Abstract
Highly efficient stereoselective syntheses of novel bis(E-2-chlorovinyl) selenides and bis(E-2-bromovinyl) selenides in quantitative yields by reactions of selenium dichloride and dibromide with alkynes were developed. The reactions proceeded at room temperature as anti-addition giving products exclusively with (E)-stereochemistry. The glutathione peroxidase-like activity of the obtained products was estimated and compounds with high activity were found. The influence of substituents in the products on their glutathione peroxidase-like activity was discussed.
1. Introduction
The term “click chemistry” was coined by K. Barry Sharpless, in 1998 and was first fully described by Sharpless, Kolb, and Finn in 2001 [1]. They believe that click chemistry reactions must be wide in scope, give very high yields, and generate only inoffensive byproducts. The required process characteristics include simple reaction conditions, readily available starting materials and reagents, high selectivity and atom economy, and simple product isolation by non-chromatographic methods. The authors also included sulfenyl halides addition reactions to carbon–carbon multiple bonds to the click chemistry [1]. Although selenenyl halides additions were not mentioned, the chemical properties of these reagents are very similar to those of sulfenyl halides, but addition reactions of selenenyl halides often proceed with higher selectivity [2,3,4,5,6].
Organylselenenyl halides are widely used in modern organic synthesis [2,3,4,5,6]. In 2003, we first applied selenium dihalides in the synthesis of organoselenium compounds [7,8,9,10]. It is known that selenium dichloride and dibromide in solutions undergo disproportionation [11,12]. However, freshly prepared in situ from elemental selenium and sulfuryl chloride or bromine, these reagents can be successfully involved in further reactions [7,8,9,10]. Since then, the application of selenium dihalides in organic synthesis is intensively developing and makes it possible to obtain new classes of organoselenium compounds and selenium-containing heterocycles [13,14,15,16,17,18,19,20,21,22,23,24,25,26].
The main methods for preparation of vinyl selenides are based on electrophilic addition of organylselenenyl halides to the triple bond as well as on nucleophilic addition of selenolate or selenide anions to acetylenes. Previously convenient methods for preparation of divinyl selenide and alkyl vinyl selenides from elemental selenium and acetylene were developed at this institute [27,28,29]. Unsubstituted divinyl selenide (1) was obtained in 80% yield from elemental selenium and acetylene in an aqueous solution of potassium hydroxide using tin dichloride as a reducing agent at 105–115 °C for 15 h (3 days, 5 h heating every day) under acetylene pressure (14 atm) in an autoclave (Scheme 1) [27,28].
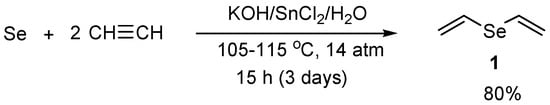
Scheme 1.
The synthesis of divinyl selenide (1) in 80% yield from elemental selenium and acetylene at 105–115 °C for 15 h (3 days, 5 h heating every day) under acetylene pressure in an autoclave.
Vinyl selenides are versatile intermediates and synthons for organic synthesis. A series of valuable products, including functionalized alkenes, ketones, (Z)-allyl alcohols, unsaturated aldehydes, and enyne derivatives, were obtained based on vinyl selenides [30,31,32,33,34,35,36,37,38].
The synthesis of resveratrol and its derivatives was realized in several stages from vinyl selenides [30]. Resveratrol and its methoxylated analogues are well known compounds due to the fact of their anti-inflammatory, anticancer, antibacterial and neuroprotective activity [30]. In addition, the cross-coupling reaction of vinyl selenides with terminal alkynes in the presence of a nickel/CuI catalyst at room temperature leading to (Z)- and (E)-enyne derivatives in good yields with retention of stereochemical configuration is very important [31]. Vinyl selenides, which exhibit antinociceptive [39], hepatoprotective [40], and antioxidant [41] activity, were found.
Previously, we developed efficient synthesis of bis(E-2-chlorovinyl) selenide (2) and bis(E-2-bromovinyl) selenide (3) by electrophilic addition of selenium dihalides to acetylene [42] (Scheme 2). Some acetylene derivatives were also involved in this reaction [43,44,45,46].
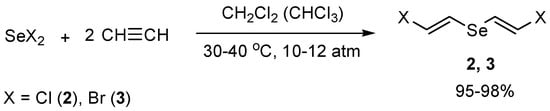
Scheme 2.
The synthesis of bis(E-2-chlorovinyl) selenide (2) and bis(E-2-bromovinyl) selenide (3) by electrophilic addition of selenium dihalides to acetylene.
A number of functionalized organoselenium compounds, including selenium heterocycles, exhibit high glutathione peroxidase-like activity [14,47,48,49,50,51,52,53,54,55,56,57]. Glutathione peroxidase, which contains selenocysteine with the selenol function, is a selenium-containing enzyme that protects human cells by the catalytic reduction of peroxides with the thiol glutathione (the catalytic cycle is shown in Scheme 3) [55,56,57]. The selenol (EnzSeH) is oxidized by peroxides to the corresponding selenenic acid (EnzSeOH), which reacts with thiol glutathione (GSH) to form a selenenyl sulfide intermediate (EnzSeSG). The glutathione then regenerates the active form of the enzyme by the reaction with EnzSeSG to produce the oxidized glutathione GSSG. It was found that a number of organoselenium compounds can act as mimetics of glutathione peroxidase and play the role of catalysts in the reduction of peroxides with the thiols [14,47,48,49,50,51,52,53,54,55,56,57].
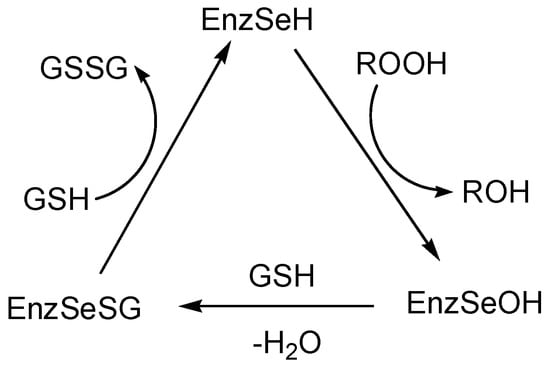
Scheme 3.
The catalytic cycle for the reduction of peroxides with the thiol glutathione (GSH) catalyzed by glutathione peroxidase (EnzSeH).
The organoselenium heterocyclic compound, ebselen, shows neuroprotective, anti-inflammatory, cytoprotective, and glutathione peroxidase-like properties [58,59,60,61,62,63]. Ebselen is used medicinally as an anti-inflammatory agent as well as for prevention of cardiovascular diseases and ischemic stroke. Furthermore, preliminary studies demonstrate that ebselen shows promising inhibitory activity against COVID-19 in cell-based assays [59]. The effect was attributed to irreversible inhibition of the main protease via a covalent bond formation with the thiol group of the active center’s cysteine (Cys-145).
Recently we developed the efficient regio- and stereoselective synthesis of the novel class of divinyl selenides, (Z,Z)-3,3′-selanediylbis(2-propenamides), based on the reaction of sodium selenide with 3-trimethylsilyl-2-propynamides [64]. Like ebselen, these products contain the amide function. The compounds with high glutathione peroxidase-like activity were found among (Z,Z)-3,3′-selanediylbis(2-propenamides) [64].
2. Results and Discussion
The goal of the present work was to develop efficient stereoselective syntheses of bis(2-chloroethyl) selenides and bis(2-bromoethyl) selenides by electrophilic addition of selenium dihalides to dialkylacetylenes (i.e., 2-butyne, 3-hexyne, 4-octyne, and 5-decyne) and the assessment of glutathione peroxidase-like activity of the obtained bis(2-halovinyl) selenides. Evaluation of the glutathione peroxidase-like activity of unsubstituted divinyl selenide 1, bis(E-2-chlorovinyl) selenide 2, and bis(E-2-bromovinyl) selenide 3 was planned, and their activity compared with that of the obtained bis(2-halovinyl) selenides. In addition, synthesis of bis(2-halovinyl) selenoxides by oxidation of corresponding bis(2-halovinyl) selenides was scheduled. The bis(2-halovinyl) selenoxides were supposed to be intermediates in the process of oxidation of dithiothreitol by tert-butyl hydroperoxide on the assessment of glutathione peroxidase-like activity of the obtained bis(2-halovinyl) selenides.
The efficient stereoselective synthesis of bis(E-2-chlorovinyl) selenides (4–7) in quantitative yields was developed by electrophilic addition of selenium dichloride to dialkylacetylenes (i.e., 2-butyne, 3-hexyne, 4-octyne, and 5-decyne). The reaction proceeded in methylene chloride or chloroform at room temperature in a stereoselective fashion as anti-addition producing products exclusively with (E)-stereochemistry (Scheme 4).
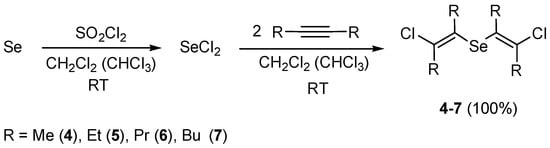
Scheme 4.
The synthesis of bis(2-chlorovinyl) selenides 4–7 by addition of selenium dichloride to dialkylacetylenes (i.e., 2-butyne, 3-hexyne, 4-octyne, and 5-decyne).
Selenium dichloride was freshly prepared in situ from elemental selenium and sulfuryl chloride and immediately involved in further reactions (Scheme 4). Removing the solvent from the reaction mixture followed by drying in vacuum led to pure products 4–7 in quantitative yields.
The reaction of selenium dibromide with 2-butyne, 3-hexyne, 4-octyne, and 5-decyne was realized in a similar manner. Selenium dibromide was produced by mixing elemental selenium and a solution of bromine in methylene chloride or chloroform. After dissolution of the selenium, the obtained solution of selenium dibromide was added dropwise to a solution of dialkylacetylene in methylene chloride or chloroform, and the reaction mixture was stirred for 1–3 h at room temperature. After removing the solvent from the reaction mixture by a rotary evaporator, the residue was dried in vacuum giving bis(E-2-bromovinyl) selenides 8–11 (quantitative yields), which did not require additional purification. The reaction proceeded in a stereoselective mode as anti-addition affording products only with (E)-configuration (Scheme 5).
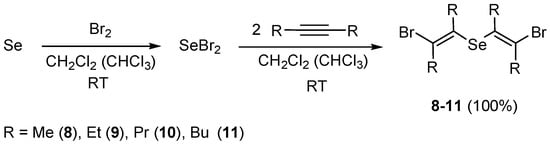
Scheme 5.
The synthesis of bis(2-bromovinyl) selenides 8–11 by addition of selenium dibromide to dialkylacetylenes (i.e., 2-butyne, 3-hexyne, 4-octyne, and 5-decyne).
Divinyl selenide 1 was obtained by a modified procedure in 91% yield from elemental selenium and acetylene in an aqueous solution of potassium hydroxide and hydrazine hydrate at 70–80 °C for 5 h under acetylene pressure in an autoclave (Scheme 6).
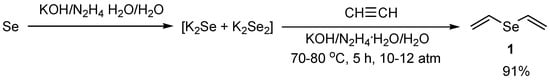
Scheme 6.
The synthesis of divinyl selenide 1 in 91% yield from elemental selenium and acetylene at 70–80 °C for 5 h under acetylene pressure in an autoclave.
The isolation of the target product did not require organic solvents for extraction: the organic phase was simply separated from the reaction mixture by a separatory funnel. This method of carrying out the reaction in water without using organic solvents can be considered as a “green chemistry method”. This procedure is superior to the earlier method [27,28] in the yield of the target product, the duration (5 h instead of 15 h, 3 days) and the temperature of the process (70–80 °C instead of 105–115 °C).
Selenide 1 was used for the glutathione peroxidase-like activity studies, and its activity was compared with that of bis(2-halovinyl) selenides 4–11.
The glutathione peroxidase-like activity of the obtained products was estimated using the model reaction of dithiothreitol oxidation by tert-butyl hydroperoxide (Scheme 7) in the presence of a catalytic number of synthesized compounds as a catalysts (10 mol%) [14,47,48,49,50,51]. The progress of this reaction was monitored by 1H NMR spectroscopy at room temperature (dithiothreitol, 0.07 mmol; tert-butyl hydroperoxide, 0.07 mmol; tested product, 0.007 mmol; deuterochloroform/CD3CD = 95/5, 0.5 mL). The control experiment was conducted under the same reaction conditions but in the absence of the catalyst.
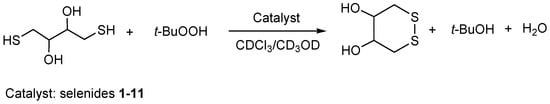
Scheme 7.
The model reaction of dithiothreitol oxidation by tert-butyl hydroperoxide in the presence of a catalytic number of synthesized compounds as catalysts (10 mol%).
It was found that unsubstituted divinyl selenide 1 showed the best activity among the tested selenides (Figure 1). The activity of bis(2-bromovinyl) selenides, in general, exceeds the activity of bis(2-chlorovinyl) selenides (Figure 1 and Figure 2). This trend can be explained in terms of electron density on the selenium atom in divinyl selenide, bis(2-chlorovinyl) selenides, and bis(2-bromovinyl) selenides. We suppose that the electron density on the selenium atom and the presence of electron-withdrawing groups, which are in conjugation with double bonds and an unshared electron pair of the selenium atom, can affect redox processes and manifestation of the glutathione peroxidase-like activity. Since bromine and especially chlorine are electronegative atoms, they can decrease the electron density on the selenium atom in bis(2-chlorovinyl) selenides and bis(2-bromovinyl) selenides. The chlorine atom was superior to the bromine atom in electronegativity and the glutathione peroxidase-like activity of bis(2-bromovinyl) selenides exceeded the activity of chloro-containing selenides (Figure 1 and Figure 2). Unsubstituted divinyl selenide does not have electronegative heteroatoms, and it showed the best activity among the tested selenides.
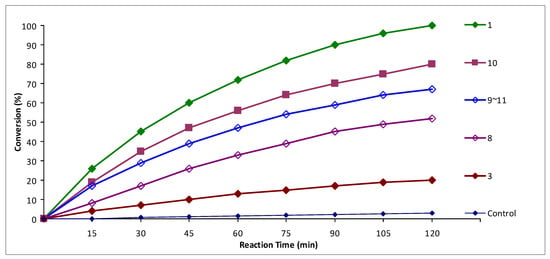
Figure 1.
The assessment of glutathione peroxidase-like activity of divinyl selenide 1 and bis(2-bromovinyl) selenides 3, 8–11 by 1H-NMR monitoring.
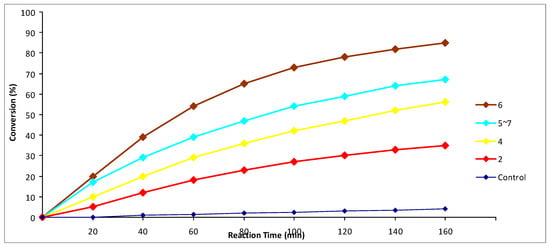
Figure 2.
The assessment of glutathione peroxidase-like activity of bis(2-chlorovinyl) selenides 2, 4–7 by 1H-NMR monitoring.
Another trend, which can be seen based on the obtained data (Figure 1 and Figure 2), was the increase in glutathione peroxidase-like activity with the increasing length of the carbon skeleton in tested molecules. However, in the case of 5-decyne derivatives 7 and 11, XC(Bu) = C(Bu)SeC(Bu) = C(Bu)X, the activity decreased and was lower than the activity of 4-octyne derivatives 6 and 10, XC(Pr) = C(Pr)SeC(Pr) = C(Pr)X (Figure 1 and Figure 2). We assume that the steric factor begins to manifest itself in the latter case, and the selenium atom in the 5-decyne derivative becomes sterically less accessible for redox processes.
It is worth noting that this is the first example of glutathione peroxidase-like activity assessment of divinyl selenide and bis(2-halovinyl) selenides, which do not contain additional heteroatoms.
Bis(2-chlorovinyl) and bis(2-bromovinyl) selenoxides were supposed to be intermediates in the catalytic process of oxidation of dithiothreitol by tert-butyl hydroperoxide on the assessment of glutathione peroxidase-like activity of the corresponding selenides.
The efficient syntheses of novel families of bis(2-chlorovinyl) selenoxides 12–15 (Scheme 8) and bis(2-bromovinyl) selenoxides 16–19 (Scheme 9) in 95–99% yields by oxidation of corresponding selenides with sodium metaperiodate or tert-butyl hydroperoxide were developed. The application of sodium metaperiodate for the oxidation of the selenides made it possible to obtain cleaner products in comparison with the use of tert-butyl hydroperoxide.
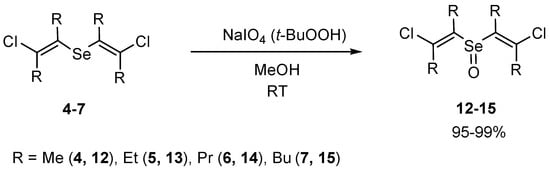
Scheme 8.
The synthesis of bis(2-chlorovinyl) selenoxides 12–15 by oxidation of selenides 4–7.
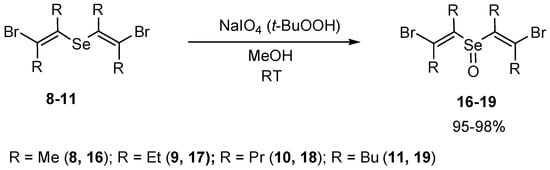
Scheme 9.
The synthesis of bis(2-bromovinyl) selenoxides 16–19 by oxidation of selenides 8–11.
As a rule, compounds with a sulfur−selenium bond are considered as intermediates in the oxidation reactions of thiols by peroxides catalyzed by organoselenium compounds [14,47,48,49,50,51]. In our case, the following scheme can be proposed to explain the catalytic effect of the obtained compounds (Scheme 10). The reaction of the formed selenoxides with dithiothreitol is assumed to lead to the heterocyclic intermediate, which undergoes conversion to the oxidized form of dithiothreitol with regeneration of the catalyst.
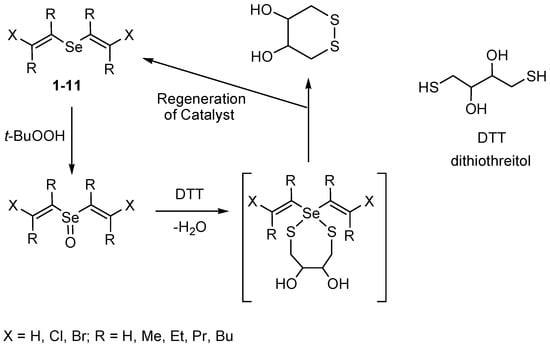
Scheme 10.
A proposed catalytic cycle to explain the catalytic effect of the obtained compounds.
Thus, stereoselective syntheses of novel bis(E-2-chlorovinyl)selenides and bis(E-2-bromovinyl)selenides in quantitative yields by electrophilic addition reactions of selenium dichloride and selenium dibromide to dialkylacetylenes were developed. The glutathione peroxidase-like activity of the obtained products was estimated and compounds with high activity were found.
3. Experimental Section
3.1. General Information
The 1H (400.1 MHz) and 13C (100.6 MHz) NMR spectra (see Supplementary Materials) were recorded on a Bruker DPX-400 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) in CDCl3 solutions and referred to the residual solvent peaks of CDCl3 (δ = 7.27 and 77.16 ppm in 1H- and 13C-NMR, respectively). Elemental analysis was performed on a Thermo Scientific Flash 2000 Elemental Analyzer (Thermo Fisher Scientific Inc., Milan, Italy). The organic solvents were dried and distilled according to standard procedures.
3.2. Synthesis of Selenides
Divinyl selenide (1). Hydrazine hydrate (7 mL) and a cold solution of KOH (85%, 9 g, 0.136 mol) in water (40 mL) were added to selenium powder (7.9 g, 0.1 mol) and the mixture was stirred overnight. The next day, the resulting mixture was heated (70–80 °C) in a 1 L rotating autoclave under the pressure of acetylene (10–12 atm) for 5 h. The lower organic phase was separated from the reaction mixture by a separatory funnel, dried over Na2SO4 and distilled at reduced pressure (85–95 mm Hg) giving divinyl selenide [27,28] (12.1 g, 91% yield), bp 45–47 °C (90–92 mm Hg).
Bis(E-2-chloro-1-methyl-1-propenyl) selenide (4). A solution of selenium dichloride (1 mmol) in methylene chloride (2 mL) was added dropwise to a solution of 2-butyne (108 mg, 2 mmol) in methylene chloride (18 mL). The mixture was stirred for 1 h at room temperature. The solvent was removed by a rotary evaporator and the residue was dried in vacuum giving compound 4 (258 mg) as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 2.49 (s, 6H, CH3), 2.54 (s, 6H, CH3). 13C-NMR (100 MHz): 23.7 (CH3), 26.3 (CH3), 121.8 (CSe, JC-Se 106.4 Hz), 130.7 (CCl). IR (KBr): λ = 2950, 2916, 2848, 1621 (C=C), 1435 cm−1.
Anal. calcd. for C8H12Cl2Se (258.05): C 37.24, H 4.69, Cl 27.48, Se 30.60%. Found: C 37.51, H 4.78, Cl 27.23, Se 30.38%.
Bis(E-2-chloro-1-ethyl-1-butenyl) selenide (5) was obtained under the same conditions as compound 4 from selenium dichloride and 3-hexyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 1.16 (t, 6H, CH3), 1.23 (t, 6H, CH3), 2,48 (q, 4H, CH2), 2.84 (q, 4H, CH2). 13C-NMR (100 MHz): 12.7 (CH3), 13.3 (CH3), 30.0 (CH2), 33.1 (CH2), 128.6 (CSe, JC-Se 106.8 Hz), 137.4 (CCl). IR (KBr): λ = 2971, 2932, 2873, 1606 (C=C), 1458 cm−1.
Anal. calcd for C12H20Cl2Se (314.15): C 45.88, H 6.42, Cl 22.57, Se 25.13%. Found: C 46.13, H 6.56, Cl 22.45, Se 24.80%.
Bis(E-2-chloro-1-propyl-1-pentenyl) selenide (6) was obtained under the same conditions as compound 4 but during 2 h from selenium dichloride and 4-octyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 1.00 (t, 6H, CH3), 1.02 (t, 6H, CH3), 1.58–1.73 (m, 8H, CH2CH2CSe, CH2CH2CCl) 2.38–2.45 (m, 4H, CH2), 2.76–2.81 (m, 4H, CH2). 13C-NMR (100 MHz): 13.2 (CH3), 13.7 (CH3), 21.3 (CH2), 21.4 (CH2), 37.8 (CH2), 40.8 (CH2), 128.3 (CSe, JC-Se 106.4 Hz), 135.9 (CCl). IR (KBr): λ = 2961, 2931, 2871, 1606 (C=C), 1461 cm−1.
Anal. calcd. for C16H28Cl2Se (370.26): C 51.90, H 7.62, Cl 19.15, Se 21.33%. Found: C 51.79, H 7.71, Cl 18.86, Se 21.50%.
Bis(E-2-chloro-1-butyl-1-hexenyl) selenide (7) was obtained under the same conditions as compound 4 but for 2 h from selenium dichloride and 5-decyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 0.91 (t, 6H, CH3), 0.94 (t, 6H, CH3), 1.28–1.40 (m, 8H, CH3CH2), 1.44–1.59 (m, 8H, CH2CH2CSe, CH2CH2CCl) 2.34–2,40 (m, 4H, CH2), 2.70–2.76 (m, 4H, CH2). 13C-NMR (100 MHz): 14.2 (CH3), 22.1 (CH2), 22.5 (CH2), 30.1 (CH2), 30.4 (CH2), 35.8 (CH2), 39.0 (CH2), 128.1 (CSe, JC-Se 107.0 Hz), 135.9 (CCl). IR (KBr): λ = 2957, 2928, 2860, 1607 (C=C), 1463 cm−1.
Anal. calcd. for C20H36Cl2Se (426.36): C 56.34, H 8.51, Cl 16.63, Se 18.52%. Found: C 56.06, H 8.75, Cl 16.42, Se 18.86%.
Bis(E-2-bromo-1-methyl-1-propenyl) selenide (8). A solution of selenium dibromide (1 mmol) in methylene chloride (2 mL) was added dropwise to a solution of 2-butyne (108 mg, 2 mmol) in methylene chloride (18 mL). The mixture was stirred for 1 h at room temperature. The solvent was removed by a rotary evaporator and the residue was dried in vacuum giving compound 8 (347 mg) as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 2.19 (s, 6H, CH3), 2.54 (s, 6H, CH3). 13C-NMR (100 MHz): 27.1 (CH3), 29.2 (CH3), 121.9 (CBr), 123.7 (CSe, JC-Se 107.5 Hz). IR (KBr): λ = 2949, 2914, 2847, 1621 (C=C), 1433 cm−1.
Anal. calcd. for C8H12Br2Se (346.95): C 27.69, H 3.49, Br 46.06, Se 22.76%. Found: C 27.91, H 3.30, Br 45.83, Se 23.02%.
Bis(E-2-bromo-1-ethyl-1-butenyl) selenide (9) was obtained under the same conditions as compound 8 but for 2 h from selenium dibromide and 3-hexyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 1.05 (t, 6H, CH3), 1.10 (t, 6H, CH3), 2,39 (q, 4H, CH2), 2.83 (q, 4H, CH2). 13C-NMR (100 MHz): 11.8 (CH3), 13.2 (CH3), 32.4 (CH2), 34.9 (CH2), 129.3 CBr), 129.7 (CSe, JC-Se 108 Hz). IR (KBr): λ = 2970, 2932, 2873, 1607 (C=C), 1456 cm−1.
Anal. calcd. for C16H28Br2SeO (403.06): C 35.76, H 5.00, Br 39.65, Se 19.59%. Found: C 36.03, H 5.17, Br 39.38, Se 19.34%.
Bis(E-2-bromo-1-propyl-1-pentenyl) selenide (10) was obtained under the same conditions as compound 8 but for 2 h from selenium dibromide and 4-octyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 0.83–0.91 (m, 12H, CH3), 1.37–1.68 (m, 8H, CH2CH2CSe, CH2CH2CBr) 2.46–2.58 (m, 8H, CH2CSe, CH2CBr). 13C-NMR (100 MHz): 13.6 (CH3), 14.2 (CH3), 21.6 (CH2), 22.4 (CH2), 41.3 (CH2), 43.5 (CH2), 128.8 (CSe JC-Se 108 Hz), 130.2 (CCl). IR (KBr): λ = 2960, 2930, 2871, 1604 (C=C), 1460 cm−1.
Anal. calcd. for C16H28Br2SeO (459.16): C 41.85, H 6.15, Br 34.80, Se 17.20%. Found: C 42.03, H 6.27, Br 35.05, Se 16.99%.
Bis(E-2-bromo-1-butyl-1-hexenyl) selenide (11) was obtained under the same conditions as compound 8 but for 3 h from selenium dibromide and 5-decyne as a light yellow oil in quantitative yield.
1H-NMR (400 MHz): 0.92 (t, 6H, CH3), 0.95 (t, 6H, CH3), 1.30–1.39 (m, 8H, CH3CH2), 1.48–1.56 (m, 8H, CH2CH2CSe, CH2CH2CBr) 2.35–2,42 (m, 4H, CH2), 2.80–2.87 (m, 4H, CH2). 13C-NMR (100 MHz): 14.1 (CH3), 14.1 (CH3), 21.9 (CH2), 22.4 (CH2), 30.0 (CH2), 31.0 (CH2), 38.9 (CH2), 41.3 (CH2), 128.5 (CBr), 129.7 (CSe, JC-Se 108.0 Hz). IR (KBr): λ = 2956, 2926, 2859, 1606 (C=C), 1462 cm−1.
Anal. calcd. for C20H36Br2Se (515.27): C 46.62, H 7.04, Br 31.01, Se 15.32%. Found: C 46.34, H 7.00, Br 30.89, Se 15.45%.
3.3. Synthesis of Selenoxides
Bis(E-2-chloro-1-methyl-1-propenyl) selenoxide (12). Sodium metaperiodate (257 mg, 1.2 mmol) was added to a solution of selenide 4 (258 mg, 1 mmol) in absolute methanol (15 mL). The mixture was stirred overnight (16 h) at room temperature. The mixture was filtered. The solvent was removed by a rotary evaporator from the filtrate and the residue was dried in vacuum giving compound 12 (266 mg) as a light yellow oil in 97% yield.
1H-NMR (400 MHz): 1.99 (s, 3H, CH3), 2.00 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.32 (s, 3H, CH3). 13C-NMR (100 MHz): 12.7 (CH3) 24.4 (CH3), 135.1 (CSe, JC-Se 106.4 Hz), 135.6 (CCl). IR (KBr): λ = 2958, 2919, 2852, 1640 (C=C), 1441, 918, 840 (Se=O) cm−1.
Anal. calcd. for C8H12Cl2SeO (274.05): C 35.06, H 4.41, Cl 25.87, Se 28.81%. Found: C 35.34, H 4.57, Cl 25.58, Se 28.61%.
Bis(E-2-chloro-1-ethyl-1-butenyl) selenoxide (13) was obtained under the same conditions as compound 12 by oxidation of selenide 5 as a light yellow oil in 95% yield.
1H-NMR (400 MHz): 1.09 (t, 6H, CH3), 1.19 (t, 6H, CH3), 251–2.75 (m, 8H, CH2CSe, CH2CCl). 13C-NMR (100 MHz): 12.4 (CH3), 13.2 (CH3), 21.1 (CH2), 30.1 (CH2), 140.1 (CSe, JC-Se 119.4 Hz), 142.2 (CCl). IR (KBr): λ = 2975, 2937, 2877, 1623 (C=C), 1458, 913, 842 (Se=O) cm−1.
Anal. calcd. for C12H20Cl2SeO (330.15): C 43.66, H 6.11, Cl 21.48, Se 23.92%. Found: C 43.44, H 5.98, Cl 21.22, Se 24.18%.
Bis(E-2-chloro-1-propyl-1-pentenyl) selenoxide (14) was obtained under the same conditions as compound 12 by oxidation of selenide 6 as a light yellow oil in 96% yield.
1H-NMR (400 MHz): 0.81–0.87 (m, 12H, CH3), 1.33–1.65 (m, 8H, CH2CH2CSe, CH2CH2CCl) 234–2.56 (m, 8H, CH2CSe, CH2CCl). 13C-NMR (100 MHz): 13.0 (CH3), 14.0 (CH3), 20.9 (CH2), 22.0 (CH2), 29.7 (CH2), 39.0 (CH2), 139.8 (CSe, JC-Se 118.6 Hz), 140.8 (CCl). IR (KBr): λ = 2963, 2932, 2873, 1621 (C=C), 1461, 911, 841 (Se=O) cm−1.
Anal. calcd. for C16H28Cl2SeO (386.26): C 49.75, H 7.31, Cl 18.36, Se 20.44%. Found: C 50.03, H 7.45, Cl 18.50, Se 20.26%.
Bis(E-2-chloro-1-butyl-1-hexenyl) selenoxide (15) was obtained under the same conditions as compound 12 by oxidation of selenide 7 as a light yellow oil in 99% yield.
1H-NMR (400 MHz): 0.76–0.82 (m, 12H, CH3), 1.19−1.58 (m, 16H, CH3CH2, CH2CH2CSe, CH2CH2CCl) 2.34–2,52 (m, 8H, CH2CSe, CH2CCl). 13C-NMR (100 MHz): 13.5 (CH3), 13.6 (CH3), 21.8 (CH2), 22.7 (CH2), 27.5 (CH2), 29.7 (CH2), 30.6 (CH2), 37.0 (CH2), 139.8 (CSe, JC-Se 120.8 Hz), 140.6 (CCl). IR (KBr): λ = 2959, 2931, 2872, 1620 (C=C), 1464, 909, 837 (Se=O) cm−1.
Anal. calcd. for C20H36Cl2SeO (442.36): C 54.30, H 8.20, Cl 16.03, Se 17.85%. Found: C 54.07, H 8.31, Cl 15.92, Se 18.09%.
Bis(E-2-bromo-1-methyl-1-propenyl) selenoxide (16) was obtained under the same conditions as compound 12 by oxidation of selenide 8 as a light yellow oil in 95% yield.
1H-NMR (400 MHz): 2.02 (s, 3H, CH3), 2.03 (s, 3H, CH3), 2.51 (s, 3H, CH3), 2.51 (s, 3H, CH3). 13C-NMR (100 MHz): 15.77 (CH3), 27.0 (CH3), 126.4 (CBr), 137.4 (CSe, JC-Se 107.5 Hz). IR (KBr): λ = 2957, 2918, 2852, 1639 (C=C), 1440, 916, 840 (Se=O) cm−1.
Anal. calcd. for C8H12Br2SeO (362.95): C 26.47, H 3.33, Br 44.03, Se 21.76%. Found: C 26.71, H 3.48, Br 43.88, Se 21.96%.
Bis(E-2-bromo-1-ethyl-1-butenyl) selenoxide (17) was obtained under the same conditions as compound 12 by oxidation of selenide 9 as a light yellow oil in 96% yield.
1H-NMR (400 MHz): 1.09 (t, 6H, CH3), 1.19 (t, 6H, CH3), 2.53–2.72 (m, 8H, CH2CSe, CH2CBr). 13C-NMR (100 MHz): 12.9 (CH3), 13.2 (CH3), 23.5 (CH2), 37.8 (CH2), 134.7 (CBr), 141.7 (CSe, JC-Se 120.6 Hz). IR (KBr): λ = 2974, 2935, 2876, 1621 (C=C), 1457, 915, 843 (Se=O) cm−1.
Anal. calcd. for C12H20Br2SeO (419.05): C 34.39, H 4.81, Br 38.14, Se 18.84%. Found: C 34.15, H 4.84, Br 37.88, Se 19.12%.
Bis(E-2-bromo-1-propyl-1-pentenyl) selenoxide (18) was obtained under the same conditions as compound 12 by oxidation of selenide 10 as a light yellow oil in 97% yield.
1H-NMR (400 MHz): 0.94–1.00 (m, 12H, CH3), 1.45–1.79 (m, 8H, CH2CH2CSe, CH2CH2CBr) 2.46–2.55 (m, 4H, CH2CSe, CH2CBr). 13C-NMR (100 MHz): 12.9 (CH3), 14.0 (CH3), 21.8 (CH2), 21.9 (CH2), 32.1 (CH2), 40.9 (CH2), 133.3 (CBr), 141.8 (CSe, JC-Se 122.0 Hz). IR (KBr): λ = 2962, 2931, 2872, 1620 (C=C), 1460, 912, 840 (Se=O) cm−1.
Anal. calcd. for C16H28Br2SeO (475.16): C 40.44, H 5.94, Br 33.63, Se 16.62%. Found: C 40.21, H 6.13, Br 33.47, Se 16.88%.
Bis(E-2-chloro-1-butyl-1-hexenyl) selenoxide (19) was obtained under the same conditions as compound 12 by oxidation of selenide 11 as a light yellow oil in 98% yield.
1H-NMR (400 MHz): 0.81–0.90 (m, 12H, CH3), 1.22–1.55 (m, 16H, CH3CH2, CH2CH2CSe, CH2CH2CBr) 2.34–2.71 (m, 8H, CH2CSe, CH2CBr). 13C-NMR (100 MHz): 13.6 (CH3), 13.8 (CH3), 21.9 (CH2), 22.8 (CH2), 30.3 (CH2), 30.6 (CH2), 30.7 (CH2), 39.3 (CH2), 134.1 (CBr), 141.0 (CSe, JC-Se 121.7 Hz). IR (KBr): λ = 2959, 2930, 2872, 1618 (C=C), 1463, 909, 837 (Se=O) cm−1.
Anal. calcd. for C20H36Br2SeO (531.27): C 45.22, H 6.83, Br 30.08, Se 14.86%. Found: C 44.87, H 6.93, Br 29.84, Se 15.12%.
4. Conclusions
The stereoselective syntheses of novel bis(E-2-chlorovinyl) and bis(E-2-bromovinyl) selenides in quantitative yields by electrophilic addition reactions of selenium dichloride and selenium dibromide to dialkylacetylenes were developed. The reactions proceeded as anti-addition producing products exclusively with (E)-stereochemistry. The reactions can be regarded as selenium dihalides click chemistry due to the quantitative yields and 100% stereoselectivity.
The glutathione peroxidase-like activity of the obtained products was estimated and compounds with high activity were found. It was revealed that unsubstituted divinyl selenide showed the best activity among the tested selenides. The activity of bis(2-bromovinyl) selenides, in general, exceeds the activity of bis(2-chlorovinyl) selenides. This trend was explained in terms of electron density on the selenium atom in divinyl selenides. The chlorine atom was superior to the bromine atom in electronegativity, and the glutathione peroxidase-like activity of bis(2-bromovinyl) selenides exceeded the activity of chloro-containing selenides. Unsubstituted divinyl selenide does not have electronegative heteroatoms, and it showed the best activity among the tested selenides.
Another observed trend was the increase in the glutathione peroxidase-like activity with the increasing length of the carbon skeleton in the tested molecules from XCH = CHSeCH = CHX to XC(Pr) = C(Pr)SeC(Pr) = C(Pr)X (X = Cl, Br). However, in the case of 5-decyne derivatives 7 and 11, the activity decreased and was lower than the activity of 4-octyne derivatives 6 and 10. The steric factor was assumed to manifest itself in the latter case, and the selenium atom in the 5-decyne derivatives became sterically less accessible for redox processes.
The synthesis of novel families of bis(2-chlorovinyl) selenoxides and bis(2-bromovinyl) selenoxides in 95–99% yields by oxidation of corresponding bis(2-halorovinyl) selenides with sodium metaperiodate was developed. The selenoxides were supposed to be intermediates in the catalytic process of oxidation of dithiothreitol by tert-butyl hydroperoxide.
Supplementary Materials
The following are available online, the NMR spectra of the obtained compounds.
Author Contributions
Methodology and experimental data processing: M.V.M.; conceptualization and the paper preparation: V.A.P.; research experiments: A.A.M.; research experiments: A.G.K.; NMR experiments: S.V.Z.; the data curation and supervision: S.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Baikal Analytical Center SB RAS for providing the instrumental equipment for structural investigations.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Organoselenium Chemistry: Synthesis and Reactions; Wirth, T., Ed.; Wiley-VCH: Weinheim, Germany, 2012; 462 p. [Google Scholar]
- Wirth, T. Organoselenium Chemistry–Modern Developments in Organic Synthesis, Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; Volume 260, p. 260. [Google Scholar]
- Nicolaou, K.C.; Petasi, N.A. Selenium in Natural Products Synthesis; CIS: Philadelphia, PA, USA, 1984; p. 300. [Google Scholar]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon: Oxford, UK, 1986; p. 463. [Google Scholar]
- Back, T.G. Organoselenium Chemistry: A Practical Approach; Oxford University Press: Oxford, UK, 1999; p. 295. [Google Scholar]
- Potapov, V.A.; Amosova, S.V.; Belozerova, O.V.; Albanov, A.I.; Yarosh, O.G.; Voronkov, M.G. Synthesis of 3,6-dihalo-4,4-dimethyl-1,4-selenasilafulvenes. Chem. Heterocycl. Comp. 2003, 39, 549–550. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Milne, J. Selenium dibromide and dichloride in acetonitrile. Polyhedron 1985, 4, 65–68. [Google Scholar] [CrossRef]
- Lamoureux, M.; Milne, J. Selenium chloride and bromide equilibria in aprotic solvents; A 77Se NMR study. Polyhedron 1990, 9, 589–595. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Kurkutov, Y.V.A.; Khabibulina, A.G.; Musalova, M.V.; Amosova, S.V.; Borodina, T.N.; Albanov, A.I. Remarkable alkene-to-alkene and alkene-to-alkyne transfer reactions of selenium dibromide and PhSeBr. Stereoselective addition of selenium dihalides to cycloalkenes. Molecules 2020, 25, 194. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Hopf, H.; Jones, P.G.; Birsa, L.M. Selenium halide-induced bridge formation in [2.2] paracyclophanes. Beilstein J. Org. Chem. 2014, 10, 2550–2555. [Google Scholar] [CrossRef]
- Arsenyan, P. A simple method for the preparation of selenopheno [3,2-b] and [2,3-b]thiophenes. Tetrahedron Lett. 2014, 55, 2527–2529. [Google Scholar] [CrossRef]
- Arsenyan, P.; Petrenko, A.; Belyakov, S. Improved conditions for the synthesis and transformations of aminomethyl selenophenothiophenes. Tetrahedron 2015, 71, 2226–2233. [Google Scholar] [CrossRef]
- Volkova, Y.M.; Makarov, A.Y.; Zikirin, S.B.; Genaev, A.M.; Bagryanskaya, I.Y.; Zibarev, A.V. 3,1,2,4-Benzothiaselenadiazine and related heterocycles. Mendeleev Commun. 2017, 27, 19–22. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Synthesis of 2,6-Dichloro-1,4-thiaselenane from Divinyl Sulfide and Selenium Dichloride. Russ. J. Org. Chem. 2009, 45, 1271–1272. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Synthesis of 4-Bromo-2-bromomethyl-1,3-diselenolane from Selenium Dibromide and Divinyl Selenide. Russ. J. Gen. Chem. 2008, 78, 1990–1991. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Annulation of phenyl propargyl ether with selenium dichloride. Russ. Chem. Bull. Int. Ed. 2010, 60, 767–768. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Rusakov, Y.Y.; Khabibulina, A.G.; Rusakova, I.L.; Amosova, S.V. Stereoselective synthesis of E-2-halovinyl tellanes, ditellanes and selenides based on tellurium tetrahalides, selenium dihalides and alkynes. J. Organomet. Chem. 2018, 867, 300–305. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V. Synthesis of a New Four-Membered Heterocycle by Reaction of Selenium Dichloride with Divinyl Sulfone. Russ. J. Org. Chem. 2010, 46, 1099–1100. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Amosova, S.V. Stereoselective Synthesis of 5-Bromo-2-bromomethyl-1,3-thiaselenolane 1,1-Dioxide by Addition of Selenium Dibromide to Divinyl Sulfone. Russ. J. Gen. Chem. 2010, 80, 1220–1221. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena [3.3.1] bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo [3.3.1] nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Trofimov, B.A.; Potapov, V.A.; Amosova, S.V.; Sinegovskaya, L.M. Reactions of Elemental Selenium with Acetylenes.1. Identification of Products of Reaction of Elemental Selenium with Acetylene. Zhurnal Org. Khimii. 1984, 20, 484–489. (In Russian) [Google Scholar]
- Potapov, V.A.; Gusarova, N.K.; Amosova, S.V.; Kashik, A.S.; Trofimov, B.A. Reactions of Chalcogen with Acetylenes. 2. Reaction of Selenium Metals with Acetylene in the HMPA and DMSO Media. Zhurnal Org. Khimii. 1986, 22, 276–281. (In Russian) [Google Scholar]
- Gusarova, N.K.; Potapov, V.A.; Amosova, S.V.; Trofimov, B.A. Alkylvinyl Selenides from Acetylene, Elemental Selenium and Alkyl Halides. Zhurnal Org. Khimii. 1983, 19, 2477–2480. (In Russian) [Google Scholar]
- Perin, G.; Barcellos, A.M.; Luz, E.Q.; Borges, E.L.; Jacob, R.G.; Lenardão, E.J.; Sancineto, L.; Santi, C. Green Hydroselenation of Aryl Alkynes: Divinyl Selenides as a Precursor of Resveratrol. Molecules 2017, 22, 327. [Google Scholar] [CrossRef]
- Silveira, C.C.; Braga, A.L.; Vieira, A.S.; Zeni, G. Stereoselective Synthesis of Enynes by Nickel-Catalyzed Cross-Coupling of Divinylic Chalcogenides with Alkynes. J. Org. Chem. 2003, 68, 662–665. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef]
- Perin, G.; Alves, D.; Jacob, R.G.; Barcellos, A.M.; Soares, L.K.; Lenardão, E.J. Synthesis of Organochalcogen Compounds using Non-Conventional Reaction Media. Chem. Select 2016, 2, 205–258. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Cella, R.; Jacob, R.G.; da Silva, T.B.; Perin, G. Synthesis and Reactivity of α-Phenylseleno-β-substituted Styrenes. Preparation of (Z)-Allyl Alcohols, (E)-α-Phenyl-α,β-unsaturated Aldehydes and α-Aryl Acetophenones. J. Braz. Chem. Soc. 2006, 17, 1031–1038. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Wolf, L. Iron-Catalyzed Coupling Reactions of Vinylic Chalcogenides with Grignard Reagents. J. Braz. Chem. Soc. 2010, 11, 2138–2145. [Google Scholar] [CrossRef]
- Tingoli, M.; Tiecco, M.; Testaferri, L.; Temperini, A. Alkynyl Phenyl Selenides as Convenient Precursors for the Synthesis of Stereodefined Trisubstituted Alkenes. Tetrahedron 1995, 51, 4691–4700. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Temperini, A.; Bagnoli, L.; Marini, F.; Santi, C. A New Synthesis of α-Phenylseleno γ- and β-Lactones from Terminal Alkynes. Synlett 2003, 655–668. [Google Scholar] [CrossRef]
- Sartori, G.; Neto, J.S.S.; Pesarico, A.P.; Back, D.F.; Nogueiraa, C.W.; Zeni, G. Bis-vinyl selenides obtained via iron (III) catalyzed addition of PhSeSePh to alkynes: Synthesis and antinociceptive activity. Org. Biomol. Chem. 2013, 11, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bortolatto, C.F.; Wilhelma, E.A.; Roman, S.S.; Nogueira, C.W. (E)-2-Benzylidene-4-phenyl-1,3-diselenole ameliorates signals of renal injury induced by cisplatin in rats. J. Appl. Toxicol. 2014, 34, 87–94. [Google Scholar] [CrossRef]
- Gonçalves, L.C.C.; Victória, F.N.; Lima, D.B.; Borba, P.M.Y.; Perin, G.; Savegnago, L.; Lenardão, E.J. CuI/glycerol mediated stereoselective synthesis of 1,2-bis-chalcogen alkenes from terminal alkynes: Synthesis of new antioxidants. Tetrahedron Lett. 2014, 55, 5275–5279. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Stereoselective synthesis of (E,E)-bis(2-halovinyl) selenides and its derivatives based on selenium halides and acetylene. Tetrahedron 2012, 68, 10567–10572. [Google Scholar] [CrossRef]
- Amosova, S.V.; Musalov, M.V.; Martynov, A.V.; Potapov, V.A. Regio- and Stereoselective Addition of Selenium Dihalogenides to Propargyl Halogenides. Russ. J. Gen. Chem. 2011, 81, 1239–1240. [Google Scholar] [CrossRef]
- Musalov, M.V.; Martynov, A.V.; Amosova, S.V.; Potapov, V.A. Stereo- and Regioselective Reaction of Selenium Dichloride and Dibromide with Ethynyl(trimethyl)silane. Russ. J. Org. Chem. 2012, 48, 1571–1573. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Reaction of selenium dichloride with trimethylpropargylsilane. Russ. Chem. Bull. Int. Ed. 2011, 60, 769–770. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P. A Novel Camphor-Derived Selenenamide That Acts as a Glutathione Peroxidase Mimetic. J. Am. Chem. Soc. 1997, 119, 2079–2083. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-C.; Kuhn, H.; Daniliuc, C.-G.; Ivanov, I.; Jones, P.G.; du Mont, W.-W. 5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors. Org. Biomol. Chem. 2010, 8, 828–834. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Remarkable Activity of a Novel Cyclic Seleninate Ester as a Glutathione Peroxidase Mimetic and Its Facile in Situ Generation from Allyl 3-Hydroxypropyl. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-Containing Proteins in Mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021, 11, 3640. [Google Scholar] [CrossRef] [PubMed]
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; p. 563. [Google Scholar]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; p. 189. [Google Scholar]
- Woollins, J.D.; Laitinen, R.S. (Eds.) Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Springer: Berlin/Heidelberg, Germany, 2011; p. 334. [Google Scholar]
- Andreev, M.V.; Potapov, V.A.; Musalov, M.V.; Amosova, S.V. (Z,Z)-Selanediylbis(2-propenamides): Novel class of organoselenium compounds with high glutathione peroxidase-like activity. Regio-and stereoselective reaction of sodium selenide with 3-trimethylsilyl-2-propynamides. Molecules 2020, 25, 5940. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).