael Addition−Cyclization Reactions beyond Cyclopropanes. Acc. Chem. Res. 2008, 41, 937–948. [Google Scholar] [CrossRef]
- Gololobov, Y.G.; Nesmeyanov, A.N.; Lysenko, V.P.; Boldeskul, I.E. Twenty-five years of dimethylsulfoxonium ethylide (Corey’s reagent). Tetrahedron 1987, 43, 2609–2651. [Google Scholar] [CrossRef]
- Li, A.-H.; Dai, L.-X.; Aggarwal, V.K. Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement. Chem. Rev. 1997, 97, 2341–2372. [Google Scholar] [CrossRef] [PubMed]
- Robiette, R. Mechanism and Diastereoselectivity of Aziridine Formation from Sulfur Ylides and Imines: A Computational Study. J. Org. Chem. 2006, 71, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef]
- Fang, F.; Li, Y.; Tian, S.-K. Stereoselective Olefination of N-Sulfonyl Imines with Stabilized Phosphonium Ylides for the Synthesis of Electron-Deficient Alkenes. Eur. J. Org. Chem. 2011, 2011, 1084–1091. [Google Scholar] [CrossRef]
- Dong, D.-J.; Li, Y.; Wang, J.-Q.; Tian, S.-K. Tunable stereoselective alkene synthesis by treatment of activated imines with nonstabilized phosphonium ylides. Chem. Commun. 2011, 47, 2158–2160. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Seng, F. Reaction of Alkylenetriphenylphosphoranes with Schiff Bases. Angew. Chem Int. Ed. 1963, 2, 393. [Google Scholar] [CrossRef]
- Russell, G.A.; Mąkosza, M.; Hershberger, J. Synthesis of Nitrocyclopropanes by Cyclization of γ-Chloro-γ-Nitrocarboxylic Esters and Nitriles. J. Org. Chem. 1979, 44, 1195–1199. [Google Scholar] [CrossRef]
- Edwards, M.G.; Paxton, R.J.; Pugh, D.S.; Taylor, R.J.K. An Improved gem-Dimethylcyclopropanation Procedure Using Triisopropylsulfoxonium Tetrafluoroborate. Synlett 2008, 521–524. [Google Scholar] [CrossRef]
- Yang, N.C.; Finnegan, R.A. A New Method for the Epoxidation of α,β-Unsaturated Ketones. J. Am. Chem. Soc. 1958, 80, 5845–5848. [Google Scholar] [CrossRef]
- Reich, V.; Shah, S.K. Organoselenium chemistry. α-Lithio selenoxides and selenides. Preparation and further transformation to olefins, dienes, and allylic alcohols. J. Am. Chem. Soc. 1975, 97, 3250–3252. [Google Scholar] [CrossRef]
- Mąkosza, M.; Nizamov, S.; Kwast, A. Vicarious Nucleophilic Substitution of Hydrogen in Electrophilic Aldimines. Synthesis of Enamines Substituted with Electron Withdrawing Groups. Mend. Commun. 1996, 6, 43–44. [Google Scholar] [CrossRef]
- Mąkosza, M.; Kwast, A. Vicarious Nucleophilic Substitution of Hydrogen in Electrophilic Alkenes. Tetrahedron 1991, 47, 5001–5018. [Google Scholar] [CrossRef]
- Mąkosza, M.; Kwast, A. Nucleophilic Substitution of Hydrogen in Electrophilic Alkenes. J. Chem. Soc. Chem. Commun. 1984, 17, 1195–1196. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Fiorini, D.; Gil, M.V.; Palmieri, A. A New, One Pot Synthesis of Alkylated Methyl Tri- and Tetracarboxylate Derivatives by Nitroalkanes. Synthesis 2004, 2004, 605–609. [Google Scholar] [CrossRef]
- Hopf, H.; Jones, P.G.; Nicolescu, A.; Bicu, E.; Birsa, L.M.; Belei, D. A Facile Synthesis of Pechmann Dyes. Chem. Eur. J. 2014, 20, 5565–5568. [Google Scholar] [CrossRef]
- Tamura, Y.; Matsushima, H.; Ikeda, M.; Sumoto, K. Syntheses and nucleophilic reactions of N-alkyldiphenylsulfilimines. Tetrahedron 1976, 32, 431–435. [Google Scholar] [CrossRef]
- Furukawa, N.; Yoshimura, T.; Ohtsu, M.; Akasaka, T.; Oae, S. One Step Synthesis of Aziridines by the Michael Addition of Free Sulfimides. Tetrahedron 1980, 36, 73–80. [Google Scholar] [CrossRef]
- Seko, S.; Miyake, K. Amination of α,β-Unsaturated γ-Dicarbonyl Compounds with Methoxyamines. Synth. Commun. 1999, 29, 2487–2492. [Google Scholar] [CrossRef]
- Lee, S.; Chataigner, I.; Piettre, S.R. Facile Dearomatization of Nitrobenzene Derivatives and Other Nitroarenes with N-Benzyl Azomethine Ylide. Angew. Chem Int. Ed. 2011, 50, 472–476. [Google Scholar] [CrossRef]
- Wang, N.; Ren, J.; Li, K. Dearomatization of Nitro(hetero)arenes through Annulation. Eur. J. Org. Chem. 2022, 18, e202200039. [Google Scholar] [CrossRef]
- Chen, C.W.; Tran, J.A.; Fleck, B.A.; Tucci, F.C.; Jiang, W.; Chen, C. Synthesis and characterization of trans-4-(4-chlorophenyl)pyrrolidine-3-carboxamides of piperazinecyclohexanes as ligands for the melanocortin-4 receptor. Bioorg. Med. Chem. Lett. 2007, 17, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, X.; Sun, J.; Niu, D.; Chruma, J.J. 2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. Chem. Rev. 2018, 118, 10393–10457. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ota, S.; Fukuta, Y.; Ueda, Y.; Sato, M. N-Heterocyclic Carbene-Catalyzed Nucleophilic Aroylation of Fluorobenzenes. J. Org. Chem. 2008, 73, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Sun, B.; Hu, Q.; Liu, K.; Li, P.; Wang, J. Recyclable polyetheretherketone fiber-supported N-heterocyclic carbene catalysts for nucleophilic acylation of fluorobenzenes. Chem. Commun. 2020, 56, 11390–11393. [Google Scholar] [CrossRef]
- Seebach, D. Methods of Reactivity Umpolung. Ang. Chem. Int. Ed. 1979, 18, 239–258. [Google Scholar] [CrossRef]
- Mąkosza, M.; Goetzen, T. Synthesis of Pure Arylketone Cyanohydrines and Arylketones from Aromatic Aldehydes. Org. Prep. Proc. Int. 1973, 5, 203–207. [Google Scholar] [CrossRef]
- Mąkosza, M.; Baran, J.; Dziewońska-Baran, D.; Goliński, J. Reactions of Nitrobenzophenones with Carbanions Containing Leaving Groups. Vicarious Nucleophilic Substitution of Hydrogen versus Darzens or Wittig Horner Reactions. Liebiegs Ann. Chem. 1989, 9, 825–832. [Google Scholar] [CrossRef]
- Rad, N.; Mąkosza, M. Simple Synthesis of Aryl p-Nitroarylacetonitriles by Vicarious Nucleophilic Substitution with Carbanions of Protected Cyanohydrins. Eur. J. Org. Chem. 2018, 2018, 376–380. [Google Scholar] [CrossRef]
- Guo, X.; Mayr, H. Quantification of the Ambident Electrophilicities of Halogen-Substituted Quinones. J. Am. Chem. Soc. 2014, 136, 11499–11512. [Google Scholar] [CrossRef]
- Mąkosza, M.; Nizamov, S.; Kwast, A. Vicarious Nucleophilic Substitution of Hydrogen versus Vinylic Substitution of Halogen in the Reactions of Carbanions of Halomethyl Aryl Sulfones with Dialkyl Halofumarates and Halomaleates. Tetrahedron 2004, 60, 5413–5421. [Google Scholar] [CrossRef]
- Chenevert, R.; Plante, R.; Voyer, N. Crown Ether Catalysis in the Synthesis of Cyanohydrin Derivatives. Synth. Commun. 1983, 13, 403–410. [Google Scholar] [CrossRef]
- Błaziak, K.; Danikiewicz, W.; Mąkosza, M. How Does Nucleophilic Substitution Really Proceed in Nitroarenes” Computational Prediction and Experimental Verification. J. Am. Chem. Soc. 2016, 138, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M.; Sulikowski, D. Multiple Reaction Pathways between the Carbanions of α-Alkoxy-α-phenylacetonitrile and o-Chloronitrobenzene. Eur. J. Org. Chem. 2011, 2011, 6887–6892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).