Abstract
Development of novel derivatives to rein in and fight bacteria have never been more demanding, as microbial resistance strains are alarmingly increasing. A multitude of new fluoroquinolones derivatives with an improved spectrum of activity and/or enhanced pharmacokinetics parameters have been widely explored. Reporting novel antimicrobial agents entails comparing their potential activity to their parent drugs; hence, parent fluoroquinolones have been used in research as positive controls. Given that these fluoroquinolones possess variable activities according to their generation, it is necessary to include parent compounds and market available antibiotics of the same class when investigating antimicrobial activity. Herein, we provide a detailed guide on the in vitro biological activity of fluoroquinolones based on experimental results published in the last years. This work permits researchers to compare and analyze potential fluoroquinolones as positive control agents and to evaluate changes occurring in their activities. More importantly, the selection of fluoroquinolones as positive controls by medicinal chemists when investigating novel FQs analogs must be correlated to the laboratory pathogen inquest for reliable results.
1. Introduction
Antimicrobial prescriptions for the treatment of infections caused in particular by Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Mycobacterium tuberculosis (M. tuberculosis) have been affected by bacterial resistance [1]. Alarmingly, the ever-increasing emergence of resistant strains has globally increased the mortality rates [2].
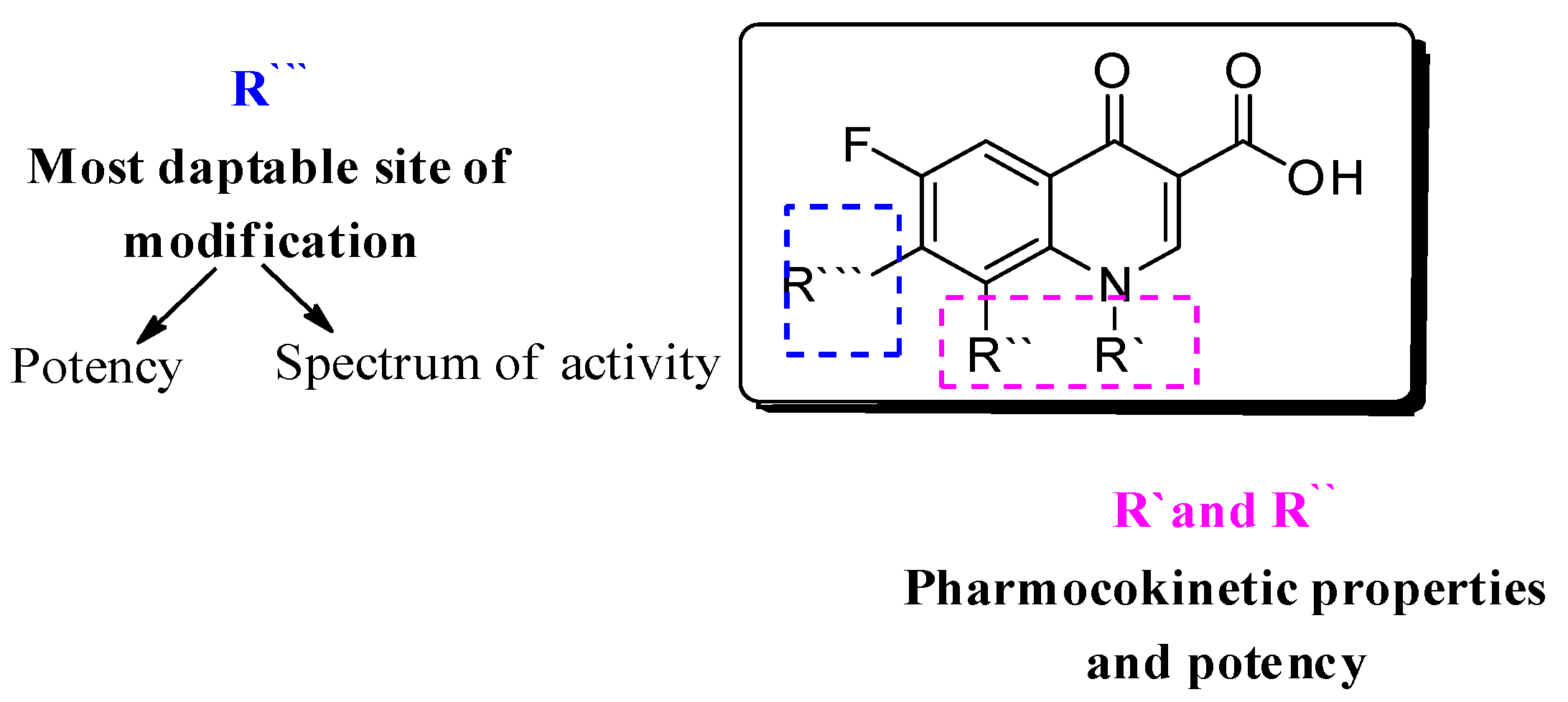
Several approaches have been followed to develop novel fluoroquinolones (FQs) with enhanced antimicrobial activity and/or to enhanced pharmacokinetic properties to tackle bacterial resistance [3,4,5,6,7,8]. With more than 500 newly introduced structural modifications on FQs’ key scaffold [9]; 1-substituted 1,4-dihydro-4-oxo-pyridine-3-carboxylic acid (Figure 1) and the recent approval of delafloxacin in 2017, researchers have focused on embracing the biological activity of FQs, particularly against resistant bacterial strains [10,11].
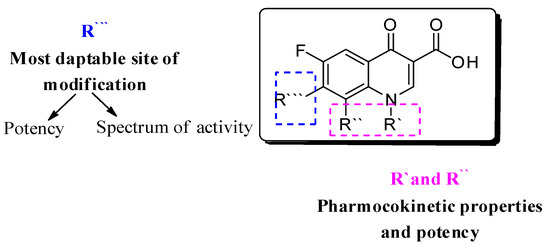
Figure 1.
Fluoroquinolone’s nucleus: 1-substituted 1,4-dihydro-4-oxo-pyridine-3-carboxylic acid; R’, R’’ are responsible for pharmacokinetic properties, and R’’’ is responsible for potency.
Additionally, literature reviews pointed out FQs’ potential activities as anticancer, antitumor, antiviral, and antifungal agents in addition to their antibacterial activity where the latter is attributed to their ability to selectively inhibit bacterial type II topoisomerases, DNA gyrase, and/or topoisomerase IV [12,13,14,15].
Currently, FQs are one of the most widely used antimicrobial drugs, with a wide range of indications, covering respiratory infections, urinary tract infections (UTIs), gastrointestinal infections, and gynecologic infections [16]. Moreover, FQs are indicated as a prophylactic treatment in immune-compromised neutropenic patients [17].
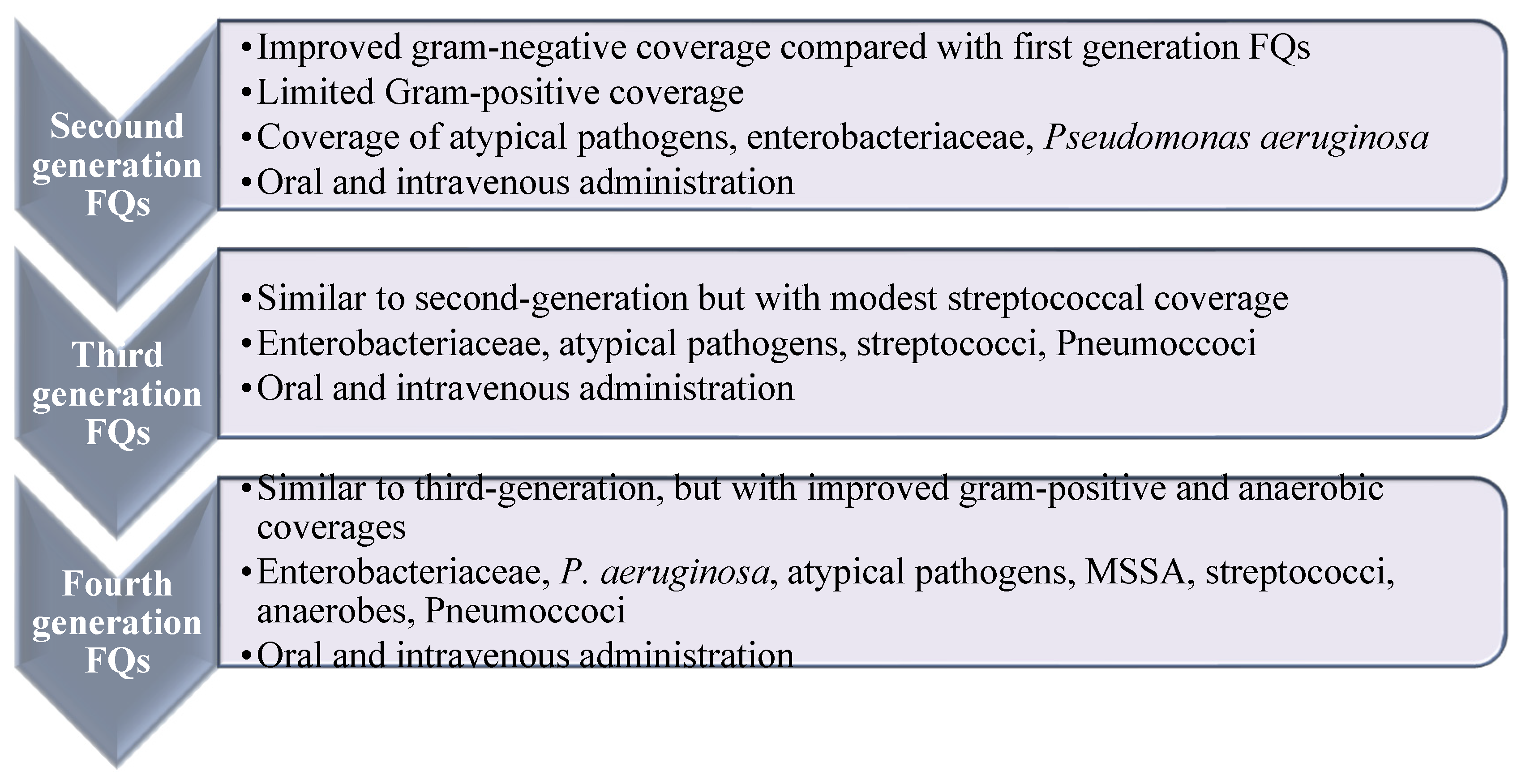
FQs are usually classified into four generations with enhanced efficacy and spectrum of activity, along with enhanced safety and pharmacokinetic characteristics (Figure 2) [18,19]. Ciprofloxacin is the most prosperous derivative, both economically and clinically [20], and the newer generations such as levofloxacin, gemifloxacin, and moxifloxacin offer enhanced activity against aerobic Gram-negative bacilli and Gram-positive bacteria over ciprofloxacin, e.g., against Streptococcus pneumoniae (S. pneumoniae) and S. aureus [20]. Ciprofloxacin and moxifloxacin retain enhanced in vitro activity against P. aeruginosa [21]. In terms of potency, moxifloxacin is more potent against Gram-positive and anaerobes than ciprofloxacin and levofloxacin. Newer generations displayed potent activity against penicillin-resistant and multidrug-resistant (MDR) pneumococcus and anaerobic bacteria. Recently, delafloxacin was granted approval in 2017 for the systemic treatment of acute bacterial skin infections [22].
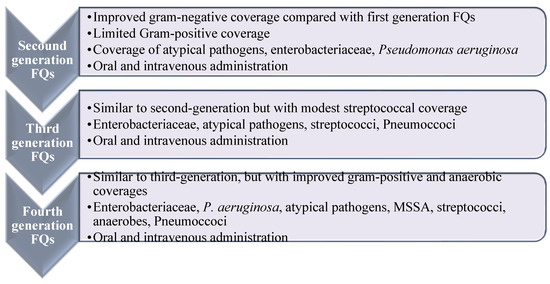
Figure 2.
Spectrum and antimicrobial activities of fluoroquinolone based on their generations. Widening of the antibacterial activity of fluoroquinolones in relation to their generation. Reproduced/adapted from ref. [13].
Appraisal of the newer FQs’ derivatives should be, in part, based on the relevant references. Herein, commonly employed FQ acting as positive controls in antimicrobial bioassays of up-to-date papers were reviewed. These results were reported in a constructive and comparative manner to facilitate the process of developing novel FQs’ analogues. The chemical structures and key physical properties of the frequently adopted standard FQs, namely norfloxacin 1, ciprofloxacin 2, levofloxacin 3, and moxifloxacin 4 are summarized in Table 1. This should provide a facile referral guide to recent research areas concerning FQs derivatives antibacterial inhibitory effect, the adopted testing protocols, and generations-based comparison between different FQs to be applied in innovative research. Choosing standard FQs will not only affect the assessment of the new counterparts, but also provide a more comprehensive and efficient performance in assays.

Table 1.
Most adopted standard fluoroquinolones, their chemical structures, and key physical properties.
2. Comparison of the In Vitro Antimicrobial Assays
A variety of methods and tactics could be adopted to evaluate the antibacterial activity of potential agents, and to draw constructive conclusions. In this regard, choosing and performing these assays varies according to the antimicrobial agents, availability of equipment, and cost-related reasons. The most known and basic standard methods are disk-diffusion [29] and broth or agar dilution methods [30]. The advantages and disadvantages of these assays are summarized in Table 2 and reviewed elsewhere [31,32], being apart from the scope of this article. In brief, standardized antimicrobial bioassays (antimicrobial susceptibility testing) are nowadays published and approved by the Clinical and Laboratory Standards Institute (CLSI) for bacteria and yeasts testing [33], herein the most commonly reported bioassays and the antimicrobial values of various FQs analogues are reported.

Table 2.
Advantages and disadvantages of commonly applied technique for the evaluation of drugs antimicrobial activity.
Dilution methods afford quantitative evaluation of the in vitro antimicrobial activity, which are usually expressed as minimum inhibitory concentration (MIC) values and represent the lowest concentration of the tested antimicrobial agent that inhibits the visible growth of tested microorganism. A number of approved guidelines for dilution antimicrobial susceptibility testing of fastidious or non-fastidious bacteria, yeast, and filamentous fungi are reported [30].
On the other hand, agar disk-diffusion method is the standard qualitative method for routine antimicrobial susceptibility testing. This method provides qualitative results by categorizing bacteria as susceptible, intermediate, or resistant based on the obtained growth zones of inhibition (ZOI) diameters. However, important parameters, including the growth media, temperature, period of incubation, and the required inoculum size should be optimized to fulfil CLSI standards [22].
Differently, measuring the inhibition of supercoiling activity (catalytic activity) of DNA gyrase or the concentration of compounds required for inhibiting 50% of gyrase supercoiling activity (IC50) has been widely reported as an alternative assay to test the antibacterial activity of different FQs derivatives, particularly if the mechanistic and catalytical activity of the developed analogues are of concern [34,35].
3. FQ’s Antibacterial Biological Activity
3.1. FQ’s Antibacterial Activity against Gram-Positive Bacteria
According to the reviewed literature in the past five years, and for the sake of including up-to-date activities on the most common FQs applied as golden antimicrobial positive controls in laboratories, herein, standard FQs and their antimicrobial activity against a panel of laboratory microbes are reported (Table 3).

Table 3.
Fluoroquinolones’ antibacterial activity against Gram-positive bacterial strains.
As reported, norfloxacin was used as a positive control in the pipeline publications, including norfloxacin derivatives synthesis. Norfloxacin MIC against Gram-positive is presented in Table 3 [1,23,24,26,28,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. In brief, norfloxacin inhibitory activity against a panel of Gram-positive bacteria regardless of the strain varied relatively. For example, norfloxacin in vitro antibacterial activity reported by Mentese et al. against E. faecalis ATCC 29212 varied from that reported by Seliem et al. (MIC ranged from <0.128 µM [46]−100.207 µM [47]). Similarly, norfloxacin MIC against S. aureus ATCC 25923 ranged from <0.128 µM [46]–156.170 µM [45] in the above-mentioned two different studies.
As illustrated in Table 3, ciprofloxacin was the most commonly adopted reference by the cited researchers against different Gram positive and negative bacterial stains, ciprofloxacin MIC against Gram-positive bacteria including B. cereus spp. ranged from 0.181 µM [46]−3.954 µM [28], S. aureus ATCC 6538 (ranged from 1.509 µM [48]−146.978 [49] µM), S. aureus ATCC 29213 (MIC ranged from 0.082 µM [67]−1.509 µM [48]), and S. aureus ATCC 25923 (MIC ranged from 0.010 [52] µM −3.954 µM [28]). Remarkably, ciprofloxacin MIC varied within similar bacterial species, one example is S. epidermidis species, according to Liu et al., strain MSSE 12-1 of S. epidermidis species was susceptible to ciprofloxacin (MIC 0.755 µM) [26], whereas it showed very limited activity against MSSE14-2 strain (MIC > 386.308 µM) [53,54]. Interestingly, discrepancy in MIC values was observed between similar bacterial strains as reported by different research groups with 100-fold MIC difference [48,49]. Minor variation between the adopted testing protocol for MIC determination, such as incubation temperature might be the driving factor for such a difference [48,49].
Considering the third FQ reference, levofloxacin was adopted by many researchers’ as a reference control, and exhibited variable antimicrobial activity against E. faecalis (MIC ranged from 1.384 µM for E. faecalis 51575 [55], 177.220 µM for E. faecalis ATCC 700221 [51]) as an example. A notable difference in levofloxacin potency against different staph strains, including methicillin-sensitive S. aureus (MSSA) [26,53,54,68,69], methicillin-resistant S. aureus (MRSA) [26,53,54,68,69], S. epidermidis, and S. pneumoniae was observed (Table 3).
Following scientific reports in the literature, levofloxacin exhibited superior antibacterial activity against Gram-positive S. epidermis strains [51,55,63] and moxifloxacin is generally the most potent amongst FQs a, gainst Gram-positive and negative bacteria [26]. Moxifloxacin was the latent agent against the food poisoning pathogen L. monocytogenes ATCC 43251 (MIC < 1.370 µM [28]) when compared with other FQs as ciprofloxacin (MIC 3.954 µM−12.072 [28,64]) and norfloxacin (MIC < 8.267 µM [28]).
3.2. FQs Antibacterial Activity against Gram-Negative Bacteria
A summary of common laboratory tested Gram-negative bacteria and standard fluoroquinolones antibiotics are presented in Table 4. It is noticeable that ciprofloxacin has potential antibacterial activity against Gram-negative bacteria as P. aeruginosa and E. coli. [28,48]. Moreover, ciprofloxacin had prospective growth inhibitory activity against H. pylori NCTC 11916 and 12 more H. pylori clinical isolates as reported by Abu-Sini et al. [72]. Ciprofloxacin broad spectrum of activity against aerobic and anaerobic Gram-negative bacteria is shown in Table 4.

Table 4.
Fluoroquinolones’ antibacterial activity against Gram-negative bacterial strains.
Nevertheless, Gorityala et al. [56] reported that ciprofloxacin potency against P. aeruginosa were superior compared to moxifloxacin. This pattern was also noticed in results published by Türe et al. and Garza et al., [28,50].
Norfloxacin inhibitory activity against a panel of Gram-negative bacterial type, and on the same bacterial strain is noted to be varied. For instance, norfloxacin in vitro antibacterial activity reported by Pardeshi et al. against E. coli ATCC 25922 varied from that reported by Leyva-Ramos et al. (MIC ranged from < 0.094 µM [24]−117.433 µM [45]). Moreover, norfloxacin and ciprofloxacin MIC against different P. aeruginosa strains ranged from 1.002 µM [1]−1565.773 µM [45] and <0.091 [62] µM−150.901 µM [42], respectively, in different studies. On the contrary, ciprofloxacin MIC against a panel of Gram–negative pathogens looks more consistent (A. haemolyticus ATCC 19002 (MIC 0.755 µM) [62], A. baumannii ATCC17961 (MIC 0.24 µM) [58], A. calcoacetious ATCC 19606 (MIC 1.509 µM) [55], and C. freundii ATCC 43864 (MIC 1.38 µM) [51]. However, a wide range in ciprofloxacin MIC against E. coli ATCC 25922 is perturbing as MIC reported ranged from 0.002 µM [24]−61.869 µM [49] in different publications. This fluctuation in ciprofloxacin antibacterial activities may explain the current abundant application of levofloxacin and moxifloxacin as positive standards by medicinal chemists when designing and synthesizing novel FQs analogues [24,28,53,54,55,68,69,70,73,74,75].
As presented in Table 4, different studies reported the use of third generation levofloxacin as a positive control against a wide range of Gram-negative organisms includes P. aeruginosa. For this infectious pathogen, MIC ranged from 5.453 µM [68] for P. aeruginosa 14–19 strain to 87.241 µM [69] for P. aeruginosa 12–14 strain. Similarly, levofloxacin MIC against K. pneumonia ranged from 0.082 µM [69] for K. pneumonia 12–4 strain to 87.241 µM [54] for K. pneumonia 14–3 strain. According to Zhang et al., [69] levofloxacin is around five hundred time more potent against K. pneumonia 12–4 strain compared to P. aeruginosa 12–14 strain, though both are Gram-negative pathogens. However, in another by Huang et al. [68], levofloxacin was more potent against P. aeruginosa for 14–19 strain compared to K. pneumonia for 14–2 strain. It is worth mentioning that the bacterial strain is the variant factor in both articles. This indeed highlights the importance of referring to the relevant standard control during laboratory investigation and comparisons.
A similar pattern of the wide range of MIC values against the same strain was observed, where the MIC of norfloxacin against E. coli ATCC-25922 ranged from <0.094 µM [24] to 117.433 µM [45].
3.3. FQs’ Antimycobacterial Activity
FQs, particularly ciprofloxacin was included as a positive control along with isoniazid and rifampicin against various Mycobacterium strains as shown in Table 5 [24,26,27,28,58,63,65,68,75,81,82]. Furthermore, levofloxacin in vitro anti-mycobacterial activity was reported and found to be comparable to ciprofloxacin [26,68]. Recent studies by Hu et al., [82] and Mohammed et al., [65] declared moxifloxacin in vitro anti-mycobacterial activity to be more potent than both ciprofloxacin 1 and levofloxacin 3.

Table 5.
Fluoroquinolones’ antimycobacterial activity.
3.4. FQs’ Antifungal, Antiparasitic, and Anticancer Activity
Apart from their antibacterial activity, FQs were also tested for their antifungal activity with little effect on most fungi. Since the late 1980s, studies revealed anti-trypanosomal activity for the quinolones prototype, nalidixic, and oxolonic acid derivatives [14]. Other studies illustrated the antiparasitic activity of norfloxacin against Plasmodium falciparum and the inhibitory effect of other fluoroquinolones against Plasmodium family [14,83,84]. Today, quinolone-amides related derivatives were used to design anti-trypanosomal compounds with many of them presenting potential in vivo activity [85].
Anticancer activity of FQs were also evaluated against a range of cancer cell lines, such as A549 Lung adenocarcinoma, HCT-116 colon cancer, MCF-7 breast cancer cell lines, and others have been determined previously and compared with the developed counterparts [48,50,61,66] as presented in Table 6.

Table 6.
Fluoroquinolones’ antifungal and anticancer activity.
3.5. FQs Inhibitory Effect as Anti-Viral Agaents against SARS-CoV-2 and HIV-1
As researchers investigate several approaches to combat COVID-19 infection, there is a wide interest in fluoroquinolones. Ciprofloxacin and Moxifloxacin were tested through in silico molecular docking and showed the potential binding capacity to SARS-CoV-2 main protease (Mpro) and low binding energy. Moreover, a recent study evaluated the potency and cellular toxicity of selected FQs (enoxacin, ciprofloxacin, levofloxacin, and moxifloxacin) against SARS-CoV-2 and MERS-COV. This study showed that a high concentration of the tested FQs should be employed to prevent viral replication with enoxacin being the superior (EC50 of 126.4) against SARS-CoV-2 [14,83,84]. Other studies evaluated FQs anti-HIV-1 activities. However, FQs standards activity were not presented [65].
4. Recommendations
Based on recently published research where FQs were used as positive controls against several microorganisms and cancer cells, it is recommended to use the most active FQ in future studies in addition to the parent drugs to compare the benefits and to have an accurate insight when reporting results.
The difference perceived in FQs’ potency according to different research articles is challenging and could be attributed to several factors, including the different testing protocols implemented by each research group, solvents or broth used in bacterial culturing, incubation time, bacterial concentration tested, bacterial growth phase, reader instrument sensitivity, etc.
Ciprofloxacin is recommended to be used as a control against Gram-negative bacteria whether resistant or susceptible. If mainly Gram-positive activity is concerned, levofloxacin or moxifloxacin might be the best choices. The wide-spectrum and potent newer generations should be compared with, when broader comparison is desired. Choose moxifloxacin if the development of newer FQs derivatives is not a biologically-based design. This should provide a proper perspective when reporting novel FQs and their activities. Working against Mycobacterium stains, moxifloxacin was found to be more active compared to the other FQs, thus it is advisable to consider it as a positive control.
Moreover, the authors spur adopting preliminary activity testing of the chosen strains before commencing biological evaluation of interest as some of the stains might not be susceptible to the reference drugs. Lastly, given that some stains exhibited varied MIC values against the same drug, we recommend revising the adopted protocols beforehand to get more accurate comparable results of the reference drug, which will be then more reliable to base the conclusions upon.
Author Contributions
Conceptualization, G.A.R.Y.S. and A.A.M.M.; resources, G.A.R.Y.S., A.A.M.M. and B.A.A.; writing—original draft preparation, A.A.M.M.; writing—review and editing, B.A.A. and G.A.R.Y.S.; supervision, G.A.R.Y.S.; funding acquisition, G.A.R.Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific research at The University of Jordan grant number [2213].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not related.
Acknowledgments
The authors would like to acknowledge The University of Jordan and The University of Petra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pintilie, L.; Stefaniu, A.; Nicu, A.I.; Maganu, M.; Caproiu, M.T. Design, synthesis and docking studies of some novel fluoroquinolone compounds with antibacterial activity. Synthesis 2018, 665, 636w. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report: 2004: Changing History; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41 (Suppl. S2), S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Suaifan, G.A.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis, and biological evaluation of 1, 8-naphthyridine glucosamine conjugates as antimicrobial agents. Drug Dev. Res. 2019, 80, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.; Mohammed, A.A.M.; Shehadeh, M.B. 1,8-naphthyridine Glucosamine Derivatives, Their Use in the Treatment of Microbial Infections, and a Method for Preparation 2020-WO202084648. Available online: https://patents.google.com/patent/WO2020084648A1/en?oq=PCT%2fJO2019%2f050010 (accessed on 13 October 2021).
- Suaifan, G.A.; Mohammed, A.A.M.; Shehadeh, M.B. Glycosylated 3-Substituted Fluoroquinolone Derivatives, Preparation Methods Thereof, and Their Use in the Treatment of Antimicrobial Infections 2020, WO2020/202239. Available online: https://patents.google.com/patent/WO2020202239A1/en?oq=WO2020202239A1 (accessed on 13 October 2021).
- Suaifan, G.A.; Mohammed, A.A.M.; Shehadeh, M.B. 1,8-naphthyridine Glucosamine Derivatives, and Uses Thereof for Treating Microbial Infections 2018. Reg. No. P/JO/2018/000097. Available online: https://patentscope.wipo.int/search/ar/detail.jsf?docId=JO320922571&_cid=P10-KZSKQB-93797-1 (accessed on 13 October 2021).
- Ezelarab, H.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.D.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. 2018, 351, 1800141. [Google Scholar]
- Suaifan, G.A.; Mohammed, A.A. Fluoroquinolones structural and medicinal developments (2013–2018): Where are we now? Bioorg. Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.; Mohammed, A.A. Erratum to “Fluoroquinolones structural and medicinal developments (2013–2018): Where are we now?”. Bioorganic Med. Chem. 2019, 27, 115072. [Google Scholar] [CrossRef]
- Markham, A. Delafloxacin: First global approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Idowu, T.; Schweizer, F. Ubiquitous nature of fluoroquinolones: The oscillation between antibacterial and anticancer activities. Antibiotics 2017, 6, 26. [Google Scholar] [CrossRef]
- Dalhoff, A. Antiviral, antifungal, and antiparasitic activities of fluoroquinolones optimized for treatment of bacterial infections: A puzzling paradox or a logical consequence of their mode of action? Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 661–668. [Google Scholar] [CrossRef]
- Ozdek, S.C.; Miller, D.; Flynn, P.M.; Flynn, H.W., Jr. In vitro antifungal activity of the fourth generation fluoroquinolones against Candida isolates from human ocular infections. Ocul. Immunol. Inflamm. 2006, 14, 347–351. [Google Scholar] [CrossRef]
- Oliphant, C.M.; Green, G. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455. [Google Scholar]
- Abidi, M.; Ledeboer, N.; Banerjee, A.; Hari, P. Morbidity and mortality attributable to Rothia bacteremia in neutropenic and nonneutropenic patients. Diagn. Microbiol. Infect. Dis. 2016, 85, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Mitscher, L.A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The new fluoroquinolones: A critical review. Can. J. Infect. Dis. Med. Microbiol. 1999, 10, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Ennis, K.; Vercaigne, L.; Walkty, A.; Gin, A.S.; Embil, J.; Smith, H.; Hoban, D.J. A critical review of the fluoroquinolones. Drugs 2002, 62, 13–59. [Google Scholar] [CrossRef] [PubMed]
- Gorityala, B.K.; Guchhait, G.; Goswami, S.; Fernando, D.M.; Kumar, A.; Zhanel, G.G.; Schweizer, F. Hybrid antibiotic overcomes resistance in P. aeruginosa by enhancing outer membrane penetration and reducing efflux. J. Med. Chem. 2016, 59, 8441–8455. [Google Scholar] [CrossRef]
- Jorgensen, S.C.; Mercuro, N.J.; Davis, S.L.; Rybak, M.J. Delafloxacin: Place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect. Dis. Ther. 2018, 7, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Panda, S.S.; Birs, A.S.; Serrano, J.C.; Gonzalez, C.F.; Alamry, K.A.; Katritzky, A.R. Synthesis and antibacterial evaluation of amino acid–antibiotic conjugates. Bioorganic Med. Chem. Lett. 2014, 24, 1856–1861. [Google Scholar] [CrossRef]
- Pardeshi, K.A.; Kumar, T.A.; Ravikumar, G.; Shukla, M.; Kaul, G.; Chopra, S.; Chakrapani, H. Targeted Antibacterial Activity Guided by Bacteria-Specific Nitroreductase Catalytic Activation to Produce Ciprofloxacin. Bioconjugate Chem. 2019, 30, 751–759. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Park, S.-E.; Abuo-Rahma, G.E.-D.A.; Sayed, M.A.; Kwon, Y. Novel N-4-piperazinyl-ciprofloxacin-chalcone hybrids: Synthesis, physicochemical properties, anticancer and topoisomerase I and II inhibitory activity. Eur. J. Med. Chem. 2013, 69, 427–438. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Wang, J.; Wang, M.; Liu, M.; Wang, B.; Guo, H.; Lu, Y. Synthesis, antimycobacterial and antibacterial evaluation of l-[(1R, 2S)-2-fluorocyclopropyl] fluoroquinolone derivatives containing an oxime functional moiety. Eur. J. Med. Chem. 2014, 86, 628–638. [Google Scholar] [CrossRef]
- Cilliers, P.; Seldon, R.; Smit, F.J.; Aucamp, J.; Jordaan, A.; Warner, D.F.; N’Da, D.D. Design, synthesis, and antimycobacterial activity of novel ciprofloxacin derivatives. Chem. Biol. Drug Des. 2019, 94, 1518–1536. [Google Scholar] [CrossRef] [PubMed]
- Türe, A.; Kulabaş, N.; Dingiş, S.İ.; Birgül, K.; Bozdeveci, A.; Karaoğlu, Ş.A.; Krishna, V.S.; Sriram, D.; Küçükgüzel, İ. Design, synthesis and molecular modeling studies on novel moxifloxacin derivatives as potential antibacterial and antituberculosis agents. Bioorganic Chem. 2019, 88, 102965. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P. Clinical and Laboratory Standards Institute (CLSI) performance standards for antimicrobial disk diffusion susceptibility tests 19th ed. approved standard. CLSI Doc. M100-S19 2009, 29, M100-S21. [Google Scholar]
- Pfaller, M.; Sheehan, D.; Rex, J. Determination of fungicidal activities against yeasts and molds: Lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- SLCI Performance Standards for Antimicrobial Disk Susceptibility Tests. Available online: https://clsi.org/standards/products/microbiology/documents/m02/ (accessed on 30 March 2020).
- Xiao, Z.-P.; Wang, X.-D.; Wang, P.-F.; Zhou, Y.; Zhang, J.-W.; Zhang, L.; Zhou, J.; Zhou, S.-S.; Ouyang, H.; Lin, X.-Y. Design, synthesis, and evaluation of novel fluoroquinolone–flavonoid hybrids as potent antibiotics against drug-resistant microorganisms. Eur. J. Med. Chem. 2014, 80, 92–100. [Google Scholar] [CrossRef]
- Towle, T.R.; Kulkarni, C.A.; Oppegard, L.M.; Williams, B.P.; Picha, T.A.; Hiasa, H.; Kerns, R.J. Design, synthesis, and evaluation of novel N-1 fluoroquinolone derivatives: Probing for binding contact with the active site tyrosine of gyrase. Bioorganic Med. Chem. Lett. 2018, 28, 1903–1910. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility test methods: Dilution and disk diffusion methods. Man. Clin. Microbiol. 2015, 1253–1273. [Google Scholar]
- Korgenski, E.K.; Daly, J.A. Evaluation of the BIOMIC video reader system for determining interpretive categories of isolates on the basis of disk diffusion susceptibility results. J. Clin. Microbiol. 1998, 36, 302–304. [Google Scholar] [CrossRef]
- Benkova, M.; Soukup, O.; Marek, J. Antimicrobial susceptibility testing: Currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 2020, 129, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Le Page, S.; van Belkum, A.; Fulchiron, C.; Huguet, R.; Raoult, D.; Rolain, J.-M. Evaluation of the PREVI® Isola automated seeder system compared to reference manual inoculation for antibiotic susceptibility testing by the disk diffusion method. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Hada, D.; Chauhan, N.P.S. 11. Antimicrobial testing methods. In Biocidal Polymers; De Gruyter: Berlin, Boston, 2019; pp. 241–262. [Google Scholar]
- Bruin, J.P.; Diederen, B.M.; IJzerman, E.P.; Den Boer, J.W.; Mouton, J.W. Correlation of MIC value and disk inhibition zone diameters in clinical Legionella pneumophila serogroup 1 isolates. Diagn. Microbiol. Infect. Dis. 2013, 76, 339–342. [Google Scholar] [CrossRef]
- Sharma, P.C.; Kumar, R.; Chaudhary, M.; Sharma, A.; Rajak, H. Synthesis and biological evaluation of novel benzothiazole clubbed fluoroquinolone derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 1–10. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, L.J. Design, synthesis, molecular docking, and antibacterial evaluation of some novel flouroquinolone derivatives as potent antibacterial agent. Sci. World J. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Chugunova, E.; Akylbekov, N.; Bulatova, A.; Gavrilov, N.; Voloshina, A.; Kulik, N.; Zobov, V.; Dobrynin, A.; Syakaev, V.; Burilov, A. Synthesis and biological evaluation of novel structural hybrids of benzofuroxan derivatives and fluoroquinolones. Eur. J. Med. Chem. 2016, 116, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Ramos, S.; de Loera, D.; Cardoso-Ortiz, J. In vitro antibacterial activity of 7-substituted-6-fluoroquinolone and 7-substituted-6, 8-difluoroquinolone derivatives. Chemotherapy 2017, 62, 194–198. [Google Scholar] [CrossRef]
- Mentese, M.; Demirbas, N.; Mermer, A.; Demirci, S.; Demirbas, A.; Ayaz, F.A. Novel azole-functionalited flouroquinolone hybrids: Design, conventional and microwave irradiated synthesis, evaluation as antibacterial and antioxidant agents. Lett. Drug Des. Discov. 2018, 15, 46–64. [Google Scholar] [CrossRef]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Nagy, Y.I.; George, R.F.; Fayad, W.; Fawzy, N.G.; Ibrahim, T.S.; Al-Mahmoudy, A.M.; Sakhuja, R. Design, synthesis, antimicrobial, and DNA gyrase inhibitory properties of fluoroquinolone–dichloroacetic acid hybrids. Chem. Biol. Drug Des. 2020, 95, 248–259. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stępień, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef]
- Mokaber-Esfahani, M.; Eshghi, H.; Akbarzadeh, M.; Gholizadeh, M.; Mirzaie, Y.; Hakimi, M.; Lari, J. Synthesis and Antibacterial Evaluation of New Pyrimidyl N-Ciprofloxacin Derivatives. ChemistrySelect 2019, 4, 8930–8933. [Google Scholar] [CrossRef]
- Garza, I.; Wallace, M.J.; Fernando, D.; Singh, A.; Lee, R.E.; Gerding, J.S.; Franklin, C.; Yendapally, R. Synthesis and Evaluation of Thiazolidine Amide and N-Thiazolyl Amide Fluoroquinolone Derivatives. Arch. Der. Pharm. 2017, 350, e201700029. [Google Scholar] [CrossRef]
- Gao, Y.; Na, L.X.; Xu, Z.; Zhang, S.; Wang, A.P.; Lü, K.; Guo, H.Y.; Liu, M.L. Design, Synthesis and Antibacterial Evaluation of 1-[(1R, 2S)-2-Fluorocyclopropyl] ciprofloxacin-1, 2, 4-triazole-5 (4H)-thione Hybrids. Chem. Biodivers. 2018, 15, e1800261. [Google Scholar] [CrossRef]
- Gao, L.-Z.; Xie, Y.-S.; Li, T.; Huang, W.-L.; Hu, G.-Q. Synthesis and antibacterial activity of novel [1, 2, 4] triazolo [3, 4-h][1, 8] naphthyridine-7-carboxylic acid derivatives. Chin. Chem. Lett. 2015, 26, 149–151. [Google Scholar] [CrossRef]
- Bykowska, A.; Starosta, R.; Jezierska, J.; Jeżowska-Bojczuk, M. Coordination versatility of phosphine derivatives of fluoroquinolones. New Cu I and Cu II complexes and their interactions with DNA. RSC Adv. 2015, 5, 80804–80815. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, W.; Liu, M.; Zhang, R.; Wang, M.; Li, L.; Wang, B.; Guo, H.; Lu, Y. Synthesis, antimycobacterial and antibacterial activity of fluoroquinolone derivatives containing an 3-alkoxyimino-4-(cyclopropylanimo) methylpyrrolidine moiety. Eur. J. Med. Chem. 2015, 104, 73–85. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Feng, L.-S.; Li, X.-N.; Huang, G.-C.; Chai, Y.; Lv, Z.-S.; Guo, H.-Y.; Liu, M.-L. Synthesis and in vitro antimycobacterial and antibacterial activity of 8-OMe ciprofloxacin-hydrozone/azole hybrids. Molecules 2017, 22, 1171. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Guchhait, G.; Fernando, D.M.; Deo, S.; McKenna, S.A.; Zhanel, G.G.; Kumar, A.; Schweizer, F. Adjuvants based on hybrid antibiotics overcome resistance in Pseudomonas aeruginosa and enhance fluoroquinolone efficacy. Angew. Chem. Int. Ed. 2016, 55, 555–559. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Rajtar, B.; Polz-Dacewicz, M. Synthesis and in vitro activity of 1, 2, 4-triazole-ciprofloxacin hybrids against drug-susceptible and drug-resistant bacteria. Eur. J. Med. Chem. 2013, 60, 128–134. [Google Scholar] [CrossRef]
- Ji, C.; Miller, P.A.; Miller, M.J. Syntheses and antibacterial activity of N-acylated ciprofloxacin derivatives based on the trimethyl lock. ACS Med. Chem. Lett. 2015, 6, 707–710. [Google Scholar] [CrossRef]
- Kassab, A.E.; Gedawy, E.M. Novel ciprofloxacin hybrids using biology oriented drug synthesis (BIODS) approach: Anticancer activity, effects on cell cycle profile, caspase-3 mediated apoptosis, topoisomerase II inhibition, and antibacterial activity. Eur. J. Med. Chem. 2018, 150, 403–418. [Google Scholar] [CrossRef]
- Rajulu, G.G.; Naik, H.S.B.; Abhilash, V.; Thiruvengadam, J.; Rajesh, K.; Ganesh, S.; Jagadheshan, H.; Kesavan, P.K. New hydroxamic acid derivatives of fluoroquinolones: Synthesis and evaluation of antibacterial and anticancer properties. Chem. Pharm. Bull. 2014, 62, 168–175. [Google Scholar] [CrossRef][Green Version]
- Rajulu, G.G.; Naik, H.S.B.; Kumar, G.C.; Ramaraj, S.; Sambasivam, G.; Koppolu, K.P. New azetidine-3-carbonyl-N-methyl-hydrazino derivatives of fluoroquinolones: Synthesis and evaluation of antibacterial and anticancer properties. Med. Chem. Res. 2014, 23, 2856–2868. [Google Scholar] [CrossRef]
- Mermer, A.; Faiz, O.; Demirbas, A.; Demirbas, N.; Alagumuthu, M.; Arumugam, S. Piperazine-azole-fluoroquinolone hybrids: Conventional and microwave irradiated synthesis, biological activity screening and molecular docking studies. Bioorganic Chem. 2019, 85, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Ha-Duong, N.-T.; Aubry, A.; Capton, E.; Fechter, P.; Plesiat, P.; Verbeke, P.; Serradji, N. In vitro activities of a new fluoroquinolone derivative highly active against Chlamydia trachomatis. Bioorganic Chem. 2019, 83, 180–185. [Google Scholar] [CrossRef]
- Marquez, B.; Pourcelle, V.; Vallet, C.M.; Mingeot-Leclercq, M.-P.; Tulkens, P.M.; Marchand-Bruynaert, J.; Van Bambeke, F. Pharmacological characterization of 7-(4-(piperazin-1-yl)) ciprofloxacin derivatives: Antibacterial activity, cellular accumulation, susceptibility to efflux transporters, and intracellular activity. Pharm. Res. 2014, 31, 1290–1301. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Abbas, S.H.; Abdelhafez, E.-S.M.; Berger, J.M.; Mitarai, S.; Arai, M.; Abuo-Rahma, G.E.-D.A. Synthesis, molecular docking, antimicrobial evaluation, and DNA cleavage assay of new thiadiazole/oxadiazole ciprofloxacin derivatives. Mon. Für Chem.-Chem. Mon. 2019, 150, 1809–1824. [Google Scholar] [CrossRef]
- Szczupak, Ł.; Kowalczyk, A.; Trzybiński, D.; Woźniak, K.; Mendoza, G.; Arruebo, M.; Steverding, D.; Stączek, P.; Kowalski, K. Organometallic ciprofloxacin conjugates with dual action: Synthesis, characterization, and antimicrobial and cytotoxicity studies. Dalton Trans. 2020, 49, 1403–1415. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.-K.; Plattner, J.J.; Mao, W.; Alley, M.; Xia, Y.; Hernandez, V.; Zhou, Y.; Ding, C.Z.; Li, J. Synthesis and antibacterial evaluation of a novel tricyclic oxaborole-fused fluoroquinolone. Bioorganic Med. Chem. Lett. 2013, 23, 963–966. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Wang, B.; Wu, Z.; Liu, M.; Feng, L.; Zhang, J.; Li, X.; Yang, Y.; Lu, Y. Synthesis, antimycobacterial and antibacterial activity of 1-(6-amino-3, 5-difluoropyridin-2-yl) fluoroquinolone derivatives containing an oxime functional moiety. Bioorganic Med. Chem. Lett. 2016, 26, 2262–2267. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Chen, S.; Liu, K.; Lin, Y.; Guo, H.; Liu, M. Synthesis and antibacterial activity of amino acid and dipeptide prodrugs of IMB-070593, a fluoroquinolone candidate. Molecules 2014, 19, 6822–6837. [Google Scholar] [CrossRef] [PubMed]
- Salunke, R.A.; Shukla, M.; Kaul, G.; Bansal, B.R.; Chopra, S.; Chhibber, M. New fluoroquinolone compounds with endo-nortropine derivatives at C-7 position show antibacterial activity against fluoroquinolone-resistant strains of Staphylococcus aureus. Chem. Biol. Drug Des. 2019, 94, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, J.; Cheng, H.; Wang, H.; Wang, J.; Wang, S.; Zhou, J.; Zhang, H. Design, Synthesis, Antibacterial Evaluation and Docking Study of Novel 2-Hydroxy-3-(nitroimidazolyl)-propyl-derived Quinolone. Chem. Biol. Drug Des. 2015, 85, 79–90. [Google Scholar] [CrossRef]
- Abu-Sini, M.; Mayyas, A.; Al-Karablieh, N.; Darwish, R.; Al-Hiari, Y.; Aburjai, T.; Arabiyat, S.; Abu-Qatouseh, L. Synthesis of 1, 2, 3-triazolo [4, 5-h] quinolone derivatives with novel anti-microbial properties against Metronidazole resistant Helicobacter pylori. Molecules 2017, 22, 841. [Google Scholar] [CrossRef]
- El-Megharbel, S.; Adam, A.; Megahed, A.; Refat, M. Synthesis and molecular structure of moxifloxacin drug with metal ions as a model drug against some kinds of bacteria and fungi. Russ. J. Gen. Chem. 2015, 85, 2366–2373. [Google Scholar] [CrossRef]
- Long, T.E.; Keding, L.C.; Lewis, D.D.; Anstead, M.I.; Withers, T.R.; Hongwei, D.Y. Anionic fluoroquinolones as antibacterials against biofilm-producing Pseudomonas aeruginosa. Bioorganic Med. Chem. Lett. 2016, 26, 1305–1309. [Google Scholar] [CrossRef]
- Charushin, V.N.; Mochulskaya, N.N.; Antipin, F.V.; Kotovskaya, S.K.; Nosova, E.V.; Ezhikova, M.A.; Kodess, M.I.; Kravchenko, M.A. Synthesis and antimycobacterial evaluation of new (2-oxo-2H-chromen-3-yl) substituted fluoroquinolones. J. Fluor. Chem. 2018, 208, 15–23. [Google Scholar] [CrossRef]
- Ross, A.G.; Benton, B.M.; Chin, D.; De Pascale, G.; Fuller, J.; Leeds, J.A.; Reck, F.; Richie, D.L.; Vo, J.; LaMarche, M.J. Synthesis of ciprofloxacin dimers for evaluation of bacterial permeability in atypical chemical space. Bioorganic Med. Chem. Lett. 2015, 25, 3468–3475. [Google Scholar] [CrossRef]
- Bartzatt, R.; Cirillo, S.L.; Cirillo, J.D. Antibacterial derivatives of ciprofloxacin to inhibit growth of necrotizing fasciitis associated penicillin resistant Escherichia coli. J. Pharm. 2013, 2013, 517638. [Google Scholar] [CrossRef]
- AA Abdel-Aal, M.; Abdel-Aziz, S.A.; Shaykoon, M.S.A.; Mohamed, M.F.; Abuo-Rahma, G.E.-D.A. Antibacterial and Urease Inhibitory activity of New Piperazinyl N-4 Functionalized Ciprofloxacin-oxadiazoles. J. Mod. Res. 2019, 1, 1–7. [Google Scholar] [CrossRef][Green Version]
- Fardeau, S.; Dassonville-Klimpt, A.; Audic, N.; Sasaki, A.; Pillon, M.; Baudrin, E.; Mullié, C.; Sonnet, P. Synthesis and antibacterial activity of catecholate–ciprofloxacin conjugates. Bioorganic Med. Chem. 2014, 22, 4049–4060. [Google Scholar] [CrossRef]
- Riahifard, N.; Tavakoli, K.; Yamaki, J.; Parang, K.; Tiwari, R. Synthesis and evaluation of antimicrobial activity of [r4w4k]-levofloxacin and [r4w4k]-levofloxacin-q conjugates. Molecules 2017, 22, 957. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Ma, T.; Xue, H.; Miao, Z.; Chen, L.; Shi, X. Ciprofloxacin-1, 2, 3-triazole-isatin hybrids tethered via amide: Design, synthesis, and in vitro anti-mycobacterial activity evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 2635–2637. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lv, Z.; Wen, J.; Zhao, S.; Xu, Z. Synthesis and in vitro evaluation of novel substituted isatin-propylene-1H-1, 2, 3-triazole-4-methylene-moxifloxacin hybrids for their anti-mycobacterial activities. Eur. J. Med. Chem. 2018, 143, 899–904. [Google Scholar] [CrossRef]
- Scroggs, S.L.; Offerdahl, D.K.; Flather, D.P.; Morris, C.N.; Kendall, B.L.; Broeckel, R.M.; Beare, P.A.; Bloom, M.E. Fluoroquinolone antibiotics exhibit low antiviral activity against SARS-CoV-2 and MERS-CoV. Viruses 2021, 13, 8. [Google Scholar] [CrossRef]
- Sarma, P. Norfloxacin: A new drug in the treatment of falciparum malaria. Ann. Intern. Med. 1989, 111, 336–337. [Google Scholar] [CrossRef]
- Hiltensperger, G.; Hecht, N.; Kaiser, M.; Rybak, J.-C.; Hoerst, A.; Dannenbauer, N.; Müller-Buschbaum, K.; Bruhn, H.; Esch, H.; Lehmann, L. Quinolone amides as antitrypanosomal lead compounds with in vivo activity. Antimicrob. Agents Chemother. 2016, 60, 4442–4452. [Google Scholar] [CrossRef]
- Allaka, T.R.; Polkam, N.; Rayam, P.; Sridhara, J.; Garikapati, N.S.; Kotapalli, S.S.; Ummanni, R.; Anireddy, J.S. Design, synthesis and biological activity evaluation of novel pefloxacin derivatives as potential antibacterial agents. Med. Chem. Res. 2016, 25, 977–993. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, X.; Sun, S.; Liu, Y. Synthesis and biological evaluation of novel quinolone derivatives dual targeting histone deacetylase and tubulin polymerization as antiproliferative agents. RSC Adv. 2018, 8, 16494–16502. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).