Occurrence and Risk Assessment of Polybrominated Diphenyl Ethers in Surface Water and Sediment of Nahoon River Estuary, South Africa
Abstract
:1. Introduction
2. Materials and Method
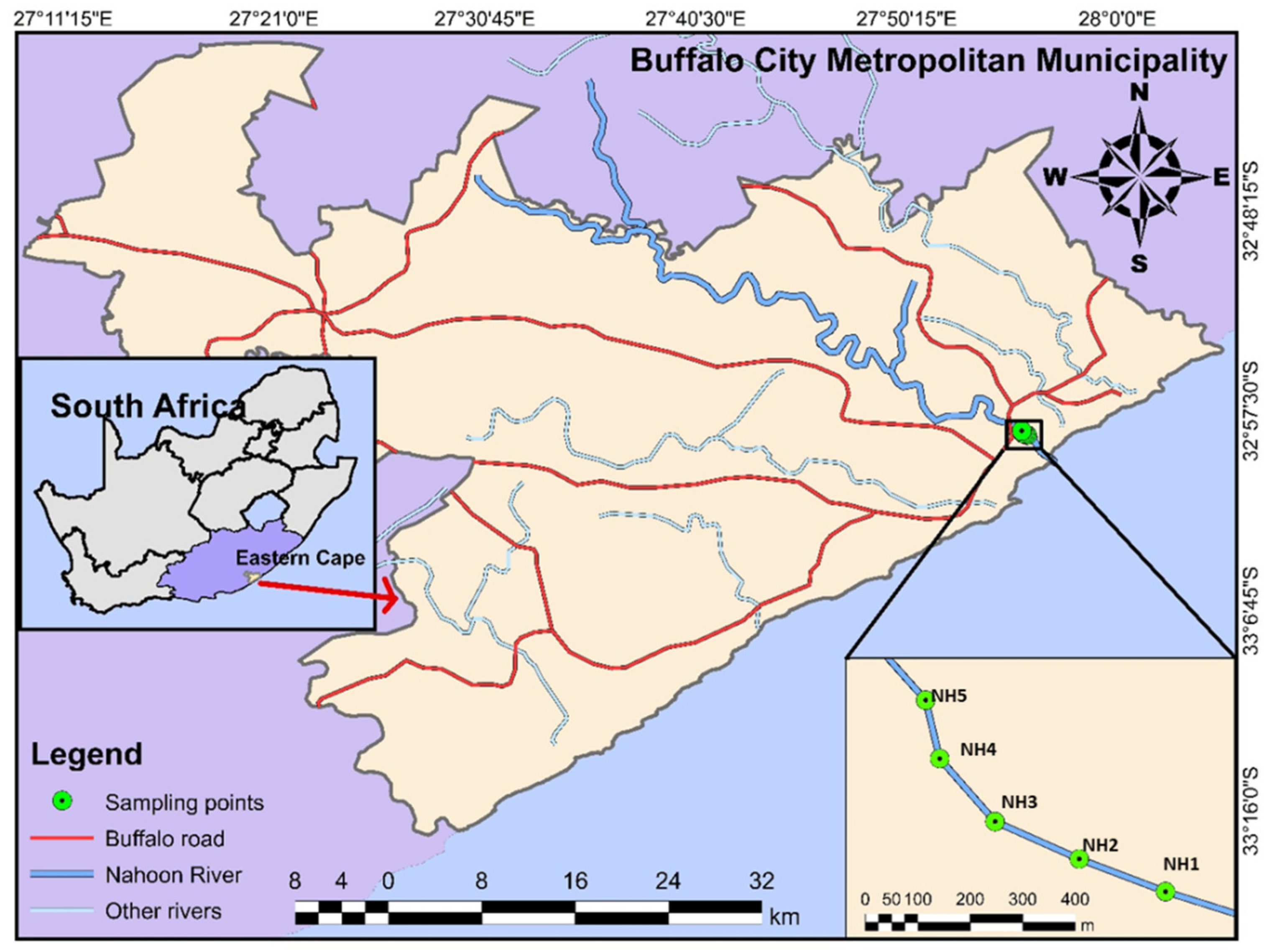
2.1. Study Area
2.2. Sampling and Sample Pretreatment
2.3. Extraction, Purification, and Instrumental Analysis
2.4. Statistical/Data Analysis
2.5. Risk Assessment
2.5.1. Estimated Daily Intake (EDI)
2.5.2. Non-Carcinogenic Risk
3. Result and Discussion
3.1. Spatial Distribution, Seasonal Variation, and Potential Sources of Pollution
3.2. The Contamination Level of PBDEs in This Current Study Compared with Other Countries
3.3. Impact of Physicochemical Parameters on CONCENTRATIONs of PBDEs in Nahoon Estuary
3.4. Correlation of PBDEs in Water and Sediment with Physicochemical Properties
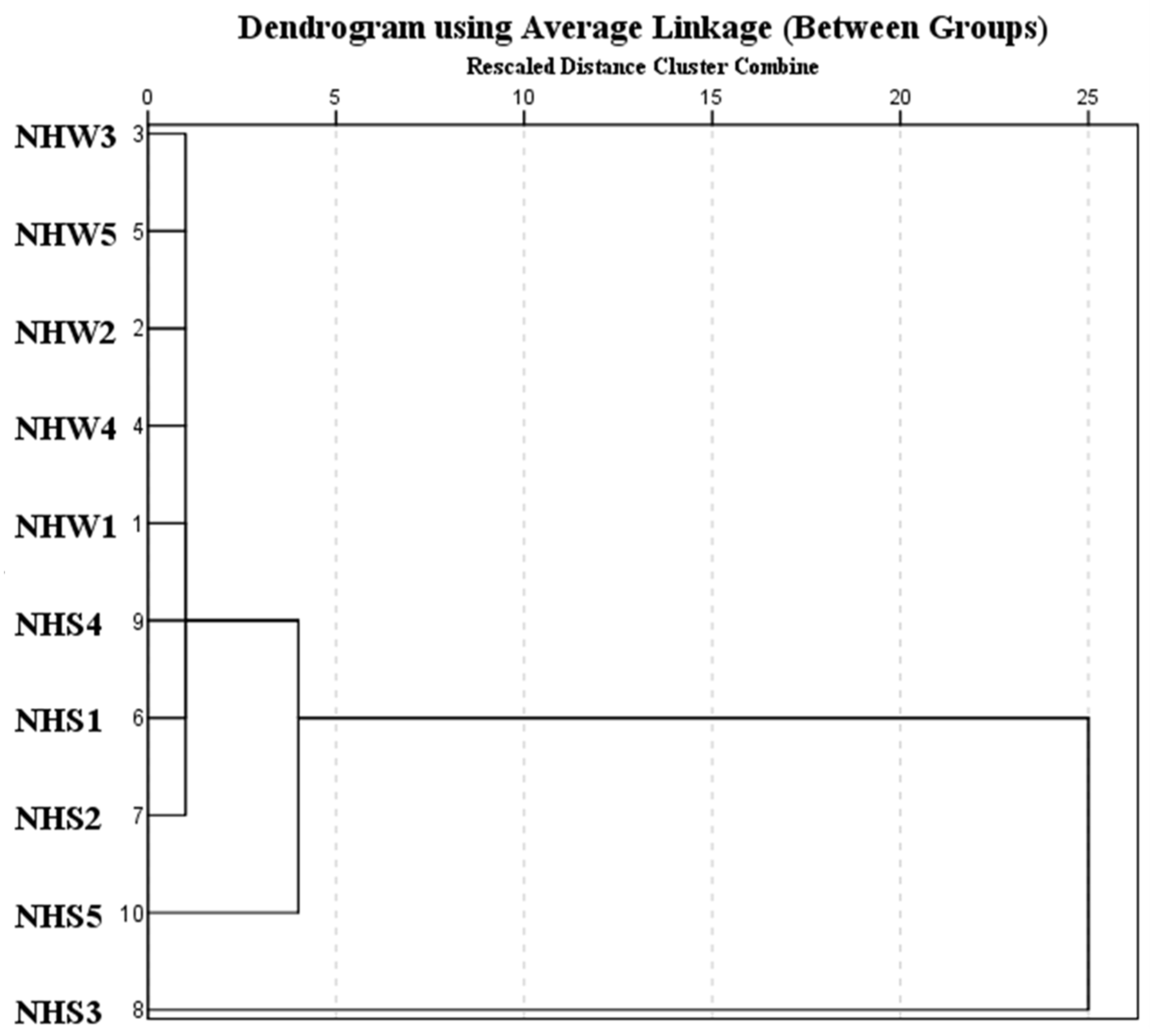
3.5. Contamination Pattern and Source Apportionment of Pollution in Nahoon Estuary Using a Dendrogram
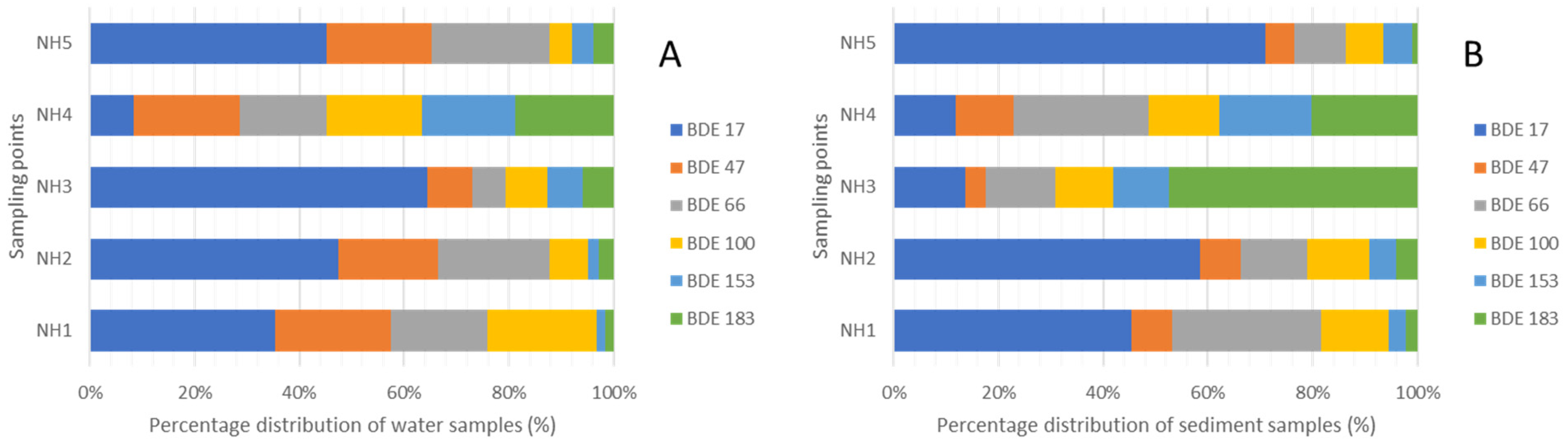
3.6. Compositional Patterns of PBDEs in the Surface Water and Sediments
3.7. Ecotoxicological Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated diphenyl ethers (PBDEs): New pollutants-old diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Robrock, K.R.; Alvarez-Cohen, L. Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs). Environ. Sci. Technol. 2006, 40, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; He, J. Reductive debromination of polybrominated diphenyl ethers by anaerobic bacteria from soils and sediments. Appl. Environ. Microbiol. 2010, 76, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robrock, K.R.; Korytár, P.; Alvarez-Cohen, L. Pathways for the anaerobic microbial denomination of polybrominated diphenyl ethers. Environ. Sci. Technol. 2008, 42, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Restrepo, B.; Kannan, K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 2009, 76, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Scheringer, M.; Von Goetz, N.; Hungerbühler, K. Total consumer exposure to polybrominated diphenyl ethers in north america and europe. Environ. Sci. Technol. 2011, 45, 2391–2397. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Lei, B.; An, J.; Zhang, X.; Yu, Y. Polybrominated diphenyl ethers in the air and comparison of the daily intake and uptake through inhalation by Shanghai residents with those through other matrices and routes. Environ. Sci. Pollut. Res. 2015, 22, 1750–1759. [Google Scholar] [CrossRef]
- Olukunle, O.; Okonkwo, J.; Kefeni, K.; Lupankwa, M. Concentrations of polybrominated diphenyl ethers in sediments from Jukskei River, Gauteng, South Africa. Bull. Environ. Contam. Toxicol. 2012, 88, 461–466. [Google Scholar] [CrossRef]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P.; Olujimi, O.O. Polybrominated diphenyl ethers (PBDEs) and 2,2′,4,4′,5, 5′-hexabromobiphenyl (BB-153) in landfill leachate in Cape Town, South Africa. Environ. Monit. Assess. 2013, 185, 431–439. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Magubane, M.N.; Abafe, O.A.; Okonkwo, J.O.; Sibiya, I.V. Levels, distributions, and ecological risk assessments of polybrominated diphenyl ethers and alternative flame retardants in river sediments from Vaal River, South Africa. Environ. Sci. Pollut. Res. 2019, 26, 7156–7163. [Google Scholar] [CrossRef] [PubMed]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Polybrominated diphenyl ethers (PBDEs) in surface water and fish tissues from Sundays and Swartkops Estuaries, Eastern Cape Province, South Africa: Levels, spatial distribution, seasonal variation and health implications. Reg. Stud. Mar. Sci. 2020, 36, 101319. [Google Scholar] [CrossRef]
- Abafe, O.A.; Martincigh, B.S. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ. Sci. Pollut. Res. 2016, 23, 7038–7049. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef] [Green Version]
- Momba, M.N.B.; Osode, A.N.; Sibewu, M. The impact of inadequate wastewater treatment on the receiving water bodies—Case study: Buffalo City and Nkokonbe Municipalities of the Eastern Cape Province. Water SA 2006, 32, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Odusanya, D.O.; Okonkwo, J.O.; Botha, B. Polybrominated diphenyl ethers (PBDEs) in leachates from selected landfill sites in South Africa. Waste Manag. 2009, 29, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Daso, A.P.; Rohwer, E.R.; Koot, D.J.; Okonkwo, J.O. Preliminary screening of polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA) flame retardants in landfill leachate. Environ. Monit. Assess. 2017, 189, 418. [Google Scholar] [CrossRef]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P.; Olujimi, O.O. Occurrence of selected polybrominated diphenyl ethers and 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153) in sewage sludge and effluent samples of a wastewater-treatment plant in Cape Town, South Africa. Arch. Environ. Contam. Toxicol. 2012, 62, 391–402. [Google Scholar] [CrossRef]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P. Polybrominated diphenyl ethers (PBDEs) and hexabromobiphenyl in sediments of the Diep and Kuils Rivers in South Africa. Int. J. Sediment Res. 2016, 31, 61–70. [Google Scholar] [CrossRef]
- De Wit, C.A. An Overview of Brominated Flame Retardants in the Environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Polder, A.; Venter, B.; Skaare, J.U.; Bouwman, H. Polybrominated diphenyl ethers and HBCD in bird eggs of South Africa. Chemosphere 2008, 73, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Olukunle, O.I.; Okonkwo, O.J.; Wase, A.G.; Sha’ato, R. Polybrominated diphenyl ethers in car dust in Nigeria: Concentrations and implications for non-dietary human exposure. Microchem. J. 2015, 123, 99–104. [Google Scholar] [CrossRef]
- Bornman, E.; Strydom, N.; Clemmesen, C. Appraisal of Warm-Temperate South African Mangrove Estuaries as Habitats to Enhance Larval Nutritional Condition and Growth of Gilchristella aestuaria (Family Clupeidae) Using RNA:DNA Ratios. Estuaries Coasts 2018, 41, 1463–1474. [Google Scholar] [CrossRef]
- Newman, B.K.; Watling, R.J. Definition of baseline metal concentrations for assessing metal enrichment of sediment from the south-eastern Cape coastline of South Africa. Water SA 2007, 33. [Google Scholar] [CrossRef] [Green Version]
- Talbot, M.M.J.F.; Branch, E.B.; Marsland, S.A.; Watling, R.J. Metal surveys in South African estuaries. X. Blind, Ihlanza, Nahoon and Quinera rivers. Water SA 1985, 11, 65–68. [Google Scholar]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Spatial, seasonal and ecological risk assessment of organohalogenated contaminants in sediments of Swartkops and Sundays Estuaries, Eastern Cape province, South Africa. J. Soils Sediments 2020, 20, 1046–1059. [Google Scholar] [CrossRef]
- Nolte, A.; Eley, M.; Schöniger, M.; Gwapedza, D.; Tanner, J.; Mantel, S.K.; Scheihing, K. Hydrological modelling for assessing spatio-temporal groundwater recharge variations in the water-stressed Amathole Water Supply System, Eastern Cape, South Africa. Hydrol. Process. 2021, 35, e14264. [Google Scholar] [CrossRef]
- Cotiyane, P.; Adams, J.; Rajkaran, A. Key factors that drive phytoplankton biomass and community composition in the urbanised Nahoon Estuary, South Africa. Afr. J. Aquat. Sci. 2017, 42, 245–257. [Google Scholar] [CrossRef]
- Geldenhuys, C.; Cotiyane, P.; Rajkaran, A. Understanding the creek dynamics and environmental characteristics that determine the distribution of mangrove and salt marsh communities at Nahoon Estuary. S. Afr. J. Bot. 2016, 107, 137–147. [Google Scholar] [CrossRef]
- Bickerton, I.B. Estuaries of the Cape: Synopses of Available Information on Individual Systems; CSIR: Pretoria, South Africa, 1991. [Google Scholar]
- Sikora, L.J.; Moore-Kucera, J. Soil Test Methods from the Southeastern United States; Southern Cooperative Series Bulletin No 419; 2014; pp. 54–58. ISBN 9798624955. Available online: https://saaesd.org/wp-content/uploads/2017/12/MethodsManualFinalSERA6-2.pdf (accessed on 9 November 2021).
- Adams, J.B.; Cowie, M.; van Niekerk, L. Assessment of Completed Ecological Water Requirement Studies for South African Estuaries and Responses to Changes in Freshwater Inflow; 2016; ISBN 9781431207466. Available online: https://www.researchgate.net/profile/Lara-Van-Niekerk/publication/329390476_Adams_J_Cowie_M_Van_Niekerk_L_2016_Assessment_of_completed_ecological_water_requirement_studies_for_South_African_estuaries_and_responses_to_changes_in_freshwater_inflow_Water_Research_Commission_Repo/links/5c064dd7a6fdcc315f9b17ef/Adams-J-Cowie-M-Van-Niekerk-L-2016-Assessment-of-completed-ecological-water-requirement-studies-for-South-African-estuaries-and-responses-to-changes-in-freshwater-inflow-Water-Research-Commission-Repo.pdf (accessed on 29 January 2021).
- Wang, Q.; Li, Y.; Wang, Y. Optimizing the weight loss-on-ignition methodology to quantify organic and carbonate carbon of sediments from diverse sources. Environ. Monit. Assess. 2011, 174, 241–257. [Google Scholar] [CrossRef]
- Kowalski, B.; Mazur, M. The simultaneous determination of six flame retardants in water samples using SPE pre-concentration and UHPLC-UV method. Water Air Soil Pollut. 2014, 225, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Spatial monitoring and health risk assessment of polybrominated diphenyl ethers in environmental matrices from an industrialized impacted canal in South Africa. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Guo, F.; Aamir, M.; Liu, Y.; Tang, M.; Liu, W. Multicenter biomonitoring of polybrominated diphenyl ethers (PBDEs) in colostrum from China: Body burden profile and risk assessment. Environ. Res. 2019, 179, 108828. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Human Health Risk Assessment Toolkit: Chemical Hazards. IPCS Harmon. Proj. Doc. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44458/9789241548076_eng.pdf?sequence=1&isAllowed=y (accessed on 21 February 2021).
- Yahaya, A.; Okoh, O.O.; Okoh, A.I.; Adeniji, A.O. Occurrences of organochlorine pesticides along the course of the Buffalo river in the eastern cape of South Africa and its health implications. Int. J. Environ. Res. Public Health 2017, 14, 1372. [Google Scholar] [CrossRef] [Green Version]
- EPA, U.S. Technical Fact Sheet—Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyls (PBBs). 2017; pp. 1–5. Available online: https://www.epa.gov/sites/default/files/2014-03/documents/ffrrofactsheet_contaminant_perchlorate_january2014_final_0.pdf (accessed on 20 July 2021).
- Ge, W.; Mou, Y.; Chai, C.; Zhang, Y.; Wang, J.; Ju, T.; Jiang, T.; Xia, B. Polybrominated diphenyl ethers in the dissolved and suspended phases of seawater from Sanggou Bay, east China. Chemosphere 2018, 203, 253–262. [Google Scholar] [CrossRef]
- Yin, H.; Tang, Z.; Meng, T.; Zhang, M. Concentration profile, spatial distributions and temporal trends of polybrominated diphenyl ethers in sediments across China: Implications for risk assessment. Ecotoxicol. Environ. Saf. 2020, 206, 111205. [Google Scholar] [CrossRef]
- Canada Environment Protection. Canadian Environmental Protection Act, 1999; Ecological Screening Assessment Report on Polybrominated Diphenyl Ethers (PBDEs); 1999; Available online: https://www.ec.gc.ca/lcpe-cepa/documents/substances/pbde/sar_pbde-eng.pdf (accessed on 17 February 2021).
- Wu, M.H.; Xu, B.T.; Xu, G.; Wang, M.N.; Ma, J.; Pan, C.Y.; Sun, R.; Han, T.; Tang, L. Occurrence and profiles of polybrominated diphenyl ethers (PBDEs) in riverine sediments of Shanghai: A combinative study with human serum from the locals. Environ. Geochem. Health 2017, 39, 729–738. [Google Scholar] [CrossRef]
- Chen, D.; Hale, R.C.; Watts, B.D.; La Guardia, M.J.; Harvey, E.; Mojica, E.K. Species-specific accumulation of polybrominated diphenyl ether flame retardants in birds of prey from the Chesapeake Bay region, USA. Environ. Pollut. 2010, 158, 1883–1889. [Google Scholar] [CrossRef]
- Wu, M.H.; Tang, L.; Xu, G.; Ma, J.; Liu, N.; Wang, L.; Lei, J.Q. Polybrominated diphenyl ethers in surface sediments from principal watersheds of Shanghai, China: Levels, distribution, influencing factors, and risk assessment. Environ. Sci. Pollut. Res. 2013, 20, 2651–2660. [Google Scholar] [CrossRef]
- Environment Canada Federal Environmental Quality Guidelines Polybrominated Diphenyl Ethers. 2013. Available online: https://www.ospar.org/documents?v=42746 (accessed on 9 November 2021).
- Luo, P.; Ni, H.G.; Bao, L.J.; Li, S.M.; Zeng, E.Y. Size distribution of airborne particle-bound polybrominated diphenyl ethers and its implications for dry and wet deposition. Environ. Sci. Technol. 2014, 48, 13793–13799. [Google Scholar] [CrossRef]
- Zhan, L.; Lin, T.; Cheng, H.; Wang, Z.; Cheng, Z.; Zhou, D.; Qin, Z.; Zhang, G. Atmospheric deposition and air–soil exchange of polybrominated diphenyl ethers (PBDEs) in a background site in Central China. Environ. Sci. Pollut. Res. 2019, 26, 31934–31944. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Odabasi, M.; Bayram, A. Wet deposition of persistent organic pollutants (POPs) in Izmir, Turkey. Environ. Sci. Pollut. Res. 2016, 23, 9227–9236. [Google Scholar] [CrossRef] [PubMed]
- Castro-Jiménez, J.; Mariani, G.; Vives, I.; Skejo, H.; Umlauf, G.; Zaldívar, J.M.; Dueri, S.; Messiaen, G.; Laugier, T. Atmospheric concentrations, occurrence and deposition of persistent organic pollutants (POPs) in a Mediterranean coastal site (Etang de Thau, France). Environ. Pollut. 2011, 159, 1948–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, B.; Chen, S.; Luo, X.; Chen, L.; Yang, Q.; Sheng, G.; Peng, P.; Fu, J.; Zeng, E.Y. Distribution of Polybrominated Diphenyl Ethers in Sediments of the Pearl River Delta and Adjacent South China Sea. Environ. Sci. Technol. 2005, 39, 3521–3527. [Google Scholar] [CrossRef]
- La Guardia, M.J.; Hale, R.C.; Harvey, E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ. Sci. Technol. 2006, 40, 6247–6254. [Google Scholar] [CrossRef]
- Trinh, M.M.; Tsai, C.L.; Chang, M.B. Characterization of polybrominated diphenyl ethers (PBDEs) in various aqueous samples in Taiwan. Sci. Total Environ. 2019, 649, 388–395. [Google Scholar] [CrossRef]
- Anh, H.Q.; Tomioka, K.; Tue, N.M.; Tri, T.M.; Minh, T.B.; Takahashi, S. PBDEs and novel brominated flame retardants in road dust from northern Vietnam: Levels, congener profiles, emission sources and implications for human exposure. Chemosphere 2018, 197, 389–398. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Polybrominated diphenyl ethers in the environmental systems: A review. J. Environ. Health Sci. Eng. 2021, 19, 1229–1247. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Wang, L.; Zhu, G.; Li, M.; Liu, J.; Li, Z.; Gong, H.; Wu, C.; Yin, G. Multiple classes of chemical contaminants in soil from an e-waste disposal site in China: Occurrence and spatial distribution. Sci. Total Environ. 2021, 752, 141924. [Google Scholar] [CrossRef]
- Nouira, T.; Risso, C.; Chouba, L.; Budzinski, H.; Boussetta, H. Polychlorinated biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) in surface sediments from Monastir Bay (Tunisia, Central Mediterranean): Occurrence, distribution and seasonal variations. Chemosphere 2013, 93, 487–493. [Google Scholar] [CrossRef]
- Hansson, K.; Cousins, A.P.; Brorström, E. Atmospheric Concentrations in Air and Deposition Fluxes of POPs at Råö and Pallas, Trends and Seasonal and Spatial Variations; 2006; Available online: https://www.diva-portal.org/smash/get/diva2:715904/FULLTEXT01.pdf (accessed on 10 February 2021).
- Khairy, M.A.; Lohmann, R. Using Polyethylene Passive Samplers to Study the Partitioning and Fluxes of Polybrominated Diphenyl Ethers in an Urban River. Environ. Sci. Technol. 2017, 51, 9062–9071. [Google Scholar] [CrossRef] [PubMed]
- Tlili, K.; Labadie, P.; Alliot, F.; Bourges, C.; Desportes, A.; Chevreuil, M. Polybrominated diphenyl ether dynamics in ambient air and atmospheric bulk/wet deposition in downtown Paris (France). Water Air Soil Pollut. 2012, 223, 1543–1553. [Google Scholar] [CrossRef]
- Fang, L.; Huang, J.; Yu, G.; Wang, L. Photochemical degradation of six polybrominated diphenyl ether congeners under ultraviolet irradiation in hexane. Chemosphere 2008, 71, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tsang, D.C.W.; Wang, Y.; Li, Y.; Yang, X. The photodegradation of polybrominated diphenyl ethers (PBDEs) in various environmental matrices: Kinetics and mechanisms. Chem. Eng. J. 2016, 297, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Jaramillo, M.; Miglioranza, K.S.B.; Gonzalez, M.; Barón, E.; Monserrat, J.M.; Eljarrat, E.; Barceló, D. Uptake, metabolism and sub-lethal effects of BDE-47 in two estuarine invertebrates with different trophic positions. Environ. Pollut. 2016, 213, 608–617. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Peng, X.; Xu, Z.; Zhang, S.; Ren, M.; Ye, Z.; Wang, X. PBDEs in sediments of the Beijiang River, China: Levels, distribution, and influence of total organic carbon. Chemosphere 2009, 76, 226–231. [Google Scholar] [CrossRef]
- Wu, M.H.; Pei, J.C.; Zheng, M.; Tang, L.; Bao, Y.Y.; Xu, B.T.; Sun, R.; Sun, Y.F.; Xu, G.; Lei, J.Q. Polybrominated diphenyl ethers (PBDEs) in soil and outdoor dust from a multi-functional area of Shanghai: Levels, compositional profiles and interrelationships. Chemosphere 2015, 118, 87–95. [Google Scholar] [CrossRef]
- Deng, W.J.; Zheng, J.S.; Bi, X.H.; Fu, J.M.; Wong, M.H. Distribution of PBDEs in air particles from an electronic waste recycling site compared with Guangzhou and Hong Kong, South China. Environ. Int. 2007, 33, 1063–1069. [Google Scholar] [CrossRef]
- Oros, D.R.; Hoover, D.; Rodigari, F.; Crane, D.; Sericano, J. Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco. Environ. Sci. Technol. 2005, 39, 33–41. [Google Scholar] [CrossRef]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P. Occurrence of polybrominated diphenyl ethers (PBDEs) and 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153) in water samples from the Diep River, Cape Town, South Africa. Environ. Sci. Pollut. Res. 2013, 20, 5168–5176. [Google Scholar] [CrossRef]
- De Boer, J.; Wester, P.G.; Van Der Horst, A.; Leonards, P.E.G. Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environ. Pollut. 2003, 122, 63–74. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Q.; Liu, X.; Wang, J. Occurrence, distribution and risk assessment of polychlorinated biphenyls and polybrominated diphenyl ethers in nine water sources. Ecotoxicol. Environ. Saf. 2015, 115, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ford, J.C.; Li, A.; Mills, W.J.; Buckley, D.R.; Rockne, K.J. Polybrominated diphlenyl ethers in the sediments of the great lakes. 1. Lake superior. Environ. Sci. Technol. 2004, 38, 3286–3293. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Han, S.; Ma, L.; Luo, M.; Yang, G.; Liu, W.; Xu, D. Polybrominated diphenyl ethers in surface waters around Beijing: Occurrence, distribution and sources. Appl. Geochemistry 2018, 98, 58–64. [Google Scholar] [CrossRef]
- Kuramochi, H.; Maeda, K.; Kawamoto, K. Physicochemical properties of selected polybrominated diphenyl ethers and extension of the UNIFAC model to brominated aromatic compounds. Chemosphere 2007, 67, 1858–1865. [Google Scholar] [CrossRef]
- Chapman, D. (Ed.) Water Quality Assessments—A Guide to Use of Biota, Sediments and Water in Environmental Monitoring, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Fontana, A.R.; Silva, M.F.; Martínez, L.D.; Wuilloud, R.G.; Altamirano, J.C. Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 4339–4346. [Google Scholar] [CrossRef]
- Xu, P.; Ge, W.; Chai, C.; Zhang, Y.; Jiang, T.; Xia, B. Sorption of polybrominated diphenyl ethers by microplastics. Mar. Pollut. Bull. 2019, 145, 260–269. [Google Scholar] [CrossRef]
- Anim, A.K.; Drage, D.S.; Goonetilleke, A.; Mueller, J.F.; Ayoko, G.A. Distribution of PBDEs, HBCDs and PCBs in the Brisbane River estuary sediment. Mar. Pollut. Bull. 2017, 120, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, X.; Li, Y.; Ya, M.; Luo, H.; Hong, H. Polybrominated diphenyl ethers, organochlorine pesticides, and polycyclic aromatic hydrocarbons in water from the Jiulong River Estuary, China: Levels, distributions, influencing factors, and risk assessment. Environ. Sci. Pollut. Res. 2017, 24, 8933–8945. [Google Scholar] [CrossRef] [PubMed]
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Olisah, C.; Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Occurrence and risk evaluation of organochlorine contaminants in surface water along the course of Swartkops and Sundays River Estuaries, Eastern Cape Province, South Africa. Environ. Geochem. Health 2019, 41, 2777–2801. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; He, W.; Qin, N.; Kong, X.Z.; He, Q.S.; Yang, B.; Yang, C.; Jorgensen, S.E.; Xu, F.L. Temporal-spatial distributions and ecological risks of perfluoroalkyl acids (PFAAs) in the surface water from the fifth-largest freshwater lake in China (Lake Chaohu). Environ. Pollut. 2015, 200, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Roscioli, C.; Guzzella, L. Decabromodiphenyl ether (BDE-209) enters the food web of the River Po and is metabolically debrominated in resident cyprinid fishes. Sci. Total Environ. 2011, 409, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tam, N.F.Y.; Cheung, S.G.; Wei, P.; Li, S.; Wu, Q. Contamination of polybrominated diphenyl ethers (PBDEs) in watershed sediments and plants adjacent to e-waste sites. J. Hazard. Mater. 2019, 379, 120788. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, J.; Xu, X.; Zhang, D.; Li, X. Polybrominated diphenyl ethers in sediments from the Southern Yellow Sea: Concentration, composition profile, source identification and mass inventory. Chemosphere 2016, 144, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.B.; Choi, M.; Yu, J.; Jung, R.H.; Choi, H.G. Contamination and potential sources of polybrominated diphenyl ethers (PBDEs) in water and sediment from the artificial Lake Shihwa, Korea. Chemosphere 2012, 88, 837–843. [Google Scholar] [CrossRef]
- Rügner, H.; Schwientek, M.; Beckingham, B.; Kuch, B.; Grathwohl, P. Turbidity as a proxy for total suspended solids (TSS) and particle facilitated pollutant transport in catchments. Environ. Earth Sci. 2013, 69, 373–380. [Google Scholar] [CrossRef]
- Zhu, X.; Beiyuan, J.; Lau, A.Y.T.; Chen, S.S.; Tsang, D.C.W.; Graham, N.J.D.; Lin, D.; Sun, J.; Pan, Y.; Yang, X.; et al. Sorption, mobility, and bioavailability of PBDEs in the agricultural soils: Roles of co-existing metals, dissolved organic matter, and fertilizers. Sci. Total Environ. 2018, 619–620, 1153–1162. [Google Scholar] [CrossRef]
- Langford, K.; Scrimshaw, M.; Lester, J. The impact of process variables on the removal of PBDEs and NPEOs during simulated activated sludge treatment. Arch. Environ. Contam. Toxicol. 2007, 53, 1–7. [Google Scholar] [CrossRef]
- Hellar-Kihampa, H.; De Wael, K.; Lugwisha, E.; Malarvannan, G.; Covaci, A.; Van Grieken, R. Spatial monitoring of organohalogen compounds in surface water and sediments of a rural-urban river basin in Tanzania. Sci. Total Environ. 2013, 447, 186–197. [Google Scholar] [CrossRef]
- Olisah, C.; Rubidge, G.; Human, L.R.D.; Adams, J.B. A translocation analysis of organophosphate pesticides between surface water, sediments and tissues of common reed Phragmites australis. Chemosphere 2021, 284, 131380. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, M.; Yun, X.; Yang, Y.; Zhang, M.; Li, Q.X.; Wang, J. Occurrence, distribution and seasonal variations of polychlorinated biphenyls and polybrominated diphenyl ethers in surface waters of the East Lake, China. Chemosphere 2014, 103, 256–262. [Google Scholar] [CrossRef] [PubMed]
| Site Code | Latitude | Longitude | Description |
|---|---|---|---|
| NH1 | 32°58′36.7″ S | 25°55′49.1″ E | Creek |
| NH2 | 32°58′34.7″ S | 27°55′43.8″ E | Open place |
| NH3 | 32°58′32.4″ S | 27°55′38.6″ E | Outdoor recreation |
| NH4 | 32°58′28.5″ S | 27°55′35.1″ E | Open air for recreation |
| NH5 | 32°58′24.9″ S | 27°55′34.3″ E | Under the bridge |
| Spring | Summer | |||||||
|---|---|---|---|---|---|---|---|---|
| Surface water | ||||||||
| Congener | Mean (n = 5) | Max | Min | DF (100%) | Mean (n = 5) | Max | Min | DF (100%) |
| BDE 17 | 140 ± 91.2 | 247 | BDL | 80 | 23.5 ± 31.5 | 70.2 | BDL | 40 |
| BDE 47 | 68.9 ± 76.7 | 190 | 4.27 | 100 | 7.92 ± 2.80 | 12.4 | 5.43 | 100 |
| BDE 100 | 44.0 ± 64.4 | 178 | 4.25 | 100 | 8.95 ± 2.96 | 11.5 | 7.36 | 100 |
| BDE 153 | 5.88 ± 75.7 | 6.39 | 4.97 | 100 | 6.59 ± 1.77 | 8.00 | 5.82 | 100 |
| BDE 183 | 5.79 ± 0.98 | 5.56 | 5.41 | 100 | 7.02 ± 1.01 | 8.80 | 5.13 | 100 |
| ∑PBDE | 329 ± 48.3 | 62.1 ± 1.50 | ||||||
| Sediment | ||||||||
| BDE 17 | 1.42 ± 1.14 | 2.77 | 0.44 | 100 | 17.4 ± 12.2 | 32.0 | 0.14 | 100 |
| DE 47 | 0.27 ± 0.03 | 0.29 | 0.24 | 100 | 3.14 ± 3.25 | 8.75 | 0.28 | 100 |
| BDE 66 | 1.47 ± 1.36 | 3.42 | 0.27 | 100 | 8.88 ± 17.8 | 31.4 | 0.29 | 100 |
| BDE 100 | 0.20 ± 0.08 | 0.41 | 0.21 | 100 | 7.25 ± 10.4 | 25.69 | 0.26 | 100 |
| BDE 153 | 0.19 ± 0.08 | 0.27 | 0.08 | 100 | 6.00 ± 10.7 | 25.1 | 0.63 | 100 |
| BDE 183 | 0.56 ± 0.07 | 0.64 | 0.47 | 100 | 22.7 ± 49.9 | 112 | 0.25 | 100 |
| ∑PBDE | 4.19 ± 0.35 | 65.4 ± 15.9 |
| Spring | Summer | |||
|---|---|---|---|---|
| Parameters | Mean ± STD | Range | Mean ± STD | Range |
| Temp. [°C] | 21.0 ± 0.28 | 20.7–21.3 | 25.9 ± 0.79 | 24.5–26.6 |
| pH | 8.48 ± 0.17 | 8.27–8.66 | 8.65 ± 0.22 | 8.33–8.94 |
| EC [mS/cm] | 45.7 ± 1.02 | 44.4–46.5 | 51.5 ± 61.0 | 51.0–51.5 |
| TDS [g/L] | 22.9 ± 0.51 | 22.2–23.3 | 25.6 ± 80.1 | 25.5–25.7 |
| Sal. [psu] | 29.7 ± 0.74 | 28.8–34.4 | 33.6 ± 0.12 | 33.5–33.8 |
| Turb. [FNU] | 17.8 ± 5.97 | 10.6–26.4 | 31.4 ± 28.8 | 3.97–73.0 |
| mVorp | 32.2 ± 13.1 | 22.7–55.1 | 55.4 ± 19.3 | 23.2–70.8 |
| RES [Ohm-cm] | 21.9 ± 0.60 | 21.0–22.7 | 19.6 ± 0.43 | 19.0–20.0 |
| DO [mg/L] | 6.84 ± 1.11 | 5.88–8.55 | 5.31 ± 0.26 | 5.00–5.59 |
| TSS [mg/L] | 8.33 ± 3.25 | 4.00–12.7 | 9.13 ± 4.21 | 4.00–15.0 |
| % MC | 63.3 ± 1.05 | 62.5–64.8 | 30.0 ±3.69 | 25.0–35.0 |
| % OC | 0.25 ± 0.02 | 0.21–0.27 | 0.29 ± 0.07 | 0.17–0.35 |
| % OM | 0.42 ± 0.04 | 0.37–0.47 | 0.50 ± 0.12 | 0.30–0.60 |
| Water | |||
|---|---|---|---|
| Congener | Mean (ng/L) | EDI (ng/L) | HQ |
| BDE 47 | 38.4 | 1.28 | 0.0 |
| BDE 66 | 36.6 | 1.22 | 0.0 |
| BDE 100 | 26.5 | 0.88 | 0.0 |
| BDE 153 | 6.23 | 0.21 | 0.0 |
| Sediment | |||
| Homologue | Mean(ng/g dw) | PNEC (ng/g) * | HQ |
| PentaBDE | 5.48 | 31 | 0.2 |
| OctaBDE | 14.7 | 9100 | 0.0 |
| This Study | ||||
|---|---|---|---|---|
| Homologue | Congener | FEQG ** | Spring | Summer |
| Water | ||||
| TetraBDE | BDE-47 | 24 | 4.27–190 | 5.43–12.4 |
| PentaBDE | BDE-100 | 0.2 | 4.25–178 | 7.36–11.5 |
| HexaBDE | BDE-153 | 120 | 4.97–6.39 | 5.82–8.00 |
| HeptaBDE | BDE-183 | 17 | 5.41–3.56 | 5.13–8.80 |
| Sediment | ||||
| TetraBDE | BDE-47 | 39 | 0.24–0.29 | 0.28–8.75 |
| PentaBDE | BDE-100 | 0.4 | 0.21–0.41 | 0.26–25.7 |
| HexaBDE | BDE-153 | 440 | 0.08–0.27 | 0.63–25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohoro, C.R.; Adeniji, A.O.; Semerjian, L.; Okoh, A.I.; Okoh, O.O. Occurrence and Risk Assessment of Polybrominated Diphenyl Ethers in Surface Water and Sediment of Nahoon River Estuary, South Africa. Molecules 2022, 27, 832. https://doi.org/10.3390/molecules27030832
Ohoro CR, Adeniji AO, Semerjian L, Okoh AI, Okoh OO. Occurrence and Risk Assessment of Polybrominated Diphenyl Ethers in Surface Water and Sediment of Nahoon River Estuary, South Africa. Molecules. 2022; 27(3):832. https://doi.org/10.3390/molecules27030832
Chicago/Turabian StyleOhoro, Chinemerem Ruth, Abiodun Olagoke Adeniji, Lucy Semerjian, Anthony Ifeanyi Okoh, and Omobola Oluranti Okoh. 2022. "Occurrence and Risk Assessment of Polybrominated Diphenyl Ethers in Surface Water and Sediment of Nahoon River Estuary, South Africa" Molecules 27, no. 3: 832. https://doi.org/10.3390/molecules27030832
APA StyleOhoro, C. R., Adeniji, A. O., Semerjian, L., Okoh, A. I., & Okoh, O. O. (2022). Occurrence and Risk Assessment of Polybrominated Diphenyl Ethers in Surface Water and Sediment of Nahoon River Estuary, South Africa. Molecules, 27(3), 832. https://doi.org/10.3390/molecules27030832








