Abstract
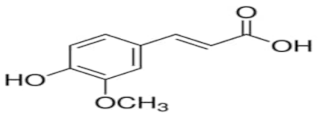
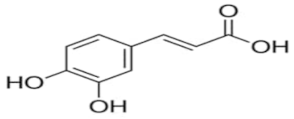
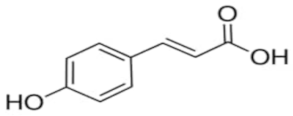
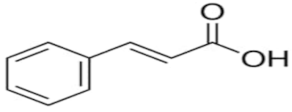
An attempt was made to evaluate the possibility of creating and assessing the stability of inclusion complexes of selected phenolic acids [trans-4-hydroxycinnamic acid (trans-p-coumaric acid), trans-3,4-dihydroxycinnamic acid (trans-caffeic acid), trans-4-hydroxy-3-methoxycinnamic acid, (trans-ferulic acid) and trans-3-phenylacrylic acid (trans-cinnamic acid)] with β-cyclodextrin and 2-HP-β-cyclodextrin in aqueous solutions in a wide temperature range 283.15 K–313.15 K. On the basis of the values of the limiting molar conductivity (ΛCDNaDod), calculated from the experimental data, the values of the formation constants and the thermodynamic functions of formation (standard enthalpy, entropy, and Gibs standard enthalpy) of the studied complexes were determined. It has been found that the stability of the studied complexes increases with lowering of the molar mass of cyclodextrin and lowering of the temperature.
1. Introduction
Cyclodextrins (CD), also called cycloamylases, cycloglucons, or Schardinger dextrins, are naturally occurring cyclic glucose oligosaccharides containing 6 (α-cyclodextrin), 7 (β-cyclodextrin) or 8 (γ-cyclodextrin) linked by an α-1.4 bond of sugar molecules in a ring [1]. Villiers obtained these compounds for the first time in 1891 by decomposing starch with Bacillus amylobacter bacteria. The structural structure of cyclodextrins was described by Schardinger in 1903. Currently, these compounds are obtained by hydrolysis of starch using the extracellular CD glucosyltransferase (CGT) enzyme produced by microorganisms, i.e., Bacillus macerans, Bacillus circulans, Bacillus coagulans, Klebsiella pneumoniae [1]. Cyclodextrins have the shape of toroidal rings whose internal diameter is equal to that of α-cyclodextrin = 4.5 Å, β = 7.0 Å, γ = 8.5 Å. Because of the different sizes of the cyclodextrin cavities, it is characterized by complex selectivity. Primary hydroxyl groups bonded to the carbon atom of glucose are located outside the ring, and the secondary hydroxyl groups linked to the glucose atoms C-2 and C-3 are located inside the torus. An important consequence of this structure is the fact that the inside of the ring is nonpolar (resulting from the presence of hydrophobic C-H groups and glycoside oxygen atoms). The outer layer is hydrophilic, resulting in good solubility of cyclodextrins in water. This arrangement of the molecule allows cyclodextrins to form stable inclusion complexes (host-guest complexes) with many ions and organic compounds in a molar ratio of 1:1 without the need to form covalent bonds [2]. This process, often called microencapsulation, can take place in both an aqueous solution and a solid phase. This enables the formation of complexes diversified in composition with a wide range of applications in many areas, including in the food [2,3,4], pharmaceutical [5,6], cosmetic [7,8] and textile [9] industries. Numerous examples of the use of cyclodextrins in production [9], environmental protection [10,11], and biocatalysis [12] have also been described. Data from the literature also show that these compounds can be used successfully to separate isomers, enantiomers, or compounds with different functional groups [13]. Due to their properties and the presence of a chiral cavity, cyclodextrins dominate the analysis of optical isomers and become called chiral stationary phases [14,15].
Commercially available cyclodextrins are sold as complexes with water. However, these complexes are unstable and water molecules can easily be replaced by guest molecules. There are also many modified cyclodextrin derivatives. Typically, they are produced by the esterification or etherification of the primary and secondary cyclodextrin hydroxyl groups. These derivatives differ from the starting compounds in the size of the hydrophobic hole. Their complexes are often characterized by better solubility and lower sensitivity to light and oxygen [3]. In the food industry, cyclodextrin inclusion complexes are used on a large scale to protect and stabilize substances sensitive to moisture, light, or oxygen, to modify the physicochemical properties of the sample, eg solubility, volatility, to mask undesirable color, smell and taste of selected products [16] or to bind volatile and highly toxic substances, which often leads to a significant extension of storage time [2]. The number of free and complexed guest molecules in the aqueous solution of cyclodextrins depends on several factors, the most important of which are the complexation constant, the temperature, and the concentration of both components. In low-temperature and high-concentration solutions, the equilibrium of this process is shifted toward the formation of inclusion complexes (and crystallization).
The most common hydroxybenzoic acids are gallic, p-hydroxybenzoic, protocatechic, vanillic, and syringic acids, and hydroxycinnamic acids: coffee, ferulic, p-coumaric, and sinapic.
In plants, phenolic acids are generally bound in the form of esters and glycosides, which are part of lignins and hydrolyzing tannins. Some of the hydroxycinnamic acids are found in ester combinations with carboxylic acids or glucose. They occur in ester combinations with the following acids: malonic, tartaric, α-hydroxy-hydrocavic, hydroxycinnamic, tartronic (HOOC-CHOH-COOH, as p-coumaroyl, feruloyl, and caffeoyl-tartronic acid), shikimic, galactaric, glucaric acid (as caffeoylglucaric acid), glucose (as feruloylgluconic acid, the main isomer of which is 2-o-feruloylgluconic acid), 4-methoxyaldaric acid (as 2-o-feruloyl-4-methoxyaldaric acid). In an acidic environment, when heated, these compounds can undergo hydrolysis, which breaks ester and glycosidic bonds, which in turn leads to an increase in the number of free phenolic acids.
On the contrary, hydroxybenzoic acids are mostly present as glycosides. Other combinations of phenolic acids, e.g., flavonoids, fatty acids, sterols, and cell wall polymers, have also been defined in plant tissues. Phenolic acids can also be components of anthocyanins or flavones.
Hydroxycinnamic acid derivatives are very widespread in plants. It has been shown that they can act as an antioxidant by removing free radicals (which is related to their ability to donate a proton and, as a result of this reaction, create a stable and not very reactive phenoxy radical). They mainly form esters with organic acids and glycosides [17]. As indicated in the literature, esterification of caffeic acid with a simple carbohydrate reduces its antioxidant properties [18]. Chen and Ho proved that chlorogenic acid showed lower antioxidant activity compared to caffeic acid contained in corn oil [19]. Caffeic acid removed alkoxy radicals (which were the result of the decomposition of methyl esters of linoleic acid in sunflower oil) much better than chlorogenic acid. Additionally, caffeic acid is more effective in preventing lipid oxidation in fish muscles than chlorogenic acid [20]. On the other hand, Sroka and Lisowski suggest that the addition of quinonic acid to caffeic acid increases antioxidant activity, but also reduces the scavenging capacity of DPPH radicals compared to caffeic acid alone [21]. Cheng, Dai, and Zhou demonstrated that both acids prevent LDL peroxidation in vitro equally [22]. Marinova’s research showed that the antioxidant activity of both acids during the oxidation of triacylglycerols in sunflower oil strongly depends on the concentration of both antioxidants. The use of both acids at a concentration of 2.8·10−4 M allowed the evidence that both have similar antioxidant activity. However, the use of both acids at concentrations above 2.8·10−4 M revealed that caffeic acid is a much more effective reaction inhibitor than chlorogenic acid. Specific interactions may explain this phenomenon between acid and hydroperoxides, as well as the possibility of participation of free radical derivatives of chlorogenic acid in more than one propagation reaction, when free radical derivatives of caffeic acid participate in only one [23].
2. Results and Discussion
The basic values of density, viscosity and dielectric permittivity for the solvent used are summarized in Supplementary Materials: Table S1. With the help of these data, it was possible to determine the values of the tested molar concentrations. The molar conductivities values of the tested phenolic acid derivatives from β-cyclodextrin and 2-HP-β-cyclodextrin are presented in Tables S2–S9. The molar concentration values presented in the tables were calculated from the dependencies presented in the article [24]. In the discussed study β-cyclodextrin and 2-HP-β-cyclodextrin with the investigated anions of phenolic acid form complexes in a 1:1 ratio according to the following relationship:
The molar conductivity of the tested solution is described by the following relationship:
where:
The constant of complex ion (DodCD−) formation is given by the equation:
Taking into account Equations (4)–(6) we get:
Thanks to the new equations proposed in the paper [24], which allow assessing the values of the formation constants and theoretical conductivity, we can analyze the correctness of these equations from the point of view of the size of cyclodexrins and complexed ions. The obtained results of the conductometric measurements allow us to determine the equilibrium constants of this process using the calculation procedures described in detail in our previous work [24]:
Thus, the complexation constant can be represented by the following relationship.
On the other hand, the values for ΛNaDod and ΛNaDodCD show dependencies:
The functions discussed have already been described and analyzed many times in the literature [24,25,26].
The molar conductivity as a function of concentration was analyzed in Tables S2–S9. These values increase as a function of concentration and as a function of temperature. The increase in molar conductivity is fully understandable based on the theory of conductivity because the mobility of the ions increases at higher temperatures. The interactions between ions and solvent molecules become weaker and weaker, which causes an increase in the values of the molar conductivity. As can be seen, an increase in the concentration of the tested ligand (i.e., β-cyclodextrin and 2-HP-β-cyclodextrin), we observe a decrease in the conductivity value. This decrease is most likely due to the formation of inclusion complexes of the tested phenolic acid anions with the tested ligands. The Kf values and the limiting molar conductivities for both studied cyclodextrins are summarized in Table 1, Table 2, Table 3 and Table 4.

Table 1.
The value of constant formation Kf [dm3/mol], theoretical conductivity ΛCDNaDod [S∙cm2/mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-cinnamic acid.

Table 2.
The value of constant formation Kf [dm3/mol], theoretical conductivity ΛCDNaDod [S∙cm2/mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans–p-coumaric acid.

Table 3.
The value of constant formation Kf [dm3/mol], theoretical conductivity ΛCDNaDod [S∙cm2/mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-caffeic acid.

Table 4.
The value of constant formation Kf [dm3/mol], theoretical conductivity ΛCDNaDod [S∙cm2/mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-ferulic acid.
As can be seen, the values of Kf increase with increasing molar mass of the tested anion (cinnamon > coumarin > coffee > ferulic) for all studied cyclodextrins, which can be seen in Figure 1.
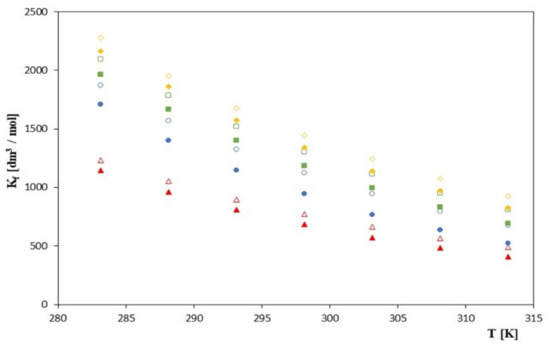
Figure 1.
The plots of dependence of the Kf [dm3/mol] formation constant in the T [K] function for β-cyclodextrin with all studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
It should be emphasized that these values decrease with an increase in the molar mass of the tested cyclodextrin. This indicates that the stability of the investigated complexes increases with the decrease in the cyclodextrin molar mass. This relationship is also valid if we take into account the Kf values previously obtained for α-cyclodextrins [24]. Thus, the analyzed values of the Kf formation constants as a function of the cyclodextrin molar mass satisfy the following relationship. Kf (α-cyclodexrin) > Kf (β-cyclodextrin) > Kf (2-hydroxypropyl-β-cyclodextrin). It should also be noted that with lowering the temperature, the stability of the analyzed complexes increases. This is evident for all tested cyclodextrins. This is most likely due to the reduction in the acids mobility of the ions tested by lowering the temperature. At high temperatures, the ion-solvent interactions are weak and the mobility of ions increases, which is not conducive to the formation of stable inclusion complexes. On the other hand, at low temperatures, the mobility of the discussed anions is relatively low and, most likely, their matching to the gap in the cone of the tested cyclodextrin is more precise, which favors the formation of the complex.
The size of the cone of the tested cyclodextrins is also very important for the process discussed. As the interior of cyclodextrin increases, the complexing power decreases. The strongest complexes are noticeable for α-cyclodextrin [24], they are weaker for β-cyclodextrin, and for 2-HP-β-CD they are the weakest. This is due to the fact that they have substituents in their structure in addition to the hydrophobic cone, which may weaken the power of the complexes formed. In the case of 2-HP-β-CD, 5 to 6 2-hydroxypropyl groups are attached to the lower cone. This may make the interior of the cyclodextrin less hydrophobic, and also it is due to the fact that the anions of the phenolic acid derivatives that attach to the analyzed cyclodextrin cannot fully fill its interior. This probably makes the complex formation process less spontaneous for both β-cyclodextrins. The fact that in the case of complexes of both β-cyclodextrins the values of the formation constants decrease can be explained by an increase in the kinetic energy of the anions and cyclodextrin molecules. However, this does not mean that the spontaneity of the formation process decreases with increasing temperature. This problem can be solved by analysing the free formation enthalpies, which are presented later in the paper.
The molar conductivities of salts composed of sodium cations and complexed phenolic anions increase monotonically with increasing temperature. Data contained in Table 1, Table 2, Table 3 and Table 4 and the relations presented in Figure 2.
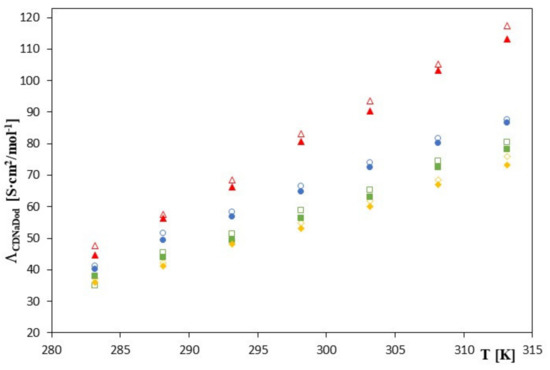
Figure 2.
The plots of the dependence of the theoretical conductivity ΛCDNaDod [S∙cm2/mol−1] in the function T [K] for β-cyclodextrin with all studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
Show that the lowest values of the formation constants of the inclusion complex and an increase in their solubility were achieved using 2HP-β-CD in the complexation reaction of the phenolic acid derivatives.
These effects are more noticeable with the use of 2HP-β-CD than with unsubstituted β-CD studies. This phenomenon may be explained by the fact that the substituents extend the cyclodextrin gap, resulting in a more efficient complexation of phenolic anions that have a fairly flat linear structure. The above data also show that cyclodextrins with larger gap sizes, such as β-cyclodextrin and its derivatives, can effectively complex the tested phenolic derivatives, thus increasing their solubility in aqueous solutions. This structure suggests the use of cyclodextrins, which dissolve well in water [27,28,29,30,31], and at the same time can increase the solubility of soluble substances by including hydrophobic groups inside the cyclodextrin gap [27,28,29,30,31].
Temperature dependences of the complex formation constant were used to determine the free enthalpy of the complex formation.
The above relationship can be presented as follows.
The enthalpy values are presented as the first derivative of the free enthalpy after temperature at constant pressure.
The enthalpy is calculated from the following relationship:
Using the parameters obtained for the values a and b, the values of ΔG0, ΔH0 and ΔS0 were determined for the formation of inclusion complexes between the phenolic acid derivatives and β-CD and 2HP-β-CD. The values of the thermodynamic functions are summarized in Table 5, Table 6, Table 7 and Table 8.

Table 5.
The values of thermodynamic functions ∆G0, ∆S0, ∆H0 [J∙mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-cinnamic acid.

Table 6.
The values of thermodynamic functions ∆G0, ∆S0, ∆H0 [J∙mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans –p-coumaric acid.

Table 7.
The values of thermodynamic functions ∆G0, ∆S0, ∆H0 [J∙mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-caffeic acid.

Table 8.
The values of thermodynamic functions ∆G0, ∆S0, ∆H0 [J∙mol−1] for β-cyclodextrin and 2-HP-β-cyclodextrin with the salt of trans-ferulic acid.
As can be seen, for both types of inclusion complexes (with β-CD and 2HP-β-CD) the values of ∆H0, ∆G0 and ∆S0 are <0, which means that the formation of inclusion complexes is exothermic and runs spontaneously. Comparing the values in Table 5, Table 6, Table 7 and Table 8, it can be seen that in the case of complexes with modified cyclodextrin, the complexation reactions are less spontaneous (the values of the thermodynamic functions are less negative).
As can be seen in Figure 3, Figure 4 and Figure 5, as the temperature increases, the spontaneity of the inclusion complex formation process increases contrary to what is shown in the analysis of changes in the formation constants. The higher energy of the anions favors the penetration of the cyclodextrin interior, and the molecules are located inside it more intensively. However, as can be seen, the energy gain in the complex formation process is clear for both cyclodextrins and much greater for unsubstituted β-cyclodextrin. The entropy of the complex formation process decreases with increasing temperature and increasing the molar mass of the tested cyclodextrin and the molar mass of the tested anions.
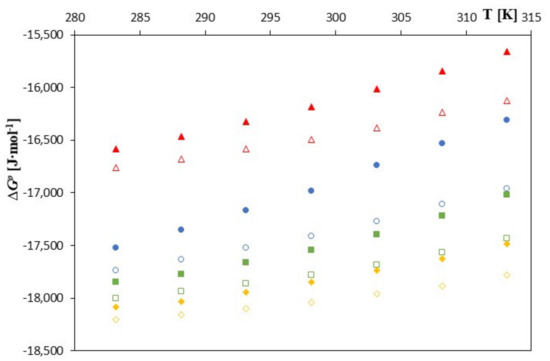
Figure 3.
The plots of dependence ∆G0 [J∙mol−1] in the function T [K] for β-cyclodextrin with all studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans –p-coumaric acid;
-trans –p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans –p-coumaric acid;
-trans –p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
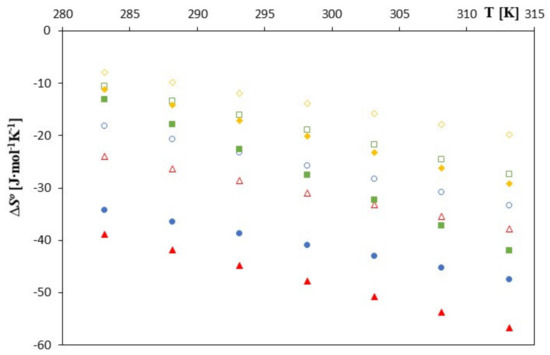
Figure 4.
The plots of the dependence ∆S0 [J∙mol−1] in the function T [K] for β-cyclodextrin with all studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
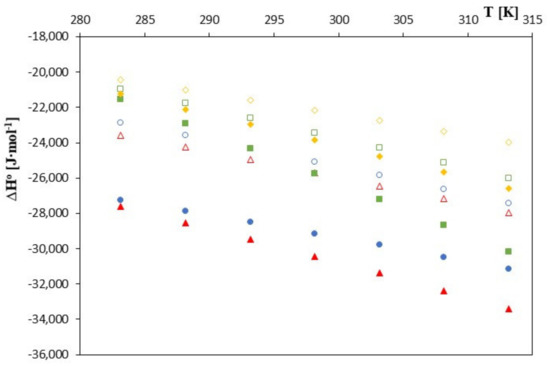
Figure 5.
The plots of dependence ∆H0 [J∙mol−1] in the function T [K] for β-cyclodextrin with all studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
 -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:
-trans-ferulic acid and 2-HP-β-cyclodextrin with the studied salts.:  -trans-cinnamic acid;
-trans-cinnamic acid;  -trans–p-coumaric acid;
-trans–p-coumaric acid;  -trans-caffeic acid;
-trans-caffeic acid;  -trans-ferulic acid.
-trans-ferulic acid.
These values are higher for β-cyclodextrin. This probably means that the total change in the dehydration effects of the hydrophobic part of the phenolic anion before its location inside cyclodextrin leads to an increase in the entropy. The larger and deeper location of the anions studied in unsubstituted cyclodextrin is associated with a greater level of hydrophobic dehydration and thus with a slightly greater increase in entropy. It is worth bearing in mind at this point that hydrophobic hydration is associated with a decrease in entropy.
The loss of hydrophobic hydration may result in a change in entropy. As can be seen, the formation of the studied complexes from the point of view of their spontaneity is determined by changes in the free enthalpy (into the results of energy gain associated with intermolecular interactions). Thermodynamic analysis of the studied complexing processes shows that in order for complexing to occur, an appropriate change in the free enthalpy must occur.
There are a number of interactions that allow the equilibrium to be shifted towards complex formation. However, the main driving force is to replace the polar-nonpolar interactions between the water and CD molecules, and the water and drug molecules, with the nonpolar-nonpolar interactions between the drug molecule and cyclodextrin.
A review of data from the literature shows that there are many research techniques (spectroscopic, electroanalytical, and separation techniques) allowing to assess the stability of cyclodextrin inclusion complexes with various types of compounds that have potential use as drugs [32]. As the authors of the works presented in the literature emphasize, none of the research techniques used separately allows for a complete structural assessment of the forming complexes [32,33,34]. They only allow one to determine the stoichiometry of the forming complexes. In the presented work, we proposed stoichiometry 1:1 for the studied complexes. Polewski et al. [33] proposed a similar stoichiometry for chlorogenic and cinnamic acids with beta-cyclodextrin based on a spectroscopic, thermodynamic and molecular modeling study. Similar conclusions can be drawn from the work of Plasson et al. [34]. In their work, affinity capillary electrophoresis was used for the simultaneous measurement of the pKa values and of the binding constants relative to the encapsulation of naturally occurring phenolic acids (rosmarinic and caffeic acids) with cyclodextrin. It should be emphasized that in the cited articles [33,34], all thermodynamic functions describing complexation processes (regardless of the research technique used) are negative, as in the case of the results presented by us. This indicates the same mechanism for the formation of inclusion complexes. The presented works (especially the work [32]) show how difficult it is to determine the exact stoichiometry and structure of the forming complexes.
The results of conductometric measurements presented in this paper only allow us to propose the stoichiometry of the forming inclusion complexes. Conductometry is one of the most accurate research techniques that allows for conducting this type of research. The innovative computational methods introduced by us in the previous work [24] and used in this work allow an accurate assessment of the complexation process taking place in the tested systems. Unfortunately, similar to other research methods used in the literature [32], it does not allow a full structural assessment of the inclusion complexes based on the results. Therefore, in order to supplement this information, we plan future spectroscopic and molecular modeling studies in the mixtures containing both α- and β-cyclodextrins.
3. Materials and Methods
3.1. Materials
High purity of trans-4-hydroxycinnamic acid (trans-p-coumaric acid), trans-3,4-dihydroxycinnamic acid (trans-caffeic acid), trans-4-hydroxy-3-methoxycinnamic acid (trans-ferulic acid), trans-3-phenylacrylic acid (trans-cinnamonic acid) and β-cyclodextrin and 2-HP-βcyclodextrin were used. The water used for the measurements was distilled twice and then passed through an ion exchanger to obtain the best purity (Behr Laboor-Technik, Düsseldorf, Germany). The water was distilled twice (specific conductivity ~ 10−6 S‧cm−1).
All the information on their purity and suppliers is presented in Table 9.

Table 9.
Specification of chemical samples.
3.2. Characterization Methods
Sodium salts of the tested phenolic acids were used for the measurements. The salts studied were obtained by mixing the appropriate amounts of acid and aqueous sodium hydroxide solution in a stoichiometric ratio of 1:1. The mixture was then heated and stirred to dissolve the acid and evaporate the solvent. All analyzed sodium salts of acids were recrystallized twice from aqueous ethanol solutions. The salts obtained were washed with acetone and dried under reduced pressure in a Büchi glass oven B-580 at T = 373.15 K until constant weight.
The exact procedure for their preparation is described in the work [24,35,36]. All sodium salts of the phenolic acids prior to measurement were dried and placed in a desiccator to avoid contact with ambient moisture.
Conductivity measurements were made on the highest-class RLC Wayne-Kerr 6430B conductometer with an uncertainty of 0.02%. The measuring cell was a three-electrode cell made of Pyrex glass containing no sodium in its composition (which would interfere with the measurement). The electrodes in this cell were made of platinum. The measuring cell in the tested temperature range 283.15–313.15 K was calibrated using KCl as the standard substance. All measurements were made at different frequencies v = 0.2, 0.5, 1.0, 1.5, 2.0, 3.0, 5.0, 10.0 and 20.0 kHz to obtain the most accurate values of the molar conductivity.
For all measurements, a BU 20F calibration thermostat (Lauda, Germany) with stability better than 0.005 K was used and the temperature was checked using an Amarell 3000TH AD thermometer (Amarell, Germany). The thermostat was connected to a DLK 25 flow cooler (Lauda, Germany). The entire measurement procedure is described in detail in [37,38,39]. All measured conductivity values, λ = 1/R∞, were the results of extrapolation of cell resistance, R∞(ν), to infinite frequency R∞ = limν→∞R(ν) using the empirical function R(ν) = R∞ + A/ν (where parameter A is specific for the cell). Taking into account the sources of errors (calibration, sample purity, measurements), the estimated uncertainty of the measured conductivity values was estimated at ±0.05%.
4. Conclusions
The paper presents conductometric measurements of phenolic acid derivatives with β and 2HP-β-CD cyclodextrin in aqueous solutions over a wide temperature range. The values of thermodynamic functions determined on the basis of experimental measurements (ΔH0 < 0, ΔS0 < 0, ΔG0 < 0) indicate the spontaneity of the inclusion of fungicides in the hydrophobic interior of the macromolecule of both cyclodextrins. The stoichiometry of the inclusion complexes of the investigated phenolic derivatives with both cyclodextrins is type 1:1. Conductometric measurements allowed us to estimate the stability of the formed complexes. It was found that the power of these complexes decreases with the size of the cyclodextrins and also with increasing temperature. This effect is most likely related to an increase in the mobility of ions of the tested phenolic acids, which significantly effects interactions of the type: the anion of studied phenolic acid, cyclodextrin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010292/s1, Table S1: Densities, viscosities and relative permittivities of pure water at temperatures T = (283.15 to 313.15)K.; Table S2: The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for β-cyclodextrin with trans-cinnamic acid sodium salt in water at all tested temperatures at pressure p = 0.1 MPa.a; Table S3. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for 2-HP-β-cyclodextrin with trans-cinnamic acid sodium salt in water at all tested temperatures; Table S4. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for β-cyclodextrin with trans-p-coumaric acid sodium salt in water at all tested temperatures; Table S5. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for 2-HP-β-cyclodextrin with trans-p-coumaric acid sodium salt in water at all tested temperatures.; Table S6. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for β-cyclodextrin with trans-caffeic acid sodium salt in water at all tested temperatures.; Table S7. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for 2-HP-β-cyclodextrin with trans-caffeic acid sodium in water at all tested temperatures.; Table S8. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for β-cyclodextrin with trans-ferulic acid sodium salt in water at all tested temperatures.; Table S9. The value of molar concentration of salt Csalt [mol/dm3], concentration of ligand Clig [mol/dm3], molar conductivity Λm [S‧cm2‧mol−1] for 2-HP-β-cyclodextrin with trans-ferulic acid sodium in water at all tested temperatures.
Funding
The author is grateful for the financial support of the Ministry of Science and Higher Education of the Republic of Poland.
Informed Consent Statement
No applicable.
Data Availability Statement
No applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Szetjli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar]
- Munzos-Botella, S.; del Castillo, B.; Martin, M.A. Cyclodextrin properties and applications of inclusion complex formation. ARS Pharm. 1995, 36, 187–198. [Google Scholar]
- Martin Del Valle, E.M. Cyclodextrin and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Fujishima, N.; Kusaka, K.; Umino, T.; Urushinata, T.; Terumi, K. Flour based foods containing highly branched cyclodextrins. Jpn. Pat. JP 2001, 136, 898. [Google Scholar]
- Bhardway, R.; Dorr, R.T.; Blanchard, J. Approaches to reducing toxicity of parenteral anticancer drug formulations using cyclodextrins. J. Pharm. Sci. Technol. 2000, 54, 233–239. [Google Scholar]
- Bibby, D.C.; Davies, N.M.; Tucker, I.G. Mechanism by which cyclodextrins modify drug release from polymeric drug delivery systems. Int. J. Pharm. 2000, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.J.; Scollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Foley, P.R.; Kaiser, C.E.; Sadler, J.D.; Burckhardt, E.E.; Liu, Z. Detergent Composition with Cyclodextrin Perfume Complexes to Mask Malodours. U.S. Patent WO2001023516A1, 29 September 2000. [Google Scholar]
- Hedges, R.A. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Baudin, C.; Pean, C.; Perly, B.; Goselin, P. Inclusion of organic pollutants in cyclodextrin and derivatives. Int. J. Environ. Anal. Chem. 2000, 77, 233–242. [Google Scholar] [CrossRef]
- Lezcano, M.; Ai-Soufi, W.; Novo, M.; Rodriguez-Nunez, E.; Tato, J.V. Complexation of several benzimidazoletype fugicides with alpha and beta- cyclodextrins. J. Agric. Food Chem. 2002, 50, 108–112. [Google Scholar] [CrossRef]
- Koukiekolo, R.; Desseaux, V.; Moreau, Y.; Marchis, M.G.; Santimone, M. Mechanism of porcine pancreatic alphaamylase inhibition of amylose and maltopentaose hydrolysis by alpha, beta- and gamma-cyclodextrins. Eur. J. Biochem. 2001, 268, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, E.; Stalcup, A.M. Cyclodextrins: A versatile tool in separation science. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y. Chiral separation of amino acids derivatives with fluoresceine-5-isothiocyanate by capillary electrophoresis and laser-induced fluorescene detection using mixed selectors of beta-cyclodextrin and sodium taurocholate. J. Chromatogr. A 2002, 955, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.K.; Kulhanek, K.M.; Smith, R. Chromatographic behaviour of selected steroids and their inclusion complexes with beta-cyclodextrin on octadecylsilica stationary phases with different carbon loads. J. Chromatogr. A 2002, 955, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrin. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Siquet, C.; Paiva-Martins, F.; Lima, J.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acid—A structure—Activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Comparison of the antioxidative activity of some acid-phenols: Structure—Activity relationship. Biosci. Biotechnol. Biochem. 1992, 56, 324–325. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.-T. Antioxidant activities of caffeic acid and its related hydroxy- cinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Medina, I.; Gallardo, J.M.; González, M.J.; Lois, A.S.; Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agric. Food Chem. 2007, 55, 3889–3895. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidant activity of hydrocinnamic acid derivative in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Kinart, Z.; Tomaš, R. Studies of the Formation of Inclusion Complexes Derivatives of Cinnamon Acid with α-Cyclodextrin in a Wide Range of Temperatures Using Conductometric Methods. Molecules 2022, 27, 4420. [Google Scholar] [CrossRef] [PubMed]
- Salomon, M.; Hefter, G.T. Mobilities of cation-macrocyclic ligand complexes. Pure Appl. Chem. 1993, 65, 1533–1540. [Google Scholar] [CrossRef][Green Version]
- Salomon, M. Conductometric study of cationic and anionic complexes in propylene carbonate. J. Solut. Chem. 1990, 19, 1225–1236. [Google Scholar] [CrossRef]
- Hoglen, N.C.; Waller, S.C.; Sipes, I.G.; Liebler, D.C. Reactions of peroxynitrite with gam-ma-tocopherol. Chem. Res. Toxicol. 1997, 10, 401–407. [Google Scholar] [CrossRef]
- Conney, R.V.; Harwood, P.J.; Franke, A.A.; Narala, K.; Sundstrom, A.K.; Berggren, P.O.; Mordan, L.J. Products of γ-tocopherol reaction with NO2 and their formation in rat insu- linoma (RINm5F) cells. Free. Radic. Biol. Med. 1995, 19, 259–269. [Google Scholar] [CrossRef]
- Conney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-pigott, V.; Custer, L.J. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef]
- Ohkatsu, Y.; Kajiyama, T.; Arai, Y. Antioxidant acivities of tocopherols. Polym. Degrad. Stab. 2001, 72, 303–311. [Google Scholar] [CrossRef]
- Williamson, K.S.; Gabbita, S.P.; Mou, S.; West, M.; Pye, Q.N.; Markesbery, W.R.; Cooney, R.V.; Grammas, P.; Reimann-Philipp, U.; Floyd, R.A.; et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide 2002, 6, 221–227. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Górnas, P.; Neunert, G.; Baczyńśki, K.; Polewski, K. Beta-cyclodextrin complexes with chlorogenic and caffeic acids from coffeebrew: Spectroscopic, thermodynamic and molecular modelling study. Food Chem. 2009, 114, 190–196. [Google Scholar] [CrossRef]
- Aksamija, A.; Tomao, V.; Dangles, O.; Plasson, R. Encapsulation of phenolic acids into cyclodextrins: A globalstatistical analysis of the effects of pH, temperature and concentrations on binding constants measured by ACE methods. Electrophoresis 2022, 43, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Swisłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Swisłocka, R. Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++G** level) studies on alkalimetal salts of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kinart, Z.; Tomaš, R. Conductivity properties of selected aliphatic monocarboxylic acid anions in water at 298.15 K. Int. J. Electrochem. Sci. 2020, 15, 10007–10027. [Google Scholar] [CrossRef]
- Bešter-Rogač, M.; Neueder, R.; Barthel, J.; Appeblat, A. Conductivity studies on aqueous solutions of stereoisomers of tartaricacids and tartrates. Part III. Acidic tartrates. J. Solut. Chem. 1998, 27, 299–307. [Google Scholar] [CrossRef]
- Barthel, J.; Feuerlein, F.; Neueder, R.; Wachter, R. Calibration of conductance cells at various temperatures. J. Solut. Chem. 1980, 9, 209–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).