Abstract
The strategy of incorporating bioactive inorganic nanomaterials without side effects as osteoinductive supplements is promising for bone regeneration. In this work, a novel biomass nanofibrous scaffold synthesized by electrospinning silica (SiO2) nanoparticles into polycaprolactone/chitosan (PCL/CS) nanofibers was reported for bone tissue engineering. The nanosilica-anchored PCL/CS nanofibrous bioscaffold (PCL/CS/SiO2) exhibited an interlinked continuous fibers framework with SiO2 nanoparticles embedded in the fibers. Compact bone-derived cells (CBDCs), the stem cells derived from the bone cortex of the mouse, were seeded to the nanofibrous bioscaffolds. Scanning electron microscopy and cell counting were used to observe the cell adhesion. The Counting Kit-8 (CCK-8) assay was used. Alkaline phosphatase (ALP), Alizarin red staining, real-time Polymerase Chain Reaction and Western blot tests were performed to confirm the osteogenesis of the CBDCs on the bioscaffolds. The research results demonstrated that the mechanical property of the PCL together with the antibacterial and hydrophilic properties of the CS are conducive to promoting cell adhesion, growth, migration, proliferation and differentiation. SiO2 nanoparticles, serving as bone induction factors, effectively promote the osteoblast differentiation and bone regeneration. This novel SiO2-anchored nanofibrous bioscaffold with superior bone induction activity provides a better way for bone tissue regeneration.
1. Introduction
The regeneration and repair of bone defects are clinical key issues that need to be addressed [1]. In the bone graft regeneration technology, an autograft transplantation technique is a priority selection due to the good biological activity and no immune rejection. However, complications such as tissue infection, necrosis, etc., hinder its widespread application [2,3]. The bone allograft is an alternative strategy but is facing limited application in clinics due to the high cost, disease transmission risk and poor host immune response [4,5].
The development of a synthetic bone graft is an advocated strategy to address the above issues [6]. The emergence of tissue engineering is to inoculate cells on a biological scaffold, where cells can adhere and grow, and finally regenerate and repair bone defects [7]. The key factor of tissue engineering technology is the characteristics of the scaffold materials. An ideal scaffold material should have suitable mechanical properties, biocompatibility, a three-dimensional structure and can promote bone tissue growth [8]. Hydrogel [9], 3D-printed scaffolds [10], nanofibers, nanoparticles and nanofilms are promising scaffolds for tissue engineering [11], which have attracted much attention due to their high surface-area-to-volume ratio, high tensile strength, high permeability associated with high biocompatibility and other characteristics [12]. Moreover, nanofibrous scaffolds exhibit a nanofibers network which can be integrated into biological systems by mimicking the environment of a natural extracellular matrix [13]. Various methods such as electrospinning [14,15], template polymerization [16] and the self-assembly method [17,18] have been used to synthesize nanofibers. Among these, nanofibers prepared by the electrospinning technique exhibit a three-dimensional porous structure and the electrospun nanofibers display similar diameters to the cells [8]. In addition, the obtained electrospun nanofibrous scaffolds loading seed cells and bone induction factors can promote cell adhesion, proliferation and differentiation and simulate an extracellular structure in vitro, which exhibit its unique advantages for a primary cell culture and subculture [19].
Biomass polymers, i.e., polylactic acid [20], collagen [21], chitosan (CS) [22], polycaprolactone (PCL) [23], gelatin [24] and bovine serum albumin [25], have been investigated as the raw materials for electrospinning nanofibrous bioscaffolds in tissue engineering. The CS has a wide application prospect as a scaffold material due to the excellent biocompatibility, antibacterial property, biodegradability and low toxicity. However, the mechanical property and poor plasticity make it difficult to maintain the structural integrity [5]. The PCL presents an excellent mechanical property and processability but a high hydrophobicity and low degradation rate [26,27]. Thus, electrospun nanofibrous scaffolds comprised of CS and PCL are expected to exhibit not only good mechanical properties but also favorable biological activity. Saderi et al. [28] added the natural hydrophilic antimicrobial CS to a PCL scaffold to resolve the poor hydrophilic and antimicrobial properties of the PCL for tissue repair scaffolds. Mahoney et al. [29] studied the influence of the PCL/CS ratio on the performance of tissue repair scaffolds. It is concluded that PCL/CS with the mass ratio of 7:3 presents both mechanical properties and hydrophilic and antibacterial properties, which are more conducive to promoting tissue repair.
The incorporation of the induction factors of bone formation into the scaffolds is an effective strategy to improve the mesenchymal stem cell (MSC) recruiting [19]. Silicon (Si) is an essential element for bone metabolism which triggers the osteogenic differentiation of MSC [30,31]. Silica modification, due to its osteoinduction activity, has become a potential alternative for silicification collagen scaffolds, and the osteogenesis silica composites have been developed [32]. Wang et al. [32] reported the resultant nanosilica-collagen (nSC) composites which possess a native-bone-like porous structure and a nanosilica coating. The osteoinductivity of the nSC scaffolds is strongly dependent on the surface roughness and Si content in the silica coating. Wang et al. [33] fabricated electrospun nanosilicates-doped nanofibers for bone tissue engineering. The results of histological and immunohistochemical assessments revealed that the nanosilicates-enriched nanofibers had a significant potential of ectopic bone formation in vivo, while the pure PCL samples only induced limited osteogenic cues.
Herein, a novel nanofibrous bioscaffold consisting of electrospun PCL/CS nanofibers-encapsulated silica (SiO2) nanoparticles was demonstrated for bone regeneration tissue engineering (Scheme 1). The PCL has good biocompatibility and provides mechanical strength and reduces the inflammation effect [34,35]. CS has good biocompatibility, degradation and absorption, a strong plastic mechanical property and can support the adhesion and differentiation of bone marrow MSCs. SiO2 nanoparticles, a bioactive inorganic nanomaterial without side effects, are selected as the induction factor of the bone formation due to the osteoinduction activity. In addition, the polymeric nanofibers loaded with SiO2 nanoparticles are capable of improving physical and mechanical properties. In addition, the SiO2 surface, which is rich in silanol groups, can effectively improve the surface hydrophilic property and form nano-active silicate materials in body fluids to combine with natural bone tissue. The released Si ions can promote the growth and proliferation of MSCs and the expression of osteogenesis genes. The synthesized PCL/CS/SiO2 bioscaffolds demonstrate high potential for clinical translation in the treatment of bone defects in bone tissue repair engineering.
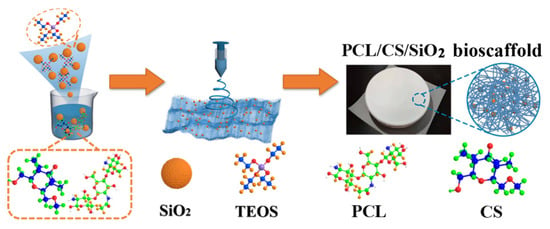
Scheme 1.
The schematic illustration of PCL/CS/SiO2 bioscaffold.
2. Results and Discussion
2.1. Structural and Morphology Characterization of the PCL/CS and PCL/Cs/SiO2 Bioscaffolds
A thermogravimetric analysis (TGA) was tested to confirm the SiO2 content in the electrospun bioscaffolds. Figure 1A shows the TG curves of the PCL/CS and PCL/CS/SiO2 bioscaffolds. The weight loss that occurred from 100 to 500 °C was ascribed to the thermal decomposition of the PCL and CS. According to the TG curves, the content of the SiO2 can be roughly calculated to be around 24.9% in the PCL/CS/SiO2. The DSC heating curve of the PCL/CS/SiO2 bioscaffold is displayed in Figure S1. The melting endothermic peak occurred at a lower temperature than the reported melting temperature (58.12 °C) of the PCL, indicating a low crystallization caused by the addition of SiO2.
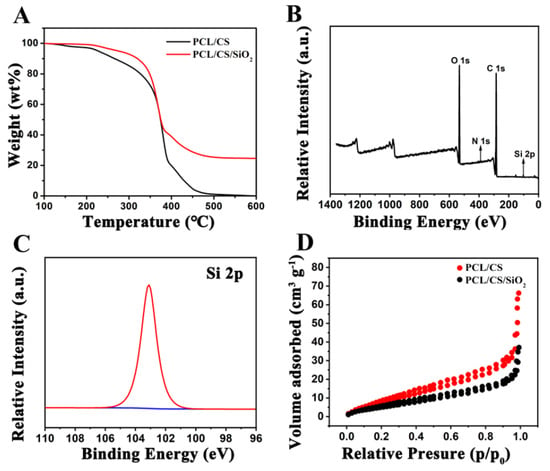
Figure 1.
TG curves (A) of PCL/CS and PCL/CS/SiO2 bioscaffolds, XPS spectra (B) and Si 2p spectrum (C) of PCL/CS/SiO2 bioscaffold, and N2 adsorption–desorption isotherms (D) of PCL/CS and PCL/CS/SiO2 bioscaffolds.
An X-ray photoelectron spectroscopy (XPS) measurement was conducted to confirm the elemental composition and the valence states in the composite. Figure 1B shows the whole spectrum of the PCL/CS/SiO2, indicating the coexistence of Si, C and O elements. The spectrum of Si 2p in Figure 1C shows that the peak located at 103 eV is attributed to the Si-O bonds which are derived from SiO2 nanoparticles [36]. In addition, the spectra of C 1s and O 1s are shown in Figure S2. The FTIR spectra of the PCL/CS and PCL/CS/SiO2 bioscaffolds are shown in Figure S3. The peaks at around 2945 and 2864 cm−1 can be ascribed to the stretching vibrations of -CH2 groups. The C=O and C–O–C stretching vibrational peaks of the PCL appeared at around 1725 and 1158 cm−1, respectively. The characteristic absorption bands corresponding to the bending vibration peak of the N–H of the CS were observed at 1589 cm−1. For the PCL/CS/SiO2 bioscaffold, the Si–O–Si antisymmetric stretching vibration peak at 1091 cm−1 is due to the infrared absorption generated by the Si–O–Si bonds of the SiO2.
The N2 adsorption–desorption properties of the PCL/CS and PCL/CS/SiO2 were analyzed by a BET measurement, and the specific surface areas are illustrated in Table S1. The PCL/CS and PCL/CS/SiO2 bioscaffolds had specific surface areas of 23.94 and 37.68 m2 g−1, which are mainly due to the void space generated by the interlinked electrospun polymer fibers [37]. The N2 adsorption–desorption isotherm curves in Figure 1D display the bioscaffolds that had a distinct hysteresis loop, indicating the existence of a mesoporous structure attributed to the void space derived from the electrospun nanofibers. The porous bioscaffolds are conductive to a better cell-fiber entanglement and provide more cell adhesion sites to increasing cell adhesion, anchoring and growth [38]. The mechanical property of the bone tissue engineering scaffolds is important for the bio-functionality. Figure S4 displays the representative stress–strain curves of the PCL/CS and PCL/CS/SiO2 bioscaffolds in Figure S4. The tensile strength was about 4.98 and 6.01 MPa and the elongation at the break was 38.03 and 23.8% for the PCL/CS and PCL/CS/SiO2 bioscaffolds, respectively. The PCL has excellent flexibility and provides good ductility, while the CS has more charged groups leading to the high breaking strength. In addition, the introduction of SiO2 into the bioscaffold also leads to the increase in breaking strength.
Figure 2A shows the electric photograph of the PCL/CS/SiO2 bioscaffold. It can be seen that the surface of the fiber membrane was smooth, and no liquid drop was seen, confirming a superior fiber-form capability. Figure 2B,C show the images of the water contact angle analysis on the nanofibrous bioscaffolds. It is clear that the PCL/CS and PCL/CS/SiO2 bioscaffolds had a good hydrophilic property with an average contact angle of 70.5°and 51.6°, respectively. Due to the poor hydrophilic and antibacterial properties of the PCL, which are unfavorable for cell adhesion growth, introducing the CS content in hybrid bioscaffolds can significantly improve the hydrophilic nature of the bioscaffold. Moreover, compared with PCL/CS, the PCL/CS/SiO2 bioscaffold presented a better hydrophilicity property due to the hydroxyl group on the surface of the SiO2. The presence of SiO2 embedded in the interlinked fibers improves the hydrophilicity of the scaffold and provides more cell adhesion sites [39]. Figure 2D displays the electrospun PCL/CS fibers that are randomly arranged and exhibit an interlinked continuous fibers framework with the diameter of around 50~100 nm and no apparent droplets are seen. The fibers have a smooth surface and exhibit an interlaced microstructure with apparent mesopores, which can facilitate cells penetrating into the pores of the bioscaffolds. Figure 2E shows the morphology of the SiO2 nanoparticles prepared by the well-established Stöber method. The SiO2 nanoparticles exhibit a uniform sphere morphology with a diameter of around 200 nm. Figure 2F shows that PCL/CS/SiO2 present obvious sphere morphology nanoparticles except for the similar fibers framework with PCL/CS. It can be inferred that SiO2 nanoparticles were successfully anchored into the bioscaffolds. From the enlarged-view SEM image (Figure 2G), it can be seen that the SiO2 nanoparticles were obviously embedded in the interlinked fibers. According to the investigation on the microstructure of the bioscaffolds, it is concluded that the induction factor of the SiO2 nanoparticles is evenly dispersed in the nanofibers, and the bioscaffolds successfully maintain the structural integrity which is conducive to cell growth, proliferation and migration and further promote bone tissue repair.
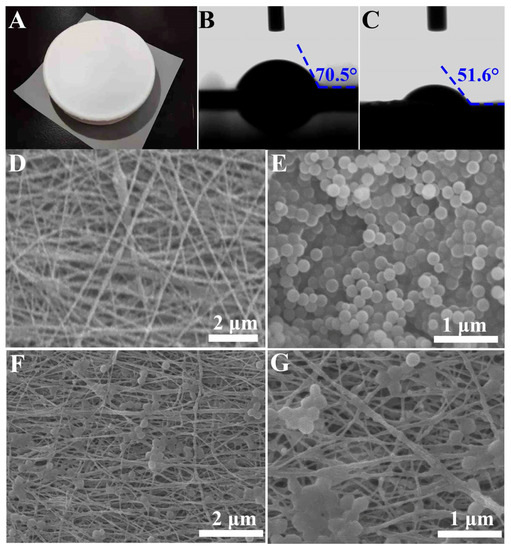
Figure 2.
Electric photograph (A) of PCL/CS/SiO2 bioscaffold, water contact angle images of PCL/CS (B) and PCL/CS/SiO2 (C) bioscaffolds, SEM images of PCL/CS bioscaffold (D), SiO2 nanoparticles synthesized by the well-established Stöber method (E), and SEM images of PCL/CS/SiO2 bioscaffold (F) and the enlarged view (G).
The degradation of the biomass nanofibrous scaffold is a key factor in bone tissue engineering. Therefore, it is of great significance to study the degradation performance of the prepared bioscaffolds for guiding the modification of the nanofibers to meet the different actual needs. The water absorption expansion rate and weight loss ratio of the PCL/CS and PCL/CS/SiO2 bioscaffolds were measured. The water absorption was measured by immersing the PCL/CS and PCL/CS/SiO2 bioscaffolds in a phosphate buffer solution (PBS) for 50 h, and the results are shown in Figure S5. The water absorption ratio increased with prolonged time and reached an equilibrium level after 40 h. Compared with PCL/CS, the PCL/CS/SiO2 bioscaffold has higher water absorption, which may be due to the enhanced hydrophilicity of the hydroxyl group on the surface of the SiO2. In addition, the degradability of the PCL/CS and PCL/CS/SiO2 bioscaffolds was evaluated at pH = 7.4. Figure S6 displays that the weight loss ratio increased as time passed over 10 days. Compared with PCL/CS, the PCL/CS/SiO2 bioscaffold exhibited a higher weight loss ratio, indicating higher biodegradability.
2.2. Biological Tests of PCL/CS and PCL/CS/SiO2 Bioscaffolds
2.2.1. Microphotographs and SEM Images of CBDCs on the PCL/CS and PCL/CS/SiO2 Bioscaffolds
Figure 3 shows the microphotographs and the SEM images of the CBDCs grown on the PCL/CS (Figure 3A,B) and PCL/CS/SiO2 (Figure 3C,D) bioscaffolds for 48 and 96 h, respectively. Compared with PCL/CS, the PCL/CS/SiO2 bioscaffold showed a better adherence with increased cell numbers and a better cell-fiber entanglement. Especially for the PCL/CS/SiO2 bioscaffold after seeding for 48 h, the CBDCs were adhered tightly and stretched well on the fibers of the bioscaffold. The cells, exhibiting a polygonal appearance on the crossed fibers, not only covered the surface but also penetrated into the pores of the fibrous bioscaffolds [40]. After 96 h of culture, the number of cells on the fibers apparently increased and the proliferating cells almost spread over the whole nanofibrous bioscaffolds. The porous electrospun nanofibrous bioscaffolds provide enough three-dimensional void space for the adhesion and proliferation of bone MSCs and deliver oxygen and nutrients through the interconnected pores to the cells on the bioscaffolds [41]. Additionally, the addition of the SiO2 induction factor improves the hydrophilicity of the bioscaffold, enhances the cell proliferation efficiency and reduces the risk of cell contamination, thus providing a better microenvironment for bone tissue regeneration [16].
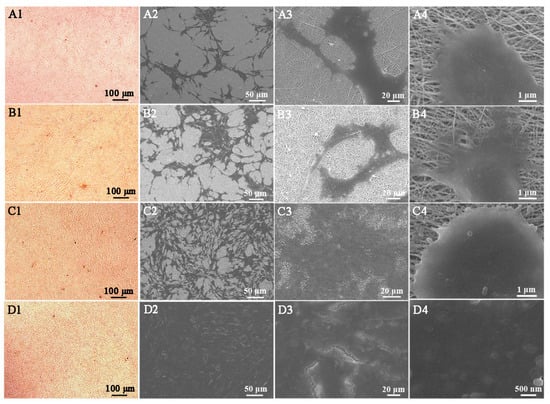
Figure 3.
Microphotographs and the corresponding SEM images of cells on PCL/CS bioscaffold for 48 h (A1–A4), 96 h (B1–B4) and on PCL/CS/SiO2 bioscaffold for 48 h (C1–C4), 96 h (D1–D4).
2.2.2. Cell Adhesion and Proliferation Assay with CBDCs
Figure 4A shows the CBDCs adhesion on the PCL/CS and PCL/CS/SiO2 bioscaffolds. After 4 h of culture, the cells adhesion rates on the surface of the PCL/CS and PCL/CS/SiO2 bioscaffolds were 66.5% and 68.3%, respectively. The cell adhesion rate of the PCL/CS and PCL/CS/SiO2 bioscaffolds increased to 69.1 and 70.4% at 12 h and 75.2 and 83.6% at 24 h, respectively. According to the CCK-8 results in Figure 4B, the CBDCs on PCL/CS/SiO2 showed superior proliferation at days 1, 4 and 7 compared with the PCL/CS bioscaffold. A light microscopic image further confirmed that the CBDCs could adhere to the bioscaffolds on day 4 (Figure 4C–E). This result is in accordance with the adhesion experiment. The cells on the PCL/CS/SiO2 bioscaffold exhibited a better adherence with increased cell numbers and a better cell-fiber entanglement at 24 h, which is perhaps attributed to the enhanced surface with better hydrophilicity and more cell adhesion sites introduced by the presence of the induction factor SiO2 nanoparticles [42,43].
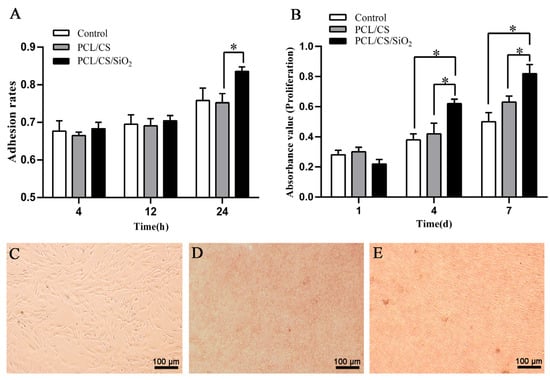
Figure 4.
Cell adhesion and proliferation rates of CBDCs on PCL/CS and PCL/CS/SiO2 bioscaffolds. (A) CBDCs adhesion after 4, 12 and 24 h seeded on the bioscaffolds (* p < 0.05, n = 3). (B) CBDCs proliferation rates on PCL/CS and PCL/CS/SiO2 bioscaffolds at 1, 4 and 7 days (* p < 0.05, n = 3). CBDCs morphology after 4 days seeded on control (C), PCL/CS (D) and PCL/CS/SiO2 (E) bioscaffolds.
2.3. Osteogenic Differentiation and Evaluation
The CBDCs after 24 h seeding on the PCL/CS and PCL/CS/SiO2 bioscaffolds were performed for osteogenic differentiation. The osteogenic activity of the CBDCs cultured was assessed via the ALP activity (Figure 5A). On days 7, 14 and 21, the metabolic activity (OD value) of the CBDCs on the surface of the PCL/CS/SiO2 was higher than that on the PCL/CS bioscaffold. Alizarin red staining was used to measure the osteogenic activity and extracellular matrix (ECM) mineralization of the CBDCs in the PCL/CS and PCL/CS/SiO2 bioscaffolds on day 21 (Figure 5B and Figure S7). Obviously, compared with PCL/CS, the PCL/CS/SiO2 bioscaffold exhibited significantly better osteogenic activity with more calcium nodules stained. A microscopy investigation on the CBDCs after the osteogenic differentiation after 48 and 96 h is shown in Figure S8, indicating osteogenic differentiation. The results confirmed that by introducing the induction factor SiO2 into the PCL/CS bioscaffold, the osteopromoting effect was significantly improved [44].
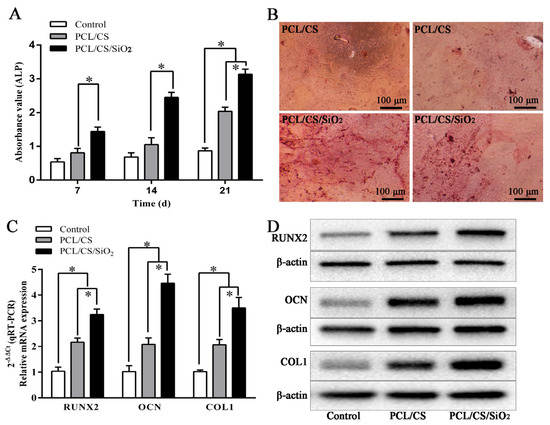
Figure 5.
Osteogenic differentiation, the osteogenic genes and protein expression of CBDCs on the PCL/CS and PCL/CS/SiO2 bioscaffolds. (A) The ALP activity of CBDCs on the bioscaffolds (* p < 0.05, n = 3). (B) Calcium nodules stained on PCL/CS and PCL/CS/SiO2 bioscaffolds by Alizarin red at day 21, n = 3. (C) The RUNX2, OCN and COL1 gene expression of day 4 (* p < 0.05, n = 3). Expression of gene was quantified relative to that of β-actin using a sequence detector software and the relative quantification (2−ΔΔCt) method. (D) The protein expression of RUNX2, OCN and COL1 increased on the bioscaffolds on day 4, n = 3.
Figure 5C shows the osteogenic genes expression evaluated after 4 days of CBDCs cultured on the PCL/CS and PCL/CS/SiO2 bioscaffolds. RUNX2, OCN and COL1 are key transcription factors associated with osteogenic differentiation and biomineralization. The RUNX2, OCN and COL1 gene expression increased on all the bioscaffolds on day 4 (2−ΔΔCt method), and PCL/CS/SiO2 exhibit the maximum upregulated. Meanwhile, Figure 5D and Figure S9 displayed the protein expression of RUNX2, OCN and COL1 on the bioscaffolds on day 4. Apparently, the protein expression levels of PCL/CS/SiO2 are superior to the PCL/CS bioscaffold. Especially, RUNX2, which is closely related to bone formation and controls the bone growth [45], can promote the early differentiation of osteoblasts and is expressed in all stages of osteogenesis [46]. OCN plays an essential role in the differentiation of preosteoblasts into mature osteoblasts [47]. COL1 accounts for more than 90% of the bone matrix, can form a collagen matrix network binding with other proteins and can provide a settling point structure for the deposition of hydroxyapatite [47]. Thus, the superiority expression of PCL/CS/SiO2 achieves facilitating the bone formation of CBDCs growth.
According to the above research results, it is clear that the excellent osteogenic induction ability of the PCL/CS/SiO2 bioscaffold is attributed to its superior morphological and microstructure characteristics [48]. The PCL/CS bioscaffold can be used as the frame of an ECM, while the induction factor SiO2 nanoparticles can regulate the biological functions of CBDCs [49]. The synthesized electrospun PCL/CS/SiO2 bioscaffolds present a three-dimensional porous microstructure with SiO2 nanoparticles uniformly dispersed in the interlinked nanofibers. The electrospun porous nanofibrous structure is similar to a natural bone scaffold and is more conducive to cell growth, proliferation and migration [44]. The introduction of SiO2 into biopolymer nanofibers can support cell attachment, promote cell proliferation, facilitate the induction of new bone formation and integrally enhance the osteoinduction ability.
3. Materials and Methods
3.1. Materials Synthesis and Characterization
Polycaprolactone (PCL, NW = 80,000, Aladdin) and chitosan (CS, Aladdin) were purchased from Sigma-Aldrich. Tetraethoxysilane (TEOS), formic/acetic acid were bought from Sinopharm Group Chemical Co., LTD, Shanghai, China. All chemicals purchased were not further treated after receipt.
3.1.1. Synthesis of PCL/CS and PCL/CS/SiO2 Bioscaffolds
A total of 210 mg PCL and 90 mg CS (mass ratio of 7:3) were dissolved in formic/acetic acid mixed solvent and stirred for 10 h. Electrospinning solution with 10% mass fraction was prepared and the electrospinning process was operated at a voltage of 22 kV with a flow rate of 0.2 mL h−1. The collected sample is denoted as PCL/CS.
SiO2 nanoparticles were prepared by the well-established Stöber method [50]. A certain amount of SiO2 nanoparticles were dispersed in the above electrospinning solution. Electrospinning process was carried out as above. The collected sample is denoted as PCL/CS/SiO2. For the subsequent cell measurement, the bioscaffolds were directly prepared for cell culture in the 24-well plates (Figure S10).
3.1.2. Materials Characterization
The morphology, constitution and physical characteristics of PCL/CS and PCL/CS/SiO2 bioscaffolds were measured by simultaneous thermal analysis (TGA and DSC, TASDT650), Fourier transform infrared spectrum (FTIR, Nicolet iS50, Thermo), X-ray photoelectron spectroscopy (XPS, ESCALab250), Brunauer–Emmett–Teller (BET, 3H-2000PS1), water contact angle analysis (OCA20), optical microscope (XDS-500C), tensile property test (YG (B) 026h-500) and scanning electron microscopy (SEM, JEM-2100F). The water absorption rate was = (Mw − M0)/M0 × 100%, where Mw is absorption mass and M0 is initial mass. The weight loss ratio = (W0 − Wd)/Wd × 100%, where W0 is the weight of the prepared samples, and Wd is the weight of drying at 40 °C after immersing for specified days.
3.2. Cell Measurement
3.2.1. Preparation of Mouse CBDCs
Mouse compact bone-derived cells (CBDCs), the MSCs derived from bone cortex of the mouse, were extracted from male C57BL/6J mice (3 weeks old, Beijing Vital River Laboratory Animal Technology Co., Ltd. Beijing, China) according to the previously published procedures [51,52]. The third passage of CBDCs were used for cell measurement of the electrospun bioscaffolds (Figure S11).
3.2.2. Cell Adhesion and Proliferation
CBDCs were seeded on the surface of the electrospun bioscaffolds. After 4, 12 and 24 h of cell culture, digested cells were counted to calculate the percentage of cell adhesion. The cell adhesion rate was = C/C0 × 100%, where C0 is the number of inoculated cells and C is the number of attached cells.
Cell proliferation was assessed by using the Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) assay. At 1, 4 and 7 days, a microplate reader was applied to measure the absorbance of solution at 450 nm to evaluate the cells’ proliferation.
3.2.3. Osteogenic Induction
Alkaline phosphatase (ALP) activity assay was measured to confirm osteogenic induction. After CBDCs were seeded on the electrospun bioscaffolds for 24 h, an osteogenic induction medium was used for the following osteogenic-related assays and cells were cultured for 21 days. On days 7, 14, 21, a BCIP/NBT color development kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was applied for ALP staining and measured at 405 nm to investigate bone formation potentiality.
Extracellular matrix (ECM) mineralization of CBDCs on the electrospun bioscaffolds were detected by Alizarin red staining (Alizarin Red S, Solarbio, Beijing, China) at 21 days.
The total RNA of CBDCs cultured for 4 days on the bioscaffolds was extracted, the osteogenic gene expression (RUNX2, OCN and COL1) was measured by real-time PCR. The primer sequences are listed in Table S2. Cellular proteins were extracted from CBDCs on the electrospun bioscaffolds and on day 4 accessed Western blotting of osteogenic proteins as previously described methods [53]. Each sample was incubated with primary antibodies: RUNX2 (1:2000; Proteintech, Chicago, IL, USA), OCN (1:2000; Proteintech, Chicago, IL, USA), COL1 (1:1000; Proteintech, Chicago, IL, USA) and β-actin (1:5000; Proteintech, Chicago, IL, USA). The band intensities were quantitated using the Image J software V1.6.0 [54].
4. Conclusions
In summary, we demonstrate a novel bioscaffold for bone tissue regeneration by electrospinning PCL/CS nanofibers anchored with bioactive SiO2 nanoparticles. The electrospun bioscaffolds demonstrate a three-dimensional porous microstructure and make use of the virtues of PCL, CS and the osteoinductive supplement SiO2. The superior microstructure together with the suitable mechanical and hydrophilic properties contributed to the cell adhesion, growth, migration, proliferation and differentiation. The key feature of the bioscaffold is to make use of bioactive SiO2 nanoparticles to improve the osteoblast differentiation and further promote the bone induction activity. In addition, the PCL/CS/SiO2 bioscaffold exhibited biodegradability in a simulated body fluid environment, indicating a future trial in vivo can be expected. Thus, this work provides a novel modified bioscaffold for bone regeneration to meet the requirements for bone tissue engineering.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248832/s1, Figure S1: The DSC heating curve of PCL/CS/SiO2 bioscaffold; Figure S2: The spectrum of C 1s (A) and O 1s (B) of PCL/CS/SiO2 bioscaffold; Figure S3: The FTIR spectra of PCL/CS and PCL/CS/SiO2 bioscaffolds; Figure S4: The stress–strain curves of PCL/CS and PCL/CS/SiO2 bioscaffolds; Figure S5: Water absorption PCL/CS and PCL/CS/SiO2 bioscaffolds as function of immersion time (n = 3); Figure S6: Weight loss ratio of PCL/CS and PCL/CS/SiO2 bioscaffolds as function of breakdown duration within PBS (pH = 7.4) at 37 °C (n = 3); Figure S7: Comparison of calcium nodules staining density after CBDCs osteogenic differentiation on the PCL/CS and PCL/CS/SiO2 bioscaffolds by Alizarin red at day 21, p < 0.05, n = 3; Figure S8: CBDCs after osteogenic differentiation under microscopy investigation after seeding on the bioscaffold of PCL/CS-48 h (A), PCL/CS-96 h (B), PCL/CS/SiO2-48 h (C) and PCL/CS/SiO2-96 h (D); Figure S9: Osteogenic protein expression of CBDCs on the PCL/CS and PCL/CS/SiO2 bioscaffolds on day 4, RUNX2 (A), OCN (B) and COL1 (C), p < 0.05, n = 3; Figure S10: The photos of the bioscaffold prepared for cell culture in the 24-well plates; Figure S11: Mouse compact bone-derived cells (CBDCs). (A) The primary culture of CBDCs. (B) The subculture of CBD1Cs; Table S1: The Brunauer–Emmett–Teller (BET) surface area, pore volume and average pore size of PCL/CS and PCL/CS/SiO2 bioscaffolds; Table S2: Primer sequences for osteogenic genes.
Author Contributions
Conceptualization, Y.F. and J.Y.; methodology, S.G.; software, C.Z. and D.J.; validation, C.D., X.Z. and Y.F.; formal analysis, S.G. and C.Z.; investigation, S.G., Y.F. and J.Y.; resources, W.S.; data curation, S.G. and D.J.; writing—original draft preparation, S.G. and Y.F.; writing—review and editing, X.Z., Y.F. and J.Y.; visualization, X.Z.; supervision, Y.F. and J.Y.; project administration, Y.F. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received the fundings of the Clinical Medicine +X Research Project (grant no. 202101040) and the Youth Foundation (grant no. 201836) of the Affiliated Hospital of Qingdao University, the National Nature Science Foundation of China (grant nos. 81702677) and the Natural Science Foundation of Shandong Province (grant nos. ZR2016HM39).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical Ethics Committee of the Affiliated Hospital of Qingdao University (protocol code AHQU-MAL20220301).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based Strategies for Maxillofacial Tumour Therapy and Bone Defect Regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Favinger, J.L.; Ha, A.S.; Brage, M.E.; Chew, F.S. Osteoarticular Transplantation: Recognizing Expected Postsurgical Appearances and Complications. Radiographics 2015, 35, 780–792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, T.; Ma, J.; Ni, S.; Liu, C.; Wang, Y.; Wu, S.; Liu, J.; Weng, Y.; Zhou, D.; Jimenez-Franco, A.; et al. Attapulgite-doped Electrospun PCL Scaffolds for Enhanced Bone Regeneration in Rat Ranium Defects. Biomater. Adv. 2022, 133, 112656. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52 (Suppl. 2), S18–S22. [Google Scholar] [CrossRef]
- Ghorbani, F.; Ghalandari, B.; Sahranavard, M.; Zamanian, A.; Collins, M.N. Tuning the Biomimetic Behavior of Hybrid Scaffolds for Bone Tissue Engineering Through Surface Modifications and Drug Immobilization. Mater. Sci. Eng. C 2021, 130, 112434. [Google Scholar] [CrossRef]
- Harimtepathip, P.; Callaway, L.F.; Sinkler, M.A.; Sharma, S.; Homlar, K.C. Progressive Osteolysis after Use of Synthetic Bone Graft Substitute. Cureus 2021, 13, e20002. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of Polylactic Acid/Carbon Nanotubes/Chitosan Composite Fibers by Electrospinning for Strawberry Preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional Neuronal Cell Growth and Differentiation on Aligned Polyhydroxyalkanoate Blend Microfibres with Varying Diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, Properties, and Applications of Gelatin-based Hydrogels (GHs) in The Environmental, Technological, and Biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial Effect of 3D Printed Mesoporous Bioactive Glass Scaffolds Doped with Metallic Silver Nanoparticles. Acta Biomater. 2022, 22, 00702–00704. [Google Scholar] [CrossRef]
- García-Valderrama, E.J.; Mamidi, N.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Angel-Sanchez, K.D.; Elías-Zúñiga, A. Engineering and Evaluation of Forcespun Gelatin Nanofibers as an Isorhamnetin Glycosides Delivery System. Pharmaceutics 2022, 6, 1116. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of Carbon Nano-onion Bioconjugates for Biomedical Applications. Mater. Sci. Eng. C 2021, 120, 111698. [Google Scholar] [CrossRef] [PubMed]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/Gelatin/Chitosan/Beta-TCP Electrospun Composite for Guided Bone Regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing Cell Infiltration of Electrospun Fibrous Scaffolds in Tissue Regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering. Int. J. Biomater. 2018, 2018, 1953636. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhong, Z.; Ma, J. Biomimetic Synthesis of Hybrid Hydroxyapatite Nanoparticles Using Nanogel Template for Controlled Release of Bovine Serum Albumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Delaine-Smith, R.M.; Hann, A.J.; Green, N.H.; Reilly, G.C. Electrospun Fiber Alignment Guides Osteogenesis and Matrix Organization Differentially in two Different Osteogenic Cell Types. Front. Bioeng. Biotechnol. 2021, 9, 672959. [Google Scholar] [CrossRef]
- Qu, X.; Wang, M.; Wang, M.; Tang, H.; Zhang, S.; Yang, H.; Yuan, W.; Wang, Y.; Yang, J.; Yue, B. Multi-mode Antibacterial Strategies Enabled by Gene-transfection and Immunomodulatory Nanoparticles in 3D-printed Scaffolds for Synergistic Exogenous and Endogenous Treatment of Infections. Adv. Mater. 2022, 34, e2200096. [Google Scholar] [CrossRef]
- Abarzua-Illanes, P.N.; Padilla, C.; Ramos, A.; Isaacs, M.; Ramos-Grez, J.; Olguin, H.C.; Valenzuela, L.M. Improving Myoblast Differentiation on Electrospun Poly(Epsilon-caprolactone) Scaffolds. J. Biomed. Mater. Res. A 2017, 105, 2241–2251. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three Dimensional Printed Macroporous Polylactic Acid/Hydroxyapatite Composite Scaffolds for Promoting Bone Formation in a Critical-Size Rat Calvarial Defect Model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef]
- Vert, M. Bioabsorbable Polymers in Medicine: An Overview. EuroIntervention 2009, 5 (Suppl. F), F9–F14. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and Comparison of New Galactosylation Methods on PCL/Chitosan Scaffolds for Enhanced Liver Tissue Engineering. Int. J. Biol. Macromol. 2021, 174, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of Nanofibrillated Chitosan into Electrospun PCL Nanofibers Makes Scaffolds with Enhanced Mechanical and Biological Properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Agarwal, T.; Das, J.; Maiti, T.K. Development of Gelatin/Carboxymethyl Chitosan/Nano-hydroxyapatite Composite 3D Macroporous Scaffold for Bone Tissue Engineering Applications. Carbohydr. Polym. 2018, 189, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qu, X.; Zhang, W.; Chen, X.; Zhang, S.; Xu, Y.; Yang, H.; Wang, Y.; Yang, J.; Yuan, W.E.; et al. Photosensitizer Nanodot Eliciting Immunogenicity for Photo-immunologic Therapy of Postoperative Methicillin-resistant Staphylococcus Aureus Infection and Secondary Recurrence. Adv. Mater. 2022, 34, e2107300. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart Electrospun Nanofibers Containing PCL/Gelatin/Graphene Oxide for Application in Nerve Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109768. [Google Scholar] [CrossRef]
- Douglas, P.; Albadarin, A.B.; Sajjia, M.; Mangwandi, C.; Kuhs, M.; Collins, M.N.; Walker, G.M. Effect of Poly Ethylene Glycol on The Mechanical and Thermal Properties of Bioactive Poly(ε-caprolactone) Melt Extrudates for Pharmaceutical Applications. Int. J. Pharm. 2016, 500, 179–186. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and Characterization of Gold Nanoparticle-doped Electrospun PCL/Chitosan Nanofibrous Scaffolds for Nerve Tissue Engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Mahoney, C.; Conklin, D.; Waterman, J.; Sankar, J.; Bhattarai, N. Electrospun Nanofibers of Poly(Epsilon-caprolactone)/Depolymerized Chitosan for Respiratory Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 611–625. [Google Scholar] [CrossRef]
- Rahmani, A.; Hashemi-Najafabadi, S.; Eslaminejad, M.B.; Bagheri, F.; Sayahpour, F.A. The Effect of Modified Electrospun PCL-nHA-nZnO Scaffolds on Osteogenesis and Angiogenesis. J. Biomed. Mater. Res. A 2019, 107, 2040–2052. [Google Scholar] [CrossRef]
- Nie, B.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone Infection Site Targeting Nanoparticle-antibiotics Delivery Vehicle to Enhance Treatment Efficacy of Orthopedic Implant Related Infection. Bioact. Mater. 2022, 16, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Jiang, D.; Zhang, Z.Z.; Chen, Y.R.; Yang, Z.D.; Zhang, J.Y.; Shi, J.; Wang, X.; Yu, J.K. Biomimetic Nanosilica-collagen Scaffolds for in situ Bone Regeneration: Toward a Cell-free, One-step Surgery. Adv. Mater. 2019, 31, e1904341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, W.; Chou, J.; Wen, S.; Sun, Y.; Zhang, H. Electrospun Nanosilicates-based Organic/Inorganic Nanofibers for Potential Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2018, 172, 90–97. [Google Scholar] [CrossRef]
- Douglas, P.; Kuhs, M.; Sajjia, M.; Khraisheh, M.; Walker, G.; Collins, M.N.; Albadarin, A.B. Bioactive PCL Matrices with a Range of Structural & Rheological Properties. React. Funct. Polym. 2016, 101, 54–62. [Google Scholar]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-onions Reinforced Poly (ε-caprolactone) Composite Nanofibers for PH-responsive Drug Release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Zhang, W.; Li, C.; Han, N.; Wang, X.; Li, Q.; Song, G.; Peng, Z.; Li, J.; Zhang, L.; et al. Low-cost Urchin-like Silicon-based Anode with Superior Conductivity for Lithium Storage Applications. J. Colloid Interface Sci. 2020, 575, 150–157. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Using Resorbable Membrane and Different Bone Substitutes: Early Histological and Molecular Events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef]
- Simonet, M.; Stingelin, N.; Wismans, J.G.F.; Oomens, C.W.J.; Driessen-Mol, A.; Baaijens, F.P.T. Tailoring the Void Space and Mechanical Properties in Electrospun Scaffolds towards Physiological Ranges. J. Mater. Chem. B 2014, 2, 305–313. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Tung, K.L.; Lin, Y.L.; Dong, C.D.; Chen, C.W.; Wu, C.H. Modifying Thin-film Composite forward Osmosis Membranes Using Various SiO2 Nanoparticles for Aquaculture Wastewater Recovery. Chemosphere 2021, 281, 130796. [Google Scholar] [CrossRef]
- Hwang, P.T.; Murdock, K.; Alexander, G.C.; Salaam, A.D.; Ng, J.I.; Lim, D.J.; Dean, D.; Jun, H.W. Poly(Varepsilon-Caprolactone)/Gelatin Composite Electrospun Scaffolds with Porous Crater-like Structures for Tissue Engineering. J. Biomed. Mater. Res. A 2016, 104, 1017–1029. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional Porous Gas-foamed Electrospun Nanofiber Scaffold for Cartilage Regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Dong, Y.; Xiao, J.; Gu, R.; Ding, M.; Huang, T.; Li, J.; Zhao, N.; Liao, H. In vivo Immuno-Reactivity Analysis of the Porous Three-dimensional Chitosan/SiO2 and Chitosan/SiO2 /Hydroxyapatite Hybrids. J. Biomed. Mater. Res. A 2018, 106, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly(Epsilon-caprolactone)-Poly(Ethylene Glycol)-Poly(Epsilon-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 735–750. [Google Scholar] [CrossRef]
- Ahmadi, T.; Monshi, A.; Mortazavi, V.; Fathi, M.H.; Sharifi, S.; Kharaziha, M.; Khazdooz, L.; Zarei, A.; Taghian Dehaghani, M. Fabrication and Characterization of Polycaprolactone Fumarate/Gelatin-based Nanocomposite Incorporated with Silicon and Magnesium Co-doped Fluorapatite Nanoparticles Using Electrospinning Method. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110172. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campo, F.M.; Santurtun, A.; Garcia-Ibarbia, C.; Pascual, M.A.; Valero, C.; Garces, C.; Sanudo, C.; Zarrabeitia, M.T.; Riancho, J.A. Osterix and RUNX2 are Transcriptional Regulators of Sclerostin in Human Bone. Calcif. Tissue Int. 2016, 99, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Alves da Silva, M.L.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. The Influence of Patterned Nanofiber Meshes on Human Mesenchymal Stem Cell Osteogenesis. Macromol. Biosci. 2011, 11, 978–987. [Google Scholar] [CrossRef]
- Shaabani, A.; Sedghi, R. Preparation of Chitosan Biguanidine/PANI-containing Self-healing Semi-conductive Waterborne Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2021, 264, 118045. [Google Scholar] [CrossRef]
- Hassan, M.I.; Sultana, N. Characterization, Drug Loading and Antibacterial Activity of Nanohydroxyapatite/Polycaprolactone (nHA/PCL) Electrospun Membrane. 3 Biotech 2017, 7, 249. [Google Scholar] [CrossRef]
- Obaid, M.; Ghouri, Z.K.; Fadali, O.A.; Khalil, K.A.; Almajid, A.A.; Barakat, N.A. Amorphous SiO2 NP-incorporated Poly(Vinylidene Fluoride) Electrospun Nanofiber Membrane for High Flux forward Osmosis Desalination. ACS Appl. Mater. Interfaces 2016, 8, 4561–4574. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, X.; Li, X.; Jia, D.; Li, D.; Ma, Z.; Li, J. Flexible Porous Silicon/Carbon Fiber Anode for High-Performance Lithium-ion Batteries. Materials 2022, 15, 3190. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A Protocol for Isolation and Culture of Mesenchymal Stem Cells from Mouse Compact Bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, X.; Li, N.; Dong, H.; Zhang, Y.; Yoshizawa, M.; Kagami, H. Spontaneously Formed Spheroids from Mouse Compact Bone-derived Cells Retain Highly Potent Stem Cells with Enhanced Differentiation Capability. Stem. Cells Int. 2019, 2019, 8469012. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cao, X.; Zhao, B.; Song, C.; Pang, B.; Hu, L.; Zhang, C.; Wang, J.; He, J.; Wang, S. Nitrate Increases Cisplatin Chemosensitivity of Oral Squamous Cell Carcinoma via REDD1/AKT Signaling Pathway. Sci. China Life Sci. 2021, 64, 1814–1828. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.B.; Frankovsky, R.; Schnick, W. Synthesis of Alkaline Earth Diazenides MAEN2 (MAE = Ca, Sr, Ba) by Controlled Thermal Decomposition of Azides under High Pressure. Inorg. Chem. 2012, 51, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).