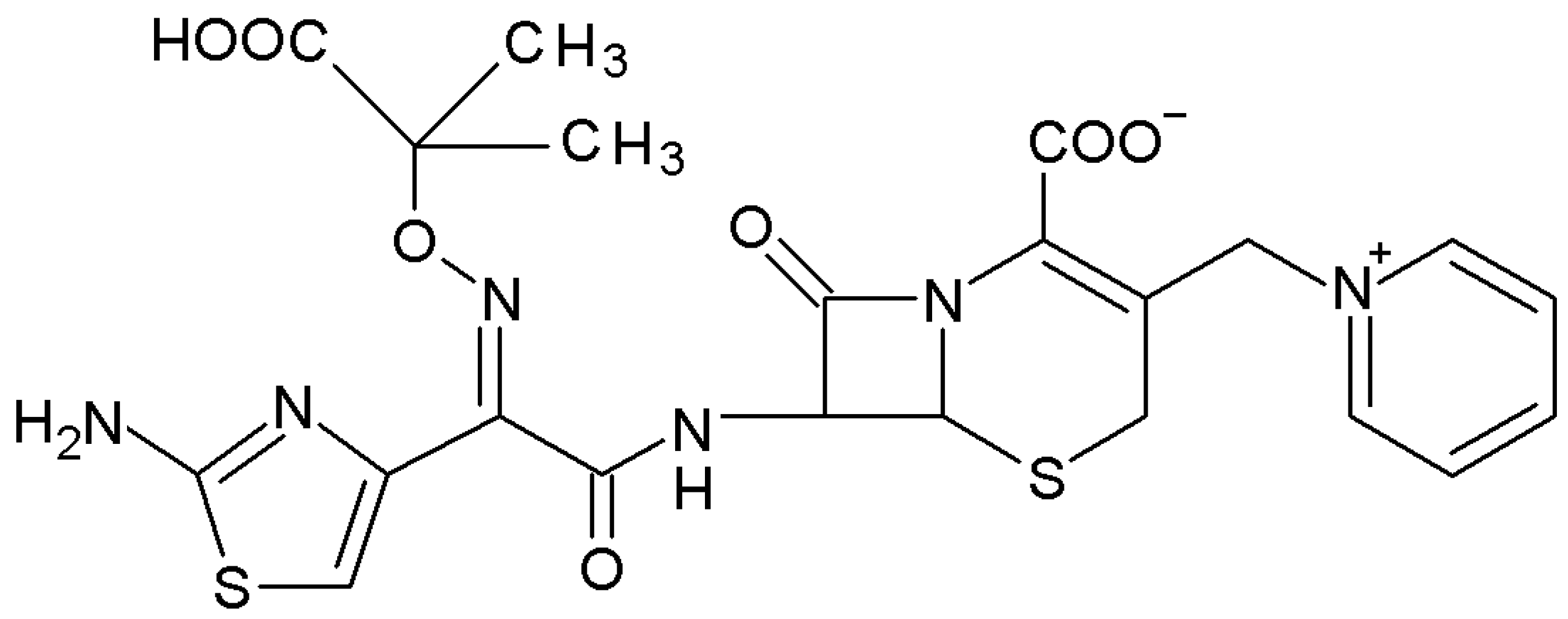
Complex-Forming Properties of Ceftazidime with Fe(III) Ions in an Aqueous Solution
Abstract
1. Introduction
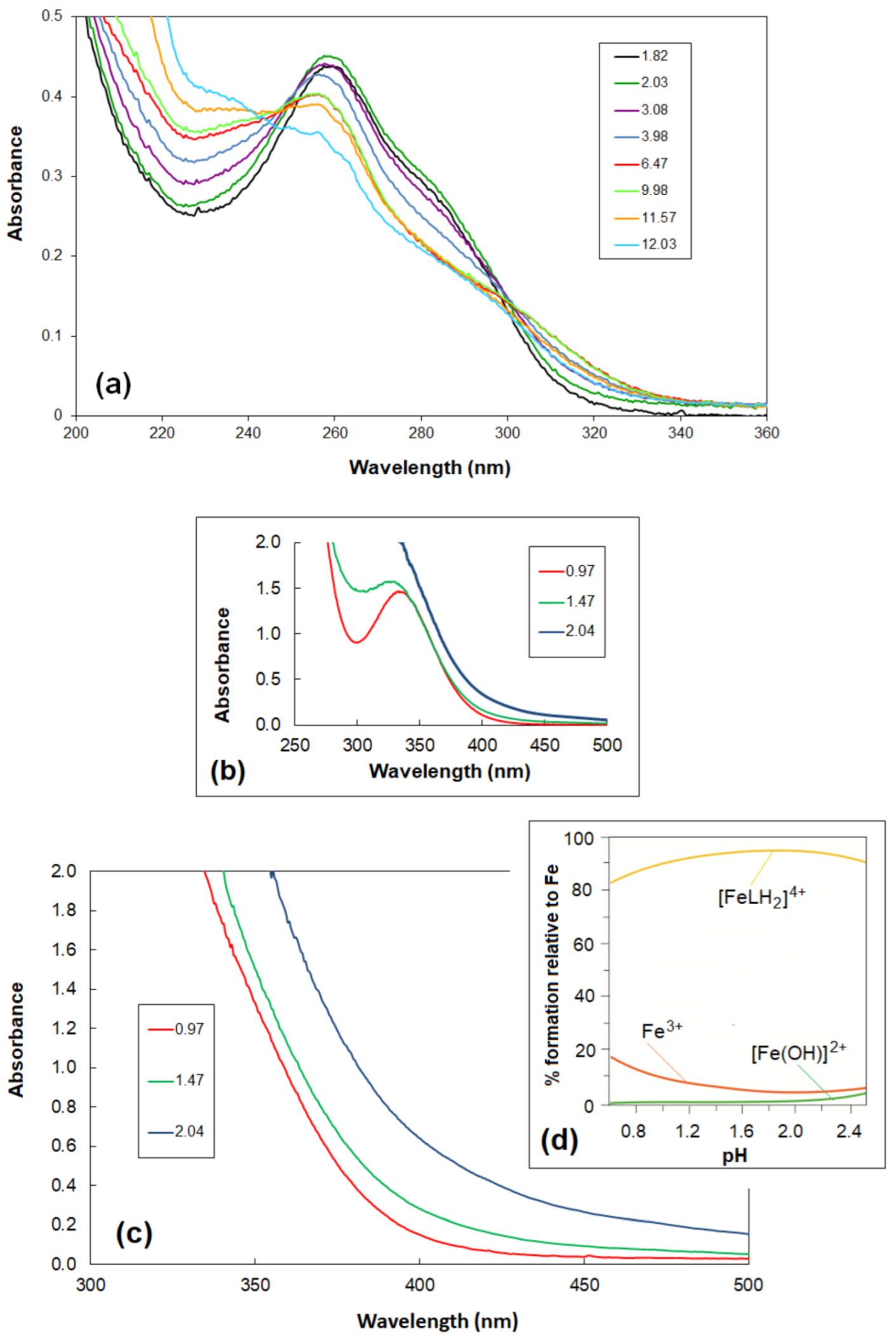
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Spectrophotometric Measurements
3.3. pH-Metric Titrations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, F.; Li, C.; Xie, Y.; Xu, H.; Gao, J. Titanium metal-organic framework nanorods for highly sensitive nitroaromatic explosives detection and nanomolar sensing of Fe3+. J. Solid State Chem. 2019, 278, 120892. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M.; Rogers, J.T.; Leedman, P.J. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int. J. Biochem. Cell Biol. 1999, 31, 1139–1152. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Woo, J.Y.; Han, C.-S. Colorimetric detection of Fe(III) ions using label-free gold nanoparticles and acidic thiourea mixture. Sens. Actuators B Chemical. 2013, 181, 114–118. [Google Scholar] [CrossRef]
- Umbreit, J. Iron deficiency: A concise review. Am. J. Hematol. 2005, 78, 225–231. [Google Scholar] [CrossRef]
- Andrews, N.C. Iron metabolism: Iron deficiency and iron overload. Annu. Rev. Genom. Human Genet. 2000, 1, 75–98. [Google Scholar] [CrossRef]
- Asbel, L.E.; Levison, M.E. Cephalosporins, Carbapenems, and Monobactams. Infect. Dis. Clin. N. Am. 2000, 14, 435–447. [Google Scholar] [CrossRef]
- Christ, W. Pharmacological Properties of Cephalosporins. Infection 1991, 19, S244–S252. [Google Scholar] [CrossRef]
- Magnet, S.; Arbeloa, A.; Mainardi, J.L.; Hugonnet, J.E.; Forgeaud, M.; Dubost, L.; Marie, A.; Delfosse, V.; Mayer, C.; Rice, L.B.; et al. Specificity of L,D-Transpeptidases from Gram-positive Bacteria Producing Different Peptidoglycan Chemotypes. J. Biol. Chem. 2007, 282, 13151–13159. [Google Scholar] [CrossRef]
- Bergan, T. Pharmacokinetic Properties of the Cephalosporins. Drugs 1987, 34, 89–104. [Google Scholar] [CrossRef]
- Anacona, J.R.; Patiño, C. Metalloantibiotics: Synthesis and antibacterial activity of ceftazidime metal complexes. J. Coord. Chem. 2009, 64, 613–621. [Google Scholar] [CrossRef]
- Tarinc, D.; Muslu, H.; Cesme, M.; Golcu, A.; Tumer, M.; Ozkan, A.S. Synthesis, structural characterization and electrochemical evaluation of Schiff base transition metal complexes with ceftazidime. Curr. Anal. Chem. 2013, 9, 319–332. [Google Scholar] [CrossRef]
- Alekseev, V.G.; Sokolova, E.M. Experimental Study and Computer Modeling of Ni(II) and Cu(II) Complexation with Ceftazidime. Russ. J. Inorg. Chem. 2016, 61, 531–534. [Google Scholar] [CrossRef]
- Sanli, S.; Sanli, N.; Gumustas, M.; Ozkan, A.S.; Karadas, N.; Aboul-Enein, H.Y. Simultaneous Estimation of Ceftazidime and Ceftizoxime in Pharmaceutical Formulations by HPLC Method. Chromatographia 2011, 74, 549–558. [Google Scholar] [CrossRef]
- Wozźniczka, M.; SŚwiątek, M.; Gądek-Sobczynńska, J.; Pasternak, B.; Kufelnicki, A. Spectroscopic Determination of Metal-Ligand Coordination by Biologically Active 2-Picolinehydroxamic Acid with Iron(III) and Oxidovanadium(IV) in Aqueous Solutions. Acta Chim. Slov. 2019, 66, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Domínguez, S.; Cerdá, M.F.; Obal, G.; Mederos, A.; Irvine, R.F.; Díaz, A.; Kremer, C. Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J. Inorg. Biochem. 2005, 99, 828–840. [Google Scholar] [CrossRef]
- Khoe, G.H.; Brown, P.L.; Sylva, R.N.; Robins, R.G. The hydrolysis of metal ions. Part 9. Iron(III) in perchlorate, nitrate, and chloride media (1 mol dm–3). J. Chem. Soc. Dalton Trans. 1986, 9, 1901–1906. [Google Scholar] [CrossRef]
- Iuliano, M.; De Tommaso, G. Interaction of Iron(III) with 2-Hydroxybenzohydroxamic Acid in Aqueous Solutions. J. Chem. Eng. Data. 2010, 55, 400–404. [Google Scholar] [CrossRef]
- Perera, W.N.; Hefter, G. Mononuclear Cyano- and Hydroxo-Complexes of Iron(III). Inorg. Chem. 2003, 42, 5917–5923. [Google Scholar] [CrossRef]
- Pettit, L.D.; Powell, K.J. Stability Constants Database, IUPAC and Academic Software; Royal Society of Chemistry: London, UK, 1993. [Google Scholar]
- Masoud, M.S.; Ali, A.E.; Elasala, G.S.; Kolkaila, S.A. Synthesis, spectroscopic, biological activity and thermal characterization of ceftazidime with transition metals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 458–466. [Google Scholar] [CrossRef]
- Schmidt, S.M. The role of iron in viral infections. Front. Biosci. 2020, 25, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kirubavathy, S.J.; Velmurugan, R.; Parameswari, K.; Chitra, S. Synthesis, Characterization and Biological Evaluation of Cu(Ii), Co(III) and Fe(III) Complexes of 2-Benzoyl-3-(Nitrophenyl)Quinoxaline. Int. J. Pharm. Sci. 2014, 5, 2508–2517. [Google Scholar] [CrossRef]
- Irving, H.; Miles, M.G.; Pettit, L.D. A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; O’Sullivan, B. GLEE, a new computer program for glass electrode calibration. Talanta 2000, 51, 33–37. [Google Scholar] [CrossRef]
- Gamov, G.A.; Yarullin, D.N.; Gudyrina, M.A.; Pogodina, E.I.; Medvedeva, A.S.; Zavalishin, M.N. Protonation of L-Ascorbic Acid in an Aqueous Solution at T = 298.2 K, p = 0.1 MPa, and I = 0.10−5.0 mol L−1 (NaCl). J. Chem. Eng. Data 2022, 67, 1358–1364. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the Hyperquad suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, M.; Woźniczka, M.; Fichna, J. Complex-Forming Properties of Ceftazidime with Fe(III) Ions in an Aqueous Solution. Molecules 2022, 27, 7226. https://doi.org/10.3390/molecules27217226
Pająk M, Woźniczka M, Fichna J. Complex-Forming Properties of Ceftazidime with Fe(III) Ions in an Aqueous Solution. Molecules. 2022; 27(21):7226. https://doi.org/10.3390/molecules27217226
Chicago/Turabian StylePająk, Marek, Magdalena Woźniczka, and Jakub Fichna. 2022. "Complex-Forming Properties of Ceftazidime with Fe(III) Ions in an Aqueous Solution" Molecules 27, no. 21: 7226. https://doi.org/10.3390/molecules27217226
APA StylePająk, M., Woźniczka, M., & Fichna, J. (2022). Complex-Forming Properties of Ceftazidime with Fe(III) Ions in an Aqueous Solution. Molecules, 27(21), 7226. https://doi.org/10.3390/molecules27217226







