Abstract
In this study, we report on the synthesis of new organoselenium derivatives, including nonsteroidal anti-inflammatory drugs (NSAIDs) scaffolds and Se functionalities (isoselenocyanate and selenourea), which were evaluated against four types of cancer cell line: SW480 (human colon adenocarcinoma cells), HeLa (human cervical cancer cells), A549 (human lung carcinoma cells), MCF-7 (human breast adenocarcinoma cells). Among these compounds, most of the investigated compounds reduced the viability of different cancer cell lines. The most promising compound 6b showed IC50 values under 10 μM against the four cancer cell lines, particularly to HeLa and MCF-7, with IC50 values of 2.3 and 2.5 μM, respectively. Furthermore, two compounds, 6b and 6f, were selected to investigate their ability to induce apoptosis in MCF-7 cells via modulation of the expression of anti-apoptotic Bcl-2 protein, pro-inflammatory cytokines (IL-2) and proapoptotic caspase-3 protein. The redox properties of the NSAIDs-Se derivatives were conducted by 2, 2-didiphenyl-1-picrylhydrazyl (DPPH), bleomycin-dependent DNA damage and glutathione peroxidase (GPx)-like assays. Finally, a molecular docking study revealed that an interaction with the active site of thioredoxin reductase 1 (TrxR1) predicted the antiproliferative activity of the synthesized candidates. Overall, these results could serve as a promising launch point for further designs of NSAIDs-Se derivatives as potential antiproliferative agents.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of active pharmaceutical ingredients (API) that are widely used in the treatment of inflammatory conditions, including pain associated with arthritis, worldwide [1,2]. Since the 1980s, when the antiproliferative effect of sulindac was first observed in reducing colon adenomas [3], a growing body of studies has evaluated the chemoprevention and antiproliferative potential of NSAIDs [4,5,6], although their exact molecular mechanism has remained elusive.
The selenium (Se) has been recognized to play an important role in human health and disease [7,8]. In recent years, a number of studies have indicated an inverse association between Se intake and cancer risk [9,10,11]. There are three main categories of Se-containing compounds (inorganic, organic, and selenoproteins) with potential pharmacological properties; the most developed and studied are organoselenium compounds, which have been shown to inhibit the initiation and postinitiation phases of chemical carcinogenesis [12]. Organic selenium compounds with diverse functional groups, including selenoesters, methylseleninic acid, isoselenocyanates, diselenides, endocyclic selenium, selenocyanates and trifluoromethyl selenides, were found to show potent antiproliferative activity [13,14,15,16,17,18] (Figure 1).
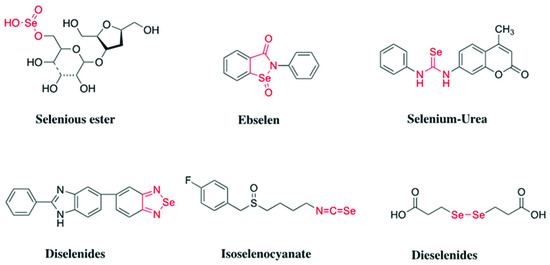
Figure 1.
Organic selenium compounds with diverse fnctional groups previously reported to exhibit antiproliferative activity.
Considering the chemo-preventive effects of NSAIDs and the antiproliferative activity of organic selenium compounds, along with reports that support the modification of NSAIDs scaffolds with Se functionalities [19,20] and our continued work to search for organoselenium derivatives with antiproliferative activity [21,22,23,24], several new NSAIDs-based derivatives, combining the NSAIDs scaffold with an organoselenium motif (Isoselenocyanate and selenoureas), were synthesized in this report (Figure 2). Their antiproliferative activities against SW480, HeLa, A549 and MCF-7 cell lines were shown using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Two compounds, 6b and 6f, were selected to test the protein expression levels of Bcl-2, IL-2 and caspase-3 biomarkers in MCF-7 cells. Furthermore, the antioxidant potential of the compounds was investigated by employing DPPH, bleomycin-dependent DNA damage and GPx-like assays. Finally, Thioredoxin Reductase (TrxR1) was selected as a docking protein to predict the target and antiproliferative activity of the prepared NSAIDs-Se hybrid compounds.
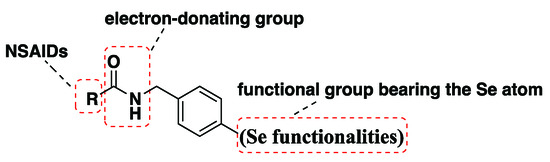
Figure 2.
Structure of NSAIDs-Se derivatives.
2. Results and Discussion
2.1. Chemistry
The synthesis of novel families of NSAIDs-based seleno derivatives as potential antiproliferative agents: Isoselenocyanates (ISCs) (5a–5h) and selenoureas (6a–6h) in this study was performed as outlined in Scheme 1.
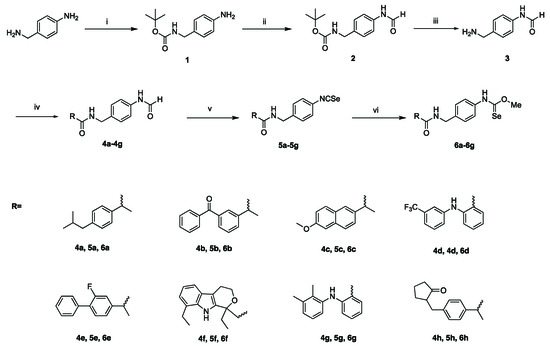
Scheme 1.
(i) (Boc)2, THF, −10 °C, 2 h, 90%; (ii) HCOOH, Si(OEt)4, CH3CN, 85 °C, 12 h, 80%; (iii) CF3COOH, DCM, −10 °C, 0.5 h, 90%; (iv) EDCI, HOBT, TEA, CH2Cl2/DMF, N2, r.t. 0.5 h, 65–80%; (v) a, Triphosgene, TEA, DCM, N2, reflux, 2 h. b, Se, N2, reflux, 12 h; (vi) MeOH, reflux, 4 h, 90%.
The synthesis of the NSAIDs-Isoselenocyanate derivatives (5a–5h) (Purity ≥ 95% by HPLC) was started from 4-aminobenzylamine protected by bis(1,1-dimethylethyl) ester to afford compound 1. Compound 1 was transformed into formamide 2 upon treatment with formic acid, with 80% yield. Next, compound 3 was obtained by deprotecting Boc-group in trifluoroacetic acid (TFA) and Dichloromethane (DCM) system. Compounds 4a–4h (Purity ≥ 95% by HPLC, except 4g) were obtained by commercially available NSAIDs and compound 3 in the presence of EDCI and HOBT as condensantion agent, in DMF as sovent and under a nitrogen atmosphere. Then, compounds 4a–4h were transformed into the NSAIDs-based isoselenocyanate (5a–5h) (Scheme 1) in a one-pot two-step procedure, which involves triphosgene-based dehydration of 4a–4h into a transient non-isolated isocyanide, followed by the addition of elemental selenium black (70% overall yield).
NSAIDs-selenourea derivatives (6a–6h) (Purity ≥ 95% by HPLC) were obtained by conducting corresponding NSAIDs-Isoselenocyanate derivatives with methanol.
2.2. Cell Viability Assay
MTT assay was conducted to evaluate the potential antiproliferative activities against human tumor cell lines derived from various human cancer types: SW480 (human colon adenocarcinoma cells), HeLa (human cervical cancer cells), A549 (human lung carcinoma cells), MCF-7 (human breast adenocarcinoma cells) of target compounds 5a–5h and 6a–6h, 5-Fu was selected as reference standard (Table 1).

Table 1.
Antiproliferative Activity of Target Compounds against four Human Cancer Cell Lines.
Overall, the IC50 values obtained and summarized in Table 1 show that allfthe tested organoselenium compounds with NSAIDs scaffolds and Se functionalities (isoselenocyanate and selenourea) exhibit growth inhibition in all cancer cell lines, while the selected patent NSAIDs (Aspirin, Ibuprofen and Naproxen) are inactive against all cells, even at the maximum dose of 50 μM.
An overview analysis of the IC50 values obtained and summarized in Table 1 showed that most of the NSAIDs-selenourea derivatives wer emore effective than NSAIDs-isoselenocyanate derivatives against all four cancer cell lines. Furthermore, the most active compounds of these two series were 6b and 6f. These two compounds show IC50 values below 10 μM in all tested cancer cell lines. Compound 6b was the most potent agent, with IC50 values below 5 μM in all cancer cell lines and remarkable antiproliferative activity against HeLa (2.3 μM) and MCF-7 (2.5 μM).
2.3. Evaluation of Bcl-2, IL-2 and Caspase-3 Molecular Biomarkers in MCF-7 Cells
To further understand the possible mechanism of the reduced cell viability of the target compounds, 6b and 6f were selected for their ability to induce apoptosis in MCF-7 cells via modulation of the expression of some apoptosis-related proteins (e.g., Bcl-2, IL-2, caspase-3). Protein levels of the anti-apoptotic marker Bcl-2 were measured using enzyme-linked immunosorbent assay (ELISA) according to the manufacturers’ instructions (Merck, Rahway, NJ, USA). Enzyme-linked immunosorbent assay was used for the quantitative detection of IL-2 and caspase-3 (Platinum ELISA).
As shown in Table 2, 6b and 6f were able to downregulate the expression of Bcl-2 and upregulate the expression of IL-2 and Caspase-3 in MCF-7 cells compared with untreated cells. Compound 6f downregulated over 50% of the expression levels of Bcl-2 compared to untreated cells. Furthermore, compound 6b modulated the IL-2 level to a 1.5-fold increase in expression when compared to the untreated control cells. Finally, compound 6b exhibited a superior activity increased the expression level of caspase-3 by 5-fold compared to untreated cells. From these results, it is likely that compounds 6b and 6f may induce apoptosis to inhibit tumor cells growth, in line with the underlying mechanism of some organoselenium compounds, which were reported to be effective against prostate and oral carcinoma cells via the estimation of potential biomarkers [15].

Table 2.
Protein expression levels of Bcl-2, IL-2 and caspase-3 in MCF-7 cells after 48 h incubation with compounds 6b and 6f at their respective IC50s compared to untreated cells.
2.4. Antioxidant Assay
Reactive oxygen species (ROS), e.g., H2O2, O2−, HO˙, and HOCl, serve important physiological roles that include signaling functions, host defense, and oxidative biosynthesis [25].
Various human diseases, including different types of cancer, are associated with a disturbed intracellular redox balance and oxidative stress (OS) [26]. Redox modulators play an important role as chemotherapeutic potential antitumor agents [27].
As a number of synthetic organoselenium compounds have been synthesized for their use as redox-modulators in recent years [28,29], the antioxidant activity of the selected synthesized compounds are further estimated by employing different biochemical assays such as DPPH, bleomycin-dependent DNA damage and Gpx-like assays [30,31,32].
2.4.1. Radical Scavenging Capacity (DPPH) Assay
The DPPH scavenging capacity assay is considered a valid and rapid colorimetric method for antioxidant property evaluation. This assay has been successfully utilized for investigating the antioxidant properties of nutritional products and organic selenides [33,34].
As depicted in Table 3, NSAIDs-selenourea derivatives 6b, 6f and 6h were the most active compounds in this assay, demonstrating a good free-radical scavenging activity compared to Vitamin C. The family of NSAIDs-selenourea derivatives is better than the corresponding NSAIDs-isoselenocyanate derivatives on this assay, except when comparing 5d and 6d.

Table 3.
Redox modulation activity of NSAID-Se hybrid compounds.
2.4.2. Bleomycin DNA Damage Assay
Bleomycin (BLM) is a family of glycopeptide antibiotics that are routinely used as antitumor agents; it is believed to oxidize DNA and induces single- and double-strand breaks [35,36]. The bleomycin-iron DNA damage assay has routinely been used as a preliminary method to test the potential of drugs and organic selenium compounds [37]. As shown in Table 3, compounds 5d, 5h and 6g induced DNA degradation significantly more than other tested compounds.
2.4.3. Glutathione Peroxidase-like Activity Assay
Glutathione peroxidase (GPx) is a well-known selenoenzyme that functions as an antioxidant [38,39]. The potential antioxidant activity of all the NSAIDs-Se derivatives were estimated using an NADPH-reductase coupled assay [40,41]. The GPx activity of the synthesized compounds was estimated by the decrease in absorbance (340 nm) due to the oxidation of NADPH to NADP+. Ebselen was used as a positive control.
As shown in Table 4, compounds 6a–6h displayed a better GPx-like activity than the respective 5a–5h derivatives. Compound 6g was the most active derivative in this assay, reaching up to 1.5-fold of the GPx mimetic ebselen.

Table 4.
GPx-like activity assay of NSAID-Se hybrid compounds in μM. Min−1.
2.5. Docking Studies
TrxR1 consists of four monimers, which have FAD and NAD binding domains at the N-terminal and the dimerization interface domain at the flexible C-terminal side. The binding mode between organoselenium compounds and Mammalian TrxR1 protein was described by docking studies. TrxR1 consists of several functional domains, including FAD- and NAD-binding domains at the N-terminal, and the dimerization interface domain at the flexible C-terminal side [42,43,44]. It has been reported that flexible docking can simulate the interaction between small molecules and TrxR1 [45]. Therefore, compounds 6b and 6f were docked into the TrxR1 protein (PDB id: 1H6V) using the Flexible Docking Protocol, as reported in the literature [45]. The distances between the selenium atom of all two compounds and Cys497/Cys498 of TrxR1 were measured and focused on because they are closely related to the accessibility of cysteine thiol attack selenides.
The pose 2 of compound 6b showed high -CDOCKER energy values (21.223 kcal/mol) and a close distance between the selenium atom and Cys498 (7.489 Å). Multiple interactions were found in this conformation, including electrostatic (Pi-Anion) between the benzene and GLU477 (4.326 Å), hydrogen bonding between the hydrogen of secondary amine of Seleno-carbamates and GLU477 (2.097 Å), and hydrogen bonding between the hydrogen of hydroxyl of Seleno-carbamates and GLU477 (2.549 Å) (Table 5, Pose 2; Figure 3). Compound 6f had multiple interactions with GLY496, which was adjacent to the key amino acid Cys497/Cys498. In pose 1, the value of -CDOCKER energy was 33.662 kcal/mol and distance between the selenium atom and Cys498 was 5.167 Å (Table 6, Pose 1). Three hydrogen bondings appear in this conformation, including the hydrogen bonding between the nitrogen of selenocarbamates and GLU477 (2.834 Å), the hydrogen bonding between the hydrogen of amide bond and GLY496 (2.776 Å) and the hydrogen bonding between the oxygen of amide bond and GLY496 (2.494 Å) (Figure 4).

Table 5.
Ligand-protein poses for compound 6b.
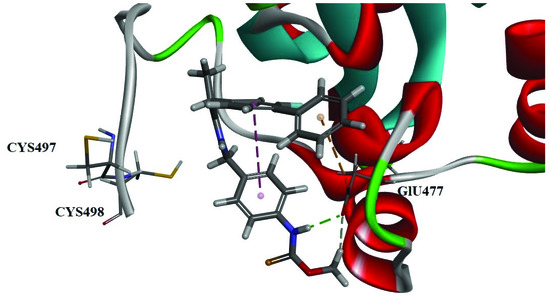
Figure 3.
The pose 2 of 6b. Three interactions were shown: electrostatic (Pi–Anion) between the benzene and GLU477 (4.326 Å), hydrogen bonding between the hydrogen of secondary amine of Seleno-carbamates and GLU477 (2.097 Å), and hydrogen bonding between the hydrogen of hydroxyl of Seleno-carbamates and GLU477 (2.549 Å).

Table 6.
Ligand-protein poses for compound 6f.
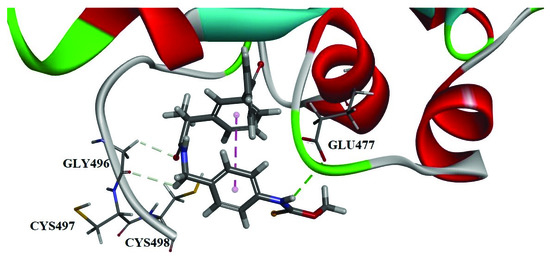
Figure 4.
The pose 1 of 6f. Three interactions are shown: hydrogen bonding between the fluorine of -SeCF3 group and Ser404 (2.80 Å); hydrogen bonding between the two hydrogens on carbonyl group α position and Ile492 (2.54 Å, 2.64 Å) or Gln494 (2.90 Å); hydrogen bonding between the oxygens of ester groups and Phe406 (2.46 Å).
3. Conclusions
In conclusion, the present study involved the synthesis of new organoselenium derivatives, including NSAIDs scaffolds and Se functionalities (isoselenocyanate and selenourea). Compound 6b exhibited the most potent activity in the MTT assay, with remarkable antiproliferative activity against HeLa (IC50 = 2.3 μM) and MCF-7 (IC50 = 2.5 μM). Compounds 6b and 6f were selected to verify if organic selenides can induce apoptosis in MCF-7 cells by modulating the expression of the Bcl-2, IL-2 and caspase-3 molecular biomarkers. The selected compounds were able to downregulate the expression of Bcl-2 and upregulate the expression of IL-2 and Caspase-3 in MCF-7 cells compared with untreated cells. Furthermore, most of the synthesized NSAIDs-Se hybrid compounds exhibited antioxidant activity in antioxidant evaluation, including DPPH, bleomycin-dependent DNA damage and Gpx-like assays.
Finally, in a flexible docking study performed on the TrxR1 enzyme, compound 6b showed promising binding energies and a promising binding mode for the distance between the selenium atom and Cys497/Cys498. At this point, compound 6b may act as a TrxR inhibitor. Further investigations of antiproliferative candidates based on these results are in progress.
4. Materials and Methods
4.1. Materials
All chemical reagents for the synthesis of the compounds were purchased from Macklin (Shanghai, China) or TCI (Shanghai, China) and used without further purification unless stated otherwise. TLCs were performed on aluminium pre-coated sheets (E. Merck Silica gel 60 F254). Melting points (uncorrected) were recorded on an Electrothermal apparatus. 1H (400 MHz), 13C (100 MHz) NMR and 19F (376 MHz) spectra were recorded at 25 °C on a Bruker Avance 400 MHz spectrometer with 5-mm PABBO probe. Chemical shifts (δ) are reported in parts per million (ppm) and the coupling constants (J) are expressed in Hertz (Hz). Mass analysis was recorded on an ESI source mass detector (Thermo LCQ FLEET).
4.2. Experimental Procedures
4.2.1. N-(4-(aminomethyl)phenyl)formamide 3
Trifluoroacetic acid (TFA, 1.2 eq.) was added to a solution of compound 2 (1.0 eq.) in DCM (5 mL) at −10 °C. The mixture was stirred at −10 °C for 1 h. TLC showed the reaction was complete. The mixture was concentrated under reduced pressure. The crude was diluted with DCM (10 mL) and the PH was adjusted to 8~9 by TEA. The mixture was used in the next step without purification.
4.2.2. General Procedure for the Synthesis of Compounds 4a–4h
1-ethyl-3(3-dimethylpropylamine) carbodiimide (EDCI, 1.2 eq.), 1-hydroxybenzotriazole (HOBT, 1.2 eq.) and TEA (3.0 eq.) was added to a solution of patent NSAIDS (1.o eq.) in DCM (5 mL) and DMF (5 mL). The mixture was stirred at 25 °C for 30 min under nitrogen atmosphere. Then, N-(4-(aminomethyl)phenyl)formamide (compound 3, 1.2 eq.) was added to the mixture. The mixture was stirred at 25 °C for 16 h under inert atmosphere. TLC showed the reaction was complete. The mixture was diluted with H2O (20 mL); the aqueous layer was extracted with DCM (15 mL × 2); the combined organic layer was washed with brine (20 mL × 3), dried over Na2SO4 and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with dichloromethane/methanol solution to obtain the desired compound.
N-(4-formamidobenzyl)-2-(4-isobutylphenyl)propenamide (4a)
Yield: 68%. White solid. Mp: 87–89 °C. 1H NMR (400 MHz, CDCl3): δ 0.86 (d, J = 8.00 Hz, 6H, 2 × -CH3), 1.34 (d, J = 8.00 Hz, 3H, -CH3), 1.77–1.84 (m, 1H, -CH), 2.41 (d, J = 8.00 Hz, 2H, -CH2), 3.63 (q, J = 8.00 Hz, 1H, -CH), 4.18–4.20 (m, 2H, -CH2), 7.06–7.09 (m, 4H, Ar-H), 7.23–7.25 (m, 2H, Ar-H), 7.46–7.48 (m, 2H, Ar-H), 8.24 (s, 1H, -NH), 8.41–8.44 (m, 1H, -NH), 10.16 (s, 1H, -CHO). 13C NMR (100 MHz, CDCl3): δ 18.9, 22.6, 30.1, 42.0, 44.7, 45.2, 117.9, 119.4, 127.5, 128.0, 128.6, 129.2, 135.2, 137.3, 139.7, 140.0, 159.9, 162.9, 173.9. MS(ESI): m/z = found 339.5 ([M + H]+).
2-(3-benzoylphenyl)-N-(4-formamidobenzyl)propenamide (4b)
Yield: 75%. White solid. Mp: 95–97 °C. 1H NMR (400 MHz, CDCl3): δ 1.40 (d, J = 8.00 Hz, 3H, -CH3), 3.80 (q, J = 8.00 Hz, 1H, -CH), 4.19–4.22 (m, 2H, -CH2), 7.08–7.10 (m, 2H, Ar-H), 7.48–7.77 (m, 11H, Ar-H), 8.24 (s, 1H, -NH), 8.55–8.58 (m, 1H, -NH), 10.17 (s, 1H, -CHO). 13C NMR (100 MHz, CDCl3): δ 18.9, 42.1, 45.4, 117.9, 119.5, 128.0, 128.6, 129.0, 130.1, 132.1, 133.2, 135.0, 137.4, 137.5, 143.2, 159.9, 162.9, 173.3, 196.3. MS(ESI): m/z = found 387.5 ([M + H]+).
N-(4-formamidobenzyl)-2-(6-methoxynaphthalen-2-yl)propenamide (4c)
Yield: 70%. White solid. Mp: 82–84 °C. 1H NMR (400 MHz, DMSO): δ1.44 (d, J = 8.00 Hz, 3H, -CH3), 3.79 (q, J = 8.00 Hz, 1H, -CH), 3.87 (s, 3H, -OCH3), 4.19–4.21 (m, 2H, -CH2), 7.07–7.16 (m, 4H, Ar-H), 7.29 (s, 1H, Ar-H), 7.46–7.49 (d, 1H, J = 8.00 Hz, Ar-H), 7.73–7.79 (m, 3H, Ar-H), 8.24 (s, 1H, -NH), 8.50 (s, 1H, -NH), 10.1 (s, 1H, -CHO). 13C NMR (100 MHz, DMSO): δ 19.0, 42.2, 45.5, 55.6, 106.1, 117.9, 119.1, 119.5, 125.8, 126.7, 128.1, 128.7, 129.6, 133.6, 135.2, 137.3, 137.9, 157.5, 159.9, 163.0, 173.8. MS(ESI): m/z = found 363.4 ([M + H]+).
N-(4-formamidobenzyl)-2-((3-(trifluoromethyl)phenyl)amino)benzamide (4d)
Yield: 75%. White solid. Mp: 77–79 °C. 1H NMR (400 MHz, DMSO): δ 4.34–4.42 (m, 2H, -CH2), 6.95–6.99 (m, 1H, Ar-H), 7.13–7.28 (m, 4H, Ar-H), 7.35–7.43 (m, 4H, Ar-H), 7.45–7.47 (m, 1H, Ar-H), 7.52–7.58 (m, 2H, Ar-H), 7.73 (brs, 1H, -NH), 8.25 (brs, 1H, -NH), 9.12 (brs, 1H, -NH), 9.69 (s, 1H, -CHO). 13C NMR (100 MHz, DMSO): δ 42.6, 114.6, 117.3, 117.5, 118.0, 119.6, 119.7, 120.4, 121.6, 122.1, 123.3, 126.0, 127.3 (q, J = 271 Hz), 128.3, 128.6, 128.9, 129.5, 130.6 (q, J = 32 Hz), 130.9, 132.4, 135.0, 137.4, 143.0, 143.6. MS(ESI): m/z = found 414.4 ([M + H]+).
2-(2-fluoro-[1,1’-biphenyl]-4-yl)-N-(4-formamidobenzyl)propenamide (4e)
Yield: 70%. White solid. Mp: 82–84 °C. 1H NMR (400 MHz, DMSO): δ1.40 (d, J = 8.00 Hz, 3H, -CH3), 3.74 (q, J = 8.00 Hz, 1H, -CH), 4.23 (s, 2H, -CH2), 7.14–7.16 (m, 2H, Ar-H), 7.25–7.27 (m, 2H, Ar-H), 7.39–7.41 (m, 1H, Ar-H), 7.46–7.55 (m, 7H, Ar-H), 8.25 (s, 1H, -NH), 8.54 (s, 1H, -NH), 10.15 (s, 1H, -CHO). 13C NMR (100 MHz, DMSO): δ 18.8, 42.3, 45.1, 115.3 (d, J = 92 Hz), 118.0, 119.5, 124.3, 126.8 (d, J = 28 Hz), 128.2, 128.7, 129.1, 129.2, 131.0, 135.1, 135.5, 137.4, 144.6, 158.1, 160.0, 160.5, 163.0, 173.2. MS(ESI): m/z = found 377.4 ([M + H]+).
2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-N-(4-formamidobenzyl) acetamide (4f)
Yield: 72%. White solid. Mp: 102–104 °C. 1H NMR (400 MHz, DMSO): δ 0.66 (t, J = 4.00 Hz, 3H, -CH3), 1.26 (t, J = 4.00 Hz, -CH3), 2.02–2.08 (m, 2H, -CH2), 2.57–2.70 (m, 2H, CH2), 2.72–2.76 (m, 1H, -CH), 2.81.2.87 (m, 2H, -CH2), 2.89–2.93 (m, 1H, -CH), 3.96–3.98 (m, 2H, -CH2), 4.17–4.32 (m, 2H, -CH2), 6.88–6.91 (m, 2H, Ar-H), 7.04–7.16 (m, 2H, Ar-H), 7.23–7.25 (m, 1H, Ar-H), 7.48–7.50 (m, 1H, Ar-H), 8.13–8.15 (m, 1H, -NH), 10.16 (s, 1H, -NH), 10.54 (s, 1H, -CHO). 13C NMR (100 MHz, DMSO): δ 8.3, 14.8, 22.4, 24.2, 31.0, 42.1, 44.4, 60.4, 76.0, 107.4, 115.9, 117.9, 119.2, 119.5, 120.1, 126.6, 127.0, 128.0, 128.5, 134.9, 137.2, 159.9, 162.9, 169.8. MS(ESI): m/z = found 420.5 ([M + H]+).
2-((2,3-dimethylphenyl)amino)-N-(4-formamidobenzyl)benzamide (4g)
Yield: 65%. White solid. Mp: 109–110 °C. 1H NMR (400 MHz, DMSO): δ 2.10 (s, 3H, -CH3), 2.27 (s, 3H, -CH3), 4.44–4.45 (m, 2H, -CH2), 6.73–6.76 (m, 1H, Ar-H), 6.83–6.86 (m, 1H, Ar-H), 6.92–6.94 (m, 1H, Ar-H), 7.04–7.08 (m, 2H, Ar-H), 7.15–7.27 (m, 2H, Ar-H), 7.29–7.32 (m, 2H, Ar-H), 7.55–7.57 (m, 1H, Ar-H), 7.72–7.74 (m, 1H, Ar-H), 8.26 (s, 1H, -NH), 9.10 (s, 1H, -NH), 9.61(s, 1H, -NH), 10.2 (s, 1H, -CHO). 13C NMR (100 MHz, DMSO): δ 14.0, 20.7, 42.5, 114.3, 117.2, 118.1, 119.6, 120.4, 125.6, 126.3, 128.3, 128.8, 129.1, 130.0, 132.5, 135.2, 137.4, 138.2, 139.7, 146.8, 159.9, 163.0, 169.5. MS(ESI): m/z = found 374.5 ([M + H]+).
N-(4-formamidobenzyl)-2-(4-((2-oxocyclopentyl)methyl)phenyl)propenamide (4h)
Yield: 68%. White solid. Mp: 107–109 °C. 1H NMR (400 MHz, CDCl3): δ 1.34 (d, J = 8.00 HZ, 3H, -CH3), 1.45–1.50(m, 1H, -CH-), 1.65–1.72 (m, 1H, -CH-), 1.83–1.95 (m, 2H, -CH2), 1.99–2.11 (m, 1H, -CH-), 2.21–2.28 (m, 1H, -CH-), 2.35–2.46 (m, 2H, -CH2), 2.93–2.97 (m, 1H, -CH-), 3.62 (q, J = 8.00 Hz, -CH), 4.18–4.20 (m, 2H, -CH2), 7.07–7.11 (m, 4H, Ar-H), 7.23–7.25 (m, 2H, Ar-H), 7.47–7,49 (m, 2H, Ar-H), 8.24 (s, 1H, -NH), 8.41–8.44 (m, 1H, -NH), 10.16 (s, 1H, -CHO). 13C NMR (100 MHz, CDCl3): δ 18.9, 20.5, 29.2, 35.0, 38.1, 42.1, 45.2, 50.5, 117.9, 119.4, 127.7, 128.0, 128.6, 129.0, 135.2, 135.4, 137.4, 138.7, 140.3, 159.9, 162.9, 173.8, 220.3. MS(ESI): m/z = found 379.5 ([M + H]+).
4.2.3. General Procedure for the Synthesis of Compounds 5a–5h
A solution of triphosgene (0.5 eq.) in CH2Cl2 (5 mL) was slowly added to a solution of compound 4 (a–h) (1.0 eq.), Et3N (4.0 equiv) and CH2Cl2 (5 mL). The resulting mixture was refluxed for 2.0 h in the dark and under a nitrogen atmosphere. Then, selenium (2.0 eq.) was added, and the mixture was refluxed for a further 12 h. TLC showed the reaction was complete. The desired compound was purified by column chromatogragraphy on silica gel.
2-(4-isobutylphenyl)-N-(4-isoselenocyanatobenzyl)propenamide (5a)
Yield: 80%. White solid. Mp: 82–84 °C. 1H NMR (400 MHz, DMSO): δ 0.86 (d, 6H, J = 8.00 Hz, 2CH3), 1.35 (d, 3H, J = 8.00 Hz, -CH3), 1.79 (q, 1H, J = 8.00 Hz, -CH), 2.41 (d, 2H, J = 8.00 Hz, -CH2), 3.64 (q, 1H, J = 8.00 Hz, -CH), 4.21–4.23 (m, 2H, -CH2), 7.08–7.10 (m, 4H, Ar-H), 7.24–7.28 (m, 4H, Ar-H), 10.06 (s, 1H, -NH). 13C NMR (100 MHz, DMSO): δ 19.0, 22.7, 30.1, 42.0, 44.7, 45.3, 125.0, 126.6, 127.5, 129.3, 136.9, 138.7, 139.8, 140.0, 173.9. MS(ESI): m/z = found 401.0 ([M + H]+).
2-(3-benzoylphenyl)-N-(4-isoselenocyanatobenzyl)propenamide (5b)
Yield: 78%. White solid. Mp: 88–90 °C. 1H NMR (400 MHz, DMSO): δ 1.41 (d, 3H, J = 8.00 Hz, -CH3), 3.81 (q, 1H, J = 8.00 Hz, -CH), 4.21–4.23 (m, 2H, -CH2), 7.10–7.12 (m, 1H, Ar-H), 7.26–7.30 (m, 2H, Ar-H), 7.52–7.60 (m, 4H, Ar-H), 7.65–7.68 (m, 2H, Ar-H), 7.73–7.78 (m, 3H, Ar-H), 8.6 (s, 1H, Ar-H), 10.07 (s, 1H, -NH). 13C NMR (100 MHz, DMSO): δ 18.9, 42.1, 45.4, 118.5, 125.1, 126.7, 127.6, 128.6, 128.9, 129.0, 130.1, 132.1, 133.2, 136.8, 137.4, 138.8, 140.9, 143.1, 170.8, 173.4, 179.1, 196.3. MS(ESI): m/z = found 448.5 ([M + H]+).
N-(4-isoselenocyanatobenzyl)-2-(6-methoxynaphthalen-2-yl)propenamide (5c)
Yield: 82%. White solid. Mp: 76–78 °C. 1H NMR (400 MHz, DMSO): δ 1.46 (d, 3H, J = 8.00 Hz, -CH3), 3.82 (q, 1H, J = 8.00 Hz, -CH), 3.87 (s, 3H, -OCH3), 4.24–4.26 (m, 2H, -CH2), 7.12–7.16 (m, 2H, Ar-H), 7.24–7.29 (m, 2H, Ar-H), 7.48–7.50 (m, 1H, Ar-H), 8.58–8.61 (m, 1H, Ar-H), 10.06 (s, 1H, -NH). 13C NMR (100 MHz, DMSO): δ 19.0, 42.2, 45.6, 55.6, 106.2, 119.1, 125.1, 125.8, 126.7, 127.0, 128.1, 128.9, 129.6, 133.6, 137.0, 137.9, 138.7, 141.0, 157.5, 173.9. MS(ESI): m/z = found 424.8 ([M + H]+).
N-(4-isoselenocyanatobenzyl)-2-((3-(trifluoromethyl)phenyl)amino)benzamide (5d)
Yield: 80%. White solid. Mp: 100–102 °C. 1H NMR (400 MHz, CDCl3): δ 4.60 (d, 2H, J = 8.00 Hz, -CH2), 6.59–6.62 (m, 1H, Ar-H), 6.82–6.86 (m, 1H, Ar-H), 7.22–7.24 (m, 1H, Ar-H), 7.31–7.48 (m, 8H, Ar-H), 9.49 (s, 1H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 43.2, 116.0, 116.4 (d, J = 4 Hz), 118.5, 118.7 (d, J = 4 Hz), 119.2, 123.1, 124.0 (q, J = 271 Hz), 126.5, 127.6, 128.8, 129.6, 129.9, 131.7 (q, J = 32 Hz), 132.8, 138.3, 142.2, 144.6, 169.3. MS (ESI): m/z = found 475.5 ([M + 1]+).
2-(2-fluoro-[1,1’-biphenyl]-4-yl)-N-(4-isoselenocyanatobenzyl)propenamide (5e)
Yield: 82%. White solid. Mp: 88–90 °C. 1H NMR (400 MHz, CDCl3): δ 1.56 (d, 3H, J = 8.00 Hz, -CH3), 3.62 (q, 1H, J = 8.00 Hz, -CH), 4.38–4.40 (m, 2H, -CH2), 5.91–5.94 (m, 1H, Ar-H), 7.10–7.21 (m, 5H, Ar-H), 7.37–7.45 (m, 4H, Ar-H), 7.51–7.54 (m, 2H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 18.5, 43.0, 46.6, 115.3 (d, J = 23 Hz), 123.6 (d, J = 4 Hz), 126.4, 127.9, 128.3 (d, J = 13Hz), 128.6 (d, J = 7 Hz), 128.8, 128.9 (d, J = 3 Hz), 129.4 (NCSe), 131.2 (d, J = 4 Hz), 135.2, 138.7, 142.4 (d, J = 7 Hz), 158.6, 161.1, 173.6. MS (ESI): m/z = found 438.7 ([M + 1]+).
2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-N-(4-isoselenocyanatobenzyl)acetamide (5f)
Yield: 78%. White solid. Mp: 107–109 °C. 1H NMR (400 MHz, CDCl3): δ 0.87 (t, J = 4.00 Hz, 3H, -CH3), 1.24 (t, J = 4.00 Hz, -CH3), 1.85–1.93 (m, 2H, -CH), 2.08–2.14 (m, 1H, -CH), 2.58–2.81 (m, 4H, 2CH2), 3.01 (s, 2H, -CH2), 4.03–4.09 (m, 2H, -CH2), 4.26 (s, 3H, -OCH3), 6.80–6.82 (m, 2H, Ar-H), 7.02–7.03 (m, 1H, Ar-H), 7.09–7.13 (m, 1H, Ar-H), 7.25–7.29 (m, 2H, Ar-H), 9.62 (s, 1H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 7.7, 14.1, 22.2, 24.1, 31.2, 42.5, 43.8, 60.4, 76.0, 107.4, 115.8, 119.8, 120.6, 126.1, 126.3, 127.0, 128.2, 128.3, 129.1, 134.8, 135.5, 138.5, 171.4. MS(ESI): m/z = found 482.1 ([M + 1]+).
2-((2,3-dimethylphenyl)amino)-N-(4-isoselenocyanatobenzyl)benzamide (5g)
Yield: 72%. White solid. Mp: 91–93 °C. 1H NMR (400 MHz, DMSO): δ 2.08 (s, 3H, -CH3), 2.26 (s, 3H, -CH3), 4.51–4.53 (m, 2H, -CH2), 6.74–6.75 (m, 1H, Ar-H), 6.83–6.85 (m, 1H, Ar-H), 6.92–6.94 (m, 1H, Ar-H), 7.04–7.09 (m, 2H, Ar-H), 7.45–7.55 (m, 3H, Ar-H), 7.71–7.76 (m, 1H, Ar-H), 9.19 (s, 1H, -NH), 9.58 (s, 1H, -NH). 13C NMR (100 MHz, DMSO): δ 14.0, 20.7, 42.4, 114.4, 116.8, 117.2, 119.6, 120.5, 125.7, 126.3, 126.8, 127.0, 128.3, 128.6, 128.9, 129.1, 130.0, 132.7, 138.2, 139.6, 142.2, 147.0, 169.7. MS(ESI): m/z = found 435.1 ([M + Na]+).
N-(4-isoselenocyanatobenzyl)-2-(4-((2-oxocyclopentyl)methyl)phenyl)propenamide (5h)
Yield: 75%. White solid. Mp: 86–88 °C. 1H NMR (400 MHz, DMSO): δ 1.36 (d, J = 8.00 Hz, 3H, -CH3), 1.45–1.51 (m, 1H, -CH), 1.66–1.73 (m, 1H, -CH), 1.82–1.93 (m, 2H, -CH2), 2.02–2.11 (m, 1H, -CH), 2.21–2.26 (m, 1H, -CH), 2.36–2.46 (m, 2H, -CH), 2.94–2.97 (m, 1H, -CH), 3.62 (q, J = 8.00 Hz, 1H, -CH), 4.46 (d, J = 8.00 Hz, 2H, -CH2), 7.11–7.13(m, 2H, Ar-H), 7.21–7.26 (m, 4H, Ar-H), 7.40–7.42 (m, 2H, Ar-H), 8.52 (s, 1H, -NH). 13C NMR (100 MHz, DMSO): δ 18.9, 20.5, 29.2, 35.0, 38.1, 42.0, 45.3, 50.5, 125.1, 126.6, 127.7, 128.1, 128.8, 129.1, 136.9, 138.8, 140.2, 140.3, 141.1, 173.9, 174.0, 179.0. MS(ESI): m/z = found 440.5 ([M + Na]+).
4.2.4. General Procedure for the Synthesis of Compounds 6a–6h
Compound 5 (a–h) (1.0 eq.) was dissolved in CH3OH (20 mL) at room temperature, then the solvent was refluxed for 4.0 h. TLC showed the reaction was complete. The desire compound was purified by column chromatogragraphy on silica gel.
O-methyl (4-((2-(4-isobutylphenyl)propanamido)methyl)phenyl) carbamoselenoate (6a)
Yield: 60%. White solid. Mp: 117–118 °C. 1H NMR (400 MHz, CDCl3): δ 0.88 (d, J = 8.00 Hz, 6H, 2CH3), 1.44 (d, 3H, J = 8.00 Hz, -CH3), 1.83 (q, 1H, J = 8.00 Hz, -CH), 2.44 (d, 2H, J = 8.00 Hz, -CH2), 3.64 (q, 1H, J = 8.00 Hz, -CH), 4.12 (s, 3H, -OCH3), 4.23–4.34 (m, 2H, -CH2), 7.07–7.12 (m, 4H, Ar-H), 7.18–7.24 (m, 4H, Ar-H), 7.51 (brs, 1H, -NH). 13C NMR (100 MHz, CDCl3): δ 17.3, 21.3, 30.1, 42.1, 44.6, 45.8, 60.7, 121.8, 126.8, 127.5, 128.9, 136.1, 136.6, 139.0, 140.2, 175.8, 191.8. MS(ESI): m/z = found 455.1 ([M + Na]+).
O-methyl (4-((2-(3-benzoylphenyl)propanamido)methyl)phenyl)carbamoselenoate (6b)
Yield: 62%. White solid. Mp: 88–90 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (d, J = 8.00 Hz, 3H, -CH3), 3.77 (q, J = 8.00 Hz, 1H, -CH), 4.12 (s, 3H, -OCH3), 4.25–4.35 (m, 2H, -CH2), 7.11–7.18 (m, 3H, Ar-H), 7.45–7.51 (m, 4H, Ar-H), 7.60–7.64 (m, 3H, Ar-H), 7.72–7.76 (m, 3H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 17.4, 42.2, 45.9, 60.7, 121.8, 124.1, 127.7, 128.2, 128.3, 128.7, 129.7, 131.4, 132.5, 135.9, 136.6, 137.3, 137.7, 142.2, 174.9, 191.8, 197.1. MS (ESI): m/z = found 503.0 ([M + Na]+).
O-methyl (4-((2-(6-methoxynaphthalen-2-yl)propanamido)methyl)phenyl)carbamoselenoate (6c)
Yield: 55%. White solid. Mp: 93–95 °C. 1H NMR (400 MHz, CDCl3): δ 1.52 (d, J = 8.00 Hz, 3H, -CH3), 3.79 (q, J = 8.00 Hz, 1H, -CH), 3.87 (s, 3H, -OCH3), 4.12 (s, 3H, -OCH3), 4.26–4.30 (m, 2H, -CH2), 7.08–7.17 (m, 5H, Ar-H), 7.40–7.43 (m, 1H, Ar-H), 7.65–7.71 (m, 3H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 17.4, 42.2, 46.1, 54.4, 60.7, 105.2, 118.5, 121.7, 124.1, 125.4, 125.8, 126.8, 127.6, 128.8, 129.0, 133.8, 136.1, 136.7, 157.7, 175.7, 191.7. MS(ESI): m/z = found 479.1 ([M + Na]+).
O-methyl (4-((2-((3-(trifluoromethyl)phenyl)amino)benzamido)methyl)phenyl)carbamoselenoate (6d)
Yield: 65%. White solid. Mp: 101–102 °C. 1H NMR (400 MHz, CDCl3): δ 4.12 (s, 3H, -OCH3), 4.46–4.48 (m, 2H, -CH2), 6.89–6.93 (m, 1H, Ar-H), 7.14–7.16 (m, 1H, Ar-H), 7.21–7.38 (m, 9H, Ar-H), 7.62–7.65 (m, 1H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 42.5, 64.8, 114.4 (J = 4.0 Hz), 116.9, 117.2 (J = 4.0 Hz), 120.0, 121.3, 121.5, 121.9, 122.9, 125.6, 127.8, 128.6, 129.8, 131.2 (q, J = 32 Hz, -CF3), 131.9, 136.29 (d, J = 58 Hz), 143.1 (d, J = 32 Hz). MS (ESI): m/z = found 530.1 ([M + Na]+).
O-methyl (4-((2-(2-fluoro-[1,1’-biphenyl]-4-yl)propanamido)methyl)phenyl)carbamoselenoate (6e)
Yield: 60%. White solid. Mp: 72–74 °C. 1H NMR (400 MHz, CDCl3): δ 1.56 (d, 3H, J = 8.00 Hz, -CH3), 3.62 (q, J = 8.00 Hz, 1H, -CH), 4.21 (s, 3H, -OCH3), 4.35–4.40 (m, 2H, -CH2), 5.91–5.97 (m, 1H, -NH), 7.10–7.18 (m, 6H, Ar-H), 7.37–7.53 (m, 6H, Ar-H), 9.22 (brs, 1H, -NH). 13C NMR (100 MHz, CDCl3): δ 18.8, 42.2, 45.1, 61.6, 115.3 (d, J = 23 Hz), 122.6, 124.3, 125.2, 126.7, 127.0, 127.7, 128.1 (d, J = 7 Hz), 128.9, 129.1, 129.2, 130.9 (d, J = 3 Hz), 135.5, 136.8 (d, J = 19 Hz), 140.9, 144.6, 158.1, 160.5, 173.2 (d, J = 16 Hz), 190.7. MS (ESI): m/z = found 493.0 ([M + Na]+).
O-methyl (4-((2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetamido)methyl)phenyl)carbamoselenoate (6f)
Yield: 62%. White solid. Mp: 83–85 °C. 1H NMR (400 MHz, CDCl3): δ 0.87 (t, J = 4.00 Hz, 3H, -CH3), 1.24 (t, J = 4.00 Hz, -CH3), 1.87–2.14 (m, 2H, -CH2), 2.63–2.80 (m, 4H, 2CH2), 3.03 (s, 2H, -CH2), 4.03–4.07 (m, 2H, -CH2), 4.26 (s, 3H, -OCH3), 6.78–6.85 (m, 3H, Ar-H), 7.00–7.13 (m, 2H, Ar-H), 7.24–7.29 (m, 2H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 8.3, 14.9, 22.4, 24.2, 31.1, 42.0, 44.3, 60.4, 61.7, 76.0, 107.4, 115.9, 119.2, 120.1, 122.5, 126.5, 127.0, 127.9, 128.7, 134.9, 137.0, 141.0, 169.9, 190.6. MS(ESI): m/z = found 536.1 ([M + Na]+).
O-methyl (4-((2-((2,3-dimethylphenyl)amino)benzamido)methyl)phenyl)carbamoselenoate (6g)
Yield: 62%. White solid. Mp: 83–85 °C. 1H NMR (400 MHz, CDCl3): δ 2.13 (s, 3H, -CH3), 2.28 (s, 3H, -CH3), 4.15 (s, 3H, -OCH3), 4.50 (s, 2H, -CH2), 6.69–6.73 (m, 1H, Ar-H), 6.79–6.81 (m, 1H, Ar-H), 6.91–6.93 (m, 1H, Ar-H), 7.02–7.05 (m, 2H, Ar-H). 7.17–7.21 (m, 1H, Ar-H), 7.25–7.32 (m, 4H, Ar-H), 7.57–7.61 (m, 1H, Ar-H). 13C NMR (100 MHz, CDCl3): δ 12.6, 19.3, 42.3, 60.7, 114.3, 116.8, 117.3, 120.6, 121.9, 125.4, 125.6, 127.7, 128.1, 130.3, 131.9, 136.3, 136.6, 137.7, 139.5, 146.8, 170.5, 191.8. MS (ESI): m/z = found 490.1 ([M + Na]+).
O-methyl (4-((2-(4-((2-oxocyclopentyl)methyl)phenyl)propanamido)methyl)phenyl)carbamoselenoate (6h)
Yield: 68%. White solid. Mp: 109–111 °C. 1H NMR (400 MHz, CD3OD): 1.34 (d, 3H, J = 8.00 Hz, -CH3), 1.45–1.50 (m, 1H, -CH-), 1.67–1.72 (m, 1H, -CH-), 1.83–1.92 (m, 2H, -CH2), 2.02–2.11(m, 1H, -CH-), 2.21–2.26 (m, 1H, -CH-), 2.36–2.44 (m, 2H, -CH2), 2.94–2.97 (m, 1H, -CH-), 3.62–3.63 (m, 1H, -CH-), 4.09 (m, 3H, -OCH3), 4.18 (s, 2H, -CH2), 7.11–7.13 (m, 4H, Ar-H), 7.23–7.27 (m, 4H, Ar-H), 8.44 (s, 1H, -NH), 11.7 (s. 1H, -NH). 13C NMR (100 MHz, CD3OD): 18.9, 20.5, 29.2, 35.0, 38.1, 42.1, 45.2, 50.5, 61.6. 122.6, 124.7, 127.7, 128.0, 129.0, 136.7, 137.1, 138.7, 140.3, 173.9, 190.7. MS (ESI): m/z = found 495.0 ([M + H]+).
4.3. Cell Lines and Growth Conditions
Exponentially growing cells were harvested and plated on 96-well plates at a concentration of 1 × 104 cells/well. After 24 h incubation at 37 °C under a humidified 5% CO2 to allow cell attachment, the cells in the wells were treated with target compounds at various concentrations for 48 h. The concentration of DMSO was always kept below 1.25%, which was found to be non-toxic to the cells. Three hours prior to experiment termination, MTT solution (20 μL of 5.0 mg/mL solution) was added to each well and incubated at 37 °C. At the termination timepoint, the medium/MTT mixtures were removed, and the formazan crystals formed by the mitochondrial dehydrogenase activity of vital cells were dissolved in 100 μL of DMSO per well. The optical densities were measured at 570 nm using a 96-well multiscanner (Dynex Technologies, MRX Revelation; Chantilly, VA, USA).
4.4. Detection of Bcl-2, IL-2 and Caspase-3 Molecular Biomarkers in MCF-7 Cells
Bcl-2, IL-2 and caspase-3 cells were evaluated in MCF-7 cells treated with the corresponding target compounds and incubated for 48 h and compared with their levels in control, untreated MCF-7 cell line. The cells were harvested by trypsinization and lysed by lysate buffer (Beyotime Biotech, Nanjing, China). Protein levels of Bcl-2, IL-2 and caspase-3 were measured using enzyme-linked immunosorbent assay (ELISA) by multifunctional enzyme marker (Molecular Devices i3, San Jose, CA, USA) at a wavelength of 570 nm.
4.5. DPPH Free Radical Scavenging Activity
DPPH free radical scavenging activity of corresponding compounds was measured according to the previously reported method with little optimization. In brief, 20 mL of test samples at different concentrations were mixed with 180 mL of or DPPH solution for 30 min in the dark. Then, the change in absorbance at 517 nm for DPPH was measured on a microplate reader. Ascorbic acid (vitamin C) and ebselen were used as a positive control; DMSO was used as a negative control.
4.6. Bleomycin-Dependent DNA Damage
The reaction mixture contained DNA (0.5 mg/mL), bleomycin sulfate (0.05 mg/mL), MgCl2 (5 mM), FeCl3 (50 mM), and tested compound in a conc. of 0.1 mg/mL. L-ascorbic acid was used as positive control. The mixture was incubated at 37 °C for 1 h. The reaction was terminated by addition of 0.05 mL EDTA (0.1 M). The color was developed by adding 0.5 mL TBA (1% w/v) and 0.5 mL HCl (25% v/v), followed by heating at 80 °C for 30 min. After cooling in ice water, the extent of DNA damage was measured by the increase in absorbance at 532 nm.
4.7. Molecular Modeling
4.7.1. Protein and Ligand Preparation
The mammalian TrxR1 protein (PDB ID: 1H6V) used for docking was obtained from Protein Data Bank. The original structure was prepared using Protein Preparation Wizard in Maestro 11.5 (Schrödinger Release 2018-1: Maestro, Schrödinger, LLC, New York, NY, USA, 2018), with all but one subunit (E) discarded, bond orders assigned, hydrogens added, ionization and tautomerization state adjusted, hydrogen bond assignment optimized, waters removed, and structure minimized.
The LigPrep utility in Maestro 11.5 was used to perform ligand preparation, applying an OPLS3 force field. The generation of tautomers and possible ionization states was mediated by Epik utility. All stereoisomers were considered to be generated, followed by minimization of the resulting 3D conformations. There was no filtration process during preparation.
4.7.2. Ligand Docking
The docking task was carried out in Discovery Studio 2018 (Dassault Systèmes BIOVIA, Discovery Studio 2018, San Diego: Dassault Systèmes, 2018). The prepared TrxR1 protein was typed in CHARMm forcefield and the docking site was defined as a sphere with center coordinates X: 27.757, Y: 6.510, Z: 33.698 and a radius of 15 Å. Using Flexible Docking protocol, the residue sidechains within the site sphere were allowed to move. Ten protein conformations were created, with a maximum alteration of 8 residues. The FAST method was adopted; up to 25 conformations per ligand were generated with an energy threshold of 20 kcal. With all other parameters as default, ligands were preliminarily docked into each protein structure. After the removal of similar poses by clustering, the remaining complexes were refined and minimized, leading to a total of 133 final poses.
4.7.3. Result Analysis
The resulting 133 poses were clustered by ligand (53 for 3 h, 40 each for 2 h and 3 h) and visualized in Maestro 11.5. For each of the poses, the distance between the compound’s selenium atom and the sulfur atom of either Cys497 or Cys498 was calculated as a measurement of covalent bonding probability. Any complex with less than 5 Å of the distance above was counted potentially reactive. For each ligand, average –CDocker energy and average selenium–sulfur distance were calculated; the latter was –CDocker energy weighted.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27144328/s1, Content of supporting information: Copies of 1H, 13C NMR and MS spectra of compounds.
Author Contributions
Conceptualization, all authors; methodology, Y.N., S.L., Y.L., M.Z., Y.Z. and X.H.; software, Y.N., S.L. and X.H.; validation, Y.N., Y.Z. and X.H.; investigation, Y.N., S.L. and Y.L.; writing, Y.N., S.L., Y.L., M.Z., X.L., Y.Z. and X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was made possible through the financial support of National Natural Science Foundation of China (Grant No.: 21302065) and Shenzhen Fushan Biological Technology Co., Ltd. (Shenzhen, China).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request. 1H, 13C NMR and MS spectra of compounds are available from the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Statistical Analysis
MTT data were given as mean ± SD of three independent experiments, graphs and curve fitting were using origin Version 8.0 (OriginLab Corporation, Northampton, MA, USA). p value less than 0.05 was considered statistically significant.
References
- Maniar, K.H.; Jones, I.A.; Gopalakrishna, R.; Vangsness, C.T. Lowering side effects of NSAID usage in osteoarthritis: Recent attempts at minimizing dosage. Expert Opin. Pharmacother. 2018, 19, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Benbow, T.; Campbell, J. Microemulsions as transdermal drug delivery systems for nonsteroidal anti-inflammatory drugs (NSAIDs): A literature review. Drug Dev. Ind. Pharm. 2019, 45, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Waddell, W.R.; Loughry, R.W. Sulindac for polyposis of the colon. J. Surg. Oncol. 1983, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Palayoor, S.T.; Tofilon, P.J.; Coleman, C.N. Ibuprofen-mediated reduction of hy- poxia-inducible factors HIF-1alpha and HIF-2alpha in prostate cancer cells. Clin. Cancer Res. 2003, 9, 3150–3157. [Google Scholar]
- Takiuchi, T.; Blake, E.A.; Matsuo, K.; Sood, A.K.; Brasky, T.M. Aspirin use and endometrial cancer risk and survival. Gynecol. Oncol. 2018, 148, 222–232. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer. prevention and cancer promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [Green Version]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A summary of new findings on the biological effects of selenium in selected animal species-a critical review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The Epidemiology of Selenium and Human Cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar]
- Vinceti, M.; Filippini, T.; del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Murdolo, G.; Bartolini, D.; Tortoioli, C.; Piroddi, M.; Torquato, P.; Galli, F. Selenium and Cancer Stem Cells. Adv. Cancer Res. 2017, 136, 235–257. [Google Scholar] [PubMed]
- El-Bayoumy, K. Overview: The late Larry C. Clark showed the bright side of the moon element (selenium) in a clinical cancer prevention trial. Nutr. Cancer 2001, 40, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Q.; Liu, J.; Liu, L.; Zhao, P.; Cao, Y.; Liu, Y.; Qi, C. Preparation of two organoselenium compounds and their induction of apoptosis to SMMC-7221 cells. Biol. Trace Elem. Res. 2013, 154, 304–311. [Google Scholar] [CrossRef]
- Desai, D.; Salli, U.; Vrana, K.E.; Amin, S. SelSA, selenium analogs of SAHA as potent histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2044–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.T.; Sinha, R.; Papp, K.; Facompre, N.D.; Desai, D.; El-Bayoumy, K. Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. Int. J. Cancer 2007, 120, 1410–1417. [Google Scholar] [CrossRef]
- Singh, U.; Null, K.; Sinha, R. In vitro growth inhibition ofmouse mammary epithelial tumor cells by methylseleninic acid: Involvement of protein kinases. Mol. Nutr. Food Res. 2008, 52, 1281–1288. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, A.; Desai, D.; Madhunapantula, S.V.; Huh, S.J.; Robertson, G.P.; Amin, S. Synthesis and antiproliferative activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J. Med. Chem. 2008, 51, 7820–7826. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Plano, D.; Lamberto, I.; Font, M.; Encio, I.; Palop, J.A.; Sanmartin, C. Sulfur and selenium derivatives of quinazoline and pyrido [2,3-d]pyrimidine: Synthesis and study of their potential cytotoxic activity in vitro. Eur. J. Med. Chem. 2012, 47, 283–298. [Google Scholar] [CrossRef]
- Desai, D.; Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Amin, S.; Sinha, R. Synthesis and antitumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int. J. Cancer 2010, 127, 230–238. [Google Scholar] [CrossRef]
- Plano, D.; Karelia, D.N.; Pandey, M.K.; Spallholz, J.E.; Amin, S.; Sharma, A.K. Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 2016, 59, 1946–1959. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Li, X.; Zhong, M.; Lu, Y.; Yang, J.; Zhang, Y.; He, X. Synthesis of NSAIDs-Se derivatives as potent anticancer agents. Med. Chem. Res. 2018, 27, 2071–2078. [Google Scholar] [CrossRef]
- Nie, Y.; Zhong, M.; Li, S.; Li, X.; Zhang, Y.; Zhang, Y.; He, X. Synthesis and potential. anticancer activity of some novel selenocyanates and diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef]
- He, X.; Zhong, M.; Li, S.; Li, X.; Li, Y.; Li, Z.; Gao, Y.; Ding, F.; Wen, D.; Lei, Y.; et al. Synthesis and biological evaluation of organoselenium (NSAIDs-SeCN and SeCF3) derivatives as potential anticancer agents. Eur. J. Med. Chem. 2020, 208, 112864. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Nie, Y.; Zhong, M.; Li, S.; Li, X.; Guo, Y.; Liu, Z.; Gao, Y.; Ding, F.; Wen, D.; et al. New organoselenides (NSAIDs-Se derivatives) as potential anticancer agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2021, 218, 113384. [Google Scholar] [CrossRef]
- Wittmann, C.; Chockley, P.; Singh, S.K.; Pase, L.; Lieschke, G.J.; Grabher, C. Hydrogen peroxide in inflammation: Messenger, guide, and assassin. Adv. Hematol. 2012, 2012, 541471. [Google Scholar] [CrossRef]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium-and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem. A Eur. J. 2010, 16, 10920–10928. [Google Scholar] [CrossRef]
- Pathania, D.; Sechi, M.; Palomba, M.; Sanna, V.; Berrettini, F.; Sias, A.; Taheri, L.; Neamati, N. Design and discovery of novel quinazolinedione-based redox modulators as therapies for pancreatic cancer. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules 2010, 15, 7292–7312. [Google Scholar] [CrossRef]
- Lagunes, I.; Begines, P.; Silva, A.; Galán, A.R.; Puerta, A.; Fernandes, M.X.; Maya, I.; Fernández-Bolaños, J.G.; López, Ó.; Padrón, J.M. Selenocoumarins as new multitarget antiproliferative agents: Synthesis, biological evaluation and in silico calculations. Eur. J. Med. Chem. 2019, 179, 493–501. [Google Scholar] [CrossRef]
- Meriane, D.; Genta-Jouve, G.; Kaabeche, M.; Michel, S.; Boutefnouchet, S. Rapid identification of antioxidant compounds of Genista saharae coss. & dur. By combination of DPPH scavenging assay and HPTLC-MS. Molecules 2014, 19, 4369–4379. [Google Scholar]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-analysis of polyphenolic compounds in gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014, 4, 273–281. [Google Scholar] [PubMed]
- Huang, D.; Qu, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of. antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Schaich, K.M. Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS(t*). J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Mira, A.; Gimenez, E.M.; Bolzan, A.D.; Bianchi, M.S.; Lopez-Larraza, D.M. Effect of thiol compounds on bleomycin-induced DNA and chromosome damage in human cells. Arch. Environ. Occup. Health 2013, 68, 107–116. [Google Scholar] [CrossRef]
- Quispe-Tintaya, W.; Lee, M.; Dong, X.; Weiser, D.A.; Vijg, J.; Maslov, A.Y. Bleomycin-induced genome structural variations in normal, non-tumor cells. Sci. Rep. 2018, 8, 16523. [Google Scholar] [CrossRef] [Green Version]
- Laffon, B.; Valdiglesias, V.; Pásaro, E.; Méndez, J. The Organic selenium compound selenomethionine modulates bleomycin-induced DNA damage and repair in human leukocytes. Biol. Trace. Elem. Res. 2010, 133, 12–19. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Rocha, J.B.T. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef]
- Hodage, A.S.; Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Synthesis, characterization and structures of 2-(3,5-dimethylpyrazol-1-yl)ethylseleno derivatives and their probable glutathione peroxidase (GPx) like activity. Org. Biomol. Chem. 2011, 9, 2992–2998. [Google Scholar] [CrossRef]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like activity of selenides and selenoxides: Experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Wessjohann, L.A.; Eubel, J.; Brandt, W. Mutational studies confirm the catalytic triad in the human selenoenzyme thioredoxin reductase predicted by molecular modeling. Chem. Biochem. 2006, 7, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Brandt, W.; Wessjohann, L.A. The functional role of selenocysteine (Sec) in the catalysis mechanism of large thioredoxin reductases: Proposition of a swapping catalytic triad including a Sec-His-Glu state. Chembiochem 2005, 6, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sandalova, T.; Zhong, L.; Lindqvist, Y.; Holmgren, A.; Schneider, G. Three dimensional structure of a mammalian thioredoxin reductase: Implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 9533–9538. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, S.; Negm, A.; Ashmawy, A.M.; Ahmed, D.M.; Wessjohann, L.A. Combinatorial synthesis, in silico, molecular and biochemical studies of tetrazole-derived organic selenides with increased selectivity against hepatocellular carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).