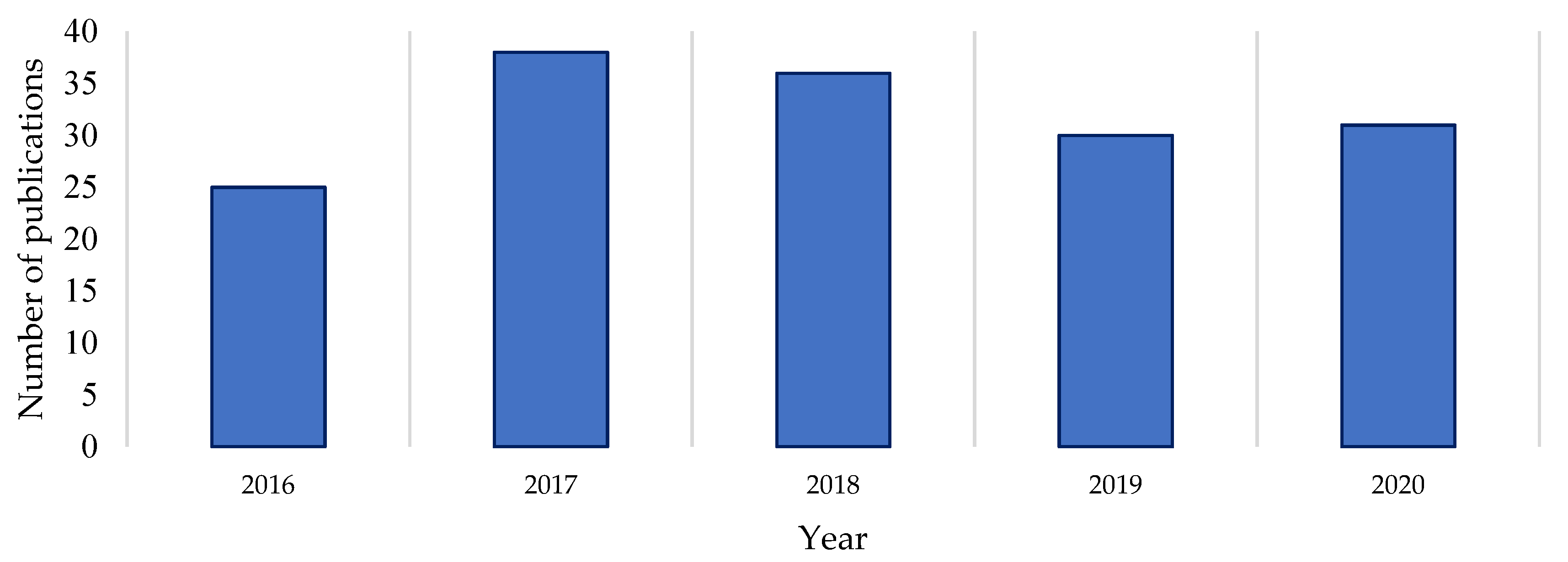1. Introduction
Analysis of
Streptomyces derived natural product discoveries from 1947 to 1997 indicates that, in terms of the number of publications, the discipline peaked in the late 1960s before tapering off through the 1970s and 1980s and significantly decreasing in the 1990s [
1]. Factors contributing to this decline include the development of combinatorial chemistry and associated high-throughput screening (HTS) techniques, a reduction in funding for traditional drug discovery pipelines, and the greater profitability of quality-of-life drugs. Had new bioinformatics technologies and methods such as genome mining not emerged to reinvigorate the field, the declining trend could have seen a cessation of work to exploit
Streptomyces as a source of new bioactives by 2020 [
1]. This is in spite of studies that conservatively estimate that the genus can synthesise some 150,000 more antimicrobial compounds than those currently known, suggesting that
Streptomyces is far from an exhausted resource [
1,
2].
A Clarivate Web of Science search was undertaken to gauge the recent level of documented drug discovery research activity in the genus [
3], using the terms
Streptomyces,
isolation,
new compound and
novel compound in the All Fields parameter of the Web of Science search engine. A review of the number of publications dedicated to novel natural product discovery from the
Streptomyces genus over the last five years reveals a reasonably consistent level of publication on the subject (
Figure 1). Comparing the raw numbers of this survey with the Watve study (2001) shows that the current levels of research output (in terms of publication numbers) are comparable to those at the start and end of the Golden Age of natural products [
1]. This suggests that
Streptomyces natural products research publications did not dissipate by 2020 as earlier trajectories may have indicated, nor has there been a major upswing in publications per year in the last five years [
1,
3]. A proportion of the publications returned in the Web of Science search (on average 7% per year) were excluded from this count because the research they describe did not yield verified novel compounds as isolated secondary metabolites of a
Streptomyces species. Similarly, reports of partial characterisation were also excluded as the natural products described therein could not be verified as novel. This indicates that a literature count with strict eligibility criteria likely underestimates the true interest in
Streptomyces drug discovery by focusing on verified novel compound elucidation, rather than simply research activity.
An insight analysis was conducted on scientific reports pertaining to the discovery of new Streptomyces secondary metabolites published in 2020. The search returned 43 references relevant to the search terms. Filtering resulted in 32 research papers that met the requirements of the review.
Each of these reports described a unique
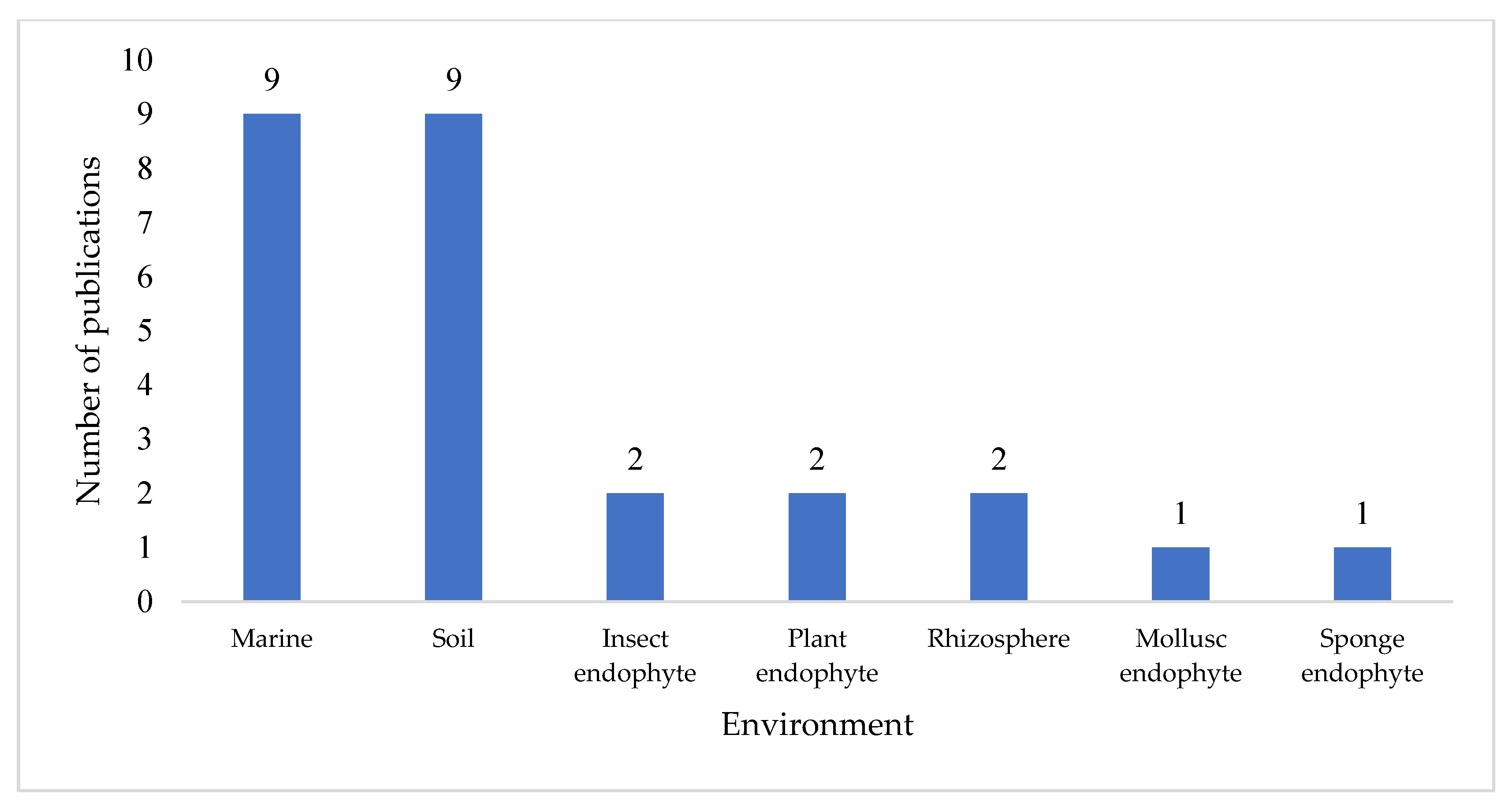
Streptomyces species or strain, and a total of 74 novel compounds were reported across the 32 papers surveyed. Further analysis revealed that marine and soil environments were the equal most common isolation locations, with 56% of these publications citing a
Streptomyces strain isolated from either a marine environment or terrestrial soil (
Figure 2). Notably, a significant proportion (19%, n = 6) of publications did not specify the environmental source of the
Streptomyces strain; where this occurred, the publication often focused on high performance liquid chromatography-UV-diode array detection (HPLC-DAD) [
4], LC-MS [
5], or genome mining [
6,
7] as a drug discovery guidance strategy.
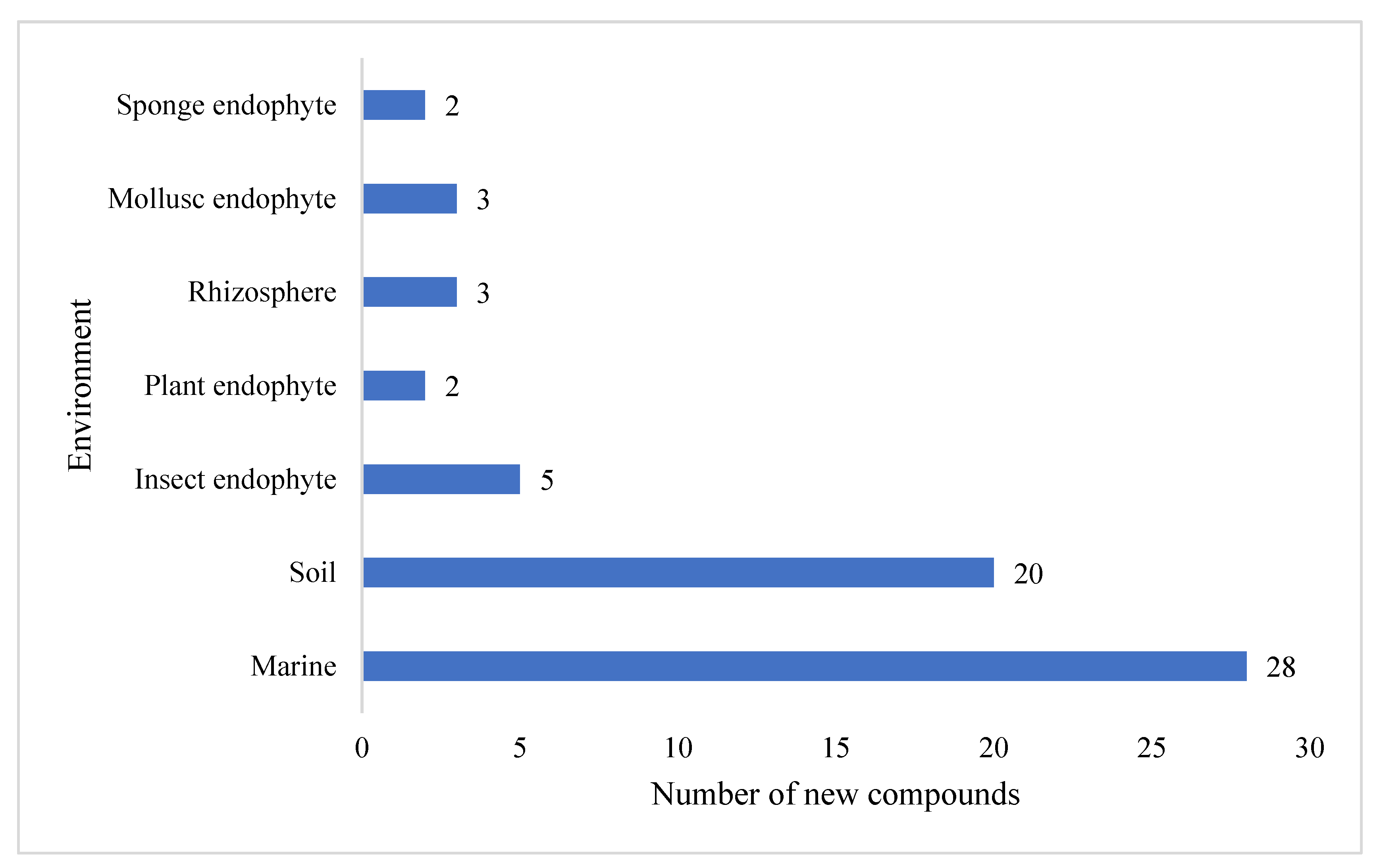
The preliminary analysis of novel secondary metabolites produced by these
Streptomyces strains also revealed that the largest proportion (39%) of compounds was isolated from marine-derived
Streptomyces, followed by terrestrial soil (27%) (
Figure 3). A further eleven secondary metabolites were isolated from unspecific environments. The compounds reported possess a wide range of chemical scaffold variability and include cyclic and linear peptides, terpenoids, macrolactams, macrolides, glycosides, polyaromatics, and linear polyketides.
Though Streptomyces species no longer generate the same level of research output as at their peak in the 1960s, the genus has continued to be the subject of interest and research activity. New genome mining strategies in addition to classic bioprospecting and bioassay-guided isolation have contributed to the plethora of unique chemical scaffolds that continue to characterise both the Streptomyces genus and natural products more generally, as an important source of drug leads.
2. Cyclic Peptides
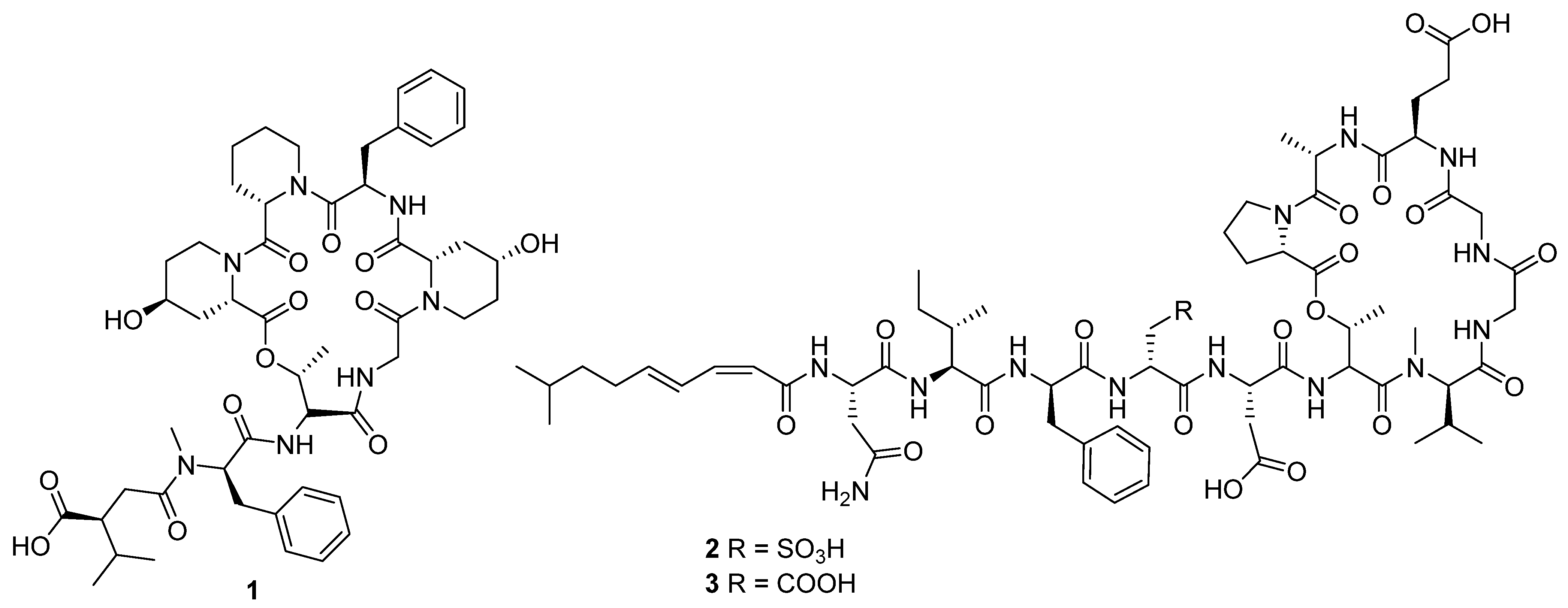
In 2020, ten novel cyclic peptides were reported across seven publications [
4,
6,
8,
9,
10,
11,
12]. The size of the peptide core ranged from two to seven amino acids, though three of these compounds, ulleungamide C (
1) [
11] and viennamycins A and B (
2 and
3) (
Figure 4) [
8], are depsipeptides, and two more, NOS-V1 (
4) and NOS-V2 (
5) (
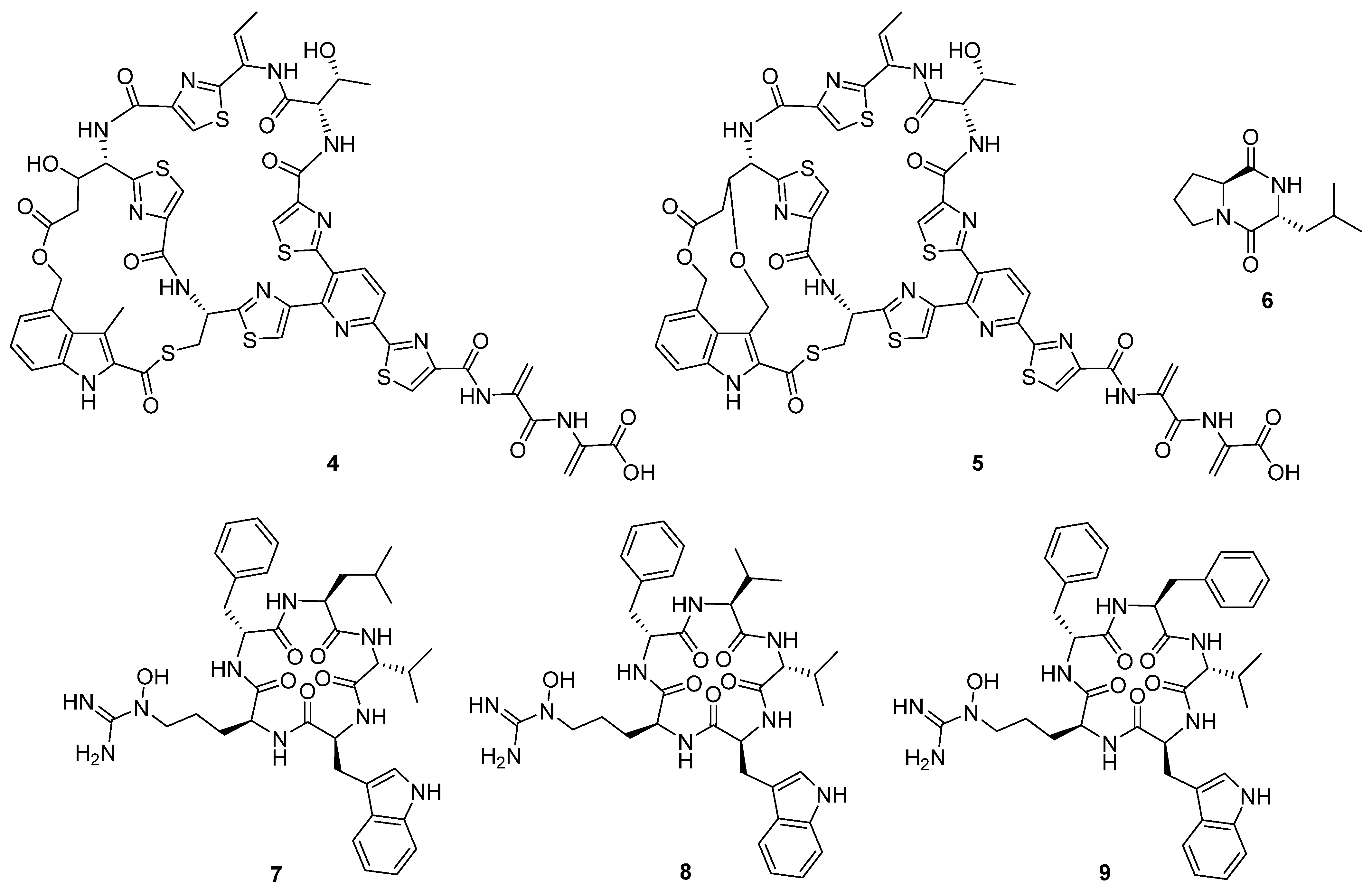
Figure 5), possess the typical di-macrocycle composition of a thiopeptide nosiheptide [
6].
These compounds originate from both non-ribosomal peptide synthetases (NRPSs) and ribosomal synthesis with post-translational modification. The most common bioactivity observed for this group of compounds was antibacterial activity, followed by cytotoxicity and antifungal proliferation. Pentaminomycin C (
7) showed activity against Gram-positive bacteria down to 16 μg mL
−1 [
4], but no activity against Gram-negative bacteria. In comparison, NOS-V1 (
4) and NOS-V2 (
5) showed activity against Gram-positive and Gram-negative cultures with a more potent effect against Gram-positive than Gram-negative bacteria [
6].
The cyclic dipeptide
l-leucyl-
l-proline (
6) showed activity against a range of bacterial and fungal targets in inhibition zone studies [
10]. The antibacterial activity of the viennamycins
2 and
3 was identified in tests against
S. aureus 209P and
B. cereus IP5812, in which these compounds were found to have comparable activity to daptomycin and vancomycin, though no activity was identified against Gram-negative bacteria [
8]. Ulleungamide C
1 displayed cytotoxic activity in vitro. In contrast, in vitro studies observed the protective effects of pentaminomycins C (
7) and D (
8) (
Figure 5) against menadione-induced cytotoxicity [
9].
3. Peptide Derivatives and Linear Peptides
Three linear peptidic compounds were reported in three publications during 2020. Spongiicolazolicins A (
10) and B (
11) (
Figure 6) are ribosomally synthesised and post-translationally modified azole-containing peptides isolated from the marine
Streptomyces sp. CWH03 (NBRC 114659) [
13]. Comprising twenty-nine (spongiicolazolicin A) and twenty-eight (spongiicolazolicin B) amino acids, the structures were deduced from 2D NMR, MS/MS experiments, and the sequence of the biosynthetic gene cluster (BGC). Despite studies of similar compounds, such as microcin B17 [
14], finding them to have antibacterial bioactivity, biological assay data did not indicate similar activity for the spongiicolazolicins against Gram-positive or Gram-negative species.
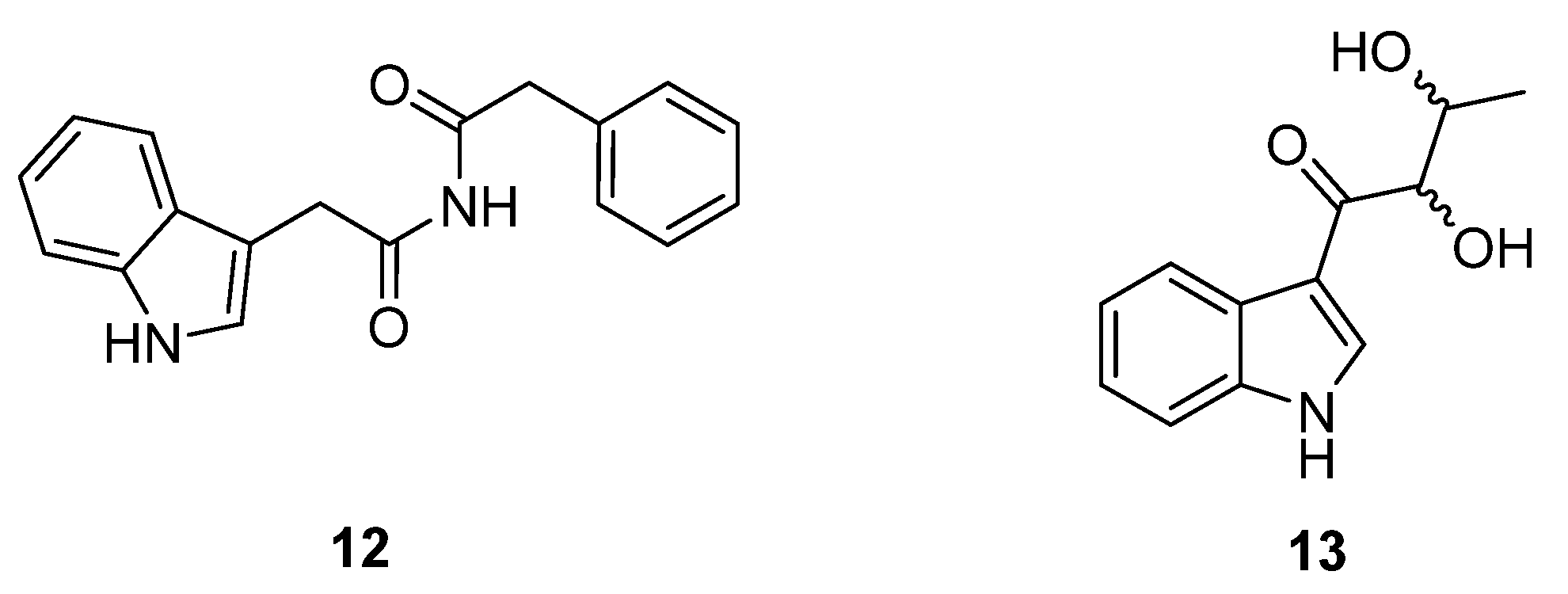
Legonimide (
12,
Figure 7) is an indole alkaloid composed of modified phenylalanine and tryptophan amino acids, and was isolated from soil-derived
Streptomyces sp. CT37 [
15]. The authors proposed that this compound is biosynthesised via a condensation reaction between phenyl-acetyl-CoA and indole-3-acetamide. The structure of legonimide was determined from a combination of 1D and 2D NMR data, and biological profiling showed that the compound has moderate antifungal activity against
Candida albicans (MIC 21.5 μg mL
−1). The final peptide derivative reported in the review period, 2,3-dihydroxy-1-(indolin-3-yl)butan-1-one (
13,
Figure 7), was isolated from
S. triticiradicis sp. strain NEAU-H2
T [
16], collected from the rhizosphere of wheat (
Triticum aestivum L.). This strain displayed antifungal effects against the mycelial growth of phytopathogenic fungi, though this activity could not be linked to the novel compound in subsequent tests [
16].
4. Linear Polyketides
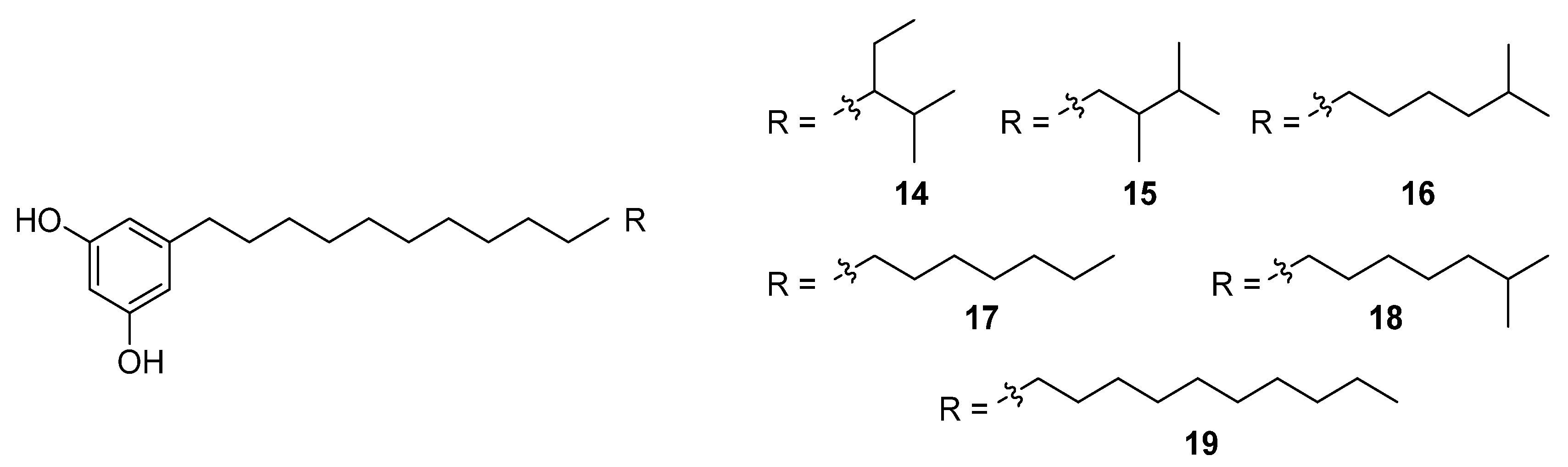
An HTS experiment looking for inhibitors of coenzyme A biosynthesis led to the isolation and spectroscopic characterisation of six new phenolic lipids, adipostatins E–J (
14–
19), from the marine-derived
S. blancoensis (
Figure 8) [
17]. These novel compounds showed activity against both Gram-positive and Gram-negative bacteria:
Enterococcus coli,
E. faecalis,
Bacillus subtilis,
B. anthracis,
Listeria monocytogenes, and
Streptococcus pneumoniae. In contrast, the compounds had low to no effect on
Staphylococcus aureus,
Salmonella enterica,
Shigella flexneri, or
Klebsiella pneumoniae. The differential in vitro activity of adipostatins E–J suggests that the biological activity is mediated by the chain length and terminal branching.
Similarly, bioactivity screening for tyrosinase inhibitors led to the serendipitous identification of trichostatic acid B (
20) from
Streptomyces sp. CA-129531 as a co-metabolite of the target molecules (
Figure 9) [
18]. Notably, trichostatic acid B did not display any cytotoxic activity up to 50 mg mL
−1. Nor was any antityrosinase activity detected for trichostatic acid B in a mushroom tyrosinase bioassay, despite trichostatin A (
21) showing strong inhibitory activity [
18], suggesting that the hydroxylamine and/or tertiary methyl group is important to its biological activity. Two additional bioassay-driven natural product discoveries were chresdihydrochalcone (
22) and chresphenylacetone (
23,
Figure 9), isolated from
S. chrestomyceticus BCC 24770 [
19]. Their molecular structures were spectroscopically characterised, and chresdihydrochalcone was assessed for haemolytic, cytotoxic, and antibacterial activity. Notably, the compound showed moderate activity against methicillin-resistant
S. aureus (MRSA, MIC 25 μg mL
−1) and a mutant
E. coli strain, and stronger activity against
S. aureus (MIC 18 μg mL
−1) and
B. subtilis (MIC 10 μg mL
−1).
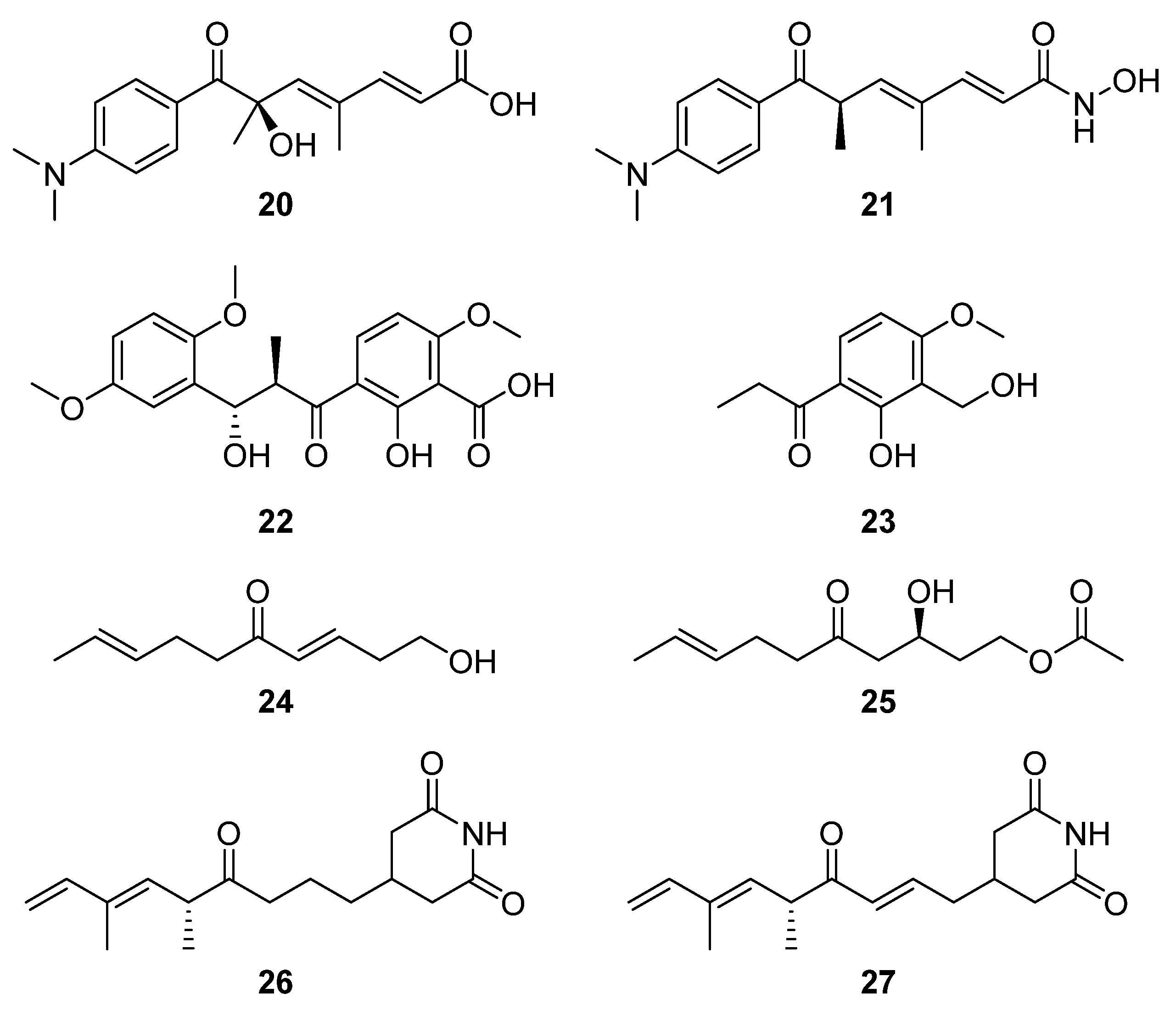
From a group of six compounds isolated from
Streptomyces sp. MJM3055 and spectroscopically characterised, two compounds, (3
E,8
E)-1-hydroxydeca-3,8-dien-5-one (
24) and (
S,E)-3-hydroxy-5-oxodec-8-en-1-yl acetate (
25), were identified as novel natural products via a Jurkat cell antiproliferation screening study (
Figure 9) [
20]. Using LCMS-guided screening of
Streptomyces sp. W3002, Lee et al. purified and characterised the new deoxy-streptimidone compounds
26 and
27 (
Figure 9) along with two glutarimide ring-opened derivatives of
27 [
5]. The new compounds, 3-(5,7-dimethyl-4-oxo-6
E,8-nonadienyl)-glutarimide (
26) and 3-(5,7-dimethyl-4-oxo 2
E,6
E,8-nonatrienyl)-glutarimide (
27), possess the characteristic glutarimide ring and alkyl chain and showed no cytotoxic activities against human cervical carcinoma, human hepatoma, or myeloid leukemia up to 100 μg mL
−1 [
21]. Analysis of the putative BGC of both glutarimide derivatives revealed that apart from module five, which contains the C-terminal thioesterase domain, the other four of the first five modules showed a strong similarity to those of the BGC that encodes 9-methylstreptimidone in
S. himastatinicus [
22]. It was proposed that the instability of the dehydratase accessory reactions catalysed by module three was responsible for the absence of the hydroxyl group from the alkyl chain (when these new structures are compared to 9-methylstreptimidone), while an oxidoreductase transforms
26 to
27 [
5].
5. Terpenoids
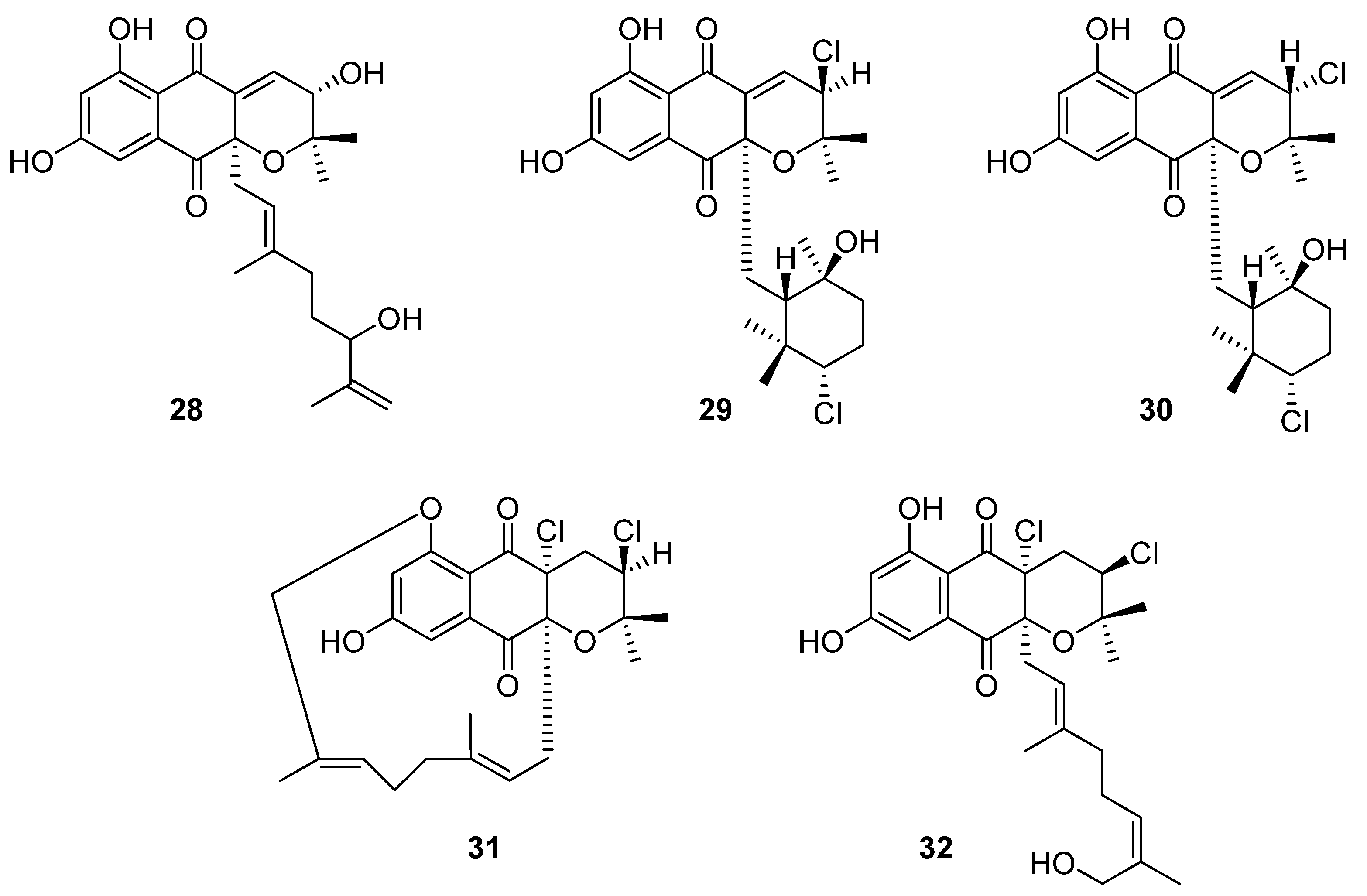
Ten novel terpenoid natural products were isolated from four
Streptomyces species in 2020. Notably, half of these compounds are napyradiomycin derivatives. Napyradiomycins are a large group of meroterpenoids that incorporate a halogenated semi-naphthoquinone moiety. This group of over fifty compounds is divided into three sub-groups based on side-chain characteristics: type A napyradiomycins possess a linear terpenoid chain, type B have a cyclohexane ring, and type C possess a 14-membered ring [
23]. In the course of reassessing secondary metabolite biosynthesis by marine-derived
Streptomyces, Carretero-Molina et al. identified four compounds from
Streptomyces sp. CA-271078: one each of types A (
28) and C (
31), and two of which were type B napyradiomycins (
29 and
30) (
Figure 10) [
23]. These compounds were spectroscopically characterised and their stereochemistry was inferred from biosynthetic relationships with previously reported napyradiomycins. These compounds all displayed weak antibacterial activity against MRSA and cytotoxicity to human liver carcinoma cell lines.
The fifth compound was also a type A napyradiomycin, and one of ten isolated from marine
Streptomyces sp. YP127: 16
Z-19-hydroxynapyradiomycin A1 (
32) [
24]. This structure was flagged as a potential drug lead through an Nrf-2-activating efficacy bioassay, which indicated that this compound would modulate the activity of Nrf-2, an essential component in the expression of antioxidant defence genes.
Three structurally related naphthoquinones were isolated from the marine-derived
Streptomyces sp. B9173: flaviogeranins B1 (
33), B (
34), and D (
35), the latter two of which are meroterpenoids (
Figure 11). These compounds differ in their prenylation pattern and presence of amino and methyl groups on the hydroxynaphthoquinone core. Flaviogeranins B1 and B showed weak to moderate activity against
S. aureus and
M. smegmatis as well as human alveolar basal epithelial adenocarcinoma and human cervical cancer cells. In contrast, flaviogeranin D displayed stronger activity against
S. aureus (MIC 9.2 μg mL
−1) and
M. smegmatis (MIC 5.2 μg mL
−1), the alveolar basal epithelial adenocarcinoma (IC
50 0.6 μg mL
−1) and human cervical cancer cell lines in vitro (IC
50 0.4 μg mL
−1).
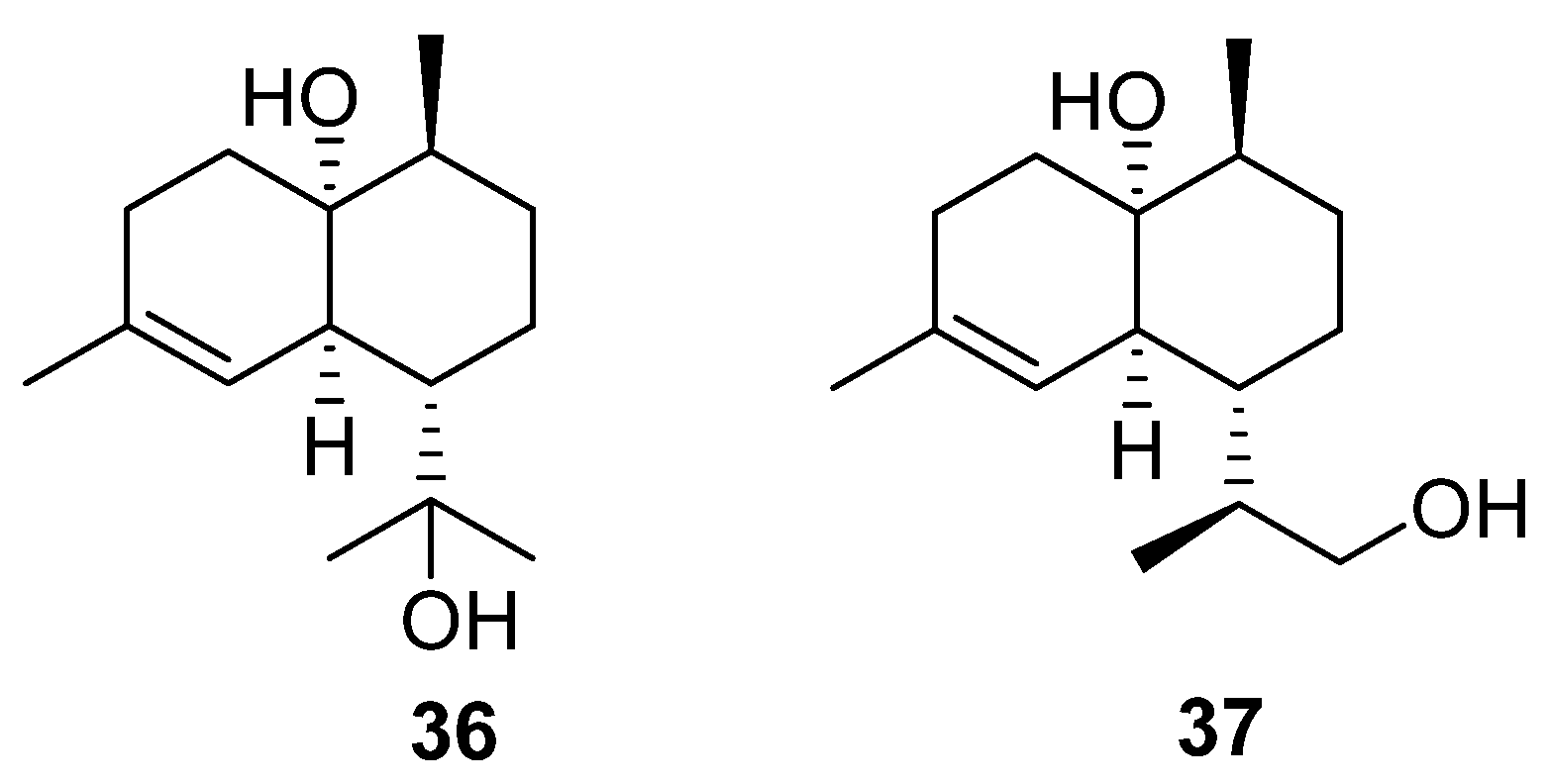
The final terpenoids isolated from the
Streptomyces genera are two cardinane sesquiterpenes (
36 and
37) isolated from marine
Streptomyces sp. ST027706 (
Figure 12), a plant endophyte taken from the mangrove
Bruguiera gymnorrihiza [
26]. Cardinanes have primarily been isolated from plant and fungal sources, with only two previous examples of the class being isolated from
Streptomyces [
27,
28]. Thus, this finding raises the possibility that bacterial fermentation could be used to obtain cardinane-derived pharmaceuticals.
6. Polyaromatics
In total, eleven polyaromatic compounds were isolated from six
Streptomyces species in 2020. Through herbicidal bioassay screenings, Kim et al. identified strong activity associated with
Streptomyces strain KRA17-580 against the weed
Digitaria ciliaris [
29]. Two compounds were spectroscopically characterised, a cinnoline-4-carboxamide, 580-H1 (
38), and a cinnoline-4-carboxylic acid, 580-H2 (
39,
Figure 13). In further phytotoxicity bioassays, the carboxamide proved to be less potent than the carboxylic acid, and electrolyte leakage assays were used to verify that the observed herbicidal activity was caused by destruction of the cell membrane [
29].
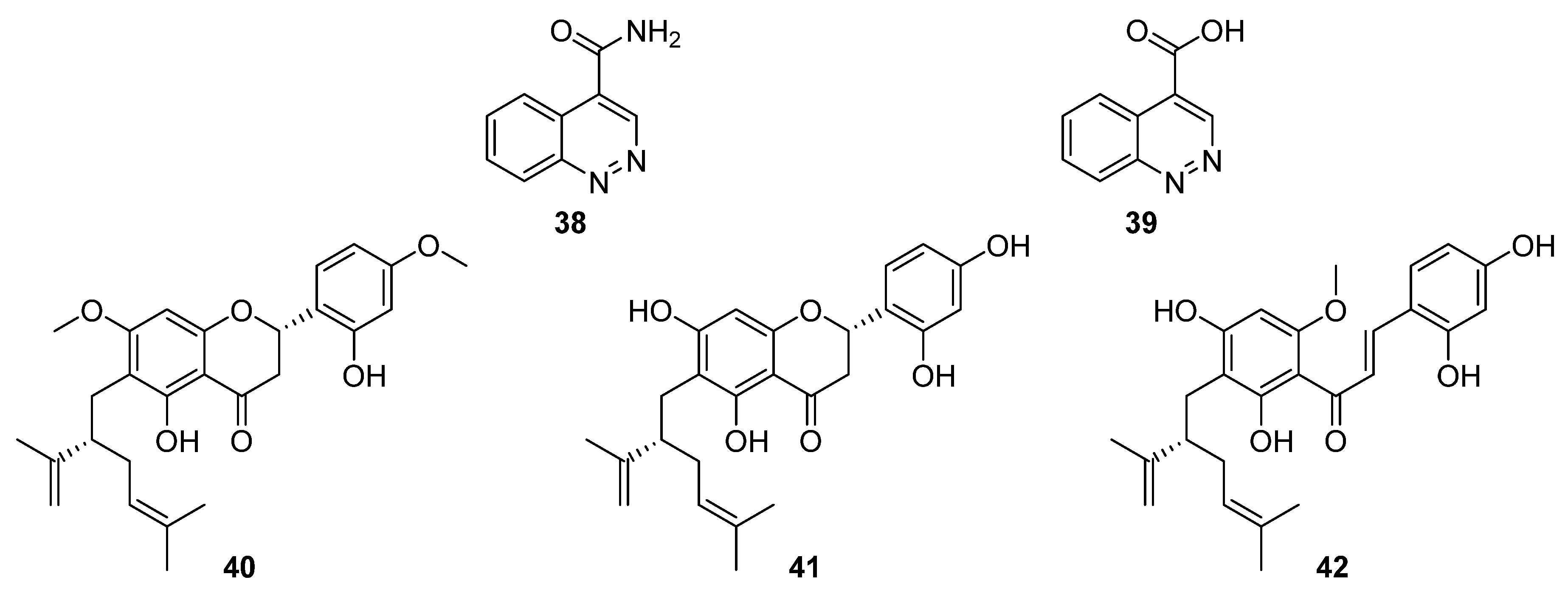
Flavonoids possess a generic structure in which two aromatic rings (A and B) are connected by a heterocyclic pyran. Investigations into bacterial endophytes of the sea sponge
Halichondria panicea led to the discovery of
Streptomyces sp. G248 and three lavandulylated flavonoid secondary metabolites: (2
S,2″
S)-6-lavandulyl-7,4′-dimethoxy-5,2′-dihydroxylflavanone (
40), (2
S,2″
S)-6-lavandulyl-5,7,2′,4′-tetrahydroxylflavanone (
41), and (2″
S)-5′-lavandulyl-2′-methoxy-2,4,4′,6′-tetrahydroxylchalcone (
42,
Figure 13) [
30]. Notably, (2
S,2″
S)-6-lavandulyl-5,7,2′,4′-tetrahydroxylflavanone showed strong antibacterial activity (IC
90 1 μg mL
−1) against
E. faecalis,
S. aureus,
B. cereus,
P. aeruginosa, and
C. albicans, suggesting that the diol on ring B and the presence of an intact pyran are essential to antibacterial activity [
30].
While investigating expression of the nybomycin BGC from
S. albus subsp.
chlorinus in heterologous host
S. albus Del14, Rodriquez Estevez et al. discovered the production of a new metabolite, benzanthric acid (
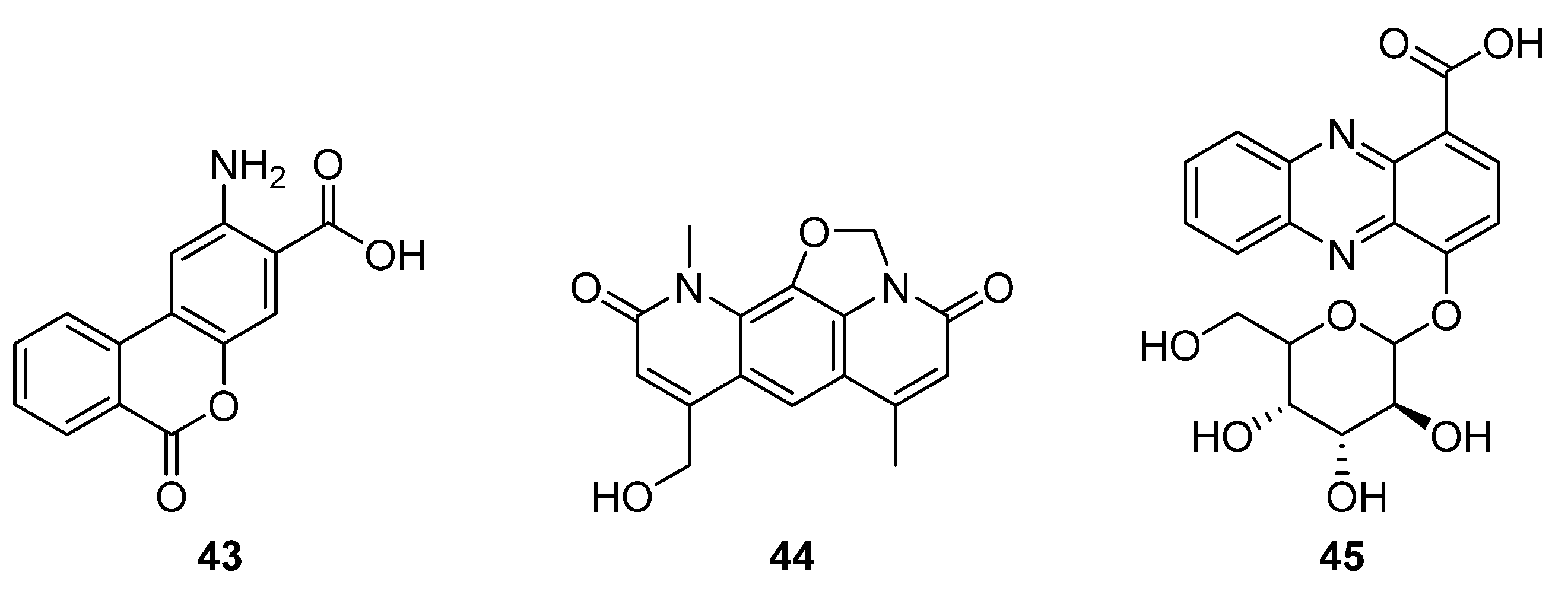
43,
Figure 14) [
31]. This new structure comprises a benzoic acid fused to an anthranilate moiety, and a
13C feeding study indicated that anthranilic acid is incorporated into
43, but not into the nybomycin scaffold (
44). Notably, benzanthric acid was not identified in either the origin strain (
chlorinus) or the unmodified host strain (Del14). Given the absence of a benzoic acid supply from the nybomycin BGC, Rodriquez Estevez et al. proposed that the benzoic acid is supplied by the host strain, possibly through a phenylalanine degradation pathway, and so too the enzyme that catalyses reaction between the anthranilate moiety and benzoic acid. This report indicated that despite the simultaneous production of nybomycin and benzanthric acid in this strain, there are substantial differences in the biosynthetic routes to the two compounds. The new benzanthric acid compound was tested for phytotoxic activity against
Agrostis stolonfera, though no activity was identified. This study demonstrates an unexpected interplay between a target BGC and host strain, once integrated.
The second fused tricyclic compound isolated in 2020 was 4-((3
S,4
R,5
S)-3,4,5-trihydroxy-6-(hydroxymethyl) tetrahydro-2Hpyran-2-yloxy)phenazine-1-carboxylic acid (
45), a phenazine that features a benzoic acid moiety and glucose pendant (
Figure 14) [
32]. The producing strain
Streptomyces sp. strain UICC B-92 is an endophyte of the large crane fly
Nephrotoma altissima. The isolated compound was found to be active against Gram-positive bacteria
S. aureus and
B. cereus in disk diffusion assays.
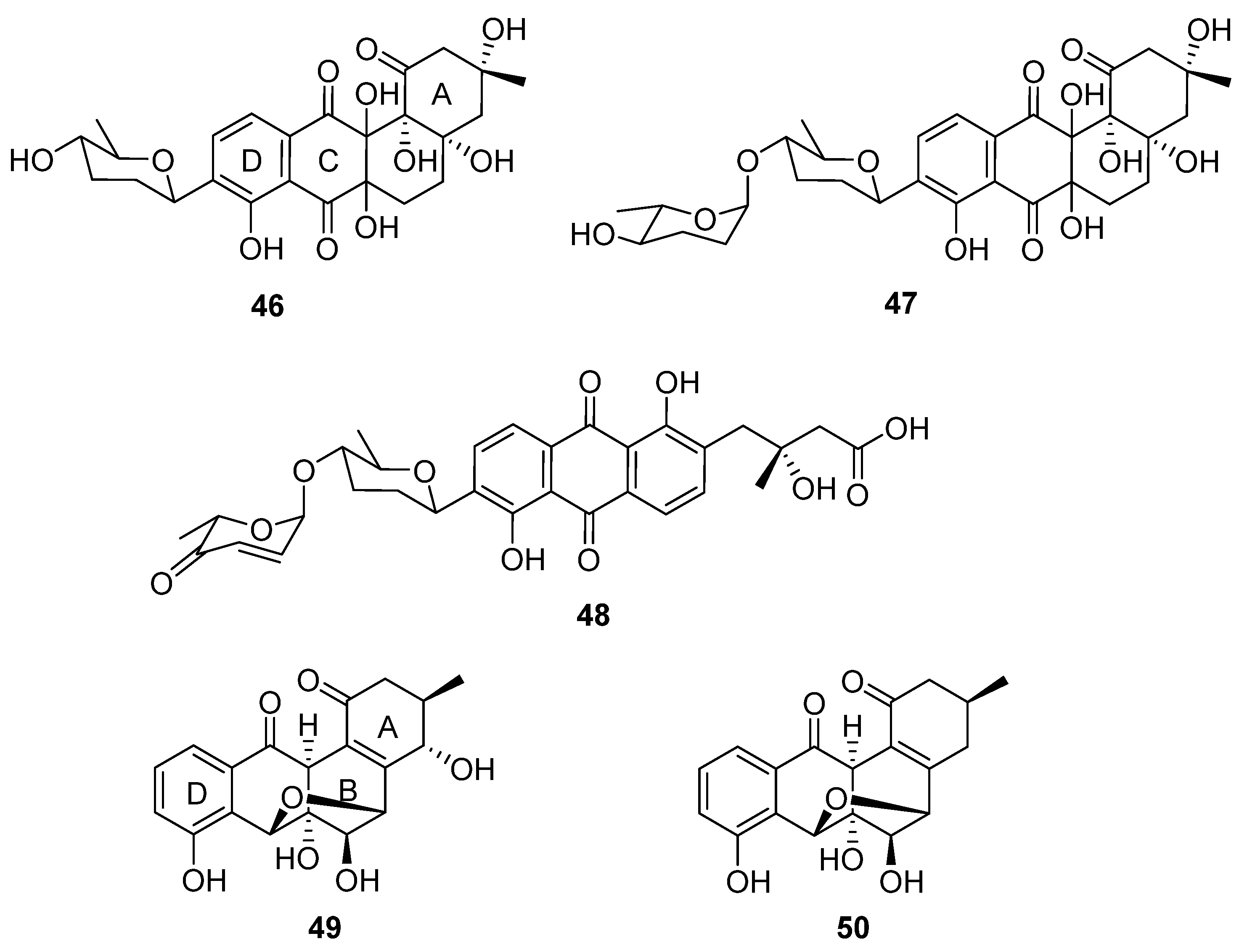
In a successful attempt to highlight the value of chemical exploration of less investigated ecological niches, Voitsekhovskaia et al. isolated anthraquinone derivatives baikalomycins A–C (
46–
48,
Figure 15) from the mollusc (
Benedictia baicalensis) endophyte
Streptomyces sp. strain IB201691-2A [
33]. Baikalomycin A was identified as an aquayamycin derivative modified with β-
d-amicetose and two additional hydroxyl groups on C-6a and C-12a of the aglycone. Baikalomycin B
47 is a disaccharide derivative of the same aglycone that incorporates epimeric α-
l-amicetose as the
O-linked second sugar. In baikalomycin C, the A ring of the parent aquayamycin structure is opened to afford an anthraquinone (from rings B, C, and D) with a 3-hydroxy-3-methyl butanoic acid side chain at C4a of the parent aglycone, and a disaccharide comprising β-
d-amicetose and α-
l-aculose at C9. The stereochemistry within the A ring of baikalomycins A (
46) and B (
47) and (by extrapolation) the sidechain of
48 was assigned by comparison with previously isolated compounds with similar biosynthetic origin, while the stereochemistry in the B ring of
46 and
47 remains uncertain. The putative type II polyketide synthase (PKS) BGC possesses typical genes responsible for angucycline core assembly, genes necessary for biosynthesis of the deoxy sugar, and three genes encoding the glycosyltransferase enzymes. Weak antibacterial and moderate cytotoxic activity were identified for baikalomycin C, while baikalomycins A and B only showed weak or no cytotoxic activity against various cell lines.
Two further tetracyclic metabolites were isolated from an endophytic
Streptomyces species: gardenomycins A and B (
49 and
50) [
34].
S. bulli GJA1 was extracted from the bark of
Gardenia jasminoides, and the molecular structures of the secondary metabolites deduced from spectroscopic analysis. Notably, gardenomycins A and B possess an unprecedented ether bridge between their B and C rings. Kim et al. proposed that an ether bridge was formed from ring opening of an α-oriented epoxide on ring C by a hydroxyl group on ring B [
34]. These compounds did not display antiproliferative or antivirulence effects in bioassays.
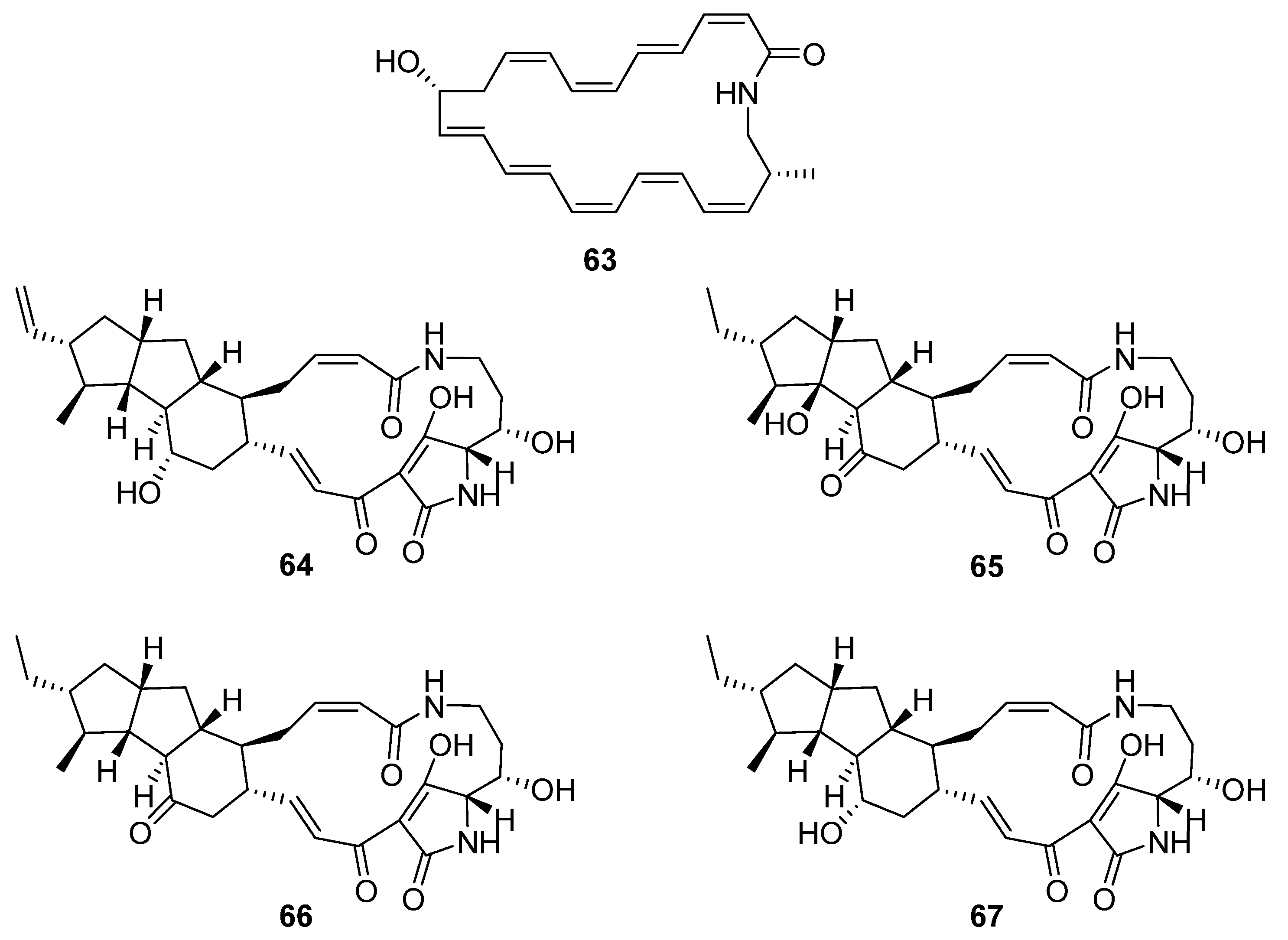
7. Macrocycles
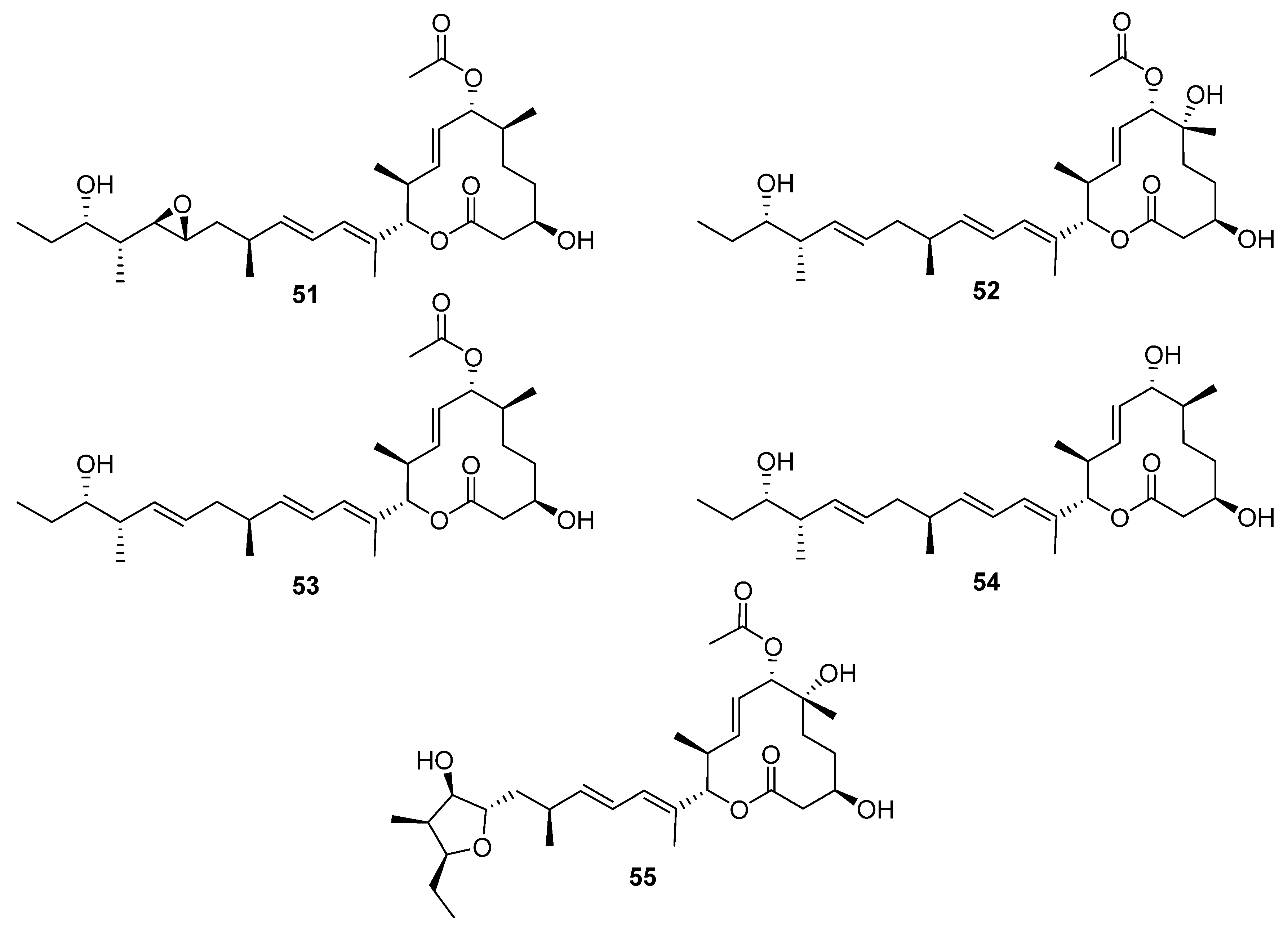
Macrocycle discoveries fall into two subgroups: macrolides (macrocyclic esters) and macrolactams (macrocyclic amides). Five macrolides (
51–
55,
Figure 16) were identified while reactivating the quiescent pladienolide B biosynthetic pathway in a laboratory strain of
S. platensis AS6200 [
35]. Unsurprisingly, the laboratory strain showed high similarity (>95% nucleotide similarity with the published
pld BGC [
36]). One notable difference was that the PKS was encoded by five genes in AS6200, while the previously reported PKS was encoded by four genes [
35,
36]. The molecular structure of these compounds was deduced from spectroscopic data, and the compounds were tested in antibacterial, antifungal, antiprotozoal, and antiproliferation bioassays. The results indicated that all the congeners have less cytotoxic activity than the parent compound pladienolide B.
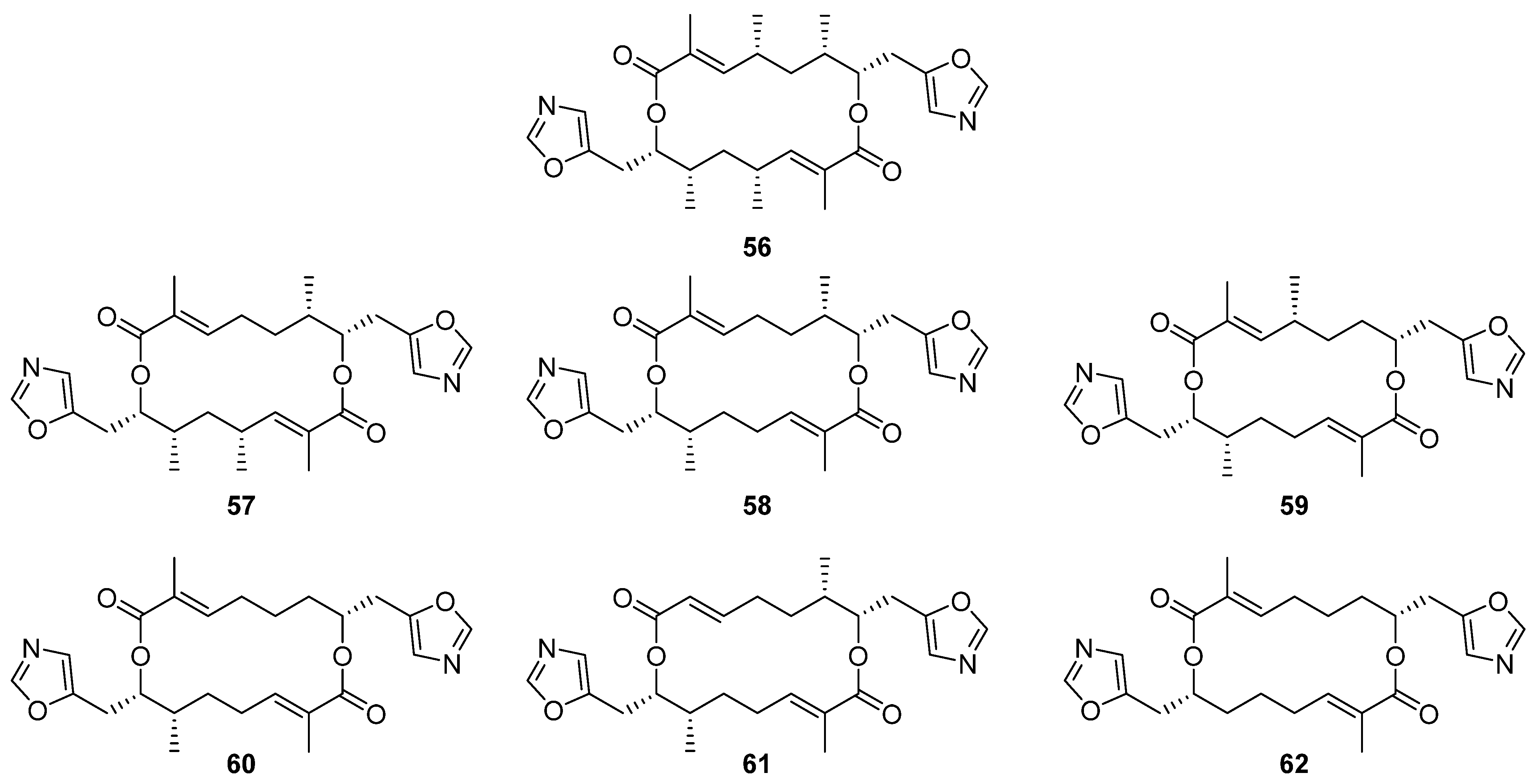
Six new analogues of oxazole-bearing macrodiolide conglobatin (
56) were isolated from the previously unreported Australian
Streptomyces strain MST-91080 (
Figure 17) [
37]. Conglobatins B–E (
57–
62) vary in the distribution of methyl groups around the macrocyclic core, proposed to arise via variable addition of methylmalonyl-CoA extender units during their biosynthesis. Three of the new conglobatins (B1
56, C1
57, and C2
58) displayed enhanced cytotoxicity against NS-1 myeloma cells, IC
50 = 0.084, 1.05, and 0.45 µg mL
−1 respectively, an increase in potency relative to the parent compound (IC
50 = 1.39 µg mL
−1).
Five macrolactams were described in two separate reports (
Figure 18). Hashimoto et al. identified a gene cluster containing a type-I PKS with glutamate mutase genes in the genome of
S. rochei IFO12908 [
7]. The BGC was expressed in a host strain derived from
S. avermitilis and produced a polyene macrolactam
63, which was subsequently named JBIR-156; its stereochemistry was predicted from the biosynthetic pathway. Given the previous reports of cytotoxicity for related macrolactam structures, the compound was tested on a range of mammalian cells and showed strong activity against human ovarian adenocarcinoma (IC
50 9.5 μM) and T lymphoma Jurkat (IC
50 3.5 μM) cell lines. The final four compounds are polycyclic tetramate macrolactams and were uncovered using a mixture of gene- and growth-condition-manipulation techniques on a cryptic BGC in the genome of
S. somaliensis SCSIO ZH66 [
39]. Somamycin A (
64) was identified spectroscopically as a polycyclic tetramate macrolactam, with the remaining compounds identified as the 10-
epi-hydroxymaltophilin (B,
65), 10-
epi-maltophilin (C,
66), and dihydro 10-
epi-maltophilin (D,
67) analogues. These compounds were tested against two plant fungal pathogens and mammalian cell lines, showing antifungal activity and cytotoxicity, with somamycin displaying the strongest antifungal activity against
Fusarium oxysporum and
Alternaria brassicae (1.56 and 3.12 μg mL
−1, respectively) and human cancer cell lines HCT116 and K562 (IC
50 0.6 and 1.5 μM, respectively) [
39].
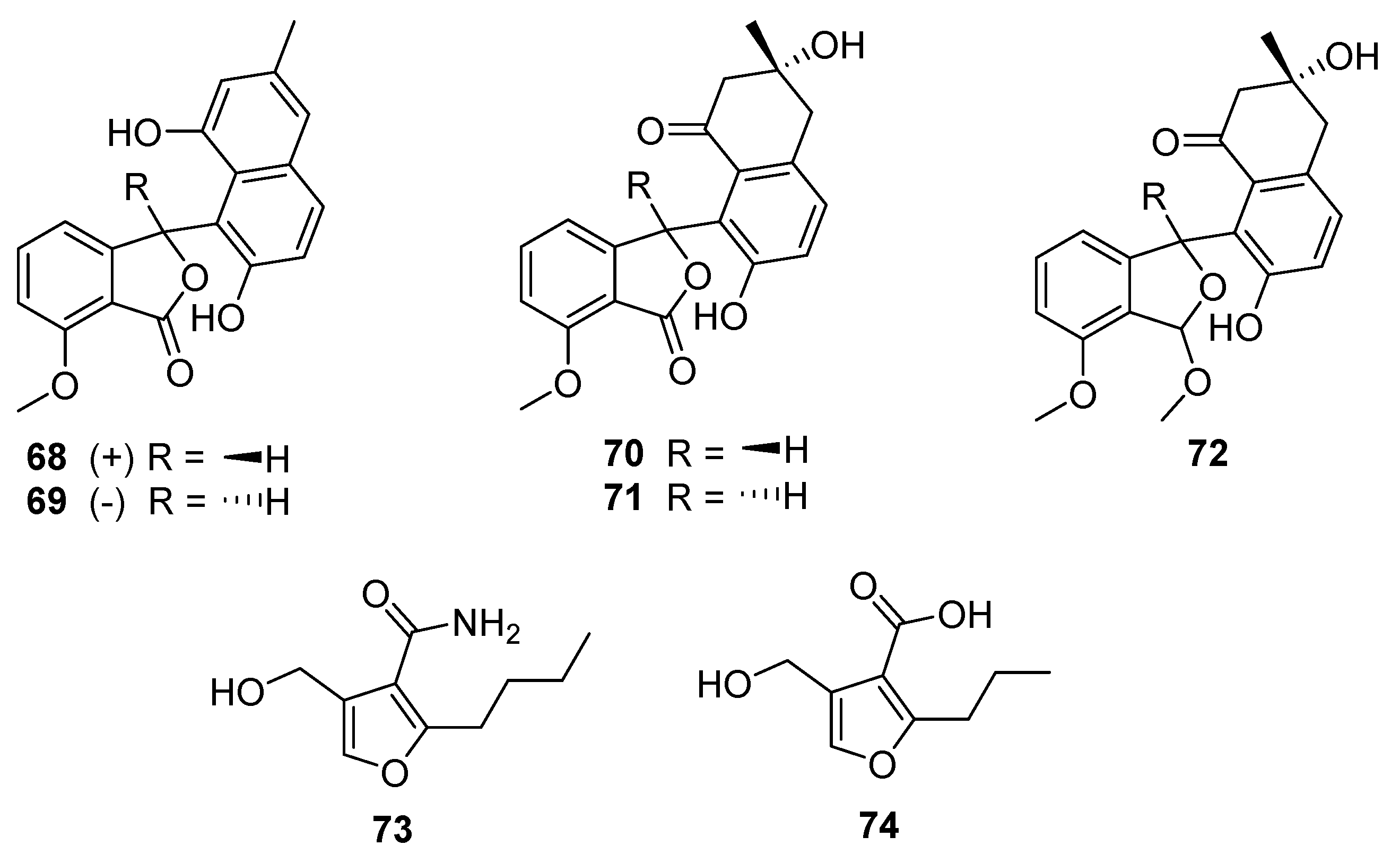
8. Furans
Five
Streptomyces-derived secondary metabolites containing modified benzofurans were isolated in 2020 from
S. pratensis strain KCB-132: (±)-pratenone A (
68 and
69), and furamycins I–III (
70–
72,
Figure 19) [
40]. The core of these compounds is a benzofuranone ring system linked to naphthalene. Pratenone A is distinguished by the presence of a 3-methylnaphthalen-1,7-diol moiety, furamycins I and II possess 3,7-dihydroxy-3-methyl-3,4-dihydronaphthalen-1(2H)-one ring systems, and furamycin III is characterised by a methoxy group in place of the carbonyl on the benzofuran(one) portion. These compounds were tested for antibacterial and cytotoxic activity. Furamycin III showed moderate activity against pancreatic cancer cells (IC
50 26.0 μg mL
−1) and hepatocellular carcinoma (IC
50 42.7 μg mL
−1) in vitro. Only pratenone A displayed antibacterial activity against
S. aureus (MIC 8 μg mL
−1) [
40].
The final furanic compound reported was 2-alkyl-4-hydroxymethylfuran carboxamide (AHFA,
73), isolated from a soil-derived
Streptomyces sp. RK44 [
41]. AHFA is a homolog of methylenomycin furan (MMF,
74) and is distinguished by the presence of an amide moiety at C-3 rather than a carboxylic acid (
Figure 19). MMFs share a common furan ring structure and are autoregulators that act in the signalling pathway that stimulates production of methylenomycin biosynthesis [
42]. Weak antiproliferative activity was observed (EC
50 89.6 μM) when AHFA was tested on a melanoma cell line, though no antibacterial or antiplasmodial activities were detected when tested.
9. Conclusions
A review of the natural products isolated from Streptomyces in 2020 reveals a diversity of novel chemical structures and a thriving and multifaceted area of drug discovery research. The level of research is on par with the end of the Golden Age of antibiotics on a publications per year basis. During the year, over seventy novel compounds were isolated from Streptomyces species using a combination of HPLC-DAD, genomics, and bioassays to guide the isolation of new compounds.
The Streptomyces species investigated derive from a wide range of environments, with marine locales being the most investigated, followed by terrestrial soil. Unsurprisingly, this resulted in most new natural products being isolated from marine and soil environments as well. A diverse range of compounds spanning many different chemical classes have been identified, including cyclic and linear peptides, linear polyketides, terpenoids, polyaromatics, macrocycles, and furans. Interestingly, the biological activity identified for these compounds was generally limited, and not tested across a consistent set of pathogenic species or cell lines, leaving an incomplete activity profile for the compound set.
Overall, the discovery of these many and different compounds demonstrates the continued potential of Streptomyces as a source of new and interesting natural products and contributes important pieces to the largely unfinished puzzle of Earth’s myriad microbes and their multifaceted chemical output.