Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl.
Abstract
1. Introduction
2. Results
2.1. Composition of Volatile Compounds in EO
2.2. Volatile Compounds Identified in the Crude Oleoresin Headspace
2.3. In Vitro Assessment of Eye and Skin Irritancy
2.4. In Chemico and In Vitro Assessment of Skin Sensitization
2.5. Cytotoxicity on HaCaT Keratinocytes
2.6. In Vitro Assessment of the Modulating Effects on Skin Wound Healing
2.7. In Vitro Assessment of Anti-Inflammatory Potential on the UV-Induced Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collection of the Crude Oleoresin
4.3. Essential Oil Extraction
4.4. Identification of Volatile Compounds in EO by GC-MS
4.5. Quantification of Volatile Compounds in EO by GC-MS
4.6. Identification of Volatile Compounds from the Crude Oleoresin by HS-SPME-GC-MS
4.7. In Vitro Assessment of Eye and Skin Irritancy
4.8. In Vitro Assessment of Skin Sensitization
4.9. In Chemico Assessment of Skin Sensitization
4.10. In Vitro Assessment of the Modulating Effects on Skin Wound Healing
4.11. In Vitro Assessment of Anti-Inflammatory Potential on the UV-Induced Inflammatory Response
- -
- 2 non stressed controls: cells incubated in 10% fetal calf serum and 10% fetal calf serum + 1% EtOH without UV exposure.
- -
- 1 stressed control: cells incubated in 10% fetal calf serum + 1% EtOH with UV exposure.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| BCA | Bicinchoninic Acid |
| BHT | Butylated Hydroxytoluene |
| CAR | Carboxen |
| DPRA | Direct Peptide Reactivity Assay |
| DSQ | Dual Stage Quadrupole |
| DVB | Divinylbenzene |
| EI | Electron Ionization |
| EO | Essential Oil (obtained from the oleoresin of Canarium schweinfurthii) |
| EtOH | Ethanol |
| GC | Gas Chromatography |
| HaCaT | Cultured Human Keratinocyte |
| HCE | Human Corneal Epithelium |
| HB-EGF | Heparin-binding Epidermal Growth Factor |
| h-CLAT | Human Cell Line Activation Test |
| HS | Headspace |
| IL-6 | Interleukin 6 |
| MS | Mass Spectrometry/Spectrometer |
| MSD | Mass-selective Detector |
| NIST | National Institute of Standards and Technology |
| NSB | No Specific Binding |
| OECD | Organization for Economic Co-operation and Development |
| PBS | Phosphate Buffered Saline |
| PDMS | Polydimethylsiloxane |
| PTFE | Polytetrafluoroethylene |
| RhCE | Reconstructed Human Cornea-like Epithelium |
| RI | Kováts Retention Index |
| SIM | Selected Ion Monitoring |
| SPME | Solid-phase Microextraction |
| THP1 | Human leukemia Monocytic Cell Line |
| TNF-α | Tumour Necrosis Factor |
| UN GHS | Globally Harmonized System of Classification and Labeling of Chemicals |
| UVB | Ultraviolet B (from 280 to 315 nm) |
References
- Vande Weghe, J.P.; Bidault, E.; Stévart, T. Les Plantes à Fleurs Du Gabon. Une Introduction à La Flore Des Angiospermes, 1st ed.; ANPN: Libreville, Gabon, 2016; pp. 446–447.
- Kuete, V. Canarium schweinfurthii. In Medicinal Spices and Vegetables from Africa, 1st ed.; Academic Press: London, UK, 2017; pp. 379–384. [Google Scholar]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible Wild Plants of Tanzania, 1st ed.; Regional Land Management Unit/Sida: Nairobi, Kenya, 2002; pp. 164–165. [Google Scholar]
- Kamtchouing, P.; Kahpui, S.M.; Dzeufiet, P.-D.D.; Tédong, L.; Asongalem, E.A.; Dimo, T. Anti-Diabetic Activity of Methanol/Methylene Chloride Stem Bark Extracts of Terminalia Superba and Canarium schweinfurthii on Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2006, 104, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Moshi, M.; Innocent, E.; Masimba, P.; Otieno, D.; Weisheit, A.; Mbabazi, P.; Lynes, M.; Meachem, K.; Hamilton, A.; Urassa, I. Antimicrobial and Brine Shrimp Toxicity of Some Plants Used in Traditional Medicine in Bukoba District, North-Western Tanzania. Tanzan. J. Health. Res. 2009, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial Activities of the Methanol Extracts of Canarium schweinfurthii and Four Other Cameroonian Dietary Plants Against Multi-Drug Resistant Gram-Negative Bacteria. Saudi J. Biol. Sci. 2016, 23, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Seukep, J.A.; Ngadjui, B.; Kuete, V. Antibacterial Activities of Fagara macrophylla, Canarium schweinfurthii, Myrianthus arboreus, Dischistocalyx grandifolius and Tragia benthamii Against Multi-Drug Resistant Gram-Negative Bacteria. SpringerPlus 2015, 4, 567. [Google Scholar] [CrossRef] [PubMed]
- Tshibangu, D.S.T.; Ngbolua, K.-N.; Moke, L.E.; Tshilanda, D.D.; Mvingu, B.M.; Iteku, J.B.; Mbala, B.M.; Mudogo, V.; Mpiana, P.T. Chemical Composition and Bioactivity of Canarium schweinfurthii Stem Bark Extrafcts from DR Congo Against Sickle Cell Disease and Associated Bacteria. J. Pharmacogn. Phytochem. 2016, 5, 181–187. [Google Scholar]
- Sokoudjou, J.B.; Chegaing Fodouop, S.P.; Djoueudam, F.G.; Kodjio, N.; Kana, J.R.; Fowa, A.B.; Kamsu, G.T.; Gatsing, D. Antisalmonellal and Antioxidant Potential of Hydroethanolic Extract of Canarium schweinfurthii Engl. (Burseraceae) in Salmonella enterica Serovar Typhimurium-Infected Chicks. Asian Pac. J. Trop. Biomed. 2019, 9, 474. [Google Scholar] [CrossRef]
- Aghoutane, Y.; Moufid, M.; Motia, S.; Padzys, G.S.; Omouendze, L.P.; Llobet, E.; Bouchikhi, B.; El Bari, N. Characterization and Analysis of Okoume and Aiele Essential Oils from Gabon by GC-MS, Electronic Nose, and Their Antibacterial Activity Assessment. Sensors 2020, 20, 6750. [Google Scholar] [CrossRef]
- Obame, L.; Koudou, J.; Kumulungui, B.; Bassolé, I.; Edou, P.; Ouattara, A.; Traore, A. Antioxidant and Antimicrobial Activities of Canarium schweinfurthii Engl. Essential Oil from Centrafrican Republic. Afr. J. Biotechnol. 2007, 6, 2319–2323. [Google Scholar] [CrossRef]
- Wahab, A.G.; Adekunle, A.I.; Elizabeth, G.A. Phytochemical Composition and Antioxidative Potential of Purple Canary (Canarium schweinfurthii) Fruit. Pharma Innov. J. 2015, 4, 49–52. [Google Scholar]
- Dongmo, P.M.J.; Tchoumbougnang, F.; Ndongson, B.; Agwanande, W.; Sandjon, B.; Zollo, P.H.A.; Menut, C. Chemical Characterization, Antiradical, Antioxidant and Anti-Inflammatory Potential of the Essential Oils of Canarium schweinfurthii and Aucoumea klaineana (Burseraceae) Growing in Cameroon. Agric. Biol. J. N. Am. 2010, 1, 606–611. [Google Scholar]
- Tcheghebe, O.T.; Seukep, J.A.; Tatong, F.N. A Review on Traditional Uses, Phytochemical Composition and Pharmacological Profile of Canarium schweinfurthii Eng. Nat. Sci. 2016, 14, 17–22. [Google Scholar]
- Murthy, K.S.R.; Reddy, M.C.; Rani, S.S.; Pullaiah, T. Bioactive Principles and Biological Properties of Essential Oils of Burseraceae: A Review. J. Pharmacogn. Phytochem. 2016, 5, 247–258. [Google Scholar]
- Helene, T.; Serge, F.; Ngadjui, B.T.; Etienne, D.; Abegaz, B.M. Phenolic Metabolites from the Seeds of Canarium schweinfurthii. Bull. Chem. Soc. Ethiop. 2000, 14, 155–159. [Google Scholar] [CrossRef]
- Kamdem, R.S.T.; Wafo, P.; Yousuf, S.; Ali, Z.; Adhikari, A.; Rasheed, S.; Khan, I.A.; Ngadjui, B.T.; Fun, H.-K.; Choudhary, M.I. Canarene: A Triterpenoid with a Unique Carbon Skeleton from Canarium schweinfurthii. Org. Lett. 2011, 13, 5492–5495. [Google Scholar] [CrossRef] [PubMed]
- Maffo, T.; Takou, M.; Tabekoueng, G.B.; Langat, M.K.; Vardamides, J.C.; Wafo, P.; Ngadjui, B.T.; Waffo, A.F.K. Triterpenoid Derivatives from Canarium schweinfurthii Engl. (Burseraceae). J. Pharmacogn. Phytochem. 2019, 8, 3064–3067. [Google Scholar]
- Sokoudjou, J.B.; Tagousop, C.N.; Khan, A.; Fodouop, S.P.C.; Atsafack, S.S.; Kodjio, N.; Atolani, O.; Gatsing, D. Canarimoic Acid: New Tirucallane Triterpene with Antisalmonellal Activity from the Stem Bark of Canarium schweinfurthii Engl. Nat. Prod. Res. 2020, 36, 2363–2369. [Google Scholar] [CrossRef]
- Koudou, J.; Abena, A.A.; Ngaissona, P.; Bessière, J.M. Chemical Composition and Pharmacological Activity of Essential Oil of Canarium schweinfurthii. Fitoterapia 2005, 76, 700–703. [Google Scholar] [CrossRef]
- Ambindei, W.A.; Ngouné, L.T.; Sameza, M.L.; Sonwa, E.T.; Nguimatsia, F.; Dongmo, P.M.J. Antifungal Activites Against Some Aspergillus Species of the Essential Oils of Canarium schweinfurthii and Aucoumea klaineana Growing in Cameroon. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 691–701. [Google Scholar]
- Nagawa, C.; Böhmdorfer, S.; Rosenau, T. Chemical Composition and Anti-Termitic Activity of Essential Oil from Canarium schweinfurthii Engl. Ind. Crops Prod. 2015, 71, 75–79. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Engonga, P.E.; Abdoul-Latif, F.M.; Obame, L.C.; Mewono, L.; Agnaniet, H. Volatile Constituents of Canarium schweinfurthii Essential Oil from Gabon. Int. J. AgriSci. 2012, 2, 200–203. [Google Scholar]
- OECD. Test No. 492: Reconstructed Human Cornea-like Epithelium (RhCE) Test Method for Identifying Chemicals Not Requiring Classification and Labelling for Eye Irritation or Serious Eye Damage; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2021. [Google Scholar]
- OECD. Test No. 442C: In Chemico Skin Sensitisation: Direct Peptide Reactivity Assay (DPRA); OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2021. [Google Scholar]
- OECD. Test No. 442E: In Vitro Skin Sensitisation: In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2018. [Google Scholar]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound Healing Activity of Terpinolene and α-Phellandrene by Attenuating Inflammation and Oxidative Stress in vitro. J. Tissue Viabil. 2019, 28, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Njoukam, R.; Peltier, R. L’aiélé (Canarium schweinfurthii Engl.): Premier Essai de Plantation Dans l’Ouest Du Cameroun. Fruits 2002, 57, 239–248. [Google Scholar] [CrossRef]

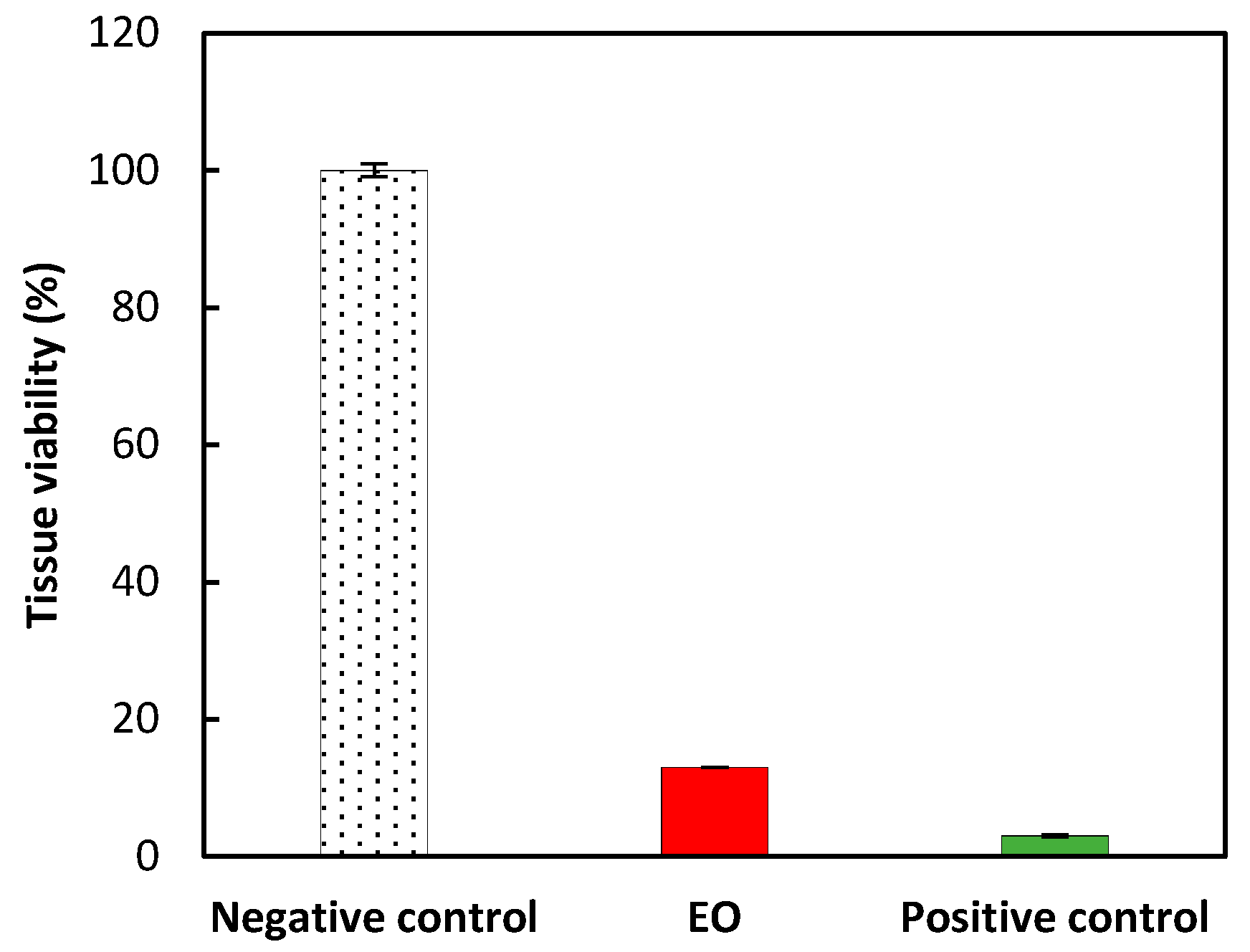
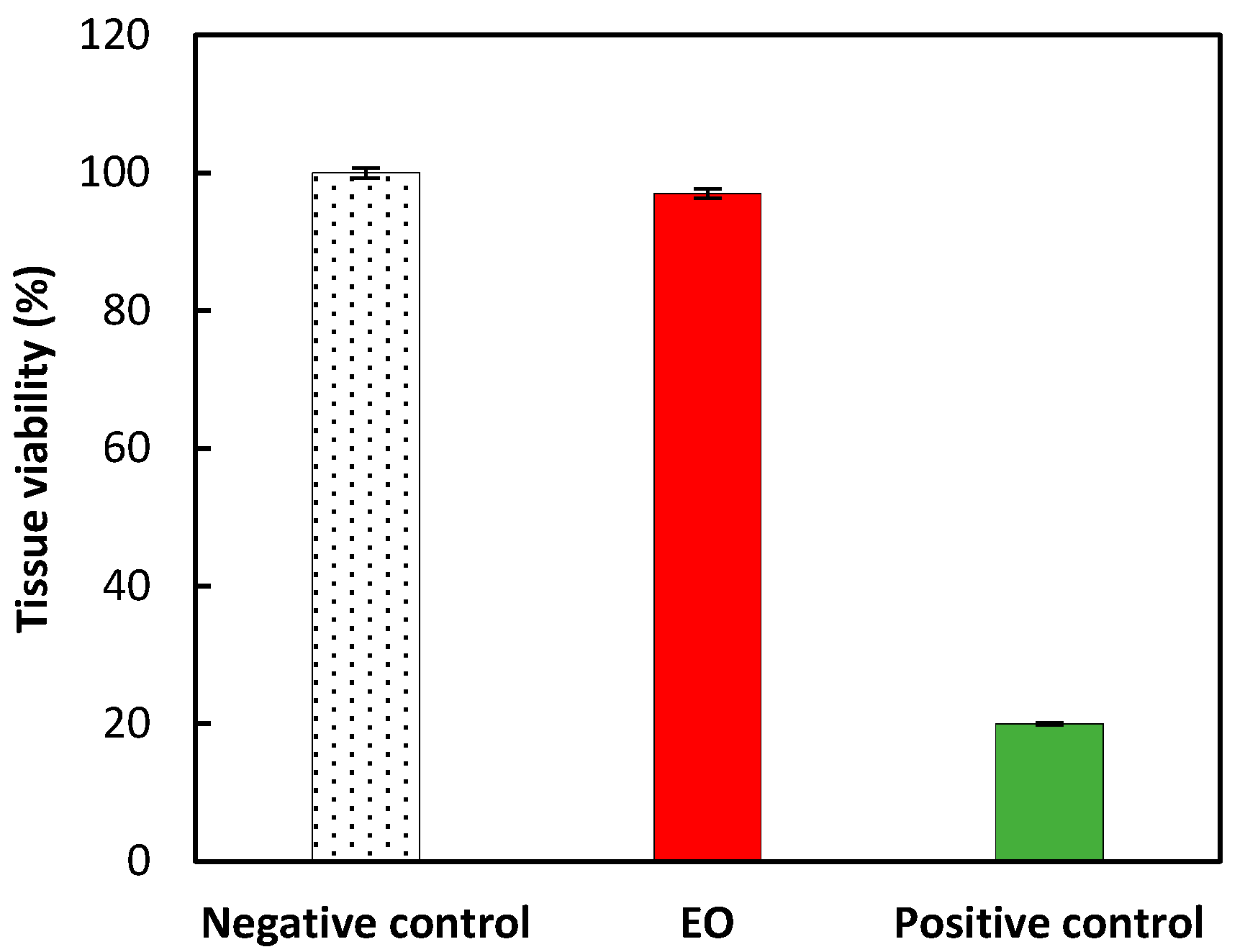
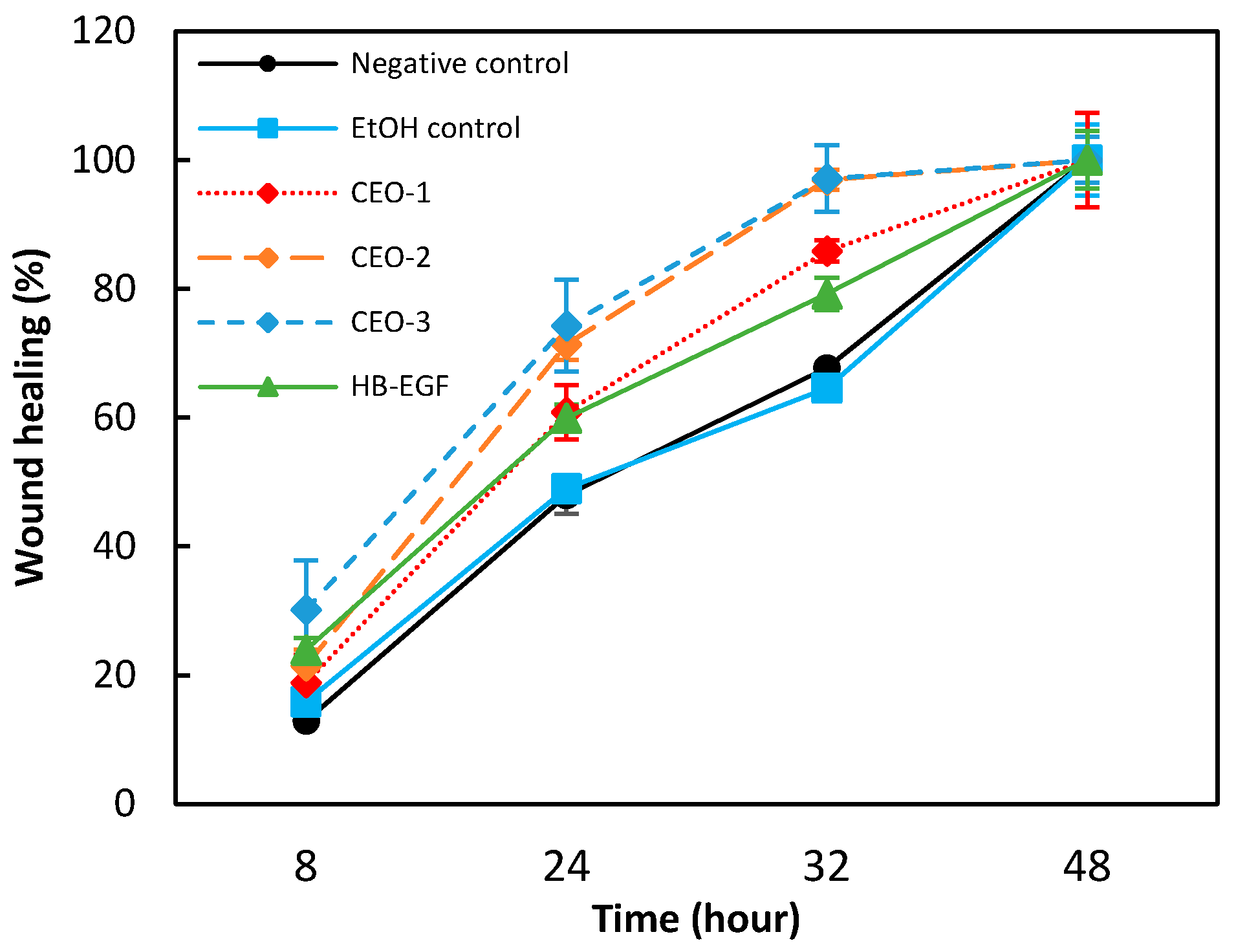
| No. | Compounds | RI Lit. (Non-Polar) | RI Calc. (Non-Polar) | RI Lit. (Polar)2 | RI Calc. (Polar) | SIM | Content in EO (%) |
|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 930 1 | 927 | nd | nd | 93 | 0.30 ± 0.09 |
| 2 | α-pinene | 939 1 | 935 | nd | nd | 93 | 5.87 ± 1.47 |
| 3 | camphene | 954 1 | 953 | nd | nd | 93 | 0.06 ± 0.02 |
| 4 | sabinene | 975 1 | 974 | 1124 ± 8 | 1124 | 93 | 7.83 ± 1.32 |
| 5 | β-pinene | 979 1 | 981 | 1112 ± 7 | 1118 | 93 | 3.77 ± 1.00 |
| 6 | 2-menthene | 1004 ± 5 2 | 1003 | nd | nd | 93 | 0.44 ± 0.12 |
| 7 | α-phellandrene | 1002 1 | 1009 | 1167 ± 9 | 1168 | 93 | 21.88 ± 2.93 |
| 8 | δ3-carene | 1011 1 | 1011 | 1147 ± 7 | 1150 | 93 | 0.39 ± 0.13 |
| 9 | α-terpinene | 1017 1 | 1019 | 1180 ± 8 | 1183 | 93 | 1.97 ± 0.50 |
| 10 | p-cymene | 1024 1 | 1027 | 1272 ± 8 | 1274 | 117 | 5.28 ± 0.90 |
| 11 | limonene | 1029 1 | 1032 | 1200 ± 7 | 1203 | 93 | 9.09 ± 1.52 |
| 12 | β-phellandrene | 1029 1 | 1034 | 1211 ± 7 | 1212 | 93 | 5.31 ± 1.44 |
| 13 | eucalyptol | 1031 1 | 1036 | nd | nd | 154 | 0.11 ± 0.06 |
| 14 | γ-terpinene | 1059 1 | 1060 | 1246 ± 9 | 1247 | 93 | 1.64 ± 0.43 |
| 15 | 4-thujanol | 1075 ± 7 2 | 1073 | 1469 | 1461 | 93 | 0.59 ± 0.20 |
| 16 | terpinolene | 1088 1 | 1088 | 1283 ± 7 | 1287 | 93 | 31.23 ± 4.86 |
| 17 | p-cymenene | 1091 1 | 1092 | 1444 ± 11 | 1439 | 117 | 0.41 ± 0.13 |
| 18 | terpinen-4-ol | 1177 1 | 1184 | 1602 ± 9 | 1601 | 93 | 1.57 ± 0.48 |
| 19 | p-cymen-8-ol | 1182 1 | 1188 | 1852 ± 13 | 1847 | 93 | 1.37 ± 0.44 |
| 20 | α-terpineol | 1188 1 | 1197 | 1697 ± 10 | 1693 | 93 | 0.88 ± 0.26 |
| No. | Compounds | RI Lit. (Non-Polar) | RI Calc. (Non-Polar) | RI Lit. (Polar) 2 | RI Calc. (Polar) | Content in Crude Oleoresin (%) | Content in EO (%) |
|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 930 1 | 929 | 1028 ± 7 | 1017 | 0.67 ± 0.30 | 0.30 ± 0.09 |
| 2 | α-pinene | 939 1 | 936 | 1028 ± 8 | 1013 | 5.68 ± 2.10 | 5.87 ± 1.47 |
| 3 | camphene | 954 1 | 953 | nd | nd | 0.07 ± 0.04 | 0.06 ± 0.02 |
| 4 | sabinene | 975 1 | 975 | 1124 ± 8 | 1115 | 12.44 ± 2.55 | 7.83 ± 1.32 |
| 5 | β-pinene | 979 1 | 980 | 1112 ± 7 | 1098 | 3.95 ± 0.68 | 3.77 ± 1.00 |
| 6 | 2-menthene | 1004 ± 5 2 | 1000 | nd | nd | 0.60 ± 0.10 | 0.44 ± 0.12 |
| 7 | α-phellandrene | 1002 1 | 1007 | 1167 ± 9 | 1160 | 23.29 ± 1.07 | 21.88 ± 2.93 |
| 8 | δ3-carene | 1011 1 | 1010 | 1147 ± 7 | 1143 | 0.19 ± 0.03 | 0.39 ± 0.13 |
| 9 | α-terpinene | 1017 1 | 1018 | 1180 ± 8 | 1174 | 1.47 ± 0.06 | 1.97 ± 0.50 |
| 10 | p-cymene | 1024 1 | 1027 | 1272 ± 8 | 1265 | 7.15 ± 0.66 | 5.28 ± 0.90 |
| 11 | limonene | 1029 1 | 1032 | 1200 ± 7 | 1191 | 11.72 ± 1.23 | 9.09 ± 1.52 |
| 12 | β-phellandrene | 1029 1 | 1033 | 1211 ± 7 | 1198 | 2.76 ± 0.27 | 5.31 ± 1.44 |
| 13 | eucalyptol | 1031 1 | 1035 | nd | nd | 0.16 ± 0.07 | 0.11 ± 0.06 |
| 14 | γ-terpinene | 1059 1 | 1060 | 1246 ± 9 | 1239 | 1.39 ± 0.10 | 1.64 ± 0.43 |
| 15 | 4-thujanol | 1075 ± 7 2 | 1073 | 1469 | 1460 | 0.11 ± 0.03 | 0.59 ± 0.20 |
| 16 | terpinolene | 1088 1 | 1087 | 1283 ± 7 | 1276 | 27.86 ± 3.27 | 31.23 ± 4.86 |
| 17 | p-cymenene | 1091 1 | 1091 | 1444 ± 11 | 1434 | 0.24 ± 0.04 | 0.41 ± 0.13 |
| 18 | terpinen-4-ol | 1177 1 | 1184 | 1602 ± 9 | 1599 | 0.12 ± 0.02 | 1.57 ± 0.48 |
| 19 | p-cymen-8-ol | 1182 1 | 1188 | 1852 ± 13 | 1845 | 0.11 ± 0.02 | 1.37 ± 0.44 |
| 20 | α-terpineol | 1188 1 | 1197 | 1697 ± 10 | 1695 | 0.03 ± 0.01 | 0.88 ± 0.26 |
| Negative Control | Positive Control | EO | ||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | |||
| CD86 (%) | 100 | 770 | 212 | 292 | 434 | 494 | 534 | 565 |
| CD54 (%) | 100 | 560 | 316 | 356 | 392 | 472 | 509 | 525 |
| EO (µg/mL) | Control 1 | 19.53 | 39.06 | 78.13 | 156.25 | 312.50 | 625.00 | 1250.00 | 2500.00 | 5000.00 |
|---|---|---|---|---|---|---|---|---|---|---|
| O.D Mean | 0.667 | 0.678 | 0.666 | 0.706 | 0.674 | 0.532 | 0.010 | 0.001 | 0.001 | 0.000 |
| St. Dev. | 0.034 | 0.028 | 0.021 | 0.028 | 0.061 | 0.027 | 0.005 | 0.001 | 0.003 | 0.000 |
| Viability (%) | 100 | 102 | 100 | 106 | 101 | 80 | 1 | 0 | 0 | 0 |
| Cytotoxicity (%) | - | 0 | 0 | 0 | 0 | 20 | 99 | 100 | 100 | 100 |
| EO (µg/mL) | Control 1 | 0.23 | 0.47 | 0.94 | 1.88 | 3.75 | 7.50 | 15.00 | 30.00 | 60.00 |
|---|---|---|---|---|---|---|---|---|---|---|
| O.D Mean | 0.542 | 0.523 | 0.556 | 0.532 | 0.525 | 0.498 | 0.493 | 0.418 | 0.210 | 0.0000 |
| St. Dev. | 0.009 | 0.009 | 0.007 | 0.014 | 0.010 | 0.010 | 0.013 | 0.015 | 0.010 | 0.0000 |
| Viability (%) | 100 | 97 | 103 | 98 | 97 | 92 | 91 | 77 | 39 | 0 |
| Cytotoxicity (%) | - | 3 | 0 | 2 | 3 | 8 | 9 | 23 | 61 | 100 |
| Wound Healing (%) | ||||
|---|---|---|---|---|
| T1 (8 h) | T2 (24 h) | T3 (32 h) | T4 (48 h) | |
| Negative control | 12.93 ± 0.76 | 47.92 ± 2.89 | 67.72 ± 0.63 | 100.00 ± 1.90 |
| EtOH control | 15.87 ± 2.71 | 48.96 ± 1.29 | 64.57 ± 1.03 | 100.00 ± 5.53 |
| CEO-1 (1.8 µg/mL) | 18.85 ± 4.32 | 60.84 ± 4.23 | 85.86 ± 1.65 | 100.00 ± 7.30 |
| CEO-2 (4.5 µg/mL) | 21.47 ± 2.51 | 71.40 ± 2.47 | 96.95 ± 1.55 | 100.00 ± 0.76 |
| CEO-3 (9.0 µg/mL) | 30.18 ± 7.59 | 74.28 ± 7.14 | 97.13 ± 5.18 | 100.00 ± 3.56 |
| HB-EGF (100 ng/mL) | 23.96 ± 1.87 | 59.94 ± 2.11 | 79.21 ± 2.52 | 100.00 ± 4.47 |
| IL-6 (pg/mg Prot.) | IL-6 Induced (pg/mg Prot.) | IL-6 Induced (%) | Stat. | ||
|---|---|---|---|---|---|
| (−)UV | Control | 63.41 ± 1.59 | |||
| (+)UV | Control | 1368.55 ± 8.20 | 1305.14 | 100 | |
| CEO-1 (1.8 µg/mL) | 635.36 ± 6.76 | 572.22 | 44 | p ≤ 0.01 | |
| CEO-2 (4.5 µg/mL) | 705.68 ± 11.37 | 642.27 | 49 | p ≤ 0.01 | |
| CEO-3 (9.0 µg/mL) | 700.44 ± 20.16 | 637.03 | 49 | p ≤ 0.01 | |
| Dexamethasone (100 µM) | 168.51 ± 5.42 | 105.10 | 8 | p ≤ 0.01 |
| TNF-α (pg/mg Prot.) | TNF-α Induced (pg/mg Prot.) | TNF-α Induced (%) | Stat. | ||
|---|---|---|---|---|---|
| (−)UV | Control | 0.57 ± 0.02 | |||
| (+)UV | Control | 6.07 ± 0.13 | 5.50 | 100 | |
| CEO-1 (1.8 µg/mL) | 4.07 ± 0.06 | 3.49 | 64 | p ≤ 0.01 | |
| CEO-2 (4.5 µg/mL) | 4.70 ± 0.14 | 4.13 | 75 | p ≤ 0.01 | |
| CEO-3 (9.0 µg/mL) | 5.31 ± 0.17 | 4.74 | 86 | p ≤ 0.01 | |
| Dexamethasone (100 µM) | 3.17 ± 0.10 | 2.59 | 47 | p ≤ 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnard, M.; Martin, E.; Parrot, I. Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl. Molecules 2022, 27, 7966. https://doi.org/10.3390/molecules27227966
Bonnard M, Martin E, Parrot I. Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl. Molecules. 2022; 27(22):7966. https://doi.org/10.3390/molecules27227966
Chicago/Turabian StyleBonnard, Michel, Enzo Martin, and Isabelle Parrot. 2022. "Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl." Molecules 27, no. 22: 7966. https://doi.org/10.3390/molecules27227966
APA StyleBonnard, M., Martin, E., & Parrot, I. (2022). Wound Healing Potential of an Oleoresin Essential Oil Chemotype from Canarium schweinfurthii Engl. Molecules, 27(22), 7966. https://doi.org/10.3390/molecules27227966




