Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the Essential Oils
2.2. Antifungal Activity—Microdilution Assay Results
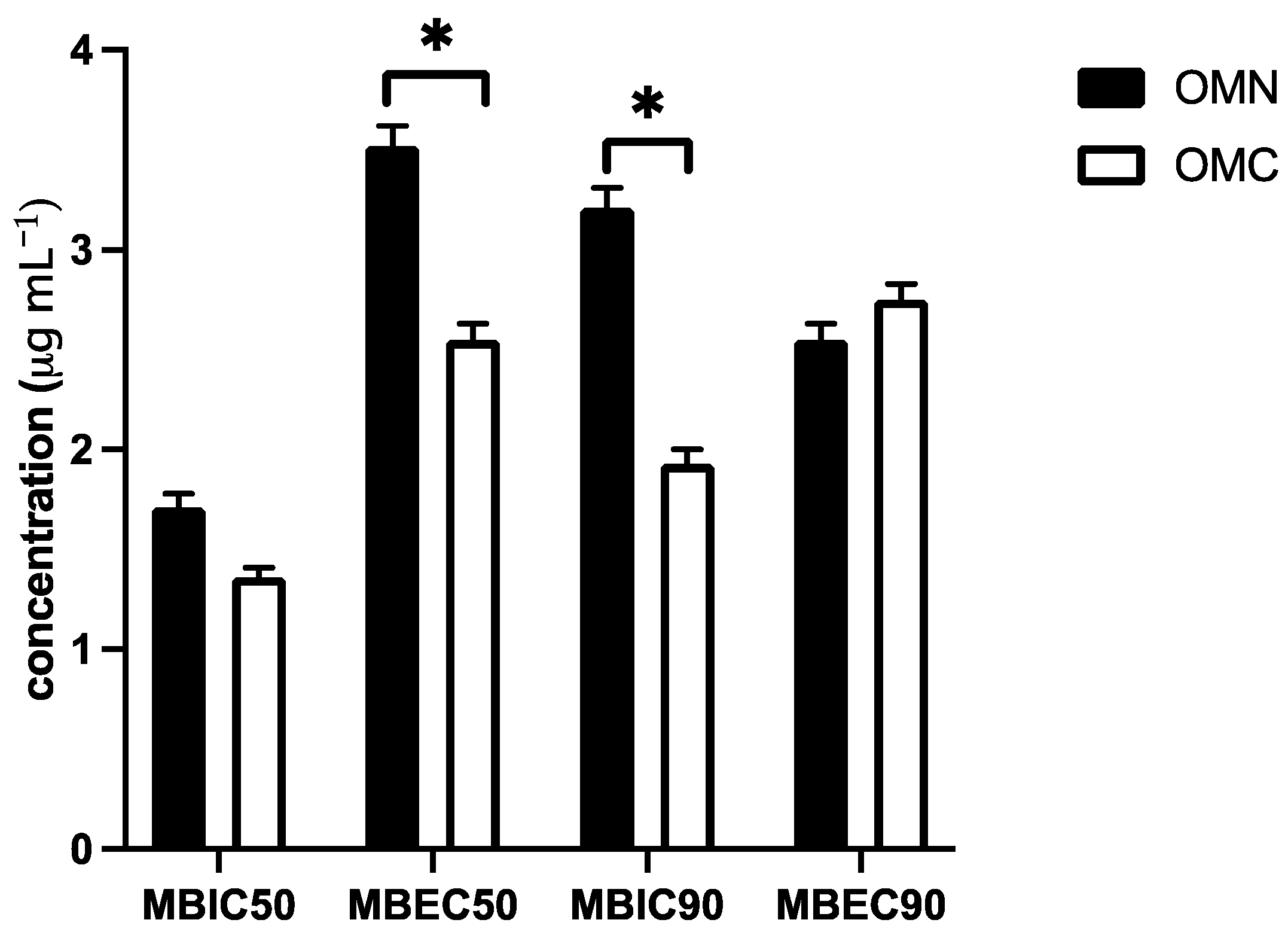
2.3. Biofilm Inhibition Assay
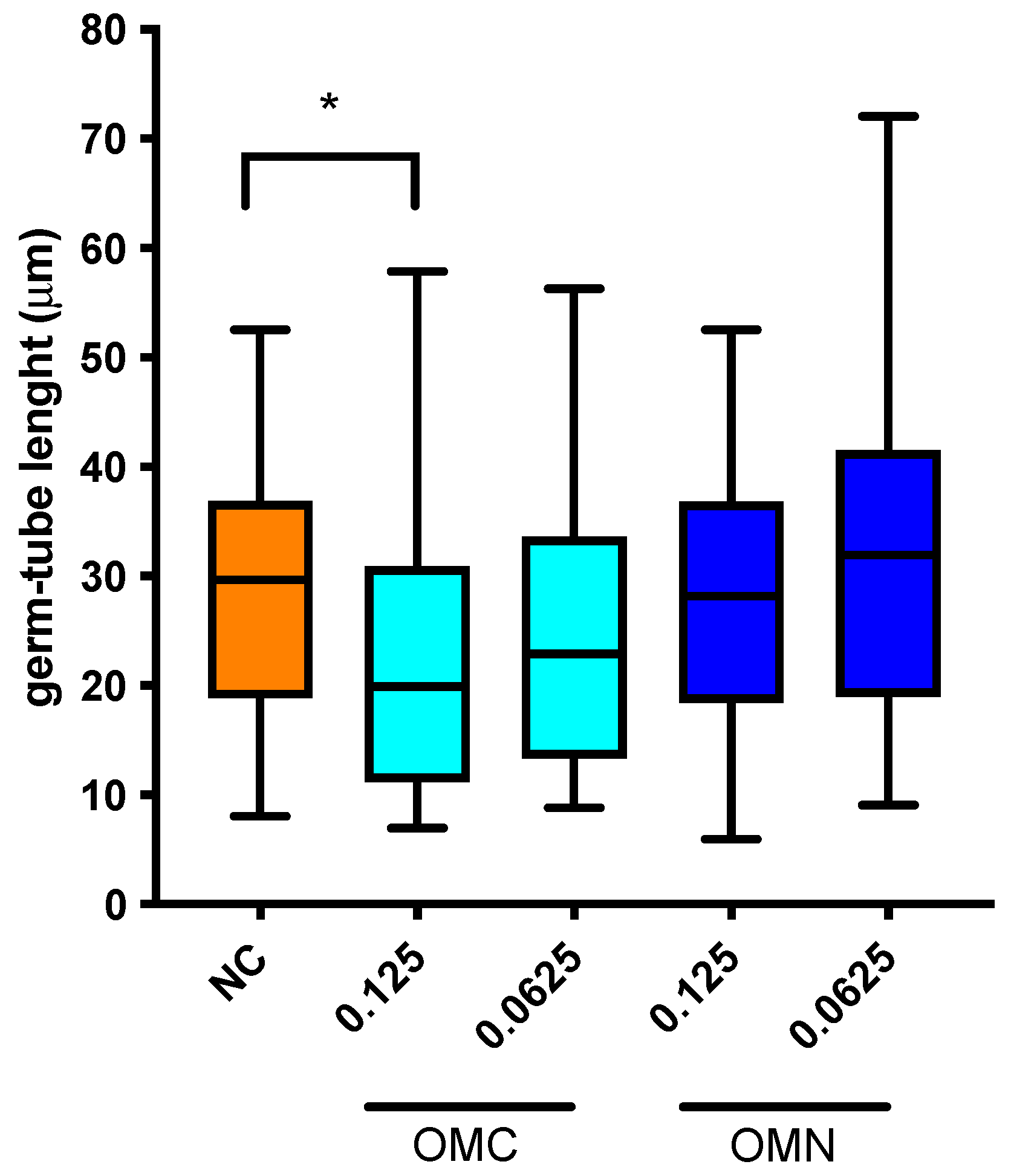
2.4. Germ-Tube Inhibition Assay and Modulation of Germ-Tube Length
2.5. Modulation of Cell Surface Hydrophobicity
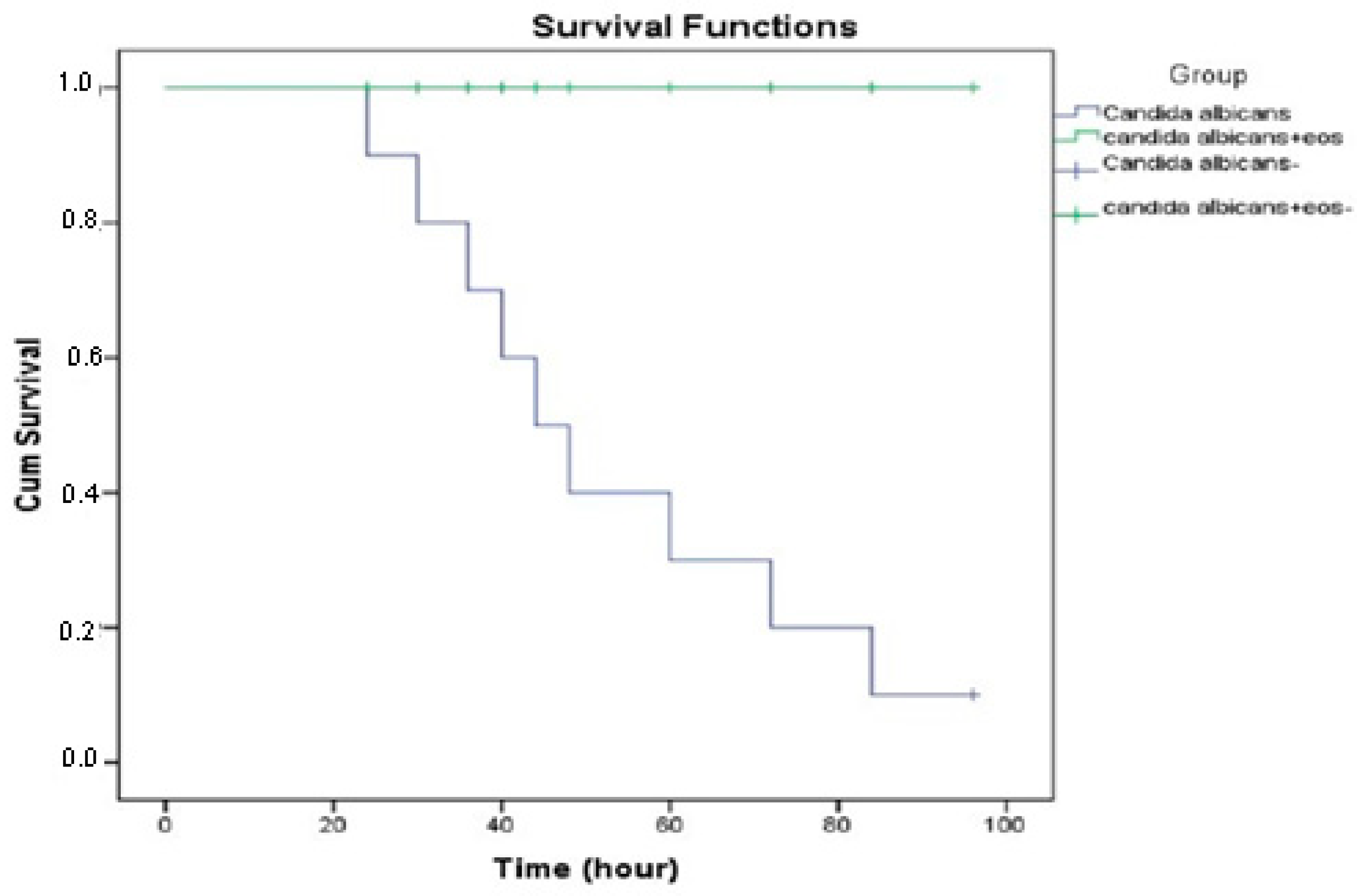
2.6. Defensive Response of Infected G. mellonella
2.7. The Effects of O. majorana EO Treatment on Infected G. mellonella
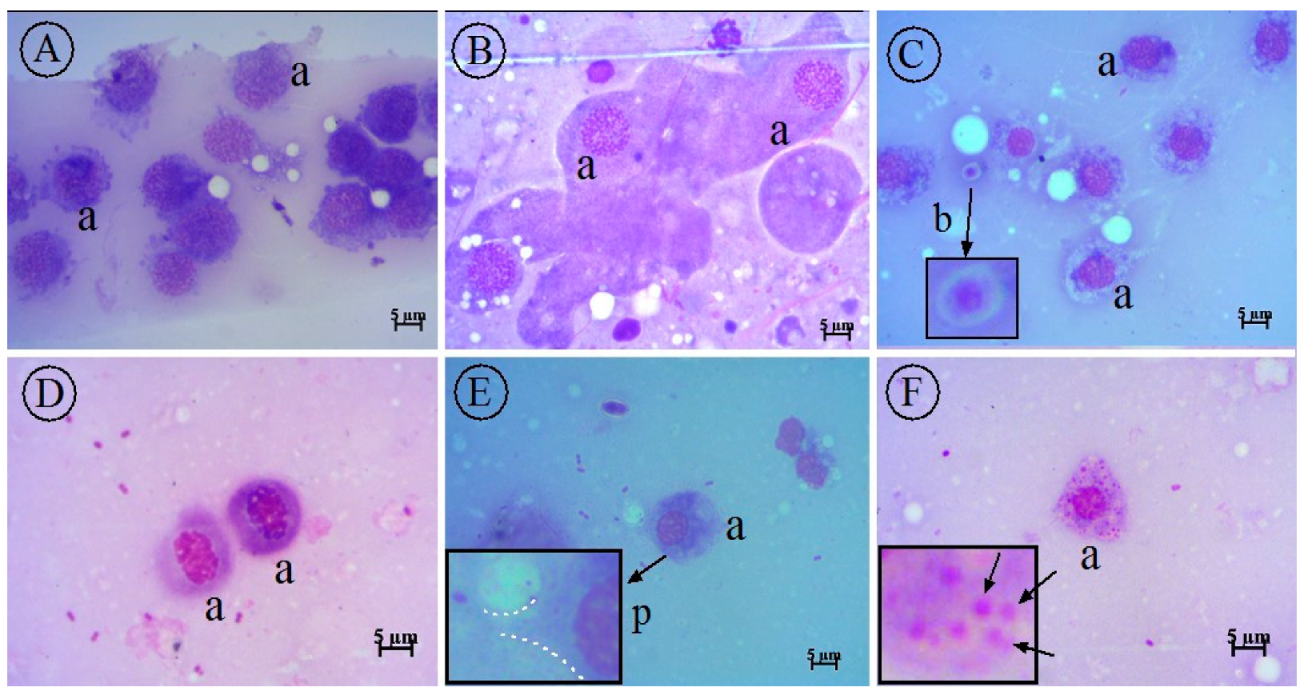
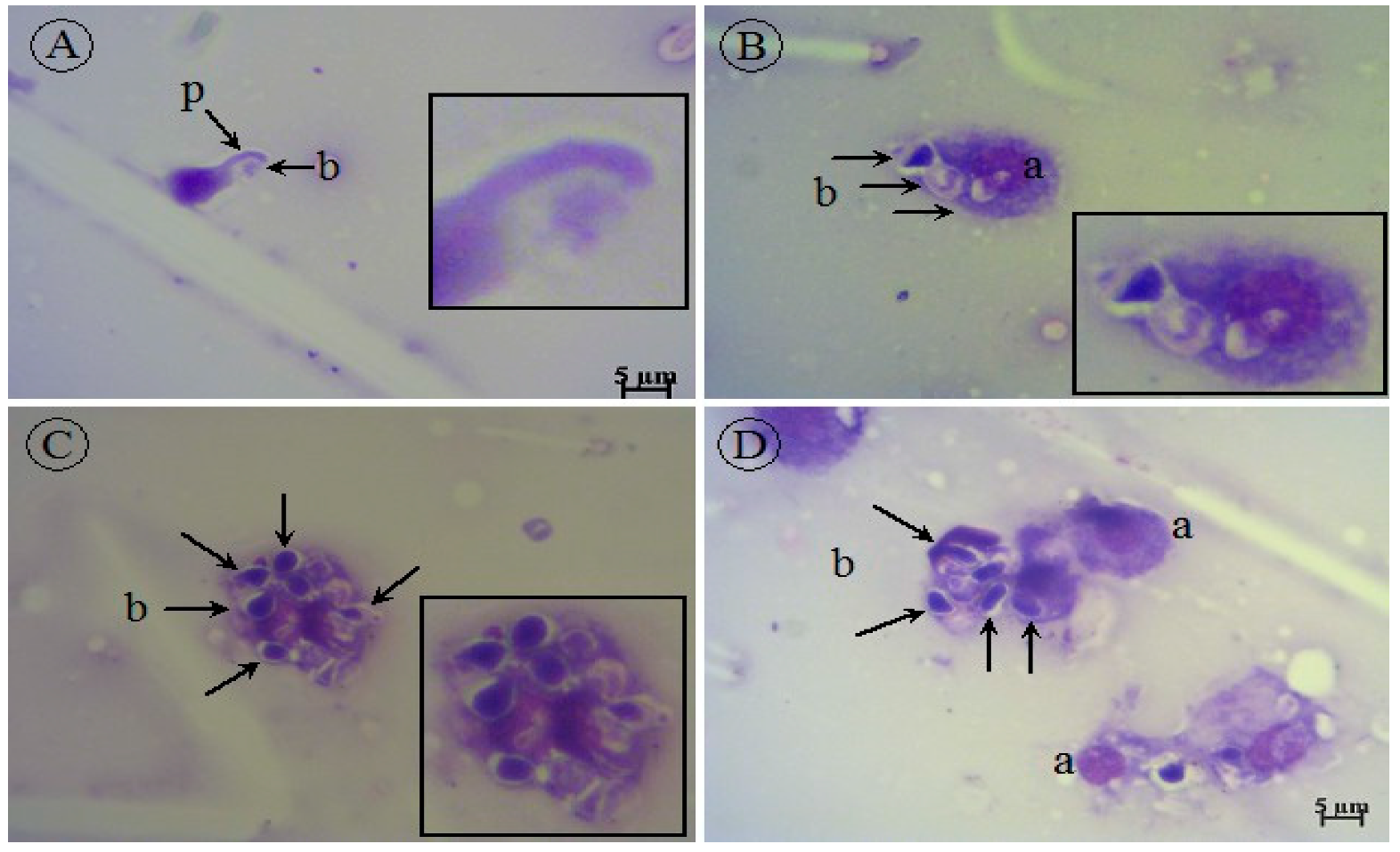
2.8. Cytology of G. mellonella Larvae
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Distillation of Essential Oils
4.3. GC and GC-MS Analysis of Essential Oils
4.4. Antimicrobial Susceptibility Testing
4.5. Inhibition of Biofilm Formation
4.6. Inhibition of Germ-Tube Formation and the Length of Germinated Cells
4.7. Modulation of Cell Surface Hydrophobicity
4.8. In Vivo Effect of O. majorana Essential Oil on G. mellonella Larvae
4.8.1. Determination of Minimum Lethal Concentration
4.8.2. Survival Assay of G. mellonella Larvae Infected with C. albicans
4.8.3. Determination of Survival Curves and Health Index Score
4.8.4. The Effects of O. majorana EO on the G. mellonella Infected with C. albicans
4.8.5. Hemolymph Collection and Preparation of G. mellonella Slides
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef]
- FDA, Food and Drug Administration. Electronic Code of Federal Regulations (eCFR). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.20 (accessed on 18 August 2018).
- Febriani, Y.; Levallois, P.; Gingras, S.; Gosselin, P.; Majowicz, S.E.; Fleury, M.D. The association between farming activities, precipitation, and the risk of acute gastrointestinal illness in rural municipalities of Quebec, Canada: A cross-sectional study. BMC Public Health 2010, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Feyaerts, A.F.; Mathé, L.; Luyten, W.; De Graeve, S.; Van Dyck, K.; Broekx, L.; Van Dijck, P. Essential oils and their components are a class of antifungals with potent vapour-phase-mediated anti-Candida activity. Sci. Rep. 2018, 8, 3958. [Google Scholar] [CrossRef]
- Roby, M.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Kirimer, N.; Başer, K.H.C.; Tümen, G. Carvacrol-rich plants in Turkey. Chem. Nat. Compd. 1995, 31, 37–41. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.; Ortega-Ramirez, L.; Leyva, J.; Siddiqui, M.W.; Valenzuela, M.R.C.; Gonzalez-Aguilar, G.; Zavala, J.F.A. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2015, 56, 1717–1727. [Google Scholar] [CrossRef]
- Tabanca, N.; Ozek, T.; Baser, K.H.C.; Tumen, G. Comparison of the essential oils of Origanum majorana L. and Origanum × majoricum Cambess. J. Essent. Oil Res. 2004, 16, 248–252. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Tümen, G.; Sezik, E. Composition of the essential oils of Turkish Origanum species with commercial importance. J. Essent. Oil Res. 1993, 5, 619–623. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kurkcuoglu, M.; Demirci, B.; Ozek, T. The essential oil of Origanum syriacum L. var. sinaicum (Boiss.) Letswaart. Flav. Fragr. J. 2003, 18, 98–99. [Google Scholar] [CrossRef]
- Erdogan, A.; Ozkan, A. Investigation of Antioxidative, Cytotoxic, Membrane-Damaging and Membrane-Protective Effects of The Essential Oil of Origanum majorana and its Oxygenated Monoterpene Component Linalool in Human-Derived Hep G2 Cell Line. Iran. J. Pharm. Res. 2017, 16, 24–34. [Google Scholar] [PubMed]
- do Socorro Barbosa Chaves, R.; Martins, R.; Rodrigues, A.B.L.; de Menezes Rabelo, E.; Farias, A.L.F.; da Conceição Vieira Araújo, C.M.; Sobral, T.F.; Galardo, A.K.R. Larvicidal Evaluation of the Origanum majorana L. Essential Oil against the Larvae of the Aedes aegypti Mosquito. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Ragab, T.I.; El Gendy, A.N.G.; Saleh, I.A.; Esawy, M.A. Chemical Composition and Evaluation of Antimicrobial Activity of the Origanum majorana Essential Oil Extracted by Microwave-assisted Extraction, Conventional Hydro-distillation and Steam distillation. J. Essent. Oil Bear. Plants 2019, 22, 563–573. [Google Scholar] [CrossRef]
- Charai, M.; Mosaddak, M.; Faid, M. Chemical Composition and Antimicrobial Activities of Two Aromatic Plants: Ori-ganum majorana L. and O. compactum Benth. J. Essent. Oil Res. 1996, 8, 657–664. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2020, 265, 113318. [Google Scholar] [CrossRef]
- Khadhri, A.; Bouali, I.; Aouadhi, C.; Lagel, M.-C.; Masson, E.; Pizzi, A. Determination of phenolic compounds by MALDI–TOF and essential oil composition by GC–MS during three development stages of Origanum majorana L. Biomed. Chromatogr. 2019, 33, e4665. [Google Scholar] [CrossRef]
- Aladağ, M.O.; Özcan, M.M.; Ergin, S. Inhibitory effect of some spice essential oils on growth of some gram-negative and gram-positive bacteria and a yeast. J. Food Process. Preserv. 2021, 45, e15264. [Google Scholar] [CrossRef]
- Omara, S.T.; El-Moez, S.I.; Mohamed, A.M. Antibacterial Effect of Origanum majorana L. (Marjoram) and Rosmarinus officinalis L. (Rosemary) Essential Oils on Food Borne Pathogens Isolated from Raw Minced Meat in Egypt. Glob. Vet. 2014, 13, 1056–1064. [Google Scholar]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Al Dhaheri, Y.; Iratni, R. Origanum majorana Essential Oil Triggers p38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimple, P.; Kadam, P.V.; Patil, M.J. Ulcer healing properties of different extracts of Origanum majorana in streptozoto-cin-nicotinamide induced diabetic rats. Asian Pac. J. Trop.Disease 2012, 2, 312–318. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio, G.P.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Cotter, G.; Doyle, S.; Kavanagh, K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol. Med. Microbiol. 2000, 27, 163–169. [Google Scholar] [CrossRef]
- Fallon, J.; Kelly, J.; Kavanagh, K. Galleria mellonella as a Model for Fungal Pathogenicity Testing. Host Fungus Interact. 2012, 845, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Sardi, J.D.C.O.; Polaquini, C.R.; Freires, I.A.; Galvao, L.C.D.C.; Lazarini, J.G.; Torrezan, G.S.; Regasini, L.O.; Rosalen, P.L. Antibacterial activity of diacetylcurcumin against Staphylococcus aureus results in decreased biofilm and cellular adhesion. J. Med. Microbiol. 2017, 66, 816–824. [Google Scholar] [CrossRef]
- Desbois, A.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, C.R.; Schroeder, G.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a Model Organism to Study Legionella pneumophila Infection. J. Vis. Exp. 2013, e50964. [Google Scholar] [CrossRef] [Green Version]
- Tuncsoy, B.S.; Tuncsoy, M.; Gomes, T.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J.; Ozalp, P. Effects of Copper Oxide Nanoparticles on Tissue Accumulation and Antioxidant Enzymes of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2019, 102, 341–346. [Google Scholar] [CrossRef]
- Lionakis, M.S. Drosophila and Galleria insect model hosts New tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Reeves, E.P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Cools, F.; Torfs, E.; Aizawa, J.; Vanhoutte, B.; Maes, L.; Caljon, G.; Delputte, P.; Cappoen, D.; Cos, P. Optimization and Characterization of a Galleria mellonella Larval Infection Model for Virulence Studies and the Evaluation of Therapeutics Against Streptococcus pneumoniae. Front. Microbiol. 2019, 10, 311. [Google Scholar] [CrossRef]
- Karaman, M.; Alvandian, A.; Bahar, H. Galleria mellonella Larva Model in Evaluating the Effects of Biofilm in Candida albicans. Mikrobiyoloji Bulteni 2017, 51, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.M.S.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Yiğit Hanoğlu, D.; Hanoğlu, A.; Güvenir, M.; Süer, K.; Demirci, B.; Başer, K.H.C.; Özkum Yavuz, D. Chemical composition and antimicrobial activity of the essential oil of Sideritis cypria Post endemic in Northern Cyprus. J. Essent. Oil Res. 2017, 29, 228–232. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Yıldız, S.S.; Kiymaci, M.E.; Yazgan, A.N.; Cesur, S.; Erdem, S.A.; Yıldız, S.S. Chemical composition and antimicrobial activity of the commercial Origanum onites L. oil against nosocomial carbapenem resistant extended spectrum beta lactamase producer Escherichia coli isolates. Acta Biol. Hung. 2017, 68, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef] [Green Version]
- Aytaç, E. Comparison Essential Oil Contents Origanum majorana L. Obtained by Clevenger and SFE. HJBC. 2020, 48, 239–244. [Google Scholar]
- Busatta, C.; Vidal, R.; Popiolski, A.; Mossi, A.; Dariva, C.; Rodrigues, M.; Corazza, F.; Corazza, M.L.; Oliveira, J.V.; Cansian, R. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiol. 2008, 25, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.; Nawwar, G. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum. Exp. Toxicol. 2011, 30, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.; Kirimer, N.; Tümen, G. Composition of the Essential Oil of Origanum majorana L. from Turkey. J. Essent. Oil Res. 1993, 5, 577–579. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, C. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Derma Vener 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Lima, I.O.; Pereira, F.D.O.; Oliveira, W.A.D.; Lima, E.D.O.; Menezes, E.A.; Cunha, F.A.; Diniz, M.D.F.F.M. Antifungal activity and mode of action of carvacrol against Candida albicans strains. J. Essent. Oil Res. 2013, 25, 138–142. [Google Scholar] [CrossRef]
- Raut, J.S.; Shinde, R.; Chauhan, N.; Karuppayil, S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2012, 29, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of antioxidant, antimicrobial, cytotoxicity and anti-acetylcholinesterase properties of Tunisian Origanum majorana L. essential oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Sarer, E.; Scheffer, J.J.C.; Janssen, A.M.; Svendsen, A.B. Composition of the essential oil of Origanum majorana grown in different localities in Turkey. In Essential Oils and Aromatic Plants; Svendsen, A.B., Scheffer, J.J.C., Eds.; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar] [CrossRef]
- Hacioglu, M.; Oyardi, O.; Kirinti, A. Oregano essential oil inhibits Candida spp. biofilms. Z. Nat. C 2021, 76, 443–450. [Google Scholar] [CrossRef]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- El-Baz, A.; Mosbah, R.; Goda, R.; Mansour, B.; Sultana, T.; Dahms, T.; El-Ganiny, A. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- McCullough, M.; Ross, B.; Reade, P. Candida albicans: A review of its history, taxonomy, epidemiology, virulence attributes, and methods of strain differentiation. Int. J. Oral Maxillofac. Surg. 1996, 25, 136–144. [Google Scholar] [CrossRef]
- Haynes, K. Virulence of Candida species. Trends Microbiol. 2001, 9, 591–596. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Virulence of Clinical Candida Isolates. Pathogens 2021, 10, 466. [Google Scholar] [CrossRef]
- Midkiff, J.; Borochoff-Porte, N.; White, D.; Johnson, D.I. Small Molecule Inhibitors of the Candida albicans Budded-to-Hyphal Transition Act through Multiple Signaling Pathways. PLoS ONE 2011, 6, e25395. [Google Scholar] [CrossRef] [Green Version]
- Palmeira-De-Oliveira, A.; Salgueiro, L.; Palmeira-De-Oliveira, R.; De Oliveira, J.M.; Pina-Vaz, C.; Queiroz, J.; Rodrigues, A.G. Anti-Candida Activity of Essential Oils. Mini Rev. Med. Chem. 2009, 9, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Bujdáková, H.; Didiášová, M.; Drahovská, H.; Černáková, L. Role of cell surface hydrophobicity in Candida albicans biofilm. Open Life Sci. 2013, 8, 259–262. [Google Scholar] [CrossRef]
- Vertyporokh, L.; Wojda, I. Immune response of Galleria mellonella after injection with non-lethal and lethal dosages of Candida albicans. J. Invertebr. Pathol. 2020, 170, 107327. [Google Scholar] [CrossRef]
- Katragkou, A.; Kruhlak, M.J.; Simitsopoulou, M.; Chatzimoschou, A.; Taparkou, A.; Cotten, C.J.; Paliogianni, F.; Diza-Mataftsi, E.; Tsantali, C.; Walsh, T.J.; et al. Interactions between Human Phagocytes and Candida albicans Biofilms Alone and in Combination with Antifungal Agents. J. Infect. Dis. 2010, 201, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.B.; Eby, J.; Nobile, C.; El Khoury, J.B.; Mitchell, A.P.; Mylonakis, E. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect. 2010, 12, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 22nd ed.; M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Zoric, N.; Horvat, I.; Kopjar, N.; Vucemilovic, A.; Kremer, D.; Tomic, S.; Kosalec, I. Hydroxytyrosol Expresses Antifungal Activity In Vitro. Curr. Drug Targets 2013, 14, 992–998. [Google Scholar] [CrossRef]
- Zuzarte, M.; Goncalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Palazzo de Mello, J.C.; Cortez, D.A.G.; Filho, B.P.D.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimi Crob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesinghe, G.K.; Maia, F.C.; De Oliveira, T.R.; De Feiria, S.N.B.; Joia, F.; Barbosa, J.P.; Boni, G.C.; Sardi, J.D.C.O.; Rosalen, P.L.; Höfling, J.F. Effect of Cinnamomum verum Leaf Essential Oil on Virulence Factors of Candida Species and Determination of the In-Vivo Toxicity with Galleria mellonella Model. Mem. Inst. Oswaldo Cruz 2020, 115, e200349. [Google Scholar] [CrossRef] [PubMed]
- Çim, S.; Altuntaş, H. Anti-oxidative, genotoxic and mutagenic effects of idiobiont, endoparasitoid, Pimpla turionellae L. (Hymenoptera: Ichneumonidae) venom on its host Galleria mellonella L. (Lepidoptera: Pyralidae). Biol. Control 2021, 158, 104595. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Ding, Y.; Yi, Y. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 2016, 48, 297–304. [Google Scholar] [CrossRef] [PubMed]


| RRI a | Compound | % Amount | ||
|---|---|---|---|---|
| OMN | OMC | |||
| 1 | 1015 | Methyl 2-methylbutyrate | 0.1 | 0.2 |
| 2 | 1023 | Methyl isovalerate | - | tr b |
| 3 | 1033 | α-Pinene | 0.5 | 0.3 |
| 4 | 1034 | α-Thujene | 1.2 | 0.8 |
| 5 | 1070 | Camphene | 0.2 | 0.1 |
| 6 | 1107 | β-Pinene | 0.1 | 0.1 |
| 7 | 1122 | Sabinene | 0.1 | - |
| 8 | 1148 | δ-3-Carene | 0.1 | 0.1 |
| 9 | 1158 | β-Myrcene | 1.9 | 1.1 |
| 10 | 1162 | α-Phellandrene | 0.3 | 0.3 |
| 11 | 1162 | α-Terpinene | 1.6 | 0.7 |
| 12 | 1197 | Limonene | 0.3 | 0.2 |
| 13 | 1204 | 1,8-Cineole | 0.2 | 0.1 |
| 14 | 1207 | β-Phellandrene | 0.5 | 0.2 |
| 15 | 1226 | (Z)-β-Ocimene | 0.2 | - |
| 16 | 1242 | γ-Terpinene | 5.1 | 2.0 |
| 17 | 1262 | p-Cymene | 7.1 | 4.8 |
| 18 | 1274 | α-Terpinolene | 0.3 | 0.1 |
| 19 | 1402 | 1-Octene-3-ol | 0.3 | 0.1 |
| 20 | 1425 | trans-Sabinene hydrate | 0.2 | 0.5 |
| 21 | 1482 | Camphor | 0.5 | - |
| 22 | 1499 | Linalool | 0.3 | 0.4 |
| 23 | 1508 | cis-Sabinene hydrate | 0.1 | 0.2 |
| 24 | 1577 | Terpinen-4-ol | 1.1 | 1.0 |
| 25 | 1596 | trans-Dihydrocarvone | 0.1 | 0.2 |
| 26 | 1615 | cis- Dihydrocarvone | - | 0.1 |
| 27 | 1673 | α-Terpineol | 0.1 | 0.7 |
| 28 | 1682 | Borneol | 0.5 | 0.6 |
| 29 | 1715 | Carvone | 0.2 | 0.2 |
| 30 | 1820 | p-Cymen-8-ol | - | 0.1 |
| 31 | 2000 | Caryophyllene oxide | - | 0.1 |
| 32 | 2147 | Thymol | 0.6 | 0.6 |
| 33 | 2186 | Carvacrol | 75.3 | 84.0 |
| Monoterpene hydrocarbons | 19.5 | 10.8 | ||
| Oxygenated monoterpenes | 79.2 | 88.7 | ||
| Sesquiterpene hydrocarbons | - | - | ||
| Oxygenated sesquiterpenes | - | 0.1 | ||
| Others | 0.4 | 0.3 | ||
| Total identified | 99.2 | 99.9 | ||
| Strains | OMN | OMC | Amphotericin B | ||
|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC90 | |
| C. albicans ATCC 90028 | 0.0625 | 0.125 | 0.0625 | 0.125 | 0.01 |
| C. albicans MFBF 10778 * | 0.0625 | 0.50 | 0.0625 | 0.50 | 0.01 |
| C. albicans MFBF 11100 ** | 0.0625 | 0.25 | 0.0625 | 0.25 | 0.01 |
| C. tropicalis ATCC 750 | 0.125 | 0.50 | 0.25 | 0.50 | 0.25 |
| C. krusei ATCC 14243 | 0.125 | 0.50 | 0.25 | 0.50 | 0.25 |
| C. dubliniensis MFBF 11098 | <0.0156 | <0.0156 | <0.0156 | <0.0156 | 0.01 |
| Media | OMN | OMC | ||
|---|---|---|---|---|
| 0.0625 µg mL−1 | 0.125 µg mL−1 | 0.0625 µg mL−1 | 0.125 µg mL−1 | |
| YPG + 10% FBS | 27 ± 2.94 | 38 ± 5.04 | 54 ± 4.36 | 51 ± 1.49 |
| N-Acetyl-D-Glucosamine | 48 ± 1.95 | 60 ± 2.78 | 58 ± 1.35 | 46 ± 2.28 |
| Spider | 81 ± 2.70 * | 75 ± 3.28 * | 83 ± 3.75 * | 83 ± 5.62 * |
| Sample | Concentration (µg mL−1) | Hydrophobicity Index | Inhibition of CSH (%) |
|---|---|---|---|
| OMN | 0.125 | 11.19 | 52.61 * |
| 0.0625 | 10.44 | 58.41 * | |
| OMC | 0.125 | 18.65 | 25.70 |
| 0.0625 | 23.13 | 7.85 | |
| Negative control | - | 25.10 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaskatepe, B.; Aslan Erdem, S.; Ozturk, S.; Safi Oz, Z.; Subasi, E.; Koyuncu, M.; Vlainić, J.; Kosalec, I. Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules 2022, 27, 663. https://doi.org/10.3390/molecules27030663
Kaskatepe B, Aslan Erdem S, Ozturk S, Safi Oz Z, Subasi E, Koyuncu M, Vlainić J, Kosalec I. Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules. 2022; 27(3):663. https://doi.org/10.3390/molecules27030663
Chicago/Turabian StyleKaskatepe, Banu, Sinem Aslan Erdem, Sukran Ozturk, Zehra Safi Oz, Eldan Subasi, Mehmet Koyuncu, Josipa Vlainić, and Ivan Kosalec. 2022. "Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model" Molecules 27, no. 3: 663. https://doi.org/10.3390/molecules27030663
APA StyleKaskatepe, B., Aslan Erdem, S., Ozturk, S., Safi Oz, Z., Subasi, E., Koyuncu, M., Vlainić, J., & Kosalec, I. (2022). Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria mellonella Larval Model. Molecules, 27(3), 663. https://doi.org/10.3390/molecules27030663








