Abstract
Realization of the one-pot Staudinger/aza-Wittig/Castagnoli–Cushman reaction sequence for a series of azido aldehydes and homophthalic anhydrides is described. The reaction proceeded at room temperature and delivered novel polyheterocycles related to the natural product realm in high yields and high diastereoselectivity. The methodology has been extended to three other cyclic anhydrides. These further unravel the potential of the Castagnoli–Cushman reaction in generating polyheterocyclic molecular scaffolds.
1. Introduction
Molecular scaffolds are the foundation of a compound’s biological activity [1]. This notion and its importance for drug discovery and the goal of generating drugs leads free from intellectual property liabilities [2] and fuels efforts in synthetic method development defined by Aktitopoulou-Zanze at Abbott as scaffold-oriented synthesis [3]. The major aim of scaffold-oriented synthesis is the deduction of synthetic pathways to novel ring systems, particularly polyheterocyclic ones, taking into account that bioactive compound [4] and natural product [5,6,7] chemical space is heavily populated with polyheterocycles.
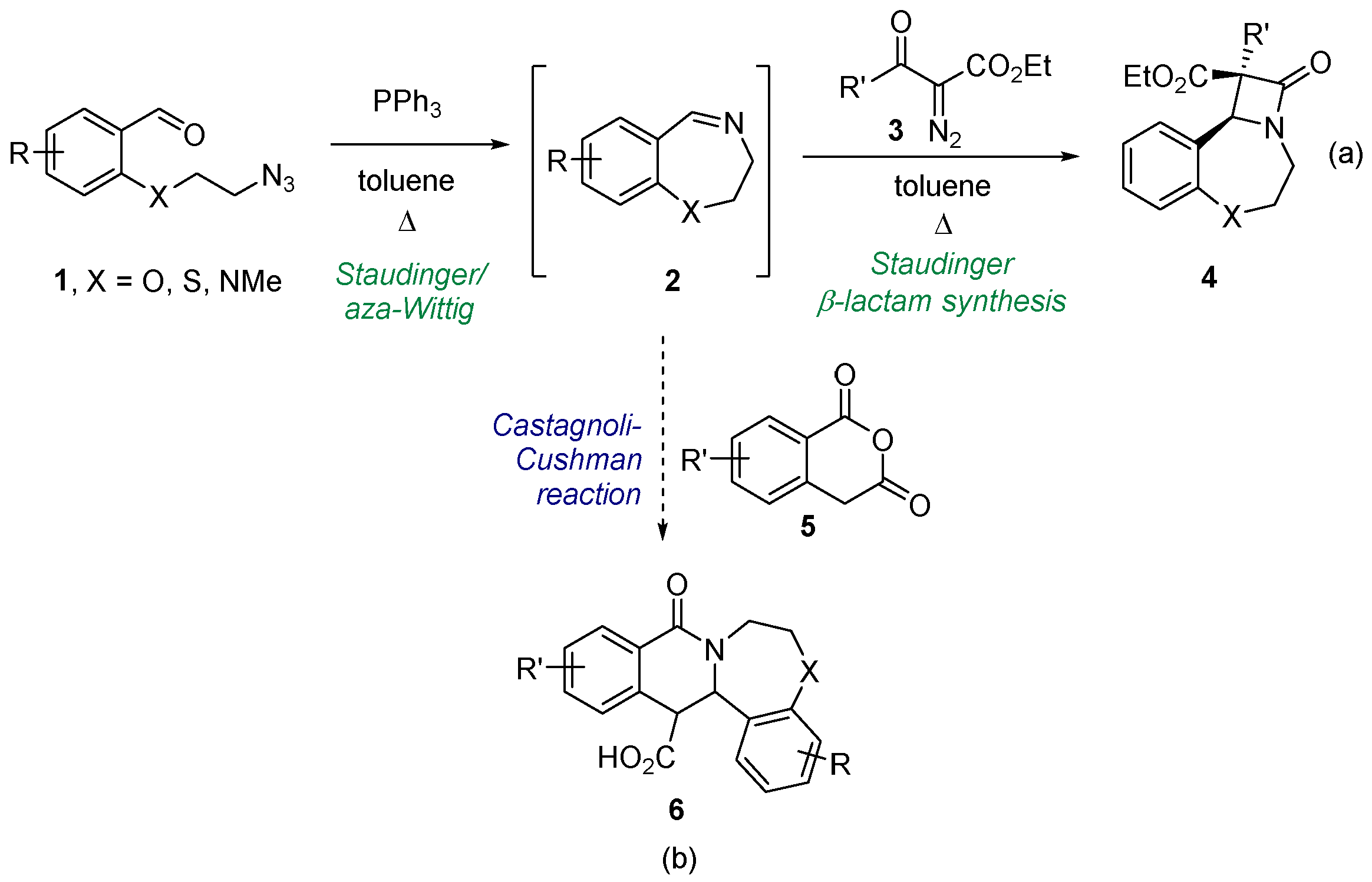
Involvement of a cyclic starting material in a ring-forming process is a proven way to create a polycyclic framework [8]. Such processes are often associated with the creation of new, sometimes multiple, stereocenters, so relying on or discovering diastereoselective approaches to the new cycle formation is key. Recently, we developed a convenient synthetic protocol to transform azide-bearing benzaldehydes 1 into cyclic seven-membered imines 2 via the Staudinger reaction with triphenyl phosphine followed by an intramolecular aza-Wittig reaction [9]. Imines 2 were involved in the Staudinger β-lactam synthesis with ketenes generated from diazo compounds 3. As the result, tricyclic β-lactams 4 fused with tetrahydrothia-, oxa- and diazepine rings were obtained with high diastereoselectivity. Encouraged by this success, we pondered if the same cyclic imine substrates could be coupled to other ring-forming reactions. Our positive recent experience [10,11,12] with the imines (cyclic and acyclic) reacting with cyclic anhydrides via the formal [4+2] cycloaddition dubbed the Castagnoli–Cushman reaction (CCR) [13,14] prompted us to investigate the possibility of employing imines 2 as substrates for the CCR. As the starting point for this investigation, we considered reacting them with homophthalic anhydrides (HPA, 5), one of the most popular and reactive anhydrides that have been involved in the CCR [15,16,17]. This, we reasoned, would provide access to a tetracyclic 6-6-7-6 ring system 6 containing two new stereogenic centers. Considering that the CCR quite often proceeds diastereoselectively [18,19,20,21,22], the choice of the CCR as the means to create the new polyheterocyclic scaffold 6 from imines 2 is quite justified (Figure 1). Herein, we present the results obtained in the course of realizing the synthetic strategy outlined above.
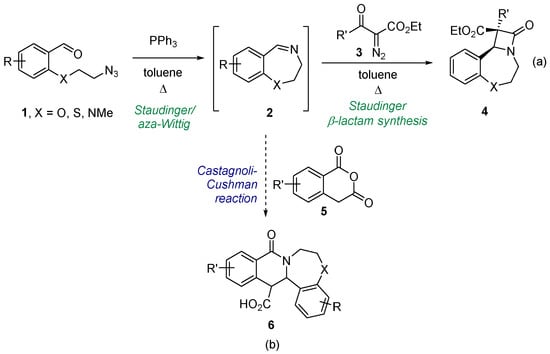
Figure 1.
Synthesis of cyclic imines 2 and their involvement in ring-forming processes: (a) recently reported and (b) investigated in this work.
2. Results and Discussion
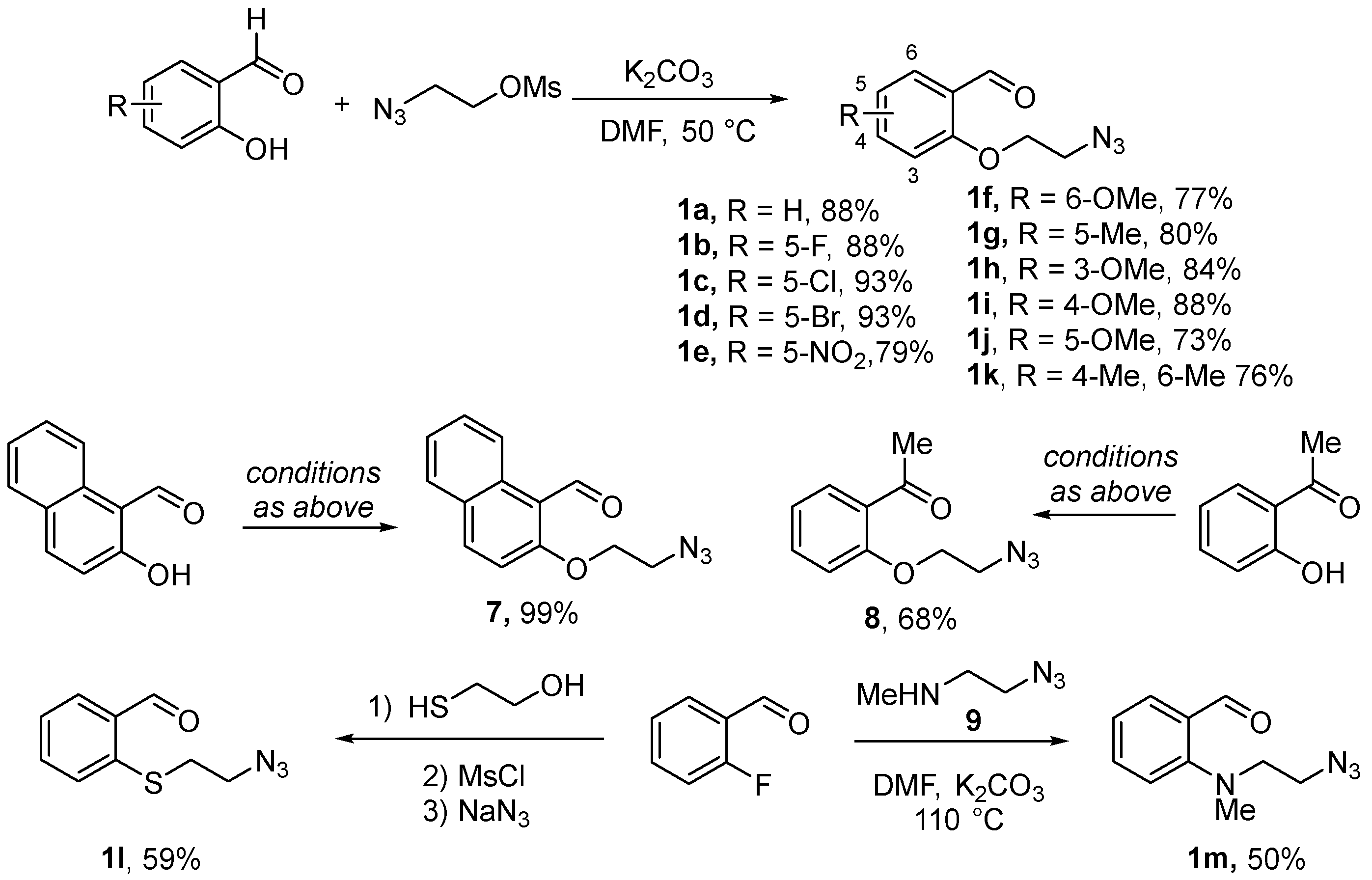
O-linked aldehydes 1 required for the realization of the planned approach were synthesized from o-hydroxybenzaldehydes via alkylation with 2-azidoethyl mesylate as described elsewhere [9]. Eleven O-(2-azidoethyl) substrates 1a–k were obtained in good to excellent yields. In addition to these, naphthalene-templated O-linked azido aldehyde 7 and azido ketone 8 were obtained, also in high yields, from the respective phenolic starting materials. S-linked azido aldehyde 1l was prepared via nucleophilic aromatic substitution of fluorine in o-fluorobenzaldehyde with 2-hydroxyethyl thiol followed by mesylation of the hydroxyl group and nucleophilic displacement of mesylate with azide. N-linked azido aldehyde 1m was prepared in a similar manner from o-fluorobenzaldehyde using 2-azidoethyl methylamine (9) as the nucleophilic agent [9] (Scheme 1).
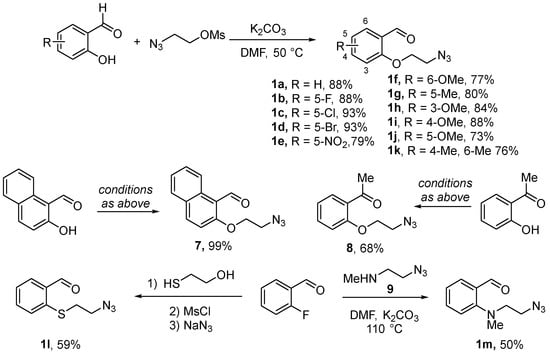
Scheme 1.
Preparation of azido aldehyde and ketone substrates for the Staudinger/aza-Wittig cyclization.
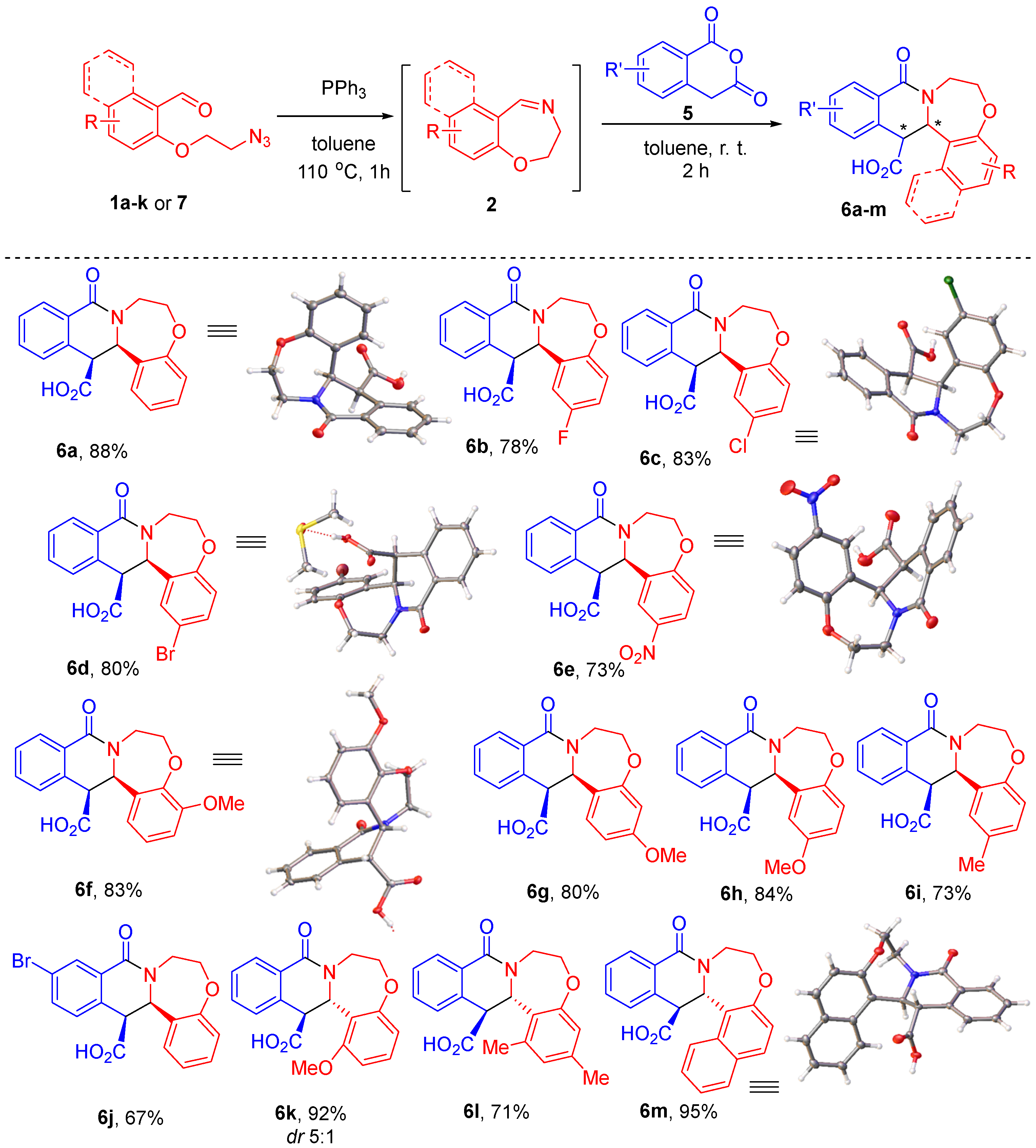
Considering that cyclic imines 2 can be generated on heating starting materials such as 1a–k or 7 in toluene and used, without the need for isolation, in the subsequent transformation, provided the latter can be conducted also in toluene (as was the case with the synthesis of b-lactams 4 [9]), we opted to attempt to couple the CCR of HPA to the preparation of 2 this way as well. The CCR of HPA can be conducted in a range of different solvents [16], depending on the solubility of the starting material (HPA itself not being the limiting factor). Fortunately, imines 2 do not precipitate from toluene even at room temperature, so after their formation and cooling the solution to ambient temperature, we added HPA (5) directly to the solution of imines 2 and continued the reaction with no heating. To our delight, in 2 h the starting materials (2 and HPA) were fully consumed and a thick precipitate of products 6 formed. The latter were filtered off, washed with ether and air-dried. NMR analysis of the products 6a–m thus isolated (in 67–95% yield) confirmed their identity and purity as greater than 95%, whereas they were not found in the filtrate. In the majority of instances (except for 6k), only one diastereomer was observed in the 1H NMR spectra (see Supplementary Materials), thus confirming an excellent case of fully diastereoselective CCR (Scheme 2).
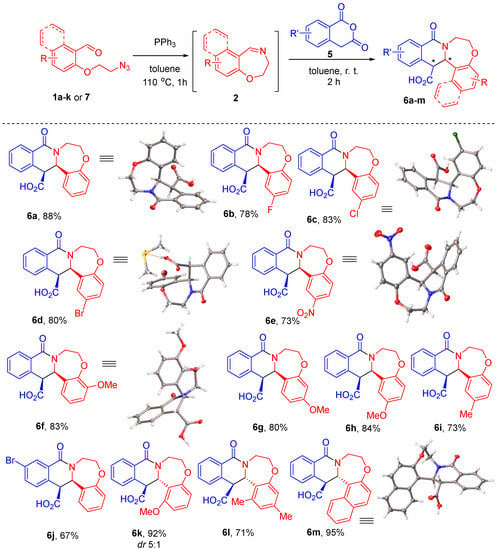
Scheme 2.
The one-pot Staudinger/aza-Wittig generation of imines 2 followed by the CCR with HPA.
The relative stereochemistry of compounds 6a–j was deemed to be cis based on the similarity of their NMR spectra and the single-crystal X-ray structures which were obtained for compounds 6a, 6c and 6d–f. The characteristic features of the 1H NMR spectra of compounds 6a–j are the significant broadening of the signals corresponding to the ex-imine aromatic portion and the bis-methylene linker (presumably, due to the conformational behavior of the seven-membered ring) as well as the vicinal 3JHH coupling constants of the signals coming from the methine protons at the asymmetric carbon atoms. In contrast, adducts 6k–m derived from aldehydes 1 where the position of the aromatic ring ortho to the carbonyl group was substituted displayed a different set of 1H NMR characteristics (no broadening and different methine 3JHH coupling constants) and were deemed to be trans-configured. Such a stereochemical outcome is not unexpected since the respective cis-configured products would be sterically congested. This stereochemistry assignment was further supported by the single-crystal X-ray structure obtained for compound 6m (see Supplementary Materials).
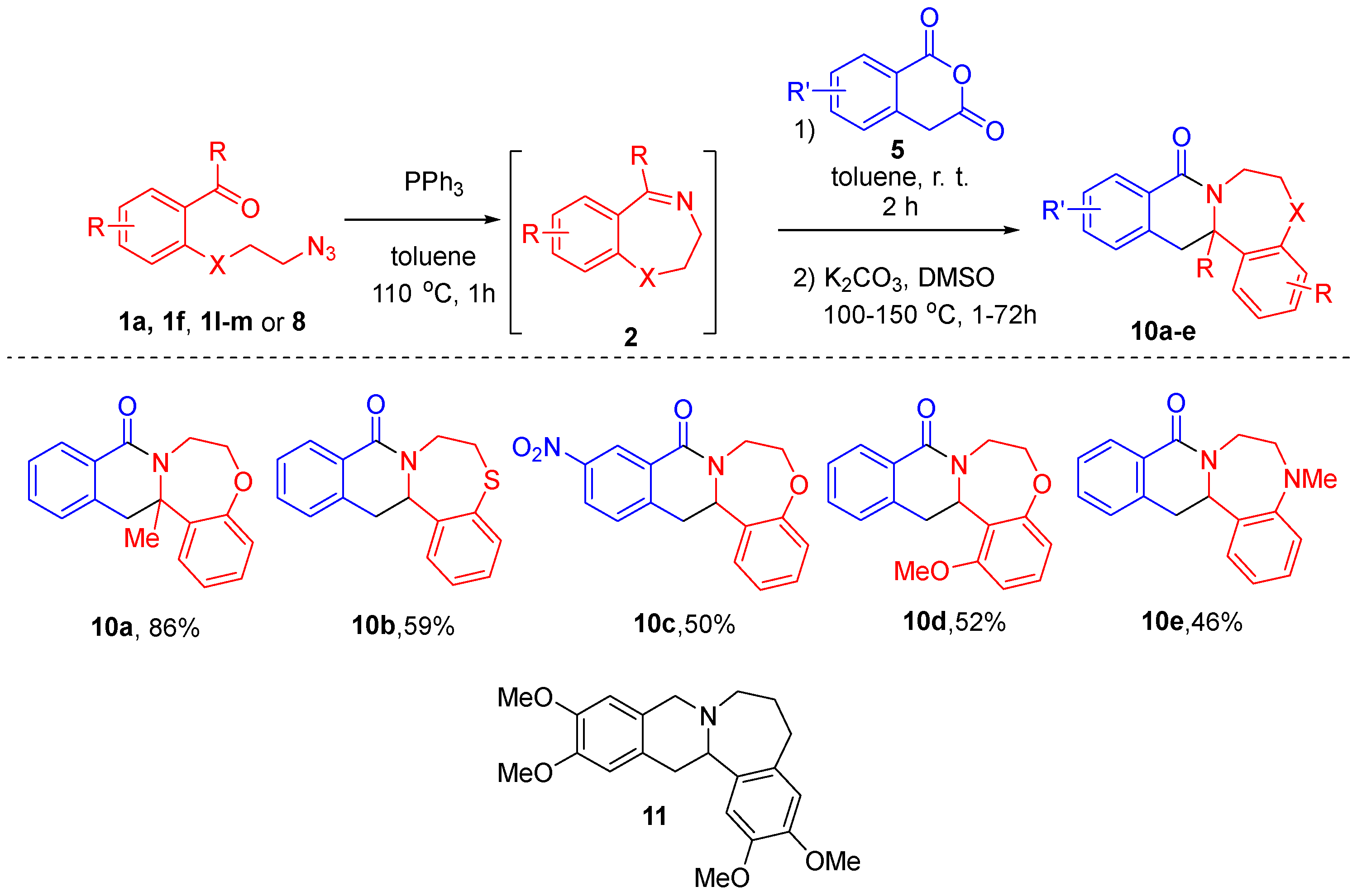
Unfortunately, involvement of imines 2 derived from azido aldehydes 1l–m and 8 in the reaction with HPA did not permit isolation of pure products 6, although it was successful in terms of starting material conversion. Likewise, isolation of the products from the reactions of 7-nitro-HPA was problematic. However, it was possible to isolate products of decarboxylation of these impure carboxylic acids. After performing the Staudinger/aza-Wittig sequence and reacting starting materials 1a, 1f, 1l–m or 8 with HPA (5), toluene was replaced with DMSO. Decarboxylation was performed at 100–150 °C in the presence of potassium carbonate. Tetracycles 10a-e were isolated in good to excellent yields over three steps. Notably, these compounds are hetero des-oxo analogs of natural product-related B-homoxylopinin (11), first reported in 1976 [23], whose congeners displayed histone acetyltransferase inhibitory activity [24] (Scheme 3).
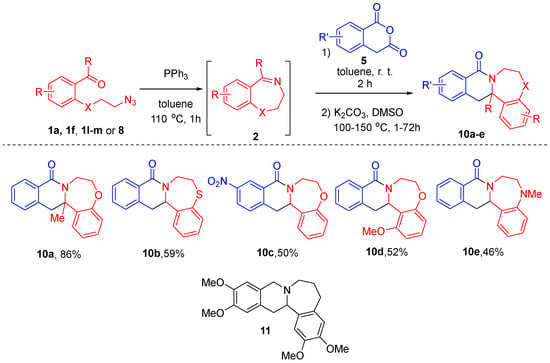
Scheme 3.
Staudinger/aza-Wittig/CCR sequence followed by decarboxylation.
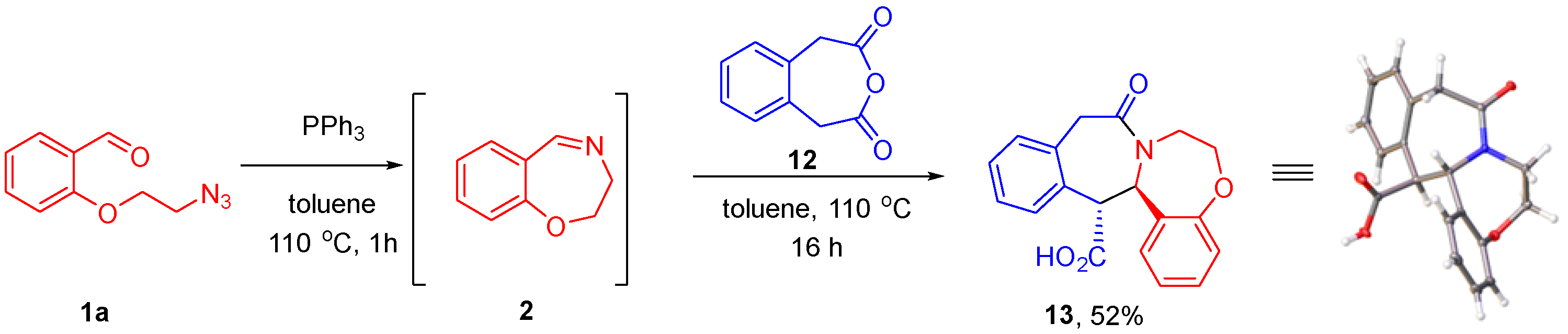
Having successfully realized our synthetic plan with HPA, we became interested in extending the scope of this approach to other cyclic anhydrides. o-Phenylenediacetic acid anhydride (12) was first introduced as a reagent for the CCR in 2017, providing an entry into ε-lactams. It was shown to be less reactive compared to HPA [25]. In line with this observation, the reaction of 12 with cyclic imine 2 generated from azido aldehyde 1a required heating at 110 °C for 16 h to go to completion. The product of this transformation (13) containing a novel 6-7-7-6 polyheterocyclic ring system was isolated in a 52% yield by simple filtration as a single diastereomer, which was shown to be trans-configured by single-crystal X-ray analysis (see Supplementary Materials) (Scheme 4).
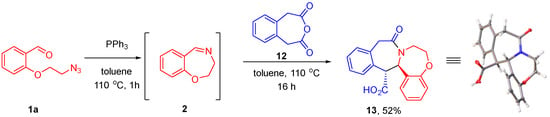
Scheme 4.
Involvement of o-phenylenediacetic acid anhydride in the Staudinger/aza-Wittig/CCR reaction sequence.
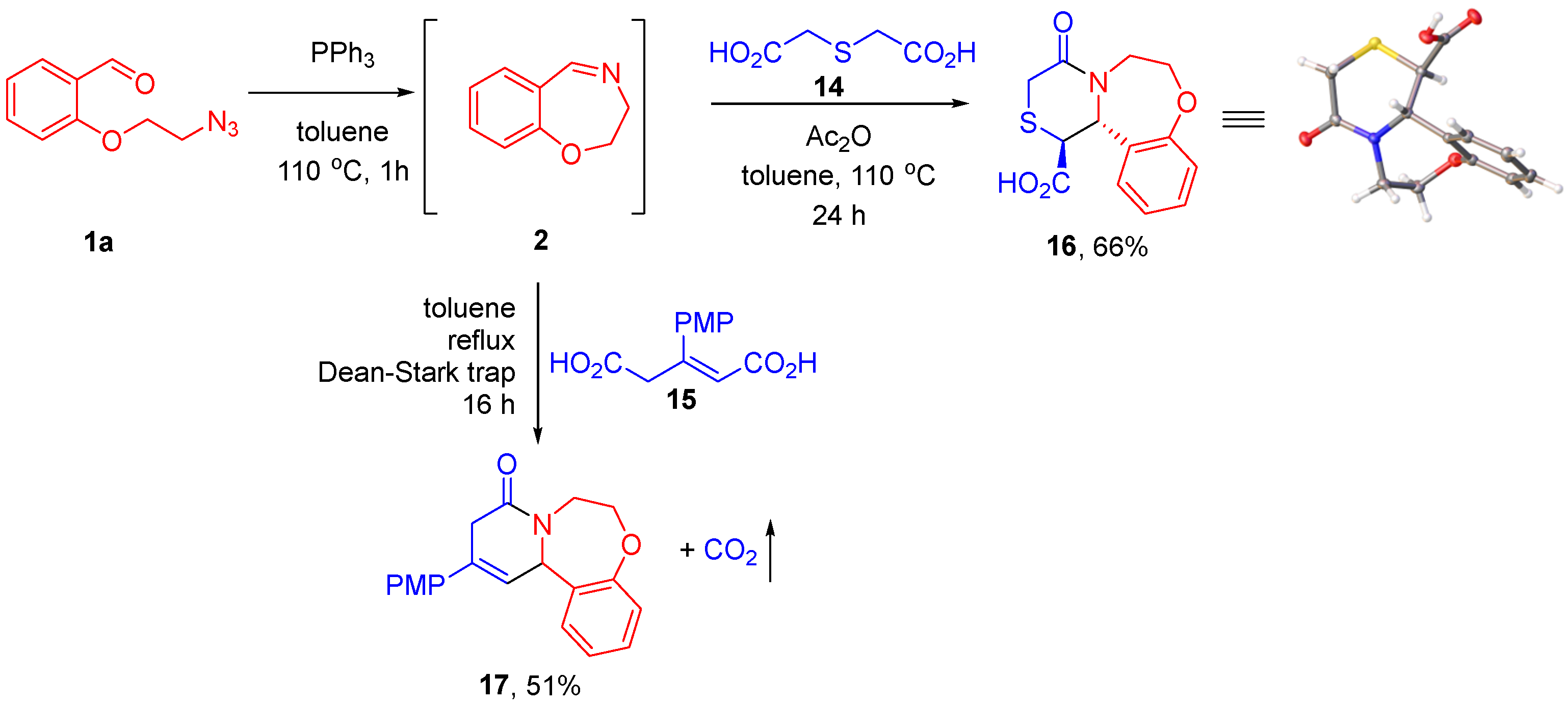
CCR can be conveniently performed with the use of dicarboxylic acids in lieu of the respective cyclic anhydrides generated from diacids using dehydrating agents [26,27,28] or more straightforward cyclodehydration by means of azeotropic removal of water [11,29]. We chose to investigate the Staudinger/aza-Wittig/CCR reaction sequence for two dicarboxylic acids—thiodiglycolic (14) and p-methoxyphenyl-substituted glutaconic acid (15). After generation of imine 2 from azido aldehyde 1a, diacid 14 and acetic anhydride (serving as the dehydrating agent) were added and the process was further conducted at 110 °C for 24 h. The CCR product 16 was obtained in a respectable 66% yield, with no need for chromatographic purification, as a single diastereomer, which was shown to be trans-configured by single-crystal X-ray analysis (see Supplementary Materials). Glutaconic acid 15 was cyclodehydrated by means of azeotropic removal of water. The CCR in this case was conducted in refluxing toluene and, as expected [11,30], was accompanied by decarboxylation and transposition of the double bond. The respective tricyclic adduct 17 was isolated in a 51% yield (Scheme 5).
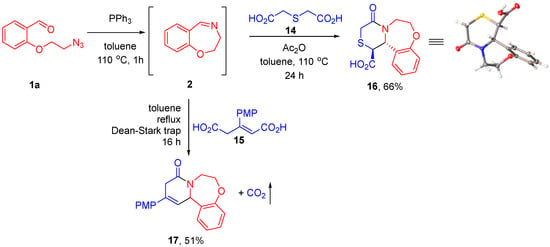
Scheme 5.
Staudinger/aza-Wittig/CCR reaction sequence conducted with dicarboxylic acids via in situ cyclodehydration.
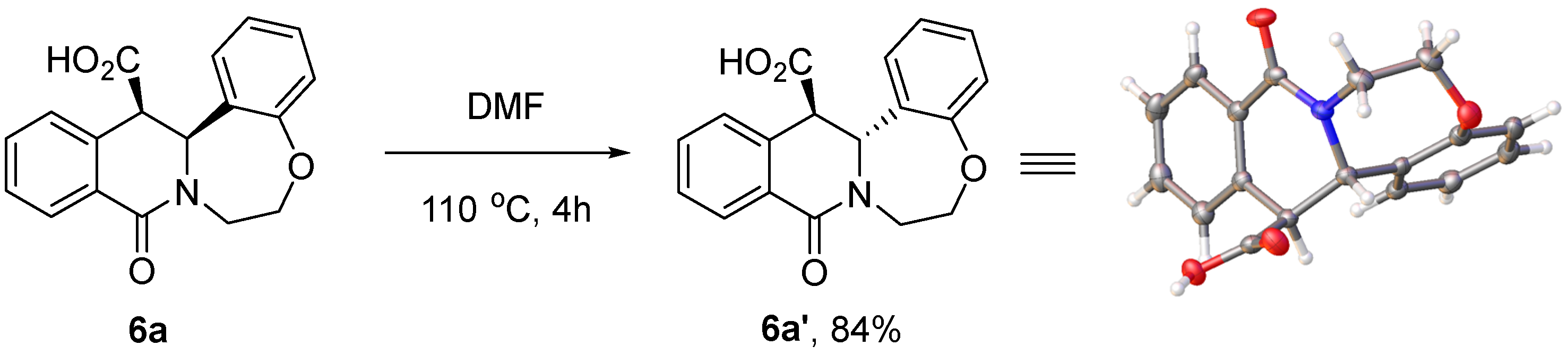
Finally, intrigued by the opposite stereochemical outcome of the reaction sequence under investigation in the case of products 6a–j and 6k–m, we reasoned that the cis-isomers of 6a–j might signify the kinetic outcome of the CCR, while trans-configured products 6k–m obtained from more sterically demanding substrates 2 are likely to be thermodynamic adducts. If such an interpretation is correct, it should be possible to isomerize the kinetic cis-configured tetracycles 6 to their trans counterparts by thermal activation or otherwise. This was indeed demonstrated for compound 6a. On heating at 110 °C in dimethylformamide for 4 h, it was completely transformed to its trans-isomer 6a′, which was isolated in an 84% yield. The relative stereochemistry of 6a′ was confirmed by single-crystal X-ray crystallography (Scheme 6).
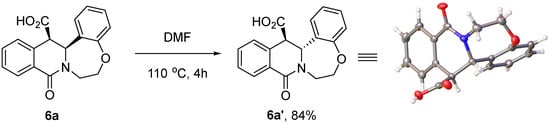
Scheme 6.
Thermal cis → trans isomerization of compound 6a.
3. Conclusions
We have described the realization of the one-pot Staudinger/aza-Wittig/Castagnoli–Cushman reaction sequence for a series of azido aldehydes and homophthalic anhydrides. The reaction proceeded at room temperature and delivered novel polyheterocycles related to the natural product realm in high yields and high diastereoselectivity. Certain substrates presented difficulty in terms of isolation of the resulting tetracyclic products, which can be overcome by thermal decarboxylation of the carboxylic acid Castagnoli–Cushman adduct in the presence of potassium carbonate. The methodology has been extended to three other cyclic anhydrides, two of which were generated from the respective dicarboxylic acids in situ. The cis-configured Castagnoli–Cushman adducts are kinetic products and can be thermally isomerized to their trans counterparts. Collectively, these findings substantially expand the scope of the Castagnoli–Cushman reaction in terms of the imine source and the molecular scaffold accessible via applying this ring-forming reaction to cyclic imines.
4. Materials and Methods
4.1. General Information
All commercial reagents were used without purification. NMR spectra were recorded using a Bruker Avance III spectrometer in CDCl3 (1H: 400.13 MHz; 13C: 100.61 MHz; 19F: 376.50 MHz); chemical shifts are reported as parts per million (δ, ppm); the residual solvent peak (CHCl3 or DMSO-d6) was used as the internal standard: 7.28 or 2.51 for 1H and 77.07 or 40.00 ppm for 13C; multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, dd = doublet of doublets, dt = doublet of triplets, ddd = doublet/doublets of doublets; coupling constants, J, are reported in Hz. Mass spectra were recorded using a Bruker microTOF spectrometer (ionization by electrospray, positive ions detection). Melting points were determined in open capillary tubes on a Stuart SMP50 Automatic Melting Point Apparatus. Analytical thin-layer chromatography was carried out on UV-254 silica gel plates using appropriate eluents. Compounds were visualized with short-wavelength UV light. Column chromatography was performed using silica gel Merk grade 60 (0.040−0.063 mm) 230−400 mesh. All reactions were conducted in the atmosphere of argon.
4.2. Preparation of Azides 1,7,8 and Their Precursors
4.2.1. 2-Azidoethan-1-ol
This compound was obtained according to a slightly modified literature procedure [31]. Sodium azide (5.85 g, 90 mmol, 3.0 eq.) was added to a solution of 2-bromoethan-1-ol (3.75 g, 30 mmol, 1.0 eq.) in water (30 mL). The mixture was stirred overnight at 80 °C. The reaction solution was extracted with diethyl ether. Combined organic layers were dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure to give 2-azidoethan-1-ol (2.5 g, 96%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 3.79 (t, J = 5.0 Hz, 2H), 3.45 (t, J = 5.0 Hz, 2H), 2.22 (s, br, 1H), in accordance with previously obtained literature data.
4.2.2. 2-Azidoethyl Methanesulfonate
This compound was obtained according to a slightly modified literature procedure [32]. Methanesulfonyl chloride (3.6 g, 31.39 mmol. 1.1 eq) was added to a solution of 2-azidoethan-1-ol (2.485 g, 28.537 mmol, 1.0 eq) and triethylamine (3.75 g, 1.3 eq) in DCM (57 mL) at 0 °C and the mixture was stirred for 30 min. One volume of saturated NaHCO3 solution was added and the mixture was stirred for 30 min. The organic layer was separated and the aqueous layer was extracted with DCM. Combined organic layers were washed with brine, dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure to give 2-azidoethyl methanesulfonate (3.94 g, 84%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 4.37 (t, J = 5.0 Hz, 2H), 3.62 (t, J = 5.0 Hz, 2H), 3.11 (s, 3H), in accordance with previously obtained literature data [33].
4.3. General Procedure for Synthesis of 1a–1k, 7, 8 (GP1)
Corresponding salicylic aldehyde (1.05 mmol, 1.05 eq), 2-azidoethyl methanesulfonate (0.165 g, 1 mmol, 1 eq) and K2CO3 (0.145 g, 1.05 mmol, 1 eq) were mixed in DMF (3 mL) and stirred at 50 °C. Reaction progress was controlled by TLC. The solvent of the resulting mixture was evaporated, and the residue was diluted with water and extracted with DCM. Combined organic layers were washed with NaOH (3%), water, brine, dried over anhydrous Na2SO4 and concentrated in vacuo to give corresponding 2-(2-azidoethoxy)benzaldehyde.
2-(2-Azidoethoxy)benzaldehyde (1a), Yield: 168 mg (88%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.54 (s, 1H), 7.89 (dd, J = 7.7, 1.4 Hz, 1H), 7.64–7.50 (m, 1H), 7.10 (t, J = 7.9 Hz, 1H), 7.00 (d, J = 7.9 Hz, 1H), 4.29 (t, J = 4.9 Hz, 2H), 3.71 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 189.3, 160.4, 135.9, 128.6, 125.1, 121.5, 112.4, 67.5, 50.2. HRMS (ESI), m/z calcd. for C9H10N3O2 [M + H]+ 192.0773; found 192.0772.
2-(2-Azidoethoxy)-5-fluorobenzaldehyde (1b), Yield: 184 mg (88%). Brown amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.46 (d, J = 3.1 Hz, 1H), 7.54 (dd, J = 8.2, 3.3 Hz, 1H), 7.32–7.23 (m, 1H), 6.97 (dd, J = 9.1, 3.8 Hz, 1H), 4.26 (d, J = 4.9 Hz, 2H), 3.69 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 188.2 (d, C-F, 4JC-F = 1.5 Hz), 157.3 (d, C-F, 1JC-F = 242.5 Hz), 156.7 (d, C-F, 3JC-F = 2.0 Hz), 125.9 (d, C-F, 3JC-F = 6.0 Hz), 122.4 (d, C-F, 2JC-F = 24.0 Hz), 114.3 (d, C-F, 2JC-F = 31.9 Hz), 114.2, 68.2, 50.2. 19F NMR (376.5 MHz, CDCl3) δ -121.32. HRMS (ESI), m/z calcd. for C9H8N3NaO2F [M + Na]+ 232.0498; found 232.0495.
2-(2-Azidoethoxy)-5-chlorobenzaldehyde (1c), Yield: 210 mg (93%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) 10.46 (s, 1H), 7.83 (d, J = 2.7 Hz, 1H), 7.52 (dd, J = 8.9, 2.7 Hz, 1H), 6.96 (d, J = 8.9 Hz, 1H), 4.28 (t, J = 4.9 Hz, 2H), 3.71 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 188.0, 158.8, 135.3, 128.2, 127.2, 126.0, 114.0, 67.9, 50.1. HRMS (ESI), m/z calcd. for C9H8ClN3NaO2 [M + Na]+ 248.0197; found 248.0196.
2-(2-Azidoethoxy)-5-bromobenzaldehyde (1d), Yield: 250 mg (93%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.45 (s, 1H), 7.98 (d, J = 2.6 Hz, 1H), 7.66 (dd, J = 8.8, 2.6 Hz, 1H), 6.90 (d, J = 8.8 Hz, 1H), 4.27 (t, J = 4.9 Hz, 2H), 3.71 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 187.9, 159.3, 138.2, 131.2, 126.4, 114.4, 67.9, 50.1. HRMS (ESI), m/z calcd. for C9H9N3O2Br [M + H]+ 269.9873; found 269.9878.
2-(2-Azidoethoxy)-5-nitrobenzaldehyde (1e), Yield: 187 mg (79%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.51 (s, 1H), 8.75 (d, J = 2.9 Hz, 1H), 8.46 (dd, J = 9.2, 2.9 Hz, 1H), 7.14 (d, J = 9.2 Hz, 1H), 4.42 (t, J = 4.9 Hz, 2H), 3.79 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 187.0, 164.1, 142.1, 130.6, 124.9, 124.7, 112.9, 68.5, 49.9. HRMS (ESI), m/z calcd. for C9H9N4O4 [M + H]+ 237.0618; found 237.0624.
2-(2-Azidoethoxy)-6-methoxybenzaldehyde (1f), Yield: 177 mg (77%). Brown oil. 1H NMR (400 MHz, CDCl3) δ 10.53 (s, 1H), 7.45 (t, J = 8.5 Hz, 1H), 6.64 (d, J = 8.5 Hz, 1H), 6.57 (d, J = 8.5 Hz, 1H), 4.22 (t, J = 5.0 Hz, 2H), 3.91 (s, 3H), 3.67 (t, J = 5.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 189.0, 161.8, 161.0, 135.7, 114.9, 104.9, 104.8, 67.8, 56.1, 50.1. HRMS (ESI), m/z calcd. for C10H12N3O3 [M + H]+ 222.0873; found 222.0875.
2-(2-Azidoethoxy)-5-methylbenzaldehyde (1g), Yield: 165 mg (80%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.50 (s, 1H), 7.68 (d, J = 2.0 Hz, 1H), 7.37 (dd, J = 8.4, 2.0 Hz, 1H), 6.89 (d, J = 8.4 Hz, 1H), 4.26 (t, J = 4.9 Hz, 2H), 3.68 (t, J = 4.9 Hz, 2H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) 189.6, 158.6, 136.5, 131.0, 128.6, 124.8, 112.4, 67.6, 50.3, 20.3. HRMS (ESI), m/z calcd. for C10H12N3O2 [M + H]+ 206.0924; found 206.0928.
2-(2-Azidoethoxy)-3-methoxybenzaldehyde (1h), Yield: 186 mg (84%). Brown oil. 1H NMR (400 MHz, CDCl3) δ 10.51 (s, 1H), 7.45 (t, J = 4.8 Hz, 1H), 7.18 (d, J = 4.8 Hz, 2H), 4.33 (t, J = 5.0 Hz, 2H), 3.93 (s, 3H), 3.66 (t, J = 5.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 190.0, 152.7, 150.7, 129.9, 124.5, 119.4, 118.1, 72.4, 56.1, 51.1. HRMS (ESI), m/z calcd. for C10H12N3O3 [M + H]+ 222.0873; found 222.0877.
2-(2-Azidoethoxy)-4-methoxybenzaldehyde (1i), Yield: 195 mg (88%). Brown amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.35 (s, 1H), 7.86 (d, J = 8.7 Hz, 1H), 6.60 (dd, J = 8.7, 1.9 Hz, 2H), 6.44 (d, J = 1.9 Hz, 1H), 4.24 (t, J = 4.9 Hz, 2H), 3.89 (s, 3H), 3.69 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 187.9, 166.0, 162.1, 130.6, 119.2, 106.4, 98.8, 67.5, 55.7, 50.1. HRMS (ESI), m/z calcd. for C10H12N3O3 [M + H]+ 222.0873; found 222.0875.
2-(2-Azidoethoxy)-5-methoxybenzaldehyde (1j), Yield: 161 mg (73%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.48 (s, 1H), 7.36 (d, J = 3.2 Hz, 1H), 7.14 (dd, J = 9.0, 3.2 Hz, 1H), 6.95 (d, J = 9.0 Hz, 1H), 4.24 (t, J = 4.9 Hz, 2H), 3.82 (s, 3H), 3.67 (t, J = 4.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 189.2, 155.2, 154.2, 125.4, 123.3, 114.4, 110.6, 68.2, 55.8, 50.3. HRMS (ESI), m/z calcd. for C10H12N3O3 [M + H]+ 222.0873; found 222.0875.
2-(2-Azidoethoxy)-4,6-dimethylbenzaldehyde (1k), Yield: 167 mg (76%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.63 (s, 1H), 6.69 (s, 1H), 6.64 (s, 1H), 4.23 (t, J = 5.0 Hz, 2H), 3.67 (t, J = 5.0 Hz, 2H), 2.57 (s, 3H), 2.36 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 191.4, 161.9, 145.6, 142.3, 125.8, 121.2, 110.6, 67.5, 50.3, 22.1, 21.5. HRMS (ESI), m/z calcd. for C11H14N3O2 [M + H]+ 220.1081; found 220.1083.
2-(2-Azidoethoxy)-1-naphthaldehyde (7), Yield: 219 mg (99%). Reddish amorphous solid. 1H NMR (400 MHz, CDCl3) δ 10.95 (s, 1H), 9.30 (d, J = 8.4 Hz, 1H), 8.08 (d, J = 9.1 Hz, 1H), 7.81 (d, J = 8.4 Hz, 1H), 7.70–7.60 (m, 1H), 7.47 (t, J = 7.1 Hz, 1H), 7.28 (d, J = 7.5 Hz, 1H), 4.41 (t, J = 5.0 Hz, 2H), 3.75 (t, J = 5.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 191.7, 162.4, 137.5, 131.5, 130.0, 129.0, 128.3, 125.1, 125.1, 117.3, 113.3, 68.4, 50.4. HRMS (ESI), m/z calcd. for C13H12N3O2 [M + H]+ 242.0930; found 242.0926.
1-(2-(2-Azidoethoxy)phenyl)ethan-1-one (8), Yield: 128 mg (68%). Brown oil. 1H NMR (400 MHz, CDCl3) δ 7.75 (dd, J = 7.6, 1.6 Hz, 1H), 7.47 (td, J = 8.4, 1.6 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.95 (d, J = 8.4 Hz, 1H), 4.23 (t, J = 5.0 Hz, 2H), 3.72 (t, J = 5.0 Hz, 2H), 2.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 199.6, 157.3, 133.5, 130.5, 128.8, 121.3, 112.4, 66.9, 50.4, 31.8. HRMS (ESI), m/z calcd. for C10H11N3NaO2 [M + Na]+ 228.0743; found 228.0746.
4.3.1. 2-((2-Azidoethyl)thio)benzaldehyde (1l)
Step 1. 2-((2-Hydroxyethyl)thio)benzaldehyde was obtained according to a slightly modified literature procedure [34]. 2-Mercaptoethanol (0.647 g, 8.3 mmol, 1 eq) was added to a solution of 2-fluorobenzaldehyde (1029 mg, 8.3 mmol, 1 eq) and K2CO3 (1.147 g, 8.3 mmol, 1 eq) in 5 mL of DMF and the mixture was stirred for 36 h at 60 °C. The reaction solution was diluted with water and extracted with chloroform. Combined organic layers were washed with water, brine and dried over anhydrous Na2SO4. Crude product was purified using column chromatography in a DCM–methanol system (0–40%) to give 2-((2-hydroxyethyl)thio)benzaldehyde (1.022 g, 68%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 10.23 (s, 1H), 7.87 (dd, J = 7.6, 1.4 Hz, 1H), 7.63 (td, J = 7.6, 1.4 Hz, 1H), 7.56 (d, J = 7.6 Hz, 1H), 7.41–7.34 (t, J = 7.6 Hz, 1H), 5.00 (t, J = 6.0 Hz, 1H), 3.64 (q, J = 6.0 Hz, 2H), 3.10 (t, J = 6.0 Hz, 2H), in accordance with previously obtained literature data [34].
Step 2. 2-((2-Formylphenyl)thio)ethyl methanesulfonate was obtained according to a slightly modified mesilation literature procedure [35]. Mesyl chloride (214.2 mg, 1.87 mmol, 1.1 eq) was added to a solution of 2-((2-hydroxyethyl)thio)benzaldehyde and triethylamine (223.7 mg, 2.21 mmol, 1.3 equiv.) in DCM (6 mL) at 0 °C. One volume of saturated NaHCO3 solution was added and the mixture was stirred for 30 min. The organic layer was separated and the aqueous layer was extracted with DCM. Combined organic layers were washed with brine, dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure to give 2-((2-formylphenyl)thio)ethyl methanesulfonate (237 mg, 91%) as a crude product that was used in the next stage directly without purification.
Step 3. Sodium azide (151 mg, 2.33 mmol, 1.5 eq.) was added to a solution of 2-((2-formylphenyl)thio)ethyl methanesulfonate (404 mg, 1.55 mmol, 1.0 eq.) in DMF (4 mL). The mixture was stirred overnight at room temperature. The reaction solution was diluted with water and extracted with chloroform. Combined organic layers were washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give 2-((2-azidoethyl)thio)benzaldehyde 1l (310 mg, 96%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 10.43 (s, 1H), 7.88 (dd, J = 7.6, 1.1 Hz, 1H), 7.61–7.53 (m, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 3.54 (t, J = 7.0 Hz, 1H), 3.16 (t, J = 7.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 191.3, 139.6, 134.8, 134.1, 132.1, 129.2, 126.5, 49.9, 32.9. HRMS (ESI), m/z calcd. for C9H9N3OSNa [M + Na]+ 230.0359; found 230.0362.
4.3.2. 2-((2-Azidoethyl)(methyl)amino)benzaldehyde (1m)
Step 1. 2-Azido-N-methylethan-1-amine (9) was obtained according to a slightly modified literature procedure [36]. Sodium azide (1.3 g, 20 mmol, 2.0 eq.) was added to a solution of N-methylamino-1-ethylbromide hydrobromide (2.19 g, 10 mmol, 1.0 eq.) in water (10 mL) and the mixture was stirred for 24 h at 80 °C. The mixture was cooled with an ice bath and NaOH (1.74 g) was added. The organic layer was separated and the aqueous layer was extracted with diethyl ether. Combined organic layers were washed with brine, dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure to give 2-azido-N-methyl-ethanamine (0.72 g, 72%) as a colorless oil. 1H NMR (400 MHz, CDCl3) 3.43 (t, J = 5.7 Hz, 2H), 2.77 (t, J = 5.7 Hz, 2H), 2.46 (s, 3H), in accordance with previously obtained literature data [36].
Step 2. 2-Azido-N-methyl ethan-1-amine (266 mg, 2.66 mmol, 1.1 eq) was added to a solution of 2-fluorobenzaldehyde (300 mg, 2.42 mmol, 1 eq) and K2CO3 (367 mg, 2.66 mmol, 1.1 eq) in 2 mL of DMF and the mixture was stirred for 16 h at 110 °C. The reaction solution was diluted with water and extracted with chloroform. Combined organic layers were washed with water, brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give 2-((2-azidoethyl)(methyl)amino)benzaldehyde 1m (251 mg, 50%) as a brown oil. 1H NMR (400 MHz, CDCl3) δ 10.33 (s, 1H), 7.83 (dd, J = 7.7, 1.6 Hz, 1H), 7.56–7.50 (m, 1H), 7.19–7.10 (m, 2H), 3.49 (t, J = 6.0 Hz, 2H), 3.37 (t, J = 6.0 Hz, 2H), 2.96 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 191.3, 154.8, 134.8, 130.6, 129.0, 122.5, 119.8, 56.2, 48.9, 43.5. HRMS (ESI), m/z calcd. for C10H12N4ONa [M + Na]+ 227.0903; found 227.0905.
4.4. General Procedure for Synthesis of 6a–6j, 6l, 6m and 13 (GP2)
Corresponding substituted 2-(2-azidoethoxy)benzaldehyde (0.315 mmol, 1.05 eq) and triphenylphosphine (82.6 mg, 0.315 mmol, 1.05 eq) were mixed in dry toluene (2 mL) in a sealed tube and heated at 110 °C for 1 h. The mixture was cooled down to room temperature. Homophthalic anhydride 5 (48.6 mg, 1 eq), 5-Br-substituted homophtalic anhydride [37] (72.3 mg, 1 eq—synthesis of compound 6j) or o-phenylenediacetic acid anhydride [25] 12 (52.9 mg, 1 eq—synthesis of compound 13) was added to the mixture and the stirring continued for 2 h, 12 h (6j) or 16 h (13) at room temperature or 110 °C (13). The precipitate was washed with toluene, then with hexane-ether (1:1) and dried at 80 °C.
(±)-(cis)-9-Oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinoline-14-carboxylic acid (6a), Yield: 82 mg (88%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.85 (s, 1H), 8.12–7.74 (m, 2H), 7.60 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.24 (t, J = 7.6 Hz, 1H), 7.14–7.06 (m, 1H), 7.04 (d, J = 7.9 Hz, 1H), 6.98 (t, J = 7.6 Hz, 1H), 5.40 (d, J = 4.4 Hz, 1H), 4.68–4.32 (m, 2H), 4.21 (d, J = 11.4 Hz, 1H), 3.79 (t, J = 10.9 Hz, 1H), 3.48 (ddd, J = 14.7, 10.9, 3.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 162.8, 157.1, 136.6, 132.5, 132.0, 129.9, 129.4, 128.4, 128.4, 127.9, 127.3, 124.3, 122.8, 70.8, 59.7, 47.1, 44.4. HRMS (ESI), m/z calcd. for C18H16NO4 [M + H]+ 310.1074; found 310.1078.
(±)-(cis)-2-Fluoro-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6b), Yield: 77 mg (78%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.93 (s, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.81 (s, 1H), 7.61 (t, J = 7.9 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.15–7.02 (m, 2H), 6.93 (s, 1H), 5.39 (d, J = 4.5 Hz, 1H), 4.49 (d, J = 14.2 Hz, 2H), 4.16 (d, J = 11.4 Hz, 1H), 3.80 (t, J = 11.3 Hz, 1H), 3.46 (t, J = 11.3 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.0, 162.8, 158.3 (d, 1JC-F = 240.6 Hz), 153.2, 136.4, 133.8, 132.6, 129.3, 128.5, 128.1, 127.3, 124.3 (d, 3JC-F = 8.5 Hz), 116.1 (d, 2JC-F = 23.0 Hz), 115.3 (d, 2JC-F = 24.1 Hz), 70.8, 59.6, 47.4, 44.1. 19F NMR (376.5 MHz, CDCl3) δ -118.90. HRMS (ESI), m/z calcd. for C18H15NO4F [M + H]+ 328.0980; found 328.0983.
(±)-(cis)-2-Chloro-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6c), Yield: 86 mg (83%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.86 (s, 1H), 7.92 (d, J = 7.6 Hz, 1H), 7.84–7.67 (m, 1H), 7.61 (td, J = 7.5, 1.5 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.32 (dd, J = 8.5, 2.6 Hz, 1H), 7.23–7.15 (m, 1H), 7.06 (d, J = 8.5 Hz, 1H), 5.39 (d, J = 4.4 Hz, 1H), 4.58–4.31 (m, 2H), 4.16 (d, J = 11.2 Hz, 1H), 3.84 (t, J = 9.7 Hz, 1H), 3.44 (ddd, J = 15.1, 11.2, 4.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.1, 162.8, 155.5, 136.4, 133.9, 132.6, 129.7, 129.4, 128.5, 128.4, 128.2, 128.1, 127.4, 124.7, 70.8, 59.8, 47.8, 43.5. HRMS (ESI), m/z calcd. for C18H15NO4Cl [M + H]+ 344.0684; found 344.0689.
(±)-(cis)-2-Bromo-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6d),Yield: 93 mg (80%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.84 (s, 1H), 7.91 (d, J = 7.4 Hz, 1H), 7.76 (s, 1H), 7.61 (td, J = 7.6, 1.5 Hz, 1H), 7.51–7.40 (m, 2H), 7.33 (s, 1H), 7.00 (d, J = 8.4 Hz, 1H), 5.38 (d, J = 4.4 Hz, 1H), 4.56–4.31 (m, 2H), 4.15 (d, J = 11.0 Hz, 1H), 3.85 (t, J = 10.8 Hz, 1H), 3.44 (ddd, J = 15.1, 11.3, 3.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.0, 162.8, 155.9, 136.5, 134.3, 132.7, 132.5, 131.4, 129.4, 128.4, 128.2, 127.4, 125.1, 116.1, 70.7, 59.7, 47.8, 43.6. HRMS (ESI), m/z calcd. for C18H15NO4Br [M + H]+ 388.0179; found 388.0182.
(±)-(cis)-2-Nitro-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6e), Yield: 78 mg (73%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.96 (s, 1H), 8.23–8.08 (m, 2H), 7.91 (d, J = 7.6 Hz, 1H), 7.76 (s, 1H), 7.62 (t, J = 7.4 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.27 (d, J = 8.7 Hz, 1H), 5.55 (d, J = 4.4 Hz, 1H), 4.64–4.41 (m, 2H), 4.31 (d, J = 11.3 Hz, 1H), 4.09–3.91 (m, 1H), 3.50 (ddd, J = 14.8, 11.0, 3.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.0, 162.8, 162.4, 143.6, 136.3, 132.8, 132.7, 129.3, 128.4, 128.3, 127.4, 125.7, 124.7, 124.3, 71.4, 59.7, 48.0, 43.2. HRMS (ESI), m/z calcd. for C18H15N2O6 [M + H]+ 355.0925; found 355.0928.
(±)-(cis)-4-Methoxy-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6f), Yield: 84 mg (83%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.80 (s, 1H), 8.03–7.79 (m, 2H), 7.59 (t, J = 7.1 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.02–6.85 (m, 2H), 6.64 (s, 1H), 5.39 (d, J = 4.3 Hz, 1H), 4.62–4.32 (m, 2H), 4.19 (d, J = 8.5 Hz, 1H), 3.76 (s, 3H), 3.70 (s, 1H), 3.49 (t, J = 10.9 Hz, 1MH). 13C NMR (101 MHz, DMSO-d6) δ 171.1, 162.8, 152.8, 145.9, 136.7, 133.4, 132.5, 129.4, 128.4, 127.9, 127.3, 124.3, 119.8, 113.2, 70.1, 59.7, 56.2, 47.2, 44.4. HRMS (ESI), m/z calcd. for C19H17NNaO5 [M + Na]+ 362.0999; found 362.1000.
(±)-(cis)-3-Methoxy-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6g), Yield: 82 mg (80%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.81 (s, 1H), 8.04–7.74 (m, 2H), 7.59 (t, J = 7.5 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 6.97 (s, 1H), 6.60 (d, J = 2.5 Hz, 1H), 6.57 (d, J = 8.0 Hz, 1H), 5.32 (d, J = 4.4 Hz, 1H), 4.64–4.32 (m, 2H), 4.20 (d, J = 9.6 Hz, 1H), 3.78 (t, J = 10.0 Hz, 1H), 3.70 (s, 3H), 3.53–3.41 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 162.8, 160.4, 158.1, 136.7, 132.4, 129.4, 129.0, 128.4, 127.9, 127.3, 123.9, 109.5, 108.6, 71.0, 59.2, 55.7, 47.3, 44.3. HRMS (ESI), m/z calcd. for C19H18NO5 [M + H]+ 340.1180; found 340.1181.
(±)-(cis)-2-Methoxy-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6h), Yield: 85 mg (84%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.88 (s, 1H), 8.04–7.78 (m, 1H), 7.61 (t, J = 7.1 Hz, 2H), 7.44 (t, J = 7.5 Hz, 1H), 6.96 (d, J = 8.7 Hz, 1H), 6.78 (dd, J = 8.6, 2.5 Hz, 2H), 6.65 (s, 1H), 5.35 (d, J = 4.3 Hz, 1H), 4.67–4.34 (m, 1H), 4.16 (d, J = 10.1 Hz, 1H), 3.71 (t, J = 9.9 Hz, 1H), 3.58 (s, 3H), 3.51–3.42 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 162.8, 155.5, 150.6, 136.6, 132.9, 132.5, 129.4, 128.5, 127.9, 127.3, 123.2, 114.5, 113.8, 71.0, 59.7, 55.6, 47.0, 44.4. HRMS (ESI), m/z calcd. for C19H18NO5 [M + H]+ 340.1180; found 340.1179.
(±)-(cis)-2-Methyl-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino-[4,5b]-isoquinoline-14-carboxylic acid (6i), Yield: 71 mg (73%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.75 (s, 1H), 7.91 (d, J = 7.6 Hz, 1H), 7.87–7.70 (m, 1H), 7.59 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.06 (dd, J = 7.8, 2.1 Hz, 1H), 6.96 (s, 1H), 6.91 (d, J = 8.0 Hz, 1H), 5.33 (d, J = 4.3 Hz, 1H), 4.59–4.25 (m, 2H), 4.10 (d, J = 10.5 Hz, 1H), 3.80 (t, J = 11.3 Hz, 1H), 3.42 (ddd, J = 14.7, 10.9, 4.0 Hz, 1H), 2.15 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 171.2, 162.8, 154.3, 136.9, 133.2, 132.4, 131.4, 130.3, 129.5, 129.1, 128.4, 128.0, 127.3, 122.5, 70.5, 60.2, 47.7, 43.5, 20.9. HRMS (ESI), m/z calcd. for C19H17NNaO4 [M + Na]+ 346.1055; found 346.1051.
(±)-(cis)-11-Bromo-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6j), Yield: 78 mg (67%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.97 (s, 1H), 8.21–7.86 (m, 2H), 7.81 (d, J = 7.4 Hz, 1H), 7.35–7.20 (m, 1H), 7.12–6.92 (m, 3H), 5.42 (d, J = 3.2 Hz, 1H), 4.76–4.34 (m, 2H), 4.22 (d, J = 9.7 Hz, 1H), 3.77 (t, J = 11.0 Hz, 1H), 3.49 (t, J = 12.3 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 170.8, 161.5, 157.2, 135.0, 135.2, 131.8, 131.4, 130.8, 130.0, 129.9, 128.2, 124.3, 122.8, 121.0, 70.8, 59.4, 46.5, 44.6. HRMS (ESI), m/z calcd. for C18H15NO4Br [M + H]+ 388.0179; found 388.0182.
(±)-(cis)-1,3-Dimethyl-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]-isoquinoline-14-carboxylic acid (6l), Yield: 72 mg (71%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.88 (s, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.57 (td, J = 7.5, 1.6 Hz, 1H), 7.48 (t, J = 7.5 Hz, 1H), 7.13 (d, J = 7.6 Hz, 1H), 6.87 (s, 1H), 6.78 (s, 1H), 5.33 (d, J = 11.4 Hz, 1H), 4.57–4.47 (m, 2H), 4.06–3.96 (m, 1H), 3.70 (dd, J = 10.6, 4.8 Hz, 1H), 2.92 (ddd, J = 14.6, 12.1, 4.9 Hz, 1H), 2.32 (s, 3H), 2.27 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 172.5, 162.6, 155.1, 140.2, 138.2, 137.7, 132.6, 129.5, 128.7, 128.5, 128.2, 126.0, 125.7, 121.4, 69.8, 57.7, 51.3, 39.7, 21.0, 20.2. HRMS (ESI), m/z calcd. for C20H20NNaO4 [M + Na]+ 360.1212; found 360.1213.
(±)-(trans)-11-Oxo-8,9,16,16a-tetrahydro-11H-naphtho [1′,2′:6,7][1,4]oxazepino [4,5b]-isoquinoline-16-carboxylic acid (6m), Yield: 102 mg (95%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.80 (s, 1H), 8.21 (d, J = 8.4 Hz, 1H), 8.10–7.97 (m, 2H), 7.94 (d, J = 7.7 Hz, 1H), 7.65–7.40 (m, 4H), 7.29 (d, J = 8.7 Hz, 1H), 7.13 (d, J = 7.4 Hz, 1H), 6.17 (d, J = 9.8 Hz, 1H), 4.73–4.46 (m, 2H), 4.21 (td, J = 10.8, 5.6 Hz, 1H), 3.87 (dd, J = 10.3, 5.1 Hz, 1H), 3.14–2.94 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 172.3, 163.1, 153.4, 137.0, 132.6, 131.9, 131.8, 131.8, 129.3, 128.9, 128.4, 128.2, 126.9, 126.8, 125.3, 124.9, 123.9, 123.2, 69.3, 57.6, 51.2, 40.6. HRMS (ESI), m/z calcd. for C22H17NO4Na [M + Na]+ 382.1050; found 382.1042.
(±)-(trans)-9-Oxo-6,7,9,10,15,15a-hexahydrobenzo[f]benzo [4,5]azepino [1,2d][1,4]- oxazepine-15-carboxylic acid (13), Yield: 48 mg (52%). White crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.56 (s, 1H), 7.46–7.35 (m, 2H), 7.34–7.20 (m, 4H), 7.16 (t, J = 7.1 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H), 5.85 (d, J = 11.6 Hz, 1H), 4.74 (d, J = 14.1 Hz, 1H), 4.48 (d, J = 11.5 Hz, 1H), 4.41 (d, J = 15.3 Hz, 1H), 4.07–3.99 (m, 1H), 3.94 (td, J = 11.6, 3.1 Hz, 1H), 3.36 (d, J = 14.2 Hz, 1H), 2.82 (ddd, J = 15.5, 12.0, 3.6 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 173.3, 171.9, 154.3, 134.7, 133.3, 131.3, 131.2, 131.1, 130.6, 129.7, 127.9, 127.9, 124.6, 122.8, 71.0, 61.4, 55.5, 42.5, 39.3. HRMS (ESI), m/z calcd. for C19H17NNaO4 [M + Na]+ 346.1050; found 346.1046.
1-Methoxy-9-oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinoline-14-carboxylic acid (6k)
This compound was obtained using a modified general procedure GP2. Precipitate did not appear at the final stage, so extraction with NaHCO3 was performed: the solvent was evaporated and the residue was diluted with DCM, and the resulting mixture was extracted with a saturated solution of NaHCO3. Combined aqueous phases were acidified with HCl (40%) to a pH lower than 7 (controlled by indicator litmus paper)—acid precipitated. The resulting suspension was extracted with DCM. Combined organic layers were washed with water, brine, dried over anhydrous Na2SO4 and concentrated in vacuo to give compound 6k (94 mg, 92%, dr = 5:1 trans:cis) as a white solid.
1H NMR (400 MHz, DMSO-d6) showed major product signals δ 12.75 (s, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.54 (t, J = 7.7 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.37–7.32 (m, 1H), 7.21 (t, J = 8.2 Hz, 1H), 6.75 (d, J = 8.4 Hz, 1H), 6.64 (d, J = 7.8 Hz, 1H), 5.63 (d, J = 5.1 Hz, 1H), 4.64–4.53 (m, 2H), 4.17–4.07 (m, 1H), 3.95 (td, J = 10.9, 4.6 Hz, 1H), 3.58 (s, 3H), 3.19 (ddd, J = 14.7, 10.3, 4.8 Hz, 1H), and minor product observed signals δ 7.97 (d, J = 7.6 Hz, 1H), 6.93 (d, J = 8.4 Hz, 1H), 4.51 (d, J = 4.7 Hz, 1H), 3.83 (s, 3H), 3.80 (t, J = 3.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) showed major product signals δ 172.7, 162.9, 158.9, 157.4, 137.4, 132.2, 130.2, 128.8, 128.0, 127.7, 120.1, 115.2, 108.8, 70.7, 57.0, 56.0, 48.6, 44.4, and minor product observed signals δ 171.1, 157.3, 132.1, 130.4, 130.2, 128.2, 127.6, 127.5, 115.6, 108.1, 69.1, 56.6, 56.0, 48.4. HRMS (ESI), m/z calcd. for C19H18NO5 [M + H]+ 340.1180; found 340.1181.
4.5. Procedure for Synthesis of 10b and 10e
Corresponding azide (0.315 mmol, 1.05 eq) and triphenylphosphine (82.6 mg, 0.315 mmol, 1.05 eq) were mixed in a sealed tube in dry toluene (2 mL) and heated to 110 °C for 1 h. The mixture was cooled down to room temperature. Homophthalic anhydride 5 (48.6 mg, 1 eq) was added to the mixture and it was stirred for 2 h (synthesis of compound 10b) or 72 h (synthesis of compound 10e) at room temperature. The precipitate was washed with toluene, hexane-ether (1:1) and dried at 80 °C. The precipitate was diluted with DSMO. K2CO3 (1.1 eq.) was added to the mixture and it was heated at 150 °C for 2 h. The mixture was diluted with water and extracted with DCM, and combined organic fractions were washed with a saturated solution of NaHCO3, water and brine, then dried over anhydrous Na2SO4 concentrated in vacuo. Crude products were purified using column chromatography in a petroleum ether–ethyl acetate system (gradient elution 5–25%).
6,7,14,14a-Tetrahydro-9H-benzo [6,7][1,4]thiazepino [4,5b]isoquinolin-9-one (10b), Yield: 50 mg (59%). White crystals. 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 7.4 Hz, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.53 (t, J = 7.4 Hz, 1H), 7.42–7.32 (m, 2H), 7.15 (td, J = 8.8, 7.3, 1.8 Hz, 1H), 7.12–7.02 (m, 2H), 5.37–5.29 (m, 1H), 5.07 (d, J = 13.7 Hz, 1H), 3.64 (dd, J = 16.3, 5.5 Hz, 1H), 3.50 (dd, J = 16.3, 3.6 Hz, 1H), 3.42 (ddd, J = 13.9, 8.5, 5.6 Hz, 1H), 2.93 (dd, J = 8.4, 3.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 163.3, 144.2, 137.5, 135.2, 134.4, 132.4, 129.1, 128.7, 128.2, 127.9, 127.6, 127.2, 127.0, 58.9, 48.1, 33.0, 32.4. HRMS (ESI), m/z calcd. for C17H16NOS [M + H]+ 282.0947; found 282.0949.
5-Methyl-6,7,14,14a-tetrahydrobenzo [5,6][1,4]diazepino [1,7b]isoquinolin-9(5H)-one (10e), Yield: 38 mg (46%). White crystals. 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.5 Hz, 1H), 7.47 (td, J = 7.4, 1.1 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 7.35–7.30 (m, 1H), 7.29–7.25 (m, 1H), 7.17 (d, J = 7.1 Hz, 1H), 7.05 (d, J = 7.9 Hz, 1H), 6.97 (t, J = 7.4 Hz, 1H), 5.01 (dd, J = 9.7, 4.4 Hz, 1H), 4.24 (dt, J = 13.9, 4.3 Hz, 1H), 3.69–3.51 (m, 2H), 3.14 (dd, J = 15.6, 4.2 Hz, 1H), 3.10–2.96 (m, 2H), 2.89 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.4, 149.8, 138.0, 132.3, 131.8, 129.4, 129.1, 128.4, 127.0, 127.0, 126.9, 122.2, 118.2, 58.4, 56.0, 42.5, 41.7, 33.2. HRMS (ESI), m/z calcd. for C18H19N2O [M + H]+ 279.1492; found 279.1493.
4.6. Procedure for Synthesis of 10a, 10c and 10d
Corresponding azide (0.315 mmol, 1.05 eq) and triphenylphosphine (82.6 mg, 0.315 mmol, 1.05 eq) were mixed in a sealed tube in dry toluene (2 mL) and heated to 110 °C for 1 h. The mixture was cooled down to room temperature. Homophthalic anhydride 5 (48.6 mg, 1 eq) or 5-NO2-substituted homophtalic anhydride [38] (62 mg, 1 eq; synthesis of compound 10c) was added to the mixture and it was stirred for 2 h or 12 h (synthesis of compound 10c) at room temperature. The solvent was evaporated and the residue was diluted with DCM, and the resulting mixture was extracted with a saturated solution of NaHCO3. Combined aqueous fractions were acidified with HCl (40%) to a pH lower than 7 (controlled by paper indicator)—acid precipitated. The resulting suspension was extracted with DCM. Combined organic layers were washed with water, brine, dried over anhydrous Na2SO4 and concentrated in vacuo. Crude product was diluted with DMSO. K2CO3 (1 eq.) was added to the mixture and it was heated to 100 °C/150 °C for 1–15 h (10d—100 °C, 15 h; 10a—150 °C, 1 h; 10c—100 °C, 1 h). The mixture was diluted with water and extracted with DCM, and combined organic fractions were washed with a saturated solution of NaHCO3, water and brine, then dried over anhydrous Na2SO4 and concentrated in vacuo. Products 10a and 10c were pure. Crude product 10d was purified using column chromatography in a petroleum ether–ethyl acetate system (gradient elution 5–25%).
14a-Methyl-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinolin-9-one (10a), Yield: 72 mg (86%). White crystals. 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 7.7 Hz, 1H), 7.48 (t, J = 7.1 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.27 (d, J = 7.6 Hz, 1H), 7.24–7.17 (m, 1H), 7.17–7.10 (m, 1H), 7.09–7.03 (m, 1H), 6.99 (t, J = 7.5 Hz, 1H), 4.78–4.65 (m, 1H), 4.17 (dd, J = 11.4, 3.2 Hz, 1H), 4.07 (td, J = 11.4, 3.4 Hz, 1H), 3.69–3.52 (m, 2H), 3.13 (d, J = 16.1 Hz, 1H), 1.81 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.9, 156.4, 137.1, 136.6, 132.2, 129.1, 128.7, 128.5, 127.1, 127.0, 126.4, 124.2, 123.6, 70.2, 61.6, 41.5, 41.3, 26.1. HRMS (ESI), m/z calcd. for C18H18NO2 [M + H]+ 280.1332; found 280.1335.
11-Nitro-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinolin-9-one (10c), Yield: 46 mg (50%). Yellow amorphous solid. 1H NMR (400 MHz, CDCl3) δ 8.86 (d, J = 2.3 Hz, 1H), 8.37 (dd, J = 8.3, 2.4 Hz, 1H), 7.60 (d, J = 8.3 Hz, 1H), 7.27–7.18 (m, 1H), 7.14–7.06 (m, 1H), 6.98–6.86 (m, 2H), 5.20–5.11 (m, 1H), 4.87 (dt, J = 14.1, 1.9 Hz, 1H), 4.46–4.36 (m, 1H), 3.89 (td, J = 11.8, 2.4 Hz, 1H), 3.73–3.46 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 161.1, 158.4, 147.6, 144.0, 132.7, 130.3, 129.7, 128.5, 126.7, 126.6, 124.2, 124.0, 122.6, 71.4, 56.4, 47.4, 31.5. HRMS (ESI), m/z calcd. for C17H15N2O4 [M + H]+ 311.1026; found 311.1029.
1-Methoxy-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinolin-9-one (10d), Yield: 46 mg (52%). White crystals. 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.4 Hz, 1H), 7.49–7.43 (m, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.32–7.25 (m, 3H), 7.21 (d, J = 7.4 Hz, 1H), 6.81–6.71 (m, 2H), 5.48 (dd, J = 13.4, 3.5 Hz, 1H), 4.76 (dd, J = 14.4, 5.1 Hz, 1H), 4.33 (ddd, J = 12.3, 10.6, 5.2 Hz, 1H), 3.91–3.82 (m, 4H), 3.41 (t, J = 14.4 Hz, 1H), 3.24 (ddd, J = 14.3, 12.5, 5.2 Hz, 1H), 2.87 (dd, J = 15.6, 3.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 163.9, 156.8, 154.7, 138.1, 131.6, 129.6, 129.5, 128.2, 127.0, 126.9, 121.5, 115.8, 107.4, 69.0, 55.8, 53.8, 40.2, 34.3. HRMS (ESI), m/z calcd. for C18H18NO3 [M + H]+ 296.1281; found 296.1286.
4.7. (±)-(trans)-4-Oxo-1,3,4,6,7,12b-hexahydrobenzo[f][1,4]thiazino [4,3d][1,4]oxazepine-1-carboxylic acid (16)
Azide 1a (60.1 mg, 0.315 mmol, 1.05 eq) and triphenylphosphine (82.6 mg, 0.315 mmol, 1.05 eq) were mixed in a sealed tube in dry toluene (2 mL) and heated to 110 °C for 1 h. The mixture was cooled down to room temperature. Thiodiglycolic acid 14 (45 mg, 0.3 mmol, 1 eq) and acetic anhydride (0.345 mmol, 1.15 eq) were added to the mixture and it was stirred for 24 h at 110 °C. The precipitate was washed with toluene, hexane-ether (1:1) and dried at 80 °C to give compound 16 (58 mg, 66%) as brownish crystals. 1H NMR (400 MHz, DMSO-d6) δ 12.93 (s, 1H), 7.44–7.32 (m, 2H), 7.18 (td, J = 7.4, 0.8 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H), 5.22 (d, J = 8.5 Hz, 1H), 4.49–4.36 (m, 1H), 4.17 (d, J = 8.5 Hz, 1H), 4.04 (dd, J = 11.2, 3.0 Hz, 1H), 3.97 (td, J = 11.3, 3.7 Hz, 1H), 3.70 (d, J = 14.5 Hz, 1H), 3.08 (d, J = 14.6 Hz, 1H), 2.93 (ddd, J = 15.3, 11.4, 4.3 Hz, 1H). 13C NMR(101 MHz, DMSO-d6) δ 172.2, 168.2, 155.3, 131.2, 130.1, 129.9, 124.9, 123.0, 71.4, 60.2, 45.3, 41.3, 28.8. HRMS (ESI), m/z calcd. for C13H14NO4S [M + H]+ 280.0638; found 280.0640.
4.8. 11-(4-Methoxyphenyl)-6,7,10,12a-tetrahydro-9H-benzo[f]pyrido [1,2d][1,4]oxazepin-9-one (17)
Azide 1a (100.4 mg, 0.525 mmol, 1.05 eq), triphenylphosphine (137.7 mg, 0.525 mmol, 1.05 eq) and p-methoxyphenyl-substituted glutaconic acid 15 (109.1 mg, 0.5 mmol, 1 eq) were mixed in dry toluene (10 mL) in a round-bottom flask equipped with a Dean-Star trap and heated to reflux for 20 h. The solvent of the resulting mixture was evaporated, and the crude product was purified using column chromatography in a hexane–ethyl acetate system (5–40%) to give compound 17 (82 mg, 51%) as white crystals. 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.8 Hz, 2H), 7.28 (td, J = 8.9, 7.6, 1.3 Hz, 1H), 7.23 (d, J = 7.6 Hz, 1H), 7.13 (d, J = 7.2 Hz, 1H), 7.08–7.02 (m, 1H), 6.98 (d, J = 8.8 Hz, 2H), 6.34 (dd, J = 5.7, 2.9 Hz, 1H), 5.50 (d, J = 4.0 Hz, 1H), 4.90 (dt, J = 14.3 Hz, 1.8, 1H), 4.50 (dt, J = 12.5, 2.3 Hz, 1H), 3.87 (s, 3H), 3.84–3.74 (m, 1H), 3.53–3.34 (m, 2H), 3.27 (dt, J = 20.2, 2.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 167.6, 159.8, 159.5, 136.0, 132.9, 130.5, 129.6, 127.1, 126.4, 124.1, 122.1, 117.8, 114.2, 72.4, 58.4, 55.4, 47.6, 35.1. HRMS (ESI), m/z calcd. for C20H20NO3 [M + H]+ 322.1438; found 322.1437.
4.9. (±)-(trans)-9-Oxo-6,7,14,14a-tetrahydro-9H-benzo [6,7][1,4]oxazepino [4,5b]isoquinoline-14-carboxylic acid (6a’)
Compound 6a (100 mg, 0.323 mmol) was dissolved in DMF heated to 110 °C for 4 h. The solvent of the resulting mixture was evaporated, and the residue was washed with water and dried at 80 °C to give compound 6a’ (84 mg, 84%) as white crystals. 1H NMR (400 MHz, DMSO-d6) δ 13.08 (s, 1H), 7.78 (d, J = 7.5 Hz, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.62 (t, J = 7.0 Hz, 1H), 7.39 (t, J = 7.2 Hz, 1H), 7.22–7.11 (m, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.96–6.82 (m, 2H), 5.50 (s, 1H), 4.80–4.66 (m, 2H), 4.50 (d, J = 12.5 Hz, 1H), 3.71–3.57 (m, 1H), 3.38–3.30 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 172.5, 161.9, 159.5, 136.0, 133.2, 132.8, 130.3, 129.5, 128.3, 128.2, 127.8, 127.4, 123.9, 122.2, 71.7, 57.9, 48.4, 45.3. HRMS (ESI), m/z calcd. for C18H16NO4 [M + H]+ 310.1074; found 310.1075.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238130/s1. Copies of NMR spectra. X-ray data [39,40,41]. Crystallographic data: CCDC 2207932 (6a), 2207933 (6c), 2207934 (6d), 2207935 (6e), 2207937 (6f), 2178410 (6m), 2207930 (13), 2207931 (16), 2207936 (6a’) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures (accessed on 17 September 2022).
Author Contributions
Conceptualization, D.D.; methodology, G.K. and D.D.; investigation, R.L. and G.K.; data curation, D.D., G.K., O.B. and M.K.; writing—original draft preparation, M.K.; writing—review and editing, O.B. and D.D.; supervision, M.K.; funding acquisition, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support from the Russian Foundation for Basic Research (project grant no. 20-03-00922) and Megagrant of the Government of Russian Federation (no. 075-15-2021-637).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request.
Acknowledgments
We thank the Research Centre for Magnetic Resonance, the Center for Chemical Analysis and Materials Research, and the Centre for X-ray Diffraction Methods of Saint Petersburg State University Research Park for obtaining the analytical data.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of the compounds 6 and 10 are available from the authors upon reasonable request.
References
- Hu, Y.; Stumpfe, D.; Bajorath, J. Computational Exploration of Molecular Scaffolds in Medicinal Chemistry. J. Med. Chem. 2016, 59, 4062–4076. [Google Scholar] [CrossRef]
- Sun, H.; Tawa, G.; Wallqvist, A. Classification of scaffold-hopping approaches. Drug Discov. Today 2012, 17, 310–324. [Google Scholar] [CrossRef]
- Akritopoulou-Zanze, I.; Darczak, D.; Sarris, K.; Phelan, K.M.; Huth, J.R.; Song, D.; Johnson, E.F.; Jia, Y.; Djuric, S.W. Scaffold oriented synthesis. Part 1: Design, preparation, and biological evaluation of thienopyrazoles as kinase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 96–99. [Google Scholar] [CrossRef]
- Abdel Hafez, N.A.; Farghaly, T.A.; Al-Omar, M.A.; Abdalla, M.M. Synthesis of bioactive polyheterocyclic ring systems as 5alpha-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 4838–4844. [Google Scholar] [CrossRef]
- Zheng, L.; Bin, Y.; Wang, Y.; Hua, R. Synthesis of Natural Product-like Polyheterocycles via One-Pot Cascade Oximation, C-H Activation, and Alkyne Annulation. J. Org. Chem. 2016, 81, 8911–8919. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. Recent Advances in Construction of Polycyclic Natural Product Scaffolds via One-Pot Reactions Involving Alkyne Annulation. Front. Chem. 2020, 8, 580355. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Iwanejko, J.; Wojaczynska, E. Cyclic imines-preparation and application in synthesis. Org. Biomol. Chem. 2018, 16, 7296–7314. [Google Scholar] [CrossRef]
- Paramonova, P.; Lebedev, R.; Bakulina, O.; Dar’in, D.; Krasavin, M. In situ generation of imines by Staudinger/aza-Wittig tandem combined with thermally induced Wolff rearrangement for one-pot three-component lactam synthesis. Org. Biomol. Chem. 2022. Submitted. [Google Scholar] [CrossRef]
- Bakulina, O.; Chizhova, M.; Dar’in, D.; Krasavin, M. A General Way to Construct Arene-Fused Seven-Membered Nitrogen Heterocycles. Eur. J. Org. Chem. 2018, 2018, 362–371. [Google Scholar] [CrossRef]
- Firsov, A.; Chupakhin, E.; Dar’in, D.; Bakulina, O.; Krasavin, M. Three-Component Castagnoli-Cushman Reaction of 3-Arylglutaconic Acids with Aromatic Aldehydes and Amines Delivers Rare 4,6-Diaryl-1,6-dihydropyridin-2(3 H)-ones. Org. Lett. 2019, 21, 1637–1640. [Google Scholar] [CrossRef]
- Guranova, N.; Bakulina, O.; Dar’in, D.; Kantin, G.; Krasavin, M. Homophthalic Esters: A New Type of Reagents for the Castagnoli-Cushman Reaction. Eur. J. Org. Chem. 2022, 2022, e202101281. [Google Scholar] [CrossRef]
- Howard, S.Y.; Di Maso, M.J.; Shimabukuro, K.; Burlow, N.P.; Tan, D.Q.; Fettinger, J.C.; Malig, T.C.; Hein, J.E.; Shaw, J.T. Mechanistic Investigation of Castagnoli-Cushman Multicomponent Reactions Leading to a Three-Component Synthesis of Dihydroisoquinolones. J. Org. Chem. 2021, 86, 11599–11607. [Google Scholar] [CrossRef]
- Bayles, T.; Guillou, C. Trifluoroethanol Promoted Castagnoli-Cushman Cycloadditions of Imines with Homophthalic Anhydride. Molecules 2022, 27, 844. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Gaikwad, N.D. Recent Advances in Synthesis of 3,4-Dihydroisoquinolin-1(2H)-one. ChemistrySelect 2020, 5, 8157–8184. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Levin, A.; Emge, T.J.; Rablen, P.R.; Floyd, D.M.; Knapp, S. N-methylimidazole promotes the reaction of homophthalic anhydride with imines. J. Org. Chem. 2014, 79, 7593–7599. [Google Scholar] [CrossRef]
- Vytla, D.; Shaw, P.; Velayuthaperumal, R.; Emmadi, J.; Mathur, A.; Roy, A. Microwave accelerated Castagnoli-Cushman reaction: Synthesis of novel 6,7,8,9-tetrahydropyrido [3′,2′:4,5]pyrrolo [1,2-a]pyrazines. Tetrahedron Lett. 2021, 68, 152943. [Google Scholar] [CrossRef]
- Polyak, D.; Phung, N.; Liu, J.; Barrows, R.; Emge, T.J.; Knapp, S. Stereochemistry and Reactivity of the HPA-Imine Mannich Intermediate. Tetrahedron Lett. 2017, 58, 3879–3883. [Google Scholar] [CrossRef]
- Pashev, A.; Burdzhiev, N.; Stanoeva, E. One-step route to tricyclic fused 1,2,3,4-tetrahydroisoquinoline systems via the Castagnoli-Cushman protocol. Beilstein J. Org. Chem. 2020, 16, 1456–1464. [Google Scholar] [CrossRef]
- Laws, S.W.; Moore, L.C.; Di Maso, M.J.; Nguyen, Q.N.N.; Tantillo, D.J.; Shaw, J.T. Diastereoselective Base-Catalyzed Formal [4 + 2] Cycloadditions of N-Sulfonyl Imines and Cyclic Anhydrides. Org. Lett. 2017, 19, 2466–2469. [Google Scholar] [CrossRef]
- Bakulina, O.; Ivanov, A.; Suslonov, V.; Dar’in, D.; Krasavin, M. A speedy route to sterically encumbered, benzene-fused derivatives of privileged, naturally occurring hexahydropyrrolo [1,2-b]isoquinoline. Beilstein J. Org. Chem. 2017, 13, 1413–1424. [Google Scholar] [CrossRef]
- Usmanova, L.; Dar’in, D.; Novikov, M.S.; Gureev, M.; Krasavin, M. Bicyclic Piperazine Mimetics of the Peptide beta-Turn Assembled via the Castagnoli-Cushman Reaction. J. Org. Chem. 2018, 83, 5859–5868. [Google Scholar] [CrossRef]
- Berney, D.; Jauner, T. 1-Aralkylated tetrahydro-2-benzazepines. Part II: Synthesis from 3-(3,4-dimethoxyphenyl)-propylamine. Helv. Chim. Acta 1976, 59, 623–626. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Chen, X.; Li, W.; Li, G.-B.; Wu, Y. Total Synthesis and Evaluation of B-Homo Palmatine and Berberine Derivatives as p300 Histone Acetyltransferase Inhibitors. Eur. J. Org. Chem. 2018, 2018, 1041–1052. [Google Scholar] [CrossRef]
- Krasavin, M.; Bakulina, O.; Dar’in, D. o-Phenylenediacetic Acid Anhydride in the Castagnoli–Cushman Reaction: Extending the Product Space to ε-Lactams. Synlett 2017, 28, 1165–1169. [Google Scholar] [CrossRef]
- Lepikhina, A.; Dar’in, D.; Bakulina, O.; Chupakhin, E.; Krasavin, M. Skeletal Diversity in Combinatorial Fashion: A New Format for the Castagnoli-Cushman Reaction. ACS Comb. Sci. 2017, 19, 702–707. [Google Scholar] [CrossRef]
- Kantin, G.; Chupakhin, E.; Dar’in, D.; Krasavin, M. Efficient cyclodehydration of dicarboxylic acids with oxalyl chloride. Tetrahedron Lett. 2017, 58, 3160–3163. [Google Scholar] [CrossRef]
- Chupakhin, E.G.; Bakulina, O.Y.; Dar’in, D.V.; Krasavin, M. 1,1′-Carbonyldiimidazole as a cyclodehydrating agent for the Castagnoli–Cushman reaction of dicarboxylic acids and imines. Mendeleev Commun. 2019, 29, 292–293. [Google Scholar] [CrossRef]
- Chupakhin, E.; Dar’in, D.; Krasavin, M. The Castagnoli-Cushman reaction in a three-component format. Tetrahedron Lett. 2018, 59, 2595–2599. [Google Scholar] [CrossRef]
- Firsov, A.; Bakulina, O.; Dar’in, D.; Guranova, N.; Krasavin, M. Further Insight into the Castagnoli-Cushman-type Synthesis of 1,4,6-Trisubstituted 1,6-Dihydropyridin-2-(3H)-ones from 3-Arylglutaconic Acid Anhydrides. J. Org. Chem. 2020, 85, 6822–6829. [Google Scholar] [CrossRef]
- Ahangarpour, M.; Kavianinia, I.; Hume, P.A.; Harris, P.W.R.; Brimble, M.A. N-Vinyl Acrylamides: Versatile Heterobifunctional Electrophiles for Thiol-Thiol Bioconjugations. J. Am. Chem. Soc. 2022, 144, 13652–13662. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Vinogradova, N.M.; Sharova, E.V.; Genkina, G.K.; Brel, V.K. Novel approach to the design of potential bioactive alkaloid anabasine conjugates using click chemistry methodology. Heteroatom Chem. 2016, 27, 245–252. [Google Scholar] [CrossRef]
- Tahtaoui, C.; Parrot, I.; Klotz, P.; Guillier, F.; Galzi, J.L.; Hibert, M.; Ilien, B. Fluorescent pirenzepine derivatives as potential bitopic ligands of the human M1 muscarinic receptor. J. Med. Chem. 2004, 47, 4300–4315. [Google Scholar] [CrossRef]
- Ginzburg, Y.; Anaby, A.; Vidavsky, Y.; Diesendruck, C.E.; Ben-Asuly, A.; Goldberg, I.; Lemcoff, N.G. Widening the Latency Gap in Chelated Ruthenium Olefin Metathesis Catalysts. Organometallics 2011, 30, 3430–3437. [Google Scholar] [CrossRef]
- Carboni, B.; Benalil, A.; Vaultier, M. Aliphatic amino azides as key building blocks for efficient polyamine syntheses. J. Org. Chem. 2002, 58, 3736–3741. [Google Scholar] [CrossRef]
- Rasale, D.; Patil, K.; Sauter, B.; Geigle, S.; Zhanybekova, S.; Gillingham, D. A new water soluble copper N-heterocyclic carbene complex delivers mild O(6)G-selective RNA alkylation. Chem. Commun. 2018, 54, 9174–9177. [Google Scholar] [CrossRef]
- Ozcan, S.; Dengiz, C.; Deliömeroglu, M.K.; Sahin, E.; Balci, M. A novel one-pot, three-component reaction for the synthesis of isocoumarin-condensed pyrazoles. Tetrahedron Lett. 2011, 52, 1495–1497. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Abdelmalak, M.; Marchand, C.; Agama, K.; Pommier, Y.; Cushman, M. Synthesis and biological evaluation of nitrated 7-, 8-, 9-, and 10-hydroxyindenoisoquinolines as potential dual topoisomerase I (Top1)-tyrosyl-DNA phosphodiesterase I (TDP1) inhibitors. J. Med. Chem. 2015, 58, 3188–3208. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).