Abstract
The pharmaceutical success of atorvastatin (ATV), a widely employed drug against the “bad” cholesterol (LDL) and cardiovascular diseases, traces back to its ability to scavenge free radicals. Unfortunately, information on its antioxidant properties is missing or unreliable. Here, we report detailed quantum chemical results for ATV and its ortho- and para-hydroxy metabolites (o-ATV, p-ATV) in the methanolic phase. They comprise global reactivity indices, bond order indices, and spin densities as well as all relevant enthalpies of reaction (bond dissociation BDE, ionization IP and electron attachment EA, proton detachment PDE and proton affinity PA, and electron transfer ETE). With these properties in hand, we can provide the first theoretical explanation of the experimental finding that, due to their free radical scavenging activity, ATV hydroxy metabolites rather than the parent ATV, have substantial inhibitory effect on LDL and the like. Surprisingly (because it is contrary to the most cases currently known), we unambiguously found that HAT (direct hydrogen atom transfer) rather than SPLET (sequential proton loss electron transfer) or SET-PT (stepwise electron transfer proton transfer) is the thermodynamically preferred pathway by which o-ATV and p-ATV in methanolic phase can scavenge DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals. From a quantum chemical perspective, the ATV’s species investigated are surprising because of the nontrivial correlations between bond dissociation energies, bond lengths, bond order indices and pertaining stretching frequencies, which do not fit the framework of naive chemical intuition.
1. Introduction
The highly radical scavenging active cholesterol-lowering drug atorvastatin (ATV) [1] is an outstanding success sale story [2]. It was patented in 1985 and approved by the Food and Drug Administration (FDA) in 1996 for medical use. Sold under the name of Lipitor by the world’s leading pharmaceutical company Pfizer, it received record high revenues of about 12.8 billion US dollars in 2006, still generated ten billion US dollars in the year of patent loss (2011) and nearly two billion US dollars in 2019. ATV, one of the most prescribed drugs in the US today, is mainly employed to prevent high risk for developing cardiovascular diseases and as treatment for abnormal lipid levels (dyslipidemia). ATV’s inhibition of the HMG-CoA (3 hydroxy-3-methylglutaryl coenzyme A) reductase is plausibly related to the high radical scavenging potency against lipoprotein oxidation.
ATV made the object of several theoretical investigations in the past [3,4]. Still, the antioxidant properties of ATV were only recently investigated from the quantum chemical perspective [5]. Unfortunately, as we drew attention recently [6], the only quantum chemical attempt of which we are aware [5] is plagued by severe flaws [6] (e.g., “prediction” of enormous, totally unrealistic O-H bond dissociation energies of eV), and this makes mandatory the effort (undertaken in the present paper) of properly reconsidering the antioxidant capacity of ATV and its ortho- and para-hydroxy metabolites in methanol. For the notoriously poor soluble ATV, this solvent is of special interest. ATV is freely soluble in methanol. In addition, antioxidant assays are mostly done in methanolic environment [5,7]. Along with quantities traditionally related to the antioxidant activity, the present study will also reports on the ATV global chemical reactivity indices, relevant bond data as well as spin densities of radical species generated by H-atom abstraction from ATV and related ortho- and para-hydroxylated derivatives (o-ATV, p-ATV, respectively).
Theoretical understanding of the differences between ATV and its ortho- and para-hydroxy metabolites, which is missing to date, is of paramount practical importance. A twenty four years old experimental study reported that atorvastatin ortho- and para-hydroxy metabolites (o-ATV and p-ATV, respectively) protect, e.g., LDL from oxidation, while the parent ATV does not [8]. Importantly for the results we are going to present in Section 3.5, the free radical scavenging activity of o-ATV and p-ATV was analyzed by the ubiquitous 1,1 diphenyl-2 picryl-hydrazyl (DPPH) assay in ref. [8]. Our study is able to provide the first theoretical explanation of this experimental finding.
2. Computational Details
The results reported below were obtained from quantum chemical calculations wherein all necessary steps (geometry optimizations, frequency calculations, and electronic energies) where conducted at the same DFT level of theory by running GAUSSIAN 16 [9] on the bwHPC platform [10]. In all cases investigated, we convinced ourselves that all frequencies are real. In all calculations we used 6-31+G(d,p) basis sets [11,12] and, unless otherwise specified (see Section 3.2 and Section 3.3), the hybrid B3LYP exchange correlation functional [13,14,15,16].
For comparative purposes, we also present results obtained by using the PBE0 [17] functional and Truhlar’s M062x [18,19] (see Section 3.2 and Section 3.3). Computations for open shell species were carried out using unrestricted spin methods (e.g., UB3LYP and UPBE0). In most radicals, employing the more computationally demanding quadratic convergence SCF methods was unavoidable. We convinced ourselves that spin contamination is not a severe issue. In all these calculations, we invariably found a value for the total spin after annihilation of the first spin contaminant, versus the exact value .
Still, to better check this aspect, for ATV’s cation and anion as well as for the ATV1H and ATV4H radicals (see Section 3.1 for the meaning of these acronyms) we also undertook the rare numerical effort (enormous for molecules with almost 80 atoms) of performing full restricted open shell (ROB3LYP) calculations; that is, not only single point calculations for electronic energy but also geometry optimization and (numerical) vibrational frequency calculations, and all these in solvent. Differences between UB3LYP and ROB3LYP were reasonably small (see Section 3.2 and Section 3.3), but they should make it clear that claims (so often formulated in the literature on antioxidation) of chemical accuracy (∼1 kcal/mol) at the B3LYP/6-31+G(d,p) are totally out of place. From experience with much smaller molecules and much simpler chemical structures (e.g., ref. [20]) we had to learn that achieving this accuracy for bond dissociation enthalpies and proton affinity (BDE and PA, quantities entering the discussion that follows) is often illusory even for extremely computationally demanding state-of-the-art compound model chemistries (CBS-QB3, CBS-APNO, G4, W1BD); see, e.g., Figure 10 of ref. [20]. DFT-calculations done by us and by others [21] revealed that, e.g., errors in ionization potential can amount up to 0.7 eV (16 kcal/mol) even when employing the functional B3LYP and the largest Pople basis set 6-311++G(3df,3pd).
Unless otherwise specified, the solvent (methanol) was accounted for within the polarized continuum model (PCM) [22] using the integral equation formalism (IEF) [23]. Although this is the “gold standard” for modeling solvents in the literature on free radical scavenging, one should be aware that this framework ignores specific solvation effects (hydrogen bonds). Because they may play an important role, e.g., in proton transfer reactions, theoretical estimates of PA may not be sufficiently accurate. While this makes comparison with experiment problematic, it should be a less critical issue when comparing among themselves PA values of various antioxidants in a given solvent (e.g., methanol). To better emphasize why we believe that solvent effects in the context of antioxidants deserve a more careful consideration, along with IEFPCM-based results, we also present results obtained in Truhlar’s SMD solvation model [24,25,26].
GABEDIT [27] was used to generate molecular geometries and spatial distributions from the GAUSSIAN output (*.log) files. To compute Wiberg bond order indices, we used the package NBO 6.0 [28] interfaced with GAUSSIAN 16. The reason why we use Wiberg bond order indices [29] rather than the heavily advertised Mayer bond order indices [30] was explained elsewhere [31]. All thermodynamic properties were calculated at K.
3. Results and Discussion
3.1. Molecular Geometries
Along with the neutral, cation and anion ATV—molecular formula C33H35FN2O5, IUPAC name (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid, CAS number 134523-00-5—and its metabolites ortho-hydroxy atorvastatin (o-ATV and para–hydroxyatorvastatin (p-ATV), we also investigated the radicals (e.g., ATVnH) generated by H-atom abstraction from their O−H and N−H groups as well as the anions ATVnH- of the latter. Here, labels the various positions of the H-atoms, as depicted in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5.
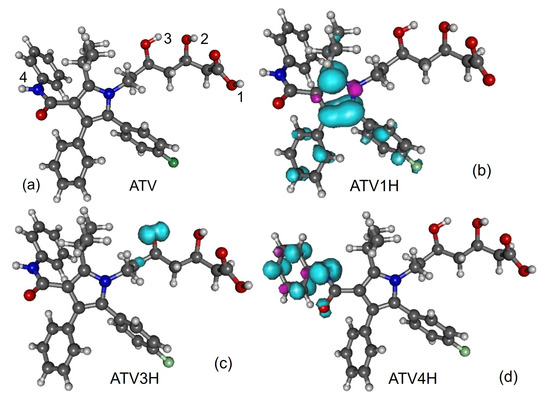
Figure 1.
(a) Optimized ATV geometry. Spin densities of neutral radicals generated from it by H-atom abstraction at positions indicated in the inset: (b) ATV1H, (c) ATV3H, and (d) ATV4H. Figure generated using GABEDIT [27].
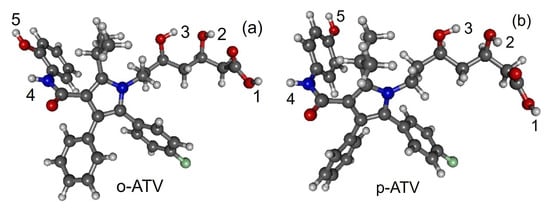
Figure 2.
Optimized geometries of atorvastatin ortho- and para-hydroxy metabolites: (a) o-ATV and (b) p-ATV. Figure generated using GABEDIT [27].
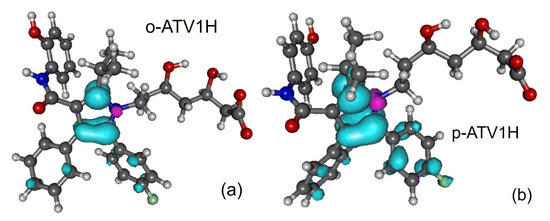
Figure 3.
Spin densities of radicals generated by H-atom abstraction at position 1-OH: (a) o-ATV1H and (b) p-ATV1H. Figure generated using GABEDIT [27].
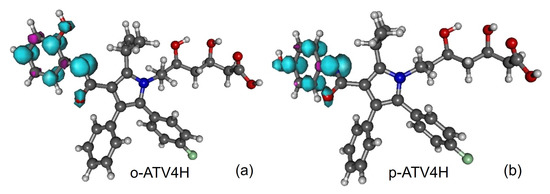
Figure 4.
Spin densities of radicals generated from atorvastatin ortho- and para-hydroxy metabolites by H-atom abstraction at position 4-NH: (a) o-ATV4H and (b) p-ATV4H. Figure generated using GABEDIT [27].
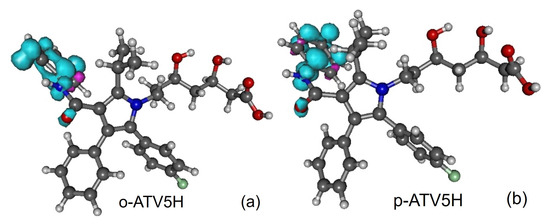
Figure 5.
Spin densities of radicals generated from atorvastatin ortho- and para-hydroxy metabolites by H-atom abstraction at position 5-OH: (a) o-ATV5H and (b) p-ATV5H. Figure generated using GABEDIT [27].
All quantities to be discussed below were calculated at the total electronic energy minima of the species listed above obtained via B3LYP/6-31+G(d,p)/IEFPCM optimization (cf. Section 2), which (with the grain of salt mentioned in Section 3.4) posed no special problems. Neither H-atom abstraction (Figure 1b–d) nor ortho- and para-O−H addition (Figure 2a,b) spectacularly modifies the molecular conformation (Figure 1a). Z-matrices for optimized geometries of representative species are presented in Table A1, Table A2, Table A3 and Table A4 and Figure 1, Figure 3, Figure 4 and Figure 5. Rather than Cartesian coordinates, we prefer to show Z-matrices because they facilitate comparison between various species and methods.
3.2. Chemical Reactivity Indices
The global chemical reactivity indices investigated in this work are listed below along with their expressions in terms of the ionization potential IP and electroaffinity EA [32,33,34,35,36]:
Here, is the fundamental (or transport) “HOMO-LUMO” gap [32,37,38]. Noteworthily, the values of IP and EA presented in this paper were calculated as enthalpy differences (cf. Equations (4a) and (6)). Estimating IP and EA using the eigenvalues of the Kohn-Sham (KS) orbitals with reversed sign (Koopmans theorem),
is unfortunately a very popular approximation, but it is totally inadequate especially in the presence of a solvent. For clarification, a comment is in order at this point.
Although both the approach using Equation (2) and the approach using Equations (4a) and (6) are based on the DFT, there is an important difference between them.
Equations (4a) and (6) rely on total energies computed via DFT. In these computations, the Kohn-Sham (KS) orbitals merely enter as eigenfunctions and eigenvalues of a mathematical (minimization) problem. They are auxiliary mathematical objects useful to compute a quantity with a clear physical meaning (namely, the total electronic energy).
However, it should be well known to any well rounded theoretician that the KS “orbitals” do not have any physical meaning; they are not real molecular orbitals [32,38,39]. What makes Equation (2) problematic is just the fact that it treats the KS-HOMO and KS-LUMO as if they were the true HOMO and LUMO of a real molecule.
To remedy the difficulty related to the KS “energies” (in reality, eigenvalues of a mathematical single-particle problem) in semiconductor physics, which translates into KS-band gaps typically amounting to about 50% of the real band gap, a so-called “scissor” operator procedure is applied [40,41], which consists of empirically shifting the KS eigenvalues. To eliminate this severe difficulty in the case of molecules immersed in solvents, we also proposed a scissor technique [42]. The important difference is that the scissor corrections proposed in ref. [42] are obtained from quantum chemical calculations rather than empirically as done in semiconductor band structure calculations.
Switching back, one may expect that the global chemical reactivity indices can give a flavor of the overall stability of a molecule and are useful in predicting how a certain chemical environment evolves in time [43,44]. In certain situations they turned out to be useful for comparing properties of different molecular species [33,45,46].
The presently calculated global chemical reactivity indices of ATV and its metabolites are collected in Table 1 and Table 2, and depicted in Figure 6. Having a chemical hardness of about 2 eV, ATV, o-ATV, and p-ATV exhibit a good chemical stability. This value lies between the values of the natural antioxidants phenol and trolox, for which our calculations at the same B3LYP/6-31+G(d,p)/IEFPCM level yielded eV and eV, respectively. For all three species, the electrophilic indices [33,45,46] are eV, a value exceeding the value of 1.50 eV, which is considered the threshold for strong electrophiles [47]. For comparison, let us again mention the values eV and eV computed by us for phenol and trolox, respectively.

Table 1.
Global chemical reactivity indices (eV) computed via B3LYP/6-31+G(d,p)/IEFPCM for atorvastatin and its ortho- and para-hydroxy metabolites and two natural oxidants in methanol.

Table 2.
Global chemical reactivity indices (eV) for ATV in methanol computed using 6-31+G(d,p) basis sets and the exchange-correlation functionals (B3LYP, PBE0, M062x) and solvent models (IEFPCM, SMD) specified above.
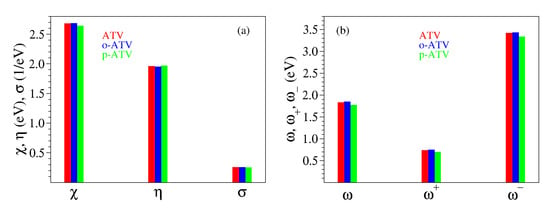
Figure 6.
Global chemical reactivity indices defined by Equation (1) for atorvastatin and its ortho- and para-hydroxy metabolites: (a) electronegativity , chemical hardness , and chemical softness ; (b) electrophilicity index , electroaccepting power , and electrodonating power .
Inspection of Table 1 reveals that, similarly to the quantities and considered above, all global chemical reactivity of ATV, o-ATV, and p-ATV are comparable to those of well known natural antioxidants. Could we then expect that ATV flavors (or other molecules) have indeed good antioxidant potency merely based on global chemical reactivity indices comparable to those of good antioxidants?
The analysis in the next section will unravel that, in fact, the global chemical indices have little relevance for assessing the antioxidant activity of a certain molecule. For the time being, let us remark that the values of Table 1 would rather suggest that ATV and o-ATV have similar antioxidant properties, and that ATV (possibly) performs (slightly) better than p-ATV.
3.3. Antioxidant Mechanisms and Pertaining Enthalpies of Reaction
As is widely discussed in the literature, an H-atom can be transferred to a free radical in one or two step processes. The three antioxidative mechanisms (HAT, SET-PT, and SPLET) and the corresponding reaction enthalpies (BDE, IP and PDE, PA and ETE, respectively) can be expressed as follows:
Direct hydrogen atom transfer (HAT) [48,49,50]
Stepwise electron transfer proton transfer (SET-PT) [51,52]
Sequential proton loss electron transfer (SPLET) [53,54]
In our specific case, X stands for an O or an N atom.
Related to the above (albeit not directly entering the aforementioned antioxidation mechanisms), the electron attachment process is quantified by the electroaffinity defined as
BDE, IP, PDE, PA, and ETE are enthalpies of reaction which can be obtained as adiabatic properties from standard -DFT prescriptions [38,55,56]. To this aim, along with the enthalpies of the various ATV-based species entering the above reactions, the enthalpies of the H-atom, proton and electron in methanol are also needed [6]. They are presented in Table 3.

Table 3.
Gas phase enthalpies and solvation enthalpies in hartree utilized in the present calculations. For the for the gas phase enthalpy of the H-atom we used the value for the B3LYP/6-31+G(d,p) electronic energy ( hartree) and the value of 1.4816 kcal/mol for thermal correction to enthalpy common for all compound model chemistries from GAUSSIAN 16.
The presently computed thermodynamic parameters are collected in Table 4 and Table 5, and depicted in Figure 7.

Table 4.
The enthalpies of reaction (in kcal/mol) needed to quantify the antioxidant activity of atorvastatin (ATV) and its ortho- and para-hydroxy metabolites (o-ATV, p-ATV).

Table 5.
Enthalpies of reaction (in kcal/mol) computed for atorvastatin (ATV) using methods indicated above and 6-31+G(d,p) basis sets. There is no difference between unrestricted (UB3LYP) and restricted open shell (ROB3LYP) methods in calculating the PA values, and for this reason the pertaining value was written in parenthesis.
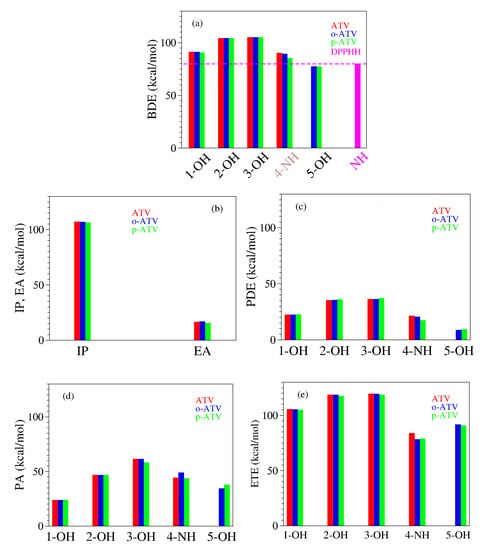
Figure 7.
Enthalpies of reaction quantifying the antioxidant activity of atorvastatin (ATV) and its ortho- (o-ATV) and para- (p-ATV) hydroxy metabolites: (a) bond dissociation; (b) ionization and electron attachment; (c) proton detachment; (d) proton affinity; (e) electron transfer. The additional information for the DPPH radical in panel (a) depicts why o-ATV and p-ATV can scavenge this radical while the parent ATV cannot.
Inspection of Table 4 and Figure 7 reveals that the additional 5-OH group has no notable impact on the O-H bond cleavage at positions 1-OH, 2-OH, and 3-OH, neither homolytic and heterolytic. BDE for H-atom abstraction at positions 1-OH, 2-OH, and 3-OH in ATV, o-ATV and p-ATV is basically the same. The differences between the values calculated by us for ATV, o-ATV, and p-ATV amounting to at most 0.5 kcal/mol are certainly irrelevant; recall that we showed recently [20] that even for much smaller molecules in vacuo DFT/B3LYP calculations with the largest Pople basis set 6-311++G(3df,3pd) are far away from “chemical” accuracy (∼1 kcal/mol). In fact, p-ATV’s numerical value of PA = kcal/mol somewhat differs from ATV’s (and o-ATV’s) PA = kcal/mol, but if heterolytic O-H bond cleavage were to occur in p-ATV, it would rather occur at position 1-OH, which has a substantially smaller value PA = kcal/mol.
With regards to position 4-NH, the extra (5-)OH-group has a qualitatively different impact on the N-H bond cleavage of o-ATV and p-ATV. Notwithstanding the different values calculated (90.2 kcal/mol versus 89.3 kcal/mol), in the above vein we cannot soberly claim that H-atom abstraction from the NH-group is facilitated by the additional OH-group of o-ATV. However, the negative impact on the heterolytic N-H bond dissociation is significant. The o-ATV’s PA = 49 kcal/mol is larger than the value PA = kcal/mol calculated for ATV. As of the heterolytic N-H bond dissociation, it is insensitively affected; the numerical difference between p-ATV’s PA = kcal/mol and ATV’s PA = kcal/mol obtained within B3LYP/6-31+G(d,p)/IEFPCM is too small to play a role in a sober analysis. Besides, similarly to what we said above, a heterolytic bond cleavage would occur at the lowest PA’s position 1-OH.
The really important effect brought about by the extra OH-group of the hydroxy metabolites is the homolytic bond dissociation at its position (5-OH). Our calculations demonstrate that this process is substantially less expensive energetically than H-atom donation from position 1-OH. The calculated BDE values for both o-ATV and p-ATV at this position are ∼77.5 kcal/mol versus the smallest value ∼91 kcal/mol for ATV at position 1-OH, respectively. Unlike the extremely similar homolytic bond dissociation, there is a certain difference between o-ATV’s and p-ATV’s heterolytic bond dissociation at position 5-OH, as expressed by the PA values (PA = kcal/mol ≠ PA = kcal/mol, respectively). However, it is unlikely that this difference in PA’s has practical consequences, again because the aforementioned values of PA are both comfortably larger than the lowest PA = kcal/mol at position 1-OH, a value that also characterizes the parent ATV molecule.
In Section 3.5 we will return to the practical implications of the above finding.
3.4. Alternative Approaches to the O-H and N-H Bond Strengths: Vibrational Frequencies and Bond Order Indices
Let us start this section with a short digression. The robustness of a single molecule diode fabricated using the scanning transmission microscopy (STM) break-junction technique [61,62] can be quantified by the maximum force that the junction subject to mechanical stretching can withstand. This rupture (pull-off) force F per molecule, which characterizes the strength of the chemical bond between electrodes and the terminal (anchoring) atom of the embedded molecule, can hardly be directly measured. To circumvent this difficulty, experimentalists use a simple mechanical model which relates F to the vibrational frequency of the pertaining stretching mode . The latter quantity can be easily measured by infrared spectroscopy [63]. To exemplify, this is the Au−S stretching mode in benchmark nanojunctions wherein molecules are anchored via thiol groups on gold electrodes.
Transposed to the present context, it is interesting to interrogate the relationship between BDE and the related stretching frequency. In the same vein, a stronger chemical X-Y bond is intuitively expected to have not only a larger BDE and a higher stretching frequency but also a shorter length and a larger bond order index.
With these in mind, let us examine the correlation of the aforementioned quantities in the presently considered molecules.
Infrared spectra calculated for ATV, o-OH-AVT, and p-ATV in methanol are depicted in Figure 8.
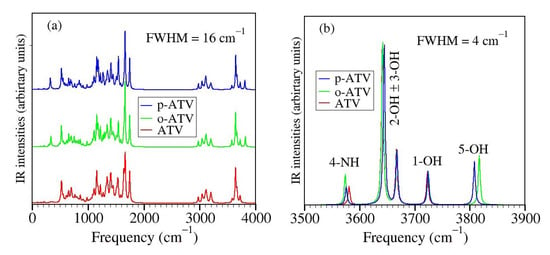
Figure 8.
Infrared spectra calculated for ATV, o-OH-AVT, and p-ATV in methanol using Lorentzian convolution of full width at half maximum (FWHM) indicated in the inset: (a) in the whole range of frequency and (b) in the range where the O-H and N-H stretching modes are active. In all species, stretching modes of 2-OH and 3-OH groups appear as linear and antilinear vibrations rather than separated vibrational modes, and this may indicate that a more adequate optimization of the radicals generated by H-atom abstraction at these positions (which appear almost degenerate energetically, see pertaining BDE values in Table 4) should be done within a multi-reference framework.
The behavior visible in Figure 8b is surprising for several reasons, e.g.,
- (i)
- although the BDE of ATV and its metabolites at position 1-OH is lower than at positions 2-OH and 3-OH, the streching mode at position 1-OH has a higher frequency than at positions 2-OH and 3-OH;
- (ii)
- although o-ATV and p-ATV have at position 5-OH a smaller BDE than for all OH-positions of the parent ATV, the 5-OH stretching mode of the metabolites is higher than those of all O-H streching mode of ATV;
- (iii)
- although o-ATV’s and ATV’s N-H BDE are equal, the frequency of the N-H of the former is smaller than that of the latter;
- (iv)
- although o-ATV’s BDE and p-ATV’s BDE are different, their N-H streching modes have the same frequency;
- (v)
- although o-ATV and p-ATV have equal BDE at position 5-OH, the o-ATV’s O-H streching frequency is higher than that of p-ATV.
Counter-intuitive aspects of the relationship BDE versus are visualized in Figure 9a and Figure 10a.

Figure 9.
Results for OH groups of atorvastatin (ATV) and its metabolites o-ATV and p-ATV: (a) bond dissociation energies versus O-H stretching frequencies; (b) bond dissociation energies versus Wiberg bond order indices; (c) Wiberg bond order indices versus bond lengths.

Figure 10.
Results similar to Figure 9 but for NH groups: (a) bond dissociation energies versus N-H stretching frequencies; (b) bond dissociation energies versus Wiberg bond order indices; (c) Wiberg bond order indices versus bond lengths.
Let us now switch to bond order indices. Our results are collected in Table 6 and Figure 9 and Figure 10.

Table 6.
Wiberg bond order indices, bond lengths (in Å), vibrational frequencies (in cm), and bond dissociation energies BDE (in kcal/mol) for atorvastatin and its metabolites.
To reiterate, based on straightforward chemical intuition, it would be obvious to expect that stronger chemical bonds (larger BDE’s) possess larger bond order indices. Figure 9b depicts that for the O-H bonds of ATV, o-ATV, and p-ATV just the opposite holds true: larger BDE’s justly correspond to smaller bond order indices. As for their N-H bonds, Figure 9b reveals that the dependence is even nonmonotonic.
To avoid misunderstanding, a clarification is in order before ending this analysis. What chemical intuition in the above example should not overlook is that a pair of atoms X and Y forming an X-Y chemical bond, do not merely interact with each other but also with the neighboring atoms in the molecular surrounding. This is also why a simple (exponential [64]) relationship between bond order indices and bond lengths can hold, e.g., for homologous molecular series [65], but cannot not hold in general; otherwise one arrives at comparing apples with oranges. Figure 9 and Figure 10 illustrate this again using the values of Table 6. BDE values corresponding to different O-H bonds of a given molecule differ from each other depending on the specific chemical environment. These differences can be visualized by inspecting the spin density landscape of the various radicals (Figure 1, Figure 3, Figure 4 and Figure 5). The stronger the delocalization in a radical, the easier is its formation, and the lower is the corresponding BDE value. Inspection of Figure 1b,c makes it clear, e.g., why ATV’s BDE at position 3-OH is higher than that at position 1-OH.
3.5. Assessing the Radical Scavenging Activity—A Specific Example
Discussion on free radical scavenging and dominant antioxidant mechanism is very often couched by comparing among themselves values the enthalpies characterizing the HAT, SET-PL, and SPLET of the specific antioxidant(s) under investigation. Every now and then publications conclude, e.g., that SPLET is the dominant pathway because a certain antioxidant has a “small” PA value or a PA substantially smaller than BDE, or that SET-PL prevails because of the small IP value. However, it is worth emphasizing that, along with the antioxidant’s properties, a proper evaluation of the antioxidant activity should mandatory consider the specific properties of the radicals to be eliminated (neutralized).
The small value BDE kcal/mol for o-ATV and p-ATV, substantially smaller than the smallest value (BDE = 90.2 kcal/mol) of the parent ATV, is perhaps the most appealing result reported in Section 3.3. Still, the “small” value mentioned above does not demonstrate per se the fact anticipated in Introduction, namely that o-ATV and p-ATV can scavenge can scavenge the ubiquitously employed 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, while the parent ATV cannot.
To demonstrate this, one should mandatory consider the pertaining DPPH property, namely the enthalpy release in DPPH’s neutralization (H-atom affinity)
Because it amounts to 80 kcal/mol [66], e.g., the reaction
is exothermic. H-atom abstraction from position 5-OH of o-ATV (or p-ATV) costs ∼77.5 kcal/mol, a value lower that the enthalpy release of 80 kcal/mol [66] in the neutralization of the DPPH radical, and this makes the HAT mechanism thermodynamically allowed. Rephrasing, because the BDE of the N−H bond of DPPHH is 80 kcal/mol [66], o-ATV (and p-ATV) can scavenge the DPPH radical through donating the H-atom at position 5-OH. On the contrary, the parent ATV cannot. The lowest ATV’s BDE (at position 1-OH) amounts to 90.2 kcal/mol (Table 4), so the HAT pathway is forbidden.
To conclude, we have presented above the first theoretical explanation of the experimental fact [8] that the antioxidant properties of atorvastatin ortho- and para-hydroxy metabolites differ from those of ATV.
By and large, there is a consensus in the literature that HAT is a possible (or even preferred) antioxidant mechanism in the gases phase but not in polar protic solvents like the presently considered methanol. In this vein, the natural question that arises is: can o-ATV and p-ATV scavenge the DPPH radical in methanol also via SPLET? Can HAT and SPLET coexist? While the large IP (Table 4) give little chances to an SET-PT pathway, SPLET would a priori be conceivable in view of the “small” value of PA, which is, although not smaller than that of ascorbic acid (as incorrectly [6] claimed in ref. [5]) at least not much larger than the latter (23.8 kcal/mol for ATV’s versus 20.5 kcal/mol for ascorbic acid, see ref. [6]).
In fact, Table 4 implicitly gives the negative answer to this question. If o-ATV and p-ATV could scavenge DPPH via SPLET, then (contrary to experiment [8]) the parent ATV could also do the job; the most favored deprotonation, implying the same enthalpy PA = 23.8 kcal/mol, occurs both for ATV and its metabolites at the same 1-OH position, where furthermore the similar spin density landscapes (compare Figure 1b with Figure 3) indicate a similar chemical reactivity.
Still, let us remain in the realm of theory and demonstrate why neither o-ATV nor p-ATV or ATV can scavenge DPPH in methanol via SPLET. To this aim suffice it to consider the first step of SPLET
where x means “o-”, “p-”, or “nothing”. Straightforward manipulation allows to express the enthalpy of this reaction as follows
Notice that the second brace in Equation (10) corresponds to the proton abstraction from the cation of the neutralized free radical DPPHH, or alternatively, the PDE pertaining to the neutralized free radical DPPHH (cf. Equation (4b)).
Equation (10) reveals that, to be thermodynamically allowed, the first SPLET step requires
Our calculations yielded kcal/mol, a value that is not larger (as the case if the first SPLET step was allowed) but smaller than kcal/mol. It now becomes clear why neither ATV, nor o-ATV or p-ATV can scavenge the DPPH radical via SPLET. Their “small” PA is not small enough to fulfill Equation (11).
4. Conclusions
We believe that the present demonstration that atorvastatin ortho- and para-hydroxy metabolites can scavenge the DPPH through donating the H-atom at the position of their extra group (5-OH), which is impossible in the parent ATV, is important not only because it theoretically explains for the first time a behavior revealed in experiment [8] but also because, from a general perspective, it provides further insight into the structure–activity relationship (SAR).
By working out a specific example (Section 3.5)—an analysis that can be straightforwardly extended to other cases—, we drew attention that an adequate approach to antioxidant’s potency should mandatory account for the thermodynamic properties of the free radicals. Equation (11) expresses a general necessary condition for thermodynamically allowed SPLET, and its application to specific cases may reveal that, even in polar solvents, free radical scavenging via this pathway is forbidden not only for ATV-based species.
In addition, our study emphasize that, while important, e.g., for modeling the temporal evolution of various molecular species interacting among themselves in a given chemical environment [65,67], the global chemical reactivity indices have no direct relevance for antioxidation. Recall that we saw in Section 3.2 that quantitative differences of ATV’s o-ATV’s, and p-ATV’s global chemical reactivity indices are minor. Furthermore, if qualitative differences in these indices were important, then, contrary to Section 3.3 and Section 3.5, o-ATV would have antioxidant properties similar to ATV rather than to p-ATV.
Last but not least, from the perspective of fundamental science, we found (Section 3.4) that properties like bond dissociation enthalpy, bond order index, bond length, and bond stretching frequency, expected after all to represent alternatives in quantifying the bond strength, are by no means correlated according to naive intuition. This finding calls for further quantum chemical efforts aiming at comprehensively characterizing ATV’s, that inherently remained beyond the scope of this study focused on ATV’s antioxidant activity. Finally, the presently reported counter-intutitve relationship between bond stretching frequency and bond strength should also be a word of caution for other communities; for example, for the molecular electronics community, wherein bond stretching frequencies (conveniently obtained via infrared spectroscopy) are used to estimate (pull-off) forces that cause the rupture of a junction subject to mechanical stretching [68].
Funding
In the initial stage, this research was funded by the German Research Foundation (DFG grant BA 1799/3-2). Computational support from the state of Baden-Württemberg through bwHPC and the German Research Foundation through Grant No. INST 40/575-1 FUGG (bwUniCluster 2.0, bwForCluster/MLS&WISO 2.0, and JUSTUS 2.0 cluster) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the author upon reasonable request.
Acknowledgments
The author is much indebted to Ederley Vélez Ortiz for providing valuable details related to her recent work [5].
Conflicts of Interest
No conflict of interest to declare.
Appendix A

Table A1.
Z−matrix of ATV.
Table A1.
Z−matrix of ATV.
| Atom | |||||||
|---|---|---|---|---|---|---|---|
| F | |||||||
| O | 1 | B1 | |||||
| O | 1 | B2 | 2 | A1 | |||
| N | 3 | B3 | 1 | A2 | 2 | D1 | 0 |
| C | 2 | B4 | 1 | A3 | 4 | D2 | 0 |
| C | 3 | B5 | 1 | A4 | 4 | D3 | 0 |
| C | 4 | B6 | 3 | A5 | 1 | D4 | 0 |
| C | 4 | B7 | 3 | A6 | 1 | D5 | 0 |
| C | 8 | B8 | 4 | A7 | 3 | D6 | 0 |
| C | 6 | B9 | 3 | A8 | 1 | D7 | 0 |
| C | 7 | B10 | 4 | A9 | 3 | D8 | 0 |
| C | 8 | B11 | 4 | A10 | 3 | D9 | 0 |
| C | 8 | B12 | 4 | A11 | 3 | D10 | 0 |
| C | 12 | B13 | 8 | A12 | 4 | D11 | 0 |
| C | 7 | B14 | 4 | A13 | 3 | D12 | 0 |
| H | 15 | B15 | 7 | A14 | 4 | D13 | 0 |
| H | 15 | B16 | 7 | A15 | 4 | D14 | 0 |
| H | 15 | B17 | 7 | A16 | 4 | D15 | 0 |
| C | 10 | B18 | 6 | A17 | 3 | D16 | 0 |
| H | 19 | B19 | 10 | A18 | 6 | D17 | 0 |
| C | 19 | B20 | 10 | A19 | 6 | D18 | 0 |
| H | 21 | B21 | 19 | A20 | 10 | D19 | 0 |
| C | 21 | B22 | 19 | A21 | 10 | D20 | 0 |
| H | 23 | B23 | 21 | A22 | 19 | D21 | 0 |
| C | 23 | B24 | 21 | A23 | 19 | D22 | 0 |
| H | 25 | B25 | 23 | A24 | 21 | D23 | 0 |
| C | 25 | B26 | 23 | A25 | 21 | D24 | 0 |
| H | 27 | B27 | 25 | A26 | 23 | D25 | 0 |
| C | 14 | B28 | 12 | A27 | 8 | D26 | 0 |
| H | 29 | B29 | 14 | A28 | 12 | D27 | 0 |
| C | 29 | B30 | 14 | A29 | 12 | D28 | 0 |
| H | 31 | B31 | 29 | A30 | 14 | D29 | 0 |
| C | 31 | B32 | 29 | A31 | 14 | D30 | 0 |
| H | 33 | B33 | 31 | A32 | 29 | D31 | 0 |
| C | 33 | B34 | 31 | A33 | 29 | D32 | 0 |
| H | 35 | B35 | 33 | A34 | 31 | D33 | 0 |
| C | 35 | B36 | 33 | A35 | 31 | D34 | 0 |
| H | 37 | B37 | 35 | A36 | 33 | D35 | 0 |
| C | 13 | B38 | 8 | A37 | 4 | D36 | 0 |
| H | 39 | B39 | 13 | A38 | 8 | D37 | 0 |
| C | 9 | B40 | 8 | A39 | 4 | D38 | 0 |
| H | 41 | B41 | 9 | A40 | 8 | D39 | 0 |
| C | 9 | B42 | 8 | A41 | 4 | D40 | 0 |
| H | 43 | B43 | 9 | A42 | 8 | D41 | 0 |
| C | 43 | B44 | 9 | A43 | 8 | D42 | 0 |
| H | 45 | B45 | 43 | A44 | 9 | D43 | 0 |
| C | 4 | B46 | 3 | A45 | 1 | D44 | 0 |
| H | 47 | B47 | 4 | A46 | 3 | D45 | 0 |
| H | 47 | B48 | 4 | A47 | 3 | D46 | 0 |
| C | 47 | B49 | 4 | A48 | 3 | D47 | 0 |
| H | 50 | B50 | 47 | A49 | 4 | D48 | 0 |
| H | 50 | B51 | 47 | A50 | 4 | D49 | 0 |
| C | 5 | B52 | 2 | A51 | 1 | D50 | 0 |
| H | 53 | B53 | 5 | A52 | 2 | D51 | 0 |
| H | 53 | B54 | 5 | A53 | 2 | D52 | 0 |
| O | 50 | B55 | 47 | A54 | 4 | D53 | 0 |
| H | 56 | B56 | 50 | A55 | 47 | D54 | 0 |
| O | 53 | B57 | 5 | A56 | 2 | D55 | 0 |
| H | 58 | B58 | 53 | A57 | 5 | D56 | 0 |
| O | 5 | B59 | 2 | A58 | 1 | D57 | 0 |
| H | 60 | B60 | 5 | A59 | 2 | D58 | 0 |
| N | 6 | B61 | 3 | A60 | 1 | D59 | 0 |
| H | 62 | B62 | 6 | A61 | 3 | D60 | 0 |
| C | 7 | B63 | 4 | A62 | 3 | D61 | 0 |
| H | 64 | B64 | 7 | A63 | 4 | D62 | 0 |
| C | 64 | B65 | 7 | A64 | 4 | D63 | 0 |
| H | 66 | B66 | 64 | A65 | 7 | D64 | 0 |
| H | 66 | B67 | 64 | A66 | 7 | D65 | 0 |
| H | 66 | B68 | 64 | A67 | 7 | D66 | 0 |
| C | 47 | B69 | 4 | A68 | 3 | D67 | 0 |
| H | 70 | B70 | 47 | A69 | 4 | D68 | 0 |
| H | 70 | B71 | 47 | A70 | 4 | D69 | 0 |
| C | 56 | B72 | 50 | A71 | 47 | D70 | 0 |
| H | 73 | B73 | 56 | A72 | 50 | D71 | 0 |
| C | 58 | B74 | 53 | A73 | 5 | D72 | 0 |
| H | 75 | B75 | 58 | A74 | 53 | D73 | 0 |

Table A2.
Elements of the Z−matrix of ATV in methanol optimized as indicated below using 6-31+G/d,p) basis sets.
Table A2.
Elements of the Z−matrix of ATV in methanol optimized as indicated below using 6-31+G/d,p) basis sets.
| Element | RB3LYP | RPBE0 |
|---|---|---|
| B1 | 7.63688039 | 7.57842858 |
| B2 | 9.51376438 | 9.49797976 |
| B3 | 4.46432846 | 4.45735736 |
| B4 | 1.22436588 | 1.22105960 |
| B5 | 1.24094609 | 1.23460721 |
| B6 | 1.38313941 | 1.37429179 |
| B7 | 1.39258446 | 1.38313248 |
| B8 | 4.26421886 | 4.25026562 |
| B9 | 2.55737607 | 2.53336720 |
| B10 | 1.39352326 | 1.38966003 |
| B11 | 1.38854352 | 1.38531627 |
| B12 | 1.48198028 | 1.47533742 |
| B13 | 1.47996148 | 1.47276739 |
| B14 | 2.54735210 | 2.53021178 |
| B15 | 1.09461863 | 1.09441258 |
| B16 | 1.09443324 | 1.09451231 |
| B17 | 1.09548377 | 1.09521443 |
| B18 | 1.40411033 | 1.39956240 |
| B19 | 1.08656542 | 1.08702976 |
| B20 | 1.39590982 | 1.39231014 |
| B21 | 1.08596263 | 1.08625533 |
| B22 | 1.39827379 | 1.39423877 |
| B23 | 1.08557774 | 1.08587383 |
| B24 | 1.39829551 | 1.39470047 |
| B25 | 1.08613361 | 1.08644255 |
| B26 | 1.39684075 | 1.39270470 |
| B27 | 1.08266424 | 1.08414373 |
| B28 | 1.40754480 | 1.40342299 |
| B29 | 1.08542085 | 1.08622867 |
| B30 | 1.39703298 | 1.39303810 |
| B31 | 1.08651573 | 1.08675442 |
| B32 | 1.39866698 | 1.39488556 |
| B33 | 1.08611015 | 1.08634947 |
| B34 | 1.39859436 | 1.39482202 |
| B35 | 1.08646176 | 1.08669302 |
| B36 | 1.39688653 | 1.39286539 |
| B37 | 1.08594257 | 1.08685598 |
| B38 | 1.40608245 | 1.40185966 |
| B39 | 1.08526008 | 1.08604603 |
| B40 | 1.38854404 | 1.38594990 |
| B41 | 1.08466653 | 1.08507152 |
| B42 | 1.38886026 | 1.38604194 |
| B43 | 1.08464963 | 1.08507327 |
| B44 | 1.39673177 | 1.39264953 |
| B45 | 1.08575599 | 1.08651262 |
| B46 | 1.47041402 | 1.45879308 |
| B47 | 1.09044919 | 1.09154067 |
| B48 | 1.08676249 | 1.08820032 |
| B49 | 3.14830814 | 3.11381339 |
| B50 | 1.09708441 | 1.09727967 |
| B51 | 1.09665251 | 1.09755904 |
| B52 | 1.51042629 | 1.50242427 |
| B53 | 1.09777914 | 1.09781828 |
| B54 | 1.09295806 | 1.09309313 |
| B55 | 2.46095421 | 2.44275249 |
| B56 | 0.97500252 | 0.97301949 |
| B57 | 2.45826509 | 2.43975864 |
| B58 | 0.97546854 | 0.97377704 |
| B59 | 1.34162105 | 1.33146502 |
| B60 | 0.97475723 | 0.97138697 |
| B61 | 1.37559401 | 1.36892180 |
| B62 | 1.01471448 | 1.01312538 |
| B63 | 1.51639092 | 1.50803841 |
| B64 | 1.09438086 | 1.09568883 |
| B65 | 1.54383200 | 1.53441635 |
| B66 | 1.09274556 | 1.09354200 |
| B67 | 1.09394603 | 1.09430081 |
| B68 | 1.09550804 | 1.09534859 |
| B69 | 1.53735984 | 1.52751574 |
| B70 | 1.09529399 | 1.09601347 |
| B71 | 1.09519081 | 1.09581652 |
| B72 | 1.43702671 | 1.42351134 |
| B73 | 1.10229127 | 1.10342439 |
| B74 | 1.44374367 | 1.43034604 |
| B75 | 1.09511016 | 1.09602489 |
| A1 | 80.07624165 | 79.42212905 |
| A2 | 36.81921669 | 36.41767374 |
| A3 | 44.89960818 | 44.80116201 |
| A4 | 69.40323071 | 68.51578435 |
| A5 | 38.40240012 | 38.36009230 |
| A6 | 74.54408034 | 74.60701401 |
| A7 | 124.00299971 | 123.75578641 |
| A8 | 142.06293646 | 143.13044343 |
| A9 | 107.05456306 | 107.03652983 |
| A10 | 108.31353197 | 108.33842834 |
| A11 | 122.99231254 | 122.86893832 |
| A12 | 126.63463731 | 126.55972259 |
| A13 | 106.44925702 | 106.31547335 |
| A14 | 89.06053861 | 89.10098490 |
| A15 | 97.89435637 | 97.80052634 |
| A16 | 142.49393445 | 142.62052327 |
| A17 | 137.34771594 | 137.15532746 |
| A18 | 119.50192439 | 119.48359463 |
| A19 | 120.38662171 | 120.33363957 |
| A20 | 119.38771650 | 119.39817314 |
| A21 | 120.33528025 | 120.33582946 |
| A22 | 120.40428227 | 120.38589644 |
| A23 | 119.22951772 | 119.27084737 |
| A24 | 120.07799529 | 120.08000208 |
| A25 | 120.85846463 | 120.80411660 |
| A26 | 119.79850536 | 119.90398307 |
| A27 | 121.00661427 | 120.96513207 |
| A28 | 119.36095172 | 119.32269019 |
| A29 | 121.04376065 | 120.97477881 |
| A30 | 119.57781238 | 119.59079132 |
| A31 | 120.32876554 | 120.32506969 |
| A32 | 120.35906970 | 120.34690449 |
| A33 | 119.30913374 | 119.33935667 |
| A34 | 120.13215959 | 120.12543035 |
| A35 | 120.27437657 | 120.26315929 |
| A36 | 119.21937981 | 119.25183302 |
| A37 | 120.20314915 | 120.00645252 |
| A38 | 119.42757095 | 119.34799906 |
| A39 | 61.14506295 | 61.02049583 |
| A40 | 120.29133823 | 120.18802069 |
| A41 | 61.56595278 | 61.54130326 |
| A42 | 120.28619011 | 120.18989970 |
| A43 | 118.21356561 | 118.29730405 |
| A44 | 118.97682184 | 119.03604297 |
| A45 | 160.61076752 | 161.02034138 |
| A46 | 107.85730043 | 108.01405941 |
| A47 | 108.36720304 | 108.53503060 |
| A48 | 155.00270587 | 155.09486109 |
| A49 | 93.02162927 | 93.36411343 |
| A50 | 65.83600761 | 65.46008045 |
| A51 | 124.63027507 | 124.38270955 |
| A52 | 106.33950994 | 106.35485078 |
| A53 | 109.65684048 | 109.75541245 |
| A54 | 62.50431090 | 62.44180079 |
| A55 | 79.14840553 | 78.61332450 |
| A56 | 94.38442272 | 93.89685773 |
| A57 | 81.94439843 | 81.33655214 |
| A58 | 122.30128587 | 122.38085758 |
| A59 | 108.69599817 | 108.46009440 |
| A60 | 118.51678659 | 118.96829710 |
| A61 | 112.14562665 | 112.66775833 |
| A62 | 125.70871686 | 125.55440414 |
| A63 | 104.10605874 | 104.05408726 |
| A64 | 115.66124171 | 115.41491837 |
| A65 | 112.85938504 | 112.90944315 |
| A66 | 111.09046713 | 111.04846793 |
| A67 | 109.12827188 | 109.12112805 |
| A68 | 112.50720874 | 112.20795577 |
| A69 | 108.93102542 | 108.90860024 |
| A70 | 109.76379473 | 109.82714850 |
| A71 | 35.55295345 | 35.64055684 |
| A72 | 108.78209604 | 109.15267108 |
| A73 | 35.76405946 | 35.85622083 |
| A74 | 104.88212372 | 105.29541039 |
| D1 | 23.44707166 | 22.86899643 |
| D2 | −143.01621213 | −145.69511584 |
| D3 | 38.42108435 | 38.12739399 |
| D4 | 167.03950592 | 166.71102018 |
| D5 | 11.19248174 | 10.26295325 |
| D6 | −166.27299883 | −167.50854474 |
| D7 | 154.43296848 | 154.87100444 |
| D8 | −24.72393257 | −23.90813959 |
| D9 | 14.87760390 | 14.33966315 |
| D10 | −166.33850331 | −167.39527774 |
| D11 | −178.68215577 | −179.05559597 |
| D12 | 116.12498790 | 117.52135961 |
| D13 | −96.95997467 | −97.21214362 |
| D14 | 10.99862661 | 10.70745397 |
| D15 | 143.71999664 | 143.27578873 |
| D16 | 53.45687997 | 56.95553385 |
| D17 | −13.05739505 | −15.48602055 |
| D18 | 166.77990428 | 164.20618170 |
| D19 | 179.92648481 | 179.96617851 |
| D20 | −0.52972049 | −0.55875352 |
| D21 | −179.66796941 | −179.58911616 |
| D22 | 0.73133137 | 0.86390555 |
| D23 | 179.16217965 | 179.06507149 |
| D24 | 0.01070889 | −0.03915718 |
| D25 | 179.03888636 | 179.05702057 |
| D26 | 48.07112323 | 45.04719980 |
| D27 | 0.74469490 | 0.91641539 |
| D28 | −179.96524676 | −179.74189717 |
| D29 | −179.85477901 | −179.85236519 |
| D30 | −0.39506461 | −0.41589868 |
| D31 | −179.79735693 | −179.79071017 |
| D32 | 0.01649873 | 0.03030576 |
| D33 | 179.92823773 | 179.93269511 |
| D34 | 0.28491317 | 0.27631314 |
| D35 | 179.18887066 | 179.19810092 |
| D36 | −116.75958591 | −117.79096246 |
| D37 | 1.86219749 | 1.56294201 |
| D38 | −115.86020598 | −117.02116664 |
| D39 | −179.58717365 | −179.74490671 |
| D40 | 63.79739074 | 62.83877093 |
| D41 | 178.95201872 | 179.06783274 |
| D42 | −0.28698798 | −0.13593353 |
| D43 | 178.22649816 | 178.07298377 |
| D44 | −148.93368537 | −152.08506467 |
| D45 | 111.42783975 | 113.18142815 |
| D46 | −4.05565899 | −2.40606604 |
| D47 | −179.15665881 | −177.51084506 |
| D48 | −12.57964549 | −12.42277636 |
| D49 | −120.44409694 | −120.19086848 |
| D50 | 98.09202903 | 96.64992442 |
| D51 | 91.15604637 | 91.57000780 |
| D52 | −153.66577605 | −153.20632535 |
| D53 | 135.69337038 | 135.76291529 |
| D54 | 154.19232954 | 153.37488771 |
| D55 | −1.14110681 | −0.79096165 |
| D56 | 4.84906073 | 4.80879280 |
| D57 | −83.86223058 | −85.19930682 |
| D58 | −1.48047919 | −1.48833544 |
| D59 | 161.82475625 | 161.81654714 |
| D60 | 8.46484636 | 8.87965902 |
| D61 | 147.77400083 | 149.20907105 |
| D62 | −178.10763280 | −178.26760363 |
| D63 | 66.16427504 | 65.99502020 |
| D64 | −71.70727202 | −71.00674962 |
| D65 | 50.12782255 | 50.90167682 |
| D66 | 168.95273147 | 169.66688376 |
| D67 | −126.11657682 | −124.36123085 |
| D68 | 54.49143257 | 54.30008219 |
| D69 | −62.36290369 | −62.63339700 |
| D70 | −66.30729976 | −66.82133358 |
| D71 | −118.50510691 | −118.64472010 |
| D72 | −128.64978340 | −128.59930105 |
| D73 | −115.36964313 | −115.74853701 |

Table A3.
Z−matrix of ATV1H.
Table A3.
Z−matrix of ATV1H.
| Atom | |||||||
|---|---|---|---|---|---|---|---|
| F | |||||||
| O | 1 | B1 | |||||
| O | 1 | B2 | 2 | A1 | |||
| N | 3 | B3 | 1 | A2 | 2 | D1 | 0 |
| C | 2 | B4 | 1 | A3 | 4 | D2 | 0 |
| C | 3 | B5 | 1 | A4 | 4 | D3 | 0 |
| C | 4 | B6 | 3 | A5 | 1 | D4 | 0 |
| C | 4 | B7 | 3 | A6 | 1 | D5 | 0 |
| C | 8 | B8 | 4 | A7 | 3 | D6 | 0 |
| C | 6 | B9 | 3 | A8 | 1 | D7 | 0 |
| C | 7 | B10 | 4 | A9 | 3 | D8 | 0 |
| C | 8 | B11 | 4 | A10 | 3 | D9 | 0 |
| C | 8 | B12 | 4 | A11 | 3 | D10 | 0 |
| C | 12 | B13 | 8 | A12 | 4 | D11 | 0 |
| C | 7 | B14 | 4 | A13 | 3 | D12 | 0 |
| H | 15 | B15 | 7 | A14 | 4 | D13 | 0 |
| H | 15 | B16 | 7 | A15 | 4 | D14 | 0 |
| H | 15 | B17 | 7 | A16 | 4 | D15 | 0 |
| C | 10 | B18 | 6 | A17 | 3 | D16 | 0 |
| H | 19 | B19 | 10 | A18 | 6 | D17 | 0 |
| C | 19 | B20 | 10 | A19 | 6 | D18 | 0 |
| H | 21 | B21 | 19 | A20 | 10 | D19 | 0 |
| C | 21 | B22 | 19 | A21 | 10 | D20 | 0 |
| H | 23 | B23 | 21 | A22 | 19 | D21 | 0 |
| C | 23 | B24 | 21 | A23 | 19 | D22 | 0 |
| H | 25 | B25 | 23 | A24 | 21 | D23 | 0 |
| C | 25 | B26 | 23 | A25 | 21 | D24 | 0 |
| H | 27 | B27 | 25 | A26 | 23 | D25 | 0 |
| C | 14 | B28 | 12 | A27 | 8 | D26 | 0 |
| H | 29 | B29 | 14 | A28 | 12 | D27 | 0 |
| C | 29 | B30 | 14 | A29 | 12 | D28 | 0 |
| H | 31 | B31 | 29 | A30 | 14 | D29 | 0 |
| C | 31 | B32 | 29 | A31 | 14 | D30 | 0 |
| H | 33 | B33 | 31 | A32 | 29 | D31 | 0 |
| C | 33 | B34 | 31 | A33 | 29 | D32 | 0 |
| H | 35 | B35 | 33 | A34 | 31 | D33 | 0 |
| C | 35 | B36 | 33 | A35 | 31 | D34 | 0 |
| H | 37 | B37 | 35 | A36 | 33 | D35 | 0 |
| C | 13 | B38 | 8 | A37 | 4 | D36 | 0 |
| H | 39 | B39 | 13 | A38 | 8 | D37 | 0 |
| C | 9 | B40 | 8 | A39 | 4 | D38 | 0 |
| H | 41 | B41 | 9 | A40 | 8 | D39 | 0 |
| C | 9 | B42 | 8 | A41 | 4 | D40 | 0 |
| H | 43 | B43 | 9 | A42 | 8 | D41 | 0 |
| C | 43 | B44 | 9 | A43 | 8 | D42 | 0 |
| H | 45 | B45 | 43 | A44 | 9 | D43 | 0 |
| C | 4 | B46 | 3 | A45 | 1 | D44 | 0 |
| H | 47 | B47 | 4 | A46 | 3 | D45 | 0 |
| H | 47 | B48 | 4 | A47 | 3 | D46 | 0 |
| C | 5 | B49 | 2 | A48 | 1 | D47 | 0 |
| H | 50 | B50 | 5 | A49 | 2 | D48 | 0 |
| H | 50 | B51 | 5 | A50 | 2 | D49 | 0 |
| C | 5 | B52 | 2 | A51 | 1 | D50 | 0 |
| H | 53 | B53 | 5 | A52 | 2 | D51 | 0 |
| H | 53 | B54 | 5 | A53 | 2 | D52 | 0 |
| O | 50 | B55 | 5 | A54 | 2 | D53 | 0 |
| H | 56 | B56 | 50 | A55 | 5 | D54 | 0 |
| O | 53 | B57 | 5 | A56 | 2 | D55 | 0 |
| H | 58 | B58 | 53 | A57 | 5 | D56 | 0 |
| O | 5 | B59 | 2 | A58 | 1 | D57 | 0 |
| N | 6 | B60 | 3 | A59 | 1 | D58 | 0 |
| H | 61 | B61 | 6 | A60 | 3 | D59 | 0 |
| C | 7 | B62 | 4 | A61 | 3 | D60 | 0 |
| H | 63 | B63 | 7 | A62 | 4 | D61 | 0 |
| C | 63 | B64 | 7 | A63 | 4 | D62 | 0 |
| H | 65 | B65 | 63 | A64 | 7 | D63 | 0 |
| H | 65 | B66 | 63 | A65 | 7 | D64 | 0 |
| H | 65 | B67 | 63 | A66 | 7 | D65 | 0 |
| C | 47 | B68 | 4 | A67 | 3 | D66 | 0 |
| H | 69 | B69 | 47 | A68 | 4 | D67 | 0 |
| H | 69 | B70 | 47 | A69 | 4 | D68 | 0 |
| C | 56 | B71 | 50 | A70 | 5 | D69 | 0 |
| H | 72 | B72 | 56 | A71 | 50 | D70 | 0 |
| C | 58 | B73 | 53 | A72 | 5 | D71 | 0 |
| H | 74 | B74 | 58 | A73 | 53 | D72 | 0 |

Table A4.
Elements of the Z−matrix of ATV1H in methanol optimized as indicated below using 6-31+G/d,p) basis sets.
Table A4.
Elements of the Z−matrix of ATV1H in methanol optimized as indicated below using 6-31+G/d,p) basis sets.
| Element | UB3LYP | ROB3LYP | UPBE0 |
|---|---|---|---|
| B1 | 8.44235812 | 8.44847690 | 8.35899747 |
| B2 | 9.48151258 | 9.48004054 | 9.44104389 |
| B3 | 4.45293368 | 4.45205992 | 4.45170279 |
| B4 | 1.28075664 | 1.28075532 | 1.27612637 |
| B5 | 1.23516216 | 1.23520893 | 1.22884511 |
| B6 | 1.39121019 | 1.39036552 | 1.38136414 |
| B7 | 1.36865245 | 1.36927061 | 1.36158073 |
| B8 | 4.22903226 | 4.22867569 | 4.21410980 |
| B9 | 2.54297346 | 2.54296359 | 2.51827392 |
| B10 | 1.41411564 | 1.41273105 | 1.41276955 |
| B11 | 1.39814133 | 1.39906265 | 1.39123927 |
| B12 | 1.45764082 | 1.45731631 | 1.45172243 |
| B13 | 1.46214878 | 1.46225268 | 1.45692318 |
| B14 | 2.52804795 | 2.52736311 | 2.50801737 |
| B15 | 1.09361662 | 1.09358464 | 1.09350906 |
| B16 | 1.09280828 | 1.09278512 | 1.09298184 |
| B17 | 1.09400195 | 1.09401889 | 1.09384406 |
| B18 | 1.40294782 | 1.40295766 | 1.39868090 |
| B19 | 1.08608989 | 1.08608772 | 1.08659742 |
| B20 | 1.39528664 | 1.39528456 | 1.39148253 |
| B21 | 1.08563236 | 1.08563462 | 1.08591759 |
| B22 | 1.39885297 | 1.39887769 | 1.39500026 |
| B23 | 1.08539151 | 1.08539203 | 1.08568270 |
| B24 | 1.39725830 | 1.39725954 | 1.39357881 |
| B25 | 1.08569301 | 1.08569202 | 1.08600487 |
| B26 | 1.39754666 | 1.39755038 | 1.39365084 |
| B27 | 1.08290419 | 1.08290428 | 1.08428078 |
| B28 | 1.41359961 | 1.41373605 | 1.40819345 |
| B29 | 1.08383502 | 1.08382196 | 1.08499596 |
| B30 | 1.39307164 | 1.39303645 | 1.38966728 |
| B31 | 1.08532894 | 1.08532521 | 1.08564174 |
| B32 | 1.39877845 | 1.39870695 | 1.39488367 |
| B33 | 1.08554363 | 1.08554068 | 1.08589662 |
| B34 | 1.40107536 | 1.40113592 | 1.39694939 |
| B35 | 1.08531301 | 1.08531095 | 1.08561477 |
| B36 | 1.39114277 | 1.39108971 | 1.38781466 |
| B37 | 1.08454080 | 1.08454217 | 1.08578418 |
| B38 | 1.41428406 | 1.41456146 | 1.40940924 |
| B39 | 1.08378663 | 1.08375477 | 1.08486309 |
| B40 | 1.39026744 | 1.39013657 | 1.38630505 |
| B41 | 1.08392114 | 1.08391505 | 1.08433610 |
| B42 | 1.39295578 | 1.39307149 | 1.39002859 |
| B43 | 1.08393715 | 1.08393322 | 1.08438619 |
| B44 | 1.38976598 | 1.38960925 | 1.38585905 |
| B45 | 1.08386009 | 1.08382658 | 1.08487947 |
| B46 | 1.48288423 | 1.48290265 | 1.46968125 |
| B47 | 1.08655848 | 1.08656906 | 1.08771365 |
| B48 | 1.08526299 | 1.08526229 | 1.08700526 |
| B49 | 3.13186572 | 3.13214456 | 3.09809470 |
| B50 | 1.09749147 | 1.09749242 | 1.09754851 |
| B51 | 1.09723187 | 1.09723227 | 1.09817660 |
| B52 | 1.54597249 | 1.54596976 | 1.53696411 |
| B53 | 1.09826990 | 1.09827142 | 1.09817381 |
| B54 | 1.09366646 | 1.09366220 | 1.09361307 |
| B55 | 2.45496563 | 2.45493465 | 2.43617051 |
| B56 | 0.98121613 | 0.98120462 | 0.98045950 |
| B57 | 2.44581966 | 2.44580722 | 2.42582935 |
| B58 | 1.00162018 | 1.00160987 | 1.00474416 |
| B59 | 1.25555505 | 1.25555586 | 1.24897137 |
| B60 | 1.36463937 | 1.36473756 | 1.35864347 |
| B61 | 1.01542708 | 1.01542689 | 1.01391007 |
| B62 | 1.50493077 | 1.50429573 | 1.49670728 |
| B63 | 1.09337036 | 1.09333772 | 1.09467810 |
| B64 | 1.54747843 | 1.54768005 | 1.53733187 |
| B65 | 1.09151271 | 1.09148630 | 1.09246030 |
| B66 | 1.09323237 | 1.09319168 | 1.09361961 |
| B67 | 1.09407784 | 1.09408957 | 1.09401377 |
| B68 | 1.53535621 | 1.53533608 | 1.52584909 |
| B69 | 1.09518094 | 1.09518217 | 1.09596063 |
| B70 | 1.09496161 | 1.09497326 | 1.09558744 |
| B71 | 1.43537977 | 1.43540753 | 1.42193713 |
| B72 | 1.10242221 | 1.10243533 | 1.10351859 |
| B73 | 1.44492447 | 1.44492195 | 1.43098159 |
| B74 | 1.09747052 | 1.09747351 | 1.09843948 |
| A1 | 76.58822830 | 76.56600564 | 76.18592634 |
| A2 | 36.60887483 | 36.62337281 | 36.65315300 |
| A3 | 40.02401754 | 40.02725714 | 40.37741291 |
| A4 | 69.22460650 | 69.21215273 | 69.11650364 |
| A5 | 37.06294802 | 37.05454543 | 37.33294209 |
| A6 | 75.97461715 | 75.97019252 | 75.54658203 |
| A7 | 125.60763591 | 125.62294801 | 125.53862522 |
| A8 | 145.46690797 | 145.43385778 | 146.47703392 |
| A9 | 107.94505539 | 107.97228749 | 107.96898197 |
| A10 | 108.38689995 | 108.36146722 | 108.27547546 |
| A11 | 124.74777225 | 124.75087571 | 124.69207445 |
| A12 | 127.97840469 | 127.96666547 | 128.15656185 |
| A13 | 105.59510463 | 105.58982134 | 105.42968231 |
| A14 | 89.06762423 | 89.07379124 | 89.13648017 |
| A15 | 98.38552433 | 98.38081510 | 98.24507700 |
| A16 | 141.42365452 | 141.39940160 | 141.62200086 |
| A17 | 135.11423751 | 135.13907569 | 134.99049648 |
| A18 | 119.61460474 | 119.61458063 | 119.55946866 |
| A19 | 120.09632412 | 120.09653690 | 120.05115014 |
| A20 | 119.42313169 | 119.42315027 | 119.43302709 |
| A21 | 120.32365928 | 120.32410586 | 120.32294735 |
| A22 | 120.29030763 | 120.28966185 | 120.26610493 |
| A23 | 119.45834121 | 119.45848930 | 119.50076102 |
| A24 | 120.18704632 | 120.18699066 | 120.19049653 |
| A25 | 120.64768821 | 120.64769441 | 120.59629785 |
| A26 | 119.56030742 | 119.56407910 | 119.66008477 |
| A27 | 120.97200577 | 120.97164632 | 120.89552896 |
| A28 | 119.95204121 | 119.93947727 | 119.96571886 |
| A29 | 120.50494184 | 120.51489097 | 120.36553094 |
| A30 | 119.59499068 | 119.60198784 | 119.61841115 |
| A31 | 120.22405430 | 120.21574498 | 120.19645130 |
| A32 | 120.10222516 | 120.09856981 | 120.06034754 |
| A33 | 119.87268934 | 119.88010650 | 119.94634847 |
| A34 | 120.16892282 | 120.16545793 | 120.17885990 |
| A35 | 120.16243769 | 120.16144382 | 120.12026330 |
| A36 | 119.28472624 | 119.28635927 | 119.38889210 |
| A37 | 119.13990753 | 119.14065136 | 118.93302300 |
| A38 | 119.93397869 | 119.91880819 | 119.92762403 |
| A39 | 120.71429714 | 120.72333881 | 120.60124236 |
| A40 | 121.46593615 | 121.47547577 | 121.48976173 |
| A41 | 61.90781157 | 61.91592578 | 61.89487363 |
| A42 | 120.24240110 | 120.24137519 | 120.17005220 |
| A43 | 118.35259175 | 118.34576673 | 118.40770516 |
| A44 | 118.86184831 | 118.86297431 | 118.93351992 |
| A45 | 158.71586453 | 158.72856275 | 159.46262187 |
| A46 | 107.97807868 | 107.98166144 | 108.16165610 |
| A47 | 107.59850751 | 107.60908685 | 107.84756046 |
| A48 | 157.42614280 | 157.44924436 | 157.60290007 |
| A49 | 94.85343455 | 94.84153771 | 95.55489296 |
| A50 | 64.95144615 | 64.95648523 | 64.45763585 |
| A51 | 116.19161990 | 116.19053946 | 115.94779395 |
| A52 | 106.86069836 | 106.86047108 | 106.91121971 |
| A53 | 109.53392738 | 109.53458463 | 109.64753221 |
| A54 | 62.07557911 | 62.08044425 | 61.96848271 |
| A55 | 76.49524293 | 76.49674331 | 75.79460665 |
| A56 | 92.76536137 | 92.76506052 | 92.30427679 |
| A57 | 73.57144767 | 73.57345073 | 72.89199367 |
| A58 | 125.45137451 | 125.45215395 | 125.49766270 |
| A59 | 120.73260155 | 120.70386918 | 121.19251908 |
| A60 | 112.50449394 | 112.51148946 | 113.02222681 |
| A61 | 125.77099330 | 125.75901888 | 125.58026516 |
| A62 | 104.23702836 | 104.25410004 | 104.23745904 |
| A63 | 115.40821162 | 115.42354499 | 115.23936294 |
| A64 | 113.24698603 | 113.25125527 | 113.34985866 |
| A65 | 111.19362011 | 111.18145366 | 111.20358677 |
| A66 | 107.92265897 | 107.90449558 | 107.87759561 |
| A67 | 112.10461860 | 112.12909104 | 111.84315602 |
| A68 | 109.13041373 | 109.12442468 | 109.15330530 |
| A69 | 110.22123305 | 110.22217326 | 110.32638994 |
| A70 | 35.66580947 | 35.66718568 | 35.77797455 |
| A71 | 108.97338188 | 108.96922402 | 109.34397229 |
| A72 | 36.23210769 | 36.23253122 | 36.38166941 |
| A73 | 106.11400594 | 106.11371323 | 106.59889713 |
| D1 | 20.32357302 | 20.28511825 | 19.87637559 |
| D2 | −162.17511087 | −162.33220490 | −162.80694109 |
| D3 | 43.63016177 | 43.60181174 | 42.29185870 |
| D4 | 164.26479778 | 164.21734263 | 164.45615232 |
| D5 | 10.77189784 | 10.75274670 | 10.05902947 |
| D6 | −166.43746347 | −166.45509545 | −167.30190503 |
| D7 | 158.44374263 | 158.23289402 | 158.80659445 |
| D8 | −28.08220344 | −28.08496724 | −26.82514391 |
| D9 | 1.91266449 | 1.90212780 | 1.62776737 |
| D10 | −165.86995934 | −165.89347603 | −166.61940143 |
| D11 | 175.37358525 | 175.35516221 | 176.13954461 |
| D12 | 113.64232131 | 113.65660017 | 114.91433970 |
| D13 | −95.76535683 | −95.82640318 | −96.19390095 |
| D14 | 12.62820769 | 12.57590279 | 12.17768961 |
| D15 | 145.45934742 | 145.38955744 | 144.82678344 |
| D16 | 54.19138739 | 54.30206745 | 57.11046377 |
| D17 | −17.85450383 | −17.82296579 | −19.82581123 |
| D18 | 161.85312253 | 161.88684573 | 159.76981057 |
| D19 | 179.94673200 | 179.94762681 | 179.95512591 |
| D20 | −0.60528557 | −0.60218788 | −0.68612039 |
| D21 | −179.59203284 | −179.59336859 | −179.50090877 |
| D22 | 0.90546409 | 0.90366406 | 1.03940801 |
| D23 | 178.93404376 | 178.93542059 | 178.83527738 |
| D24 | 0.05603713 | 0.05403935 | 0.03326550 |
| D25 | 177.82860566 | 177.83470660 | 177.66605428 |
| D26 | −137.16331958 | −137.09552508 | −138.22471461 |
| D27 | 0.43265772 | 0.51931825 | 0.61777653 |
| D28 | 178.15552655 | 178.21539172 | 178.43299456 |
| D29 | −178.95651780 | −178.95670070 | −178.99276897 |
| D30 | 0.79493565 | 0.77240094 | 0.77488924 |
| D31 | 179.51072096 | 179.53026575 | 179.50412377 |
| D32 | −0.68679275 | −0.67915786 | −0.68858323 |
| D33 | 179.67314075 | 179.67322011 | 179.71709289 |
| D34 | −0.20109695 | −0.20087651 | −0.18063234 |
| D35 | 179.30402096 | 179.29000187 | 179.32243696 |
| D36 | −130.28427078 | −130.51938776 | −130.58633908 |
| D37 | 1.84741835 | 1.91470640 | 1.67453246 |
| D38 | 179.74200519 | 179.74365748 | 179.60779307 |
| D39 | −178.45418197 | −178.42099743 | −178.44652258 |
| D40 | 51.73180726 | 51.51509192 | 51.55090486 |
| D41 | 178.78350784 | 178.76463222 | 178.80834185 |
| D42 | −0.73877044 | −0.75662840 | −0.65322109 |
| D43 | 177.62465046 | 177.60834940 | 177.40162657 |
| D44 | −156.11290080 | −156.22713857 | −158.14021510 |
| D45 | 116.06952548 | 116.07911206 | 117.63013987 |
| D46 | 0.99099890 | 1.01912297 | 2.42551191 |
| D47 | −171.42587327 | −171.42390819 | −169.95676548 |
| D48 | −15.20357679 | −15.21183395 | −14.84818756 |
| D49 | −122.34742832 | −122.36008992 | −121.73630690 |
| D50 | 88.06897802 | 87.95008988 | 88.85753223 |
| D51 | 83.56556644 | 83.56187427 | 83.14352949 |
| D52 | −160.76875154 | −160.77109993 | −161.02867903 |
| D53 | 132.32183428 | 132.32174256 | 132.43569018 |
| D54 | 149.97524265 | 149.98218167 | 148.79908686 |
| D55 | −9.90827522 | −9.91152885 | −10.13040806 |
| D56 | 9.74285779 | 9.73770241 | 9.81321453 |
| D57 | −93.97703409 | −94.06732013 | −93.02985254 |
| D58 | 160.87867630 | 160.75346463 | 161.09930653 |
| D59 | 6.66281917 | 6.73900407 | 7.18339300 |
| D60 | 145.35128108 | 145.39894308 | 146.79423835 |
| D61 | −177.00884009 | −177.06601025 | −177.27755993 |
| D62 | 67.06790752 | 66.97955721 | 66.63767044 |
| D63 | −71.82425328 | −71.79410371 | −71.15947356 |
| D64 | 50.84104068 | 50.87713734 | 51.65171208 |
| D65 | 169.00058929 | 169.03088651 | 169.71030546 |
| D66 | −120.64476837 | −120.63362997 | −119.11115232 |
| D67 | 51.89044308 | 51.87027494 | 51.66320139 |
| D68 | −65.58155270 | −65.59104262 | −65.93846062 |
| D69 | −66.67428542 | −66.66835138 | −67.19000316 |
| D70 | −118.76410502 | −118.75497303 | −118.87135263 |
| D71 | −132.76879862 | −132.76775335 | −132.41059726 |
| D72 | −117.45364759 | −117.45138885 | −117.81963777 |
References
- Roth, B.D. The Discovery and Development of Atorvastatin, A Potent Novel Hypolipidemic Agent. Prog. Med. Chem. 2002, 40, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mikulic, M. Worldwide revenue of Pfizer’s Lipitor from 2003 to 2019. 2021. Available online: https://www.statista.com/statistics/254341/pfizers-worldwide-viagra-revenues-since-2003/ (accessed on 11 July 2022).
- Alnajjar, R.; Mohamed, N.; Kawafi, N. Bicyclo[1.1.1]Pentane as Phenyl Substituent in Atorvastatin Drug to improve Physicochemical Properties: Drug-likeness, DFT, Pharmacokinetics, Docking, and Molecular Dynamic Simulation. J. Mol. Struct. 2021, 1230, 129628. [Google Scholar] [CrossRef]
- Hoffmann, M.; Nowosielski, M. DFT study on hydroxy acid-lactone interconversion of statins: The case of atorvastatin. Org. Biomol. Chem. 2008, 6, 3527–3531. [Google Scholar] [CrossRef]
- Duque, L.; Guerrero, G.; Colorado, J.H.; Restrepo, J.A.; Velez, E. Theoretical Insight into mechanism of antioxidant capacity of atorvastatin and its o-hydroxy and p-hydroxy metabolites, using DFT methods. Comput. Theor. Chem. 2022, 1214, 113758. [Google Scholar] [CrossRef]
- Bâldea, I. Critical analysis of radical scavenging properties of atorvastatin in methanol recently estimated via density functional theory. arXiv 2022, arXiv:2206.13990. [Google Scholar] [CrossRef]
- Portes, E.; Gardrat, C.; Castellan, A. A comparative study on the antioxidant properties of tetrahydrocurcuminoids and curcuminoids. Tetrahedron 2007, 63, 9092–9099. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis 1998, 138, 271–280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- bwHPC. bwHPC 2.0 (JUSTUS cluster_2.0, bwUniCluster_2.0, MLS&WISO_2.0) Supported by the State of Baden-Württemberg Supported by the State of Baden-Württemberg and the German Research Foundation (DFG) through Grant no INST 40/575-1 FUGG. Available online: https://wiki.bwhpc.de/e/Category:BwForCluster_JUSTUS_2 (accessed on 12 June 2022).
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-Shell Atoms and Hydrides of the First-Row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A Complete Basis Set Model Chemistry. II. Open-Shell Systems and the Total Energies of the First-Row Atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, J.F.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functional for Spectroscopy: No Long-Range Self-Interaction Error, Good Performance for Rydberg and Charge-Transfer States, and Better Performance on Average than B3LYP for Ground States. J. Phys. Chem. A 2006, 110, 13126–13130. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Bâldea, I. Comprehensive Quantum Chemical Characterization of the Astrochemically Relevant HCnH Chain Family. An Attempt to Aid Astronomical Observations. Adv. Theor. Simul. 2022, 2200244. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Sun, B.J.; Lin, H.M.; Chang, A.H.H.; Mebel, A.M.; Kostko, O.; Ahmed, M. An Experimental and Theoretical Study on the Ionization Energies of Polyynes H–(C≡C)n–H; n = 1–9). Astrophys. J. 2010, 719, 1884. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. A Universal Approach to Solvation Modeling. Acc. Chem. Res. 2008, 41, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Perspective on Foundations of Solvation Modeling: The Electrostatic Contribution to the Free Energy of Solvation. J. Chem. Theory Comput. 2008, 4, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Allouche, A.R. Gabedit: A Graphical User Interface For Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Glendening, E.; Badenhoop, J.; Reed, A.; Carpenter, J.; Bohmann, J.; Morales, C.; Weinhold, F. NBO Code Version 6.0. 2012. Available online: https://nbo6.chem.wisc.edu/ (accessed on 7 April 2022).
- Wiberg, K.B. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Mayer, I. Bond Order and Valence Indices: A Personal Account. J. Comput. Chem. 2007, 28, 204–221. [Google Scholar] [CrossRef]
- Bâldea, I. Chemical bonding in representative astrophysically relevant neutral, cation, and anion HCnH chains. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Clarendon, UK, 1989; p. 149. [Google Scholar]
- Gázquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and Electroaccepting Powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Rajan, V.K.; Hasna, C.K.; Muraleedharan, K. The natural food colorant Peonidin from cranberries as a potential radical scavenger—A DFT based mechanistic analysis. Food Chem. 2018, 262, 184–190. [Google Scholar] [CrossRef]
- Bâldea, I. Impact of Molecular Conformation on Transport and Transport-Related Properties at the Nanoscale. Appl. Surf. Sci. 2019, 487, 593–600. [Google Scholar] [CrossRef]
- Bâldea, I. Alternation of Singlet and Triplet States in Carbon-Based Chain Molecules and Its Astrochemical Implications: Results of an Extensive Theoretical Study. Adv. Theory Simul. 2019, 2, 1900084. [Google Scholar] [CrossRef]
- Burke, K. Perspective on density functional theory. J. Chem. Phys. 2012, 136, 150901. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, I. A Quantum Chemical Study from a Molecular Transport Perspective: Ionization and Electron Attachment Energies for Species Often Used to Fabricate Single-Molecule Junctions. Faraday Discuss. 2014, 174, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Chem. Phys. 1996, 100, 12974–12980, Notice that after eq. (19), the authors of this reference state: “The individual eigenfunctions and eigenvalues, φj and εj, of the Kohn-Sham equations have no strict physical significance…”. [Google Scholar] [CrossRef] [Green Version]
- Godby, R.W.; Schlüter, M.; Sham, L.J. Self-energy operators and exchange-correlation potentials in semiconductors. Phys. Rev. B 1988, 37, 10159–10175. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, V.; Baldereschi, A. Dielectric Scaling of the Self-Energy Scissor Operator in Semiconductors and Insulators. Phys. Rev. B 1995, 51, 17196–17198. [Google Scholar] [CrossRef]
- Bâldea, I. Demonstrating Why DFT-Calculations For Molecular Transport in Solvents Need Scissor Corrections. Electrochem. Commun. 2013, 36, 19–21. [Google Scholar] [CrossRef]
- Bâldea, I. Profiling C4N Radicals of Astrophysical Interest. Mon. Not. R. Astron. Soc. 2020, 493, 2506–2510. [Google Scholar] [CrossRef]
- Bâldea, I. Profiling Astrophysically Relevant MgC4H Chains. An Attempt to Aid Astronomical Observations. Mon. Not. R. Astron. Soc. 2020, 498, 4316–4326. [Google Scholar] [CrossRef]
- Zhou, Z.; Parr, R.G. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar]
- Gázquez, J.L. Perspectives on the Density Functional Theory of Chemical Reactivity. J. Mex. Chem. Soc. 2008, 52, 3–10. [Google Scholar]
- Domingo, L.R.; Aurell, M.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Burton, G.W.; Doba, T.; Gabe, E.; Hughes, L.; Lee, F.L.; Prasad, L.; Ingold, K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985, 107, 7053–7065. [Google Scholar] [CrossRef]
- de Heer, M.I.; Mulder, P.; Korth, H.G.; Ingold, K.U.; Lusztyk, J. Hydrogen Atom Abstraction Kinetics from Intramolecularly Hydrogen Bonded Ubiquinol-0 and Other (Poly)methoxy Phenols. J. Am. Chem. Soc. 2000, 122, 2355–2360. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for “fuzzy” atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 1996, 2, 2497–2504. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstractions. 1. The Reactions of Phenols with 2,2-Diphenyl-1-picrylhydrazyl (DPPH•) in Alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef] [Green Version]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 2. Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Jones, R.O.; Gunnarsson, O. The Density Functional Formalism, Its Applications and Prospects. Rev. Mod. Phys. 1989, 61, 689–746. [Google Scholar] [CrossRef]
- Bâldea, I. Extending the Newns-Anderson Model to Allow Nanotransport Studies Through Molecules with Floppy Degrees of Freedom. Europhys. Lett. 2012, 99, 47002. [Google Scholar] [CrossRef] [Green Version]
- Fifen, J.J. Thermodynamics of the Electron Revisited and Generalized. J. Chem. Theory Comput. 2013, 9, 3165–3169. [Google Scholar] [CrossRef] [PubMed]
- Fifen, J.J.; Dhaouadi, Z.; Nsangou, M. Revision of the Thermodynamics of the Proton in Gas Phase. J. Phys. Chem. A 2014, 118, 11090–11097. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Tosovic, J.; Milenkovic, D.; Markovic, S. Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput. Theor. Chem. 2016, 1077, 11–17. [Google Scholar] [CrossRef]
- Rimarcik, J.; Lukes, V.; Klein, E.; Ilcin, M. Study of the solvent effect on the enthalpies of homolytic and heterolytic N-H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J. Mol. Struct. THEOCHEM 2010, 952, 25–30. [Google Scholar] [CrossRef]
- Xu, B.; Tao, N.J. Measurement of Single-Molecule Resistance by Repeated Formation of Molecular Junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Xiao, X.; Tao, N.J. Measurements of Single-Molecule Electromechanical Properties. J. Am. Chem. Soc. 2003, 125, 16164–16165. [Google Scholar] [CrossRef]
- Bruot, C.; Hihath, J.; Tao, N. Mechanically controlled molecular orbital alignment in single molecule junctions. Nat. Nano 2011, 7, 35–40. [Google Scholar] [CrossRef]
- Pauling, L. Atomic Radii and Interatomic Distances in Metals. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
- Bâldea, I. Long Carbon-Based Chains of Interstellar Medium Can Have a Triplet Ground State. Why Is This Important for Astrochemistry? ACS Earth Space Chem. 2019, 3, 863–872. [Google Scholar] [CrossRef]
- Luo, Y.R. (Ed.) Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2003; p. 239. [Google Scholar] [CrossRef]
- Bâldea, I. Extensive Quantum Chemistry Study of Neutral and Charged C4N Chains: An Attempt to Aid Astronomical Observations. ACS Earth Space Chem. 2020, 4, 434–448. [Google Scholar] [CrossRef]
- Li, Y.; Haworth, N.L.; Xiang, L.; Ciampi, S.; Coote, M.L.; Tao, N. Mechanical Stretching-Induced Electron-Transfer Reactions and Conductance Switching in Single Molecules. J. Am. Chem. Soc. 2017, 139, 14699–14706. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).