Abstract
The 1,3-oxazolidine-2-thiones (OZTs) are important chiral molecules, especially in asymmetric synthesis. These compounds serve as important active units in biologically active compounds. Herein, carbohydrate anchored OZTs were explored to develop a copper-catalyzed C-S bond formation with aryl iodides. Chemoselective S-arylation was observed, with copper iodide and dimethylethylenediamine (DMEDA) as the best ligand in dioxane at 60–90 °C. The corresponding chiral oxazolines were obtained in reasonable to good yields under relatively mild reaction conditions. This approach is cheap, as using one of the cheapest transition metals, a simple protocol and various functional group tolerance make it a valuable strategy for getting S-substituted furanose-fused OZT. The structures of the novel carbohydrates were confirmed by NMR spectroscopy and an HRMS analysis.
1. Introduction
Carbohydrate derivatives are an important class of bioactive compounds with a high density of functional groups [1]. Carbohydrates annulated with heterocycles demonstrate interesting biological properties, including fructose transporter protein GLUT5 inhibition and the selective inhibition of glycoside hydrolase O-GlcNAcase (OGA) or the dual inhibition of OGA and cholinesterases, which are relevant for new therapeutic candidates [2,3,4].
In the past few decades, chemists have paid considerable attention to modifying carbohydrate molecules, which has resulted in the development of innumerable techniques to obtain heteroannulated sugars. Amongst the various reported methods, the most common have used 1,2-annulation strategies such as metal-catalyzed reactions, Michael addition reactions, cycloaddition methods, radical-mediated reactions, or nucleophilic attacks [1]. For example, the formation of 1,2-annulated sugars, having substituted tetrahydropyran and tetrahydrofuran moieties, was investigated via Lewis acid-catalyzed silyl-Prins/alkyne-Prins reactions from appropriately substituted sugar alcohols [5]. Recently, a simple Pd(OAc)2-catalyzed strategy was presented using glucose and galactose for the synthesis of sugar-fused indolines via C-H activation/cyclization using the oxalyl group as an auxiliary protecting group [6]. Werz and co-workers explored a convenient approach to obtain carbohydrate-based chromans and isochromans using an intermolecular Pd-catalyzed domino reaction with the catalytic systems Pd(PPh3)4, [(tBu)3PH]BF4, and Cs2CO3 or HN(iPr)2, as well as with microwave irradiation [7].
On the other hand, sulfur-containing compounds play an important role in organic synthesis and have been found in various natural products; they also appear in pharmaceuticals, agrochemicals, and functional materials [8,9]. Notably in asymmetric synthesis, a small heterocycle, 1,3-oxazolidine-2-thione (OZT), is a key molecule when chiral. Its parent heterocycle, 1,3-oxazolidine-2-one (OZO), is a chiral template that is well known in the Evans asymmetric methodology and that was specifically modified by Crimmins for the production of acylated OZT [10,11,12,13,14]. This small heterocycle is also well known as a degradation product from natural thioglucosides. The glucosinolates progoitrin, epiprogoitrin, and glucobarbarin are chiral OZTs resulting from the glucohydrolase action of a specific enzyme, myrosinase [15,16,17,18,19,20,21]. These molecules, as part of the defense mechanism of plants, have been shown to have several biological impacts—most notably on feedstock, but also on human health as inhibitors of tyrosine kinase [22,23]. They are also known for various biological activities such as anticancer, antibacterial, antifertility, and insecticidal activities [24]. A few representative biologically relevant OZT-based molecules are shown in Figure 1.
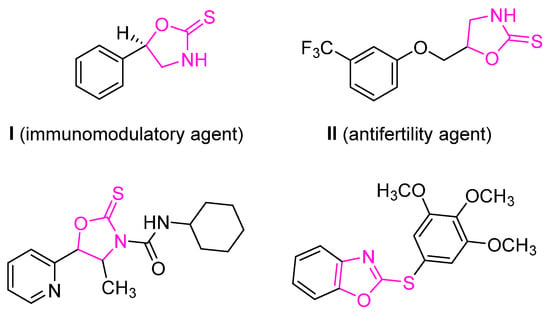
Figure 1.
Biologically active compounds (I–IV) bearing the OZT scaffold [9,25,26,27].
Chemists have developed different methods of synthesis for OZT or have analyzed its potential as an asymmetric inducer [24,28,29,30,31,32,33]. OZT connected to a carbohydrate backbone has been the subject of various studies to control the geometry of the carbohydrate structure using the specific chemistry of such heterocycles as well as to access new biologically active compounds [34]. The reactivity of OZT anchored on carbohydrates has been studied through classical reactions such as acylation, sulfonylation, and alkylation, as well as oxidation. OZT connected to carbohydrate templates were studied as precursors of 2-alkyl or 2-aryl 1,3-oxazoline following a copper-promoted palladium-catalyzed cross-coupling reaction called the Liebeskind–Srogl reaction, which uses a range of organoboronyl and organostannyl reagents [35,36,37]. Recently, carbohydrate-anchored OZTs were explored for the formation of iminosugars as inhibitors of glycosidases [38].
Herein, we present our preliminary results on the exploration of a chemoselective copper-catalyzed S-arylation reaction of aryl iodides with these unusual chiral oxazolidine-2-thiones anchored onto carbohydrate backbones. Copper-catalyzed S-arylation is a well-known process, but to our knowledge, it has not been applied to chiral OZT, or connected to carbohydrate backbones, in which the metal-catalyzed C-S bond formation has been scarcely explored [39,40,41]. Only a few examples have described the synthesis of S-arylated oxazolidinethiones, either through a one-pot procedure using isocyanide dichloride in a two-step approach [42] or using the reactivity of transient arynes with 2-oxazolidinethiones to obtain selective S-arylation [43].
2. Results and Discussion
The oxazolidine-2-thione moiety has two potential nucleophilic sites: sulfur and nitrogen atoms. Accordingly, both S-arylation and N-arylation may take place due to the existence of two tautomeric thione–thiol forms (Scheme 1). Sulfur, as a soft nucleophile, can prevail in the formation of S-arylated products in a cross-coupling reaction [44,45,46].
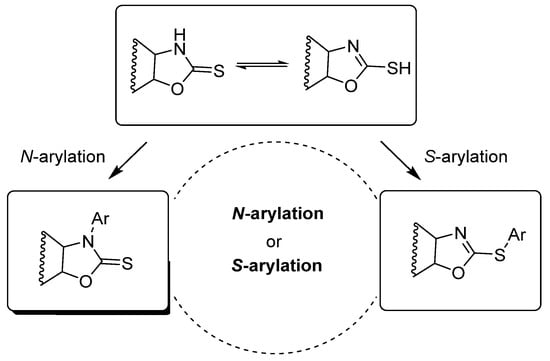
Scheme 1.
Possible sites of arylation of oxazolidine-2-thiones.
D-arabinofuranose-, D-xylofuranose-, and D-ribofuranose-derived oxazolidine-2-thiones 1–3 can easily be obtained from the corresponding carbohydrates using potassium thiocyanate under acidic conditions [2,36,47]. D-arabinose was chosen as the starting precursor in our study [48]. The protection of the hydroxyl groups was achieved with acetyl (Ac) or tert-butyldimethylsilyl (TBDMS) groups, allowing the selective protection of the alcohols obtained without modifying the oxazolidinethione group [47,49,50] (Scheme 2). The reaction of D-arabinose OZT 1 with acetic anhydride in pyridine resulted in the pre-acetylated product-derivative 4 in a quantitative yield without any requirement for purification. The crude product was selectively deacetylated in a mixture of methanol and pyridine. These conditions resulted in an efficient selective deacetylation of the acyl-protected 1,3-oxazolidine-2-thione vs. the ester group. The silylation process directly produced a selective protection of the hydroxyl groups of the three pentose OZTs 1, 2, and 3. The corresponding silyl- protected OZTs (6–8) were obtained in very good yields of 96%, 98%, and 95%, respectively.
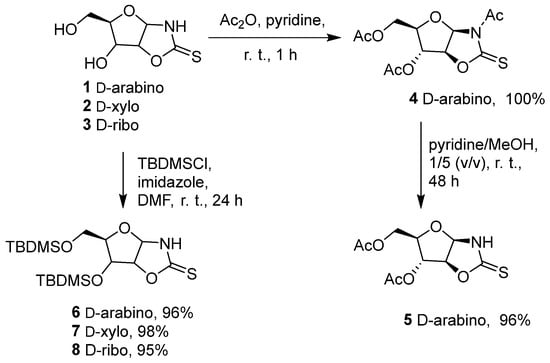
Scheme 2.
Protection of hydroxyl groups of carbohydrates 1–3.
We decided to convert OZT 5 into the corresponding oxazolidin-2-one (OZO) derivative 10 (Scheme 3), which could then be tested with a Cu-catalyzed cross-coupling reaction. We have previously reported that the direct conversion of OZT derivatives to OZO derivatives by oxidation is not efficient [2]. Prior to oxidation, OZT template 5 required S-alkylation and was converted into the corresponding 2-benzylsulfanyloxazoline 9 at a 92% yield. After oxidation, OZO 10 was obtained at a 57% yield.
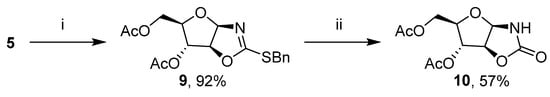
Scheme 3.
Synthesis of 1,3-oxazolidin-2-one derivative 10. Alkylation conditions i: BnBr, Et3N, DCM, r. t., 48 h. Oxidation conditions ii: m-CPBA, NaHCO3, DCM, 0 °C, 2 h.
Copper-catalyzed coupling reactions was performed by applying standard conditions with various copper ligands and copper iodide, which was chosen for its air stability [51,52,53]. We first chose the D-arabino derivative 5 with 4-iodoanisole as a model substrate to optimize the catalytic S-arylation (Scheme 4, Table 1). As a control experiment (Table 1, entry 1), no product was observed if the reaction conditions were carried out in the absence of the copper ligand. When the reaction was performed with 20 mol% of CuI and 40 mol% of dimethylethylenediamine (DMEDA) under basic conditions (Cs2CO3), C-S coupling was observed with a better, but lower, yield of 20% (Table 1, entry 3). A reduced or increased ratio of CuI and DMEDA did not improve the yields. A reduction in the temperature to 60 °C maintained the results at 35% (Table 1, entry 5). Thus, the 1:2 ratio was maintained and proved to be similar to other copper-catalyzed cross-coupling reactions [54].
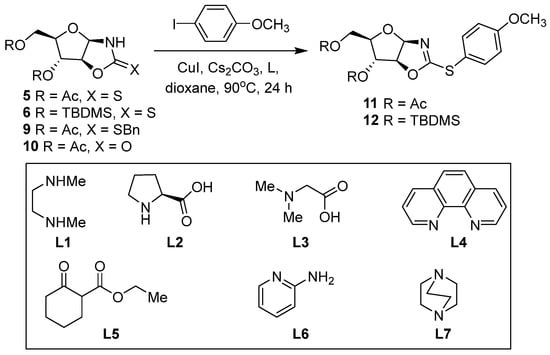
Scheme 4.
Model reactions of the C-S coupling using different ratios of CuI/ligand and different ligands.

Table 1.
Comparative studies utilizing various ligands and ratios of catalyst and ligand [a].
Applying these conditions (Table 1, entry 6) to the silyl-protected OZT 6 resulted in a low yield of 22%. Pushing the conditions with a higher temperature (Table 1, entry 7) gave a much better yield of 69%. The ligands, including L-proline, DABCO, 2-aminopyridine, ethyl 2-oxocyclohexane-1-carboxylate, and 9,10-phenanthroline, were tested to enhance the C-S coupling reaction between OZT 6 and 4-iodoanisole using the conditions of entry 7 (Table 1, entries 8–13). None of them were effective for this coupling reaction. The coupling reaction with ethyl 2-oxocyclohexane-1-carboxylate L5 and DABCO L7 did not perform at all (Table 1, entries 11 and 13); in other cases, a degradation of the starting compound was observed (Table 1, entries 1, 8, 9 and 12). Only the 9,10-phenanthroline L4 showed reactivity, but its efficiency was worse (Table 1, entry 10). Of all these ligands, only DMEDA L1 was efficient in the C-S coupling reaction. As expected with benzylsulfanyloxazoline 9 and oxazolidinone 10, no coupling reaction was observed (Table 1, entries 14–15).
Notably, only the S-arylation was observed; no N-arylation was detected, although the N-arylation of aryl halides has been shown to proceed under similar conditions [55,56,57]. In this case, S-arylation was preferred compared with N-arylation. Sulfur, as a soft nucleophile, favors an interaction with copper and occurs in the formation of S-arylated products in a cross-coupling reaction [44]. Furthermore, this can be explained by the mechanism of the copper-assisted nucleophilic substitution reaction [58]. In the intermediate catalysis step, the mercapto group mainly attacks the copper complex, which is a soft metal atom. Sulfur is a stronger nucleophile than nitrogen due to its nature of high polarizability, a large size, and more electron lone pairs [58].
Although the exact mechanism remains unknown for this Cu-catalyzed S-arylation process, there are valuable experimental and theoretical mechanistic studies of Cu-catalysed N-arylation [59,60,61]. Moreover, there are investigations that the formation of the Cu(I)-complex nucleophile and the coupling product depends on the concentration of the ligand [62,63]. Applying optimized reaction conditions (Table 1, entry 7), the S-arylation reaction of 6 was explored with various functionalized aryl iodides (Scheme 5). The results are summarized in Table 2. Aryl iodides were chosen for their enhanced reactivity compared with other aryl halides. As shown in Table 2, D-arabinose derivative 6 reacted with various substituted aryl iodides, leading to the corresponding products 12–19 with low to good yields (Figure 2).
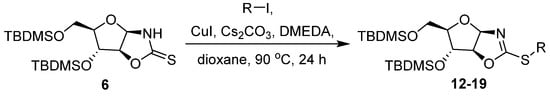
Scheme 5.
Synthesis of compounds 12–19.

Table 2.
Reactivity of d-arabinose derivative 6 with various aryl iodides [a].
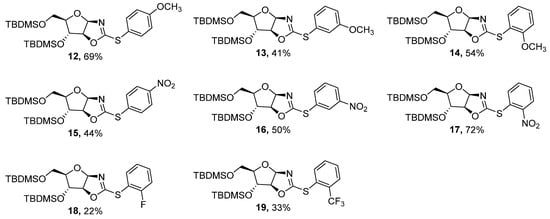
Figure 2.
Synthesized compounds 12–19.
The aryl iodides with electron-donating groups (such as the methoxy group) and electron-withdrawing groups (such as the nitro group) obtained the desired S-aryl oxazolines in moderate to good yields (Table 2, entries 1–6). No clear evidence of the electronic effect could be extracted from these results. Whether the substitution of iodobenzene was at the para-, meta-, or ortho-position, the C–S bond formation resulted in moderate yields and no effect of steric hindrance was clearly visible. On the contrary, the comparison of the ortho-substitution with ortho-fluorobenzene and ortho-trifluoromethyl benzene showed a significant drop in yields. The relatively poor yields obtained by performing the coupling reaction with ortho-substituted iodobenzene containing CF3 and F groups could be attributed to the instability of the compounds (Table 2, entries 7–8). Degradation could have occurred through the hydrolysis of phenylsulfanyloxazoline. When left under basic conditions, the formation of the corresponding oxazolidinone was detected.
The application of this cross-coupling reaction on D-xylose oxazolidine-2-thione 7 and D-ribose oxazolidine-2-thione 8 was explored using ortho-substituted iodobenzene derivatives (Scheme 6).
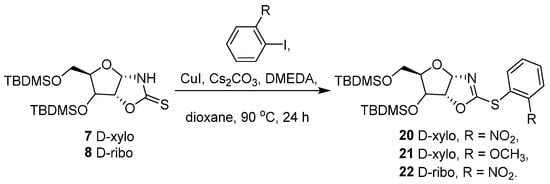
Scheme 6.
Reactivity of D-xylose 7 and D-ribose 8 with ortho-substituted iodobenzenes.
Both compounds proved that the method was effective on other carbohydrate anchored 1,3-oxazolidine-2-thiones but with reduced yields. The derivatives D-xylo 20–21 and D-ribo 22 were obtained with 58%, 45%, and 46% yields, respectively.
3. Materials and Methods
3.1. General Information
Reactions in anhydrous conditions were performed under an argon atmosphere in pre-dried flasks, using anhydrous solvents (distilled, when necessary, according to D. D. Perrin, W. L. F. Armarego and D. R. Perrin in Purification of Laboratory Chemicals, Pergamon, Oxford, 1986). Molecular sieves were activated prior to use by heating for 4 h at 500 °C. All reagents were obtained from commercial chemical suppliers and used without further purification. The course of the reactions was monitored by an initial TLC analysis on precoated aluminum foil-backed plates (Merck Kieselgel 60 F254, Darmstadt, Germany). TLC results were visualized using standard visualization techniques or agents: UV fluorescence (254 nm) and staining with a 1% aq potassium permanganate solution or a heat treatment with a 10/85/5 mixture of sulfuric acid/ethanol/water. Flash column chromatography was performed with Silica Gel 60 Å (230–400 µm, Merck KGaA, Darmstadt, Germany). The melting points (°C) were measured with a Thermo Scientific 9200 capillary apparatus and are uncorrected. The NMR spectra were recorded on a 400 MHz Bruker Avance 2 spectrometer (Bruker BioSpin AG, Fallanden, Switzerland) (400 MHz (1H), 100 MHz (13C), and 376 MHz (19F)). The chemical shifts are expressed in parts per million (ppm) downfield from the TMS internal standard. The coupling patterns for 1H NMR are designated as s = singlet, d = doublet, t = triplet, and m = multiplet, (a few 13C NMR are designated as d = doublet, and q = quartet); the coupling constants are given in Hz. The NMR peak assignments were elucidated via DEPT, COSY, and HSQC techniques for all reported compounds. The IR spectra from the samples in a neat form were measured with a Thermo Scientific Nicolet iS10 FT-IR spectrophotometer. IR absorption frequencies are given in cm–1. Optical rotations were measured at 20 °C with a Perkin Elmer 341 polarimeter and were given in g–1⋅cm3⋅dm–1. Low-resolution mass spectra (MS) were recorded with a Perkin–Elmer Sciex API 300 spectrometer [ionspray (IS) mode] (PerkinElmer Inc., Waltham, MA, USA). High-resolution mass spectra (HRMS) were recorded with a Bruker MaXis spectrometer [electrospray ionization (ESI) mode] (Bruker DaltonikGmbH, Bremen, Germany). 1H, 13C NMR spectra and HRMS data of all new compounds are provided in Supplementary Materials as Figures S1–S38.
3.2. Synthesis of N-acetyl-4,5-dihydro(3′,5′-di-O-acetyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazolidine-2-thione (4)
To a solution of compound 1 (500 mg, 2.61 mmol) in dry pyridine (8 mL), acetic anhydride (2 mL) was added dropwise to a well-stirred solution at 0 °C under argon for 10 min. Then the resulting reaction mixture was stirred at room temperature for 1 h. The reaction mixture was diluted with ethyl acetate and washed with 10% acetic acid, then water and sat. aq. NaHCO3 solution, and finally dried over MgSO4. After filtration, the solvent was removed under vacuum. The obtained compound 4 was a colorless oil of very good purity (830 mg, quantitative yield); = −69 (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 2.08 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.79 (s, 3H, CH3(C=O)N), 4.03 (dd, 1H, J5′b,4′ = 4.6 Hz, J5′b,5′a = 11.9 Hz, 5′b-H), 4.30 (dd, 1H, J5′a,4′ = 5.8 Hz, J5′a,5′b = 11.9 Hz, 5′a-H), 4.35−4.38 (m, 1H, 4′-H), 5.06 (d, 1H, J2′,1′ = 5.6 Hz, 2′-H), 5.32 (d, 1H, J3′,4′ = 1.0 Hz, 3′-H), 6.50 (d, 1H, J1′,2′ = 5.6 Hz, 1′-H) ppm. 13C NMR (100 MHz, CDCl3): δ = 20.8 (CH3), 20.9 (CH3), 26.2 (CH3(C=O)N), 63.1 (C-5′), 77.0 (C-3′), 84.0 (C-4′), 85.5 (C-1′), 90.9 (C-2′), 169.7 (C=O), 170.7 (C=O), 170.8 (C=O), 183.7 (C=S) ppm. IR (NEAT): ν = 1740, 1716 (C=O), 1366, 1324, 1214, 1162 (C-O, C-N) cm−1. MS (IS): m/z = 318 [M + H]+, 340 [M + Na]+. HRMS (ESI): m/z [M + H]+ calcd. for C12H16NO7S: 318.06420; found: 318.06432. HRMS (ESI): m/z [M + Na]+ calcd. for C12H15NNaO7S: 340.04614; found: 340.04654.
3.3. Synthesis of 4,5-dihydro(3′,5′-di-O-acetyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazolidine-2-thione (5)
Under argon, to a cooled solution of compound 4 (830 mg, 2.61 mmol) in dry pyridine (4 mL) at 0 °C, methanol (1 mL) was added dropwise. The reaction mixture was stirred at 0 °C for 15 min, then was allowed to reach room temperature and stirred for 48 h. The reaction mixture was diluted with ethyl acetate (60 mL) and washed with 10% cold acetic acid solution (20 mL), cold water (20 mL), and then sat. aq. NaCl solution (20 mL), and finally dried over MgSO4. After filtration, the solvent was removed under reduced pressure. The residue was purified by flash chromatography (eluent: PE/EtOAc, 1:1, Rf = 0.27) to give 4 (689 mg, 96%) as a white solid; mp 157−159 °C, = −34 (c = 0.49, CHCl3). 1H NMR (400 MHz, DMSO-d6): δ = 2.06 (s, 3H, CH3), 2.08 (s, 3H, CH3), 3.96 (dd, 1H, J5′b,4′ = 6.5 Hz, J5′b,5′a = 11.9 Hz, 5′b-H), 4.06 (dd, 1H, J5′a,4′ = 5.1 Hz, J5′a,5′b = 11.9 Hz, 5′a-H), 4.31−4.34 (m, 1H, H-4′), 5.20 (s, 1H, H-3′), 5.37 (d, 1H, J2′,1′ = 5.7 Hz, H-2′), 5.91 (d, 1H, J1′,2′ = 5.7 Hz, H-1′), 11.06 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ = 20.5 (CH3), 20.6 (CH3), 62.9 (C-5′), 76.7 (C-3′), 81.3 (C-4′), 88.7 (C-1′), 89.9 (C-2′), 169.5 (C=O), 170.1 (C=O), 188.0 (C=S). IR (NEAT): ν = 3263 (N-H), 1746, 1704 (C=O), 1503, 1313, 1212, 1165, 1048 (C-O, C-N) cm−1. MS (IS): m/z = 276 [M + H]+, 298 [M + Na]+. HRMS (ESI): m/z [M + H]+ calcd. for C10H14NO6S: 276.05363; found: 276.05369. HRMS (ESI): m/z [M + Na]+ calcd. for C10H13NNaO6S: 298.03558; found: 298.03584.
3.4. Synthesis of 2-benzylsulfanyl-4,5-dihydro(3′,5′-di-O-acetyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (9)
To a solution of compound 5 (0.3 g, 1.09 mmol) in dry dichloromethane (5 mL), triethylamine (0.46 mL, 0.73 mmol) and benzyl bromide (0.26 mL, 2.18 mmol) were added dropwise. The reaction mixture was stirred at room temperature under argon for 24 h. It was then diluted with dichloromethane (60 mL) and washed with 1M HCl (20 mL), NaHCO3 sat. (20 mL), and NaCl sat. (20 mL) solutions, and finally dried over MgSO4. After filtration, the solvent was evaporated in vacuo. The obtained residue was purified by flash chromatography (eluent: PE/EtOAc, 7:1, Rf = 0.26) to give 5 (368 mg, 92%) as colorless oil; = −69 (c = 1, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 2.07 (s, 3H, CH3), 2.10 (s, 3H, CH3), 3.94 (dd, 1H, J5′b,4′ = 6.3 Hz, J5′b,5′a = 11.7 Hz, 5′b-H), 4.05 (dd, 1H, J5′a,4′ = 6.5 Hz, J5′a,5′b = 11.7 Hz, 5′a-H), 4.26 (dd, 1H, J4′,3′ = 2.0 Hz, J4′,5′b = 6.3 Hz, H-4′), 4.30 (s, 2H, SCH2), 4.94 (d, 1H, J2′,1′ = 5.9 Hz, H-2′), 5.18 (d, 1H, J = 2,0 Hz, H-3′). 6.12 (d, 1H, J1′,2′ = 5.9 Hz, H-1′), 7,26−7,33 (m, 3H, HAr); 7,37−7,39 (m, 2H, HAr) ppm. 13C NMR (100 MHz, CDCl3): δ = 20.8 (2×CH3), 36.5 (SCH2), 63.2 (C-5′), 78.2 (C-3′), 81.7 (C-4′), 87.7 (C-2′), 101.4 (C-1′), 127.8 (CH), 128.7 (2×CH), 129.1 (2×CH), 136.1 (Cq), 169.8 (Cq), 170.2 (C=O), 170.5 (C=O) ppm. IR (NEAT): ν = 2959, 1740 (C=O), 1596, 1366, 1212, 1134, 1029 (C-O, C-N) cm−1. MS (IS): m/z = 366 [M + H]+, 388 [M + Na]+. HRMS (ESI): m/z [M + H]+ calcd. for C17H20NO6S: 366,10058; found: 366,10104. HRMS (ESI): m/z [M + Na]+ calcd. for C17H19NNaO6S: 388,08253; found: 388,08296.
3.5. Synthesis of 4,5-dihydro(3′,5′-di-O-acetyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazolidine-2-one (10)
To a solution of compound 9 (270 mg, 0.75 mmol) in dry dichloromethane (5 mL), dry NaHCO3 (188 mg, 2.24 mmol) was added. Then, the reaction mixture was cooled to 0 °C temperature under argon for 10 min, then m-CPBA (75%, 490 mg, 2.84 mmol) was added slowly. The resulting reaction mixture was stirred at 0 °C for 2 h. Then the reaction mixture was diluted with dichloromethane and washed with sat. aq. Na2S2O5 solution, then sat. aq. NaHCO3 solution, and finally dried over MgSO4. After filtration, the solvent was evaporated in vacuo. The obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 4:6, Rf = 0.22) to give 10 (110 mg, 57%) as white solid; mp 115–116 °C; = −63 (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 2.09 (s, 3H, CH3), 2.10 (s, 3H, CH3), 3.96 (dd, 1H, J5′b,4′ = 2.9 Hz, J5′b,5′a = 10.4 Hz, 5′b-H), 4.25−4.31 (m, 2H, 5′a-H, 4′-H), 4.98 (d, 1H, J2′,1′ = 5.7 Hz, 2′-H), 5.25 (s, 1H, 3′-H), 5.77 (d, 1H, J1′,2′ = 5.7 Hz, 1′-H), 6.45 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ = 20.7 (CH3), 20.9 (CH3), 63.8 (C-5′), 78.0 (C-3′), 83.3 (C-4′), 84.4 (C-2′), 87.3 (C-1′), 156.8 (C=O), 169.9 (C=O), 171.0 (C=O) ppm. IR (NEAT): ν = 3316 (N-H), 1788, 1749, 1711 (C=O), 1377, 1267, 1223, 1212, 1092, 1051 (C-O, C-N) cm−1. MS (IS): m/z = 260 [M + H]+; 282 [M + Na]+. HRMS (ESI): m/z [M + H]+ calcd. for C10H14NO7: 260.07648; found: 260.07669. HRMS (ESI): m/z [M + Na]+ calcd. for C10H13NNaO7: 282.05842; found: 282.05856.
3.6. Synthesis of 4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazolidine-2-thione (6)
Compound 1 (400 mg, 2.08 mmol) was dissolved in dry DMF (5 mL). Imidazole (712 mg, 10.47 mmol) and tert-butyldimethylsilyl chloride (792 mg, 2.52 mmol) were then added at 0 °C temperature. Then, the reaction mixture was stirred at room temperature for 15 h. The reaction mixture was diluted with dichloromethane (100 mL) and washed three times with water (3 × 30 mL), then brine (30 mL), and finally dried over MgSO4. After filtration, the solvent was removed by evaporation in vacuo. The obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 95:5 after 9:1, Rf = 0.2) to give 6 (841 mg, 96%) as white solid; mp 64–65 °C; = −52 (c = 1.1, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.05 (s, 3H, CH3Si), 0.06 (s, 3H, CH3Si), 0.11 (s, 3H, CH3Si), 0.12 (s, 3H, CH3Si), 0.87 (s, 9H, 3 × CH3), 0.88 (s, 9H, 3×CH3), 3.40−3.45 (m, 1H, 5′b-H), 3.63 (dd, 1H, J5′a,4′ = 5.5 Hz, J5′a,5′b = 10.5 Hz, 5′a-H), 4.04−4.07 (m, 1H, 4′-H), 4.53 (s, 1H, 3′-H), 5.01 (d, 1H, J2′,1′ = 5.6 Hz, 2′-H), 5.82 (d, 1H, J1′,2′ = 5.6 Hz, 1′-H), 7.40 (br s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.7 (2×CH3Si), 18.2 (Cq), 18.5 (Cq), 25.9 (3 × CH3), 26.1 (3 × CH3), 62.5 (C-5′), 76.2 (C-3′), 88.2 (C-4′), 89.5 (C-1′), 93.2 (C-2′), 189.0 (C=S) ppm. MS (IS): m/z = 420 [M + H]+. HRMS (ESI): m/z [M + Na]+ calcd. for C18H37 NNaO4SSi2: 442.18740; found: 442.18742.
3.7. Synthesis of 4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-α-D-xylofuranoso)-[1,2-d]-oxazolidine-2-thione (7)
Compound 2 (600 mg, 3.14 mmol) was dissolved in dry DMF (6 mL). Imidazole (1079 mg, 15.85 mmol) and tret-butyldimethylsilyl chloride (1192 mg, 7.90 mmol) were then added at 0 °C temperature. Then, the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with dichloromethane and washed with water, then brine, and finally dried over MgSO4. After filtration, the solvent was removed by evaporation in vacuo. The obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 95:5 after 9:1, Rf = 0.35) to afford 7 (1.3 g, 98%) as white solid; mp 106−107 °C; = −22 (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.03 (s, 3H, CH3Si), 0.04 (s, 3H, CH3Si), 0.09 (s, 3H, CH3Si), 0.11 (s, 3H, CH3Si), 0.86(s, 9H, 3 × CH3), 0.86 (s, 9H, 3 × CH3), 3.74−3.81 (m, 2H, 5′a,b-H), 3.94 (dt, 1H, J4′,5′a = 2.9 Hz, J4′,5′b = 8.7 Hz, 4′-H), 4.40 (d, 1H, J3′,4′ = 2.6 Hz, 3′-H), 4.95 (d, 1H, J2′,1′ = 5.4 Hz, 2′-H), 5.83 (d, 1H, J1′,2′ = 5.4 Hz, 1′-H), 7.86 (s, 1H, NH) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.2 (CH3Si), -5.0 (CH3Si), -4.7 (CH3Si), 18.2 (Cq), 18.5 (Cq), 25.8 (3 × CH3), 26.1 (3 × CH3), 60.3 (C-5′), 74.4 (C-3′), 81.3 (C-4′), 88.6 (C-1′), 91.2 (C-2′), 189.4 (C=S) ppm. IR (NEAT): ν = 3286 (N-H), 2954, 2929 (C-HAl), 1502, 1254, 1153, 1003, 833 (C-O, C-N) cm−1. MS (IS): m/z = 420 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C18H38NO4SSi2: 420.20546; found: 420.0565.
3.8. General Procedure: Copper-catalyzed S-arylation of Compounds 11–21
One of the appropriate starting oxazolidine-2-thiones 5, 6, 7 or 8 (1 equiv), Cs2CO3 (2 equiv), the copper iodide (0.2 equiv), and the iodide derivative (1.5 equiv) were dissolved in anhydrous dioxane (2 mL) in a round-bottom flask under argon. After 10 min, DMEDA (0.4 equiv) was added dropwise and the reaction was stirred at 60 °C or 90 °C for 24 h. The reaction was then allowed to cool to room temperature, and poured into a sat. aq NaCl (40 mL) solution and extracted with EtOAc (2 × 25 mL), then with H2O (1 × 10 mL), and dried over MgSO4. After the evaporation of the solvent, the residue was purified by flash chromatography using PE/EtOAc as an eluent to produce the desired products. The data for the selected compounds are described below.
3.8.1. 2-[(4-Methoxyphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-acetyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (11)
Prepared from 5 (100 mg, 0.36 mmol) and 4-iodoanisole (127 mg, 0.54 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 1:1, Rf = 0.27) to give 11 (48 mg, 35%) as a colorless oil; = −64 (c = 1.2, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 2.07 (s, 3H, CH3), 2.10 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 4.03 (dd, 1H, J5′b,4′ = 7.0 Hz, J5′b,5′a = 11.6 Hz, 5′b-H), 4.10 (dd, 1H, J5′a,4′ = 6.4 Hz, J5′a,5′b = 11.6 Hz, 5′a-H), 4.23–4.26 (m, 1H, 4′-H), 4.90 (d, 1H, J2′,1′ = 5.8 Hz, 2′-H), 5.19 (s, 1H, 3′-H), 6.05 (d, 1H, J1′,2′ = 5.8 Hz, 1′-H), 6.90 (d, 2H, J3,2 = J5,6 = 8.8 Hz, 3-HPh, 5-HPh), 7.49 (d, 2H, J2,3 = J6,5 = 8.8 Hz, 2-HPh, 6-HPh) ppm. 13C NMR (100 MHz, CDCl3): δ = 20.9 (CH3), 21.0 (CH3), 55.6 (OCH3), 63.4 (C-5′), 78.3 (C-3′), 81.8 (C-4′), 87.7 (C-2′), 101.8 (C-1′), 115.3 (2 × CPh), 116.9 (Cq), 127.1 (Cq), 137.0 (2 × CPh), 161.4 (C-S), 169.9 (C=O), 170.8 (C=O) ppm. IR (NEAT): ν = 1740 (C=O), 1592, 1494, 1215, 1129, 1025 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 382 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C17H20NO7S: 382.09550; found: 382.09618. HRMS (ESI): m/z [M + Na]+ calcd. for C17H19NNaO7S: 404.07744; found: 404.07785.
3.8.2. 2-[(4-Methoxyphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (12)
Prepared from 6 (100 mg, 0.24 mmol) and 4-iodoanisole (84 mg, 0.36 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.18) to give 12 (86 mg, 69%) as a colorless oil; = −67 (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.06 (s, 3H, CH3Si), 0.07 (s, 6H, 2 × CH3Si), 0.08 (s, 3H, CH3Si), 0.86 (s, 9H, 3 × CH3), 0.90 (s, 9H, 3 × CH3), 3.42 (dd, 1H, J5′b,4′ = 8.5 Hz, J5′b,5′a = 10.5 Hz, 5′b-H), 3.63 (dd, 1H, J5′a,4′ = 4.6 Hz, J5′a,5′b = 10.6 Hz, 5′a-H), 3.79 (s, 3H, OCH3), 3.87−3.91 (m, 1H, 4′-H), 4.37 (br s, 1H, 3′-H), 4.71 (d, 1H, J2′,1′ = 5.9 Hz, 2′-H), 5.96 (d, 1H, J1′,2′ = 5.9 Hz, 1′-H), 6.89 (d, 2H, J3,2 = J5,6 = 8.6 Hz, 3-HPh, 5-HPh), 7.45 (d, 2H, J2,3 = J6,5 = 8.6 Hz, 2-HPh, 6-HPh) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.7 (CH3Si), -4.6 (CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 55.6 (OCH3), 62.3 (C-5′), 77.1 (C-3′), 86.1 (C-4′), 91.0 (C-2′), 101.0 (C-1′), 115.2 (2 × CPh), 117.4 (Cq), 136.8 (2 × CPh), 161.2 (C-O), 169.9 (N=C-S) ppm. IR (NEAT): ν = 2953 (C-HAl), 1594, 1249, 1107, 1001, 837 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 526.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C25H44NO5SSi2: 526.24732; found: 526.24786.
3.8.3. 2-[(3-Methoxyphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (13)
Prepared from 6 (100 mg, 0.24 mmol) and 3-iodoanisole (0.043 mL, 0.36 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.25) to give 13 (51 mg, 41%) as a colorless oil; = −57 (c = 0.95, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.06 (s, 3H, CH3Si), 0.07 (s, 3H, CH3Si), 0.07 (s, 3H, CH3Si), 0.08 (s, 3H, CH3Si), 0.86 (s, 9H, 3×CH3), 0.89 (s, 9H, 3×CH3), 3.43 (dd, 1H, J5′b,4′ = 8.3 Hz, J5′b,5′a = 10.5 Hz, 5′b-H), 3.63 (dd, 1H, J5′a,4′ = 4.6 Hz, J5′a,5′b = 10.6 Hz, 5′a-H), 3.78 (s, 3H, OCH3), 3.87–3.91 (m, 1H, 4′-H), 4.37 (br s, 1H, 3′-H), 4.72 (dd, 1H, J2′,3′ = 1.2 Hz, J2′,1′ = 6.0 Hz, 2′-H), 5.99 (d, 1H, J1′,2′ = 6.0 Hz, 1′-H), 6.92 (dd, 1H, J4,2 = 2.5 Hz, J4,5 = 8.3 Hz, 4-H), 7.10 (br s, 1H, 2-H), 7.14 (d, 1H, J6,5 = 7.7 Hz, 6-H), 7.25–7.28 (m, 1H, 5-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.7 (CH3Si), -4.6 (CH3Si), 18.2 (Cq), 18.6 (Cq), 26.0 (3 × CH3), 26.2 (3 × CH3), 55.6 (OCH3), 62.3 (C-5′), 77.1 (C-3′), 86.1 (C-4′), 90.9 (C-2′), 101.0 (C-1′), 116.1 (C-4), 120.1 (C-2), 127.1 (C-6), 127.8 (Cq), 130.3 (C-5), 160.0 (C-3), 168.9 (N=C-S) ppm. IR (NEAT): ν = 2953 (C-HAl), 1591, 1250, 1106, 1002, 837 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 526.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C25H44NO5SSi2: 526.24732; found: 526.24783.
3.8.4. 2-[(2-Methoxyphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (14)
Prepared from 6 (90 mg, 0.21 mmol) and 2-iodoanisole (0.04 mL, 0.321 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 85/:15, Rf = 0.25) to give 14 (61 mg, 54%) as a white solid; mp 82−84 °C; = −39 (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.06 (s, 9H, 3 × CH3Si), 0.07 (s, 3H, CH3Si), 0.86 (s, 9H, 3 × CH3), 0.89 (s, 9H, 3 × CH3), 3.45 (dd, 1H, J5′b,4′ = 9.1 Hz, J5′b,5′a = 10.3 Hz, 5′b-H), 3.63 (dd, 1H, J5′a,4′ = 4.8 Hz, J5′a,5′b = 10.6 Hz, 5′a-H), 3.79 (s, 3H, OCH3), 3.88−3.91 (m, 1H, 4′-H), 4.35 (br s, 1H, 3′-H), 4.67 (d, 1H, J2′,1′ = 5.9 Hz, 2′-H), 5.97 (d, 1H, J1′,2′ = 5.9 Hz, 1′-H), 6.92–6.96 (m, 2H, 3-H, 5-H), 7.39 (td, 1H, J4,6 = 1.2 Hz, J4,3 = J4,5 = 7.8 Hz, 4-H), 7.54 (dd, 1H, J6,4 = 1.4 Hz, J6,5 = 7.5 Hz, 6-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.1 (CH3Si), -5.1 (CH3Si), -4.7 (CH3Si), -4.6 (CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 56.2 (OCH3), 62.5 (C-5′), 77.2 (C-3′), 86.4 (C-4′), 90.8 (C-2′), 101.2 (C-1′), 111.9 (CHPh), 115.1 (Cq), 121.3 (CHPh), 132.2 (CHPh), 137.0 (CHPh), 159.7 (C-O), 168.7 (N=C-S) ppm. IR (NEAT): ν = 2930 (C-HAl), 1604, 1462, 1249, 1105, 1060, 1004, 814 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 526.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C25H44NO5SSi2: 526.24732; found: 526.24789.
3.8.5. 2-[(4-Nitrophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (15)
Prepared from 6 (100 mg, 0.24 mmol) and 1-iodo-4-nitrobenzene (89 mg, 0.36 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 95:5, Rf = 0.15) to give 15 (57 mg, 44%) as a yellow oil; = −88 (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.04 (s, 6H, 2×CH3Si), 0.08 (s, 3H, CH3Si), 0.09 (s, 3H, CH3Si), 0.87 (s, 9H, 3×CH3), 0.88 (s, 9H, 3×CH3), 3.44 (dd, 1H, J5′b,4′ = 7.6 Hz, J5′b,5′a = 10.7 Hz, 5′b-H), 3.63 (dd, 1H, J5′a,4′ = 4.4 Hz, J5′a,5′b = 10.7 Hz, 5′a-H), 3.87-3.90 (m, 1H, 4′-H), 4.39 (d, 1H, J3′,2′ = 1.4 Hz, 3′-H), 4.78 (dd, 1H, J2′,3′ = 1.4 Hz, J2′,1′ = 6.0 Hz, 2′-H), 5.99 (d, 1H, J1′,2′ = 6.0 Hz, 1′-H), 7.79 (d, 2H, J2,3 = J6,5 = 8.6 Hz, 2-H, 6-H), 8.20 (d, 2H, J3,2 = J5,6 = 8.6 Hz, 3-H, 5-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.7 (2 × CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3 × CH3), 26.1 (3 × CH3), 62.0 (C-5′), 76.8 (C-3′), 85.9 (C-4′), 91.3 (C-2′), 100.6 (C-1′), 124.3 (C-3, C-5), 134.3 (C-2, C-6), 135.9 (Cq), 148.3 (Cq), 166.7 (N=C-S) ppm. IR (NEAT): ν = 2953 (C-HAl), 1522 (N-O), 1343 (N-O), 1253, 1108, 1060, 1004, 816 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 541.5 [M+H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 541.22184; found: 541.22229. HRMS (ESI): m/z [M + Na]+ calcd. for C24H40N2NaO6SSi2: 563.20378; found: 563.20394.
3.8.6. 2-[(3-Nitrophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (16)
Prepared from 6 (90 mg, 0.21 mmol) and 1-iodo-3-nitrobenzene (80 mg, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.3) to give 16 (58 mg, 50%) as a white solid; mp 85−86 °C; = −74 (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.05 (s, 3H, CH3Si), 0.06 (s, 3H, CH3Si), 0.08 (s, 3H, CH3Si), 0.08 (s, 3H, CH3Si), 0.87 (s, 9H, 3 × CH3), 0.89 (s, 9H, 3 × CH3), 3.45 (dd, 1H, J5′b,4′ = 7.6 Hz, J5′b,5′a = 10.7 Hz, 5′b-H), 3.64 (dd, 1H, J5′a,4′ = 4.4 Hz, J5′a,5′b = 10.7 Hz, 5′a-H), 3.85-3.89 (m, 1H, 4′-H), 4.38 (d, 1H, J3′,2′ = 1.7 Hz, 3′-H), 4.78 (d, 1H, J2′,1′ = 6.0 Hz, 2′-H), 5.97 (d, 1H, J1′,2′ = 6.0 Hz, 1′-H), 7.57 (t, 1H, J = 8.0 Hz, 5-H), 7.91 (d, 1H, J6,5 = 7.8 Hz, 6-H), 8.24-8.26 (m, 1H, 4-H), 8.44 (s, 1H, 2-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.7 (2 × CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3 × CH3), 26.1 (3 × CH3), 62.1 (C-5′), 76.9 (C-3′), 85.9 (C-4′), 91.5 (C-2′), 100.6 (C-1′), 124.8 (C-4), 129.5 (C-2), 129.7 (Cq), 130.3 (C-5), 140.5 (C-6), 148.6 (Cq), 167.4 (N=C-S) ppm. IR (NEAT): ν = 2930 (C-HAl), 1533 (N-O), 1347 (N-O), 1255, 1114, 1061, 1000, 814 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 541.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 541.22184; found: 541.22241. HRMS (ESI): m/z [M + Na]+ calcd. for C24H40N2NaO6SSi2: 563.20378; found: 563.20412.
3.8.7. 2-[(2-Nitrophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (17)
Prepared from 6 (90 mg, 0.21 mmol) and 1-iodo-2-nitrobenzene (80 mg, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.18) to give 17 (83 mg, 72%) as a yellow solid; mp 111–113 °C; = −74 (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.03 (s, 6H, 2 × CH3Si), 0.08 (s, 3H, CH3Si), 0.09 (s, 3H, CH3Si), 0.86 (s, 9H, 3×CH3), 0.87 (s, 9H, 3×CH3), 3.42 (dd, 1H, J5′b,4′ = 7.9 Hz, J5′b,5′a = 10.7 Hz, 5′b-H), 3.62 (dd, 1H, J5′a,4′ = 4.6 Hz, J5′a,5′b = 10.7 Hz, 5′a-H), 3.87−3.91 (m, 1H, 4′-H), 4.37 (d, 1H, J3′,4′ = 1.6 Hz, 3′-H), 4.76 (dd, 1H, J2′,3′ = 1.2 Hz, J2′,1′ = 6.0 Hz, 2′-H), 6.01 (d, 1H, J1′’,2′ = 6.0 Hz, 1′-H), 7.48−7.52 (m, 1H, 4-H), 7.58−7.62 (m, 1H, 5-H), 8.02 (d, 2H, J = 8.0 Hz, H-3, 6-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.2 (CH3Si), -4.7 (2 × CH3Si), 18.2 (Cq), 18.5 (Cq), 25.9 (3 × CH3), 26.1 (3 × CH3), 62.3 (C-5′), 77.0 (C-3′), 86.2 (C-4′), 91.0 (C-2′), 100.8 (C-1′), 124.6 (Cq), 125.5 (C-3), 129.8 (C-4), 133.3 (C-5), 136.0 (C-6), 150.2 (Cq), 166.7 (N=C-S) ppm. IR (NEAT): ν = 2930 (C-HAl), 1525 (N-O), 1343 (N-O), 1254, 1109, 1064, 815 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 541.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 541.22184; found: 541.22238. HRMS (ESI): m/z [M + Na]+ calcd. for C24H40N2NaO6SSi2: 563.20378; found: 563.20400.
3.8.8. 2-[(2-Fluorophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-di-deoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (18)
Prepared from 6 (90 mg, 0.21 mmol) and 2-fluoroiodobenzene (0.04 mL, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 95:5, till 9:1, Rf = 0.30) to give 18 (24 mg, 22%) as a colorless oil; = −46 (c = 0.85, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.06 (s, 3H, CH3Si), 0.07 (s, 9H, 3×CH3Si), 0.86 (s, 9H, 3 × CH3), 0.90 (s, 9H, 3 × CH3), 3.41−3.46 (m, 1H, 5′b-H), 3.61 (dd, 1H, J5′a,4′ = 4.5 Hz, J5′a,5′b = 10.6 Hz, 5′a-H), 3.89−3.92 (m, 1H, 4′-H), 4.38 (s, 1H, 3′-H), 4.73 (d, 1H, J2′,1′ = 6.0 Hz, 2′-H), 5.98 (d, 1H, J1′,2′ = 6.0 Hz, 1′-H), 7.13−7.17 (m, 2H, 3-H, 6-H), 7.40–7.45 (m, 1H, 4-H), 7.54−7.57 (m, 1H, 5-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.2 (CH3Si), -4.7 (CH3Si), -4.6 (CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 62.3 (C-5′), 77.1 (C-3′), 86.4 (C-4′), 91.3 (C-2′), 101.1 (C-1′), 114.4 (d, 2JC-F = 18.4 Hz, C-1), 116.70 (d, 2JC-F = 22.5 Hz, C-3), 125.0 (d, 3JC-F = 3.9 Hz, C-6), 132.9 (d, 3JC-F = 8.1 Hz, C-4), 137.0 (C-5), 162.7 (d, 1JC-F = 251.0 Hz, C-2), 167.5 (N=C-S) ppm. 19F NMR (376 MHz, CDCl3): δ = -104.91 (s, F) ppm. IR (NEAT): ν = 2953, 2930 (C-HAl), 1606, 1475, 1255, 1107 (C-F), 1062, 1001, 815 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 514.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41FNO4SSi2: 514.22734; found: 514.22782. HRMS (ESI): m/z [M + Na]+ calcd. for C24H40 FNNaO4SSi2: 536.20928; found: 536.20943.
3.8.9. 2-[(2-Trifluoromethylphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethyl-silyl-1′,2′-dideoxy-β-D-arabinofuranoso)-[1,2-d]-oxazole (19)
Prepared from 6 (90 mg, 0.21 mmol) and o-iodotrifluoromethylbenzene (0.05 mL, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.27) to give 19 (40 mg, 33%) as a white solid; mp 98–100 °C; = −49 (c = 1.04, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.03 (s, 3H, CH3Si), 0.06 (s, 6H, 2x(CH3Si)), 0.07 (s, 3H, CH3Si), 0.85 (s, 9H, 3 × CH3), 0.89 (s, 9H, 3 × CH3), 3.41 (dd, 1H, J5′b,4′ = 8.5 Hz, J5′b, 5′a = 10.6 Hz, 5′b-H), 3.61 (dd, 1H, J5′a,4′ = 4.6 Hz, J5′a,5′b = 10.6 Hz, 5′a-H), 3.85–3.89 (m, 1H, 4′-H), 4.35 (br s, 1H, 3′-H), 4.73 (dd, 1H, J2′,3′ = 1.2 Hz, J2′,1′ = 6.0 Hz, 2′-H), 5.96 (d, 1H, J1′,2′ = 6.0 Hz, 1′-H), 7.51–7.58 (m, 2H, 4-H, 5-H), 7.74−7.77 (m, 2H, 3-H, 6-H) ppm. 13C NMR (100 MHz, CDCl3): δ = −5.3 (CH3Si), −5.2 (CH3Si), −4.7 (CH3Si), -4.7 (CH3Si), 18.2 (Cq), 18.6 (Cq), 25.9 (3×CH3), 26.1 (3×CH3), 62.3 (C-5′), 77.0 (C-3′), 86.2 (C-4′), 91.3 (C-2′), 100.9 (C-1′), 123.1 (q, 1JC-F = 273.9 Hz, CF3), 125.7 (C-1), 127.4 (q, 3JC-F = 5.3 Hz, C-3), 130.5 (C-4), 132.6 (C-5), 133.5 (q, 2JC-F = 30.2 Hz, C-2), 139.5 (C-6), 168.0 (N=C-S) ppm. 19F NMR (376 MHz, CDCl3): δ = −59.87 (s, 3F) ppm. IR (NEAT): ν = 2930 (C-HAl), 1605, 1312, 1254, 1164 (C-F), 1131, 1001, 812 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 564 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C25H41F3NO4SSi2: 564.22414; found: 564.22464. HRMS (ESI): m/z [M + Na]+ calcd. for C25H40 F3NNaO4SSi2: 586.20609; found: 586.20630.
3.8.10. 2-[(2-Nitrophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-α-D-xylofuranoso)-[1,2-d]-oxazole (20)
Prepared from 7 (90 mg, 0.21 mmol) and 1-iodo-2-nitrobenzene (80 mg, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.16) to give 20 (67 mg, 58%) as a yellow oil; = +56 (c = 0.57, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.04 (s, 3H, CH3Si), 0.05 (s, 3H, CH3Si), 0.09 (s, 3H, CH3Si), 0.11 (s, 3H, CH3Si), 0.87 (s, 9H, 3 × CH3), 0.88 (s, 9H, 3 × CH3), 3.68–3.72 (m, 1H, 4′-H), 3.76–3.84 (m, 2H, 5′-H), 4.25 (d, 1H, J3′,4′ = 3.1 Hz, 3′-H), 4.69 (d, 1H, J2′,1′ = 5.5 Hz, 2′-H), 6.06 (d, 1H, J1′,2′ = 5.5 Hz, 1′-H), 7.48–7.52 (m, 1H, 4-H), 7.59−7.62 (m, 1H, 5-H), 8.04 (d, 2H, J = 8.1 Hz, 3-H, 6-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.9 (CH3Si), -4.9 (CH3Si), 18.3 (Cq), 18.5 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 60.1 (C-5′), 75.0 (C-3′), 80.3 (C-4′), 88.9 (C-2′), 100.1 (C-1′), 124.7 (Cq), 125.7 (C-3), 129.8 (C-4), 133.4 (C-5), 136.0 (C-6), 150.2 (Cq), 166.7 (N=C-S) ppm. IR (NEAT): ν = 2930 (C-HAl), 1528 (N-O), 1346 (N-O), 1254, 1102, 1004, 814 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 541.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 541.22184; found: 541.22158. HRMS (ESI): m/z [M + Na]+ calcd. for C24H40N2NaO6SSi2: 563.20378; found: 563.20327.
3.8.11. 2-[(2-Methoxyphenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-α-D-xylofuranoso)-[1,2-d]-oxazole (21)
Prepared from 7 (90 mg, 0.21 mmol) and 1-iodo-2-nitrobenzene (80 mg, 0.32 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.17) to give 21 (50 mg, 45%) as a colorless oil; = +18 (c = 0.86, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 0.04 (s, 3H, CH3Si), 0.04 (s, 3H, CH3Si), 0.07 (s, 3H, CH3Si), 0.09 (s, 3H, CH3Si), 0.87 (s, 18H, 6 × CH3), 3.69−3.73 (m, 1H, 4′-H), 3.76−3.83 (m, 2H, 5′-H), 3.86 (s, 3H, OCH3), 4.21 (d, 1H, J3′,4′ =2.9 Hz, 3′-H), 4.63 (d, 1H, J2′,1′ = 5.4 Hz, 2′-H), 6.00 (d, 1H, J1′,2′ = 5.4 Hz, 1′-H), 6.92−6.97 (m, 2H, 3-H, 5-H), 7.39−7.41 (m, 1H, 4-H), 7.57 (dd, 1H, J = 1.6 Hz, J = 7.6 Hz, 6-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.9 (CH3Si), -4.6 (CH3Si), 18.3 (Cq), 18.5 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 56.2 (OCH3), 60.2 (C-5′), 75.1 (C-3′), 79.9 (C-4′), 88.7 (C-2′), 100.2 (C-1′), 111.9 (C-3), 115.4 (C-4), 121.4 (Cq), 132.1 (C-5), 136.6 (C-6), 159.6 (Cq), 168.5 (N=C-S) ppm. IR (NEAT): ν = 2930 (C-HAl), 1603, 1474, 1252, 1105, 1065, 1007, 812 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 526.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 526.24732; found: 526.24718.
3.8.12. 2-[(2-Nitrophenyl)sulfanyl]-4,5-dihydro(3′,5′-di-O-tert-butyldimethylsilyl-1′,2′-dideoxy-β-D-ribofuranoso)-[1,2-d]-oxazole (22)
Prepared from 8 (120 mg, 0.28 mmol) and 1-iodo-2-nitrobenzene (107 mg, 0.43 mmol), the obtained residue was purified by flash chromatography (eluent: petroleum ether/EtOAc, 9:1, Rf = 0.16) to give 22 (53 mg, 46%) as a yellowish solid. 1H NMR (400 MHz, CDCl3): δ = 0.04 (s, 3H, CH3Si), 0.04 (s, 3H, CH3Si), 0.08 (s, 3H, CH3Si), 0.11 (s, 3H, CH3Si), 0.87 (s, 9H, 3 × CH3), 0.88 (s, 9H, 3 × CH3), 3.67-3.70 (m, 1H, 4′-H), 3.75-3.83 (m, 2H, 5′-H), 4.26 (d, 1H, J = 3.2 Hz, 3′-H), 4.73 (d, 1H, J = 5.5 Hz, 2′-H), 6.03 (d, 1H, J = 5.5 Hz, 1′-H), 7.58 (t, 1H, J = 8.0 Hz, 5-H), 7.96 (d, 1H, J = 7.8 Hz 6-H), 8.24 (d, 1H, J = 7.7 Hz, 4-H), 8.46 (br s, 1H, 2-H) ppm. 13C NMR (100 MHz, CDCl3): δ = -5.2 (CH3Si), -5.1 (CH3Si), -4.9 (CH3Si), -4.6 (CH3Si), 18.3 (Cq), 18.5 (Cq), 25.9 (3 × CH3), 26.2 (3 × CH3), 60.2 (C-5′), 75.2 (C-3′), 80.3 (C-4′), 89.4 (C-2′), 100.0 (C-1′), 124.7 (CH), 129.4 (CH), 129.8 (Cq), 130.3 (CH), 140.5 (CH), 148.6 (Cq), 167.4 (N=C-S) ppm. IR (NEAT): ν = 2929 (C-HAl), 1532, 1341 (C-NO2), 1251, 1094, 1059, 810 (C-O, C-N, C=C, C=N) cm−1. MS (IS): m/z = 541.5 [M + H]+. HRMS (ESI): m/z [M + H]+ calcd. for C24H41N2O6SSi2: 541.22184; found: 541.22238.
4. Conclusions
In summary, our continuous efforts to study chiral 1,3-oxazolidine-2-thione anchored onto carbohydrate templates allowed us to describe the application of a copper-catalyzed carbon–sulfur bond formation. Despite a few yield limitations, this methodology provided an alternative chemical tool for 1,3-oxazolidine-2-thione functionalization, and to the best of our knowledge, a unique method to link aromatic rings onto chiral thioamide-derived heterocycles. The structures of all of the synthesized compounds were confirmed by detailed NMR spectroscopy and HRMS investigations. A further investigation to broaden the scope of the reactions and their selectivity to other templates as well as coupling reactions is currently being undertaken.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27175597/s1. Figure S1: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 4, Figure S2: HRMS (ESI) of compound 2, Figure S3: 1H NMR (400 MHz, DMSO-d6) and 13C NMR (100 MHz, DMSO-d6) spectrums of compound 5, Figure S4: HRMS (ESI) of compound 5, Figure S5: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 9, Figure S6: HRMS (ESI) of compound 9, Figure S7: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 10, Figure S8: HRMS (ESI) of compound 10, Figure S9: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 6, Figure S10: HRMS (ESI) of compound 6, Figure S11: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 7, Figure S12: HRMS (ESI) of compound 7, Figure S13: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 11, Figure S14: HRMS (ESI) of compound 11, Figure S15: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 12, Figure S16: HRMS (ESI) of compound 12, Figure S17: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 13, Figure S18: HRMS (ESI) of compound 13, Figure S19: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 14, Figure S20: HRMS (ESI) of compound 14, Figure S21: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 15, Figure S22: HRMS (ESI) of compound 15, Figure S23: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 16, Figure S24: HRMS (ESI) of compound 16, Figure S25: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 17, Figure S26: HRMS (ESI) of compound 17, Figure S27: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 18, Figure S28: HRMS (ESI) of compound 18, Figure S29: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 19, Figure S30: HRMS (ESI) of compound 19, Figure S31: 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectrums of compound 20, Figure S32: HRMS (ESI) of compound 20, Figure S33: 1H NMR (400 MHz, CDCl3) spectrum of compound 21, Figure S34: 13C NMR (100 MHz, CDCl3) spectrum of compound 21, Figure S35: HRMS (ESI) of compound 21, Figure S36: 1H NMR (400 MHz, CDCl3) spectrum of compound 22, Figure S37: 13C NMR (100 MHz, CDCl3) spectrum of compound 22, Figure S38: HRMS (ESI) of compound 22.
Author Contributions
Conceptualization, A.T. and A.Š.; methodology, J.R.; validation, V.K.; formal analysis, V.K.; investigation V.K.; data curation, J.R.; writing—original draft preparation, V.K., M.S.; writing—review and editing, M.S., J.R., A.Š. and A.T.; visualization V.K.; resources, A.T.; supervision, A.T and A.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the Doctoral Fund of Kaunas University of Technology No. A-410, approved 26 June 2019, and also the FEDER FSE(2014-2020) projects Mistic EX003242, ChemBio EX003677, Techsab-EX011313, the RTR Motivhealth (2019-00131403), and the Labex programs SYNORG (ANR-11-LABX-0029) and IRON (ANR-11-LABX-0018-01) for their financial support of ICOA, UMR 7311, University of Orléans, CNRS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Awan, S.I.; Werz, D.B. Syntheses of 1,2-annulated and 1-spiroannulated carbohydrate derivatives: Recent developments. Bioorg. Med. Chem. 2012, 20, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Girniene, J.; Tatibouet, A.; Sackus, A.; Yang, J.; Holman, G.D.; Rollin, P. Inhibition of the D-Fructose Transporter Protein GLUT5 by Fused-Ring Glyco-1,3-Oxazolidin-2-Thiones and -Oxazolidin-2-Ones. Carbohydr. Res. 2003, 338, 711–719. [Google Scholar] [CrossRef]
- Igual, M.O.; Nunes, P.S.G.; da Costa, R.M.; Mantoani, S.P.; Tostes, R.C.; Carvalho, I. Novel glucopyranoside C2-derived 1,2,3-triazoles displaying selective inhibition of O-GlcNAcase (OGA). Carbohydr. Res. 2019, 471, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Velueta-Viveros, M.; Martínez-Bailén, M.; Puerta, A.; Romero-Hernández, L.L.; Křen, V.; Merino-Montiel, P.; Montiel-Smith, S.; Fernandes, M.X.; Moreno-Vargas, A.J.; Padrón, J.M.; et al. Carbohydrate-derived bicyclic selenazolines as new dual inhibitors (cholinesterases/OGA) against Alzheimer’s disease. Bioorganic Chem. 2022, 127, 105983–105990. [Google Scholar] [CrossRef] [PubMed]
- Chennaiah, A.; Dubbu, S.; Parasuraman, K.; Vankar, Y.D. Stereoselective Synthesis of 1,2-Annulated Sugars Having Substituted Tetrahydropyran/(-furan) Scaffolds Using the PrinsReaction. Eur. J. Org. Chem. 2018, 2018, 6706–6713. [Google Scholar] [CrossRef]
- Verma, A.K.; Chennaiah, A.; Dubbu, S.; Vankar, Y.D. Palladium catalyzed synthesis of sugar-fused indolines via C(sp2)-H/N-H activation. Carbohydr. Res. 2019, 473, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Leibeling, M.; Milde, B.; Kratzert, D.; Stalke, D.; Werz, D.B. Intermolecular Twofold Carbopalladation/Cyclization Sequence to Access Chromans and Isochromans from Carbohydrates. Chem.—A Eur. J. 2011, 17, 9888–9892. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, S.; Li, H.; Zhang, Z.; Xu, T. Pd-Catalyzed Decarboxylative Cycloaddition of Vinylethylene Carbonates with Isothiocyanates. J. Org. Chem. 2020, 85, 8773–8779. [Google Scholar] [CrossRef]
- Vessally, E.; Mohammadi, R.; Hosseinian, A.; Didehban, K.; Edjlali, L. S-arylation of 2-mercaptobenzazoles: A comprehensive review. J. Sulfur Chem. 2018, 39, 443–463. [Google Scholar] [CrossRef]
- Crimmins, M.T.; King, B.W.; Tabet, E.A. Pav. Asymmetric Aldol Additions with Titanium Enolates of Acyloxazolidinethiones: Dependence of Selectivity on Amine Base and Lewis Acid Stoichiometry. J. Am. Chem. Soc. 1997, 119, 7883–7884. [Google Scholar] [CrossRef]
- Crimmins, M.T.; King, B.W.; Tabet, E.A.; Chaudhary, K. Asymmetric Aldol Additions: Use of Titanium Tetrachloride and (−)-Sparteine for the Soft Enolization of N-Acyl Oxazolidinones, Oxazolidinethiones, and Thiazolidinethione. J. Org. Chem. 2001, 66, 894–902. [Google Scholar] [CrossRef]
- Crimmins, M.T.; McDougall, P.J. Anti-Selective Aldol Reactions with Titanium Enolates of N-Glycolyloxazolidinethiones. Org. Lett. 2003, 5, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Carter, R.G.; Yokochi, A.F.T. Unified Synthesis of C19-C26 Subunits of Amphidinolides B1, B2, and B3 by Exploiting Unexpected Stereochemical Differences in Crimmins’ and Evans’ Aldol Reactions. J. Org. Chem. 2004, 69, 2569–2572. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T.; Shamszad, M. Highly Selective Acetate Aldol Additions Using Mesityl-Substituted Chiral Auxiliaries. Org. Lett. 2007, 9, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Olsen, C.E. Glucosinolate Structures in Evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Rollin, P.; Tatibouët, A. Glucosinolates: The Synthetic Approach. C. R. Chim. 2011, 14, 194–210. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mishra, A. Prospects and Problems of Dietary Glucosinolates in Animal Feeding. Adv. Dairy Res. 2017, 5, 1000180. [Google Scholar] [CrossRef]
- Rollin, P.; Tatibouet, A. Sulfur Metabolites in Brassicales: From Daily Vegetables to Thiofunctional Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 1130–1136. [Google Scholar] [CrossRef]
- Xie, Z.; Shi, Y.; Wang, Z.; Wang, R.; Li, Y. Biotransformation of Glucosinolates Epiprogoitrin and Progoitrin to (R)- and (S)-Goitrin in Radix Isatidis. J. Agric. Food Chem. 2011, 59, 12467–12472. [Google Scholar] [CrossRef]
- Ishikawa, S.; Maruyama, A.; Yamamoto, Y.; Hara, S. Extraction and Characterization of Glucosinolates and Isothiocyanates from Rape Seed Meal. J. Oleo Sci. 2014, 63, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Agerbirk, N.; Olsen, C.E.; Cipollini, D.; Orgaard, M.; Linde-Laursen, I.; Chew, F.S. Specific Glucosinolate Analysis Reveals Variable Levels of Epimeric Glucobarbarins, Dietary Precursors of 5-Phenyloxazolidine-2-Thiones, in Watercress Types with Contrasting Chromosome Numbers. J. Agric. Food Chem. 2014, 62, 9586–9596. [Google Scholar] [CrossRef]
- Seo, B.; Yun, J.; Lee, S.; Kim, M.; Hwang, K.; Kim, J.; Min, K.R.; Kim, Y.; Moon, D. Barbarin as a New Tyrosinase Inhibitor from Barbarea Orthocerus. Planta Med. 1999, 65, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Mithofer, A.; Boland, W. Plant Defense against Herbivores: Chemical Aspects. Annu Rev. Plant. Biol 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.B.; Agrahari, A.K.; Tiwari, V.K. One-pot synthesis of oxazolidine-2-thione and thiazolidine-2-thione from sugar azido-alcohols. Carbohydr. Res. 2017, 450, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.S.; Todorovska, M.M.; Zlatković, D.B.; Stojanović, N.M.; Randjelović, P.J. Two goitrogenic 1,3-oxazolidine-2-thione derivatives from Brassicales taxa: Challenging identification, occurrence and immunomodulatory effects. Food Chem. Toxicol. 2017, 110, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sansinenea, E. The synthetic versatility of oxazolidinethiones. J. Sulfur Chem. 2007, 28, 109–147. [Google Scholar] [CrossRef]
- Li, G.; Qian, X.; Cui, J.; Huang, Q.; Zhang, R.; Guan, H. Synthesis and Herbicidal Activity of Novel 3-Aminocarbonyl-2-oxazolidinethione Derivatives Containing a Substituted Pyridine Ring. J. Agric. Food Chem. 2006, 54, 125–129. [Google Scholar] [CrossRef]
- Morales-Nava, R.; Fernandez-Zertuche, M.; Ordonez, M. Microwave-Assisted Improved Synthesis of Oxazolidin-2-Ones, Oxazolidine-2-Thiones and Thiazolidine-2-Thione Chiral Auxiliaries. Molecules 2011, 16, 8803–8814. [Google Scholar] [CrossRef]
- Cano, I.; Gomez-Bengoa, E.; Landa, A.; Maestro, M.; Mielgo, A.; Olaizola, I.; Oiarbide, M.; Palomo, C. N-(Diazoacetyl)oxazolidin-2-thiones as Sulfur-Donor Reagents: Asymmetric Synthesis of Thiiranes from Aldehydes. Angew. Chem. Int. Ed. 2012, 51, 10856–10860. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Chen, W.-B.; Han, W.-Y.; Wu, Z.-J.; Zhang, X.-M.; Yuan, W.-C. Highly Efficient and Stereoselective Construction of Dispiro-[Oxazolidine-2-Thione]Bisoxindoles and Dispiro[Imidazolidine-2-Thione]Bisoxindoles. Org. Lett. 2012, 14, 490–493. [Google Scholar] [CrossRef]
- Munive, L.; Rivas, V.M.; Ortiz, A.; Olivo, H.F. Oxazolidine-2-Thiones and Thiazolidine-2-Thiones as Nucleophiles in Intermolecular Michael Additions. Org. Lett. 2012, 14, 3514–3517. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Qiao, C. Synthesis of Epigoitrin from (R)-(+)-4-Hydroxy-γ-Butyrolactone. J. Heterocycl. Chem. 2013, 50, 1290–1293. [Google Scholar] [CrossRef]
- Saygili, N.; Ozalp, M.; Yildirim, L.T. Synthesis, X-Ray Analysis, and Biological Activities of Novel Oxazolidinethiones. J. Heterocycl. Chem. 2014, 51, 1264–1269. [Google Scholar] [CrossRef]
- Simao, A.C.; Rousseau, J.; Silva, S.; Rauter, A.P.; Tatibouet, A.; Rollin, P. Thionocarbamates on Carbohydrate Scaffolds–from Synthesis to Bioactivity. Carbohydr. Chem. 2009, 35, 127–172. [Google Scholar] [CrossRef]
- Leconte, N.; Pellegatti, L.; Tatibouet, A.; Suzenet, F.; Rollin, P.; Guillaumet, G. Benzylsulfanyloxazolines in Palladium-Catalyzed Cross-Coupling Reactions: A Novel Approach to Chiral Oxazolines. Synthesis 2007, 857–864. [Google Scholar] [CrossRef]
- Silva, S.; Sylla, B.; Suzenet, F.; Tatibouet, A.; Rauter, A.P.; Rollin, P. Oxazolinethiones and Oxazolidinethiones for the First Copper-Catalyzed Desulfurative Cross-Coupling Reaction and First Sonogashira Applications. Org. Lett. 2008, 10, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Tardy, S.; Routier, S.; Suzenet, F.; Tatibouet, A.; Rauter, A.P.; Rollin, P. 1,3-Oxazoline- and 1,3-Oxazolidine-2-Thiones as Substrates in Direct Modified Stille and Suzuki Cross-Coupling. Tetrahedron Lett. 2008, 49, 5583–5586. [Google Scholar] [CrossRef]
- Domingues, M.; Jaszczyk, J.; Ismael, M.I.; Figueiredo, J.A.; Daniellou, R.; Lafite, P.; Schuler, M.; Tatibouet, A. Conformationally Restricted Oxazolidin-2-One Fused Bicyclic Iminosugars as Potential Glycosidase Inhibitors. Eur. J. Org. Chem. 2020, 2020, 6109–6126. [Google Scholar] [CrossRef]
- Shen, C.; Xia, H.; Yan, H.; Chen, X.; Ranjit, S.; Xie, X.; Tan, D.; Lee, R.; Yang, Y.; Xing, B.; et al. Concise, Efficient Synthesis of Sugar-Based Benzothiazoles through Chemoselective Intramolecular C-S Coupling. Chem. Sci. 2012, 3, 2388–2393. [Google Scholar] [CrossRef]
- Brachet, E.; Brion, J.-D.; Alami, M.; Messaoudi, S. Nickel-Catalyzed Arylation, Alkenylation, and Alkynylation of Unprotected Thioglycosides at Room Temperature. ChemEur. J. 2013, 19, 15276–15280. [Google Scholar] [CrossRef]
- Brachet, E.; Brion, J.-D.; Alami, M.; Messaoudi, S. Stereoselective Palladium-Catalyzed Alkenylation and Alkynylation of Thioglycosides. Adv. Synth. Catal. 2013, 355, 2627–2636. [Google Scholar] [CrossRef]
- Soeta, T.; Matsumoto, A.; Sakata, Y.; Ukaji, Y. Development of a One-Pot Synthetic Method for Multifunctional Oxazole Derivatives Using Isocyanide Dichloride. J. Org. Chem. 2017, 82, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-H.; Lu, Y.; Zhang, Q.; Lu, R.; Bao, L.-Q.; Shen, M.-H.; Xu, H.-D. Selective S-Arylation of 2-Oxazolidinethiones and Selective N-Arylation of 2-Benzoxazolinones/2-Benzimidazolinones. Org. Biomol. Chem. 2017, 15, 4058–4063. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Kriščiūnienė, V.; Matulevičiūtė, G.; Paliulis, O.; Rollin, P.; Šačkus, A. Conversion of 2-thioxo-2,3-dihydroquinazolin-4(1H)-ones to N(3)-unsubstituted 2-(het)arylquinazolin-4(3H)-ones by copper-mediated Pd-catalysed cross-coupling reactions. Heterocycles 2016, 93, 150–163. [Google Scholar] [CrossRef]
- Jha, M.; Enaohwo, O.; Guy, S. Yttrium Triflate-Catalyzed Efficient Chemoselective S-Benzylation of Indoline-2-Thiones Using Benzyl Alcohols. Tetrahedron Lett. 2011, 52, 684–687. [Google Scholar] [CrossRef]
- Gosselin, G.; Bergogne, M.C.; De Rudder, J.; De Clercq, E.; Imbach, J.L. Systematic Synthesis and Biological Evaluation of α- and β-D-Xylofuranosyl Nucleosides of the Five Naturally Occurring Bases in Nucleic Acids and Related Analogs. J. Med. Chem. 1986, 29, 203–213. [Google Scholar] [CrossRef]
- Girniene, J.; Gueyrard, D.; Tatibouet, A.; Sackus, A.; Rollin, P. Base-Modified Nucleosides from Carbohydrate Derived Oxazolidinethiones: A Five-Step Process. Tetrahedron Lett. 2001, 42, 2977–2980. [Google Scholar] [CrossRef]
- Tatibouet, A.; Lawrence, S.; Rollin, P.; Holman, G.D. Selective Formation of 1,3-Oxazolidine-2-Thiones on Ketohexose Templates. Synlett 2004, 11, 1945–1948. [Google Scholar] [CrossRef]
- Girniene, J.; Apremont, G.; Tatibouet, A.; Sackus, A.; Rollin, P. Small Libraries of Fused Quinazolinone-Sugars. Access to Quinazolinedione Nucleosides. Tetrahedron 2004, 60, 2609–2619. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, W.; Ma, D. L -Proline-Promoted CuI-Catalyzed C-S Bond Formation between Aryl Iodides and Thiols. Synthetic Commun. 2007, 37, 25–35. [Google Scholar] [CrossRef]
- Kwong, F.Y.; Klapars, A.; Buchwald, S.L. Copper-Catalyzed Coupling of Alkylamines and Aryl Iodides: An Efficient System Even in an Air Atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kederienė, V.; Jaglinskaitė, I.; Voznikaitė, P.; Rousseau, J.; Rollin, P.; Šačkus, A.; Tatibouët, A. Mild copper-catalyzed, l-proline-promoted cross-coupling of methyl 3-amino-1-benzothiophene-2-carboxylate. Molecules 2021, 26, 6822. [Google Scholar] [CrossRef] [PubMed]
- Surry, D.S.; Buchwald, S.L. Diamine Ligands in Copper-Catalyzed Reactions. Chem. Sci. 2010, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Klapars, A.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.-F.; Correa, A.; Carril, M.; Norrby, P.-O.; Bolm, C. Copper-Catalyzed Cross-Couplings with Part-per-Million Catalyst Loadings. Angew. Chem. Int. Ed. 2009, 48, 5691–5693. [Google Scholar] [CrossRef]
- Larsson, P.-F.; Bolm, C.; Norrby, P.-O. Kinetic Investigation of a Ligand-Accelerated Sub-Mol % Copper-Catalyzed C-N Cross-Coupling Reaction. Chem. Eur. J. 2010, 16, 13613–13616. [Google Scholar] [CrossRef]
- Sekar, R.; Srinivasan, M.; Marcelis, A.T.M.; Sambandam, A. S-arylation of mercaptobenzimidazoles using Cu(I) catalysts—experimental and theoretical observations. Tet. Lett. 2011, 52, 3347–3352. [Google Scholar] [CrossRef]
- Strieter, E.R.; Bhayana, B.; Buchwald, S.L. Mechanistic studies on the Copper-catalyzed N-arylation of amides. JACS. 2009, 131, 78–88. [Google Scholar] [CrossRef]
- Jones, G.O.; Liu, P.; Houk, K.N.; Buchwald, S.L. Computational Explorations of Mechanisms and Ligand-Directed Selectivities of Copper-Catalyzed Ullmann-Type Reactions. J. Am. Chem. Soc. 2010, 132, 6205–6213. [Google Scholar] [CrossRef]
- Tye, J.W.; Weng, Z.; Giri, R.; Hartwig, J.F. Copper(I) phenoxide complexes in the etherification of aryl halides. Angew. Chem. Int. Ed. 2010, 49, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Tye, J.W.; Weng, Z.; Jons, A.M.; Incarvito, C.D.; Hartwig, J.F. Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides. J. Am. Chem. Soc. 2008, 130, 9971–9983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-L.; Liu, L.; Fu, Y.; Guo, Q.-X. Theoretical study on Copper(I)-catalyzed cross-coupling between aryl halides and amides. Organometallics 2007, 26, 4546–4554. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).