Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content (TPC)
2.2. Total Flavonoid Content (TFC)
2.3. Antioxidant Capacity
2.3.1. DPPH Radical Scavenging Assay
2.3.2. Ferric Reducing Antioxidant Power Assay (FRAP)
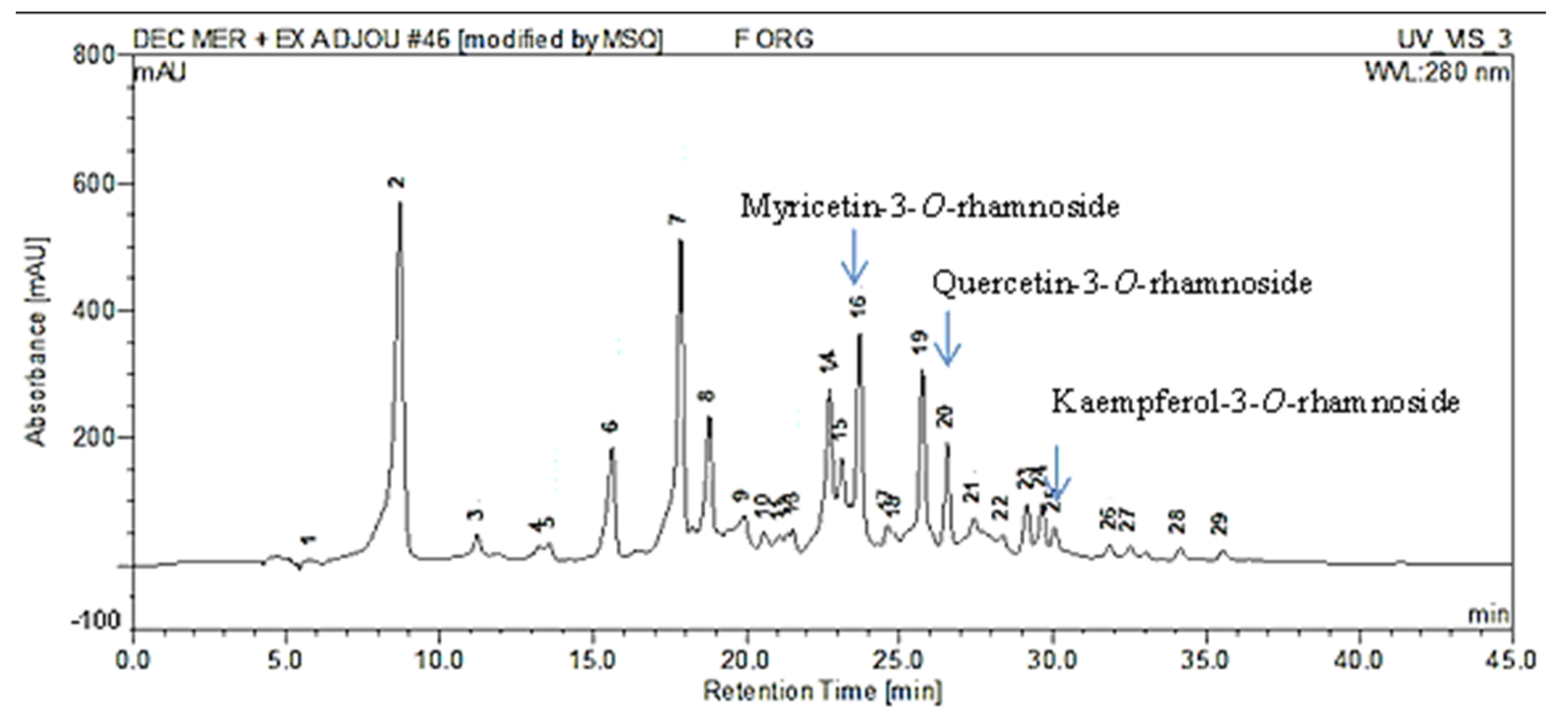
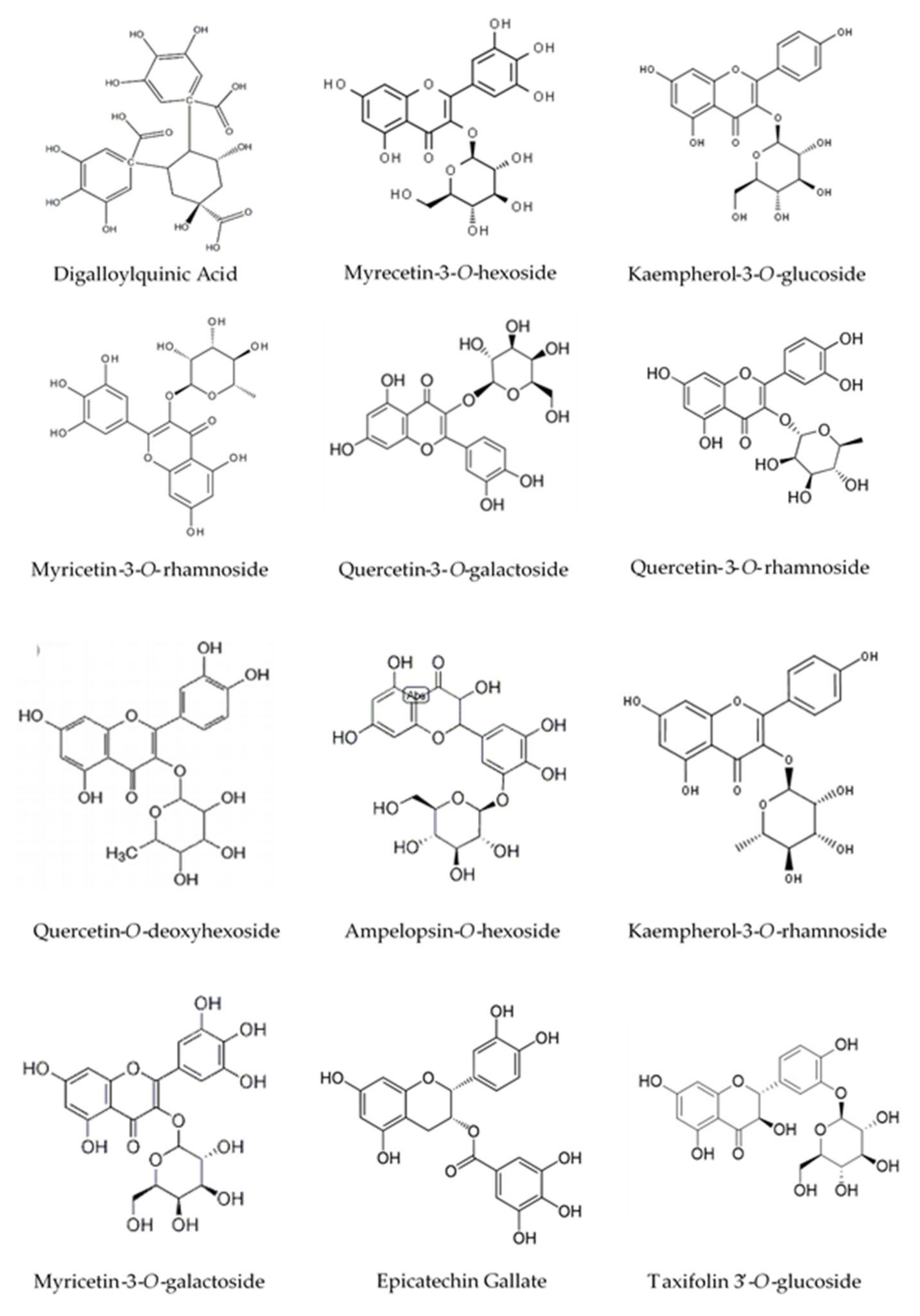
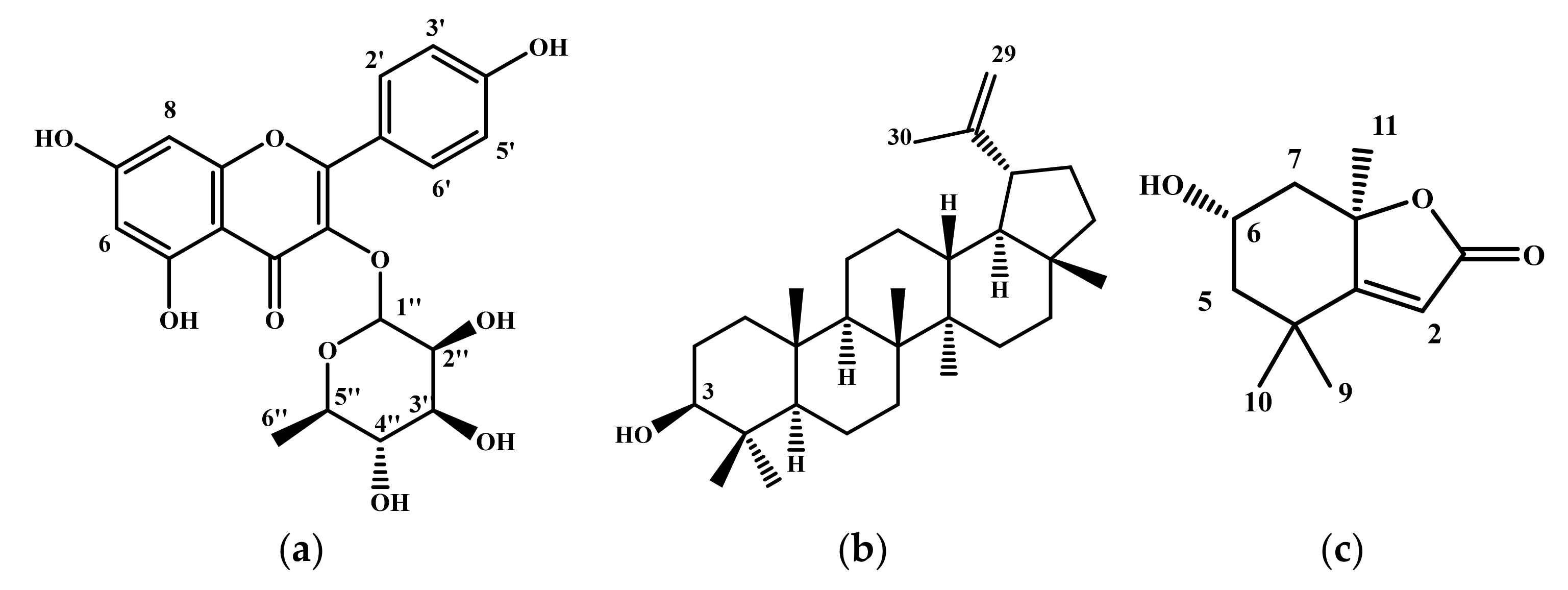
2.4. High-Pressure Liquid Chromatography Coupled to Mass Spectrometry (HPLC-MS) Analysis
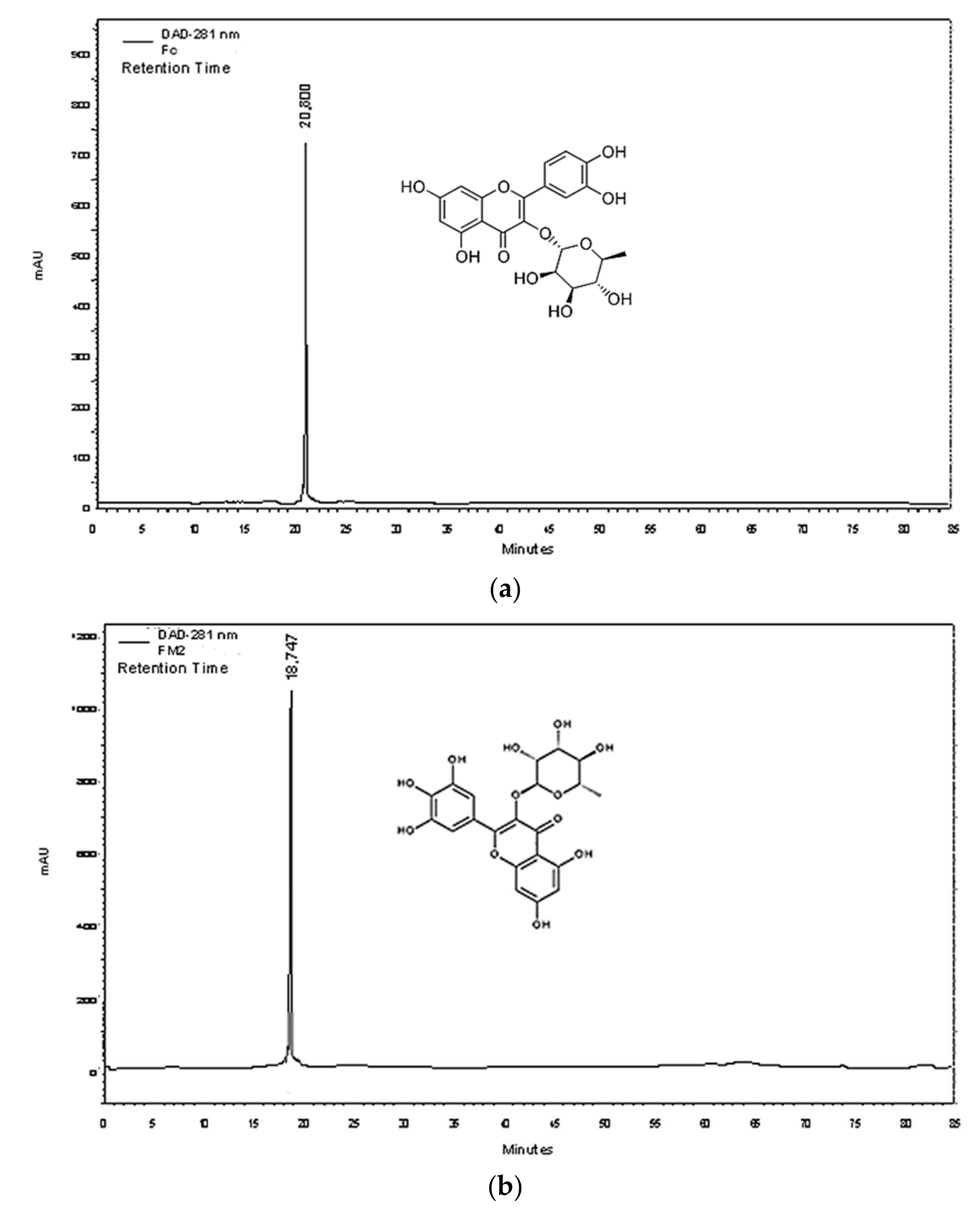
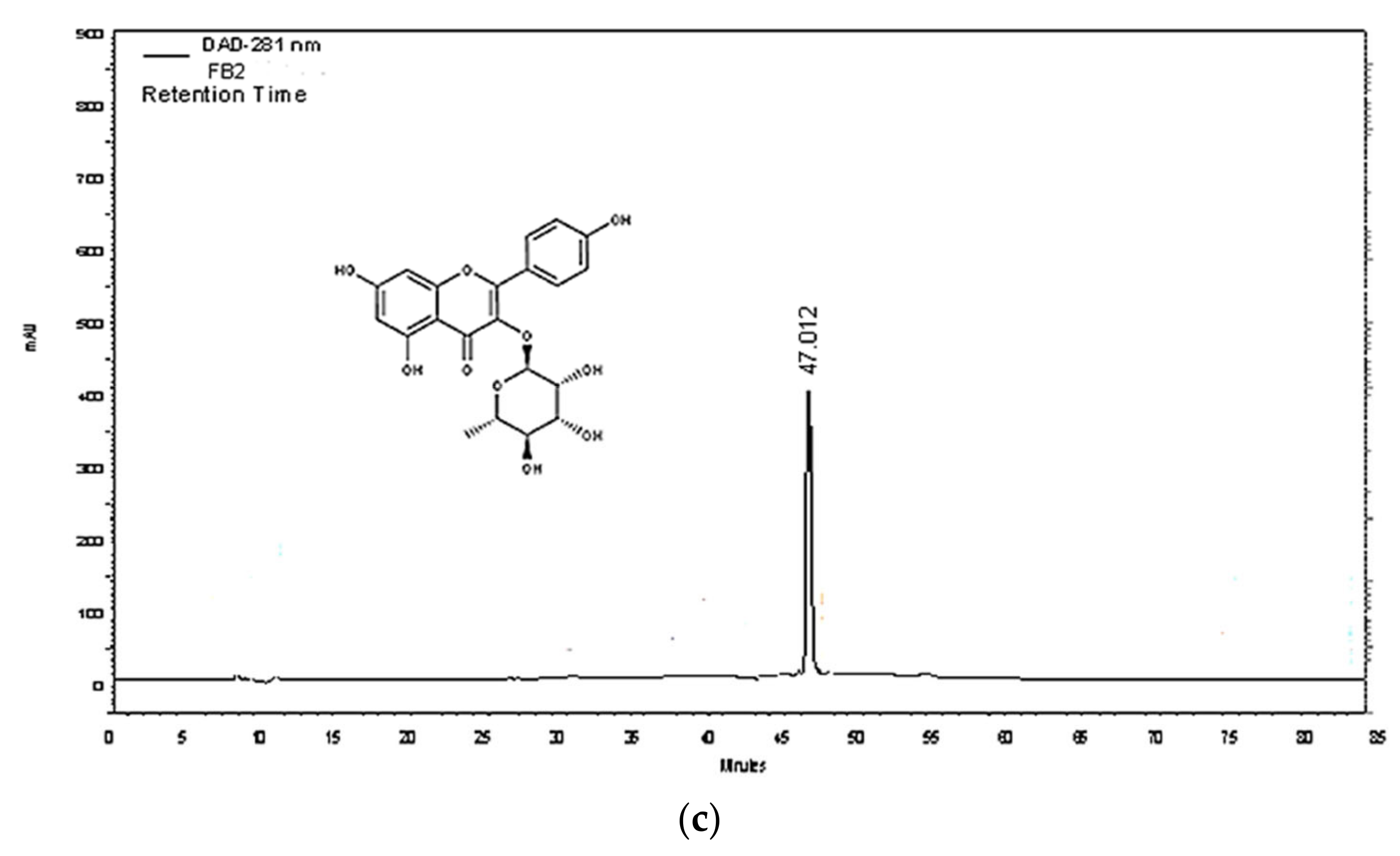
2.5. Purification and Quantification of Phenolic Compounds
2.6. Transdermal Diffusion
2.7. Enzymatic Inhibition Activities In Vitro
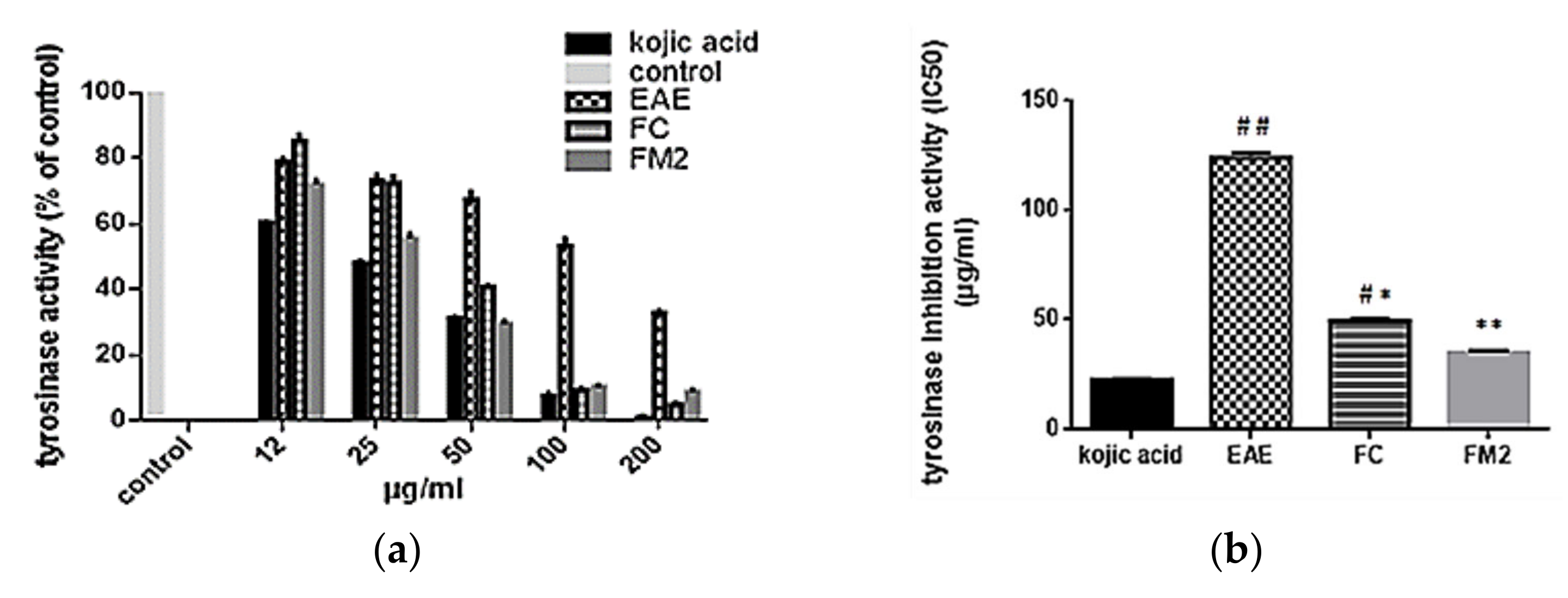
2.7.1. Tyrosinase Inhibition Assay
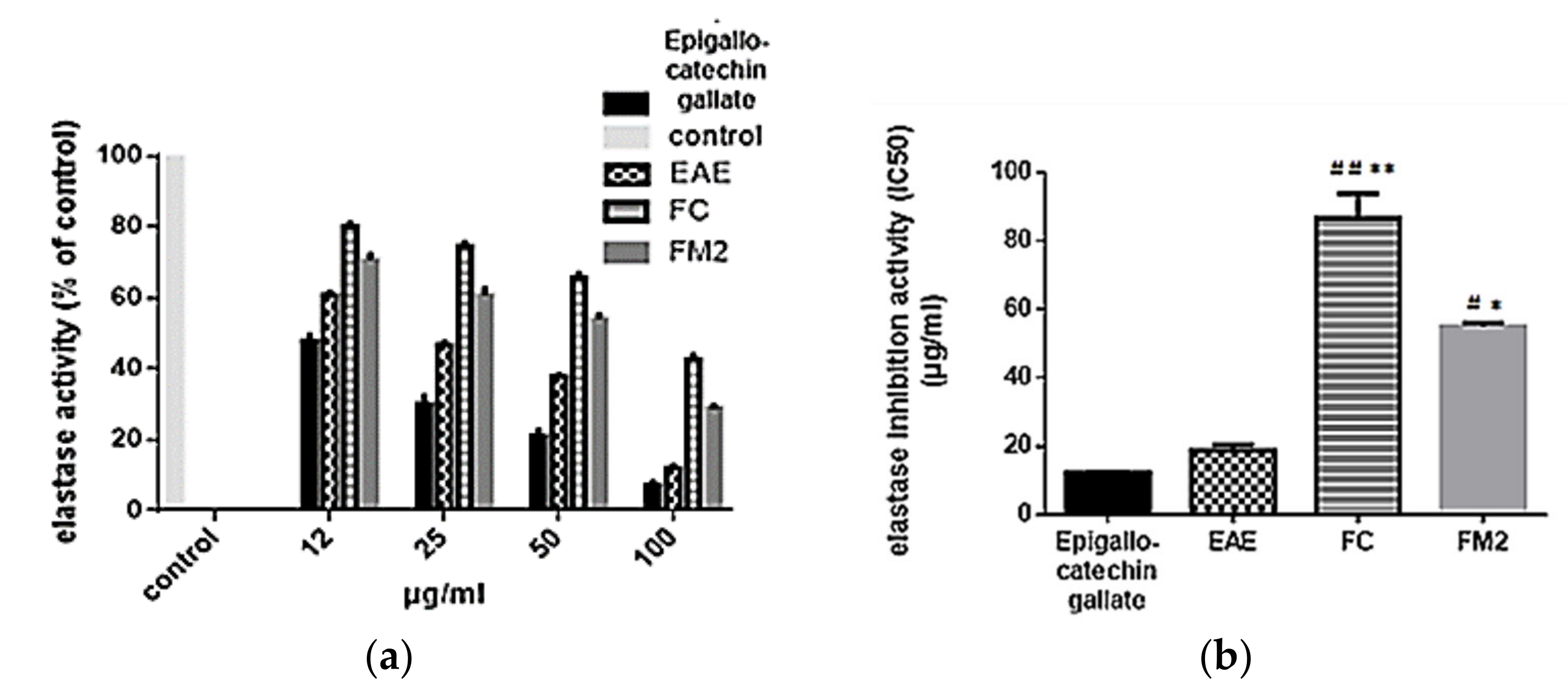
2.7.2. Elastase Inhibition Assay
2.8. Cellular Tyrosinase Inhibition Activity on B16 Cells
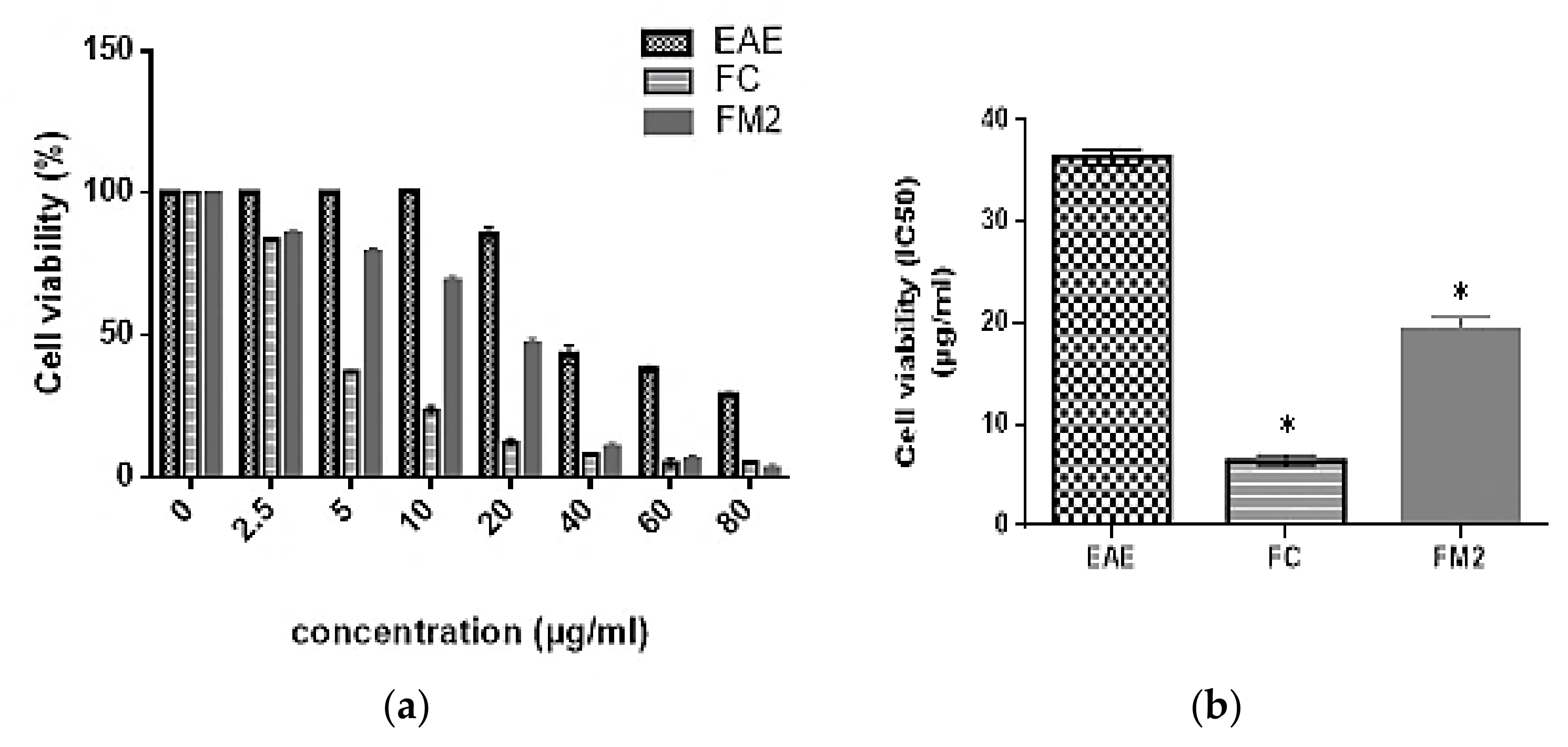
2.8.1. Cell Viability
2.8.2. Cellular Tyrosinase Inhibition Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Preparation
4.3. Extracts Characterization
4.3.1. PSE Procedure
4.3.2. Total Phenolic Content
4.3.3. Total Flavonoid Content
4.3.4. Antioxidant Activities
DPPH Radical Scavenging Assay
FRAP Assay
4.4. Identification of Phenolic Compounds
4.4.1. HPLC-DAD and HPLC-MS Procedures
4.4.2. CPC Separation
4.4.3. MPLC Separation
4.4.4. Preparative HPLC
4.4.5. Determination of Isolated Compounds Structure
4.4.6. Validation of HPLC-DAD Method and Quantification of Purified Molecules
4.5. Transdermal Diffusion Test
4.6. In Vitro Enzymatic Activities
4.6.1. Tyrosinase Inhibition
4.6.2. Elastase Inhibition
4.7. Cell Culture Conditions
4.7.1. Cell Viability Analysis
4.7.2. Cellular Tyrosinase Activity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Espin, J.C.; Wichers, H.J. Effect of captopril on mushroom tyrosinase activity in vitro. Biochim. Biophys. Acta 2001, 1544, 289–300. [Google Scholar] [CrossRef]
- Passeron, T.; Ballotti, R.; Ortonne, J.P. Mélanogénèse. EMC-Dermatol.-Cosmétologie 2005, 2, 204–216. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; Varon, R.; Tudela, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Generation of hydrogen peroxide in the melanin biosynthesis pathway. Biochim. Biophys. Acta 2009, 1794, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.C.; Chen, Y.C. Identification of tyrosinase inhibitors from traditional Chinese medicines for the management of hyperpigmentation. Springerplus 2015, 4, 184. [Google Scholar] [CrossRef] [Green Version]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003, 16, 629–638. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G. Analysis of initial melanogenesis including tyrosinase transfer and melanosome differentiation through interrupted melanization by glutathione. J. Investig. Dermatol. 1989, 93, 100–107. [Google Scholar] [CrossRef] [Green Version]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market–A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Zrira, S.; Elamrani, A.; Benjilali, B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco—A seasonal variation. Flavour Frag. J. 2003, 18, 475–480. [Google Scholar] [CrossRef]
- Ierapetritis, D.G. The Geography of the Chios Mastic Trade from the 17th through to the 19th Century. Ethnobot. Res. Appl. 2010, 8, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Le, F.H.E.; Schoenenberger, A.; Nabli, A.; Valdeyron, M.A. Biologie et écologie des principaux taxons. In Essai de Synthèse sur la Végétation et la Phytoécologie Tunisienne. I. Eléments de Botanique et de Phytoécologie; Nabli, A., Ed.; Fac. Sci. Tunis, Imprimante Officielle de la République Tunisienne: Rades, Tunisia, 1989; pp. 49–193. [Google Scholar]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000, 72, 191–205. [Google Scholar] [CrossRef]
- Boukef, K.; Souissi, H.R. Contribution à l’étude des plantes médicinales en médecine populaire en Tunisie. Rev. Soc. Pham. 1982, 2, 34–35. [Google Scholar]
- Piluzza, G.; Virdis, S.; Serralutzu, F.; Bullitta, S. Uses of plants, animal, and mineral substances in Mediterranean ethno-veterinary practices for the care of small ruminants. J. Ethnopharmacol. 2015, 168, 87–99. [Google Scholar] [CrossRef]
- Remila, S.; Atmani-Kilani, D.; Delemasure, S.; Connat, J.L.; Azib, L.; Richard, T.; Atmani, D. Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med. 2015, 7, 274–286. [Google Scholar] [CrossRef]
- Yemmen, M.; Landolsi, A.; Mégraud, F. Antioxidant activities, anticancer activity and polyphenolics profile, of leaf, fruit and stem extracts of Pistacia lentiscus from Tunisia. Cell. Mol. Biol. 2017, 63, 87–95. [Google Scholar] [CrossRef]
- Botsaris, G.; Orphanides, A.; Yiannakou, E.; Gekas, V.; Goulas, V. Antioxidant and antimicrobial effects of Pistacia lentiscus L. extracts in pork sausages. Food Technol. Biotechnol. 2015, 53, 472–478. [Google Scholar] [CrossRef]
- Boulebda, N.; Belkhiri, A.; Belfadel, F.; Bensegueni, A.; Bahri, L. Dermal Wound Healing Effect of Pistacia Lentiscus Fruit’s Fatty Oil. Pharmacogn. Res. 2009, 1, 66. [Google Scholar]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F.J.I.C. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Balan, K.V.; Prince, J.; Han, Z.; Dimas, K.; Cladaras, M.; Wyche, J.H.; Pantazis, P. Antiproliferative activity and induction of apoptosis in human colon cancer cells treated in vitro with constituents of a product derived from Pistacia lentiscus L. var. chia. Phytomedicine 2007, 14, 263–272. [Google Scholar] [CrossRef]
- Tassou, C.C.; Nychas, G.J.E. Antimicrobial activity of the essential oil of mastic gum (Pistacia lentiscus var. chia) on Gram positive and Gram negative bacteria in broth and in Model Food System. Int. Biodeter. Biodegr. 1995, 36, 411–420. [Google Scholar] [CrossRef]
- Azaizeh, H.; Halahleh, F.; Abbas, N.; Markovics, A.; Muklada, H.; Ungar, E.D.; Landau, S.Y. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastro-intestinal nematode larvae. Vet. Parasitol. 2013, 191, 44–50. [Google Scholar] [CrossRef]
- Dedoussis, G.V.; Kaliora, A.C.; Psarras, S.; Chiou, A.; Mylona, A.; Papadopoulos, N.G.; Andrikopoulos, N.K. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis 2004, 174, 293–303. [Google Scholar] [CrossRef]
- Bourgou, S.; Beji, R.S.; Medini, F.; Ksouri, R. Effet du solvant et de la méthode d’extraction sur la teneur en composés phénoliques et les potentialités antioxydantes d’Euphorbia helioscopia. J. New Sci. 2016, 28, 1649–1655. [Google Scholar]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Ben Hadj KILANI, H. Unité de Traitement et de Transformation des Produits Forestiers non Ligneux. Master’s Thesis, Université Virtuelle de Tunis, Tunis, Tunisia, 2019. [Google Scholar]
- de Brito, E.S.; de Araújo, M.C.P.; Lin, L.Z.; Harnly, J. Determination of the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chem. 2007, 105, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, S.; Piccolella, S.; Marciano, S.; Galasso, S.; Nocera, P.; Piscopo, V.; Monaco, P. LC-MS/MS profiling of a mastic leaf phenol enriched extract and its effects on H2O2 and Aβ (25–35) oxidative injury in SK-B-NE (C)-2 cells. J. Agr. Food Chem. 2014, 62, 11957–11966. [Google Scholar] [CrossRef] [PubMed]
- Aderogba, M.A.; Kgatle, D.T.; McGaw, L.J.; Eloff, J.N. Isolation of antioxidant constituents from Combretum apiculatum subsp. Apiculatum. S. Afr. J. Bot. 2012, 79, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Katekhaye, S.D.; Paul, A.; Laddha, K.S. Lupane analogue from bark of Pithecellobium dulce and in vitro α-glucosidase and α-amylase enzyme inhibition assay of extract for potential antidiabetic activity. Chem. Nat. Compd. 2016, 52, 359–362. [Google Scholar] [CrossRef]
- Hiraga, Y.; Taino, K.; Kurokawa, M.; Takagi, R.; Ohkata, K. (−)-Loliolide and other germination inhibitory active constituents in Equisetum arvense. Nat. Prod. Lett. 1997, 10, 181–186. [Google Scholar] [CrossRef]
- Braca, A.; Bilia, A.R.; Mendez, J.; Morelli, I. Myricetin glycosides from Licania densiflora. Fitoterapia 2001, 72, 182–185. [Google Scholar] [CrossRef]
- Xu, Y.; Tao, Z.; Jin, Y.; Yuan, Y.; Dong, T.T.; Tsim, K.W.; Zhou, Z. Flavonoids, a Potential New Insight of Leucaena leucocephala Foliage in Ruminant Health. J. Agric. Food Chem. 2018, 66, 7616–7626. [Google Scholar] [CrossRef]
- ISO. ISO Report. In Biological Evaluation of Medical Devices, Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef]
- Gardeli, C.; Vassiliki, P.; Athanasios, M.; Kibouris, T.; Komaitis, M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: Evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008, 107, 1120–1130. [Google Scholar] [CrossRef]
- Ben Ahmed, Z.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Vander Heyden, Y. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. Leaves. Pharm. Biol. 2017, 55, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, I.; Daniel, M.; Sabnis, S.D. Chemotaxonomic studies on some members of Anacardiaceae. Plant Sci. 1988, 98, 205–208. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.J.; Yang, C.R.; Xu, M. Phenolic antioxidants from green tea produced from Camellia crassicolumna Var. multiplex. J. Agric. Food Chem. 2009, 57, 586–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [Green Version]
- Schühly, W.; Fabian, C.; Jahangir, S.; Fabian, W. Structure and absolute configuration of loliolide isomers determined by comparison of calculated and experimental CD spectra. In Proceedings of the 11th International Electronic Conference on Synthetic Organic Chemistry, Basel, Switzerland, 1–30 November 2007. [Google Scholar]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Halabalaki, M. Traditional uses, phytochemistry, and pharmacology of Chios mastic gum (Pistacia lentiscus var. chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Mulinacci, N.; Tattini, M. Identification and quantification of galloyl derivatives, flavonoid glycosides, and anthocyanins in leaves of Pistacia lentiscus L. Phytochem. Anal. 2002, 3, 79–86. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Byun, J.C.; Bandl, A.K.R.; Hyun, C.G.; Lee, N.H. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J. Med. Plant Res. 2009, 3, 914–920. [Google Scholar]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef]
- Ijaz, S.; Khan, H.M.S.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Seo, Y.; Kong, C.S. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (Thunb.) Bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Zbytek, B.; Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 2009, 124, 1470–1477. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: An update, Comprehensive Reviews. Res. Pharm. Sci. 2012, 11, 378–398. [Google Scholar]
- Kishore, N.; Twilley, D.; Blom van Staden, A.; Verma, P.; Singh, B.; Cardinali, G.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef]
- Eghbali-Feriz, S.; Taleghani, A.; Al-Najjar, H.; Emami, S.A.; Rahimi, H.; Asili, J.; Tayarani-Najaran, Z. Anti-melanogenesis and anti-tyrosinase properties of Pistacia atlantica subsp. mutica extracts on B16F10 murine melanoma cells. Res. Pharm. Sci. 2018, 13, 533. [Google Scholar]
- Tu, P.T.B.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, Y.; Matsuoka, Y.; Saito, K.; Ikekita, M.; Higuchi, S.; Shinomiya, T. Coordinate involvement of cell cycle arrest and apoptosis strengthen the effect of FTY720. Jpn. J. Cancer Res. 2001, 92, 680–687. [Google Scholar] [CrossRef]
- Dilworth, L.L.; Riley, C.K.; Stennett, D.K. Plant constituents: Carbohydrates, oils, resins, balsams, and plant hormones. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 61–80. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT—Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Bahorun, T. Substances naturelles actives: La flore mauricienne, une source d’approvisionnement potentielle. In Proceedings of the 2nd Annual Meeting of Agricultural Scientists, Reduit, Mauritius, 12–13 August 1998; Volume 83. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.Y.; Jalil, J.; Ng, P.Y.; Ng, S.F. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Ortiz, S.; Tavares, R.P.M.; Mandova, T.; Araújo, E.R.D.; Andrade, A.W.L.; Zucolotto, S.M. Bryophyllum pinnatum markers: CPC isolation, simultaneous quantification by a validated UPLC-DAD method, and biological evaluations. J. Pharm. Biomed. Anal. 2021, 193, 113682. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Filho, P.A.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In vitro and in vivo evaluation of nanoemulsion containing vegetable extracts. Cosmetics 2017, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Chomwal, R.; Kumar, P.; Sawal, R. Anti-inflammatory and wound healing activity of Curcuma aromatica salisb extract and its formulation. J. Chem. Pharm Res. 2009, 1, 304–310. [Google Scholar]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Angelis, A.; Hubert, J.; Aligiannis, N.; Michalea, R.; Abedini, A.; Nuzillard, J.M.; Renault, J.H. Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 2016, 211, 1586. [Google Scholar] [CrossRef] [Green Version]
- Elloumi, W.; Jebali, A.; Maalej, A.; Chamkha, M.; Sayadi, S. Effect of Mild Salinity Stress on the Growth, Fatty Acid, and Carotenoid Compositions, and Biological Activities of the Thermal Freshwater Microalgae Scenedesmus sp. Biomolecules 2020, 10, 1515. [Google Scholar] [CrossRef]
- Maalej, A.; Forte, M.; Bouallagui, Z.; Donato, S.; Mita, L.; Mita, D.G.; Sayadi, S. Olive compounds attenuate oxidative damage induced in HEK-293 cells via MAPK signaling pathway. J. Funct. Foods. 2017, 39, 18–27. [Google Scholar] [CrossRef]
- Kamei, Y.; Otsuka, Y.; Abe, K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology 2009, 59, 183. [Google Scholar] [CrossRef] [Green Version]


| Extracts | Total Phenolic (mg GAE/g of Extract) | Total Flavonoids (mg QE/g of Extract) |
|---|---|---|
| ME | 156.43 ± 1.08 | 3.52 ± 1.00 |
| EAE | 152.92 ± 0.05 | 3.48 ± 0.58 |
| AE | 148.11 ± 0.05 * | 2.30 ± 0.56 ** |
| Extracts | DPPH IC50 (µg/mL) | FRAP (mg AEAC/g of Extract) |
|---|---|---|
| ME | 19.62 | 467.29 ± 21.77 ** |
| EAE | 18.07 | 522.76 ± 22.99 |
| AE | 19.52 | 421.91 ± 15.48 ** |
| Ascorbic acid | 13.85 | - |
| Peak No. | Rt (min) | [M − H]− (m/z) | λmax (nm) | Identification |
|---|---|---|---|---|
| 2 | 8.70 | 479 | 260/355 | Myricetin-3-O-galactoside |
| 3 | 11.23 | 441 | 217/276 | Epicatechin Gallate |
| 7 | 17.88 | 495 | 219/276 | Digalloylquinic Acid |
| 8 | 18.87 | 495 | 217/278 | Digalloylquinic Acid |
| 14 | 22.71 | 479 | 260/350 | Myricetin-3-O-hexoside |
| 15 | 23.1 | 447 | 260/355 | Kaempferol-3-O-glucoside |
| 16 | 23.69 | 463 | 263/350 | Myricetin-3-O-rhamnoside |
| 17 | 24.59 | 463 | 269/351 | Quercetin-3-O-galactoside |
| 19 | 25.74 | 465 | 280/340 | Taxifolin 3′-O-glucoside |
| 20 | 26.5 | 447 | 258/351 | Quercetin-3-O-rhamnoside |
| 21 | 27.42 | 447 | 272/345 | Quercetin-O-deoxyhexoside |
| 23 | 29.15 | 615 | 267/354 | Myricetin-O-galloyl-deoxyhexoside |
| 24 | 29.64 | 481 | 280/350 | Ampelopsin-O-hexoside |
| 26 | 31.85 | 431 | 267/348 | Kaempferol-3-O-rhamnoside |
| Compounds | Linear Regression Equation | LOQ (mg/mL) | LOD (mg/mL) | r2 | Repeatability (% RSD) | ||
| Low Level | Medium Level | High Level | |||||
| FC | Y = 143.1X + 1.24 | 0.311 | 0.102 | 0.993 | 2.039 | 0.936 | 1.920 |
| FM2 | Y = 165.2X + 1.30 | 0.029 | 0.009 | 0.997 | 3.150 | 2.190 | 2.241 |
| FB2 | Y = 102.5X + 0.19 | 0.026 | 0.008 | 0.998 | 3.950 | 0.645 | 0.275 |
| Intermediate Precision (% RSD) | Accuracy % | ||||||
| Low Level | Medium Level | High Level | Low Level | Medium Level | High Level | ||
| FC | 1.656 | 0.995 | 0.712 | 100.932 | 96.174 | 102.950 | |
| FM2 | 1.829 | 1.805 | 0.907 | 115.927 | 115.927 | 110.60 | |
| FB2 | 1.630 | 0.922 | 0.321 | 123.449 | 123.449 | 99.651 | |
| mg of Pure Molecule/g Dry Weight | µg of Crossed Molecule/g of 5% Hydro-cream | ||||||
| FC | 10.471 ± 0.261 | 3.161 ± 0.006 | |||||
| FM2 | 12.173 ± 0.742 | 52.102 ± 0.001 | |||||
| FB2 | 4.530 ± 0.592 | - | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules 2022, 27, 855. https://doi.org/10.3390/molecules27030855
Elloumi W, Maalej A, Ortiz S, Michel S, Chamkha M, Boutefnouchet S, Sayadi S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules. 2022; 27(3):855. https://doi.org/10.3390/molecules27030855
Chicago/Turabian StyleElloumi, Wiem, Amina Maalej, Sergio Ortiz, Sylvie Michel, Mohamed Chamkha, Sabrina Boutefnouchet, and Sami Sayadi. 2022. "Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities" Molecules 27, no. 3: 855. https://doi.org/10.3390/molecules27030855
APA StyleElloumi, W., Maalej, A., Ortiz, S., Michel, S., Chamkha, M., Boutefnouchet, S., & Sayadi, S. (2022). Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules, 27(3), 855. https://doi.org/10.3390/molecules27030855







