Abstract
Four dioxidovanadium(V) complexes with Schiff-base ligands based on 2-hydroxybenzhydrazide with four different substituted salicylaldehydes (5-chlorosalicylaldehyde, 3,5-dichlorosalicylaldehyde, 5-nitrosalicylaldehyde, 3-bromo-5-chlorosalicylaldehyde) were synthesized and described, by using V2O5 and triethylamine. The single crystal X-ray structure measurements as well as elemental analyses and IR spectra confirmed the formulas of the ionic complexes with a protonated triethylamine acting as counterion, HTEA[VO2(L)] (HL = Schiff-base ligand). The kinetic stability of the complexes at pH = 2 and 7 was discussed with respect to the neutral vanadium(V) complexes previously studied as potential insulin-mimetic agents. A correlation between the substituents in an aromatic ring of the Schiff-base ligands with crystal packing, and also with the stability of the compounds, was presented.
1. Introduction
Vanadium compounds are widely investigated because of their biological role including insulin-mimetic, anticancer, anti-inflammatory, or antibacterial activity [1,2,3,4,5,6,7]. Similarly, Schiff-base ligands have potential application in pharmacotherapy in view of their antidiabetic, antimicrobial, and anticancer activity [8,9,10]. The role of vanadium in insulin-mimetic compounds for type I and type II diabetes treatment was recognized in 1899 and intense studies were started in the 1990s. In this field the bis(maltolato)oxidovanadium(IV) (BMOV), and its bis(ethylmaltolato)oxido-vanadium(IV) (BEOV) analogue have been the most intensively studied compounds, which were used in clinical tests [11,12,13]. Our previous studies focused on the biological activity of the vanadium Schiff-base complexes, where Schiff bases were composed of aldehydes and relatives of amines—hydrazides, to form hydrazone type ligands. These complexes can be potentially used as insulin-mimetic compounds. The vanadium complexes with Schiff bases obtained up until now have manifested problems—most often for their cytotoxicity, very low solubility in water, instability at pH = 2 and in transport to cells, and, last but not least, difficulties in determining the crystal structure. Therefore, it is worth searching for new organic vanadium compounds in order to optimize their pharmaceutical activity. So far, we have tested plenty of vanadium(III, IV and V) complexes with Schiff bases, controlling both the starting compound for the synthesis of complexes, such as [VO(acac)2], VOSO4, [V(acac)3], and V2O5, as well as changing the substituents in the aromatic ring of aldehyde and hydrazide—Schiff-base components [14,15,16,17,18,19,20]. The obtained compounds were highly soluble in organic solvents, but insoluble in water. Thus, we used a DMSO–H2O solvent mixture to test the stability of the complexes. In our last publication, we described the synthesis of a vanadium(V) complex with triethylamine (TEA) as countercation—HTEA[VO2(L)] (where L = Schiff base formed by the reaction of 5-bromosalicylaldehyde with 2-hydroxybenzhydrazide) [21]. In the formed ionic complexes, we were able to obtain single crystals and determine the molecular structure where a hydrogen interaction appears between the cation and the complex anion. The ionic structure of such compounds should increase the solubility of the complexes in water. Moreover, the ionic vanadium(V) complexes with Schiff-base ligands show antibacterial and anticancer activities [22,23]. In this work, we present a series of vanadium(V) complexes with hydrazone Schiff-base ligand-type, and protonated triethylamine as cation, along with the structural and physicochemical characterization. In particular, the influence of substituents in the Schiff-base components on the structure and stability of complexes at pH = 2 and 7 was investigated.
2. Experimental
2.1. Materials and Methods
V2O5, triethylamine (TEA), 2-hydroxybenzhydrazide, 5-chlorosalicylaldehyde, 3,5-dichlorosalicylaldehyde, 5-nitrosalicylaldehyde, 3-bromo-5-chlorosalicylaldehyde were of analytical grade (Aldrich) and were used as received. Ethanol (98%) of pharmaceutical grade was from Polmos (Poland), all other solvents were of analytical grade and were used as supplied. Microanalysis of carbon, hydrogen and nitrogen was performed using Elementar Vario MICRO Cube elemental analyzer. Electronic absorption spectra were recorded with a Shimadzu UV-3600 UV-vis-NIR spectrophotometer equipped with a CPS-240 temperature controller. IR spectra were recorded on a Nicolet iS5 FT-IR spectrophotometer.
2.2. Syntheses of HTEA[VO2(Ln)] (1–4)
All the compounds were obtained as result of the following synthetic procedure: 1 mmol of 2-hydroxybenzhydrazide and 1 mmol of the respective salicylaldehyde, namely 5-chloro-salicylaldehyde, 3,5-dichloro-salicylaldehyde, 5-nitro-salicylaldehyde, 3-bromo-5-chloro-salicylaldehyde (as indicated in Table 1), were refluxed in 50 mL of methanol for 15 min. To the resulting yellow solution, 0.5 mmol of V2O5 was added and the mixture was refluxed for additional 5 min. The color of the solution changed to dark red. Then, 2 mL of triethylamine (TEA) was added and the mixture was refluxed for additional 5 min and left for crystallization. After several days, crystals suitable for X-ray diffraction measurement were taken directly from the solution. The residual of the precipitate formed was filtered off, washed with methanol, and dried in air. The yield of individual syntheses was almost quantitative (close to 85–95%). The formulas and elemental analysis results of the diamagnetic complexes are given in Table 1.

Table 1.
The formulas of compounds 1–4 with elemental analysis results and components of Schiff-base ligands used in synthesis.
2.3. Crystallographic Data Collection and Structure Refinement
Diffraction intensity data for compounds 1–4 were collected on a Rigaku XtaLAB Synergy-S diffractometer with mirror-monochromated Cu-Kα radiation (λ = 1.54184 Å). Cell refinement and data reduction were performed using CrysAlisPro firmware [24]. The positions of all non-hydrogen atoms were determined by direct methods using SHELXL-2019/2 [25,26]. All non-hydrogen atoms were refined anisotropically using weighted full-matrix least-squares on F2, and refinements were carried out using SHELXL-2019/2 [25,26]. All hydrogen atoms were positioned at idealized positions, except those of the OH group and NH of protonated trimethylamine, which were freely refined.
The crystal data and structure refinement parameters for complexes 1–4 are collected in Table 2.

Table 2.
Crystal data and details of structure refinement for complexes 1–4.
CCDC 2161295, 2161337, 2161365 and 2161385 contain the supplementary crystallographic data for 1–4, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif accessed on 26 September 2022.
The XRD powder diffractograms of 2–4 (crystallinity tests) were recorded at 294 K on a Philips X’Pert-Pro diffractometer equipped with a Ni-filtered Cu-Kα radiation (λ = 1.54059 Å) over 2θ range from 5 to 50° with a counting time of 1 s and a step size of 0.04°. The X-ray source was operated at 30 mA and 40 kV.
3. Results and Discussion
3.1. Crystal Structure Description
The X-ray structural analyses show that complexes 1–4 have a close comparable structure and, in particular, complexes 1 and 3 present isomorphous structures and similarly 2 and 4, as can be evidenced from the unit cell parameters (Table 2). An ORTEP view of the crystallographic independent unit for all complexes is shown in Figure 1, and a selection of bond lengths and angles is reported in Table 3. The XRD powder diffractograms of 2–4 are presented in Figures S1–S3 and show that the polycrystalline sample is similar to the monocrystalline one.
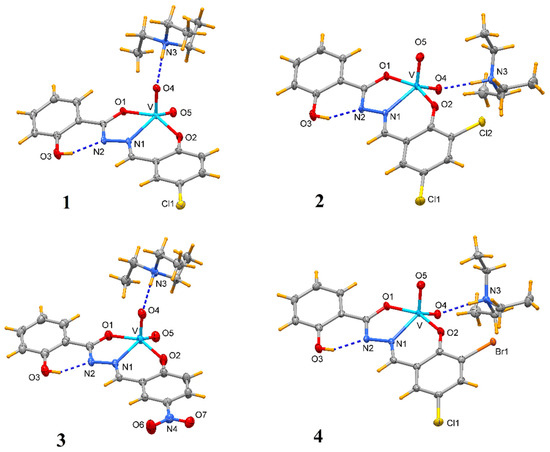
Figure 1.
Molecular structure of complexes 1–4 (ellipsoid probability at 50%).

Table 3.
Selected bond lengths (Å) and angles (°) for complexes 1–4.
In each anionic [VO2(L)]− complex, the vanadium atom is pentacoordinated by two oxido groups and by the anionic tridentate chelating ONO Schiff-base ligand through the phenoxo oxygen O1, the imino nitrogen N1 and the carboxylate oxygen O2. The V-O1 bond distances that fall in the narrow range 1.9874(10)–1.9899(15) Å are systematically shorter by ca. 0.07 Å with respect to V-O2 ones (range 1.9052(10)–1.9264(15) Å). The V-N1 bond lengths vary from 2.1391(17) to 2.1740(13) Å. The V=O4 bond distances (of oxo atom involved in hydrogen bond with HTEA, see below) have a mean value of 1.645 Å, systematically slightly longer than the V = O5 bonds that average to 1.618 Å. However, the different substituents on the phenol ring do not seem to affect the electronic properties of the donor atoms and thus the coordination sphere of vanadium.
The vanadium in the isomorphous structures of 1 and 3 exhibits a slightly distorted square–pyramidal coordination sphere, as ascertained by the τ parameter [27] of 0.151 and 0.122, respectively. On the other hand, the metal in 2 and 4 presents a coordination environment in between a square–pyramidal and trigonal bipyramidal (τ = 0.495 and 0.470, respectively, being τ = 0 for an ideal square pyramid and 1 for an ideal trigonal bipyramid). These findings are confirmed also by the program SHAPE 2.1 [28], which indicates a square pyramid geometry for 1 and 3, while there is a trigonal bipyramid geometry for 2 and 4 (Table S2).
These bonding parameters are in agreement with those detected in other dioxovanadium complexes [23,29,30]. In all [VO2(L)]− anions, a strong intramolecular H-bond is realized between the O3-H and nitrogen N2 with O…N distance of ca. 2.58 Å (Table 4).

Table 4.
Selected hydrogen bonds for complexes 1–4 [Å/°].
In all compounds, the protonated triethylamine is hydrogen bound to oxygen O4 of the vanadium complex, with N3…O4 distance in between 2.686(2)–2.787(2) Å (Figure 1, Table 4).
The crystal packing of compound 1 is shown in Figure 2, where complexes are interacting by π-stacking interactions between phenol rings (centroid-to-centroid distance of 4.185(1) Å. A similar crystal packing is observed for 3, with slightly longer π-stacking interactions 4.411(1) Å. The crystal packing of compounds 2 and 4 exhibits pairs of complexes centrosymmetrically related to form reciprocal π-stacking interactions with centroid distances of 3.606(1) and 3.656(1) Å, respectively (Figure 3). Finally, numerous unconventional hydrogen bonds of type C-H…O, C-H…Cl and C-H…Br, as indicated in Table 4 and Table S1 (Supplementary Materials), as well as C-H…π interactions (Table 5) reinforce the crystal packing of all the complexes.
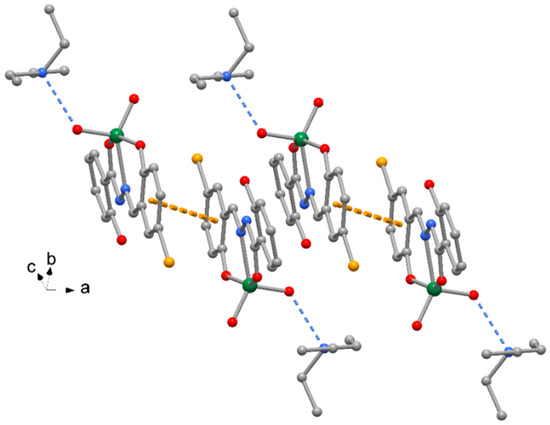
Figure 2.
Crystal packing of complex 1 with indication of π-stacking between complex pairs with indication of the orientation with respect to cell axes (orange dotted lines = π-stacking interactions, blue dotted lines = H-bonds). The same crystal packing is exhibited by complex 3.
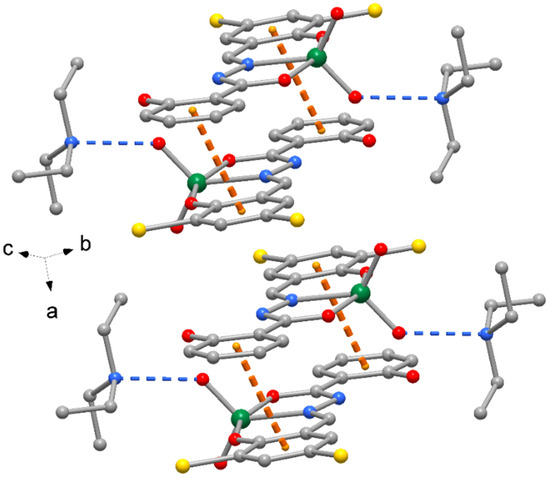
Figure 3.
Crystal packing of complex 2 with indication of π-stacked centrosymmetric complex pairs with indication of the orientation with respect to cell axes (orange dotted lines = π-stacking interactions, blue dotted lines = H-bonds). The same crystal packing is exhibited by complex 4.

Table 5.
The X-H∙∙∙Cg(π) interaction in 1 and 3 [Å/°].
3.2. Spectroscopic Characteristics
The IR spectra of 1–4 were measured in the 400–4000 cm−1 range and presented in Figure 4. As the complexes differ only in the substituent in the fragment derived from the aldehyde, the spectra are very similar. The position of ν(V=O) stretching frequency is 939, 942, 936, 944 cm−1 (for 1–4, respectively). For 1 and 3 as well as for 2 and 4 the position is very similar, as these pairs of compounds show an isomorphous structure. These bands were also observed in other dioxido vanadium compounds [31,32,33]. The bands related to the stretching frequencies of -C=N are observed in the range of 1610–1630 cm−1. The stretching vibration of the -OH group in the Schiff-base ligand is observed at ca. 2980 cm−1, and that of the -NH vibration in the cation at 2450–2600 cm−1.
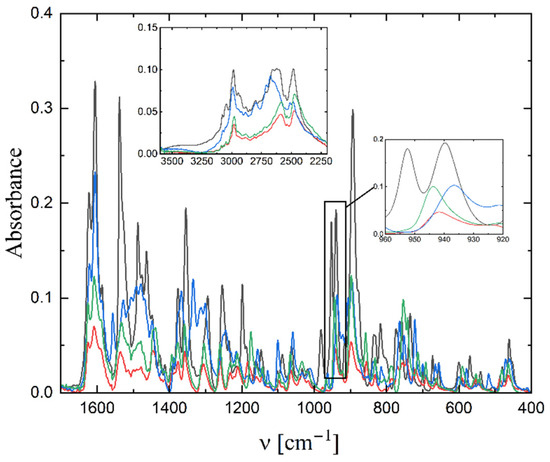
Figure 4.
IR spectra of complex 1 (black line), 2 (red), 3 (blue) and 4 (green).
The UV-Vis spectra of all the complexes were recorded in EtOH, MeOH, MeCN, DMSO and water (see Figure 5).
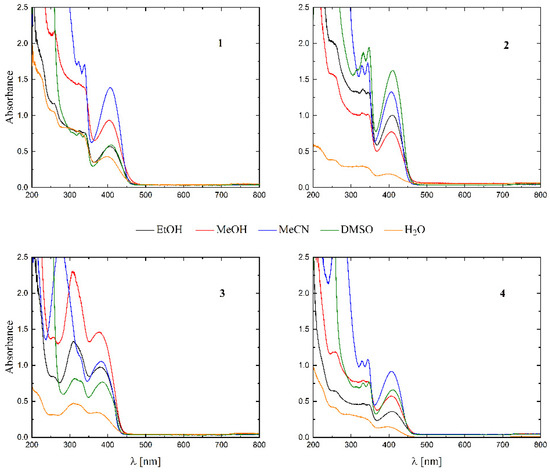
Figure 5.
UV-Vis spectra of complexes 1–4 in various solvents (black—EtOH, red—MeOH, blue—MeCN, green—DMSO and orange—H2O).
The solubility of 1–4 in different organic solvents was very good, while in water the complexes exhibited partial solubility that increased with increasing temperature. The complexes 1, 2 and 4 show intense bands in the range of 400–410 nm, which can be assigned to ligand-to-metal charge transfer (LMCT). Compound 3 shows this band at higher energy (ca. 380–390 nm) and presents also a well-formed band at ca. 308 nm, being the only compound that does not have a halogen atom as a substituent. The stability of the complexes was proved in DMSO (Figure 6), in water (pH ca. 7) as well as in 0.01 M HCl (pH ca. 2, imitating the environment in the stomach). All measurements were performed at 37 °C. As the compounds are stable in DMSO, this is an important feature, since this solvent can be used as a solvent to transport molecules across cell membranes like the skin. Since these compounds were soluble in water, we had the opportunity to determine for the first time their stability in water. By using a DMSO/H2O mixture, the complexes were shown to be stable at pH = 7, but were unstable at pH = 2 [16,17,18,19,20]. In Figure 7 and Figure 8 a similar behavior can be observed: at pH = 7 the band at 400 nm does not change with time (Figure 7). Acidification of the solution causes the formation of a suspension (raised background in the entire range as shown in Figure 8). The disappearance of the bands at ca. 400 nm, combined with an increase in the intensity of the bands at ca. 300 nm, suggests the decomposition of Schiff bases into components.
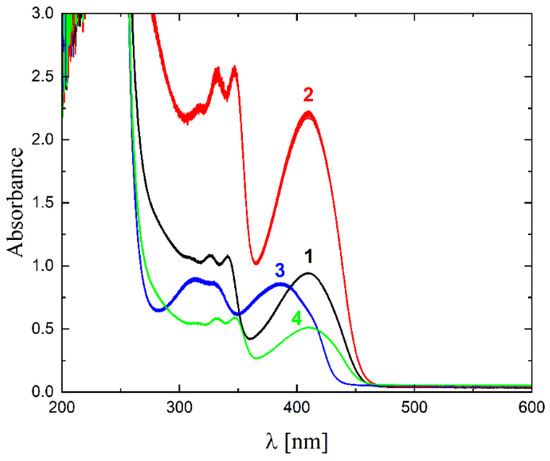
Figure 6.
UV-Vis spectra of complexes 1–4 in DMSO (black, red, blue and green lines, respectively), T = 37 °C, d = 1 cm, 15 spectra measured in 780 s intervals.
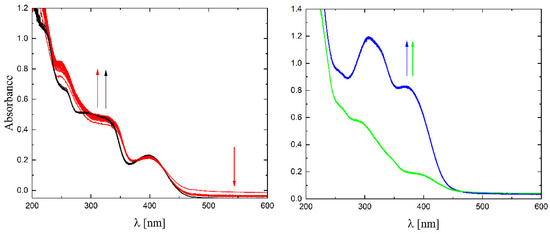
Figure 7.
UV-Vis spectra of complexes 1–4 in water (pH ≈ 7) (black, red, blue and green lines, respectively), T = 37 °C, d = 1 cm, 15 spectra measured in 360 s intervals. The arrows show direction of changes.
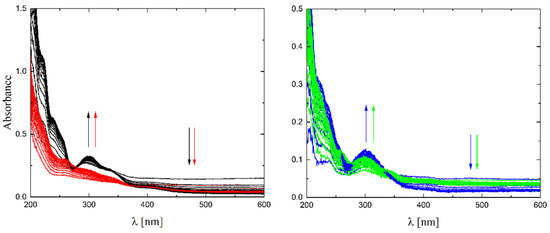
Figure 8.
UV-Vis spectra of complexes 1–4 (black, red, blue and green lines, respectively) in 0.01M HCl (pH ≈ 2) T = 37 °C, d = 1 cm, 15 spectra measured in 360 s intervals. The arrows show direction of changes.
4. Conclusions
Four complexes of vanadium(V) with Schiff-base ligands were isolated and structurally characterized. The addition of triethylamine caused deprotonation of the Schiff-base ligand and isolation of ionic complexes with Et3NH+ as cation. The structural data indicate that complexes 1 and 3 have an isomorphous structure, and similarly 2 and 4. These complexes differ only in the number and type of substituents on the ring constituting the part of the aldehyde component of the Schiff-base ligand. The introduction of an additional substituent on the salicylaldehyde ring induces a decrease in the symmetry of the unit cell for complexes 2 and 4, which crystallize in the triclinic system, in contrast to compounds 1 and 3 crystallizing in the monoclinic system. The present ionic complexes display a better solubility in water with respect to similar neutral complexes of vanadium(V) previously reported, and this represents an important aspect from the point of view of their potential biological applications. Complex 2 showed the best solubility, due to the additional chlorine substituent. The stability of complexes in DMSO and water is very good; however, they are unstable at pH = 2. Research on improving compound stability in the acidic environment by changing the cation is in progress.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/molecules27206942/s1. Table S1: Hydrogen bonds for complexes 1–4 [Å/°]. Table S2: Results from Continuous Shape Measures calculation SHAPE v2.1. Figures S1–S3: Powder diffractograms for 2–4.
Author Contributions
A.J.: Conceptualization, methodology, investigation, writing—original draft, writing—review & editing, visualization, supervision. J.S.: critical review, commentary or revision—including pre- or post-publication stages. M.H.: X-ray crystal structure measurements. W.S.: investigation. E.Z.: writing—original draft, visualization. G.M.: writing—original draft, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
The open-access publication of this article was funded under the program “Excellence Initia-tive-Research University” at the Jagiellonian University in Kraków.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Samples of the compounds are available from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Orvig, C.; Thompson, K.H.; Battell, M.; McNeill, J.H. Vanadium compounds as insulin mimics. Met. Ions Biol. Syst. 1995, 31, 575–594. [Google Scholar]
- Thompson, K.H.; McNeill, J.H.; Orvig, C. Vanadium compounds as insulin mimics. Chem. Rev. 1999, 99, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health benefits of vanadium and its potential as an anticancer agent. Met. Ions Life Sci. 2018, 18, 251–279. [Google Scholar]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic or anticancer drug: A clinical and historical perspective. Met. Ions Life Sci. 2019, 19, 203–230. [Google Scholar]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301, 87–105. [Google Scholar] [CrossRef]
- Singh, K.; Patel, P.; Goswami, A.K. Anti-inflammatory activity of Hydroxytriazenes and their vanadium complexes. E-J. Chem. 2008, 5, 1144–1148. [Google Scholar] [CrossRef]
- Rudbari, H.A.; Iravani, M.R.; Moazam, V.; Askari, B.; Khorshidifard, M.; Habibi, N.; Bruno, G. Synthesis, characterization, X-ray crystal structures and antibacterial activities of Schiff base ligands derived from allylamine and their vanadium (IV), cobalt (III), nickel (II), copper (II), zinc (II) and palladium (II) complexes. J. Mol. Struct. 2016, 1125, 113–120. [Google Scholar] [CrossRef]
- Torabi, S.; Mohammadi, M.; Shirvani, M. Antidiabetic, antioxidant, antibacterial, and antifungal activities of vanadyl Schiff base complexes. Trends Pharmacol. Sci. 2018, 4, 1–8. [Google Scholar]
- Tadele, K.T.; Tsega, T.W. Schiff Bases and their metal complexes as potential anticancer candidates: A review of recent works. Anti-Cancer Agents Med. Chem. 2019, 19, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Caravan, P.; Gelmini, L.; Glover, N.; Herring, F.G.; Li, H.; McNeill, J.H.; Rettig, S.J.; Setyawati, I.A.; Shuter, E.; Sun, Y.; et al. Reaction chemistry of BMOV, bis (maltolato) oxovanadium (IV), a potent insulin mimetic agent. J. Am. Chem. Soc. 1995, 117, 12759–12770. [Google Scholar]
- Peters, K.G.; Davis, M.G.; Howard, B.W.; Pokross, M.; Rastogi, V.; Diven, C.; Greis, K.D.; Eby-Wilkens, E.; Maier, M.; Evdokimov, A.; et al. Mechanism of insulin sensitization by BMOV (bis maltolato oxo vanadium); unliganded vanadium (VO4) as the active component. J. Inorg. Biochem. 2003, 96, 321–330. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Gryboś, R.; Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M. Properties, structure and stability of V(IV) hydrazide Schiff base ligand complex. J. Mol. Struct. 2018, 1171, 880–887. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Gryboś, R.; Matoga, D. Role of co-ligand and solvent on properties of V(IV) oxido complexes with ONO Schiff bases. J. Mol. Struct. 2019, 1180, 839–848. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Gryboś, R.; Kruczała, K.; Głuch-Lutwin, M.; Kazek, G. Vanadium complexes with salicylaldehyde based Schiff base ligands—Structure, properties and biological activity. J. Coord. Chem. 2020, 73, 986–1008. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Matoga, D.; Kruczała, K.; Kazek, G.; Mordyl, B.; Sapa, J.; Papież, M. Synthesis, coordination properties and biological activity of vanadium complexes with hydrazone Schiff base ligands. Polyhedron 2020, 185, 114589. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Głuch-Lutwin, M.; Sapa, J. Ligand role on insulin-mimetic properties of vanadium complexes. Structural and biological studies. Inorg. Chim. Acta 2021, 516, 120135. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Głuch-Lutwin, M.; Sapa, J.; Papież, M. Tridentate ONO ligands in vanadium(III-V) complexes—Synthesis, characterization and biological activity. J. Mol. Struct. 2021, 1224, 129205. [Google Scholar] [CrossRef]
- Jasińska, A.; Szklarzewicz, J.; Jurowska, A.; Hodorowicz, M.; Kazek, G.; Mordyl, B.; Głuch-Lutwin, M. V(III) and V(IV) Schiff base complexes as potential insulin-mimetic compounds—Comparison, characterization and biological activity. Polyhedron 2022, 215, 115682. [Google Scholar] [CrossRef]
- Jurowska, A.; Serafin, W.; Hodorowicz, M.; Kruczała, K.; Szklarzewicz, J. Vanadium precursors and the type of complexes formed with Schiff base ligand composed of 5-bromosalicylaldehyde and 2-hydroxybenzhydrazide—Structure and characterization. Polyhedron 2022, 222, 115903. [Google Scholar] [CrossRef]
- Khosravan, M.; Abdolahi, L.; Ebrahimipour, S.Y. A novel anionic di-oxido vanadium (V) Schiff base complex: Synthesis, spectral characterization, X ray crystal structure, catalytic activity for the preparation of tetrahydro-4H-chromene derivatives and antibacterial properties. Inorg. Chem. Commun. 2021, 128, 108561. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Kautz, A.C.; Ameri, M.; Pasban-Aliabadi, H.; Amiri Rudbari, H.; Bruno, G.; Janiak, C. Mono- and dioxido-vanadium(V) complexes of a tridentate ONO Schiff base ligand: Synthesis, spectral characterization, X-ray crystal structure, and anticancer activity. Polyhedron 2015, 93, 99–105. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlisPro; Version. 1.171.36.20; Rigaku Oxford Diffraction: Yarnton, Oxfordshire, UK, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELX2017, Programs for Crystal Structure Determination; Universität Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Alvarez, S.; Llunell, M. Continuous symmetry measures of penta-coordinate molecules: Berry and non-Berry distortions of the trigonal bipyramid. J. Chem. Soc. Dalton Trans. 2000, 3288–3303. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Asthana, M.; Syiemlieh, I.; Lywait, A.A.; Longchara, M.; Lal, R.A. New dioxido-vanadium(V) complexes containing hydrazone ligands: Syntheses, crystal structure and their catalytic application toward C-H bond functionalization. J. Organomet. Chem. 2018, 876, 10–16. [Google Scholar] [CrossRef]
- Kurbah, S.D. Dioxido-vanadium(V) complex catalyzed oxidation of alcohols and tandem synthesis of oximes: A simple catalytic protocol for C–N bond formation. J. Coord. Chem. 2021, 74, 905–918. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.R.; Mukherjee, G.; Drew, M.G.B.; Ghosh, S. Oxidoalkoxidovanadium(V) complexes: Synthesis, characterization and comparison of X-ray crystal structures. Polyhedron 2012, 34, 92–101. [Google Scholar] [CrossRef]
- Mohanty, M.; Maurya, S.K.; Banerjee, A.; Patra, S.A.; Maurya, M.R.; Crochet, A.; Brzezinski, K.; Dinda, R. In vitro cytotoxicity and catalytic evaluation of dioxidovanadium(V) complexes in an azohydrazone ligand environment. New J. Chem. 2019, 43, 17680–17695. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.; Carabineiro, S.A.; Guedes da Silva, M.F.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J. Oxidovanadium (V) Complexes Anchored on Carbon Materials as Catalysts for the Oxidation of 1-Phenylethanol. ChemCatChem 2016, 8, 2254–2266. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).