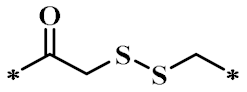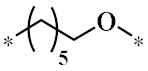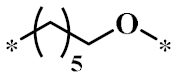Abstract
A series of novel paclitaxel derivatives modified by boronic acid according to the characteristics of the interaction between RB(OH)2 and different strapping agents of intraliposomal aqueous phase were designed and synthesized, which were then used to develop remote poorly water-soluble drugs loading into liposomes. Meanwhile, we screened nineteen paclitaxel boronic acid derivatives for their cytotoxic activities against three cancer cell lines (A549, HCT-116 and 4T1) and one normal cell line (LO2), and performed liposome formulation screening of active compounds. Among all the compounds, the liposome of 4d, with excellent drug-encapsulated efficiency (>95% for drug-to-lipid ratio of 0.1 w/w), was the most stable. Furthermore, the liposomes of compound 4d (8 mg/kg, 4 times) and higher dose of compound 4d (24 mg/kg, 4 times) showed better therapeutic effect than paclitaxel (8 mg/kg, 4 times) in the 4T1 tumor model in vivo, and the rates of tumor inhibition were 74.3%, 81.9% and 58.5%, respectively. This study provided a reasonable design strategy for the insoluble drugs to improve their drug loading into liposomes and anti-tumor effect in vivo.
1. Introduction
Paclitaxel, a natural product with effectiveness, is one of the most widely used clinical anticancer agents [1,2] and shows commendable antitumor activity against various cancers, especially breast cancer [3,4]. The mechanism of paclitaxel is to inhibit the proliferation of tumor cells by stabilizing the microtubules and inhibiting the G2 or M phases of the cell cycle [5]. It has been reported that Taxol® is the first marketed preparation of paclitaxel, but histamine may be produced with the degradation of its cosolvent Cremophor EL(CrEL), which results in allergic reactions in patients [6,7]. Similar to Taxol®, and although other marketed paclitaxel formulations have shown potent antitumor activity against various cancers, there are still problems which limit their clinical applications, such as poor water solubility and low bioavailability, as well as drug resistance [8,9,10,11].
The advantage of a drug carrier is to improve the targeting ability, water solubility and PK properties of a drug [12,13,14,15,16,17]. Paclitaxel, as a non-ionizable drug, is difficult to stably encapsulate in a drug carrier. Fascicle chemical modification or derivatization of drugs is effective in developing remote poorly water-soluble drugs loading into liposomes. In our previous study, we found that weak acidic or basic derivatives of insoluble drugs can be actively encapsulated into liposomes by pH gradients [18,19,20]. In addition to pH gradients, there are many other remote loading gradients, such as copper ion gradients, which can complex with and encapsulate drugs into liposomes [21]. Boronic acid RB(OH)2 can both spontaneously complex with copper ion and undergo dynamic covalent reactions with diols, such as catechol, sugar and mannitol. Inspired by the characteristics of boronic acid RB(OH)2, we expected that the boronic acid derivatives would form precipitates by the complexation after entering the inner phase of liposomes, so as to achieve the active drug loading in liposomes.
Therefore, we designed and synthesized nineteen boric-acid-modified paclitaxel derivatives and evaluated their cytotoxic activities against three cancer cell lines (lung cancer cell line A549, colon cancer cell line HCT-116, breast cancer cell line 4T1) and one normal cell line (hepatocyte cell line LO2). In addition, the selected compounds 4a, 4d, 13a, 17a and 17c were further screened by liposome prescription. As a result, compound 4d was chosen as the preferred prodrug as it possessed better loading efficiencies (>95% for drug-to-lipid ratio of 0.1 w/w). Additionally, liposomes loaded with compound 4d showed the best therapeutic effect in the 4T1 tumor model in vivo.
2. Results and Discussion
2.1. Design the Combination Strategy of Paclitaxel Derivatives
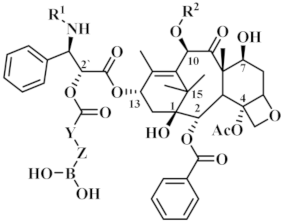
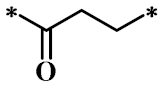
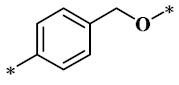
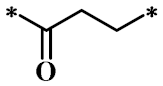
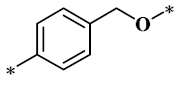
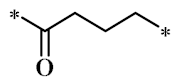
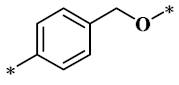
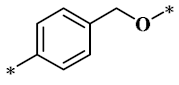
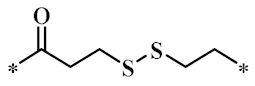
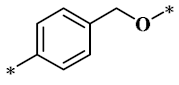
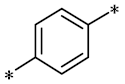
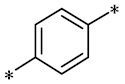
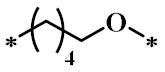
According to the structure-activity relationship of paclitaxel [22], the 2’-OH of paclitaxel is both an active site and a common structural modification site. Therefore, we introduced boronic acid into 2’-OH by using the combination strategy. There are three key parts in the designed derivatives (Figure 1): (i) Boronic acid group; (ii) Linker fragment; (iii) Paclitaxel drug. Boronic acid groups interact with the trapping agents of the intraliposomal aqueous phase to achieve the purpose that derivatives are encapsulated by liposomes. The Linker fragment is a breakable ester bond or carbonate bond.
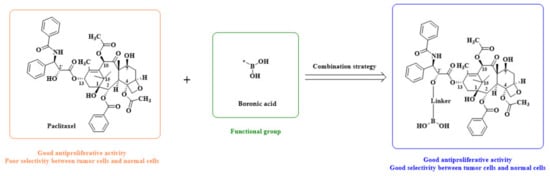
Figure 1.
Combination strategy of designing paclitaxel derivatives by linking paclitaxel (as a parent drug) with a boronic acid (as the functional group) via ester bond or carbonate bond.
2.2. Chemistry
The synthetic routes of the target compounds are illustrated in Scheme 1. Anhydrides 1a–1d were obtained via a dehydration reaction of alkyldicarboxylic acids, then reacted with 4-(hydroxymethyl)benzeneboronic acid pinacol ester to obtain intermediates 2a–2d, and then esterified with paclitaxel or docetaxel to obtain key intermediates 3a–3e. Finally, the target compounds 4a–4e and 6a–6b were obtained by oxidation hydrolysis.
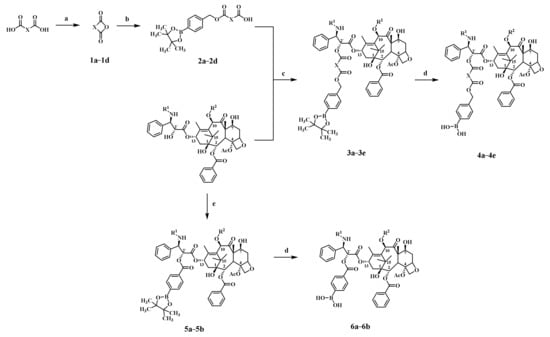
Scheme 1.
Reagents and conditions: a. Ac2O, r.t.; b. (4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol, DMAP, dry DCM, r.t.; c. EDCI, DMAP, dry DCM, r.t.; d. NaIO4, NH4OAc, H2O, acetone, r.t.; e. 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzoic acid, EDCI, DMAP, dry DCM, r.t.
The key intermediates 7a–7b were obtained by Arbuzov reaction of bromoalkyl alcohols, and then reacted with 4-nitrophenyl chloroformate to give 8a–8b, followed by esterification and oxidative hydrolysis to give the target compounds 10a–10c (Scheme 2). The synthetic routes of the target compounds 13a–13b and 17a–17g were similar to those of the compounds 10a–10c. The structures of target compounds, as listed in Table 1, were confirmed by 1H-NMR, 13C-NMR and HRMS.
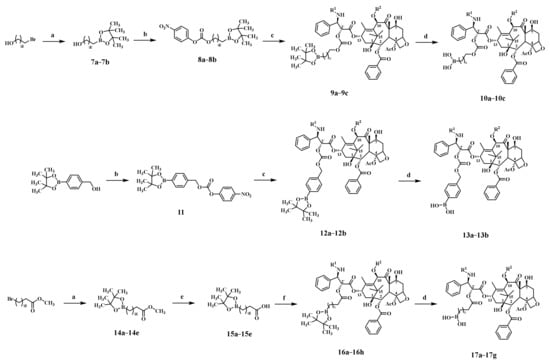
Scheme 2.
Reagents and conditions: a. bis(pinacolato)diboron, CuCl, PCy3, t-BuOK, dry THF, N2, 0 °C; b. 4-Nitrophenyl chloroformate, Py, dry DCM, −20 °C; c. paclitaxel or docetaxel, DMAP, dry DCM, r.t.; d. NaIO4, NH4OAc, H2O, acetone, r.t.; e. NaOH, MeOH, r.t.; f. paclitaxel or docetaxel, EDCI, DMAP, dry DCM, r.t.

Table 1.
The chemical structures of compounds 4a–4e, 6a–6b, 10a–10c, 13a–13b and 17a–17g.
2.3. In Vitro Cytotoxicity Studies
MTT assay was applied to verify the rationality of the molecular combination strategy. The cytotoxicity of the compounds was evaluated on lung cancer cell line A549, colon cancer cell line HCT-116, breast cancer cell line 4T1 and normal hepatocyte cell line LO2. Paclitaxel was used as a positive drug. The results showed that, compared with LO2, the tested compounds were more sensitive to HCT-116, A549 and 4T1, which suggested the cell selectivity of these compounds. The therapeutic index (TI) for LO2/HCT-116, LO2/A549 and LO2/4T1 of active compounds 4a, 4d, 13a, 17a, 17c and paclitaxel are shown in Table 2.

Table 2.
Inhibitory activity against tumor cells A549, HCT-116, 4T1 and normal cell LO2.
According to the results of cytotoxicity, we roughly summarized the structure-activity relationship (SAR): (a) Compound 4d with disulfide bonds showed better selectivity for tumor cells than compound 4a without disulfide bonds. (b) The selectivity of aromatic boronic acid compound 13a was much better than that of alkyl boronic acid compounds (10a–10c). (c) Compound 17c (n = 3) showed the best selectivity, but when the carbon chain was further increased, the compounds 17d–17e (n = 4, 5) showed no selectivity.
2.4. Design and Optimization of Compound 4d Liposome Formulation
Since boronic acid RB(OH)2 is not only a weak acid, but also a structure that can complex with copper ions, ATP and saccharides, we investigated whether different inner phases could be employed to encapsulate compounds (4a, 4d, 13a, 17a, 17c) with good therapeutic index. Firstly, the encapsulated efficiency of calcium acetate gradients was systematically explored and it was found that it was not feasible with the amount of sediment precipitated after loading (Supplementary Materials: Table S1).
The reasons may be that logD (4a:2.466; 4d: 2.413; 13a: 2.540; 17a: 2.309; 17c: 2.559) and pKa (4a: 12.302; 4d: 12.301; 13a: 12.297; 17a: 12.302; 17c: 12.302) of the compounds (4a, 4d, 13a, 17a, 17c) were not suitable for calcium acetate gradients (−2.5 < logD < 2; pKa < 11) [23]. Vyxeos® is a liposomal formulation of a fixed molar ratio (1:5) combination of the antineoplastic drugs daunorubicin (DN) and cytarabine (Cyt) [24]. Daunorubicin is subsequently actively encapsulated with high efficiency through complexation with intra-liposomal copper. The copper gluconate buffer forms a complex with daunorubicin at a ratio of 1:1 to 1:2 [25]. Referring to Vyxeos®, copper ion was selected as a complexed metal ion in the inner phase. In addition to compound 4d, most derivatives (4a, 13a, 17a, 17c) failed in loading into the liposomes, showing obvious sedimentation. Therefore, compound 4d was selected to investigate the effect of the anions on the loading efficiency. Among them, only the neutral gluconate copper was a suitable inner phase to facilitate the encapsulation of compound 4d (Table 3).

Table 3.
Encapsulation efficiency of copper ion gradients.
The reason for the stability of the neutral formulations was potentially due to the fact that PBA was effectively protonated and interacted with copper more tightly in the neutral condition compared with the acidic condition [26]. Thus, the neutral Cu-gluconate was selected as the inner phase. The concentration of copper ions was also investigated, showing that the higher the copper concentration, the higher the EE. Considering the safety of copper ions, 300 mM was chosen. For lipid composition, the content of Chol was 45% (molar ratio), which revealed higher EE. The EE of compound 4d active loading liposome was more than 95% with copper ion gradient. Furthermore, we also evaluated the ATP and saccharides as remote gradients with the optimal formulation of copper ion gradients. The results of encapsulation efficiency are shown in Table 4.

Table 4.
Encapsulation efficiency of ATP and saccharides gradients.
The inner phase of ATP was not successful, which may be caused by the weak binding ability between PBA and ATP. The results of different saccharides gradients showed that saccharides could be selected as the inner phase of liposomes to load PBA-modified derivates, but the EE of compound 4d was not high enough. Thus, the copper ion gradient was chosen for further experiments.
The optimal formulation was shown in Figure 2. The drug to lipid ratio was more than 1:10, drug loading efficiency was up to 9.02% and the prepared liposomes remained stable for more than two weeks at 4 °C.
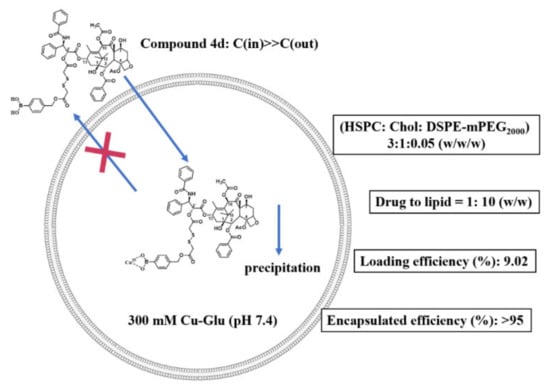
Figure 2.
The structure of compound 4d and its optimal formulations.
The morphology of compound 4d liposome is shown in Figure 3 and Figure 4, with spherical particles, a narrow size distribution at ~110 nm and zeta potential at ~−15 mV (Table S2).
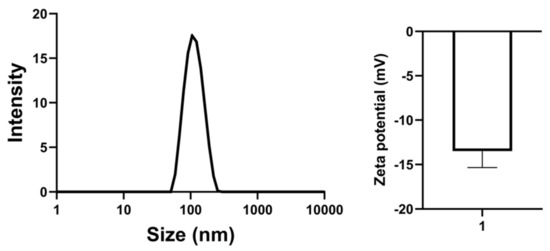
Figure 3.
Particle size and zeta potential of compound 4d liposome.
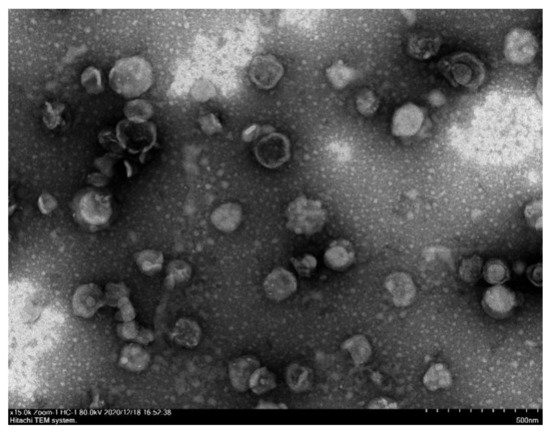
Figure 4.
TEM image of compound 4d liposome.
2.5. In Vitro Release of PTX Derivative 4d Liposomes
In vitro release study of compound 4d liposome (Figure 5) showed that compound 4d was a ROS-sensitive paclitaxel derivative. When the content of H2O2 increased from 0 to 10 mmol/L, the paclitaxel released by compound 4d significantly increased. Paclitaxel release at high H2O2 content (1 mM and 10 mM) for 24 h (75.3% and 67.7%) was much better than that at low H2O2 content (0.1 μM and 1 μM, 19.1% and 29.6%). The results showed that compound 4d liposome was stable in plasma and could reduce the toxicity of paclitaxel in systemic circulation, indicating that compound 4d liposome could effectively release paclitaxel in tumor tissue to achieve targeted anti-tumor effect.
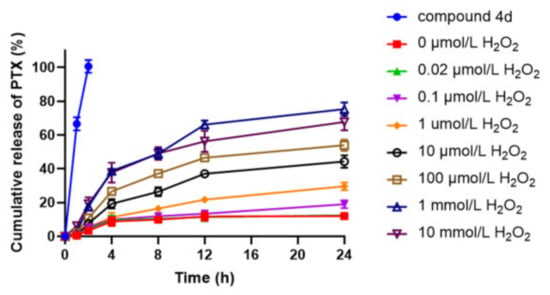
Figure 5.
In vitro ROS-sensitive release studies of PTX.
2.6. Study of Palictaxel Derivative Loaded Liposomes In Vivo
A549 and HCT-116 cells are human tumor cells, while 4T1 cells are mouse tumor cells. Based on the availability of the tumor model, mice bearing a 4T1 xenograft tumor were used to study the in vivo antitumor efficacy of the compound 4d liposome. As depicted in Figure 6a, there was difference between compound 4d (8 mg/kg) and the paclitaxel control group (8 mg/kg). Furthermore, compound 4d liposome (8 mg/kg) significantly inhibited tumor growth, which may be due to its better stability, better drug delivery advantages and more effective drug release from tumor cells. When administered with a higher dose of compound 4d liposome (24 mg/kg), the tumor volume further decreased, showing obviously anti-tumor effect in vivo. In addition, according to changes in animal weight, all liposome preparations showed no obvious toxic effects (Figure 6b).
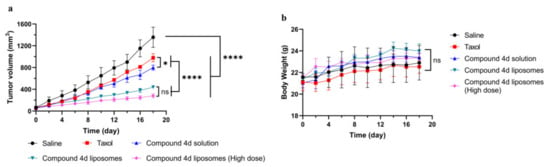
Figure 6.
Compound 4d liposome against 4T1 xenograft tumor in vivo. (a) Tumor growth profiles. (b) Body weight. All data are presented as mean ± SD (n = 5), * p < 0.05 and **** p < 0.0001 by two-tailed Student’s t test. “ns” mean “no significance”.
3. Materials and Methods
3.1. Materials
All chemical materials and solvents were obtained from commercial suppliers without further purification. Intermediates were purified by silica gel column chromatography (200–300 mesh). Nuclear magnetic resonance (NMR) spectra were generated on a Bruker Avance III-600 instrument (600 MHz for 1H and 150 MHz for 13C), using TMS as an internal standard. High-resolution mass spectra experiments (HR-MS) were performed on an Agilent Accurate-Mass Q-TOF 6530 instrument. Hydrogenated soybean phosphatidylcholine (HSPC), cholesterol (for injection) (chol), and 2-distearoyl-snglycero-3-phosphoethanolamine-N-[methyl(polyethyleneglycol)-2000 (DSPE-PEG2000) were purchased from Shanghai Advanced Vehicle Technology Pharmaceutical Ltd. (Shanghai, China).
3.2. Chemistry
3.2.1. General Synthesis Procedure for Intermediates 1a–1d
Compounds 1a–1d were prepared according to the literature [27]. Diacids (2.89 mmol) were added in acetic anhydride (5 mL), followed by nitrogen protection. The reaction was stirred at room temperature for 2 h. Then, dry toluene (10 mL) was added and the solvent was removed by reduced pressure. The product did not need to be purified.
3.2.2. General Synthesis Procedure (A) for Esterification
(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol (2.03 mmol), acid anhydride (6.09 mmol) and DMAP (0.61 mmol) were added to dry DCM (10 mL), followed by nitrogen protection. The reaction was stirred at room temperature for 3 h and monitored by TLC. The mixture was washed with 2 M HCl solution and extracted with DCM three times. The combined organic layer was dried over Na2SO4 and evaporated in a vacuum. The crude product was purified by silica gel column chromatography (petroleum ether: acetone = 10:1) to obtain the product.
3.2.3. General Synthesis Procedure (B) for Esterification
Carboxylic acid (0.35 mmol) and DMAP (0.04 mmol) were added to dry DCM (10 mL). The mixture was stirred at −20 °C for 10 min, followed by adding EDCI (0.35 mmol). Then, the reaction was stirred at −20 °C for 30 min, followed by adding paclitaxel or docetaxel (0.12 mmol). Then, the reaction was stirred at −20 °C for 60 min, and transferred to room temperature for 5–8 h. The mixture was washed with 2 M HCl solution and extracted with DCM three times. The combined organic layer was dried over Na2SO4 and evaporated in a vacuum. The crude product was purified by thin layer silica gel column chromatography (dichloromethane: methanol = 20:1) to obtain the product.
3.2.4. General Synthesis Procedure (C) for the Hydrolysis Reaction
To a solution of boronic acid ester (0.13 mmol) in acetone (5 mL), a solution of sodium periodate (0.52 mmol) and ammonium acetate (0.65 mmol) in water (5 mL) was added and the reaction was stirred at room temperature for 8 h. The solid was precipitated after acetone was evaporated in a vacuum. The crude product was purified by thin layer silica gel column chromatography (dichloromethane: methanol = 20:1) to obtain the product.
3.2.5. General Synthesis Procedure (D) for the Arbuzov Reaction
CuCl (0.39 mmol), bis(pinacolato)diboron (1.97 mmol) and PCy3 (0.39 mmol) were added to DCM (20 mL), followed by nitrogen protection. Then, t-BuOK (1.97 mmol) was added and the reaction was at 0 °C for 30 min. Finally, bromide (1.31 mmol) was added and the reaction was at 0 °C for 30 min. The mixture was washed with water and extracted with DCM three times. The combined organic layer was dried over anhydrous sodium sulfate and evaporated in a vacuum. The crude product was purified by silica gel column chromatography (petroleum ether: acetone = 100:1) to obtain the product.
3.2.6. General Synthesis Procedure (E) for Esterification
Alcohol (5.13 mmol), pyridine (14.89 mmol) and 4-nitrophenyl chloroformate (7.66 mmol) were added to dry DCM (10 mL), followed by nitrogen protection. The reaction was stirred at −20 °C for 5 h. The mixture was washed with 2 M HCl solution and extracted with DCM three times. The combined organic layer was dried over Na2SO4 and evaporated in a vacuum. The crude product was purified by silica gel column chromatography (petroleum ether: acetone = 100:3) to obtain the product.
3.2.7. General Synthesis Procedure (F) for Esterification
Paclitaxel or docetaxel (0.12 mmol), DMAP (0.36 mmol) and ester (0.36 mmol) were added to dry DCM (10 mL). Then, the reaction was stirred at room temperature for 2 h. The mixture was washed with 2 M HCl solution and extracted with DCM three times. The combined organic layer was dried over Na2SO4 and evaporated in a vacuum. The crude product was purified by thin layer silica gel column chromatography (dichloromethane: methanol = 20:1) to obtain the product.
3.2.8. General Synthesis Procedure (G) for the Hydrolysis Reaction
To a solution of methyl carboxylate (2.20 mmol) in methanol (5 mL), 2 N sodium hydroxide solution (10 mL) was added. The reaction was stirred at room temperature for 3 h. The mixture was added to 2 M HCl solution, adjusted to pH = 3, and extracted with DCM three times. The combined organic layer was dried over Na2SO4 and evaporated in a vacuum. The product did not need to be purified.
3.2.9. Preparation of Compound 4a–4e (Exemplified by 4a)
4-oxo-4-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy)butanoic acid (2a).
Following general procedure A, compound 2a was obtained from compound 1a and 4-hydroxymethyl phenylborate pinacol ester (0.53g, 78%).1H-NMR (600 MHz, DMSO-d6) δH 12.20 (s, 1H), 7.66 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 7.8 Hz, 2H), 5.12 (s, 2H), 2.59–2.57 (m, 2H), 2.49–2.46 (m, 2H), 1.29 (s, 12H).
2’-O-[4-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl))oxy)-4-oxobutyryl] taxol (3a).
Following general procedure B, compound 3a was obtained from compound 2a and palictaxel (0.13g, 24%). 1H-NMR (600 MHz, CDCl3) δH 8.14 (d, J = 7.2 Hz, 2H), 7.78 (d, J = 7.2 Hz, 4H), 7.60 (t, J = 7.2 Hz, 1H), 7.53–7.47 (m, 3H), 7.42–7.37 (m, 6H), 7.34–7.31 (m, 1H), 7.25–7.24 (m, 2H), 7.06 (d, J = 9.2 Hz, 1H), 6.29 (s, 1H), 6.24 (t, J = 8.4 Hz, 1H), 5.99 (dd, J = 9.1, 3.0 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 3.1 Hz, 1H), 4.99 (s, 2H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.44 (dd, J = 11.0, 6.6 Hz, 1H), 4.31 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.81 (d, J = 7.0 Hz, 1H), 2.77–2.75 (m, 2H), 2.66–2.64 (m, 2H), 2.52–2.50 (m, 1H), 2.44 (s, 3H), 2.39–2.35 (m, 2H), 2.22 (s, 3H), 1.93 (s, 3H), 1.88 (ddd, J = 14.7, 11.0, 2.3 Hz, 1H), 1.68 (s, 3H), 1.33 (s, 12H), 1.23 (s, 3H), 1.13 (s, 3H).
2’-O-[4-((4-boronobenzyl)oxy)-4-oxobutyryl]taxol (4a).
Following general procedure C, compound 4a was obtained from compound 3a (40 mg, 33%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 7.1 Hz, 2H), 7.78 (d, J = 7.1 Hz, 2H), 7.74 (d, J = 7.8 Hz, 2H), 7.61 (t, J = 7.2 Hz, 1H), 7.53–7.51 (m, 4H), 7.42–7.38 (m, 6H), 7.32–7.31 (m, 2H), 7.04 (d, J = 9.0 Hz, 1H), 6.32 (s, 1H), 6.22 (t, J = 9.7 Hz, 1H), 5.92 (dd, J = 9.1, 3.2 Hz, 1H), 5.69 (d, J = 6.8 Hz, 1H), 5.45 (d, J = 3.3 Hz, 1H), 5.08 (s, 2H), 4.97 (d, J = 9.7 Hz, 1H), 4.43 (dd, J = 11.0, 6.9 Hz, 1H), 4.32 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.81 (d, J = 7.2 Hz, 1H), 2.81–2.77 (m, 2H), 2.67–2.65 (m, 2H), 2.45–2.43 (m, 1H), 2.41 (s, 3H), 2.38–2.33 (m, 2H), 2.24 (s, 3H), 1.89 (s, 3H), 1.88–1.85 (m, 1H), 1.69 (s, 3H), 1.23 (s, 3H), 1.15 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 203.7, 171.9, 171.5, 171.2, 169.9, 168.0, 167.2, 167.0, 142.7, 138.5, 136.9, 133.9 (2C), 133.7, 133.5, 132.8, 132.0, 130.2 (2C), 130.2, 129.1 (2C), 128.7 (4C), 128.5, 127.3 (2C), 127.2 (3C), 126.6 (2C), 84.4, 81.1, 79.1, 76.4, 75.7, 75.0, 74.2, 71.9, 71.8, 66.4, 58.5, 52.8, 45.7, 43.2, 35.7, 35.5, 29.7, 29.2, 29.0, 26.7, 22.6, 20.9, 14.7, 9.6. HR-MS (ESI): m/z calcd for C58H63BNO19+ ([M+H]+) 1088.4082, found: 1088.4093.
Compounds 4b–4e and 6a–6b were prepared by the same method.
2’-O-[4-((4-boronobenzyl)oxy)-4-oxobutyryl]docetaxel (4b).
Following general procedure A, B and C, compound 4b was obtained (35 mg, 6%). 1H-NMR (600 MHz, CDCl3) δH 8.09 (d, J = 7.5 Hz, 2H), 7.74 (d, J = 7.5 Hz, 2H), 7.60 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 7.6 Hz, 2H), 7.36 (d, J = 7.6 Hz, 2H), 7.30–7.27 (m, 5H), 6.14 (t, J = 9.1 Hz, 1H), 5.65 (d, J = 6.7 Hz, 1H), 5.57 (d, J = 9.5 Hz, 1H), 5.43 (s, 1H), 5.28 (d, J = 9.5 Hz, 1H), 5.24 (s, 1H), 5.11 (s, 2H), 4.95 (dd, J = 8.8, 2.0 Hz, 1H), 4.31 (d, J = 8.8 Hz, 1H), 4.25 (dd, J = 11.0, 6.7 Hz, 1H), 4.18 (d, J = 8.4 Hz, 1H), 3.88 (d, J = 7.0 Hz, 1H), 2.78–2.75 (m, 1H), 2.70–2.66 (m, 2H), 2.56–2.53 (m, 2H), 2.40 (s, 3H), 2.32–2.28 (m, 2H), 1.99 (s, 3H), 1.97–1.96 (m, 1H), 1.83 (s, 3H), 1.33 (s, 9H), 1.19 (s, 3H), 1.09 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 211.2, 172.0, 171.8, 171.4, 168.0, 167.1, 155.3, 138.3, 138.3, 134.0 (2C), 133.7, 130.2 (2C), 128.9 (2C), 128.8 (2C), 128.7 (2C), 128.7, 128.2, 127.4, 127.3, 126.4 (3C), 84.2, 82.1, 80.4, 78.8, 77.9, 76.5, 75.9, 74.9, 74.7, 71.8, 66.5, 57.6, 57.2, 46.4, 43.0, 29.7, 29.1, 28.8, 28.1 (3C), 26.3, 22.6, 20.8, 16.8, 14.2, 9.9. HR-MS (ESI): m/z calcd for C54H64BNO19Na+ ([M+Na]+) 1064.4058, found: 1064.4065.
2’-O-[5-((4-boronobenzyl)oxy)-5-oxopentanoyl]taxol (4c).
Following general procedure A, B and C, compound 4c was obtained (43 mg, 7%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 7.3 Hz, 2H), 7.73 (d, J = 7.7 Hz, 2H), 7.71 (d, J = 7.8 Hz, 2H), 7.60 (t, J = 7.5 Hz, 1H), 7.52–7.46 (m, 3H), 7.41–7.35 (m, 6H), 7.33–7.29 (m, 3H), 7.13 (d, J = 9.2 Hz, 1H), 6.30 (s, 1H), 6.23 (t, J = 9.0 Hz, 1H), 5.96 (dd, J = 9.2, 3.4 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.49 (d, J = 3.5 Hz, 1H), 5.10 (s, 2H), 4.96 (dd, J = 9.7, 2.3 Hz, 1H), 4.42 (dd, J = 10.9, 6.7 Hz, 1H), 4.30 (d, J = 8.4 Hz, 1H), 4.20 (d, J = 8.5 Hz, 1H), 3.80 (d, J = 7.1 Hz, 1H), 2.56–2.52 (m, 1H), 2.49–2.46 (m, 2H), 2.43 (s, 3H), 2.39–2.33 (m, 2H), 2.21 (s, 3H), 2.04–1.96 (m, 4H), 1.92 (s, 3H), 1.89–1.87(m, 1H), 1.67 (s, 3H), 1.22 (s, 3H), 1.13 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 203.8, 172.7, 171.9, 171.3, 169.8, 168.2, 167.4, 167.0, 142.6, 138.3, 136.8, 134.0, 133.7, 133.5, 132.8, 132.0, 130.2 (2C), 129.2, 129.0 (2C), 128.7 (4C), 128.5, 127.2, 127.1 (4C), 127.1, 126.5, 126.5, 84.4, 81.0, 79.0, 76.4, 75.6, 75.0, 74.1, 72.0, 71.8, 66.1, 58.4, 52.8, 45.6, 43.1, 35.5, 35.5, 32.8, 32.6, 26.7, 22.6, 22.0, 20.8, 20.0, 14.8, 9.6. HR-MS (ESI): m/z calcd for C59H65BNO19+ ([M+H]+) 1102.4238, found: 1102.4249.
2’-O-[2-((2-((4-boronobenzyl)oxy)-2-oxoethyl)disulfaneyl)acetyl]taxol (4d).
Following general procedure A, B and C, compound 4d was obtained (44 mg, 7%). 1H-NMR (600 MHz, CDCl3) δH 8.14 (d, J = 7.2 Hz, 2H), 7.76 (d, J = 7.0 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H), 7.60 (t, J = 7.2 Hz, 1H), 7.53–7.48 (m, 4H), 7.43–7.41 (m, 6H), 7.33 (d, J = 7.0 Hz, 2H), 7.16 (d, J = 9.1 Hz, 1H), 6.29 (s, 1H), 6.23 (t, J = 8.4 Hz, 1H), 5.97 (dd, J = 9.0, 3.4 Hz, 1H), 5.69 (d, J = 7.1 Hz, 1H), 5.50 (d, J = 2.9 Hz, 1H), 5.16–5.15 (m, 2H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.43–4.41 (m, 1H), 4.32 (d, J = 8.6 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.80 (d, J = 7.4 Hz, 1H), 3.50–3.49 (m, 4H), 2.57–2.52 (m, 1H), 2.42 (s, 3H), 2.35–2.31 (m, 2H), 2.22 (s, 3H), 1.89 (s, 3H), 1.88–1.85 (m, 1H), 1.68 (s, 3H), 1.22 (s, 3H), 1.13 (s, 3H). 13C-NMR (150 MHz, CDCl3) δC 203.8, 171.5, 170.0, 169.6, 168.4, 167.8, 167.5, 167.0, 142.7, 137.7, 136.7, 134.0 (2C), 133.7 (2C), 132.8, 132.0, 130.2 (2C), 129.2, 129.1 (2C), 128.8 (2C), 128.7 (3C), 128.6, 127.7 (2C), 127.2 (2C), 126.8 (2C), 84.4, 81.2, 79.1, 76.5, 75.7, 75.0 (2C), 72.1, 72.0, 67.4, 58.5, 53.0, 45.7, 43.2, 41.4, 40.3, 35.6, 35.5, 26.8, 22.7, 22.0, 20.9, 14.8, 9.6. HR-MS (ESI): m/z calcd for C58H63BNO19S2+ ([M+H]+) 1152.3523, found: 1152.3539.
2’-O-[3-((3-((4-boronobenzyl)oxy)-3-oxopropyl)disulfaneyl)propionyl]taxol (4e).
Following general procedure A, B and C, compound 4e was obtained (49 mg, 9%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 8.1 Hz, 2H), 7.74 (d, J = 8.1 Hz, 2H), 7.70 (d, J = 6.8 Hz, 2H), 7.61 (t, J = 6.7 Hz, 1H), 7.53–7.52 (m, 5H), 7.41–7.37 (m, 6H), 7.32–7.30 (m, 1H), 7.05 (d, J = 10.8 Hz, 1H), 6.29 (s, 1H), 6.23 (t, J = 9.0 Hz, 1H), 5.68 (d, J = 7.1 Hz, 1H), 5.61 (d, J = 7.5 Hz, 1H), 5.53 (d, J = 3.5 Hz, 1H), 5.30 (s, 2H), 4.96 (dd, J = 11.7, 2.4 Hz, 1H), 4.42 (dd, J = 10.9, 6.7 Hz, 1H), 4.31 (d, J = 8.6 Hz, 1H), 4.20 (d, J = 8.3 Hz, 1H), 3.80 (d, J = 6.9 Hz, 1H), 2.90–2.88 (m, 2H), 2.86–2.81 (m, 4H), 2.76–2.72 (m, 2H), 2.58–2.52 (m, 1H), 2.43 (s, 3H), 2.37–2.31(m, 2H), 2.17 (s, 3H), 1.88–1.85 (m, 1H), 1.79 (s, 3H), 1.68 (s, 3H), 1.21 (s, 3H), 1.12 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 203.8, 171.5, 170.0, 169.6, 168.4, 167.8, 167.4, 167.0, 142.7, 137.7, 136.7, 134.0 (2C), 133.7 (2C), 133.6, 132.8, 132.0, 130.2 (2C), 129.1, 128.8 (2C), 128.7 (3C), 128.6, 128.5, 127.7 (2C), 127.2 (2C), 126.8 (2C), 84.4, 81.2, 79.1, 76.5, 75.7, 75.0 (2C), 72.1, 72.0, 67.4, 58.5, 53.0, 45.7, 43.2, 35.6 (2C), 35.5, 30.8, 26.8 (2C), 26.5, 22.7, 22.0, 20.9, 14.8, 9.6. HR-MS (ESI): m/z calcd for C60H67BNO19S2+ ([M+H]+) 1180.3836, found: 1180.3837.
2’-O-[4-boronobenzoyl]taxol (6a).
Following general procedure B and C, compound 6a was obtained (44 mg, 10%). 1H-NMR (600 MHz, CDCl3) δH 8.12 (d, J = 6.8 Hz, 2H), 7.99 (d, J = 7.9 Hz, 2H), 7.85 (d, J = 7.8 Hz, 2H), 7.76 (d, J = 7.8 Hz, 2H), 7.60 (t, J = 7.2 Hz, 1H), 7.53–7.50 (m, 3H), 7.46–7.40 (m, 6H), 7.34–7.32 (m, 1H),7.06 (d, J = 9.1 Hz, 1H), 6.29 (s, 1H), 6.26 (t, J = 9.7 Hz, 1H), 6.04 (dd, J = 9.0, 3.9 Hz, 1H), 5.70 (d, J = 3.9 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 4.97 (d, J = 9.7 Hz, 1H), 4.45 (dd, J = 11.0, 6.6 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.19 (d, J = 8.3 Hz, 1H), 3.81 (d, J = 7.1 Hz, 1H), 2.57–2.53 (m, 1H), 2.43 (s, 3H), 2.35–2.31 (m, 2H), 2.23 (s, 3H), 1.96 (s, 3H), 1.91–1.86 (m, 1H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 203.8, 171.3 (2C), 169.8, 167.1 (2C), 165.6, 142.8, 136.9, 134.2, 133.9, 133.7, 132.7, 132.0, 130.2 (2C), 130.0, 129.7 (2C), 129.6 (2C), 129.5 (2C), 129.1 (2C), 128.8 (4C), 127.1 (2C), 126.7 (2C), 84.4, 81.0, 79.2, 76.4, 75.6, 75.1, 74.8, 72.1, 67.8, 58.5, 53.8, 45.5, 43.2, 35.5, 31.9, 29.7, 26.8, 22.7, 20.8, 14.1, 9.6. HR-MS (ESI): m/z calcd for C54H57BNO17+ ([M+H]+) 1002.3714, found: 1002.3721.
2’-O-[4-boronobenzoyl]docetaxel (6b).
Following general procedure B and C, compound 6b was obtained (34 mg, 9%). 1H-NMR (600 MHz, CDCl3) δH 8.10 (d, J = 8.2 Hz, 2H), 7.97 (d, J = 7.6 Hz, 2H), 7.88 (d, J = 7.6 Hz, 1H), 7.82 (d, J = 7.6 Hz, 2H), 7.62 (d, J = 7.4 Hz, 2H), 7.52–7.50 (m, 2H), 7.38–7.37 (m, 3H), 6.23 (t, J = 9.0 Hz, 1H), 5.68 (d, J = 7.9 Hz, 1H), 5.54 (s, 2H), 5.46 (d, J = 7.0 Hz, 1H), 5.22 (s, 1H), 4.96 (d, J = 9.4 Hz, 1H), 4.31 (d, J = 7.8 Hz, 1H), 4.27 (dd, J = 7.3, 5.7 Hz, 1H), 4.19 (d, J = 8.7 Hz, 1H)), 3.93 (d, J = 6.6 Hz, 1H), 2.60–2.54 (m, 1H), 2.42 (s, 3H), 2.33–2.28 (m, 2H), 1.97 (s, 3H), 1.91–1.88 (m, 1H), 1.74 (s, 3H), 1.34 (s, 9H), 1.21 (s, 3H), 1.11 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 211.2, 173.3, 169.9, 167.9, 167.0, 155.3, 139.0, 137.3 (2C), 135.6, 133.7, 130.2 (2C), 130.1 (2C), 130.0, 129.2, 128.8 (2C), 128.7 (2C), 128.2, 126.7, 126.4 (3C), 84.3, 81.1, 78.8, 76.5, 75.0, 74.4, 74.3, 72.1, 72.0, 71.7, 57.5, 57.2, 46.5, 43.1, 35.7, 29.3, 28.1 (3C), 26.3, 22.6, 20.8, 14.2, 9.9. HR-MS (ESI): m/z calcd for C50H59BNO17+ ([M+H]+) 956.3871, found: 956.3862.
3.2.10. Preparation of Compounds 10a–10c and 13a–13b (Exemplified by 10b)
6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)hexan-1-ol (7b).
Following general procedure D, compound 7b was obtained from bromide (0.77 g, 65%).
4-nitrophenyl (6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)hexyl) carbonate (8b).
Following general procedure E, compound 8b was obtained from 7b (0.51 g, 77%). 1H-NMR (600 MHz, CDCl3) δH 8.21 (d, J = 9.1 Hz, 2H), 7.31 (d, J = 9.1 Hz, 2H), 4.21 (t, J = 6.7 Hz, 2H), 1.71–1.66 (m, 2H), 1.39–1.33 (m, 4H), 1.31–1.27 (m, 2H), 1.18 (s, 12H), 0.72 (t, J = 7.7 Hz, 2H).
2’-O-[6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)hexyl carbonate]taxol (9b).
Following general procedure F, compound 9b was obtained from 8b and paclitaxel (0.13 g, 33%).
2’-O-[6-boronohexyl carbonate]taxol (10b).
Following general procedure C, compound 10b was obtained from 9b (53 mg, 43%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 7.1 Hz, 2H), 7.74 (d, J = 7.1 Hz, 2H), 7.60 (t, J = 7.1 Hz, 1H), 7.52–7.49 (m, 3H), 7.42–7.39 (m, 6H), 7.36 -7.34 (m, 1H), 7.07 (d, J = 9.3 Hz, 1H), 6.30 (s, 1H), 6.25 (t, J = 9.1 Hz, 1H), 5.97 (dd, J = 9.1, 3.7 Hz, 1H), 5.69 (d, J = 7.2 Hz, 1H), 5.44 (d, J = 3.1Hz, 1H), 4.97 (dd, J = 9.6, 2.4 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 4.13–4.10 (m, 1H), 3.81 (d, J = 7.1 Hz, 1H), 2.57–2.52 (m, 1H), 2.45 (s, 3H), 2.40–2.36 (m, 2H), 2.22 (s, 3H), 2.00–1.97 (m, 2H), 1.92 (s, 3H), 1.91–1.86 (m, 1H), 1.68 (s, 3H), 1.67–1.63 (m, 2H), 1.37–1.29 (m, 6H), 1.23 (s, 3H), 1.14 (s, 3H), 0.73 (t, J = 7.6 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 203.7, 171.3, 169.9, 168.1, 167.3, 167.0, 154.2, 142.5, 136.7, 133.7, 133.5, 132.8, 132.0, 130.2 (2C), 129.2, 129.1 (2C), 128.7 (4C), 128.5, 127.2 (2C), 126.6 (2C), 84.4, 81.1, 79.1, 76.6, 76.4, 75.6, 75.0, 72.1, 72.0, 69.4, 58.4, 52.8, 45.6, 43.2, 35.6, 35.5, 31.4, 29.7, 28.1, 26.8, 25.2, 23.9, 22.6, 22.1, 20.8, 14.8, 9.6. HR-MS (ESI): m/z calcd for C54H65BNO18+ ([M+H]+) 1026.4289, found: 1026.4290.
Compounds 10a, 10c and 13a–13b were prepared by the same method.
2’-O-[5-boronopentyl carbonate]taxol (10a).
Following general procedure D, E, F and C, compound 10a was obtained (54 mg, 7%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 6.7 Hz, 2H), 7.74 (d, J = 6.7 Hz, 2H), 7.61 (t, J = 6.7 Hz, 1H), 7.53–7.50 (m, 3H), 7.43–7.39 (m, 5H), 7.37–7.34 (m, 2H), 7.02 (d, J = 9.3 Hz, 1H), 6.30 (s, 1H), 6.22 (t, J = 9.7 Hz, 1H), 5.98 (dd, J = 9.3, 3.1 Hz, 1H), 5.69 (d, J = 7.1 Hz, 1H), 5.45 (d, J = 3.2 Hz, 1H), 4.97 (dd, J = 9.6, 2.4 Hz, 1H), 4.43 (dd, J = 10.9, 6.7 Hz, 1H), 4.32 (d, J = 8.5 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.81 (d, J = 7.1 Hz, 1H), 2.58–2.53 (m, 1H), 2.45 (s, 3H), 2.40–2.35 (m, 2H), 2.23 (s, 3H), 2.22–2.19 (m, 2H), 1.92 (s, 3H), 1.89–1.87 (m, 1H), 1.68 (s, 3H), 1.65–1.62 (m, 2H), 1.41–1.35 (m, 4H), 1.23 (s, 3H), 1.14 (s, 3H), 0.78 (t, J = 7.5 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 203.7, 171.3, 169.9, 168.1, 167.1, 167.0, 154.2, 142.5, 136.8, 133.7, 133.6, 132.8, 132.0, 130.2 (2C), 129.1 (3C), 128.7 (4C), 128.5, 127.2 (2C), 126.7 (2C), 84.4, 81.1, 79.2, 76.6, 76.4, 75.6, 75.0, 72.1, 72.0, 69.3, 58.5, 52.8, 45.7, 43.2, 35.6, 31.9, 29.7, 28.1 (2C), 26.8, 23.6, 22.6, 22.1, 20.8, 14.8, 9.6. HR-MS (ESI): m/z calcd for C53H63BNO18+ ([M+H]+) 1012.4133, found: 1012.4160.
2’-O-[6-boronohexyl carbonate] docetaxel (10c).
Following general procedure D, E, F and C, compound 10c was obtained (36 mg, 6%).1H-NMR (600 MHz, CDCl3) δH 8.11 (d, J = 7.9 Hz, 2H), 7.60 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 7.5 Hz, 2H), 7.41–7.38 (m, 2H), 7.35–7.31 (m, 3H), 6.25 (t, J = 8.9 Hz, 1H), 5.68 (d, J = 6.7 Hz, 1H), 5.62–5.52 (m, 1H), 5.45 (s, 1H), 5.29–5.23 (m, 2H), 4.96 (d, J = 9.0 Hz, 1H), 4.32 (d, J = 8.8 Hz, 1H), 4.21–4.18 (m, 1H), 4.10–4.06 (m, 1H), 3.92 (d, J = 7.1 Hz, 1H), 2.57–2.51 (m, 1H), 2.43 (s, 3H), 2.35–2.31 (m, 2H), 2.25–2.20 (m, 2H), 1.94 (s, 3H), 1.87–1.85 (m, 1H), 1.74 (s, 3H), 1.68–1.63 (m, 2H), 1.39–1.36 (m, 6H), 1.33 (s, 9H), 1.25 (s, 3H), 1.23 (s, 3H), 0.77 (t, J = 7.3 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 202.2, 169.7, 168.0, 167.0, 167.0, 154.2, 137.3, 133.6, 130.2 (2C), 128.9 (2C), 128.8 (2C), 128.7 (2C), 128.2, 126.5 (3C), 84.3, 81.0, 80.6, 79.3, 78.9, 76.6, 76.1, 74.7, 74.5, 71.8, 69.2, 58.3, 57.5, 46.4, 45.3, 43.6, 43.1, 41.7, 40.9, 35.6, 29.7, 28.1 (3C), 26.9, 26.3, 21.5, 20.9, 14.1, 9.9. HR-MS (ESI): m/z calcd for C50H67BNO18+ ([M+H]+) 980.4446, found: 980.4452.
2’-O-[4-boronobenzyl carbonate]taxol (13a).
Following general procedure E, F and C, compound 13a was obtained (36 mg, 13%). 1H-NMR (600 MHz, CDCl3) δH 8.14 (d, J = 7.2 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H), 7.60 (t, J = 7.2 Hz, 1H), 7.51–7.48 (m, 5H), 7.43–7.35 (m, 9H), 6.93 (d, J = 9.3 Hz, 1H), 6.30 (s, 1H), 6.25 (t, J = 8.4 Hz, 1H), 5.99 (dd, J = 9.0, 3.4 Hz, 1H), 5.69 (d, J = 7.1 Hz, 1H), 5.48 (d, J = 2.9 Hz, 1H), 5.28–5.22 (m, 2H), 4.98 (dd, J = 9.7, 2.3 Hz, 1H), 4.44–4.42 (m, 1H), 4.32 (d, J = 8.6 Hz, 1H), 4.20 (d, J = 8.4 Hz, 1H), 3.82 (d, J = 7.4 Hz, 1H), 2.59–2.53 (m, 1H), 2.46 (s, 3H), 2.40–2.38 (m, 2H), 2.22 (s, 3H), 1.97–1.94 (m, 1H), 1.87 (s, 3H), 1.68 (s, 3H), 1.23 (s, 3H), 1.14 (s, 3H). 13C-NMR (100 MHz, CDCl3) δc 203.8, 171.3, 169.9, 167.8, 167.2, 167.0, 154.1, 142.5, 136.6, 134.1, 133.7, 133.5, 132.8, 132.0, 130.2 (2C), 129.2, 129.1 (2C), 128.7 (2C), 128.7 (3C), 128.6, 127.6, 127.1 (4C), 126.7, 126.6 (2C), 84.4, 81.1, 79.1 (2C), 77.1, 76.5, 75.6, 75.1, 72.2, 70.6, 58.5, 52.8, 45.7, 43.2, 35.6, 29.7, 27.2, 26.8, 22.6, 20.8, 14.8, 9.6. HR-MS (ESI): m/z calcd for C55H59BNO18+ ([M+H]+) 1032.3820, found: 1032.3833.
2’-O-[4-boronobenzyl carbonate] docetaxel (13b).
Following general procedure E, F and C, compound 13b was obtained (36 mg, 11%). 1H-NMR (600 MHz, CDCl3) δH 8.21 (d, J = 8.2 Hz, 2H), 8.09 (d, J = 8.2 Hz, 2H), 7.71 (t, J = 7.6 Hz, 1H), 7.59 (d, J = 7.6 Hz, 2H), 7.49 (d, J = 7.3 Hz, 2H), 7.39–7.36 (m, 2H), 7.33–7.30 (m, 3H), 6.20 (t, J = 9.0 Hz, 1H), 5.66 (d, J = 7.9 Hz, 1H), 5.51 (s, 1H), 5.45 (d, J = 7.0 Hz, 1H), 5.30 (s, 1H), 5.18 (s, 1H), 5.08 (d, J = 7.0 Hz, 1H), 4.96 (d, J = 9.6 Hz, 1H), 4.31 (d, J = 8.7 Hz, 1H), 4.26–4.23 (m, 1H), 4.18 (d, J = 8.7 Hz, 1H)), 3.88 (d, J = 6.6 Hz, 1H), 2.59–2.53 (m, 1H), 2.42 (s, 3H), 2.35–2.21 (m, 2H), 1.88 (s, 3H), 1.87–1.82 (m, 1H), 1.73 (s, 3H), 1.26 (s, 9H), 1.21 (s, 3H), 1.09 (s, 3H). 13C-NMR (150 MHz, CDCl3) δc 211.2, 173.3, 169.9, 167.9, 167.0, 155.3, 139.0, 135.6, 133.7 (2C), 130.2 (2C), 130.1, 129.2, 129.1, 128.8 (3C), 128.7 (2C), 128.2, 127.2, 126.7, 126.4 (3C), 84.3, 81.1, 78.8, 76.5, 75.0, 74.4, 74.3, 72.1, 72.0, 71.7, 67.9, 57.5, 57.2, 46.5, 43.1, 35.7, 29.3, 28.1 (3C), 26.3, 22.6, 20.8, 14.2, 9.9. HR-MS (ESI): m/z calcd for C51H61BNO18+ ([M+H]+) 986.3976, found: 986.3981.
3.2.11. Preparation of Compound 17a–17g (Exemplified by 17b)
Methyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (14b).
Following general procedure D, compound 14b was obtained (0.32 g, 54%). 1H-NMR (600 MHz, CDCl3) δH 3.65 (s, 3H), 2.32 (t, J = 7.6 Hz, 2H), 1.77–1.72 (m, 2H), 1.24 (s, 12H), 0.81 (t, J = 7.9 Hz, 2H).
4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoic acid (15b).
Following general procedure G, compound 15b was obtained from compound 14b (0.25 g, 85%).
2’-O-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoyl]taxol (16b).
Following general procedure B, compound 16b was obtained from compound 15b and paclitaxel (0.15 g, 44%).
2’-O-[4-boronobutanoyl]taxol (17b).
Following general procedure C, compound 17b was obtained from compound 16b (36 mg, 26%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 8.3 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 7.61 (t, J = 8.3 Hz, 1H), 7.53–7.49 (m, 3H), 7.43–7.37 (m, 6H), 7.35–7.32 (m, 1H), 6.98 (d, J = 9.1 Hz, 1H), 6.29 (s, 1H), 6.23 (t, J = 8.4 Hz, 1H), 5.96 (dd, J = 9.2, 3.6 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 5.54 (d, J = 3.7 Hz, 1H), 4.97 (dd, J = 9.5, 2.2 Hz, 1H), 4.43 (dd, J = 10.9, 6.6 Hz, 1H), 4.31 (d, J = 8.4 Hz, 1H), 4.19 (d, J = 8.4 Hz, 1H), 3.80 (d, J = 7.3 Hz, 1H), 2.58–2.53 (m, 1H), 2.45 (s, 3H), 2.43–2.40 (m, 2H), 2.36–2.31 (m, 2H), 2.22 (s, 3H), 1.92 (s, 3H), 1.88 (ddd, J = 14.7, 11.0, 2.3 Hz, 1H), 1.76–1.74 (m, 2H), 1.68 (s, 3H), 1.22 (s, 3H), 1.13 (s, 3H), 0.77 (t, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δc 203.7, 173.0, 171.2, 169.9, 168.5, 167.2, 167.0, 142.6, 136.9, 133.7, 133.6, 132.9, 132.0, 130.2 (2C), 129.2, 129.1(2C), 129.0, 128.7 (3C), 128.5, 127.1 (4C), 84.4, 81.1, 79.1, 76.4, 75.6, 75.1, 73.9, 72.1, 72.0, 58.5, 52.9, 45.6, 43.2, 35.7, 35.5, 29.7, 26.8, 22.7, 22.1, 20.8, 19.7, 14.8, 14.1, 9.6. HR-MS (ESI): m/z calcd for C51H58BNO17Na+ ([M+Na]+) 990.3690, found: 990.3698.
Compounds 17a, 17c–17g were prepared by the same method.
2’-O-[3-boronopropanoyl]taxol (17a).
Following general procedure D, G, B and C, compound 17a was obtained (37 mg, 5%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 8.3 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 7.61 (t, J = 8.3 Hz, 1H), 7.53–7.50 (m, 3H), 7.42–7.37 (m, 7H), 7.06 (d, J = 9.3 Hz, 1H), 6.29 (s, 1H), 6.24 (t, J = 8.4 Hz, 1H), 5.98 (dd, J = 9.2, 3.3 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 5.53 (d, J = 3.3 Hz, 1H), 4.98 (dd, J = 9.5, 2.2 Hz, 1H), 4.43 (dd, J = 10.9, 6.6 Hz, 1H), 4.31 (d, J = 7.8 Hz, 1H), 4.20 (d, J = 8.3 Hz, 1H), 3.80 (d, J = 7.0 Hz, 1H), 2.58–2.53 (m, 1H), 2.45 (s, 3H), 2.43–2.41 (m, 2H), 2.39–2.34 (m, 2H), 2.22 (s, 3H), 1.92 (s, 3H), 1.87 (ddd, J = 14.7, 11.0, 2.3 Hz, 1H), 1.68 (s, 3H), 1.22 (s, 3H), 1.13 (s, 3H), 0.81 (t, J = 8.4 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 210.8, 171.9, 171.1, 169.9, 168.5, 167.5, 166.0, 140.6, 134.2, 130.2 (2C), 129.6, 129.5 (2C), 128.9, 128.9, 128.7 (3C), 128.5, 127.0 (4C), 126.8, 120.7, 115.3, 84.4, 83.3, 79.9, 74.9, 71.2, 70.2, 69.5, 67.8, 58.3, 53.8, 52.7, 45.6, 38.9, 31.7, 30.6, 29.7, 29.3, 29.0, 24.0, 23.0, 22.7, 14.0, 11.1. HR-MS (ESI): m/z calcd for C50H56BNO17Na+ ([M+Na]+) 976.3534, found: 976.3550.
2’-O-[5-boronopentanoyl]taxol (17c).
Following general procedure D, G, B and C, compound 17c was obtained (45 mg, 6%).1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 8.3 Hz, 2H), 7.74 (d, J = 8.3 Hz, 2H), 7.60 (t, J = 8.3 Hz, 1H), 7.53–7.50 (m, 3H), 7.41–7.38 (m, 6H), 7.35–7.32 (m, 1H), 6.97 (d, J = 9.2 Hz, 1H), 6.29 (s, 1H), 6.25 (t, J = 8.4 Hz, 1H), 5.97 (dd, J = 9.2, 3.6 Hz, 1H), 5.68 (d, J = 7.5 Hz, 1H), 5.54 (d, J = 3.7 Hz, 1H), 4.97 (dd, J = 9.5, 2.2 Hz, 1H), 4.44 (dd, J = 10.9, 6.6 Hz, 1H), 4.30 (d, J = 8.4 Hz, 1H), 4.19 (d, J = 8.4 Hz, 1H), 3.81 (d, J = 7.3 Hz, 1H), 2.58–2.53 (m, 1H), 2.46 (s, 3H), 2.40–2.38 (m, 2H), 2.36–2.34 (m, 2H), 2.22 (s, 3H), 2.13–2.10 (m, 2H), 1.93 (s, 3H), 1.88 (ddd, J = 14.7, 11.0, 2.3 Hz, 1H), 1.68 (s, 3H), 1.62–1.58 (m, 2H), 1.23 (s, 3H), 1.13 (s, 3H), 0.75 (t, J = 8.4 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 203.7, 173.1, 171.2, 169.8, 168.4, 167.2, 167.0, 142.6, 136.9, 133.7, 133.6, 132.8, 132.0, 130.2 (2C), 129.1 (2C), 129.0, 128.7 (3C), 128.5, 127.1 (4C), 126.6, 84.4, 81.0, 79.1, 76.4, 75.6, 75.0, 73.9, 72.0, 71.9, 58.4, 53.4, 45.6, 43.1, 35.5, 33.4, 29.7, 26.8, 26.8, 23.3, 22.7, 22.0, 20.8, 14.8, 10.3, 9.6. HR-MS (ESI): m/z calcd for C52H61BNO17+ ([M+H]+) 982.4027, found: 982.4025.
2’-O-[6-boronohexanoyl]taxol (17d).
Following general procedure D, G, B and C, compound 17d was obtained (42 mg, 6%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 7.1 Hz, 2H), 7.74 (d, J = 7.2 Hz, 2H), 7.61 (t, J = 7.2 Hz, 1H), 7.53–7.50 (m, 3H), 7.43–7.37 (m, 5H), 7.35–7.32 (m, 2H), 6.90 (d, J = 9.2 Hz, 1H), 6.30 (s, 1H), 6.23 (t, J = 9.7 Hz, 1H), 5.94 (dd, J = 9.1, 3.9 Hz, 1H), 5.68 (d, J = 7.0 Hz, 1H), 5.54 (d, J = 3.9 Hz, 1H), 4.97 (dd, J = 9.7, 2.3 Hz, 1H), 4.44 (q, J = 6.4 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.19 (d, J = 8.5 Hz, 1H), 3.81 (d, J = 7.1 Hz, 1H), 2.57–2.53 (m, 1H), 2.44 (s, 3H), 2.42–2.38 (m, 2H), 2.35–2.30 (m, 2H), 2.23 (s, 3H), 2.17–2.13 (m, 2H), 1.93 (s, 3H), 1.91–1.88 (m, 1H), 1.87–1.85 (m, 2H), 1.75–1.72 (m, 2H), 1.68 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H), 0.74 (t, J = 8.6 Hz, 2H). 13C NMR (150 MHz, CDCl3) δc 203.8, 172.9, 171.3, 169.8, 168.2, 167.1, 167.0, 142.7, 136.9, 133.7 (2C), 132.8, 132.0, 130.2 (2C), 129.2, 129.1 (2C), 128.7 (4C), 128.5, 127.1 (2C), 126.6 (2C), 84.4, 81.1, 79.2, 76.4, 75.6, 75.0, 73.8, 72.1, 71.8, 58.5, 53.0, 45.6, 43.1, 35.6, 33.5, 31.3, 29.7, 26.8, 24.3, 23.7, 22.7, 22.6, 22.0, 20.8, 14.8, 9.6. HR-MS (ESI): m/z calcd for C53H62BNO17Na+ ([M+Na]+) 1018.4003, found: 1018.4024.
2’-O-[7-boronoheptanoyl]taxol (17e).
Following general procedure D, G, B and C, compound 17e was obtained (52 mg, 7%). 1H-NMR (600 MHz, CDCl3) δH 8.13 (d, J = 6.9 Hz, 2H), 7.74 (d, J = 7.1 Hz, 2H), 7.61 (t, J = 7.2 Hz, 1H), 7.53–7.50 (m, 3H), 7.43–7.38 (m, 6H), 7.35–7.32 (m, 1H), 6.93 (d, J = 9.3 Hz, 1H), 6.30 (s, 1H), 6.22 (t, J = 9.7 Hz, 1H), 5.93 (dd, J = 9.2, 4.0 Hz, 1H), 5.68 (d, J = 7.2 Hz, 1H), 5.54 (d, J = 3.9 Hz, 1H), 4.97 (dd, J = 9.7, 2.4 Hz, 1H), 4.43 (dd, J = 11.0, 6.9 Hz, 1H), 4.31 (d, J = 8.5 Hz, 1H), 4.19 (d, J = 8.4 Hz, 1H), 3.80 (d, J = 7.1 Hz, 1H), 2.58–2.54 (m, 1H), 2.43 (s, 3H), 2.41–2.37 (m, 2H), 2.35–2.30 (m, 2H), 2.23 (s, 3H), 2.16–2.12 (m, 2H), 1.93 (s, 3H), 1.91–1.86 (m, 1H), 1.68 (s, 3H), 1.61–1.56 (m, 2H), 1.36–1.32 (m, 2H), 1.22 (s, 3H), 1.13 (s, 3H), 0.75 (t, J = 8.6 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 203.7, 172.9, 171.3, 169.8, 168.2, 167.1, 167.0, 142.6, 136.9, 133.7, 133.6, 132.8, 132.0, 130.2 (2C), 129.2, 129.0 (2C), 128.7 (3C), 128.5, 127.1 (4C), 126.6, 84.4, 81.1, 79.1, 76.4, 75.6, 75.0, 73.8, 72.0, 71.8, 58.5, 53.0, 45.6, 43.1, 35.6, 35.5, 33.7, 31.5, 29.7, 28.4, 26.8, 24.5, 23.9, 22.6, 22.0, 20.8, 14.8, 9.6. HR-MS (ESI): m/z calcd for C54H64BNO17Na+ ([M+Na]+) 1032.4160, found: 1032.4185.
2’-O-[4-boronobutanoyl]docetaxel (17f).
Following general procedure D, G, B and C, compound 17f was obtained (32 mg, 4%). 1H-NMR (600 MHz, CDCl3) δH 8.10 (d, J = 7.5 Hz, 2H), 7.60 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 6.9 Hz, 2H), 7.39–7.37 (m, 2H), 7.32–7.29 (m, 3H), 6.20 (t, J = 9.1 Hz, 1H), 5.67 (d, J = 6.5 Hz, 1H), 5.54 (d, J = 9.2 Hz, 1H), 5.46 (s, 1H), 5.38 (d, J =10.9 Hz, 1H), 5.23 (s, 1H), 4.96 (d, J = 9.8 Hz, 1H), 4.31 (d, J = 8.9 Hz, 1H), 4.26 (dd, J = 11.0, 6.7 Hz, 1H), 4.19 (d, J = 7.8 Hz, 1H), 3.89 (d, J = 7.2 Hz, 1H), 2.55–2.53 (m, 1H), 2.42 (s, 3H), 2.36–2.32 (m, 2H), 2.31–2.29 (m, 2H), 1.92 (s, 3H), 1.88–1.86 (m, 1H), 1.85–1.82 (m, 2H), 1.74 (s, 3H), 1.34 (s, 9H), 1.21 (s, 3H), 1.11 (s, 3H), 0.74 (t, J = 8.8 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 211.2, 173.3, 169.9, 168.6, 167.0, 155.3, 135.6, 133.7, 130.2, 130.1 (2C), 129.2, 128.8 (2C), 128.7 (2C), 128.2, 126.4 (3C), 84.3, 81.1, 78.8, 76.5, 75.0, 74.4, 74.3, 72.1, 72.0, 71.7, 57.5, 57.2, 46.5, 43.1, 35.7, 29.7, 28.1 (3C), 27.2, 26.3, 25.8, 22.6, 20.8, 19.5, 14.2, 9.9. HR-MS (ESI): m/z calcd for C47H61BNO17+ ([M+H]+) 922.4027, found: 922.4022.
2’-O-[6-boronohexanoyl]docetaxel (17g).
Following general procedure D, G, B and C, compound 17g was obtained (32 mg, 4%). 1H-NMR (600 MHz, CDCl3) δH 8.10 (d, J = 7.7 Hz, 2H), 7.61 (t, J = 7.5 Hz, 1H), 7.50 (d, J = 7.6 Hz, 2H), 7.38 (d, J = 7.5 Hz, 2H), 7.30–7.28 (m, 3H), 6.21 (t, J = 9.0 Hz, 1H), 5.67 (d, J = 7.4 Hz, 1H), 5.49 (s, 1H), 5.45–5.43 (m, 1H), 5.39–5.37 (m, 1H), 5.22 (s, 1H), 4.96 (d, J = 9.0 Hz, 1H), 4.31 (d, J = 8.8 Hz, 1H), 4.27–4.25 (m, 1H), 4.19 (d, J = 8.5 Hz, 1H)), 3.91 (d, J = 7.1 Hz, 1H), 2.59–2.52 (m, 1H), 2.42 (s, 3H), 2.33–2.31 (m, 2H), 2.22–2.14 (m, 2H), 1.93 (s, 3H), 1.88–1.84 (m, 1H), 1.78–1.77 (m, 2H), 1.74 (s, 3H), 1.60–1.55 (m, 2H), 1.34 (s, 9H), 1.27–1.25 (m, 2H), 1.21 (s, 3H), 1.11 (s, 3H), 0.76 (t, J = 7.7 Hz, 2H). 13C-NMR (150 MHz, CDCl3) δc 211.4, 173.0, 169.8, 168.3, 167.0, 155.2, 135.5, 133.7, 130.2 (2C), 130.0, 129.2, 128.8 (2C), 128.7 (2C), 128.2, 126.4 (3C), 84.3, 81.0, 80.4, 78.9, 76.5, 75.0, 74.4, 74.3, 74.2, 71.9, 71.8, 57.5, 46.5, 43.1, 33.5, 31.2, 29.7, 29.3, 28.1 (3C), 26.3, 24.3, 23.6, 22.6, 14.2, 14.2, 14.1, 9.9. HR-MS (ESI): m/z calcd for C49H64BNO17Na+ ([M+Na]+) 972.4160, found: 972.4160.
3.3. MTT Assay In Vitro
Cells were inoculated in 96-well plates (cell density 2 × 104 cells/well) followed by incubation for 24 h at 37 °C. Paclitaxel and target compounds were added to the corresponding well. Subsequently, the cultures were incubated for 72 h. After the MTT solution (50 µL, 2 mg/mL) was added to cultures, the plates were incubated for an additional 4 h. Purple colored formazan crystals were dissolved in 200 µL DMSO, and the absorbance was recorded at 570 nm using Synergy HT, Bio-Tek (Winooski, VT, USA).
3.4. Liposome Preparation
Blank liposomes were prepared for membrane hydration. HSPC, chol and DSPE-PEG2000 were dissolved using chloroform at a mass ratio of 3:1:0.02 and then spin-evaporated at 37 °C. The obtained film was then hydrated with 300 mM Cu-Glu (pH 7.4) at 65 °C for 30 min. The multi-layer vesicles were subsequently put into an extruder and passed through polycarbonate membranes with pore sizes of 400 nm, 200 nm and 100 nm 12 times at 65 °C containing 20 mM HEPES, 15 mM EDTA and 300 mM sucrose for removing the un-encapsulated Cu2+, and then EDTA was removed with 20 mM HEPES and 300 mM sucrose buffer (pH 7.4, adjusted with triethylamine). Compound 4d was dissolved in DMSO and slowly added into the liposome at 65 °C for 30 min and then quenched in an ice bath for 5 min (the drug to lipid ratio was 1:10). The encapsulation efficiency and drug loading efficiency of compound 4d were determined using HPLC. The morphologies of liposomes were characterized using a transition electron microscope (TEM, Hitachi HT7700, Tokyo, Japan); then, their size, PDI and Zeta potential were measured using a Zetasizer Nano ZS90 (Malvern, UK).
3.5. In Vitro Drug Release Study
The in vitro drug release of compound 4d liposome was carried out by dialysis method at 37 °C under a shaking bed (100 rpm). An amount of 1 mL of compound 4d liposome was added into a dialysis bag and suspended in 25 mL PBS (pH 7.4) containing 15% ethanol (v/v) and 10 mmol/L EDTA. The release behaviors for redox response were also accomplished as described above, except for the addition of 0, 0.02 μmol/L, 0.1 μmol/L, 1 μmol/L, 10 μmol/L, 100 μmol/L, 1 mmol/L and 10 mmol/L H2O2. At the predetermined time points, 200 μL samples were taken for analysis and an equal volume of medium was replenished. The concentrations of paclitaxel were determined by HPLC.
3.6. In Vivo Antitumor Efficacy and Toxicity
The antitumor effect was evaluated by the nude mice armpit tumor model bearing breast cancer 4T1 cells. When the average tumor volume reached 100 mm3, the treatment was initiated at a 3-day interval for 12 days. The mice were randomly divided into five groups (n = 5 per group) receiving different palictaxel at 8 mg/kg as follows: saline (the control group), taxol, compound 4d, compound 4d liposome, high dose 4d liposome (3 times the dose, immediately 24 mg/kg). Tumor volume (V = length × width2/2) and body weight were recorded. At the end of the experiment, mice were sacrificed. The obtained tumors were accurately weighed to calculate the tumor burden (Wtumor/Wbody) and tumor inhibition rate [TIR= (Wcontrol tumor − Wsample tumor)/Wcontrol tumor].
3.7. Statistical Analysis
Statistics data were presented as mean ± SD and were representative of at least three independent trials. Multiple-group comparisons were performed using a one-way ANOVA followed by Dunnett’s t-test. Two group comparisons were performed using Student’s t-test. The treatment effect was considered significant at p < 0.05.
4. Conclusions
By using a molecular combination strategy to link paclitaxel with boronic acid compounds, a series of new paclitaxel derivatives were designed and synthesized. The antitumor activities of these compounds against A549, HCT-116, 4T1 and LO2 cells in vitro were also determined. As a result, compound 4d, one of the most effective compounds, showed considerable selectivity to tumor cells. The optimal formulation of compound 4d liposome, with a drug-lipid ratio of 1:10, was obtained by screening liposomes prescriptions, and the encapsulated efficiency was more than 95%. Meanwhile, compound 4d liposome (8 mg/kg, four times) and a higher dose of compound 4d liposome (24 mg/kg, four times) showed better therapeutic effect than paclitaxel (8 mg/kg, four times) in 4T1 tumor models in vivo, and tumor inhibition rates were 74.3%, 81.9% and 58.5%, respectively. In summary, compound 4d, which connected paclitaxel with boronic acid by using a combination strategy, showed the potential for further research and laid a foundation for exploring the universality of active drug loading of PBA-modified insoluble drugs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227967/s1, Table S1. Encapsulation efficiency of calcium acetate gradients; Table S2. Parameters of compound 4d liposome; Figure S1. Tumor burden of compound 4d liposome against 4T1 xenograft tumor in vivo; Figure S2. 1H-NMR spectra of compound 2a; Figure S3. 1H-NMR spectra of compound 3a; Figure S4. 1H-NMR spectra of compound 8b; Figure S5. 1H-NMR spectra of compound 14b; Figure S6–Figure S81: 1H-NMR, 13C-NMR, HR-MS spectra and HPLC chromatograms of the title compounds.
Author Contributions
D.L., Y.W. and L.Z. conceived the experiments and provided guidance. X.X. designed and synthesized novel compounds. Y.L. studied the in vivo antitumor efficacy of the compound. S.S., J.L., Z.X., Y.Y. assisted in the synthetic and biology research. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82273878, funder: Yongjun Wang), Natural Science Foundation of Liaoning Province, China (No. 2020-MS-189; funder: Dan Liu), Chinese Pharmaceutical Society-Yiling Biomedical Innovation Fund (CPAYLJ201906; funder: Yongjun Wang), as well as the NMR Key Laboratory of Eco-Environmental Science and Technology, China (No. MEEST-2020-7; funder: Dan Liu).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Laboratory Animal Center of Ningxia Medical University (protocol code: IACUC-NYLAC-2022-118 and date of approval: 13 May 2022). for studies involving animals.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; De Diego Puente, T. A Compressive Review about Taxol: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. A new endophytic taxol- and baccatin III-producing fungus isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2011, 10, 16379–16386. [Google Scholar] [CrossRef]
- Aglawe, S.; Gayke, A.; Kadam, P.; Kanawade, S.; Garud, Y. Taxol as an anticancer agent: A review. Int. J. Res. Ayurveda Pharm. 2019, 10, 15–17. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Ying, Z.; Yang, Q.; Kai, W.; Tao, L.; Xiang, Z. Advances in tumor microenvironmentally responsive antitumor prodrugs. Pharm. Chem. 2017, 43, 184–186. [Google Scholar]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical Translation of Nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Tsai, C.-Y.; Cheng, Y.-M.; Nieh, S.-W.; Yeh, T.-K.; Chen, C.P.; Wang, M.-H.; Chou, L.-H.; Chiu, T.-Y.; Liu, L.; et al. Impacts of Intralipid on Nanodrug Abraxane Therapy and on the Innate Immune System. Sci. Rep. 2020, 10, 2838. [Google Scholar] [CrossRef]
- Li, W.; Wilson, G.C.; Bachmann, M.; Wang, J.; Mattarei, A.; Paradisi, C.; Edwards, M.J.; Szabo, I.; Gulbins, E.; Ahmad, S.A.; et al. Inhibition of a Mitochondrial Potassium Channel in Combination with Gemcitabine and Abraxane Drastically Reduces Pancreatic Ductal Adenocarcinoma in an Immunocompetent Orthotopic Murine Model. Cancers 2022, 14, 2618. [Google Scholar] [CrossRef]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: Inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin. Drug Deliv. 2016, 14, 783–796. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Zhang, X.; Guo, M.; Sun, M.; Yu, H.; Chen, Q.; Yang, W.; Wang, M.; Zuo, S.; et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Zheng, Q.; Zhao, Q.; Zhang, H.; Ma, Y.; Fallon, J.K.; Fu, Q.; Haynes, M.T.; Lin, G.; et al. Disulfide Bond Bridge Insertion Turns Hydrophobic Anticancer Prodrugs into Self-Assembled Nanomedicines. Nano Lett. 2014, 14, 5577–5583. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, X.; Xia, R.; Liu, S.; Pei, Q.; Chen, G.; Xie, Z.; Jing, X. A Paclitaxel Prodrug Activatable by Irradiation in a Hypoxic Microenvironment. Angew. Chem. Int. Ed. 2020, 59, 23198–23205. [Google Scholar] [CrossRef]
- Mu, J.; Zhong, H.; Zou, H.; Liu, T.; Yu, N.; Zhang, X.; Xu, Z.; Chen, Z.; Guo, S. Acid-sensitive PEGylated paclitaxel prodrug nanoparticles for cancer therapy: Effect of PEG length on antitumor efficacy. J. Control. Release 2020, 326, 265–275. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Yang, Z.; Chi, D.; Wang, Q.; Guo, X.; Lv, Q.; Wang, Y. Improved antitumor activity and tolerability of cabazitaxel derived remote-loading liposomes. Int. J. Pharm. 2020, 589, 119814. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, S.; Li, J.; Wang, Y.; Su, Y.; Chi, D.; Wang, J.; Wang, X.; He, Z.; Lin, G.; et al. Simple weak-acid derivatives of paclitaxel for remote loading into liposomes and improved therapeutic effects. RSC Adv. 2020, 10, 27676–27687. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Yu, J.; Yang, L.; Kuang, X.; Wang, Z.; Wang, Y.; Liu, H.; Lin, G.; He, Z.; et al. A facile and universal method to achieve liposomal remote loading of non-ionizable drugs with outstanding safety profiles and therapeutic effect. Acta Pharm. Sin. B 2020, 11, 258–270. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.; Yu, J.; Cai, W.; Yang, Y.; Kuang, X.; Liu, H.; He, Z.; Wang, Y. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J. Control. Release 2021, 335, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-S.; Liang, X.T. Recent Progress in Structure Activity Relationship and Mechanistic Studies of Taxol Analogues. Mini-Rev. Med. Chem. 2005, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome drugs’ loading efficiency: A working model based on loading conditions and drug’s physicochemical properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
- Mayer, L.D.; Tardi, P.; Louie, A.C. CPX-351: A nanoscale liposomal co-formulation of daunorubicin and cytarabine with unique biodistribution and tumor cell uptake properties. Int. J. Nanomed. 2019, 14, 3819–3830. [Google Scholar] [CrossRef]
- Tang, W.-L.; Szeitz, A.; Kulkarni, J.; Cullis, P.; Li, S.-D. Systemic study of solvent-assisted active loading of gambogic acid into liposomes and its formulation optimization for improved delivery. Biomaterials 2018, 166, 13–26. [Google Scholar] [CrossRef]
- Murphy, A.C.; Gao, S.-S.; Han, L.-C.; Carobene, S.; Fukuda, D.; Song, Z.; Hothersall, J.; Cox, R.J.; Crosby, J.; Crump, M.P.; et al. Biosynthesis of thiomarinol A and related metabolites of Pseudoalteromonas sp. SANK 73390. Chem. Sci. 2013, 5, 397–402. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).