2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells
Abstract
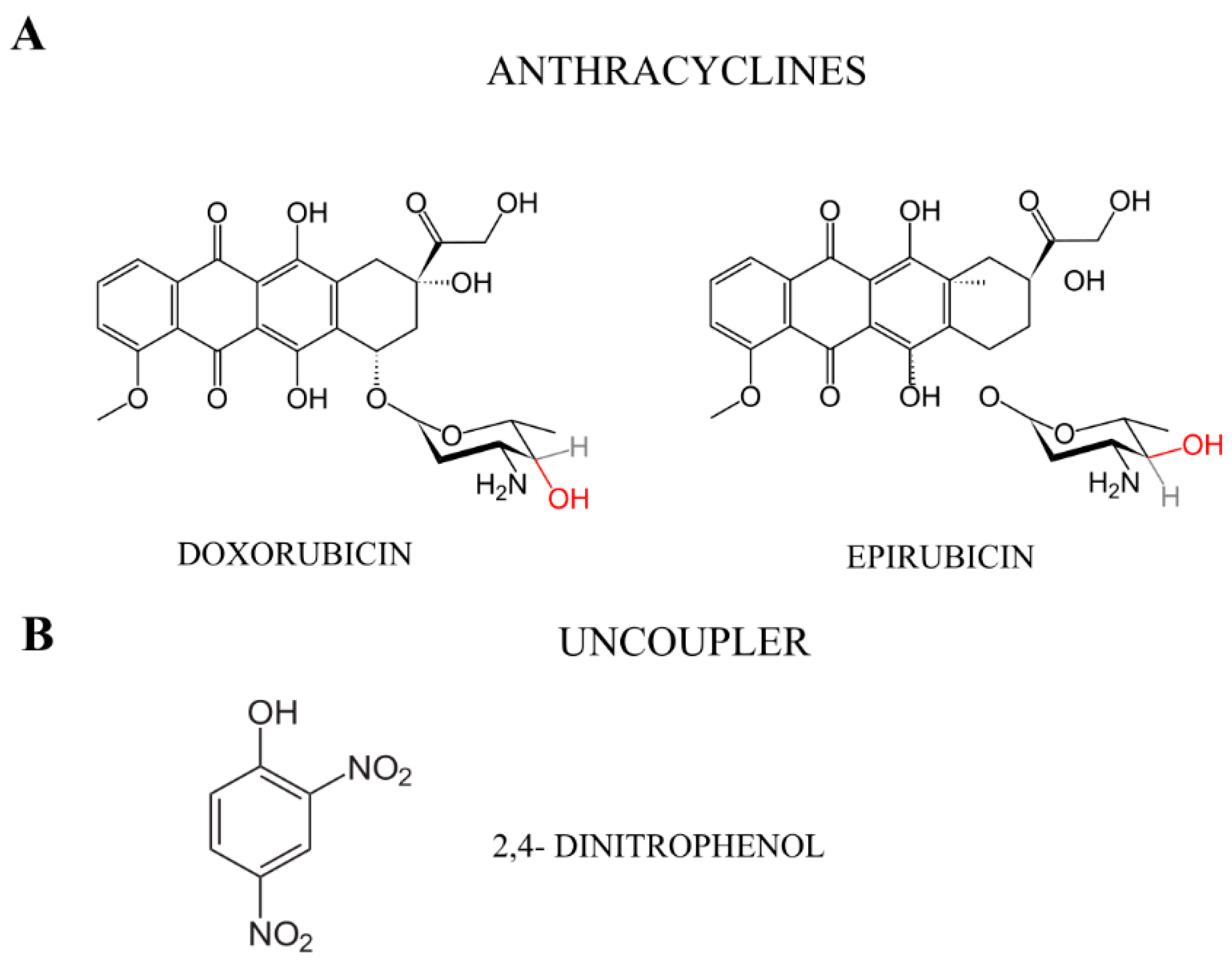
1. Introduction
2. Result
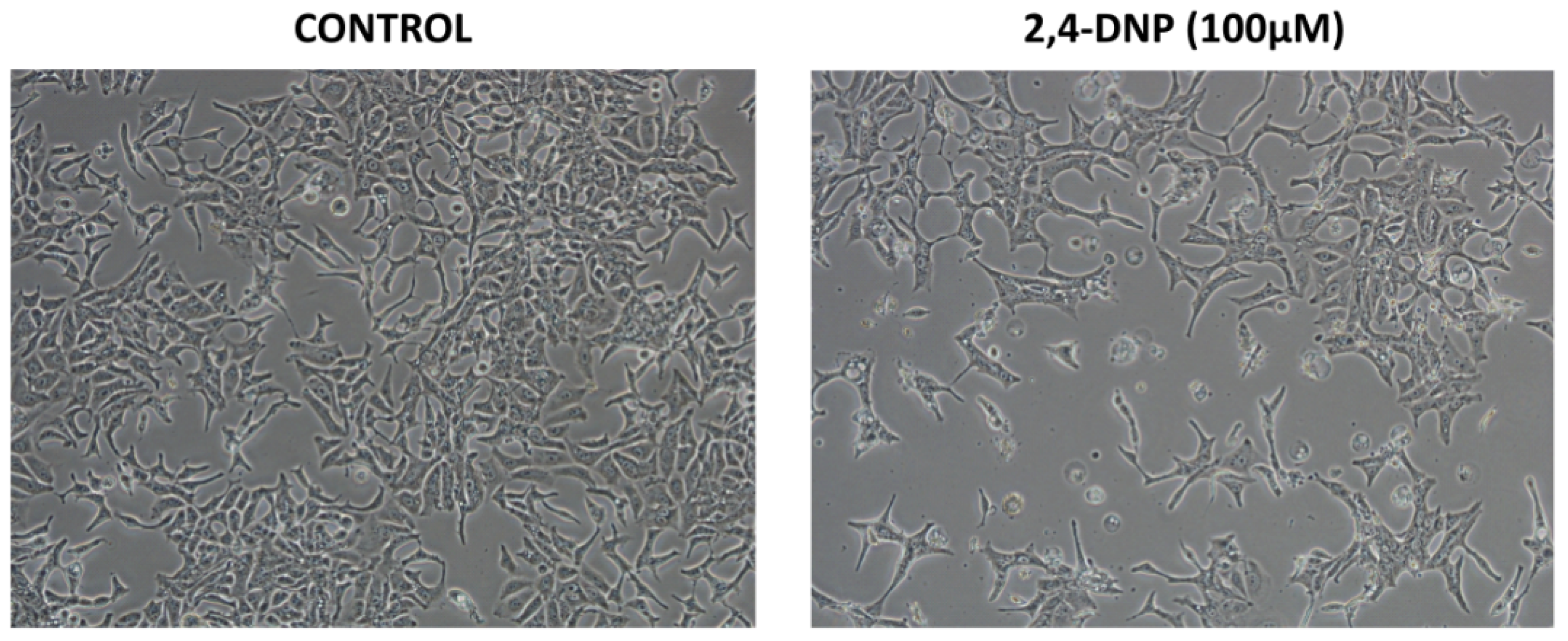
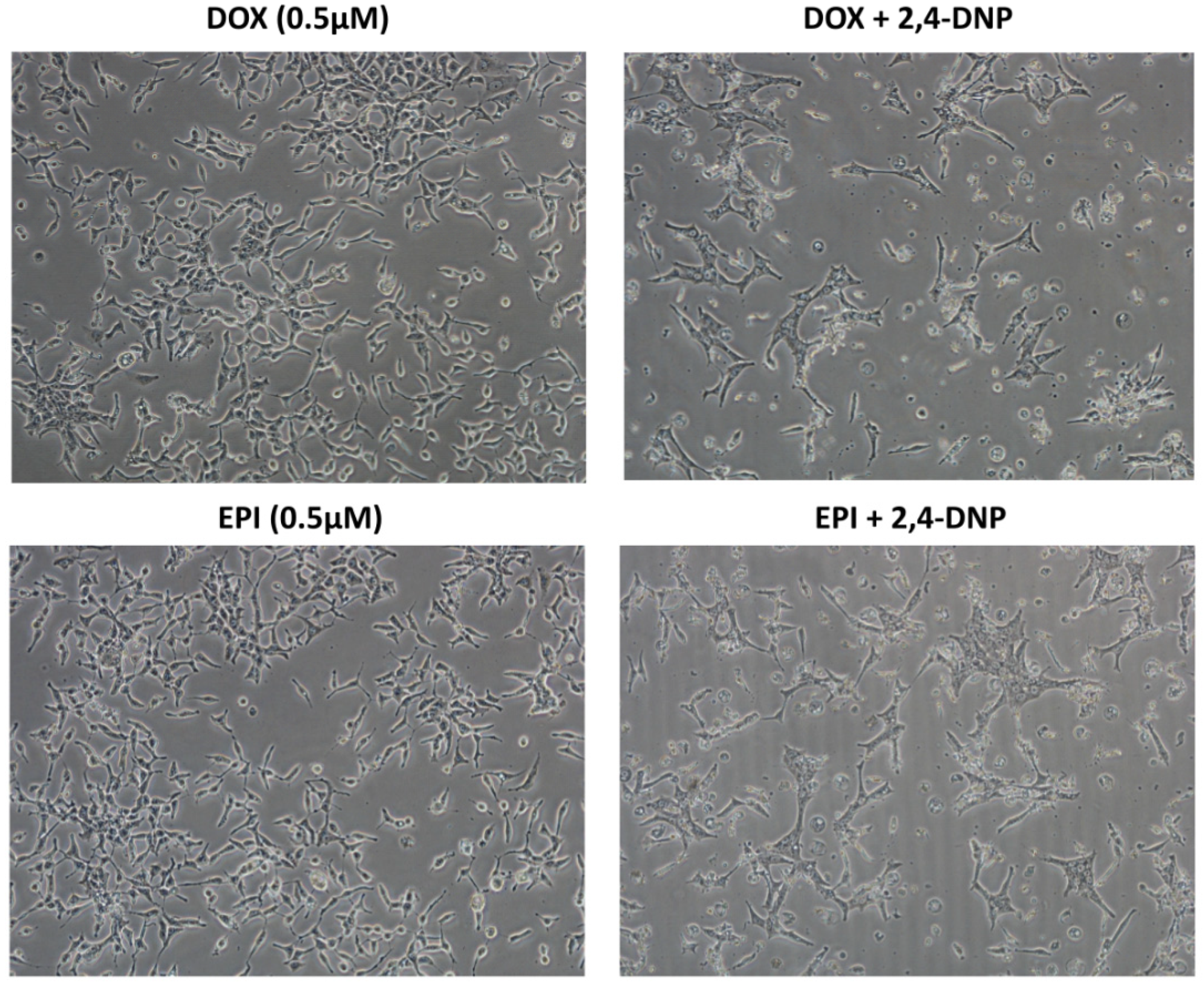
2.1. Cytotoxic Analysis
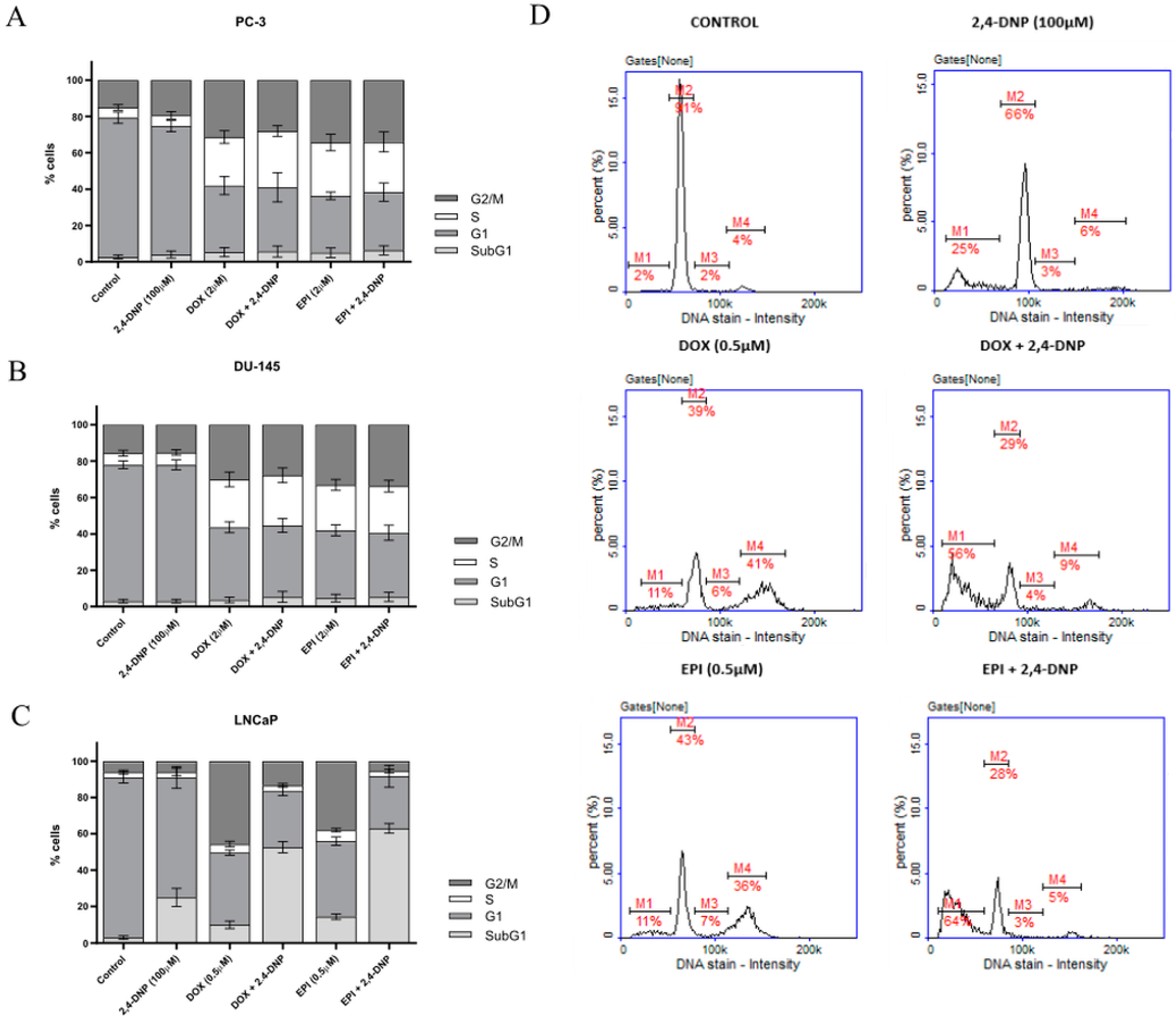
2.2. Cell Cycle Analysis
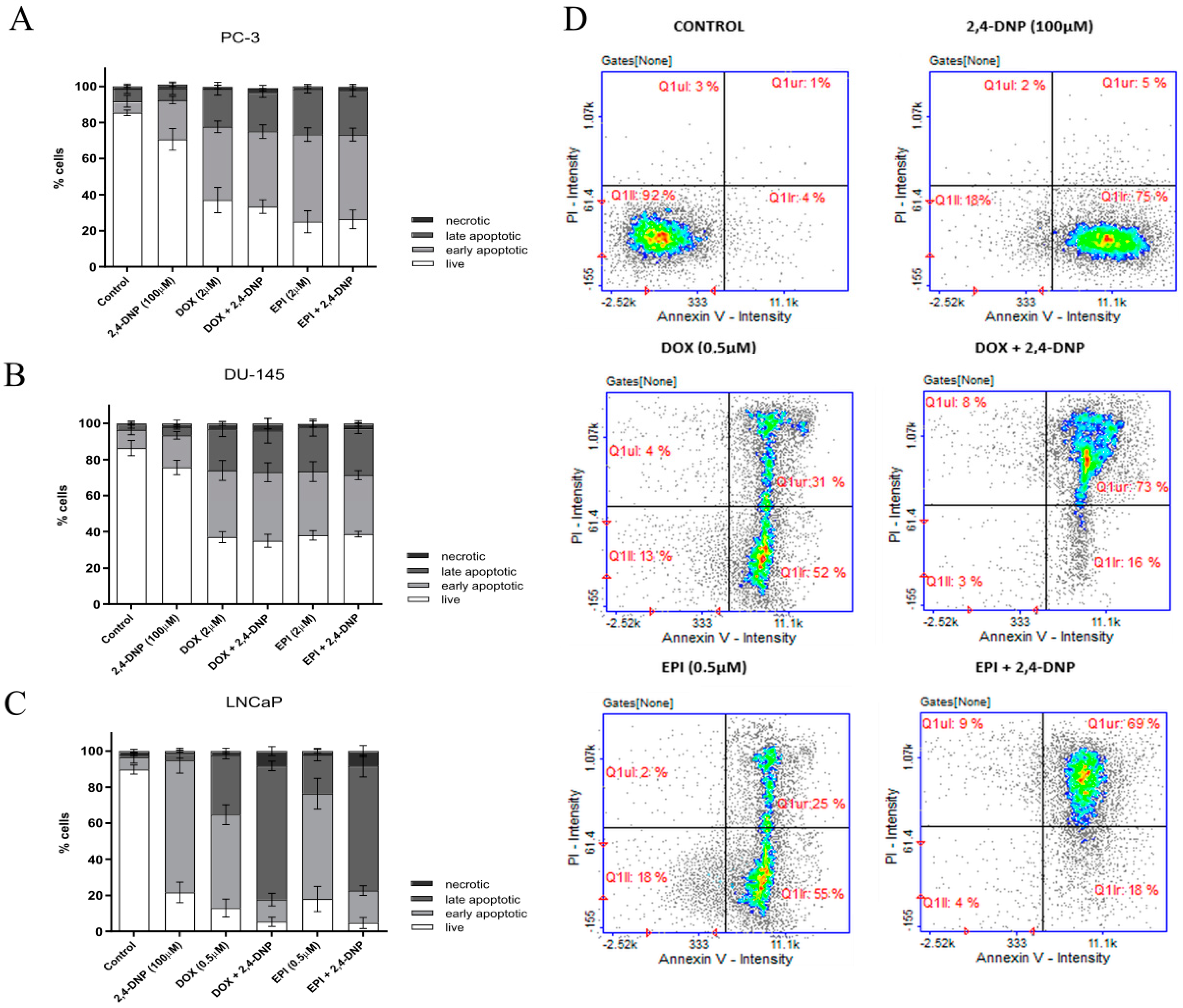
2.3. Detection of Apoptosis
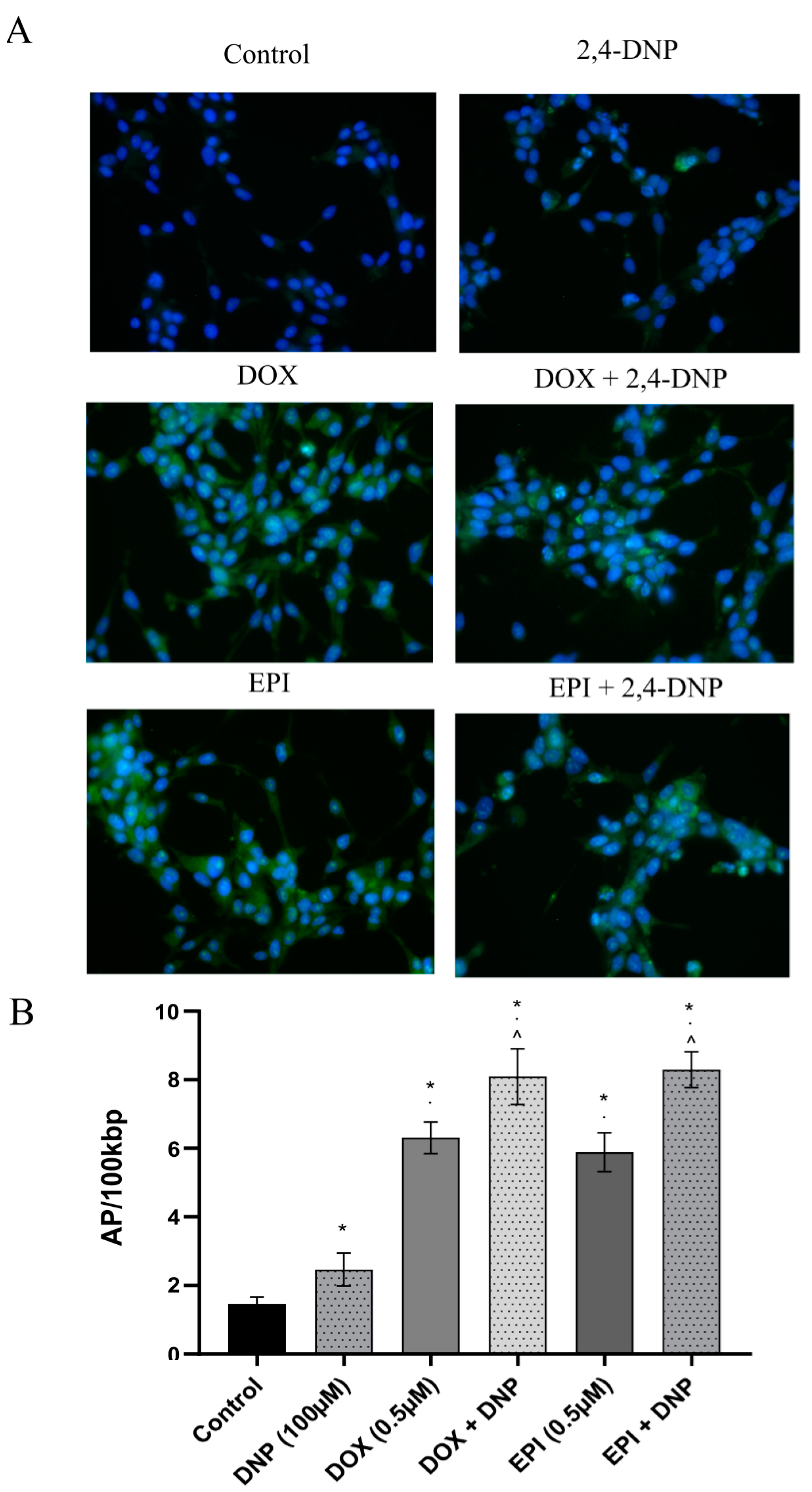
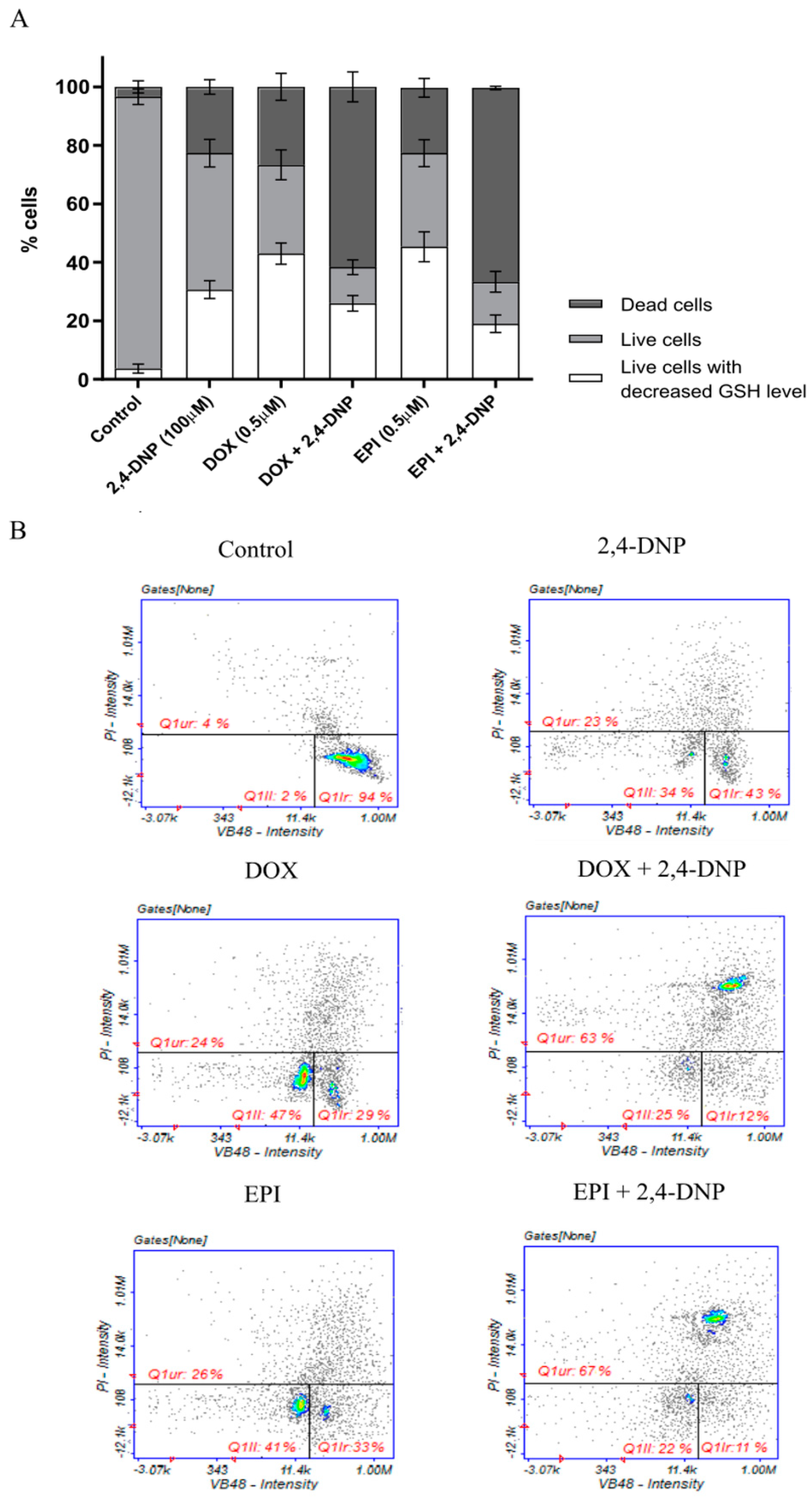
2.4. Detection of Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. MTT Assay
4.3. Assessment of Cells Morphology
4.4. Cell Cycle Analysis
4.5. Detection of Apoptosis
4.6. Determination of DNA Oxidative Damage
4.7. Oxidative Stress Detection
4.8. The Level of Cellular Thiols as a Determinant of Oxidative Stress
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer (IARC) TIA for R on. Global Cancer Observatory. Available online: http://gco.iarc.fr/today/home (accessed on 2 January 2022).
- Kalyanaraman, B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017, 12, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.L. Multiclonal tumor origin: Evidence and implications. Mutat. Res. Rev. Mutat. Res. 2018, 777, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Serpa, J. Metabolic Remodeling as a Way of Adapting to Tumor Microenvironment (TME), a Job of Several Holders. Adv. Exp. Med. Biol. 2020, 1219, 1–34. [Google Scholar] [CrossRef]
- Geisler, J.G. 2,4 Dinitrophenol as Medicine. Cells 2019, 8, 280. [Google Scholar] [CrossRef]
- Rui, L. New Antidiabetes Agent Targeting Both Mitochondrial Uncoupling and Pyruvate Catabolism: Two Birds with One Stone. Diabetes 2019, 68, 2195–2196. [Google Scholar] [CrossRef]
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J. Med. Toxicol. 2011, 7, 205–212. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.W.; Kim, S.H.; Kim, S.Z.; Park, W.H. 2,4-Dinitrophenol induces G1 phase arrest and apoptosis in human pulmonary adenocarcinoma Calu-6 cells. Toxicol. Vitr. 2008, 22, 659–670. [Google Scholar] [CrossRef]
- Venkatesh, P.; Kasi, A. Anthracyclines. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Szuławska, A.; Czyz, M. Molecular mechanisms of anthracyclines action. Postepy Hig. Med. Dosw. 2006, 60, 78–100. [Google Scholar]
- Chaiswing, L.; St Clair, W.H.; St Clair, D.K. Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer. Antioxid. Redox Signal. 2018, 29, 1237–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Guan, Y.-D.; Chen, X.-S.; Yang, J.-M.; Cheng, Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Menendez, P.; Hevia, D.; Mayo, J.C.; Sainz, R.M. The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas? Int. J. Cancer 2018, 142, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 2014, 3, e02242. [Google Scholar] [CrossRef]
- Sena, P.; Mancini, S.; Benincasa, M.; Mariani, F.; Palumbo, C.; Roncucci, L. Metformin Induces Apoptosis and Alters Cellular Responses to Oxidative Stress in Ht29 Colon Cancer Cells: Preliminary Findings. Int. J. Mol. Sci. 2018, 19, 1478. [Google Scholar] [CrossRef]
- Alimova, I.N.; Liu, B.; Fan, Z.; Edgerton, S.M.; Dillon, T.; Lind, S.E.; Thor, A.D. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009, 8, 909–915. [Google Scholar] [CrossRef]
- Higgins, L.; Withers, H.; Garbens, A.; Love, H.; Magnoni, L.; Hayward, S.; Moyes, C. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Biophys. Acta 2009, 1787, 1433–1443. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Jankovich, A.D. Glutathione Levels and Susceptibility to Chemically Induced Injury in Two Human Prostate Cancer Cell Lines. Molecules 2015, 20, 10399–10414. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Bourdeau-Heller, J.M.; Zhong, W.; Oberley, T.D. Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radic. Biol. Med. 2007, 43, 202–215. [Google Scholar] [CrossRef]
- Childs, S.; Haroune, N.; Williams, L.; Gronow, M. Determination of cellular glutathione:glutathione disulfide ratio in prostate cancer cells by high performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2016, 1437, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Johnson, E.; Byrne, F.L. Exploring the therapeutic potential of mitochondrial uncouplers in cancer. Mol. Metab. 2021, 51, 101222. [Google Scholar] [CrossRef] [PubMed]
- De Campos, E.G.; Fogarty, M.; De Martinis, B.S.; Logan, B.K. Analysis of 2,4-Dinitrophenol in Postmortem Blood and Urine by Gas Chromatography–Mass Spectrometry: Method Development and Validation and Report of Three Fatalities in the United States. J. Forensic Sci. 2020, 65, 183–188. [Google Scholar] [CrossRef]
- IC50 Calculator, AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 25 May 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczuk, G.; Humeniuk, E.; Adamczuk, K.; Grabarska, A.; Dudka, J. 2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells. Molecules 2022, 27, 7227. https://doi.org/10.3390/molecules27217227
Adamczuk G, Humeniuk E, Adamczuk K, Grabarska A, Dudka J. 2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells. Molecules. 2022; 27(21):7227. https://doi.org/10.3390/molecules27217227
Chicago/Turabian StyleAdamczuk, Grzegorz, Ewelina Humeniuk, Kamila Adamczuk, Aneta Grabarska, and Jarosław Dudka. 2022. "2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells" Molecules 27, no. 21: 7227. https://doi.org/10.3390/molecules27217227
APA StyleAdamczuk, G., Humeniuk, E., Adamczuk, K., Grabarska, A., & Dudka, J. (2022). 2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells. Molecules, 27(21), 7227. https://doi.org/10.3390/molecules27217227





