Abstract
The 2-imidazoline nitroxide derivatives of cymantrene—2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (NNMn) and 2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl (INMn) were synthesized. It was shown that NNMn and INMn exhibit a sufficiently high kinetic stability both in solids and in solutions under normal conditions. Their structural characteristics, magnetic properties and electrochemical behavior are close to Re(I) analogs. This opens the prospect of using paramagnetic cymantrenes as prototypes in the design of Re(I) half-sandwiched derivatives for theranostics, where therapy is combined with diagnostics by magnetic resonance imaging due to the contrast properties of nitroxide radicals.
1. Introduction
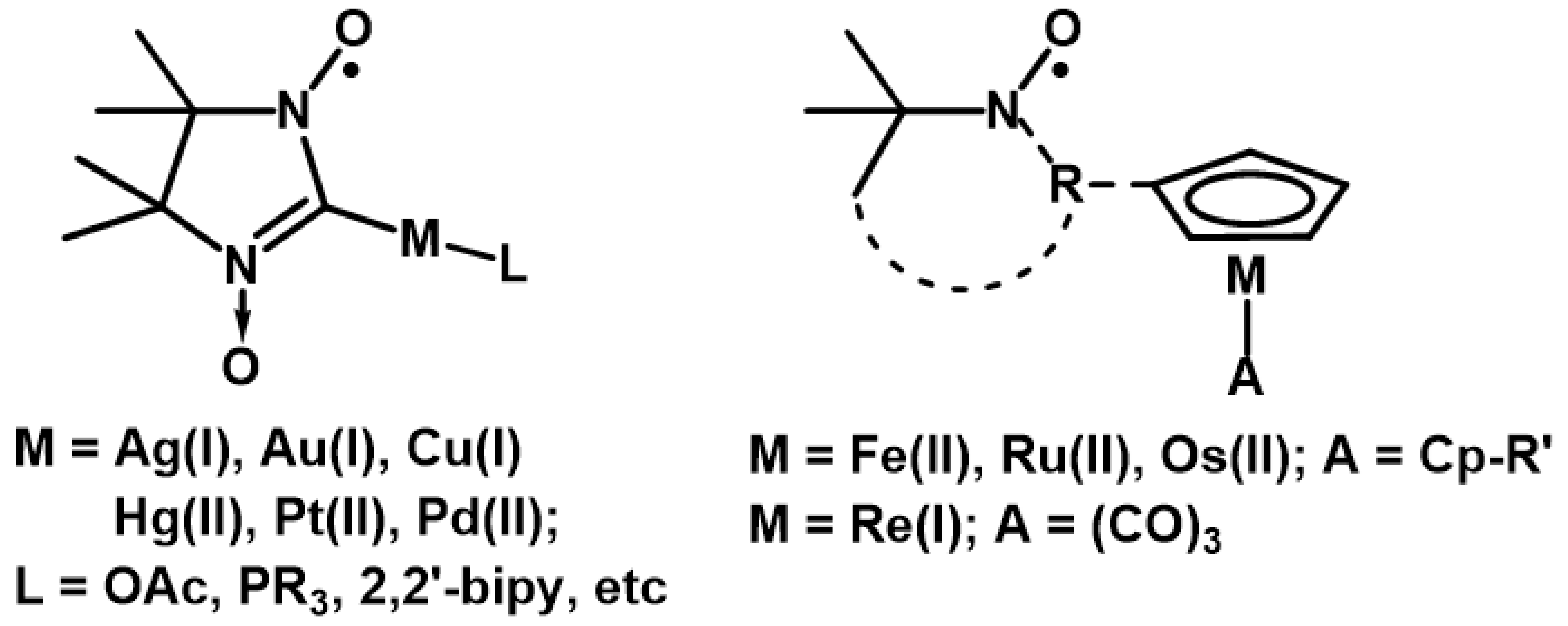
The design of organometallic derivatives of nitroxide radicals [1,2,3] has a high potential for the development of new organic synthesis principles and the study of the mechanisms of catalytic reactions [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] as well as for the creation of polyfunctional magnetoactive materials exhibiting diverse intra- and intermolecular interactions and spin-exchange coupling [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], redox properties [34,35,36,37,38,39,40], and specific biological activity [1,2,3,39]. A number of studies have shown that organometallic nitroxides based on 2-imidazoline nitronyl nitroxides containing an M–C bond in the second position of the heterocycle [11,12,13,14,15,16,17,18,19,20,21,22,23] and sandwich/half-sandwich compounds bearing various nitroxide substituents in the cyclopentadienyl ring proved to be the most stable (Scheme 1) [28,29,30,31,32,33,34,35,36,37,38,39,40,41].
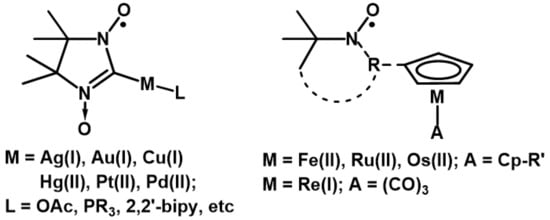
Scheme 1.
Organometallic nitroxide-substituted derivatives.
High efficiency of Pd-catalyzed cross-coupling reactions of organogold carbene-like derivatives of 2-imidazoline nitronyl nitroxides with aryl- and vinyl-halides has been demonstrated as a reliable methodological tool for the obtaining of nitroxide types unavailable by other synthetic routes [12,13,14,15,16,17]. It has been shown that the introduction of various nitroxide substituents into ferrocene can also serve as a fruitful approach by which peculiar types of molecular magnets [28,29,30,31,32,33,34,35,36,37,38,39,40,41], spin labels with tunable spacing between different types of paramagnetic centers [36,37], miniature fast-charging electrochemical elements [38,39], and paramagnets with antioxidant activity have been obtained [40].
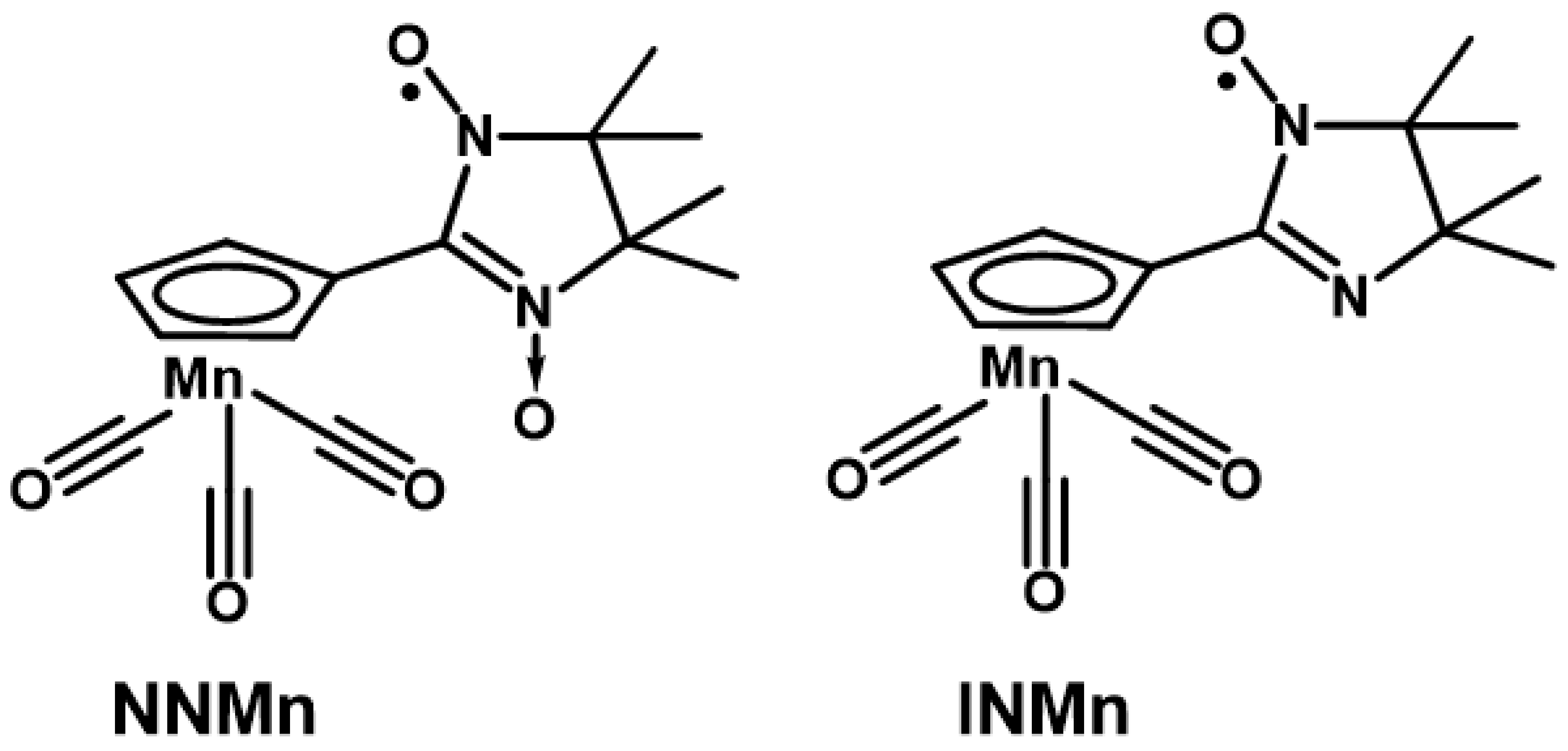
We would like to pay attention to the recently obtained first nitroxide-substituted derivative of cyrhetrene [41], that actually opened the opportunity of developing theranostics medicines on their basis. The therapeutic effect of radiological treatment with 186/188Re [42,43] isotopes in such compounds can be combined with monitoring and diagnosis by magnetic resonance imaging (MRI), where a paramagnetic organic fragment acts as a contrast agent [44,45]. In this regard, the development of methods for the synthesis and study of the corresponding nitroxide-substituted cymantrene derivatives becomes of essential importance. Paramagnetic cymantrenes can also be very promising due to their photodynamic therapeutic activity [46,47]; on the other hand, cymantrene analogs are more commercially available, and therefore it is more reasonable to use them to work out some synthetic steps when developing the design of target Re(I) or Tc(I) compounds [42,43]. This prompted us to synthesize 2-imidazoline nitroxide derivatives of cymantrene (Scheme 2) and study their structure, magnetic properties, and electrochemical behavior.
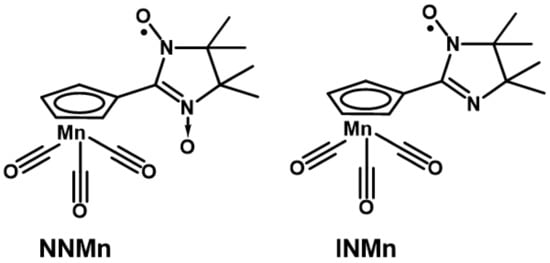
Scheme 2.
Nitronyl nitroxide NNMn and imino nitroxide INMn.
2. Results and Discussion
Nitroxide-substituted cymantrenes were obtained by synthetic procedures similar to those for the previously studied cyrhetrene analogs [41]. The possibility of using commercially available cymantrene as a starting reagent on a larger scale allowed us to increase the yield of the target paramagnetic half-sandwiched nitroxides by ~1.5–2 times compared to cyrhetrene derivatives. Except for the need to protect NNMn and INMn from visible and UV light, they are stable in both solids and solutions and do not require an inert atmosphere, decreasing temperature or other special precautions for storage and handling in common laboratory conditions.
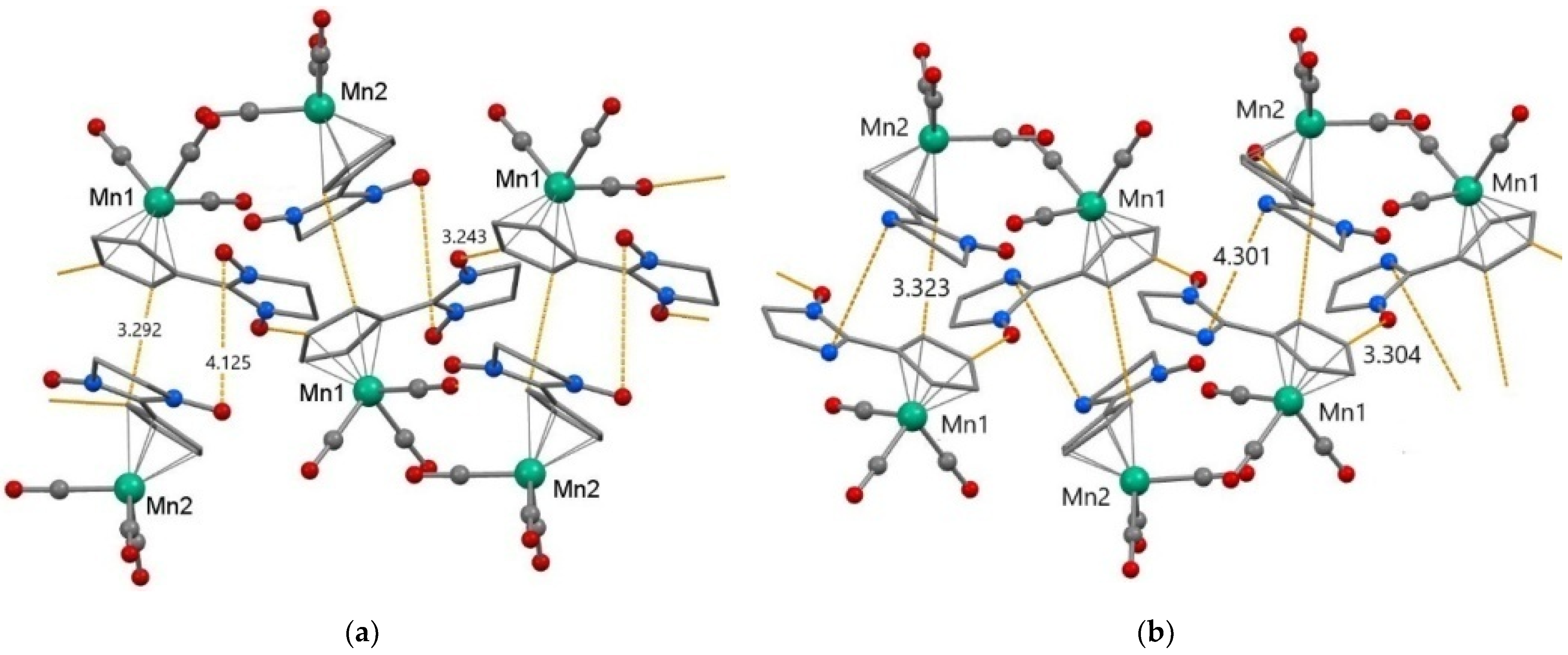
According to the data of SC XRD study, the bond lengths in the 2-imidazoline substituents and in the {Mn(CCp)5(CCO)3} coordination units of NNMn and INMn are in the intervals typical of nitroxides and cymantrenes: N-O—1.24–1.28 Å, Mn-CCp—2.12–2.15 Å, Mn-CCO—1.75–1.81 Å (Table S2) [48]. In the crystal structure of NNMn/INMn there are two crystallographically independent molecules—A and B (Figure 1, Table S2); in the INMn type B molecule, the NO group is disordered in two positions with a weight of 0.8/0.2. In type A molecules, the angle between the nitroxide fragment {O●-N-C = N(→O)} and the Cp cycle is 5.3° for NNMn and 6.0° for INMn, i.e., their planes are almost coplanar, whereas in type B molecules the angle between cycles is somewhat larger—19.8 and 13.8° for NNMn and INMn, respectively.
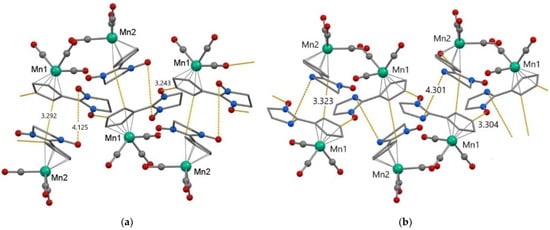
Figure 1.
Fragments of crystal structure and the shortest intermolecular distances (Å) in NNMn (a) and INMn (b). Color scheme: Mn—green, O—red, C—grey, and N—blue. The H atoms, the geminal CH3 groups and disordered O atoms of NO groups in positions with a weight of 0.2 for INMn are omitted for clarity.
Mutual arrangement of NNMn and INMn molecules in crystalline structures is similar (Figure 1, Table S3). Neighboring molecules A and B are combined into {AB} dimers with short distances between C atoms of cyclopentadienyl rings (NNMn: CCp···CCp 3.29 Å; INMn: CCp···CCp 3.32 Å). For nitronyl nitroxide NNMn this orientation relative to each other of molecules A and B is favorable for the formation of close contacts between NO groups (N···ONO 3.96/4.01 and ONO···ONO 4.13 Å), whereas for imino nitroxide INMn the nearest distances are realized between Nim atoms of adjacent molecules (Nim···Nim 4.30 Å). Type A molecules for both NNMn and INMn are linked together in chains by H-bonds CCp–H···ONO (NNMn: C···O 3.24, H···O 2.62 Å; INMn: C···O 3.30, H···O 2.58 Å).
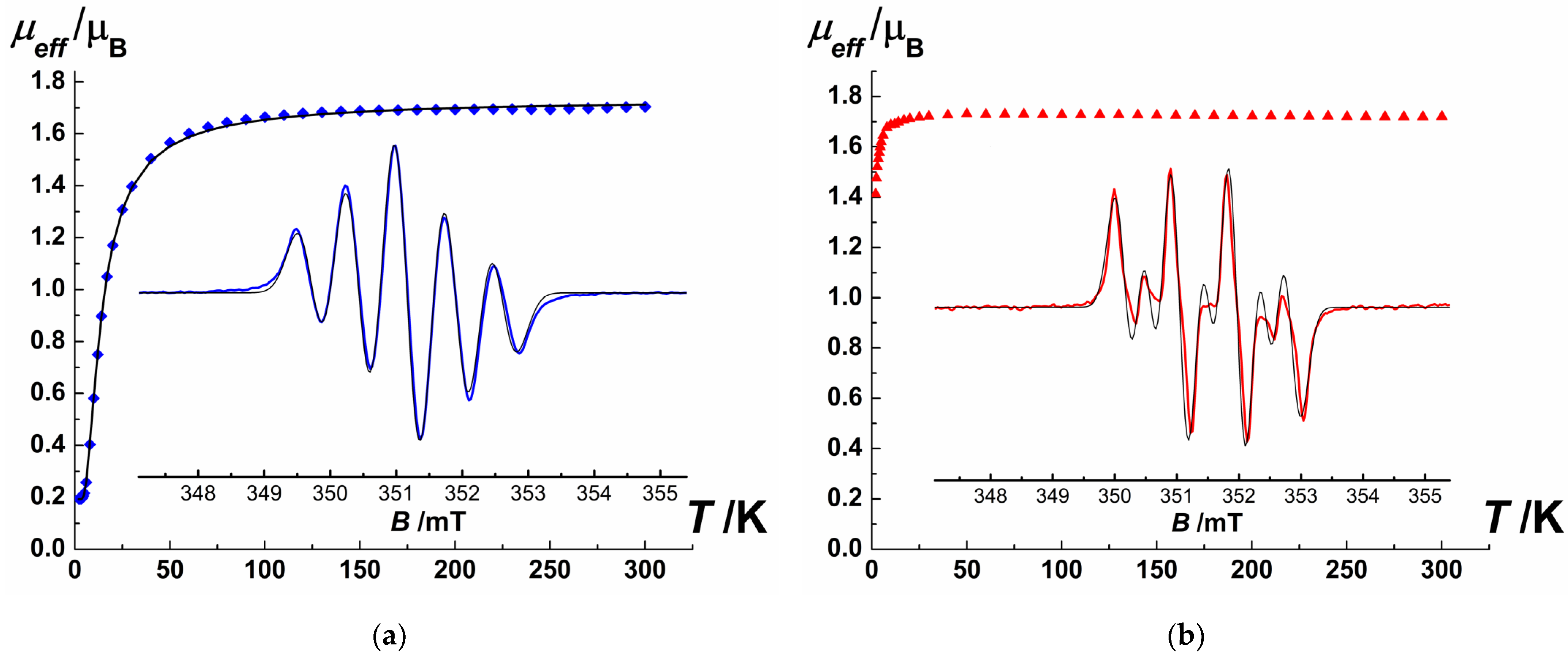
SQUID magnetometry measurements showed that NNMn and INMn (Figure 2, Table S4) are paramagnets: µeff magnitude at 50–300 K is close to the theoretical spin-only value of 1.73 µB for the monoradical; the monotonic decrease in µeff with further lowering temperature from 30 to 2 K indicates the presence of antiferromagnetic exchange interactions. For NNMn nitronyl nitroxide, the optimal parameters of magnetic exchange interactions obtained by fitting the experimental dependence µeff(T) within the model of exchange-coupled dimers (H = −2JSR1SR2, SR1 = SR2 = ½) [49] consist of JNNMn = −12.1 cm−1 and gNNMn = 2.01. These results agree with the data of periodic quantum-chemical calculations and are confirmed by an independent molecular DFT calculation (JQE mark—Quantum Espresso 6.2 package [50] and JORCA mark—ORCA 5.0 quantum chemistry package [51]; Table S4). The calculated values of intermolecular magnetic exchange interactions occurring in {AB} dimers with closely arranged NO groups (ONO···ONO 4.13 Å) are JQE-NNMnNO···ON = −3.0 cm−1 and JORCA-NNMnNO···ON = −3.15 cm−1. At the same time, the efficiency of the chain-wise magnetic exchange channel formed upon the H-bond of ONO atom with the cyclopentadienyl ring (C···O = 3.24 Å) is much weaker: JQE-NNMnCp···ON ~0 cm−1 and JORCA-NNMnCp···ON = −0.01 cm−1. The fitting of the µeff(T) curve for INMn using the same dimer model yielded a much lower value of the magnetic exchange interactions—JINMn = −0.8 cm−1 (gINMn = 2.00). Unfortunately, in this case we failed to reproduce the weak antiferromagnetic exchange-coupling by either the molecular DFT method or the periodic DFT method used above. The much weaker magnetic exchange interactions for INMn as compared with NNMn is consistent with the larger distances between the {O●-N-C = N(→O)} fragments, where almost the entire spin density is concentrated (e.g., the closest N···N distances are 4.30 Å in INMn versus 4.08 Å in NNMn; Tables S3 and S5).
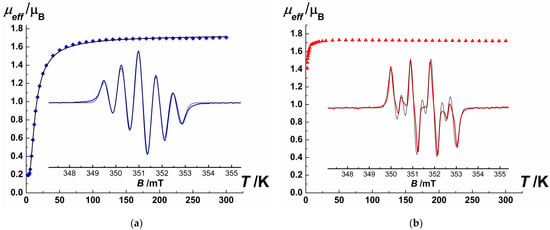
Figure 2.
Experimental dependences µeff(T) for the nitronyl nitroxide NNMn (a—blue squares; solid thick black line — fitted data based on optimized parameters, Table S4) and imino nitroxide INMn (b—red triangles). Insets: experimental EPR spectra for NNMn (a—blue curve) and INMn (b—red curve) recorded at room temperature in a degassed and diluted toluene solution; solid thin black curves—their modeling (Table S5).
The EPR spectra of dilute solutions of NNMn and INMn (Figure 2) show characteristic five- and seven-line patterns for aromatically-substituted 2-imidazoline nitronyl and imino nitroxides, respectively [1,2,3]. Modeling parameters of EPR spectra used spin Hamiltonian in the form (S = 1/2, =1) [52] gave giso = 2.009 for both compounds and hyperfine coupling constants = = 0.721 mT for NNMn and = 0.915 mT, = 0.418 mT for INMn.
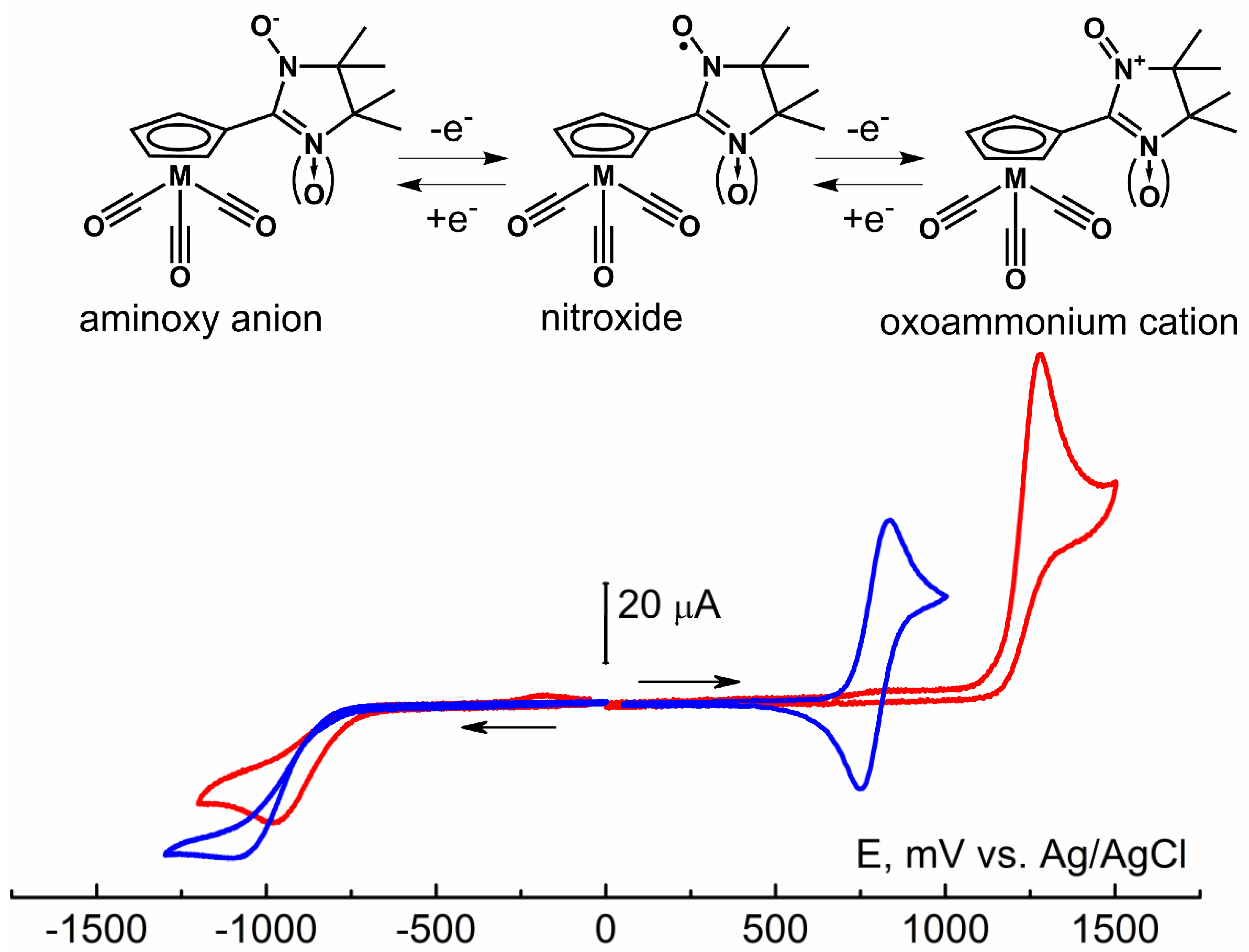
According to cyclic voltammetry (CV) data, for nitroxide-substituted cymantrenes, electron transfer appears only on the nitroxide fragments {NN}/{IN}, while the cymantrenous part {(Cp)M(CO)3} of the molecules remains unchanged in a wide range of electrochemical potentials (Figure 3, Figures S2 and S3, Table S5). NNMn and INMn exhibit the irreversible reduction process characteristic of nitroxide radicals [53] when the potentials reach −1096 and −982 mV, respectively. The oxidation of nitronyl nitroxide NNMn at 793 mV is a one-electron completely reversible process, while the oxidation of imino nitroxide INMn occurs at higher potentials at 1284 mV and is irreversible. The distinction in the oxidation and reduction potentials for NNMn and INMn is consistent with the difference in the calculated energies of their α-SOMO and β-LUMO orbitals (ORCA 5.0 quantum chemistry package [51] with range-separated LC-BLYP functional and def2-TZVP basis set; Table S5). The higher calculated magnitude of the orbital energy α-SOMO of −8.068 eV for NNMn than −8.560 eV for INMn agrees well with the lower value of its oxidation potential. The higher reduction potential for NNMn indicates its lower electron affinity, which corresponds to a higher calculated magnitude of the β-LUMO orbital energy: −0.466 eV for NNMn and −0.644 eV for INMn.
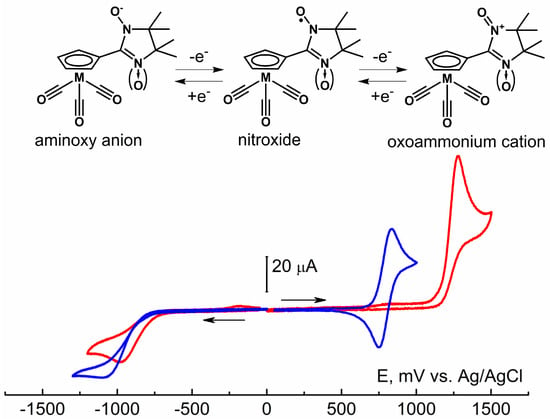
Figure 3.
Redox processes of the NNMn/INMn molecules and CV curves of oxidation and reduction in NNMn (blue line) and INMn (red line) 5.0 × 10−3 M solutions in MeCN (a GC disk electrode d = 1.7 mm, supporting electrolyte 0.1 M Bu4NBF4/MeCN, the scan rate 100 mV s−1, T = 298 K).
A comparison of the structure, magnetic, and electrochemical properties for the obtained nitroxide-substituted cymatrenes with the previously reported cyrhetrene [41] derivatives showed that they are complete analogs. The crystal structures of NNMn and INMn are isostuctural to one of the polymorphic modifications of the nitronyl nitroxide cyrhetrene NNRe-III (Supplementary Materials Tables S1–S3). The similarity of the network of intermolecular contacts for NNMn and NNRe-III results in almost complete coincidence of the dependences µeff(T) for them, as well as the parameters of intermolecular magnetic exchange interactions calculated by quantum chemistry [50] and fitted from the experimental data [49] (JQE-NNMnNO···ON = −3.0 cm −1, JNNMn = −12.1 cm −1 and gNNMn = 2.01; JQE-NNReNO···ON = −5.3 cm−1, JNNRe-III = −12.3 cm−1 and gNNRe-III = 2.03; Table S4, Figure S1). Substitution Re(I) by Mn(I) in {(Cp)M(CO)3} moiety according to the results of quantum chemical calculations [51] and EPR spectroscopy data did not affect the character of the spin density spread in {O●-N-C = N→O}/{O●-N-C = N} fragments for the corresponding nitronyl and imino nitroxide derivatives of cymantrene and cyrhetrene (Table S5). The calculated values of the orbital energies α-SOMO and β-LUMO for the cymantrene and cyrhetrene nitroxides are also close to each other, which agrees with the similar magnitudes of their electrochemical potentials from CV measurements (Figure 3, Table S5).
In summary, the first persistent manganeseorganic nitroxide-substituted compounds NNMn and INMn were synthesized and characterized. They are kinetically stable in both solids and solutions and do not require any special precautions for their storage and handling. The physicochemical properties of NNMn and INMn are similar to those of the cyrhetrene analogues NNRe and INRe [41]. Taking into account that cymantrene derivatives are more commercially available, some synthetic steps can initially be developed on their basis and then adapted to produce the target Re(I) or Tc(I) compounds. Functionalized derivatives of both NNMn/INMn and NNRe/INRe could potentially be used to create new medicines where therapy with cymantrene or cyrhetrene-based moieties is combined with MRI diagnosis due to the contrast properties of nitroxide radicals.
3. Materials and Methods
3.1. General Procedures
The 2,3-bis(hydroxyamino)-2,3-dimethylbutane BHA [54], (η5-formylcyclopentadienyl)tricarbonylmanganese(I) [(CHOCp)Mn(CO)3] [55,56] were synthesized by the known procedures. The commercially available reagents and solvents for synthesis under Ar, electrochemical measurements and EPR study were purified, dried and degassed following standard literature methods [57] and/or using an MBRAUN MB SPS-800 system. The synthesis, purification and storage of cymantrene derivatives were carried out with darkening due to their light sensitivity. The reactions were monitored by TLC using «Alugram SIL G/UV254» and «POLYGRAM ALOX N/UV254» (“Macherey-Nagel”) sheets. Column chromatography was carried out with the use of SiO2 0.04–0.0063 mm/230–400 mersh ASTM for column chromatography (“Macherey-Nagel”) and Al2O3 of chromatographic grade purchased from the Donetsk Plant of Chemical Reagents. The IR spectra of the samples pelletized with KBr were recorded on a «VECTOR-22» (Bruker, Karlsruhe, Germany). The melting points were determined on a melting point apparatus «Stuart» (SMP3). The microanalyses were performed on a «EURO EA3000» CHNS analyzer (HEKAtech, Webberg, Germany) at the Chemical Analytical Center of the Novosibirsk Institute of Organic Chemistry SB RAS.
3.2. Synthesis of Spin-Labeled Cymantrenes (Scheme 3)
- 2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (NNMn). The mixture of freshly obtained [(CHOCp)Mn(CO)3] (0.580 g; 2.50 mmol) and BHA (0.555 g; 3.75 mmol) in dry EtOH (10 mL) was kept under Ar at room temperature for 72 h. Then, EtOH was evaporated, and the colorless residue was purified by column chromatography (Al2O3 1.5 × 15 cm, Et2O as an eluent). After evaporation it was dried in a vacuum to obtain 0.760 g whitish powder of 2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethylimidazolidine-1,3-diole (BHAMn). MnO2 (1.00 g) was added to a solution of BHAMn (0.760 g) in toluene (~15 mL) and the mixture was stirred for ~40 min at the 20 °C water bath. Then, the resultant dark-blue solution was filtered, the filtrate was evaporated, and the residue was purified by column chromatography (Al2O3 1.5 × 15 cm, Et2O as an eluent). Gradual concentration of an Et2O:n-C6H14 = 1:5 solution by solvents evaporation from open flask at 15 °C gave aggregates of dark-blue elongated prismatic crystals. Yield: 0.570 g (75% per [(CHOCp)Mn(CO)3)]. Rf ~0.50 (Et2O) on the Aluminium oxide N/UV254 plates. NNMn is soluble in aromatic hydrocarbons, halogen-substituted hydrocarbons, acetone, Et2O and alcohols, moderately soluble in saturated hydrocarbons, and insoluble in water. IR spectrum (KBr) ν: 2994, 2023, 1934, 1560, 1453, 1429, 1398, 1372, 1143, 1037, 869, 631, 542 cm−1. Mp 139–141 °C (decomp). Found (%): C, 50.22; H, 4.83; N, 7.75. Calculated for C15H16MnN2O5 (%): C, 50.15; H, 4.49; N, 7.80.
- 2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl (INMn). Oxidation of the condensation product of [(CHOCp)Mn(CO)3] (0.235 g; 1.01 mmol) and BHA (0.300 g; 2.03 mmol) omitting its purification by column chromatography resulted in INMn as a main product and NNMn as an admixture. INMn was separated by column chromatography (Al2O3 1.5 × 15 cm, Et2O as an eluent) and recrystallized from an Et2O:n-C6H14 mixture. Yield: 0.073 g (25% per [(CHOCp)Mn(CO)3]), orange elongated plates. INMn soluble in aromatic hydrocarbons, halogen-substituted hydrocarbons, saturated hydrocarbons, acetone, Et2O, alcohols, insoluble in water. Rf ~0.95 (Et2O) on the Aluminium oxide N/UV254 plates. Mp 89–92 °C (decomp). IR spectrum (KBr) ν: 2982, 2023, 1933, 1587, 1437, 1413, 1377, 1165, 1038, 843, 633, 542 cm−1. Found (%): C, 52.47; H, 4.65; N, 8.11. Calculated for C15H16MnN2O5 (%): C, 52.49; H, 4.70; N, 8.16.
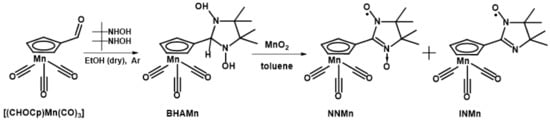
Scheme 3.
Synthesis of NNMn and INMn.
Scheme 3.
Synthesis of NNMn and INMn.
3.3. Single Crystals X-ray Crystallography
The intensity data for single crystals were collected on Bruker AXS diffractometers—a SMART APEX II (Mo Kα radiation) and an APEX DUO (Cu Kα radiation) with a Cobra low-temperature accessory (Oxford Cryosystem). The structures were solved by direct methods and refined by full-matrix least-squares in an anisotropic approximation for all non-hydrogen atoms. The H atoms were calculated geometrically and refined in a riding model. All calculations on structure solution and refinement were performed with SHELXL (2016/4 and 2018/3) software. Crystallographic data, selected bond lengths, angles and intermolecular distances can be found in Tables S1–S3. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, deposition numbers 2,182,686 (NNMn) and 2,182,685 (INMn). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, accessed on 27 October 2022 (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
3.4. Variable-Temperature SQUID Magnetometry
Magnetic measurements were carried out on MPMSXL SQUID magnetometer (Quantum Design) in the temperature range 2−300 K in a magnetic field of up to 5 kOe. The paramagnetic components of the magnetic susceptibility χ were determined with allowance for the diamagnetic contribution evaluated from the Pascal constants [58]. The effective magnetic moment was calculated as μeff = ((3k/NAμB2)χT)1/2 ≈ (8χT)1/2.
3.5. Quantum-Chemical Calculations
The isotropic exchange parameters (Table S4) were estimated within the broken-symmetry approach of Yamaguchi and co-workers [59]. All periodic DFT + U calculations were performed using the crystallographically determined geometries and an approach based on calculations of similar systems [60] utilizing pseudo-potential PW-SCF code of Quantum Espresso 6.2 package [50]. We used the nonlinear core-corrected ultrasoft pseudo-potentials of type X.pbe-van_ak.UPF (X—is a symbol of chemical element) with the PBE exchange-correlation functional. The kinetic energy cutoffs for wave functions and charge density are 50 and 400 Ry, respectively. The integration in the k space was performed over the mesh 2 × 2 × 2 in the first Brillouin zone as in Monkhorst−Pack scheme [61] with a displacement of k-grid at the center of the Brillouin zone and the Gaussian smoothing of 0.136 eV. The Hubbard correlations on Mn and O sites were taken into account within the framework of the Dudarev version of GGA + U approach [62] with the values Ud(Mn) = 5.0 eV [63], Up(O) = 5.0 eV [64]. The results of periodic DFT + U calculations are confirmed by independent molecular DFT calculations that were performed by ORCA 5.0 quantum chemistry package [51] using the TPSSh hybrid functional in ma-def2-TZVP basis set, augmented by s-, p-diffuse functions, which is important for correct calculation of distance interactions.
The total Mulliken spin density values localized on atoms of {O●-N-C = N→O} or {O●-N-C = N} fragments and metal centers and energy values of α-SOMO and β-LUMO orbitals for NNM and INM (M = Mn, Re) were estimated by molecular DFT calculations with optimized geometries of the complexes, carried out with ORCA 5.0 quantum chemistry package [51] using range-separated LC-BLYP functional and def2-TZVP basis set (Table S5).
3.6. EPR Spectroscopy
Continuous wave (CW) X-band (9.88 GHz) EPR measurements were carried out on a Bruker EMX spectrometer at room temperature. Spectra were recorded at a microwave power of ~2 mW and a modulation amplitude of 0.01 mT at 100 kHz. Diluted toluene solutions of NNMn and INMn (~10−3 mM) were studied; samples were preliminarily degassed by freeze–pump–thaw cycles. EPR data were modeled with the EasySpin toolbox [52].
3.7. Cyclic Voltammetry
Cyclic voltammetry (CV) measurements were performed with a PC-piloted digital potentiostat IPC-Pro-MF (Econix). The experiments were carried out in a 10-mL five-neck glass conic electrochemical cell equipped with a water jacket for thermostating. As a working electrode, a glass carbon (GC) disk (d = 1.7 mm) was used, polished before each run; a platinum wire was used as an auxiliary electrode. The potentials are referred to the AgCl/KClsat electrode separated from the analyte by an electrolytic bridge filled with supporting electrolyte (0.1 M Bu4NBF4/MeCN). Solution deaeration was carried out by purging them with highly pure Ar before recording every CV curve and Ar passing over it for the duration of the experiments. The approximation to zero current of the potentials of cathodic and anodic peaks to the determination of half-wave potentials E0′ was carried out as described elsewhere [53,65].
Supplementary Materials
The following are available at https://www.mdpi.com/article/10.3390/molecules27217545/s1, Table S1: Crystal data and experimental details; Table S2: Selected bond lengths (Å) and angles (°);Table S3: Selected intermolecular bond lengths (Å) and angles (°); Table S4: The intermolecular magnetic exchange coupling parameters (cm−1) according to results of analysis and fitting of magnetochemical data and periodical quantum-chemical calculations; Table S5: The calculated Mulliken spin density values localized on selected atoms, giso and Aiso (mT) from modeling of EPR data, calculated α-SOMO and β-LUMO orbitals energy values (eV), oxidation ′ and reduction potentials (mV) from CV experiments for nitroxide-substituted cymantrenes and cyrhetrenes; Figure S1: Experimental and fitted dependences µeff(T) for the nitronyl nitroxides NNMn and NNRe-III; Figure S2: CV curves of oxidation of NNMn and INMn solutions; Figure S3: CV curves of oxidation of NNMn solution at different scan rates of potential application.
Author Contributions
Conceptualization, V.O. and K.M.; methodology and supervision, G.R., V.O. and M.E.; synthesis and characterization of NNMn and INMn, K.M.; SC XRD experiment, refinement the X-ray data and solving structures, G.L. and G.R.; magnetochemistry measurements and analysis, A.B.; quantum chemical calculations, V.M.; EPR spectroscopy study, S.T., S.V. and M.F.; CV measurements and analysis, E.S., M.S. and M.E.; writing—original draft preparation, K.M., V.M. and G.R.; writing—review and editing, V.O. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Foundation for Basic Research (Grant No. 19-29-08005), the Russian Science Foundation (Grant No. 18-13-00380, XRD study), and the Ministry of Education and Science of the Russian Federation (state contract no. 075-15-2021-580, EPR study).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds NNMn and INMn are available from the authors.
References
- Ovcharenko, V. 13. Metal-Nitroxide Complexes: Synthesis and Magnetostructural Correlations. In Radicals: Fundamentals and Applied Aspects of Odd Electron Compounds; Hicks, R.G., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 461–506. [Google Scholar]
- Tretyakov, E.V.; Ovcharenko, V.I.; Terent’ev, A.O.; Krylov, I.B.; Magdesieva, T.V.; Mazhukin, D.G.; Gritsan, N.P. Conjugated nitroxides. Russ. Chem. Rev. 2022, 91, RCR5025. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I. The chemistry of nitroxide radicals in the molecular design of magnets. Russ. Chem. Rev. 2009, 78, 971–1012. [Google Scholar] [CrossRef]
- Boocock, D.G.B.; Darcy, R.; Ullman, E.F. Studies of Free Radicals. II. Chemical Properties of Nitronylnitroxides. A Unique Radical Anion. J. Am. Chem. Soc. 1968, 90, 5945–5946. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Romanenko, G.V.; Stass, D.V.; Mareev, A.V.; Medvedeva, A.S.; Ovcharenko, V.I. Key influence of the nature of the substituent in the propynal molecule on the outcome of its reaction with vicinal di(N-hydroxyamine). Russ. Chem. Bull. 2008, 57, 601–607. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Chupakhin, O.N.; Kovalev, I.S.; Tretyakov, E.V.; Romanenko, G.V.; Stass, D.V. SNH Reaction of lithiated nitronyl nitroxide with quinoline N-oxide. Russ. Chem. Bull. 2008, 57, 2227–2229. [Google Scholar] [CrossRef]
- Suzuki, S.; Furui, T.; Kuratsu, M.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Nitroxide-Substituted Nitronyl Nitroxide and Iminonitroxide. J. Am. Chem. Soc. 2010, 132, 15908–15910. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Tolstikov, S.E.; Romanenko, G.V.; Bogomyakov, A.S.; Stass, D.V.; Maryasov, A.G.; Gritsan, N.P.; Ovcharenko, V.I. Method for the synthesis of a stable heteroatom analog of trimethylenemethane. Russ. Chem. Bull. 2011, 60, 2608–2612. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. Synthesis and Magnetic Properties of (Pyrrolidin-1-oxyl)–(Nitronyl Nitroxide)/(Iminonitroxide)-Dyads. Chem. Asian J. 2019, 14, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Suzuki, S.; Kanzaki, Y.; Shiomi, D.; Sato, K.; Takui, T.; Tanaka, R.; Okada, K.; Kozaki, M. Heteroatom-incorporated Trimethylenemethane: Synthesis and Properties of Triphenylphenylnitroxide-(Nitronyl Nitroxide) Dyad. Chem. Lett. 2022, 51, 458–460. [Google Scholar] [CrossRef]
- Zhang, X.; Suzuki, S.; Kozaki, M.; Okada, K. NCN Pincer−Pt Complexes Coordinated by (Nitronyl Nitroxide)-2-ide Radical Anion. J. Am. Chem. Soc. 2012, 134, 17866–17868. [Google Scholar] [CrossRef]
- Tanimoto, R.; Suzuki, S.; Kozaki, M.; Okada, K. Nitronyl Nitroxide as a Coupling Partner: Pd-Mediated Cross-coupling of (Nitronyl nitroxide-2-ido)(triphenylphosphine)gold(I) with Aryl Halides. Chem. Lett. 2014, 43, 678–680. [Google Scholar] [CrossRef]
- Suzuki, S.; Kira, S.; Kozaki, M.; Yamamura, M.; Hasegawa, T.; Nabeshima, T.; Okada, K. An efficient synthetic method for organometallic radicals: Structures and properties of gold(I)-(nitronyl nitroxide)-2-ide complexes. Dalton Trans. 2017, 46, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, M.; Tretyakov, E.; Gritsan, N.; Romanenko, G.; Gorbunov, D.; Bogomyakov, A.; Maryunina, K.; Suzuki, S.; Kozaki, M.; Shiomi, D.; et al. (Azulene-1,3-diyl)-bis(nitronyl nitroxide) and bis(iminonitroxide) and Their Copper Complexes. Chem. Asian J. 2017, 12, 2929–2941. [Google Scholar] [CrossRef]
- Slota, M.; Keerthi, A.; Myers, W.K.; Tretyakov, E.; Baumgarten, M.; Ardavan, A.; Sadeghi, H.; Lambert, C.J.; Narita, A.; Müllen, K.; et al. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature 2018, 557, 691–695. [Google Scholar] [CrossRef]
- Shu, C.; Pink, M.; Junghoefer, T.; Nadler, E.; Rajca, S.; Casu, M.B.; Rajca, A. Synthesis and Thin Films of Thermally Robust Quartet (S = 3/2) Ground State Triradical. J. Am. Chem. Soc. 2021, 143, 5508–5518. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Zhivetyeva, S.I.; Petunin, P.V.; Gorbunov, D.E.; Gritsan, N.P.; Bagryanskaya, I.Y.; Bogomyakov, A.S.; Postnikov, P.S.; Kazantsev, M.S.; Trusova, M.E.; et al. Ferromagnetically Coupled S = 1 Chains in Crystals of Verdazyl-Nitronyl Nitroxide Diradicals. Angew. Chem. Int. Ed. 2020, 59, 20704–20710. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Kraut, N. Dinitroxide Carbenes, A New Class of Carbenes with Autoumpolung Character: Preparation in Solution and Stabilization in Transition Metal Complexes. Angew. Chem. Int. Ed. 2002, 41, 311–314. [Google Scholar] [CrossRef]
- Fokin, S.V.; Romanenko, G.V.; Baumgarten, M.; Ovcharenko, V.I. Crystal structure of Cu(hfac)2 complexes with organomercury binitroxide. J. Struct. Chem. 2003, 44, 864–869. [Google Scholar] [CrossRef]
- Tanimoto, R.; Yamada, K.; Suzuki, S.; Kozaki, M.; Okada, K. Group 11 Metal Complexes Coordinated by the (Nitronyl Nitroxide)-2-ide Radical Anion: Facile Oxidation of Stable Radicals Controlled by Metal–Carbon Bonds in Radical Metalloids. Eur. J. Inorg. Chem. 2018, 1198–1203. [Google Scholar] [CrossRef]
- Suzuki, S.; Wada, T.; Tanimoto, R.; Kozaki, M.; Shiomi, D.; Sugisaki, K.; Sato, K.; Takui, T.; Miyake, Y.; Hosokoshi, Y.; et al. Cyclic Triradicals Composed of Iminonitroxide–Gold(I) with Intramolecular Ferromagnetic Interactions. Angew. Chem. Int. Ed. 2016, 55, 10791–10794. [Google Scholar] [CrossRef]
- Kira, S.; Miyamae, T.; Yoshida, K.; Kanzaki, Y.; Sugisaki, K.; Shiomi, D.; Sato, K.; Takui, T.; Suzuki, S.; Kozaki, M.; et al. Aurophilic Interactions in Multi-Radical Species: Electronic-Spin and Redox Properties of Bis- and Tris-[(Nitronyl Nitroxide)-Gold(I)] Complexes with Phosphine-Ligand Scaffolds. Chem. Eur. J. 2021, 27, 11450–11457. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, R.; Wada, T.; Okada, K.; Shiomi, D.; Sato, K.; Takui, T.; Suzuki, S.; Naota, T.; Kozaki, M. A Molecule Having 13 Unpaired Electrons: Magnetic Property of a Gadolinium(III) Complex Coordinated with Six Nitronyl Nitroxide Radicals. Inorg. Chem. 2022, 61, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Kokhanov, Y.V.; Rozantsev, É.G.; Nikolenko, L.N.; Maksimova, L.A. Synthesis of some hetrocyclic radicals of the iminoxyl class. Chem. Heterocycl. Compd. 1971, 7, 1421–1423. [Google Scholar] [CrossRef]
- Rendina, L.M.; Vittal, J.J.; Puddephatt, R.J. Stable Organoplatinum(IV) Complexes with Pendant Free Radicals. Organometallics 1995, 14, 2188–2193. [Google Scholar] [CrossRef]
- Stroh, C.; Mayor, M.; von Hänisch, C.; Turek, P. Intramolecular exchange interaction in twofold spin-labelled platinum complexes. Chem. Commun. 2004, 18, 2050–2051. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Peng, Z.; Huang, B.; Zhao, X.-L.; Sun, D.; Shi, X.; Yang, H.-B. TEMPO Radical-Functionalized Supramolecular Coordination Complexes with Controllable Spin−Spin Interactions. J. Am. Chem. Soc. 2021, 143, 433–441. [Google Scholar] [CrossRef]
- Forrester, A.R.; Hepburn, S.P.; Dunlop, R.S.; Mills, H.H. t-Butylferrocenylnitroxide, a Stable Ferrocenyl Radical. J. Chem. Soc. D 1969, 13, 698–699. [Google Scholar] [CrossRef]
- Owtscharenko, W.I.; Huber, W.; Schwarzhans, K.E. Synthesis of 4,4,5,5-Tetramethyl-3-oxide-2-ferrocenyl-imidazoline-1-oxyl. Zeitschrift für Naturforschung B 1986, 41, 1587–1588. [Google Scholar] [CrossRef]
- Owtscharenko, W.I.; Huber, W.; Schwarzhans, K.E. Synthesis of a spinlabel-ferrocene. Monatsh. Chem. 1987, 118, 955–957. [Google Scholar] [CrossRef]
- Sporer, C.; Ruiz-Molina, D.; Wurst, K.; Kopacka, H.; Veciana, J.; Jaitner, P. Ferrocene substituted nitronyl nitroxide and imino nitroxide radicals. Synthesis, X-ray structure and magnetic properties. J. Organomet. Chem. 2001, 637–639, 507–513. [Google Scholar] [CrossRef]
- Jürgens, O.; Vidal-Gancedo, J.; Rovira, C.; Wurst, K.; Sporer, C.; Bildstein, B.; Schottenberger, H.; Jaitner, P.; Veciana, J. Transmission of Magnetic Interactions through an Organometallic Coupler: A Novel Family of Metallocene-Substituted α-Nitronyl Aminoxyl Radicals. Inorg. Chem. 1998, 37, 4547–4558. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Mochida, T.; Moriyama, H. Structure and Magnetic Properties of Biferrocenyl Nitronyl Nitroxide Radicals. Mol. Cryst. Liq. Cryst. 2000, 343, 211–214. [Google Scholar] [CrossRef]
- Le Poul, P.; Caro, B.; Cabon, N.; Guen, F.R.-L.; Golhen, S. Synthesis and characterization of novel nitroxide organometallic Fischer-type carbene complexes. J. Organomet. Chem. 2013, 745–746, 57–63. [Google Scholar] [CrossRef]
- Nakamura, Y.; Koga, N.; Iwamura, H. Synthesis and Characterization of 2-Ferrocenyl-4,4,5,5-tetramethyl-2-imidazolin-1-oxyl 3-Oxide and Its CT-Complex with DDQ. Chem. Lett. 1991, 20, 69–72. [Google Scholar] [CrossRef]
- Gurskaya, L.Y.; Polienko, Y.F.; Rybalova, T.V.; Gritsan, N.P.; Dmitriev, A.A.; Kazantsev, M.S.; Zaytseva, E.V.; Parkhomenko, D.A.; Beregovaya, I.V.; Zakabluk, G.A.; et al. Multispin Systems with a Rigid Ferrocene-1,1’-diylSubstituted 1,3-Diazetidine-2,4-diimine Coupler: A General Approach. Eur. J. Org. Chem. 2022, e202101234. [Google Scholar] [CrossRef]
- Yi, S.; Captain, B.; Ottaviani, M.F.; Kaifer, A.E. Controlling the Extent of Spin Exchange Coupling in 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) Biradicals via Molecular Recognition with Cucurbit[n]uril Hosts. Langmuir 2011, 27, 5624–5632. [Google Scholar] [CrossRef]
- Fujiwara, K.; Akutsu, H.; Yamada, J.; Satoh, M.; Nakatsuji, S. Structures and charge–discharge properties of spin-carrying ferrocene derivatives. Tetrahedron Lett. 2011, 52, 6655–6658. [Google Scholar] [CrossRef]
- Nakatsuji, S.; Fujiwara, K.; Akutsu, H.; Yamada, J.; Satoh, M. Structures and properties of ferrocene derivatives with different kinds of nitroxide radicals. New J. Chem. 2013, 37, 2468–2472. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, H.; Lan, M. Novel ferrocenyl nitroxides: Synthesis, structures, electrochemistry and antioxidative activity. J. Organomet. Chem. 2009, 694, 3958–3964. [Google Scholar] [CrossRef]
- Maryunina, K.; Letyagin, G.; Bogomyakov, A.; Morozov, V.; Tumanov, S.; Veber, S.; Fedin, M.; Saverina, E.; Syroeshkin, M.; Egorov, M.; et al. Re(I)-nitroxide complexes. RSC Adv. 2021, 11, 19902–19907. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Lepareur, N.; Lacoeuille, F.; Bouvry, C.; Hindré, F.; Garcion, E.; Chérel, M.; Noiret, N.; Garin, E.; Knapp, F.F.R. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.I.; Fursova, E.Y.; Tolstikova, T.G.; Sorokina, K.N.; Letyagin, A.Y.; Savelov, A.A. Imidazol-4-yl 2-Imidazoline Nitroxide Radicals, a New Class of Promising Contrast Agents for Magnetic Resonance Imaging. Dokl. Chem. 2005, 404, 171–173. [Google Scholar] [CrossRef]
- Artyukhova, N.A.; Romanenko, G.V.; Fursova, E.Y.; Ovcharenko, V.I. Method of producing nitronylnitroxyl radical 2-(5-methyl-1N-imidazole-4-yl)-4,5-bis(spiropentane)-4,5-dihydro-1N-imidazol-3-oxid-1-oxyl. Patent RF RU2642468C2, 25 January 2018. [Google Scholar]
- Jabłoński, A.; Matczak, K.; Koceva-Chyła, A.; Durka, K.; Steverding, D.; Jakubiec-Krześniak, K.; Solecka, J.; Trzybiński, D.; Woźniak, K.; Andreu, V.; et al. Cymantrenyl-Nucleobases: Synthesis, Anticancer, Antitrypanosomal and Antimicrobial Activity Studies. Molecules 2017, 22, 2220. [Google Scholar] [CrossRef]
- Huentupil, Y.; Chung, P.; Novoa, N.; Arancibia, R.; Roussel, P.; Oyarzo, J.; Klahn, A.H.; Silva, C.P.; Calvis, C.; Messeguer, R.; et al. Novel multifunctional and multitarget homo-(Fe2) and heterobimetallic-[(Fe,M) with M = Re or Mn] sulfonyl hydrazones. Dalton Trans. 2020, 49, 12249–12265. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Structural Database; Version 5.43; University of Cambridge: Cambridge, UK, 2021.
- Boča, R. A Handbook of Magnetochemical Formulae; Elsevier Inc.: Amsterdam, The Netherlands, 2012; 1010p. [Google Scholar]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009, 21, 395502. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Mendkovich, A.S.; Luzhkov, V.B.; Syroeshkin, M.A.; Sen’, V.D.; Khartsii, D.I.; Rusakov, A.I. Influence of the nature of solvent and substituents on the oxidation potential of 2,2,6,6-tetramethylpiperidine 1-oxyl derivatives. Russ. Chem. Bull. 2017, 66, 683–689. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Romanenko, G.V.; Korobkov, I.V.; Rey, P. Synthesis of vicinal bishydroxylamine. Russ. Chem. Bull. 1999, 48, 1519–1525. [Google Scholar] [CrossRef]
- Kolobova, N.E.; Valueva, Z.P.; Solodova, M.Y. Synthesis of formylcyclopentadienyltricarbonylrhenium and some of its properties. Russ. Chem. Bull. 1980, 29, 1701–1705. [Google Scholar] [CrossRef]
- Heldt, J.-M.; Fischer-Durand, N.; Salmain, M.; Vessières, A.; Jaouen, G. Preparation and characterization of poly(amidoamine) dendrimers functionalized with a rhenium carbonyl complex and PEG as new IR probes for carbonyl metallo immunoassay. J. Organomet. Chem. 2004, 689, 25–4775. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Boudreaux, E.A.; Mulay, L.N. Theory and Application of Molecular Paramagnetism; John Wiley & Sons: New York, NY, USA, 1976; 491p. [Google Scholar]
- Shoji, M.; Koizumi, K.; Kitagawa, Y.; Kawakami, T.; Yamanaka, S.; Okumura, M.; Yamaguchi, K. A general algorithm for calculation of Heisenberg exchange integrals J in multispin systems. Chem. Phys. Lett. 2006, 432, 343–347. [Google Scholar] [CrossRef]
- Streltsov, S.V.; Petrova, M.V.; Morozov, V.A.; Romanenko, G.V.; Anisimov, V.I.; Lukzen, N.N. Interplay between lattice, orbital, and magnetic degrees of freedom in the chain-polymer Cu(II) breathing crystals. Phys. Rev. B 2013, 87, 024425. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Nakamura, K.; Arita, R.; Yoshimoto, Y.; Tsuneyuki, S. First-principles calculation of effective onsite Coulomb interactions of 3d transition metals: Constrained local density functional approach with maximally localized Wannier functions. Phys. Rev. B 2006, 74, 235113. [Google Scholar] [CrossRef]
- Morozov, V.A.; Petrova, M.V.; Lukzen, N.N. Exchange coupling transformations in Cu (II) heterospin complexes of “breathing crystals” under structural phase transitions. AIP Adv. 2015, 5, 087161. [Google Scholar] [CrossRef]
- Sen’, V.D.; Tikhonov, I.V.; Borodin, L.I.; Pliss, E.M.; Golubev, V.A.; Syroeshkin, M.A.; Rusakov, A.I. Kinetics and thermodynamics of reversible disproportionation–comproportionation in redox triad oxoammonium cations–nitroxyl radicals–hydroxylamines. J. Phys. Org. Chem. 2015, 28, 17–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).