Sustainable Diesel from Pyrolysis of Unsaturated Fatty Acid Basic Soaps: The Effect of Temperature on Yield and Product Composition
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Preparation of Unsaturated Fatty Acids
2.3. Preparation of Mixed Metal Hydroxides
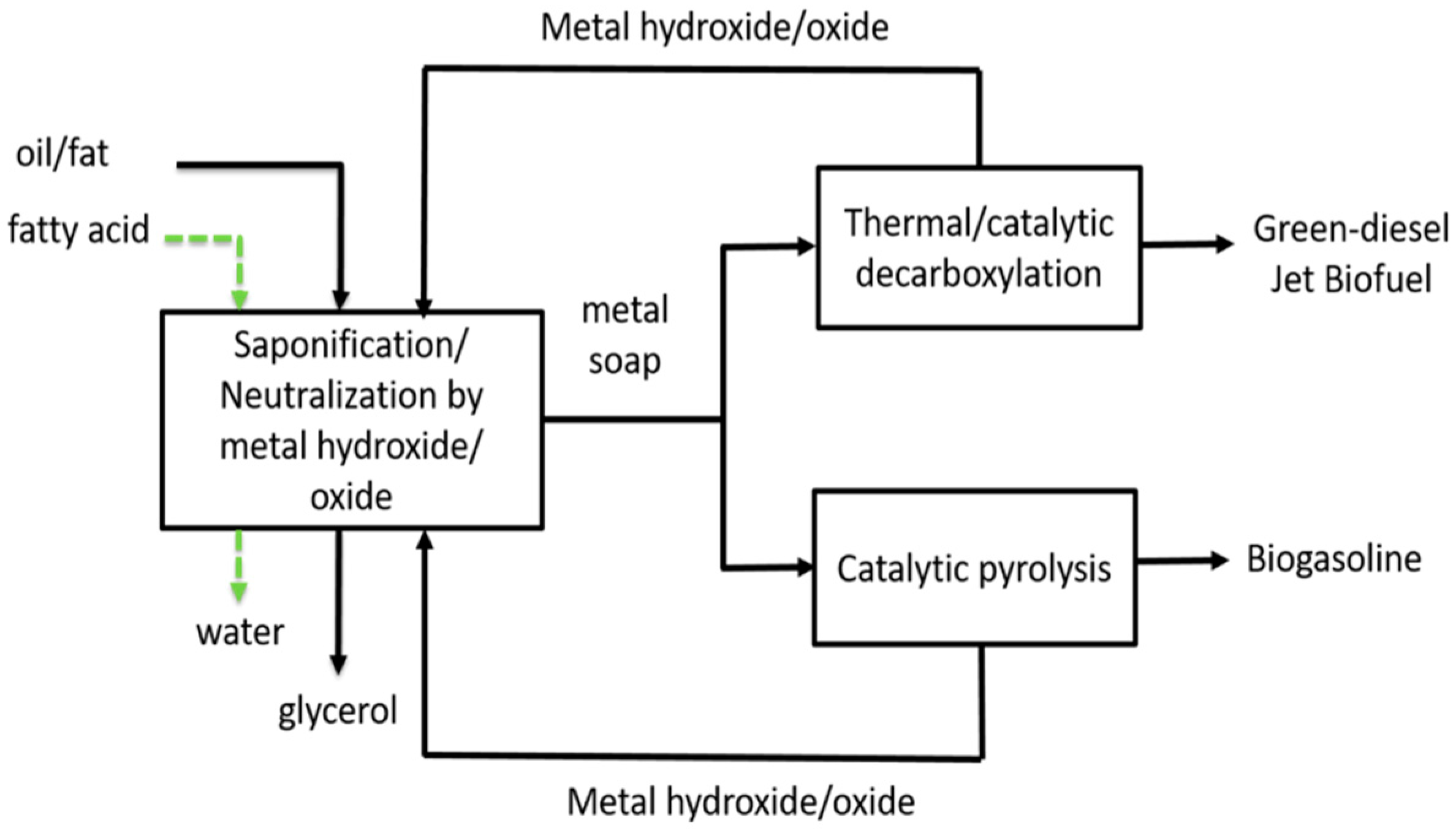
2.4. Preparation of Basic Soap
2.5. Pyrolysis of Basic Soap
2.6. Measurement Values and Units
3. Results and Discussion
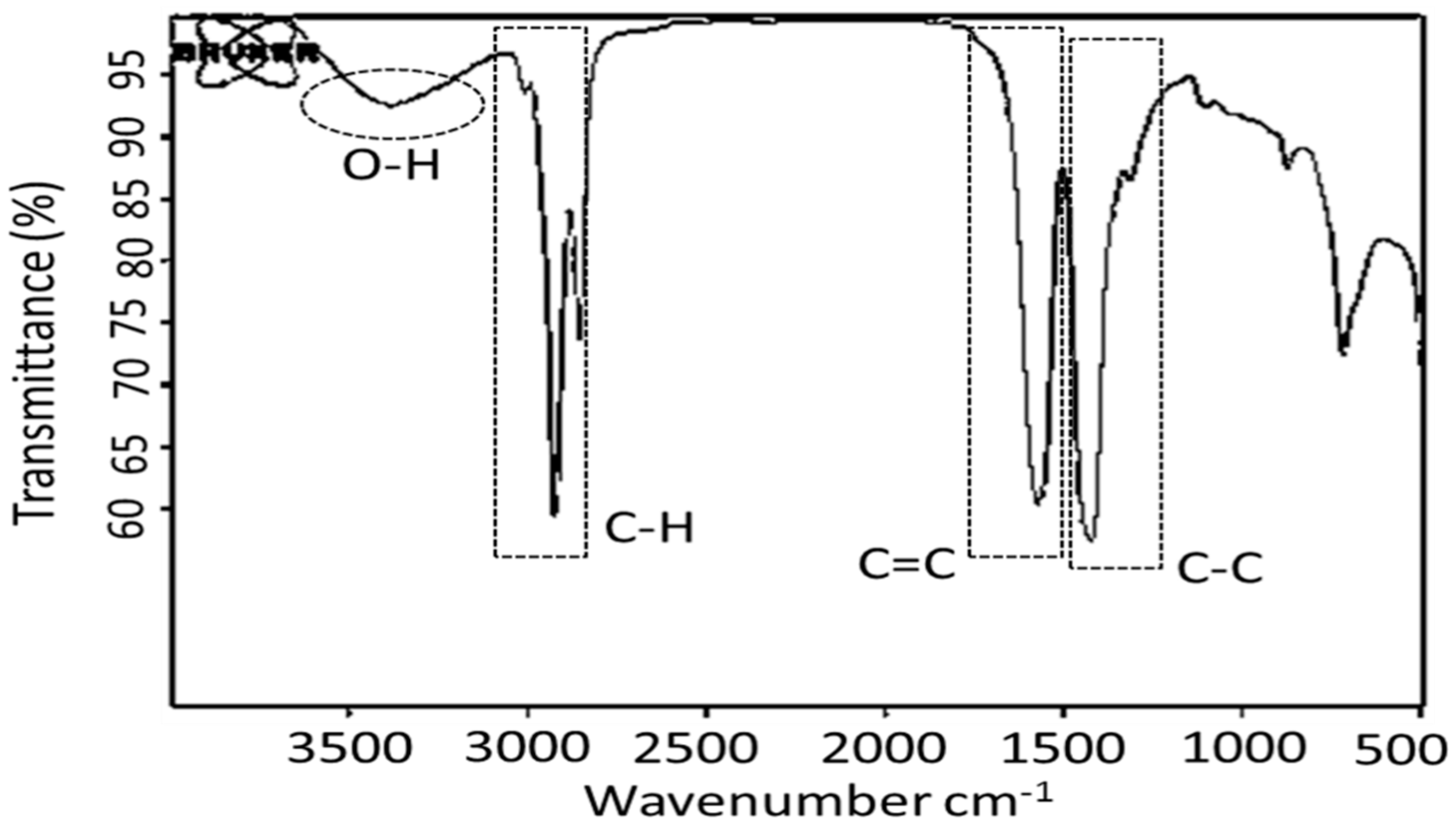
3.1. Unsaturated Fatty Acids and Mixed Metal Basic Soap Analysis
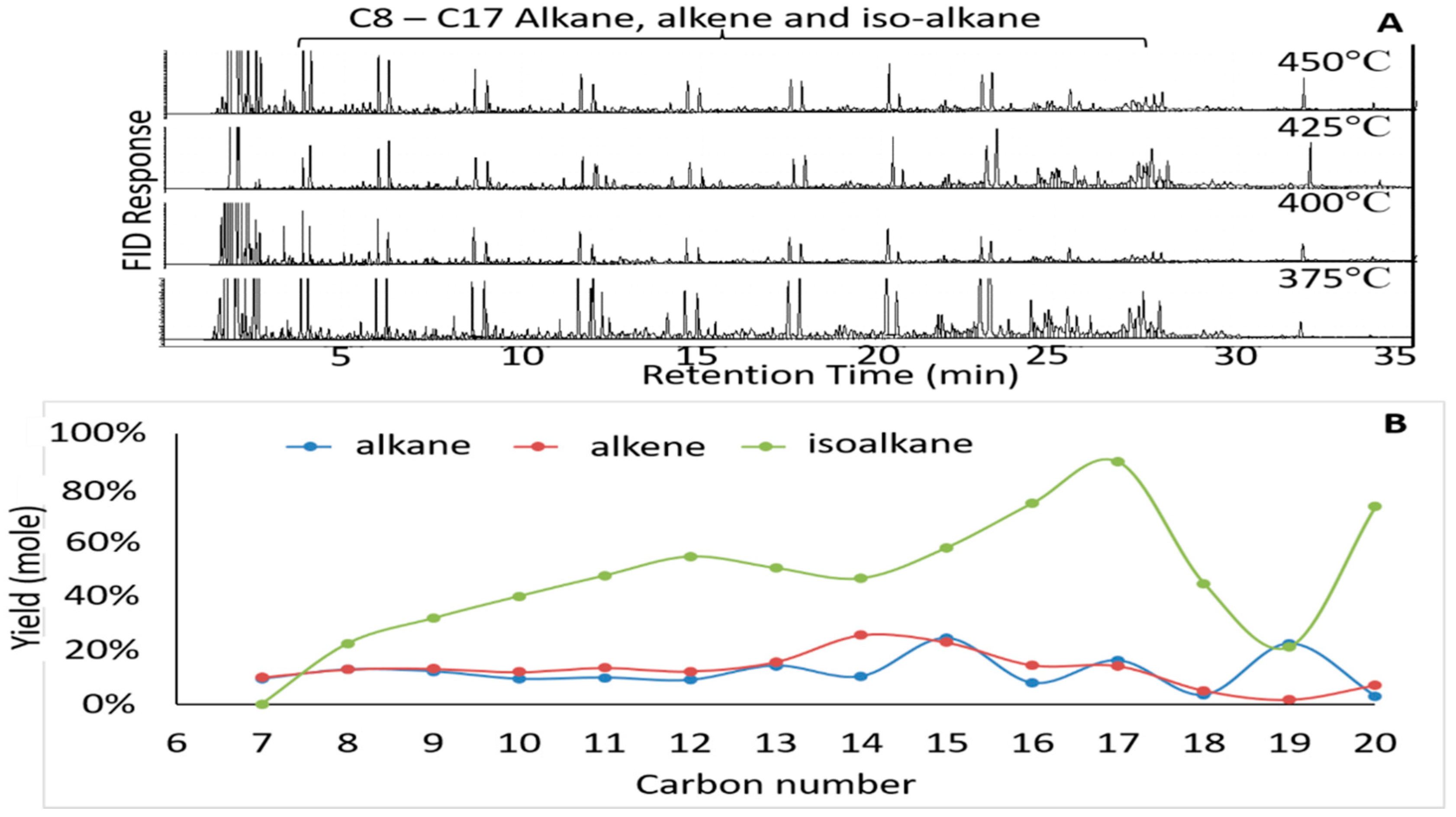
3.2. Identification of Product in the Liquid Fraction
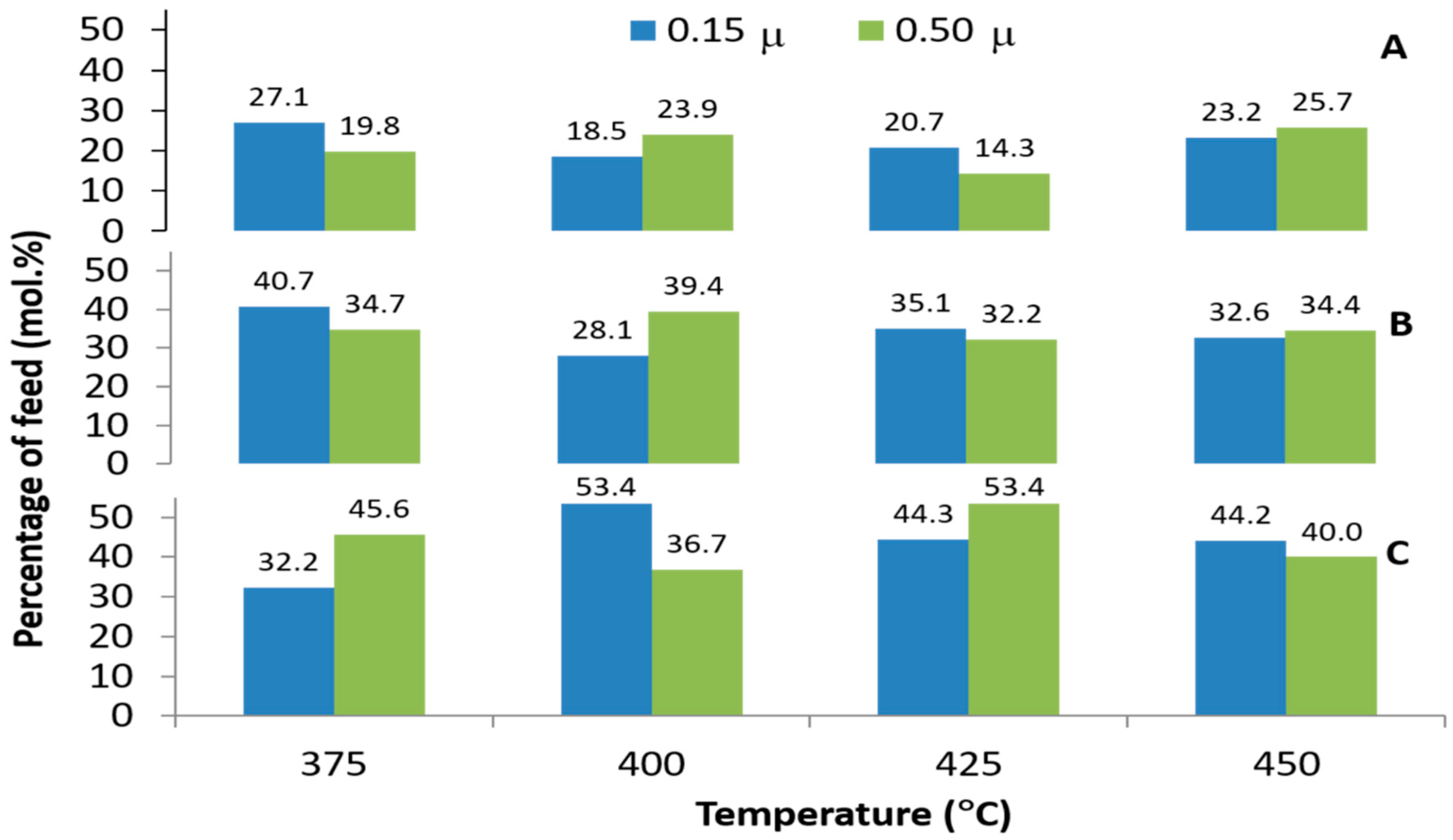
3.3. Effects of Temperature on the Composition of Alkane and Alkene Compounds in the Liquid Products
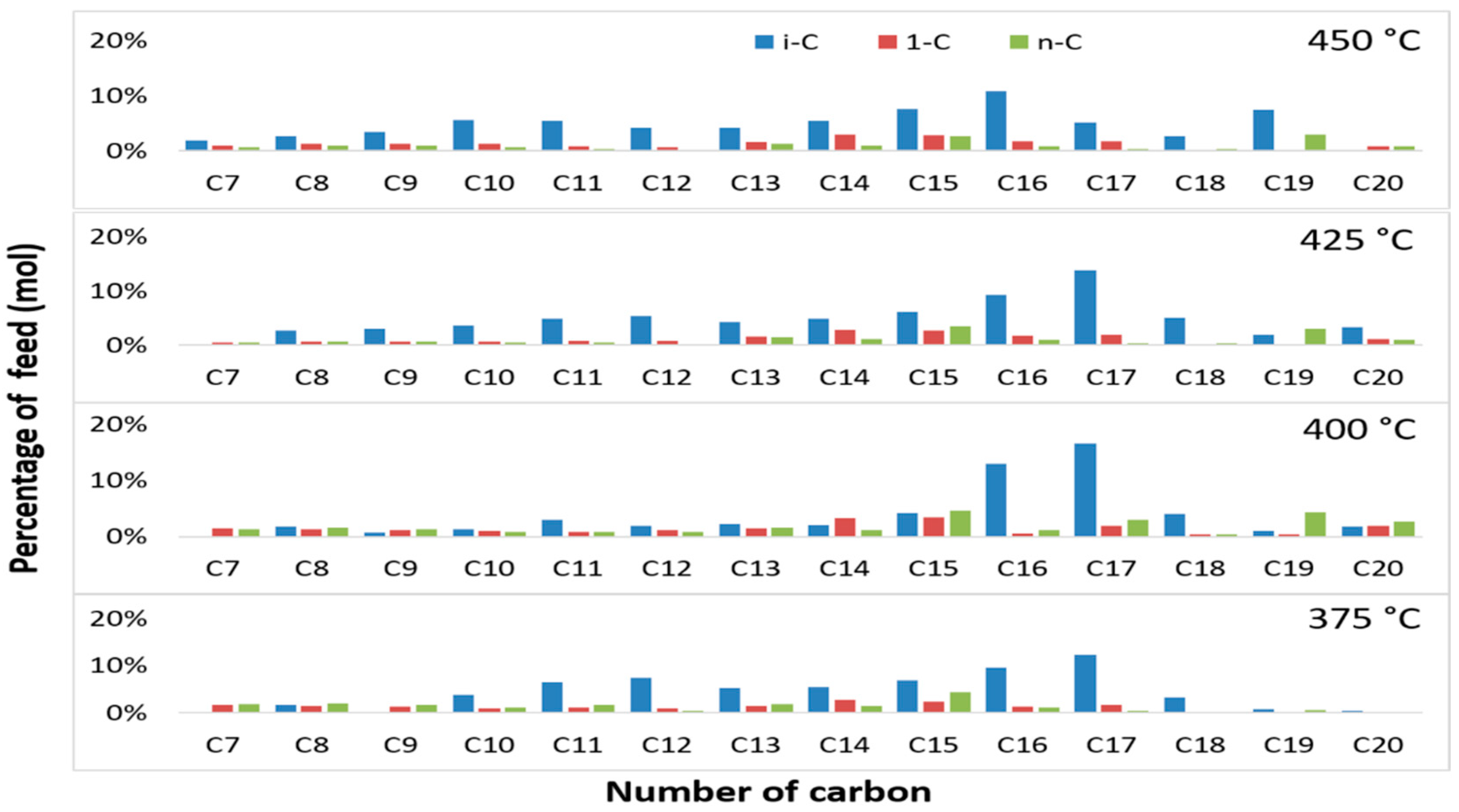
3.4. Effect of Temperature on Iso-Alkane Compounds in the Liquid Products
Metal-oleic basic soap → Heptadecene + Mixed-metal carbonate
Heptadecene + Hydrogen → Heptadecane
Heptadecane → heptadecane isomers
3.5. Effects of Temperature on Yield of Gasoline, Avture and Diesel Hydrocarbon Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neonufa, G.F.; Pratiwi, M.; Soerawidjaja, T.H.; Prakoso, T. High Selectivity of Alkanes Production by Calcium Basic Soap Thermal Decarboxylation. In Proceedings of the 24th Regional Symposium on Chemical Engineering (RSCE), Semarang, Indonesia, 15–16 November 2017; p. C-62. [Google Scholar]
- Cardoso, A.R.T.; Conrado, N.M.; Krause, M.C.; Bjerk, T.R.; Krause, L.C.; Caramão, E.B. Chemical characterization of the bio-oil obtained by catalytic pyrolysis of sugarcane bagasse (industrial waste) from the species Erianthus Arundinaceus. J. Environ. Chem. Eng. 2019, 7, 102970. [Google Scholar] [CrossRef]
- Ghosh, D.; Dasgupta, D.; Agrawal, D.; Kaul, S.; Adhikari, D.; Kurmi, A.K.; Arya, P.K.; Bangwal, D.; Negi, M.S. Fuels and Chemicals from Lignocellulosic Biomass: An Integrated Biorefinery Approach. Energy Fuels 2015, 29, 3149–3157. [Google Scholar] [CrossRef]
- Lesmana, D.; Wu, H.-S. Pyrolysis of waste oil in the presence of a spent catalyst. J. Environ. Chem. Eng. 2015, 3 Pt A, 2522–2527. [Google Scholar] [CrossRef]
- Suchamalawong, P.; Pengnarapat, S.; Reubroycharoen, P.; Vitidsant, T. Biofuel preparation from waste chicken fat using coal fly ash as a catalyst: Optimization and kinetics study in a batch reactor. J. Environ. Chem. Eng. 2019, 7, 103155. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- de Morais Araújo, A.M.; de Oliveira Lima, R.; Gondim, A.; Diniz, J.; di Souza, L.; Araujo, A.S. Thermal and catalytic pyrolysis of sunflower oil using AlMCM-41. Renew. Energy 2017, 101, 900–906. [Google Scholar] [CrossRef]
- Naik, S.N.; Goudbc, V.V.; Routb, P.K.; Dalaib, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.; Khalid, M.; Abdullah, E.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 103219. [Google Scholar] [CrossRef]
- Baladincz, P.; Tóth, C.; Hancsók, J. Production of diesel fuel via hydrogenation of rancid lard and gas oil mixtures. In Proceedings of the CHISA 2012—20th International Congress of Chemical and Process Engineering and PRES 2012—15th Conference PRES, Prague, Czech Republic, 25–29 August 2012. [Google Scholar]
- Solymosi, P.; Eller, Z.; Hancsók, J. Motor fuel purpose hydrogenation of used cooking oils. Chem. Eng. Trans. 2013, 35, 1351–1356. [Google Scholar]
- Keskin, A.; Gürü, M.; Altiparmak, D.; Aydin, K. Using of cotton oil soapstock biodiesel–diesel fuel blends as an alternative diesel fuel. Renew. Energy 2008, 33, 553–557. [Google Scholar] [CrossRef]
- Onay, O.; Kockar, O. Slow, fast and flash pyrolysis of rapeseed. Renew. Energy 2003, 28, 2417–2433. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Lu, Y.; Chen, J. Liquid hydrocarbon fuels obtained by the pyrolysis of soybean oils. Bioresour. Technol. 2009, 100, 4867–4870. [Google Scholar] [CrossRef]
- Yigezu, Z.; Muthukumar, K. Catalytic cracking of vegetable oil with metal oxides for biofuel production. Energy Convers. Manag. 2014, 84, 326–333. [Google Scholar] [CrossRef]
- Biswas, S.; Mohanty, P.; Sharma, D. Studies on co-cracking of jatropha oil with bagasse to obtain liquid, gaseous product and char. Renew. Energy 2014, 63, 308–316. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Zainuddin, N.F.; Chong, C.T.; Lee, C.L.; Lam, W.-H.; Poh, S.C.; Lam, S.S. Conversion of waste shipping oil into diesel-like oil via microwave-assisted pyrolysis. J. Environ. Chem. Eng. 2017, 5, 5836–5842. [Google Scholar] [CrossRef]
- Chiappero, M.; Do, P.T.M.; Crossley, S.; Lobban, L.L.; Resasco, D.E. Direct conversion of triglycerides to olefins and paraffins over noble metal supported catalysts. Fuel 2011, 90, 1155–1165. [Google Scholar] [CrossRef]
- Li, H.; Shen, B.; Kabalu, J.; Nchare, M. Enhancing the production of biofuels from cottonseed oil by fixed-fluidized bed catalytic cracking. Renew. Energy 2009, 34, 1033–1039. [Google Scholar] [CrossRef]
- Idem, R.O.; Katikaneni, S.P.R.; Bakhshi, N.N. Thermal Cracking of Canola Oil: Reaction Products in the Presence and Absence of Steam. Energy Fuels 1996, 10, 1150–1162. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Lappi, H.; Alén, R. Production of vegetable oil-based biofuels—Thermochemical behavior of fatty acid sodium salts during pyrolysis. J. Anal. Appl. Pyrolysis 2009, 86, 274–280. [Google Scholar] [CrossRef]
- Santos, M.; Lourenço, R.; de Abreu, D.; Pereira, A.; de Castro, D.; Pereira, M.; Almeida, H.; Mâncio, A.; Lhamas, D.; da Mota, S.; et al. Gasoline-like hydrocarbons by catalytic cracking of soap phase residue of neutralization process of palm oil (Elaeis guineensis Jacq). J. Taiwan Inst. Chem. Eng. 2017, 71, 106–119. [Google Scholar] [CrossRef]
- Hiebert, D.R. Decarboxylation and Hydrogenation of Safflower and Rapeseed Oils and Soaps to Produce Diesel Fuels. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 1985. [Google Scholar]
- Joonwichien, S.; Duangchan, A. The study of preparation of biodiesel from pyrolysis of palm stearin and soap of palm stearin over catalyst. In Proceedings of the International Conference on Green and Sustainable Innovation, Chiang Mai, Thailand, 1 December 2006. [Google Scholar]
- Kaisha, N.G.K.K. A Method of Manucafturing Hydrocarbon Oils from Oils, Fats or Fatty Acids. U.S. Patent No GB175974, 1923. [Google Scholar]
- Hites, R.A.; Biemann, K. Mechanism of ketonic decarboxylation. Pyrolysis of calcium decanoate. J. Am. Chem. Soc. 1972, 94, 5772–5777. [Google Scholar] [CrossRef]
- Kufeld, S.E. Production of Diesel from Safflower Oil by a Soap-Pyrolysis Process. Ph.D. Thesis, Montana State University (MSU), Bozeman, MT, USA, 1 September 1988. [Google Scholar]
- Wenzel, R.N. Fatty Acids, Their Chemistry, Properties, Production and Uses. J. Am. Chem. Soc. 1961, 83, 4303. [Google Scholar]
- Hoerr, C.W. Separation of Oleic Acid from Stearic and Palmitic Acids. U.S. Patent 2.705.723, 21 April 1951. [Google Scholar]
- Zhao, R.; Yin, C.; Zhao, H.; Liu, C. Synthesis, characterization, and application of hydotalcites in hydrodesulfurization of FCC gasoline. Fuel Process. Technol. 2003, 81, 201–209. [Google Scholar] [CrossRef]
- Hirsch, A.; Fleischer, E. Process for the Production of Basic Soaps of Divalent Metals in Powder Form. U.S. Patent 4.927.548, 22 May 1990. [Google Scholar]
- Rogers, R.H.; Blew, W.R. Manufacture of Metal Soaps. U.S. Patent 2.890.232, 31 May 1956. [Google Scholar]
- McAskie, W. Ruminant Feedstuffs, Their Production and Apparatus for Use There. U.S. Patent 4.826.694, 2 May 1989. [Google Scholar]
- Puspawiningtiyas, E.; Pratiwi, M.; Purwadi, R.; Istyami, A.N.; Elizabeth, L.; Prakoso, T.; Subagjo; Soerawidjaja, T.H. The effect of Ca/Mg/Zn mixing ratio on the research octane number of bio-gasoline during basic soap pyrolysis. Heliyon 2021, 7, 08314. [Google Scholar] [CrossRef]
- Pratiwi, M.; Muraza, O.; Neonufa, G.F.; Purwadi, R.; Prakoso, T.; Soerawidjaja, T.H. Production of Sustainable Diesel via Decarboxylation of Palm Stearin Basic Soaps. Energy Fuels 2019, 33, 11648–11654. [Google Scholar] [CrossRef]
- Beis, S.; Onay, Ö.; Koçkar, Ö.M. Fixed-bed pyrolysis of safflower seed: Influence of pyrolysis parameters on product yields and compositions. Renew. Energy 2002, 26, 21–32. [Google Scholar] [CrossRef]
- Neonufa, G.F. Drop-in Fuel Production Technology of Green Diesel and Bioavtur Type via Catalytic Thermal Decarboxylation of Basic Soap Base on Magnesium and Transition Metal Combination. Ph.D. Thesis, Institut Teknologi Bandung, Jawa Barat, Indonesia, 2017. [Google Scholar]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Thermal deoxygenation and pyrolysis of oleic acid. J. Anal. Appl. Pyrolysis 2014, 105, 1–7. [Google Scholar] [CrossRef]
- Puspawiningtiyas, E.; Pratiwi, M.; Neonufa, G.F.; Purwadi, R.; Isyami, A.N.; Elizabeth, L.; Soerawidjaja, T.H.; Subagjo; Prakoso, T. Effect of metal type on basic soap pyrolysis produce bio-gasoline. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012027. [Google Scholar] [CrossRef]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Pyrolysis of polyunsaturated fatty acids. Fuel Process. Technol. 2014, 120, 89–95. [Google Scholar] [CrossRef]
- Fontaine, M.F.; Riordan, M.D.; Jack, R. Treatment of Reformed Hydrocarbons with a Zinc Oxide-Zinc Chromite Catalyst. U.S. Patent 2.967.143, 3 January 1961. [Google Scholar]
- Masudi, A.; Muraza, O. Vegetable Oil to Biolubricants: Review on Advanced Porous Catalysts. Energy Fuels 2018, 32, 10295–10310. [Google Scholar] [CrossRef]
- Shim, J.-O.; Jeong, D.-W.; Jang, W.-J.; Jeon, K.-W.; Jeon, B.-H.; Cho, S.-Y.; Roh, H.-S.; Na, J.-G.; Ko, C.H.; Oh, Y.-K.; et al. Deoxygenation of oleic acid over Ce(1–x)Zr(x)O2 catalysts in hydrogen environment. Renew. Energy 2014, 65, 36–40. [Google Scholar] [CrossRef]
- Maher, K.D.; Kirkwood, K.M.; Gray, M.R.; Bressler, D.C. Pyrolytic Decarboxylation and Cracking of Stearic Acid. Ind. Eng. Chem. Res. 2008, 47, 5328–5336. [Google Scholar] [CrossRef]
- Zhenga, Y.; Wanga, J.; Liua, C.; Lua, Y.; Lina, X.; Lia, W.; Zheng, Z. Efficient and stable Ni-Cu catalysts for ex situ catalytic pyrolysis vapor upgrading of oleic acid into hydrocarbon: Effect of catalyst support, process parameters and Ni-to-Cu mixed ratio. Renew. Energy 2020, 154, 797–812. [Google Scholar] [CrossRef]


| Component | PFAD | UFA |
|---|---|---|
| tetradecanoic acid (C14:0) | 1.7 | 3 |
| hexadecanoic acid (C16:0) | 40.2 | 17.6 |
| 9,12-octadecadienoic acid (C18:2) | 5.2 | 5.4 |
| 9-octadecenoic acid (C18:1) | 40.3 | 59.9 |
| octadecanoic acid (C18:0) | 7.8 | 5.3 |
| methyl 18-methylnonadecanoate | 1.3 | -- |
| Octadecanoic acid, 10-oxo- | -- | 1.9 |
| Methyl 10D-hydroxyoctadecanoate | -- | 2.2 |
| others | 3.6 | 4.7 |
| Temperature (°C) | Yield of Product (wt.%) | ||
|---|---|---|---|
| Liquid Bio-Hydrocarbon | Solid Residues | Others (Include Water and Gas) | |
| 375 | 51.6 | 20.7 | 27.7 |
| 400 | 55.9 | 22.5 | 21.7 |
| 425 | 58.4 | 23.7 | 17.9 |
| 450 | 50.1 | 13.9 | 36.0 |
| Temperature (°C) | Acid Value (mg KOH/100 g Sample) |
|---|---|
| 375 | 0.66 |
| 450 | 0.39 |
| Temperature (°C) | CO2 | CH4 | N2 | O2 |
|---|---|---|---|---|
| 375 | √ | - | - | - |
| 425 | √ | √ | √ | √ |
| 450 | - | - | √ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puspawiningtiyas, E.; Muraza, O.; Devianto, H.; Pratiwi, M.; Subagjo; Prakoso, T.; Krisnawan; Zaki, U.; Elizabeth, L.; Soerawidjaja, T.H.; et al. Sustainable Diesel from Pyrolysis of Unsaturated Fatty Acid Basic Soaps: The Effect of Temperature on Yield and Product Composition. Molecules 2022, 27, 667. https://doi.org/10.3390/molecules27030667
Puspawiningtiyas E, Muraza O, Devianto H, Pratiwi M, Subagjo, Prakoso T, Krisnawan, Zaki U, Elizabeth L, Soerawidjaja TH, et al. Sustainable Diesel from Pyrolysis of Unsaturated Fatty Acid Basic Soaps: The Effect of Temperature on Yield and Product Composition. Molecules. 2022; 27(3):667. https://doi.org/10.3390/molecules27030667
Chicago/Turabian StylePuspawiningtiyas, Endar, Oki Muraza, Hary Devianto, Meiti Pratiwi, Subagjo, Tirto Prakoso, Krisnawan, Usamah Zaki, Lidya Elizabeth, Tatang H. Soerawidjaja, and et al. 2022. "Sustainable Diesel from Pyrolysis of Unsaturated Fatty Acid Basic Soaps: The Effect of Temperature on Yield and Product Composition" Molecules 27, no. 3: 667. https://doi.org/10.3390/molecules27030667
APA StylePuspawiningtiyas, E., Muraza, O., Devianto, H., Pratiwi, M., Subagjo, Prakoso, T., Krisnawan, Zaki, U., Elizabeth, L., Soerawidjaja, T. H., Situmorang, Y. A., & Indarto, A. (2022). Sustainable Diesel from Pyrolysis of Unsaturated Fatty Acid Basic Soaps: The Effect of Temperature on Yield and Product Composition. Molecules, 27(3), 667. https://doi.org/10.3390/molecules27030667







