Removal of Amoxicillin from Aqueous Media by Fenton-like Sonolysis/H2O2 Process Using Zero-Valent Iron Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Zero-Valent Iron Nanoparticles
2.3. Characterization of Zero-Valent Iron Nanoparticles
3. Experimental Section
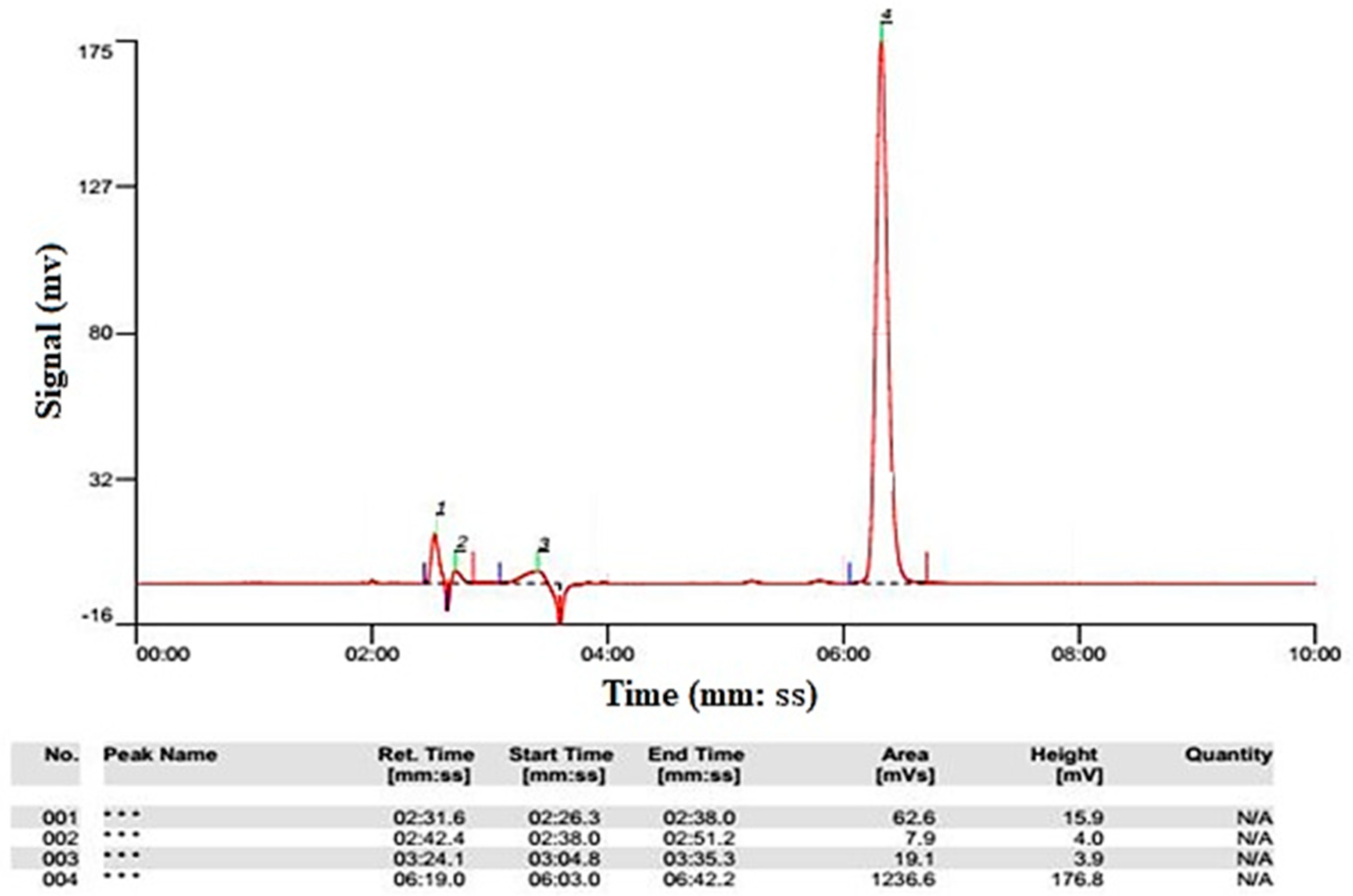
Analysis of Samples
4. Results
4.1. Properties of the Synthesized Nanoparticles
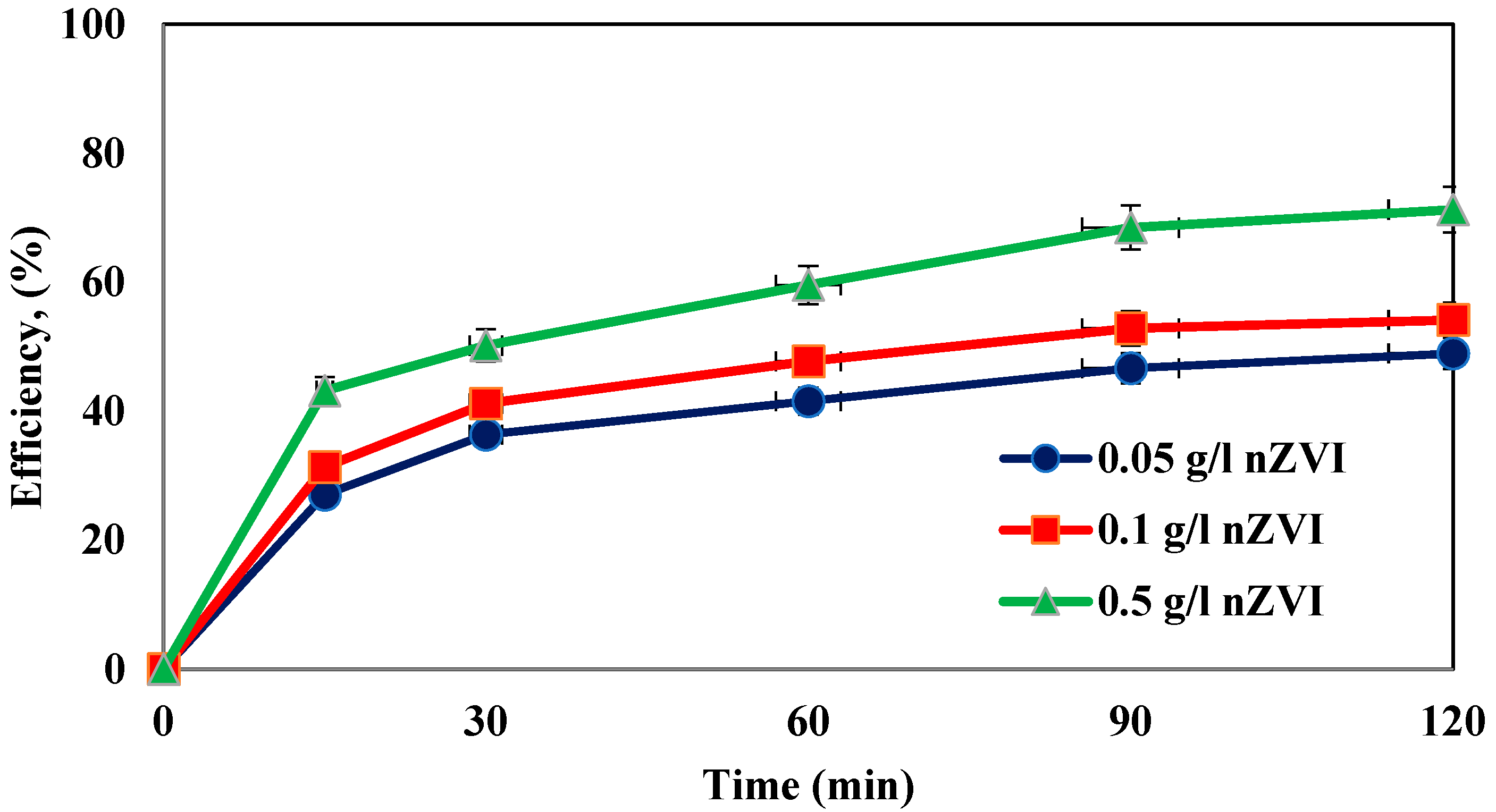
4.2. Investigation of the Effect of Different Dosages of Iron Nanoparticles
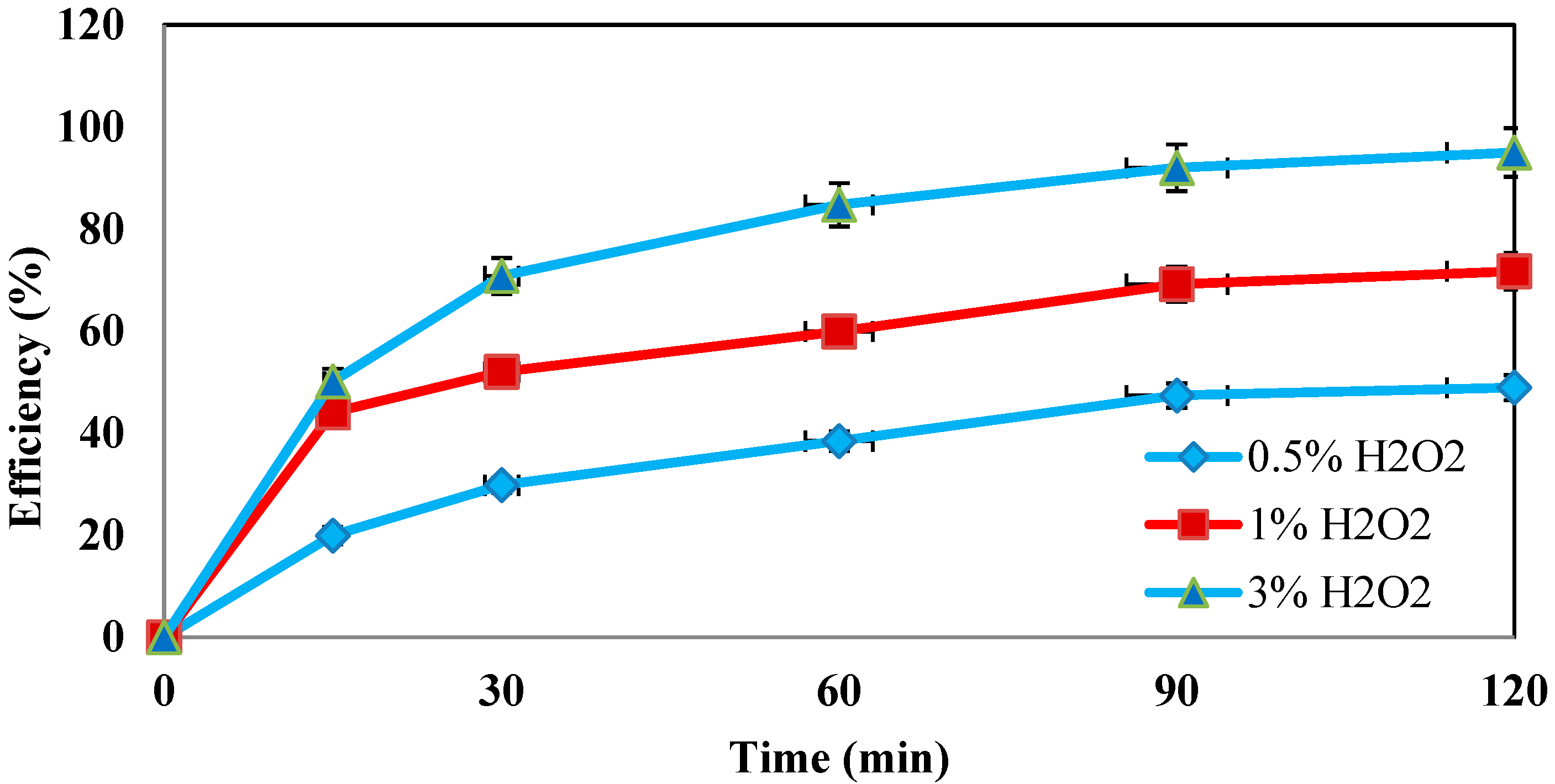
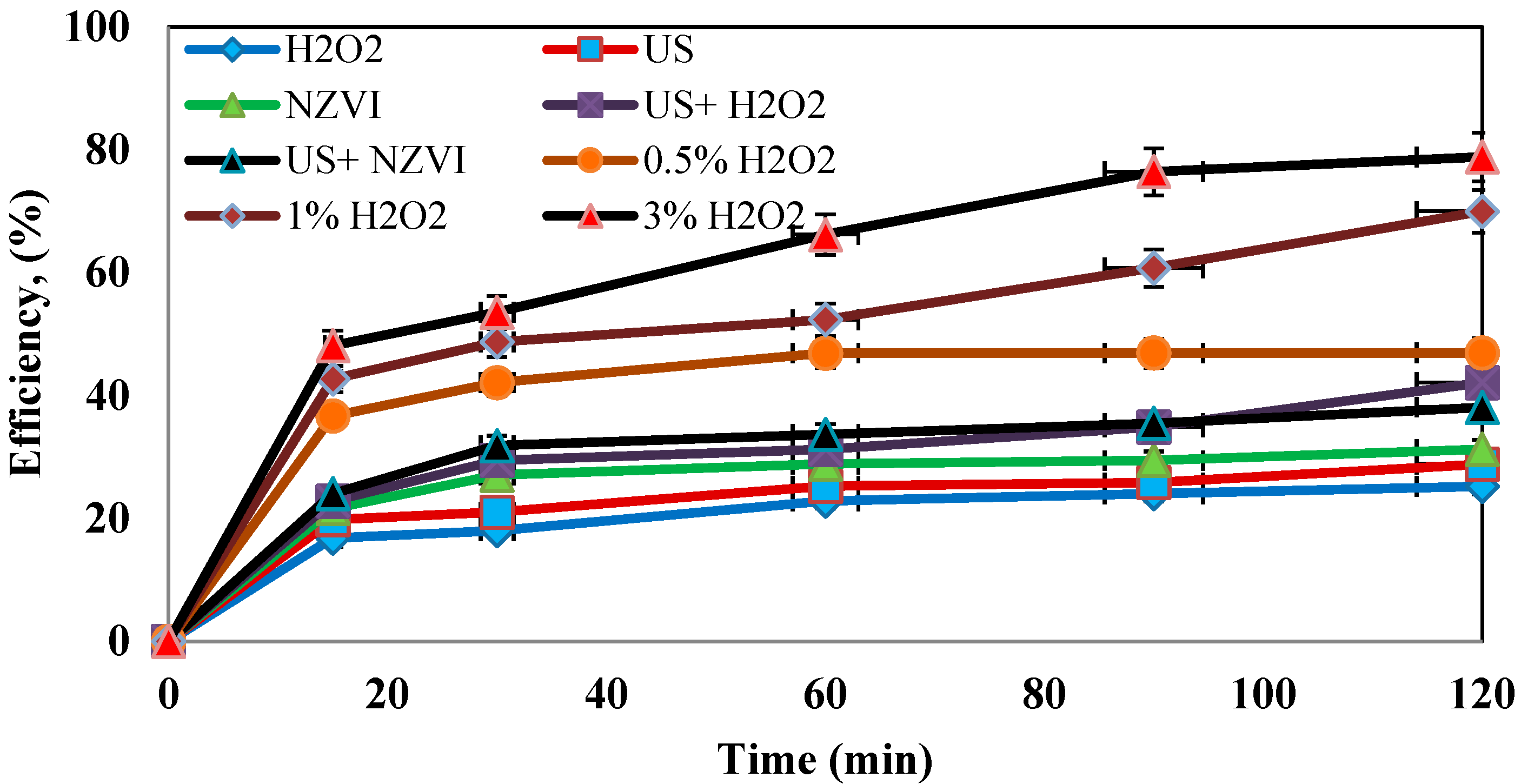
4.3. Determination of the Optimum Concentration of Hydrogen Peroxide
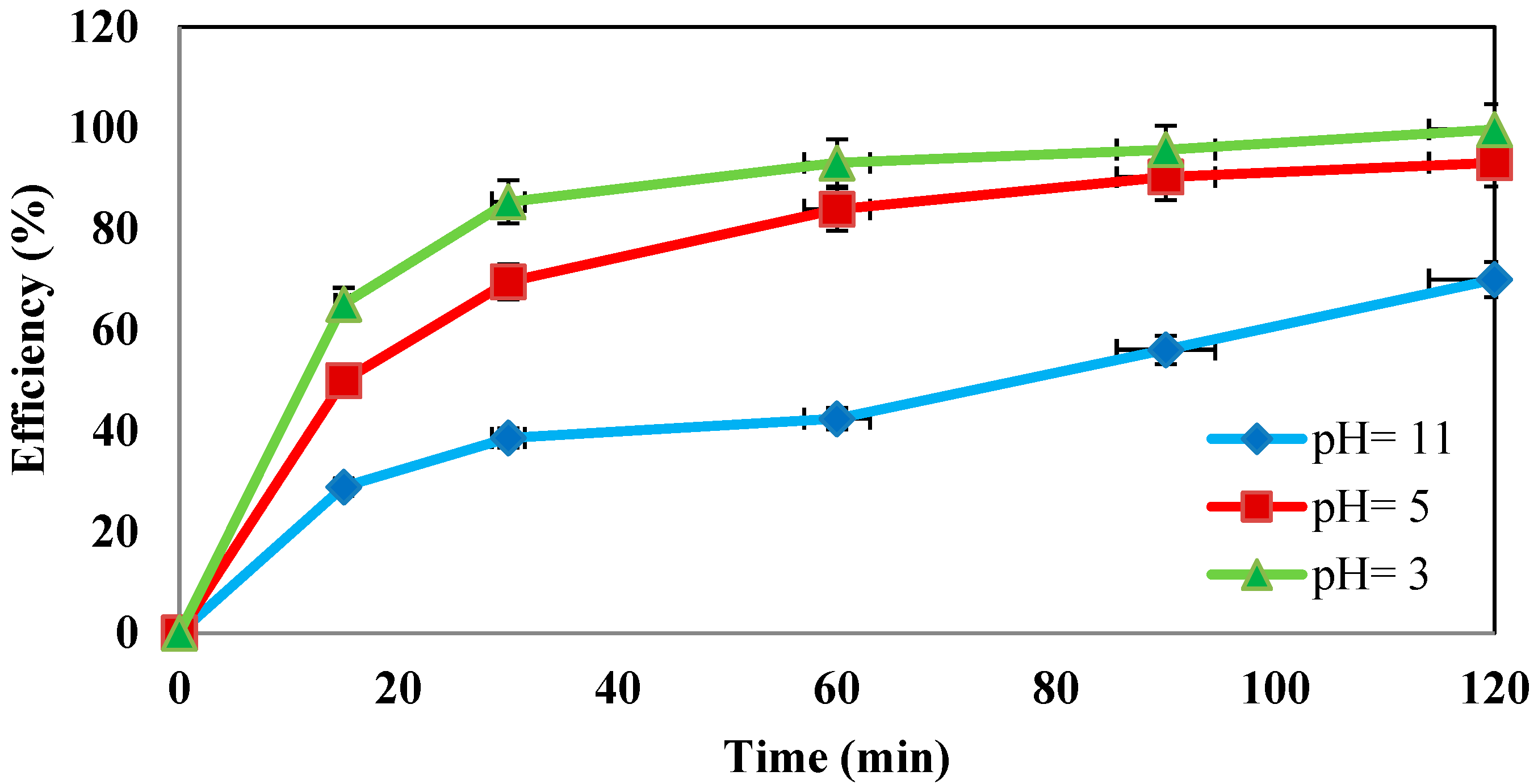
4.4. Determination of the Effect of pH Changes
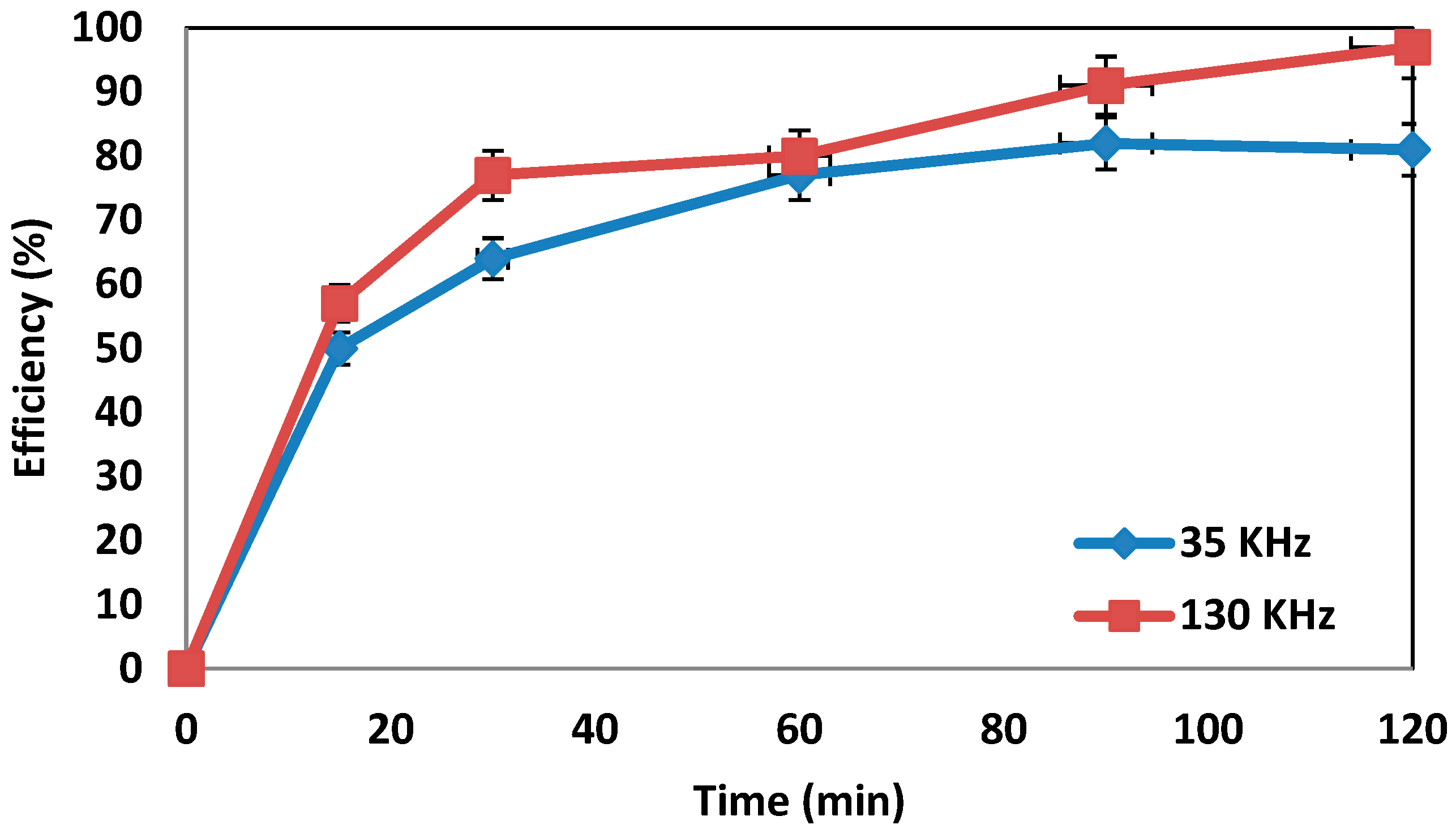
4.5. Determination of the Effect of Frequency Changes on the System’s Efficiency
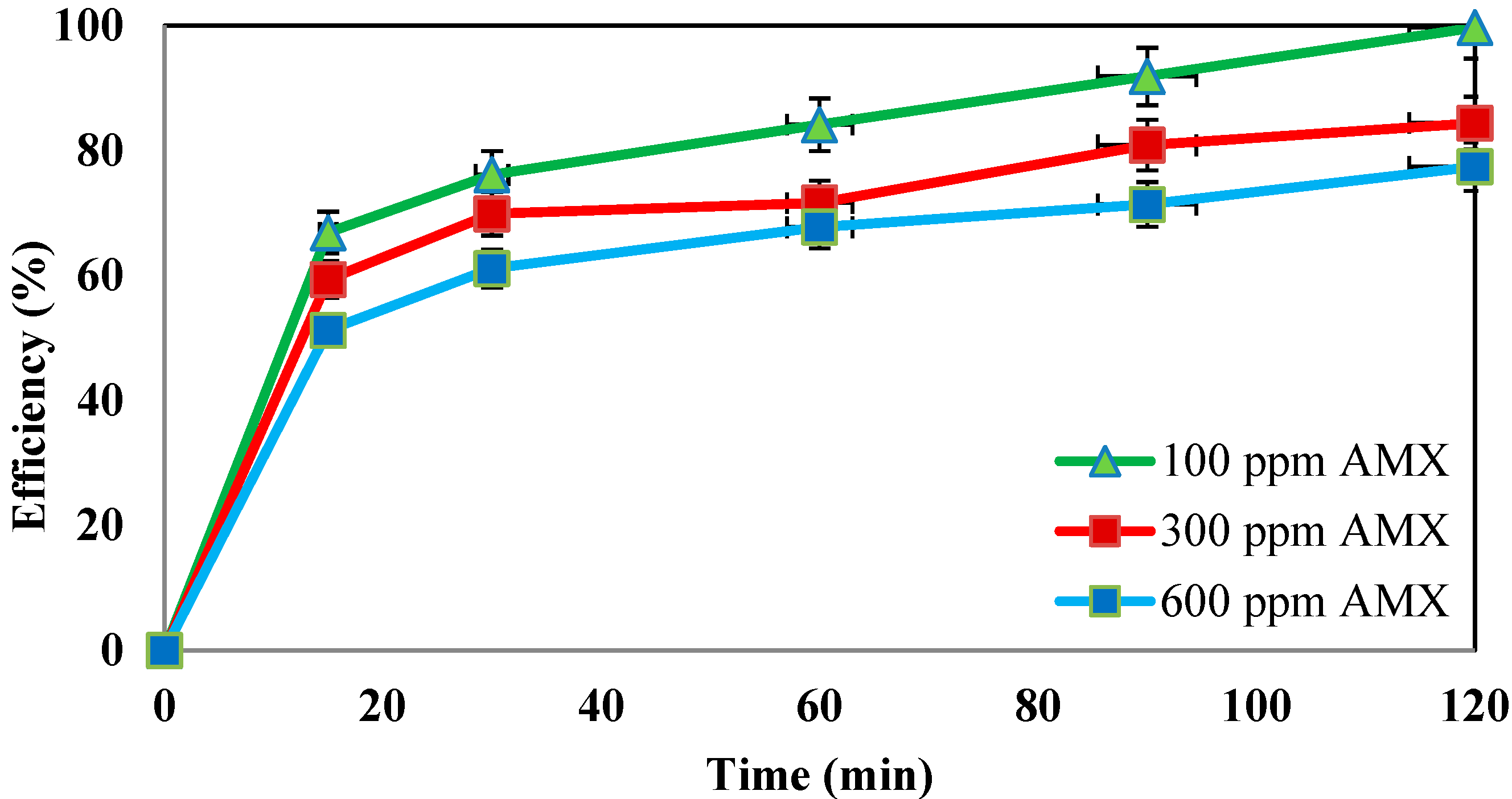
4.6. Effect of Different Concentrations of Amoxicillin Antibiotic
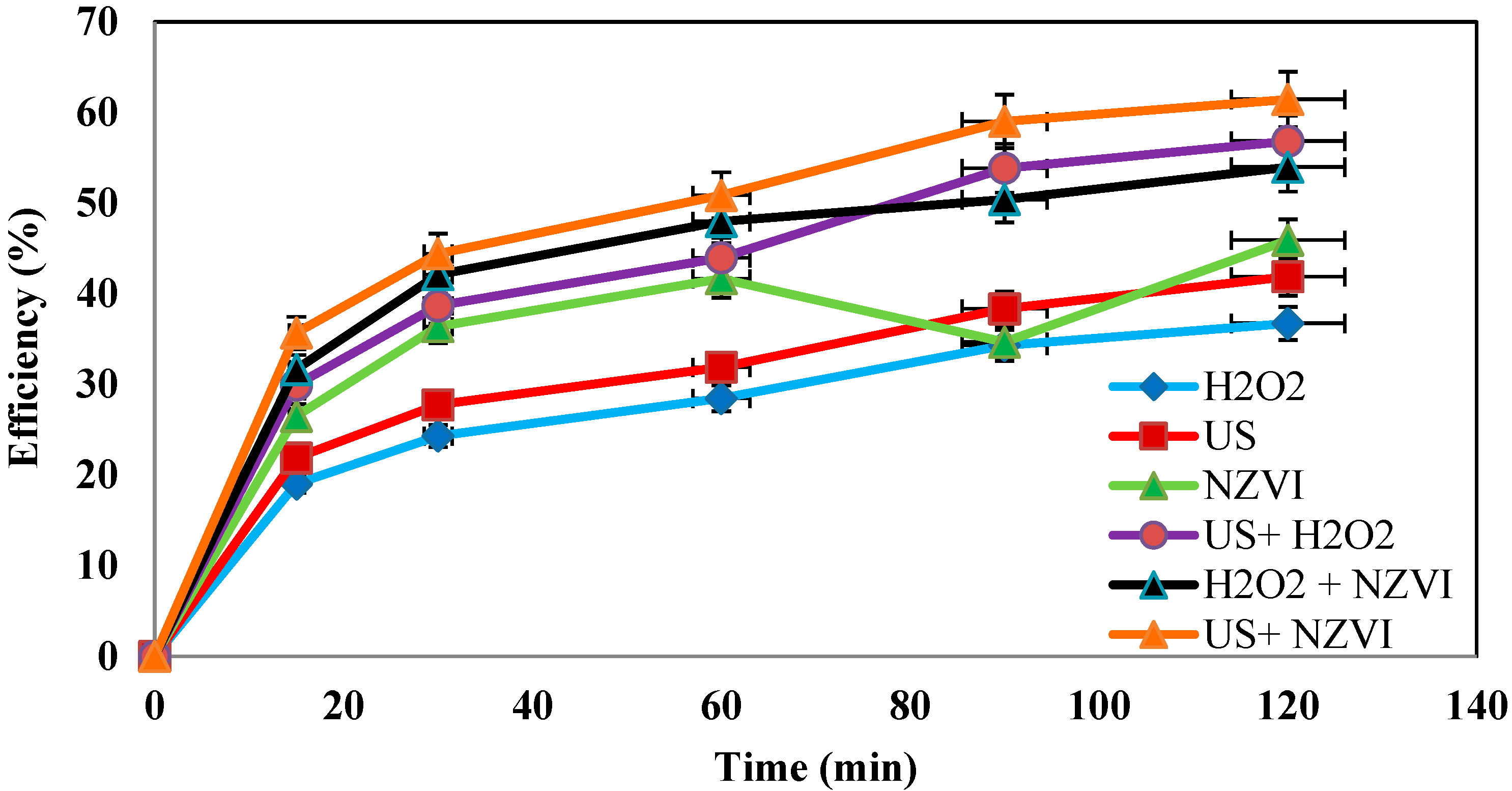
4.7. Determination of the Efficiency of the Process in the Absence of One of the Parameters
4.8. Determination of the System Performance in COD Removal
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS Analysis of Bioactive Pharmaceuticals and Personal-Care Products in Environmental Matrices. TrAC Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and Diclofenac: Removal in Wastewater Treatment Plants and Occurrence in Water Bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of Drugs in German Sewage Treatment Plants and Rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Miao, X.-S.; Metcalfe, C.D. Determination of Carbamazepine and Its Metabolites in Aqueous Samples Using Liquid Chromatography−Electrospray Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Ashtarinezhad, A.; Panahyab, A.; Mohamadzadehasl, B.; Shirazi, F.H. Characterization of Miconazole effects on Mice fetus liver tissue using FTIR-MSP. Iran. J. Pharm. Res. IJPR 2017, 16, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Tunç, Ö. Application of Biosorption for Penicillin G Removal: Comparison with Activated Carbon. Process Biochem. 2005, 40, 831–847. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Prados-Joya, G.; Ferro-García, M.; Bautista-Toledo, I. Removal of Tinidazole from Waters by Using Ozone and Activated Carbon in Dynamic Regime. J. Hazard. Mater. 2010, 174, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of Some Antibiotics, Elimination of the Genotoxicity and Affection of Wastewater Bacteria in A Simple Test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Githinji, L.J.M.; Musey, M.K.; Ankumah, R.O. Evaluation of the Fate of Ciprofloxacin and Amoxicillin in Domestic Wastewater. Water Air Soil Pollut. 2010, 219, 191–201. [Google Scholar] [CrossRef]
- Xing Zha, S.; Zhou, Y.; Jin, X.; Chen, Z. The Removal of Amoxicillin from Wastewater Using Organobentonite. J. Environ. Manag. 2013, 129, 569–576. [Google Scholar] [CrossRef]
- Ghauch, A.; Baydoun, H.; Dermesropian, P. Degradation of Aqueous Carbamazepine in Ultrasonic/Fe0/H2O2 Systems. Chem. Eng. J. 2011, 172, 18–27. [Google Scholar] [CrossRef]
- Ghafoori, S.; Mowla, A.; Jahani, R.; Mehrvar, M.; Chan, P.K. Sonophotolytic Degradation of Synthetic Pharmaceutical Wastewater: Statistical Experimental Design and Modeling. J. Environ. Manag. 2015, 150, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, E.; Torabian, A.; Nabi-Bidhendi, G. Research Article Carbamazepine Removal from Groundwater: Effectiveness of the Tio2/UV, Nanoparticulate Zero-Valent Iron, and Fenton (NZVI/H2O2) Processes. CLEAN–Soil Air Water 2013, 41, 1062–1072. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, H.; He, J.; Yang, P.; Wang, D.; Ma, T.; Xia, H.; Xu, X. Removal of Norfloxacin Using Coupled Synthesized Nanoscale Zero-Valent Iron (Nzvi) With H2O2 System: Optimization of Operating Conditions and Degradation Pathway. Sep. Purif. Technol. 2016, 172, 158–167. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Hu, X. Novel Zero-Valent Co–Fe Encapsulated in Nitrogen-Doped Porous Carbon Nanocomposites Derived from Cofe2o4@ZIF-67 For Boosting 4-Chlorophenol Removal Via Coupling Peroxymonosulfate. J. Colloid Interface Sci. 2020, 575, 206–219. [Google Scholar] [CrossRef]
- Vahidi, H.; Rahmani, S. Amoxicillin Removal from Aqueous Solutions Using US/H2O2/NZVI process. J. Environ. Health Eng. 2018, 5, 184–196. [Google Scholar] [CrossRef][Green Version]
- Fazlzadeh, M.; Rahmani, A.; Nasehinia, H.R.; Rahmani, H.; Rahmani, K. Degradation of Sulfathiazole Antibiotics in Aqueous Solutions by Using Zero Valent Iron Nanoparticles and Hydrogen Peroxide. Koomesh 2016, 18, 350–356. [Google Scholar]
- Valentine, R.L.; Wang, H.C.A. Iron Oxide Surface Catalyzed Oxidation of Quinoline by Hydrogen Peroxide. J. Environ. Eng. 1998, 124, 31–38. [Google Scholar] [CrossRef]
- Gholami, M.; Rahmani, K.; Rahmani, A.; Rahmani, H.; Esrafili, A. Oxidative Degradation of Clindamycin in Aqueous Solution Using Nanoscale Zero-Valent Iron/H2O2/US. Desalination Water Treat. 2016, 57, 13878–13886. [Google Scholar] [CrossRef]
- Molina, R.; Martínez, F.; Melero, J.A.; Bremner, D.H.; Chakinala, A.G. Mineralization of Phenol by a Heterogeneous Ultrasound/Fe-SBA-15/H2O2 Process: Multivariate Study by Factorial Design of Experiments. Appl. Catal. B Environ. 2006, 66, 198–207. [Google Scholar] [CrossRef]
- Ibrahem, A.K.; Moghny, T.A.; Mustafa, Y.M.; Maysour, N.E.; Dars, F.M.S.E.D.E.; Hassan, R.F. Degradation of Trichloroethylene Contaminated Soil by Zero-Valent Iron Nanoparticles. ISRN Soil Sci. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, J.; Qiu, X.; Qiu, X.; Cheng, W.; Zhu, L. Effective Removal of Antibiotic Metronidazole from Water by Nanoscale Zero-Valent Iron Particles. Desalination 2011, 268, 60–67. [Google Scholar] [CrossRef]
- Reichert, J.S.; McNeight, S.A.; Rudel, H.W. Determination of Hydrogen Peroxide and Some Related Peroxygen Compounds. Ind. Eng. Chem. Anal. Ed. 1939, 11, 194–197. [Google Scholar] [CrossRef]
- Talinli, I.; Anderson, G. Interference of Hydrogen Peroxide on the Standard Cod Test. Water Res. 1992, 26, 107–110. [Google Scholar] [CrossRef]
- Wu, T.; Englehardt, J.D. A New Method for Removal of Hydrogen Peroxide Interference in the Analysis of Chemical Oxygen Demand. Environ. Sci. Technol. 2012, 46, 2291–2298. [Google Scholar] [CrossRef]
- Carranzo, I.V. Standard Methods for Examination of Water and Wastewater. In Anales De Hidrología Médica; Universidad Complutense de Madrid: Madrid, Spain, 2012; Volume 5, p. 185. [Google Scholar]
- Dong, H.; Zhao, F.; He, Q.; Xie, Y.; Zeng, Y.; Zhang, L.; Tang, L.; Zeng, G. Physicochemical Transformation of Carboxymethyl Cellulose-Coated Zero-Valent Iron Nanoparticles (Nzvi) In Simulated Groundwater Under Anaerobic Conditions. Sep. Purif. Technol. 2016, 175, 376–383. [Google Scholar] [CrossRef]
- Weng, X.; Cai, W.; Lin, S.; Chen, Z. Degradation Mechanism of Amoxicillin Using Clay Supported Nanoscale Zero-Valent Iron. Appl. Clay Sci. 2017, 147, 137–142. [Google Scholar] [CrossRef]
- Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezade, A.; Fazlzadeh, M. Green Preparation of Activated Carbon from Pomegranate Peel Coated with Zero-Valent Iron Nanoparticles (Nzvi) And Isotherm and Kinetic Studies of Amoxicillin Removal in Water. Environ. Sci. Pollut. Res. 2020, 27, 36732–36743. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, S.; Shahlaei, M.; Wang, X.; Farhadian, N. A New Composite of Nano Zero-Valent Iron Encapsulated in Carbon Dots for Oxidative Removal of Bio-Refractory Antibiotics from Water. J. Clean. Prod. 2018, 209, 1523–1532. [Google Scholar] [CrossRef]
- Alvani, V.; Nabizadeh, R.; Ansarizadeh, M.; Mahvi, A.H.; Rahmani, H. Predicting TOC Removal Efficiency in Hybrid Biological Aerated Filter Using Artificial Neural Network. Desalination Water Treat. 2015, 57, 20283–20291. [Google Scholar] [CrossRef]
- Zare, M.R.; Amin, M.; Nikaeen, M.; Bina, B.; Rahmani, A.; Borji, S.H.; Rahmani, H. Acute Toxicity of Hg, Cd, and Pb Towards Dominant Bacterial Strains of Sequencing Batch Reactor (SBR). Environ. Monit. Assess. 2015, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Çatalkaya, E.Ç.; Şengül, F. Application of Box–Wilson Experimental Design Method for the Photodegradation of Bakery’s Yeast Industry with UV/H2O2 and UV/H2O2/Fe (II) Process. J. Hazard. Mater. 2006, 128, 201–207. [Google Scholar] [CrossRef]
- Manousaki, E.; Psillakis, E.; Kalogerakis, N.; Mantzavinos, D. Degradation of Sodium Dodecylbenzene Sulfonate in Water by Ultrasonic Irradiation. Water Res. 2004, 38, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.; Palanivelu, K. The Role of Ferrous Ion in Fenton and Photo-Fenton Processes for the Degradation of Phenol. Chemosphere 2004, 55, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Seid-Mohammadi, A.; Asgarai, G.; Ghorbanian, Z.; Dargahi, A. The removal of cephalexin antibiotic in aqueous solutions by ultrasonic waves/hydrogen peroxide/nickel oxide nanoparticles (US/H2O2/NiO) hybrid process. Sep. Sci. Technol. 2020, 55, 1558–1568. [Google Scholar] [CrossRef]
- Shu, H.-Y.; Chang, M.-C. Decolorization and Mineralization of a Phthalocyanine Dye C.I. Direct Blue 199 Using UV/H2O2 Process. J. Hazard. Mater. 2005, 125, 96–101. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M.; Eltoukhy, M.M. The Use of Artificial Neural Network (ANN) for Modeling of COD Removal from Antibiotic Aqueous Solution by the Fenton Process. J. Hazard. Mater. 2010, 179, 127–134. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, L.; Kamani, H.; Asghari, A.; Mohammadpour, A.; Golaki, M.; Rahdar, A.; Kyzas, G.Z. Removal of Amoxicillin from Aqueous Media by Fenton-like Sonolysis/H2O2 Process Using Zero-Valent Iron Nanoparticles. Molecules 2022, 27, 6308. https://doi.org/10.3390/molecules27196308
Mohammadi L, Kamani H, Asghari A, Mohammadpour A, Golaki M, Rahdar A, Kyzas GZ. Removal of Amoxicillin from Aqueous Media by Fenton-like Sonolysis/H2O2 Process Using Zero-Valent Iron Nanoparticles. Molecules. 2022; 27(19):6308. https://doi.org/10.3390/molecules27196308
Chicago/Turabian StyleMohammadi, Leili, Hossein Kamani, Abolfazl Asghari, Amin Mohammadpour, Mohammad Golaki, Abbas Rahdar, and George Z. Kyzas. 2022. "Removal of Amoxicillin from Aqueous Media by Fenton-like Sonolysis/H2O2 Process Using Zero-Valent Iron Nanoparticles" Molecules 27, no. 19: 6308. https://doi.org/10.3390/molecules27196308
APA StyleMohammadi, L., Kamani, H., Asghari, A., Mohammadpour, A., Golaki, M., Rahdar, A., & Kyzas, G. Z. (2022). Removal of Amoxicillin from Aqueous Media by Fenton-like Sonolysis/H2O2 Process Using Zero-Valent Iron Nanoparticles. Molecules, 27(19), 6308. https://doi.org/10.3390/molecules27196308







