Abstract
As one of the abundant and inexpensive metals on the earth, copper has demonstrated broad applications in synthetic chemistry and catalysis. Among these copper-catalyzed advances, copper carbenes are versatile and reactive intermediates that can mediate a variety of transformations, which have attracted much attention in the past decades. The present review summarizes two different reaction models that take place between a copper carbene intermediate and alkyne species, including the cross-coupling reaction of copper carbene intermediate with terminal alkyne, and the addition of copper carbene intermediate onto the C–C triple bond. This article will cover the profile from 2010 to 2021 by placing emphasis on the detailed catalytic models and highlighting the synthetic applications offered by these practical and mild methods.
1. Introduction
Transition-metal-catalyzed transformations of carbon-carbon triple bond have been presented as one of the most effective and prominent tools for the construction of functionalized molecules and fine chemicals [1,2,3,4]. Alkynes have low Csp–H pKa (~25), and the carbon-carbon triple bond could be selectively activated by π-acidic transition metals. These unique characteristics have rendered nucleophilic and/or electrophilic properties of alkynes, which make them versatile building blocks in the synthesis of natural products and biologically active molecules [5,6]. In this area, only a few metal complexes based on late transition metals such as platinum and coinage metals could enable the activation of alkynes [7,8,9].
On the other hand, metal carbenes, which are generated in situ via transition-metal-catalyzed transformations from different carbene precursors, are powerful reaction intermediates in organic synthesis [10,11,12,13]. Catalytic functionalization of alkynes with metal carbene species has attracted substantial attention in both academic research and industry during the past decades [14,15,16,17,18,19]. In comparison to the catalytic transformations with precious metal catalysts (e.g., gold, rhodium, and platinum complexes) [20,21,22,23,24], the use of copper catalysts is much more appealing, either for the formation of a copper carbene intermediate or for the activation of alkyne species, because of its lower cost, less toxicity, and easier accessibility. The typical transformations in this area include alkynylation [25,26,27,28,29], cycloaddition [30,31,32,33,34], allene formation [35,36,37,38,39], and many others [40,41,42,43,44].
The copper carbene species, first identified by Roy et al. in 1906 [45], were generated from the decomposition of ethyl diazoacetate in the presence of copper dust above 80 °C heating conditions. However, the development in this area was sluggish during the last century (Figure 1). Until 2000, with abundant copper salts such as Cu(acac)2, Cu(hafaca)2,
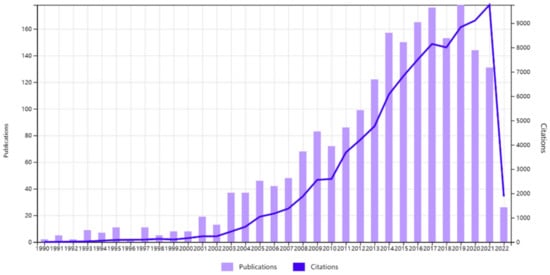
Figure 1.
Collected publications and citations report of copper carbene from Web of Science (1990–2021).
CuOTf, Cu(OTf)2, CuPF6 (see Appendix A, Table A1 for details), and others, suitable for the development of copper carbene chemistry, a rapid growth has been achieved in this area [46,47,48]. In addition, the advancement made in modern era ligand design further accelerated the progress of copper carbene chemistry [49,50,51,52,53,54,55,56,57,58], especially in catalytic asymmetric versions. A variety of stereoselective reactions have been realized with copper carbene intermediates, including X–H insertion [59,60,61,62,63,64], cyclopropanation [65,66], cycloaddition [67,68,69], ylide formation [70,71,72], and others [73,74]. The representative advances in this area have been summarized by Doyle [75,76,77], Zhou [78,79], Wang [80,81,82], Pérez [83,84], and Davies [85,86]. However, no topic review article on the copper carbene reaction with alkyne has been reported so far. This review article will summarize and discuss two distinct reaction modes. Firstly, the cross-coupling reaction of a copper carbene intermediate with terminal alkynes delivers alkynoate or allenoate copper intermediates, and each of these intermediates could be followed by protonation, nucleophilic substitution, electrophilic addition, or elimination process, yielding functionalized alkynes (Scheme 1, paths a and b) and allenes (Scheme 1, paths c and d), respectively. Secondly, the addition of a copper carbene intermediate onto the C–C triple bond, which involves cyclopropenation or carbene/alkyne metathesis process, forms complex molecules with structural diversity through cascade transformations (Scheme 1, paths e and f). This article will cover the profile from 2010 to 2021 by placing emphasis on the detailed catalytic models and highlighting the synthetic applications offered by these practical and useful methods.
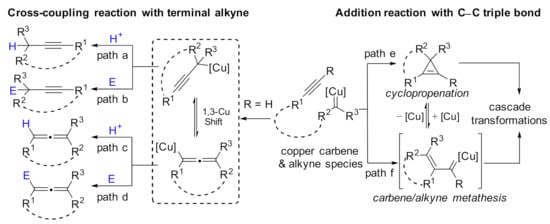
Scheme 1.
Catalytic transformations of copper carbene species with alkyne.
2. Cross-Coupling Reaction of Copper Carbene Intermediate with Terminal Alkyne
Copper-catalyzed cross-coupling reaction of a copper carbene intermediate with terminal alkynes was one of the most powerful protocols for the construction of C–C bonds [87]. However, in early works, a mixture of alkynoates and allenoates was generated in combined moderate yields under harsh reaction conditions [88]. Until 2004, Fu reported the first example of the copper-catalyzed coupling reaction of terminal alkynes with diazo esters or diazo amides to yield 3-alkynoate or 3-butynamide products selectively with minimal amount of allene byproducts under no-basic conditions [89]. Consequently, a variety of copper-catalyzed coupling reactions of terminal alkynes with various carbene precursors have been developed independently.
2.1. Alkynylation
2.1.1. Alkynylation Terminated by Protonation
In 2012, Wang reported a copper-catalyzed cross-coupling of N-tosylhydrazones 1 with trialkylsilylethynes 2, leading to the alkynylated products 4 via the formation of C(sp)–C(sp3) bonds (Scheme 2). Mechanism study shows that migratory insertion of copper carbene species gives the alkynoate copper intermediate 3, and sequential protonation affords the target products 4. This coupling reaction proceeded efficiently with N-tosylhydrazones derived from aromatic and aliphatic aldehydes or ketones in moderate to excellent yields without detecting the formation of allene byproducts 5. However, when a tert-butyl substituted alkyne 7 was employed, the corresponding allene product 8 was formed selectively [90].
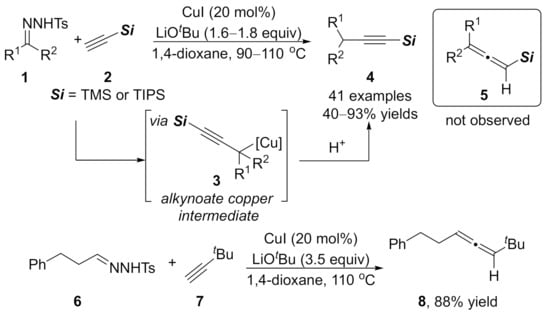
Scheme 2.
Cross-coupling reaction of N-tosylhydrazones with terminal alkynes.
Later in 2014, Zhou reported a copper-catalyzed coupling reaction using dialkoxycarbenes 9 as carbene precursor [91], which provides unsymmetrical propargylic acetals 11 in moderate to good yields (Scheme 3).
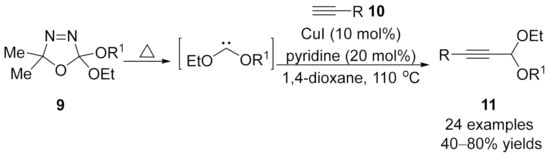
Scheme 3.
Cross-coupling reaction of dialkoxycarbenes with terminal alkynes.
In 2018, an asymmetric coupling reaction of N-tosylhydrazones 12 with terminal alkynes 13 was achieved by Uozumi and co-workers using chiral copper(I)/phosphoramidite complex as the chiral catalyst (Scheme 4), and optically active alkynylated product 14 was generated in moderate to good yields and enantioselectivities [92].
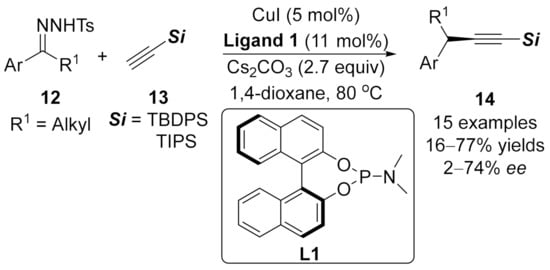
Scheme 4.
Chiral Cu(I)/phosphoramidite complex catalyzed asymmetric coupling reaction.
2.1.2. Alkynylation Terminated by Electrophilic Addition
Beyond the copper-catalyzed alkynylation terminated by protonation, the alkynoate copper intermediates, formed in situ from copper carbene species and terminal alkynes, could be intercepted through a nucleophilic substitution or electrophilic addition process. In 2015, Wang and co-workers contributed a three-component cross-coupling reaction of terminal alkyne with α-diazo ester and alkyl halide or Michael acceptor [93]. In this transformation, α-diazoesters 15 react firstly with the (triisopropylsilyl)acetylene 16 through a migratory insertion process to form the alkynoate copper intermediate 17, followed by a nucleophilic substitution with alkyl halides 18 or Michael addition with electron-deficient alkenes 20 to produce the three-component products 19 and 21, respectively (Scheme 5). This transformation represents a highly efficient method for the construction of alkynylation products with an all-carbon quaternary center in moderate to high yields. Notably, the copper catalyst works as the only catalyst to install two new C–C bonds on the carbenic carbon in this reaction.
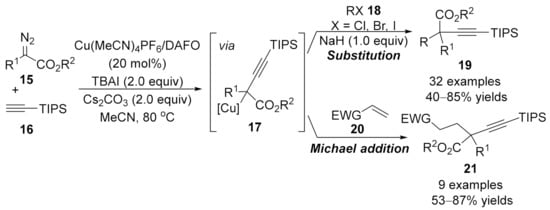
Scheme 5.
Copper-catalyzed alkynylation terminated by nucleophilic substitution/Michael addition.
In 2018, Hu and co-workers reported a copper-catalyzed three-component [1+2+2]-cycloaddition of trifluoromethyl diazo compounds 22 with terminal alkynes 23 and nitrosoarenes 24 [94]. With this method, a series of trifluoromethyl-substituted dihydroisoxazoles 26 were obtained in high yields under mild conditions. Mechanistically, electrophilic trapping of the alkynoate copper intermediate by nitrosobenzenes was proposed as the key step in this cascade transformation, which forms a proposed intermediate 25, followed by a copper catalyzed intramolecular annulation to deliver the target products 26 (Scheme 6).
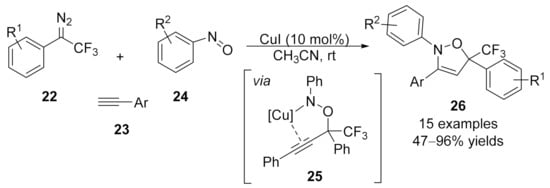
Scheme 6.
Three-component reaction of diazo compound with terminal alkyne and nitrosobenzene.
One year later, the same group reported a copper-catalyzed three-component reaction of terminal alkynes 10 with α-diazoamides 27 and isatin ketimines 28 in 2019 [95]. A series of alkynyl-containing 3,3-disubstituted oxindoles 30 were efficiently formed in high yields and diastereoselectivities through a Mannich type trapping of an in situ generated alkynoate copper intermediate 29 (Scheme 7).
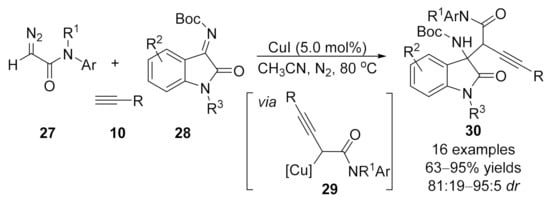
Scheme 7.
Copper-catalyzed three-component reaction of α-diazoamide with terminal alkyne and isatintetimine.
2.1.3. Alkynylation Terminated by β-Elimination
Besides the intermolecular trapping reactions of the alkynoate copper intermediate, β-H elimination could occur with this reactive species, providing a variety of conjugated enynes. Representative advances in this area have been reported by Wang’s group [96,97,98]. In 2015, they reported a copper-catalyzed cross-coupling reaction of terminal alkynes 9 with trifluoromethyl ketone N-tosylhydrazones 31, which provides an efficient synthesis of 1,1-difluoro-1,3-enyne derivatives 33 under mild reaction conditions [96]. Mechanistically, the alkynoate copper intermediate 32 was generated in situ through a migratory insertion of the copper carbene intermediate, followed by β-F elimination, leading to the gem-difluoroolefination products 33 in moderate to high yields [Scheme 8, Eq. (a)]. Later, the author reported a copper-catalyzed three-component reaction of a (triisopropylsilyl)acetylene 16 with Ethyl diazoacetate 34 (EDA) and aldehydes 35 that provided an efficient method for the synthesis of α-alkynyl-α, β-unsaturated esters 37 [97]. In this cascade reaction, nucleophilic aldol addition of an alkynoate copper intermediate with aldehyde 35 formed product 36, which delivered the desired products 37 as a single (E)-stereoisomers through an elimination process in good to excellent yields [Scheme 8, Eq. (b)]. In the same year, an analogous cross-coupling reaction with α-diazo phosphonates 38 instead of EDA through a sequential alkynylation/aldol addition/Horner−Wadsworth−Emmons (HWE) type reaction was disclosed by the same group [98]. This method provided straightforward access to conjugated enynes 40 with good stereoselectivity and excellent functional group compatibility [Scheme 8, Eq. (c)]. Moreover, one C–C bond and one C=C bond were formed successively in a one-pot manner, making those novel enynes synthesis methods practically useful.
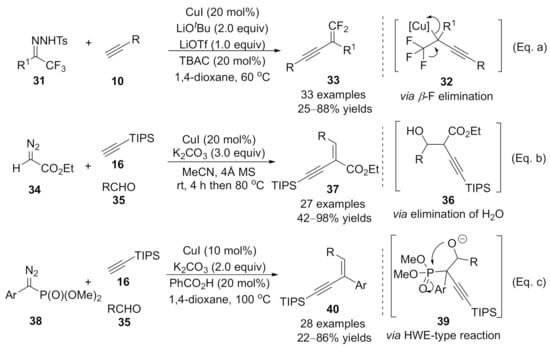
Scheme 8.
Copper-catalyzed alkynylation terminated by β-elimination. (a) Coupling reaction of N-tosylhydrazone with alkyne. (b) Coupling reaction of diazoacetate with alkyne. (c) Coupling reaction of α-diazo phosphonate with alkyne.
2.2. Allenylation
2.2.1. Allenylation Terminated by Protonation
As a complementary to Fu’s method for the selective synthesis of alkynoates [89], in 2011, Fox’s group reported a selective coupling reaction of α-substituted-α-diazoesters 41 with terminal alkynes 10 to the syntheses of allenoates in the presence of Cu(II)(trifluoroacetylacetonate)2 and 3,6-di(2-pyridyl)-s-tetrazine L2 in DCE [99]. As a result, allenoates 43 were obtained as the main products with slight traces of the alkynoates. Key to the development of this selective method was the recognition of an adventitious base, potassium carbonate, which improved the selectivity of isomerization to form the allenoate products (Scheme 9).
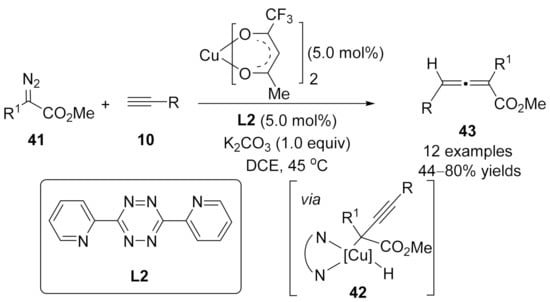
Scheme 9.
Cross-coupling reaction of copper carbene with terminal alkynes for the synthesis of allenoates.
Later in 2013, an efficient copper-catalyzed cross-coupling between diazoacetamides 44 and terminal alkynes 10 under ligand-free conditions was developed by Sun [100]. This method provided a practical method for the assembly of substituted 3-butynamides 45 and dienamides 46. Interestingly, when sodium carbonate was added to the reaction mixture, the allenic compounds 46 were obtained as the main products. However, alkyne products 45 were generated as the major products in the absence of this base (Scheme 10). Moreover, the alkynoate compounds 45 could be smoothly converted into the isomeric allenes 46 in the presence of sodium carbonate without assistance of the copper catalyst.
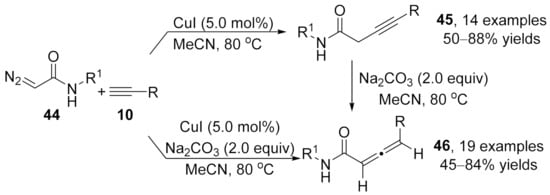
Scheme 10.
Copper-catalyzed divergent cross-coupling reaction of diazoacetamides with terminal alkynes.
In the same year, Wang and co-workers developed a series of synthetic methods to form substituted allenes under optimized conditions in the presence of copper(I) complexes [101,102,103,104]. Diffident types of substituted diazo compounds and N-tosylhydrazones were employed as the carbene precursors for the coupling with various terminal alkynes, delivering the allenoic derivatives in good yields with a wide range of functional group tolerance (Scheme 11). Notably, ethyne 54 was also a compatible substrate for this reaction, which leads to a new synthetic method for the synthesis of terminal allenes 55 in moderate to excellent yields [104]. However, using one equivalent amount of CuI with DMF as the solvent is critical to the success of this transformation [Scheme 11, Eq. (d)].
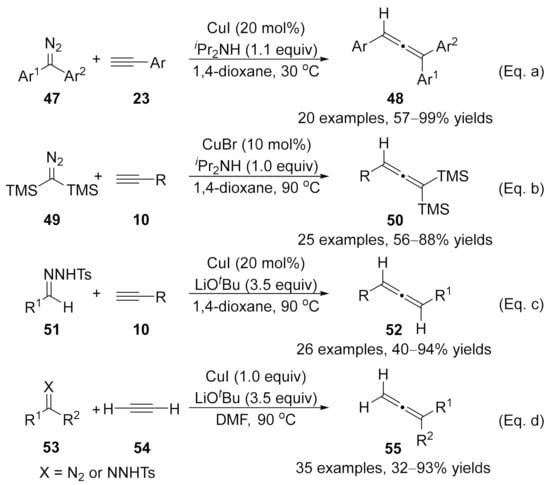
Scheme 11.
Copper-catalyzed cross-coupling reaction for the syntheses of substituted allenes. (a) Cross-coupling reaction of diaryl diazo compound with alkyne. (b) Cross-coupling reaction of diazo compound 49 with alkyne. (c) Cross-coupling reaction of N-tosylhydrazone 51 with alkyne. (d) Cross-coupling reaction of 53 with acetylene.
In 2015, a copper-catalyzed coupling reaction between flow-generated unstabilized diazo compounds and terminal alkynes was reported by Ley’s group, providing a practical method for the synthesis of di- and tri-substituted allenes 59 in high yields under mild conditions [105]. The unstable diazo compounds 57 were generated in situ from hydrazones 56 through oxidation with activated MnO2. Then, the above solution was injected directly into the other reaction mixture, which contained terminal alkynes 58, base, and CuI catalyst. The reaction delivered the allene products 59 in good to excellent yields. To highlight the selectivity and functional group compatibility of this protocol, norethindrone and propargylated quinine were successfully applied to the optimal reaction conditions, generating the corresponding products in 63% and 82% yields, respectively (Scheme 12).
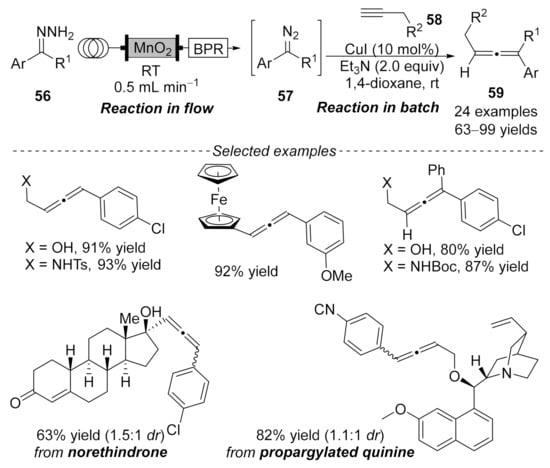
Scheme 12.
Copper-catalyzed cross-coupling reaction via flowing chemistry.
In addition to the diazo compounds, conjugated eneyne ketones 60 were introduced as carbene precursors by Wang and co-workers in 2016 in a copper-catalyzed cross-coupling reaction with terminal alkynes [106]. This reaction afforded trisubstituted allenes 61 in high yields with broad functional group tolerance under mild reaction conditions (Scheme 13).
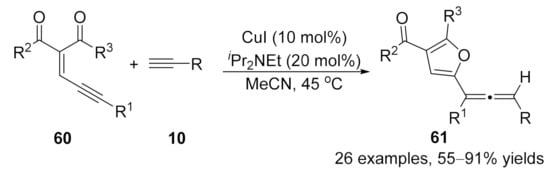
Scheme 13.
Copper-catalyzed cross-coupling reaction of conjugated eneyne ketones with terminal alkynes.
In 2015, Feng and Liu contributed an asymmetric cross-coupling of α-diazoesters 15 with terminal alkynes 10 using chiral Cu(I)/ guanidine complex as the catalyst [107]. Notably, no additional base was necessary for this transformation, providing optically active 2,4-disubstituted allenoates 62 under mild reaction conditions in good to high yields (up to 99 %) with good to excellent enantioselectivities (Scheme 14).
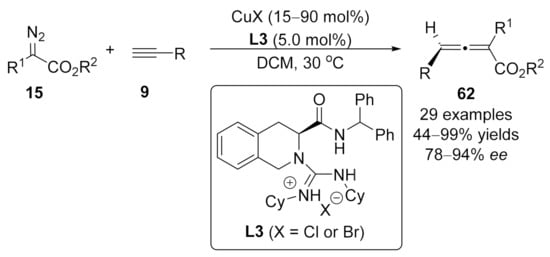
Scheme 14.
Asymmetric cross-coupling reaction catalyzed by copper/guanidine complex.
In 2016, Wang and co-workers reported a highly enantioselective copper-catalyzed cross-coupling of aryldiazoalkanes 57 with terminal alkynes 10 [108]. By utilizing chiral Cu(I)/bisoxazoline ligand L4, this reaction delivered a series of trisubstituted allenes 63 in moderate to high yields (up to 96%) with excellent enantioselectivities (up to 98% ee). Unlike the previous works using CuI as the catalyst, Cu(MeCN)4PF6 complex was used as the optimal metal catalyst to enable high reactivity and stereoselectivity in this reaction (Scheme 15).
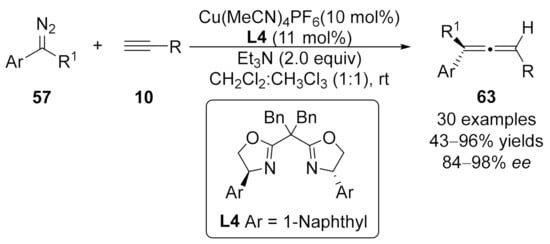
Scheme 15.
Asymmetric cross-coupling reaction catalyzed by copper/Box complex.
One year later, Ley’s group reported their continuous flow strategy for the asymmetric coupling reaction of unstabilized diazo compounds 65 with propargyl amines 66 in the presence of chiral Cu(I)/PyBIM complex [109]. This method generated the amino-substituted chiral allenoates 67 in moderate yields (up to 57%) with high enantioselectivities (up to 96% ee) in a fast reaction rate (10–20 min) with a variety of functional group compatibility (Scheme 16).
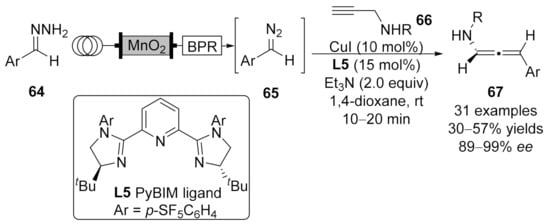
Scheme 16.
Asymmetric cross-coupling reaction catalyzed by copper/PyBIM complex through flowing chemistry.
2.2.2. Allenylation Terminated by Electrophilic Addition
In 2018, a one-pot copper-catalyzed asymmetric three-component reaction of diazoesters 15 with terminal alkynes 10 and isatins 68 was reported by Liu’s group [110]. Axially chiral tetra-substituted allenoates 70 bearing a stereogenic center were obtained under a chiral Cu(I)/guanidinium salt/YBr3 catalytic system with high diastereo- and enantioselectivities. In this work, the aldol type addition of allenoate-copper intermediates 69 with isatins 68 has been proposed as the key step in this reaction. Moreover, convincing experimental evidence for the formation of allenoate-copper intermediate 69 was provided through the synthesis of chiral allenoate, which was generated from the C–H insertion reaction of α-diazoester with alkyne. The author found that additional acids improved the catalyst efficiency of the chiral copper complex. The intramolecular nucleophilic trapping reaction of allenoate-copper intermediate with embedded aldehyde species was also successful, generating the cyclic allenoate product 73, albeit the yield and stereoselectivity were moderate (Scheme 17).
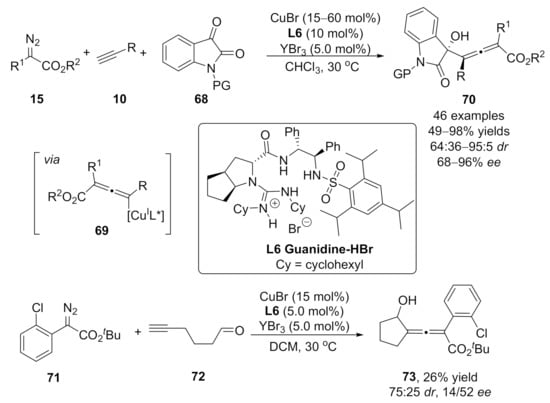
Scheme 17.
Asymmetric three-component reaction for the synthesis of tetra-substituted allenoates.
Recently, Sun’s group has realized an enantioselective intramolecular nucleophilic aldol addition of in situ formed allenoate-copper intermediate with aldehyde using chiral Cu(II)/Box complex [111]. Distinct from the previous version with copper(I) catalysts, this protocol used copper(II) salt as an optimal catalyst in this asymmetric cross-coupling reaction. The tetra-substituted allenoates 75 containing both central and axial chiralities have been obtained in moderate to good yields with good to excellent stereoselectivities. (Scheme 18).
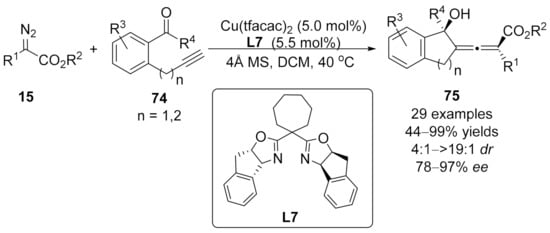
Scheme 18.
Copper-catalyzed asymmetric allenylation terminated by aldol addition.
2.2.3. Allenylation Terminated by Allylation
In 2016, Wang and co-workers realized the synthesis of allyl-substituted allenes through trapping of allenoate-copper intermediate with allyl bromide through a nucleophilic substitution process [106]. In this reaction, conjugated eneyne ketones 60 have been used as the carbene source. Mechanistically, the cooper-(2-furyl) carbene intermediate was generated in situ from eneyne in the presence of CuI, followed by a migratory insertion process to afford nucleophilic alkynoate copper intermediate 77 that was trapped by allyl halide 76 (Scheme 19). In this method, the choice of the base was pivotal for the reaction outcomes when K2CO3 was employed as the base, affording 2-furyl substituted allenes 78 in generally good yields.
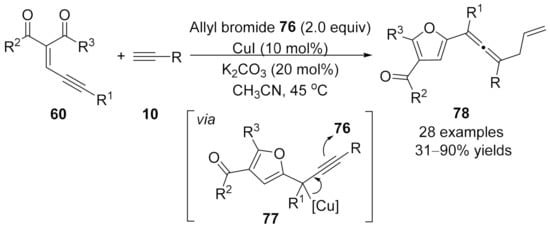
Scheme 19.
Copper-catalyzed allenylation terminated by allylation.
2.2.4. Cascade Transformations Involving Allenylation Process
Nucleophilic addition with allenoic ester or its isomeric compound is one of the generally used synthetic strategies for the expeditious construction of highly functionalized carbocycle or heterocycle structures [112,113,114,115,116,117,118,119,120]. Thus, a variety of inter- or intra-molecular cascade reactions have been developed through different nucleophilic addition processes of the allene derivatives that were generated from cross-coupling between alkynes and copper carbenes.
In 2011, a one-pot synthesis of phenanthrenes 81 via ligand-free CuBr2-catalyzed coupling reaction/intramolecular cyclization of terminal alkynes 23 with N-tosylhydrazones 79 derived from o-formyl biphenyls was developed by Wang and co-workers [121]. In this cascade reaction, allene intermediates 85 were initially generated through a cross-coupling reaction of N-tosylhydrazones 79 with terminal alkynes 23, followed by a 6π-cyclization and isomerization to deliver the phenanthrene products 81 with broad functional group compatibility (Scheme 20).
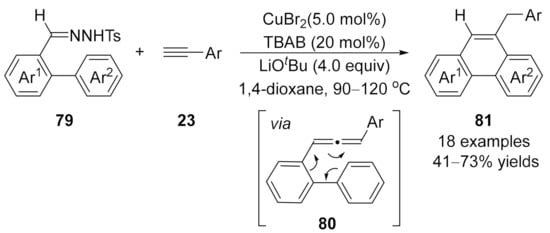
Scheme 20.
Copper-catalyzed allenylation followed by 6π-cyclization.
Later in the same year, instead of using o-aryl substituted N-tosylhydrazones, o-hydroxy- or o-amino-substituted N-tosylhydrazones were introduced by the same group as carbene precursors in an analogous cascade transformation, a ligand-free CuBr-catalyzed coupling reaction/intramolecular cyclization sequence, achieving the synthesis of benzofuran or indole derivatives 84 in moderate to excellent yields [122]. The initially formed allene intermediates 83 were trapped through a nucleophilic addition by the embedded o-hydroxy- or o-amino group to afford the cyclized products 84 (Scheme 21).
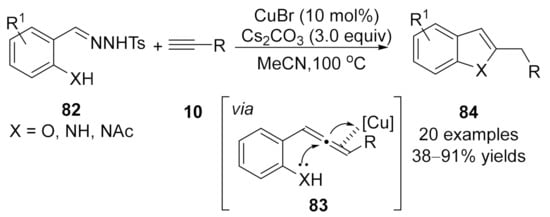
Scheme 21.
Copper-catalyzed allenylation followed nucleophilic addition.
In 2011, a similar catalytic strategy was developed by Balakishan’s group. They reported a simple procedure for the synthesis of aza- and oxa-cycles via a copper-catalyzed coupling reaction of functionalized terminal alkynes 85 with diazoesters 86 [123]. Initially, the allene intermediates were formed in the presence of CuI, followed by an intramolecular aza- or oxa-Michael cycloaddition and isomerization to generate the cyclized five- or six-membered products 87 in generally good yields (Scheme 22).
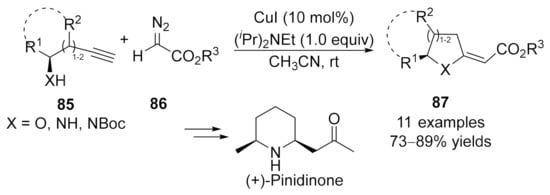
Scheme 22.
Copper-catalyzed allenylation followed by cyclization.
In 2015, a stereo-divergent synthesis of five-membered heterocycles was developed by Sun’s group [124]. This work described a copper-catalyzed cross-coupling reaction and annulation cascade reaction of amino alkynes 88 with diazo compounds 15. The proposed reaction mechanism involves trapping in situ formed allene intermediates, yielding 2-methylenes 89 (when PG = Bn) and 2,3-dihydropyrroles 90 (when PG = Ts) in good yields with broad functional group tolerance under mild conditions. Control experimental results show that N-benzyl amino alkynes were more likely to form 2-methylenespyrroles derivatives 89 through 5-exo-dig cycloaddition, while 2,3-dihydropyrroles 90 generated from N-tosylamino alkynes through 5-endo-dig cycloaddition would be more favorable (Scheme 23).
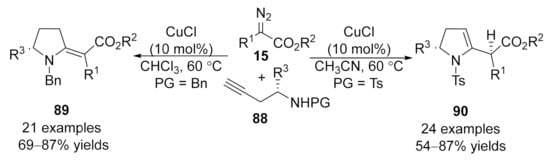
Scheme 23.
Copper-catalyzed allenylation followed by divergent annulation.
In 2018, Sun and co-workers expanded the above chemistry to the synthesis of the four- to six-membered heterocycles with N-substituted prop-2-yn-1-amines 91 and diazoacetates 15 [125]. Generated allenoic species 92 have been proven as the key intermediates for the subsequent diverse annulations under optimized conditions toward functionalized heterocycle in moderate to good yields. Treatment of allenoates 92 with sodium phenolates led to six-membered products 93; silver nitrate and triethylamine yielded five-membered products 94; and what’s more, four-membered products 95 were generated under lithium tert-butoxide conditions (Scheme 24).
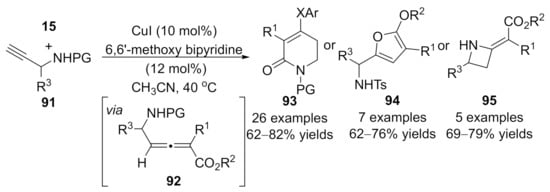
Scheme 24.
Divergent synthesis of four- to six-membered heterocycles involving an allenylation process.
In addition to the cyclization through addition with a heteroatom, carbon-based nucleophilic species could also be served as the nucleophile to addition with these allenes, forming the C–C bond instead of the C–X bond [126,127]. In 2015, Kumaraswamy’s group developed a cooper catalyzed cross-coupling reaction/intramolecular Michael addition cascade reaction [128], achieving the formation of indene and dihydronaphthalene derivatives 97 in good yields with broad functional group tolerance [Scheme 25, Eq. (a)]. Later in 2017, Sun’s group reported an analogous approach toward five- or six-membered carbo-/heterocycles with diazo compounds 15 and alkyne-substituted malonates 98 [129]. In this reaction, the ligand significantly enhanced the reaction yields and inhibited the Conia-ene side reaction. As a result, the polyfunctionalized cyclohexenes, tetrahydropyridines, and dihydropyrans have been prepared in moderate to high yields under mild reaction conditions [Scheme 25, Eq. (b)].
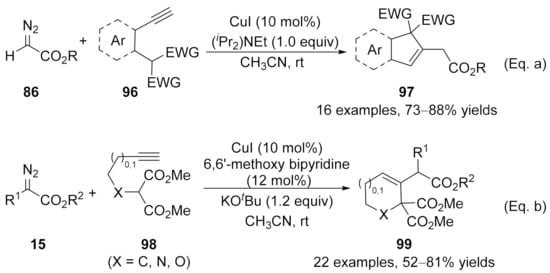
Scheme 25.
Copper-catalyzed allenylation followed by Michael addition. (a) Synthesis of indenes. (b) Synthesis of five- or six-membered carbo-/heterocycles.
In 2015, a Cu(I)-catalyzed denitrogenative annulation reaction of pyridotriazoles 100 with terminal alkynes 10 was developed by Gevorgyan’s group [130]. Initially, α-pyridyl copper carbenes were generated from pyridotriazoles 100 in the presence of the copper catalyst, followed by a cross-coupling reaction with terminal alkyne to form either propargylic or allenoic intermediates 101, which were terminated by copper-catalyzed cycloisomerization to furnish the indolizines 102 in moderate to excellent yields (Scheme 26).
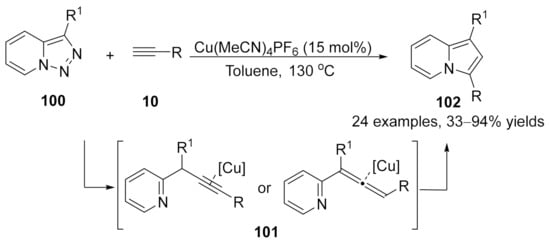
Scheme 26.
Copper-catalyzed allenylation followed by cycloisomerization.
In 2018, Wang and co-workers reported a copper-catalyzed geminal difunctionalization reaction of terminal alkynes [131]. The key step in this cascade reaction is trapping the in situ generated allenoic species 105 with a sulfonyl anion to form the carbon-sulfur bond, providing a variety of vinyl sulfones 106 in good yields with excellent stereoselectivities under mild reaction conditions. It was noted that the excellent stereoselectivities might be due to the influence of steric hindrance, and no ligand and additive was required in this transformation (Scheme 27).
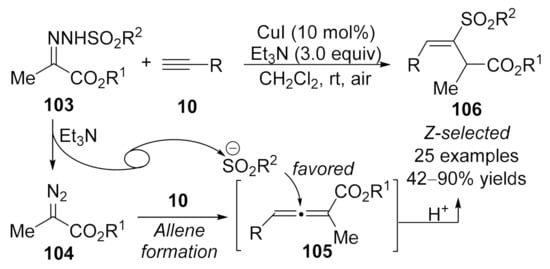
Scheme 27.
Copper-catalyzed allenylation followed by sulfonylation.
Recently, Sun and co-workers have demonstrated a copper-catalyzed three-component reaction of terminal alkynes with diazo compounds and B2pin2 for the synthesis of trisubstituted alkenylboronates [132]. In this alkyne difunctionalization transformation, the copper catalyst plays dual roles in the whole process. Initially, copper catalyzed the cross-coupling to form an allenoic intermediate, followed by a copper-catalyzed stereoselective boration reaction with B2pin2. When diazo compounds 53 were used as carbene precursors, the steric interaction forced the boron group to attack the β-carbon from the opposite side of the γ-phenyl group on the allenoic species 107, leading to the favored (Z)-isomers 108 as major products. Whereas, in the case with N-tosylhydrones 51 as carbene precursors, the addition of Cu-Bpin complex to corresponding allenoic species 109 provided allyl copper intermediate, which was more favored to form a six-membered ring transition state with the association of MeOH, finally furnishing the more thermodynamically stable (E)-products 110 (Scheme 28).
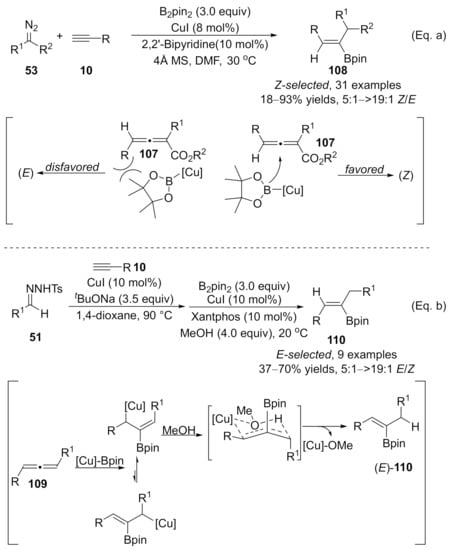
Scheme 28.
Copper-catalyzed allenylation followed by boroalkylation. (a) Reaction of diazo compound with alkyne. (b) Reaction of N-tosylhydrone with alkyne.
3. Copper Carbene Intermediate Addition onto C–C Triple Bond
3.1. Cyclopropenation
Cyclopropenation is a well-known reaction of metal carbene intermediate with alkynes. This widely used reaction could be catalyzed by rhodium [133,134,135,136,137], cobalt [138], gold [139], silver [140,141] and many others [142,143,144]. Herein, selected examples related to copper catalysis will be discussed.
In 2010, a new tridentate coordination copper complex, Cu[Ms(CH2SCN)3]BAr’4 (BAr’4 = tetra(3,5-bis(trifluoromethylphenyl)borate), was designed by Miguel and co-workers by using [Cu(OTf)]2•C6H6 and an alkylthiocyanate ligand [145]. This catalyst promoted the cyclopropenation of ethyl diazoacetate 34 (EDA) with a wide range of internal alkynes 111, providing cyclopropenes 112 in moderate yields (Scheme 29). The same cyclopropenation work was achieved by Dias, in which unique bis(pyrazolyl)borate ligand supported [(CF3)2Bp]Cu(NCMe) catalyst was used, yielding cyclopropene products in moderate to high yields [146].
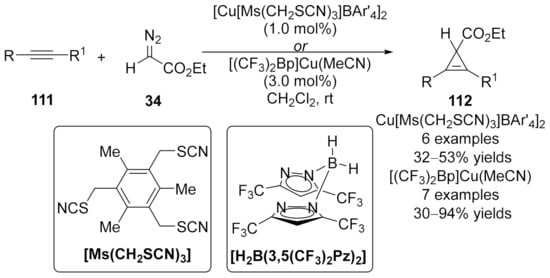
Scheme 29.
Copper/alkylthiocyanate complex catalyzed cyclopropenation.
In 2016, a Cu(I)/N-heterocyclic carbene (CuNHC) complex catalyzed cyclopropenation of internal alkynylsilanes 113 with diazoacetate 15 was reported by Coleman’s group [147]. A series of 1,2,3-trisubstituted and 1,2,3,3-tetrasubstituted cyclopropenylsilane compounds 114 were isolated in moderate to good yields (Scheme 30). An interesting regioselective and chemodivergent reaction pathway occurred to furnish a tetra-substituted furan through an intramolecular cyclopropane and ring-opening cascade process in the case of electron-rich diazoacetate.
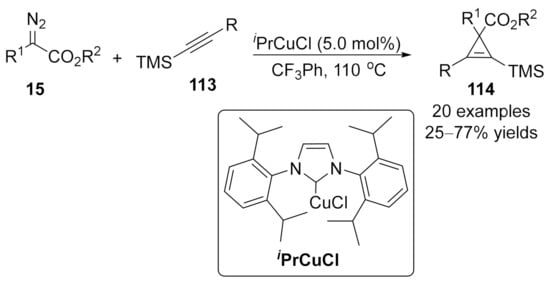
Scheme 30.
Copper/N-heterocyclic carbene (CuNHC) complex catalyzed cyclopropenation.
3.2. Cascade Reaction Involving Carbene/Alkyne Metathesis Process
Carbene/alkyne metathesis (CAM) refers to the processes where a metal carbene reacts with an alkyne, generating a new vinyl metal carbene intermediate, which was difficult to access with other carbene precursors [148,149,150]. This in situ generated vinyl metal carbene intermediate could be involved in typical metal carbene reactions, such as [3+2]-cycloaddition [151], cyclopropanation [152,153,154], C–H bond insertion [155,156,157,158], and others [159,160,161]. Herein, we summarized recent works on the copper-mediated cascade transformations involving carbene/alkyne metathesis.
It is a general protocol for the synthesis of furan derivatives through transition metal-catalyzed formal [3+2]-cycloaddition of α-diazocarbonyl compounds with alkynes [162,163,164,165]. However, the cases under copper carbenes mediated were limited. In 2014, Wang’s group developed a copper-catalyzed formal [3+2]-cycloaddition reaction of terminal alkynes with β-keto α-diazoesters 115 (X = O), offering an operationally simple and applicable method for the synthesis of trisubstituted furans 116 (X = O) with a wide substrate scope [Scheme 31, Eq. (a)] [166]. This reaction has also been applied to ethyl (E)-2-diazo-3-(methoxyimino)butanoate 115 (X = NOMe) for the synthesis of 2,3,5-trisubstituted N-methoxypyrroles (X = NOMe). Later in 2016, a Cu(I)-catalyzed cycloaddition of diazoacetates 15 with electron-rich internal aryl alkynes 117 was discovered by Coleman and co-workers [167]. Tetra-substituted furans 118 were generated in moderate isolated yields with high chemoselectivities and regioselectivities [Scheme 31, Eq. (b)].
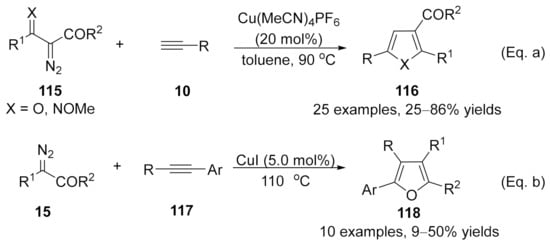
Scheme 31.
Copper-catalyzed carbene/alkyne metathesis (CAM) for the synthesis of furan derivatives. (a) The CAM cascade reaction of diazo compound with internal alkynes. (b) The CAM cascade reaction of diazo compound with terminal alkynes.
In 2016, Xu’s group developed a chemo-divergent copper-catalyzed cascade reaction of alkynyl-tethered α-iminodiazoacetates 119, providing polycyclic and multi-substituted pyrroles in high yields with a broad substrate scope [168]. Especially, the tetra-substituted 3-formylpyrroles 124, which were difficult to access by alternate approaches. Mechanistic studies indicated that the α-imino carbene 120 is the key common intermediate in this divergent reaction, which was generated by metal-catalyzed carbene/alkyne metathesis of the alkynyl-tethered diazo compounds 121. When R1, R2 was imbedded with an aromatic ring, polycyclic pyrroles 122 were formed as the major products through a [3+2]-cyclization and aromatization process. Whereas, for substrates with a methoxy group on the nitrogen (R2 = OMe), the carbene intermediate underwent an N–O insertion/alkoxy migration/alcoholysis sequence, giving the 3-formylpyrrole products 124 in generally good to excellent yields (Scheme 32).
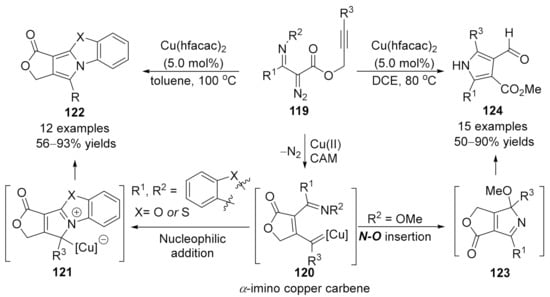
Scheme 32.
Copper-catalyzed carbene/alkyne metathesis for the synthesis of pyrroles.
At the same time, Xu and co-workers also developed a copper-catalyzed carbene/alkyne metathesis cascade reaction with alkyne-tethered diazo compounds 125 [169]. This transformation provided a rapid access for the construction of multi-substituted 4-carboxyl quinoline derivatives 127 in high to excellent yields. In this cascade reaction, one C=N and one C=C bond were formed with the assistance of the copper catalyst under mild reaction conditions [Scheme 33, Eq. (a)]. Later in 2017, Ye’s group reported an analogous protocol by using ynamides 128 as carbene precursor [170]. In this work, a copper carbene was generated in situ through a catalytic oxidation process in the presence of quinoline N-oxide, followed by a CAM process and terminated by carbene reaction with an embedded azide group, providing a wide range of pyrrolo [3,4-c]quinolin-1-ones 130 in good yields [Scheme 33, Eq. (b)]. These works represented a practical method for the dual functionalization of alkynes.
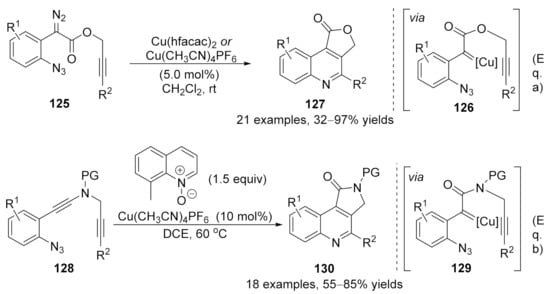
Scheme 33.
Copper-catalyzed carbene/alkyne metathesis for the synthesis of quinolines. (a) Synthesis of multi-substituted 4-carboxyl quinoline derivatives 127. (b) Synthesis of pyrrolo [3,4-c]quinolin-1-ones 130.
In addition to the nucleophilic addition of the in situ formed copper carbene intermediates, electrophilic aromatic substitution or C(sp2)–H bond functionalization is another useful terminating transformation for the direct construction of polycyclic fused frameworks. In 2017, Doyle’s group reported a copper-catalyzed intramolecular cascade reaction of diazo compounds 131. This transformation went through a CAM process followed by a carbene C(sp2)–H bond functionalization cascade, yielding the fused indeno-furanone derivatives 133 in excellent yields under mild reaction conditions [Scheme 34, Eq. (a)] [171]. Instead of terminating the reaction through C–H functionalization, a selective Buchner insertion reaction occurred as the terminating step in Xu’s work when the ortho-aniline substituted propargyl diazoacetates 134 were employed, selectively affording the dihydrocyclohepta[b]indole derivatives 136 in moderate to high yields [Scheme 34, Eq. (b)]. Notably, this reaction described a rare example of the Buchner reaction with donor/donor type metal carbene species [172].
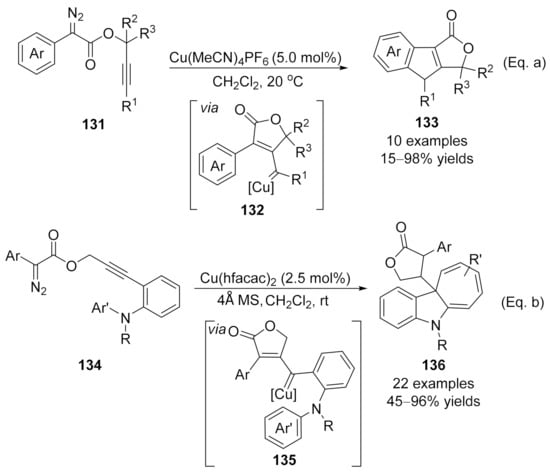
Scheme 34.
Copper-catalyzed carbene/alkyne metathesis for the synthesis of tri-cyclic molecules. (a) The CAM cascade reaction terminated by C-H functionalization. (b) The CAM cascade reaction terminated by Buchner reaction.
In 2018, Xu and co-workers developed an intermolecular copper-catalyzed formal CAM process [173], which underwent a copper promoted [3+2]-cycloaddition/dinitrogen exclusion/nucleophilic addition process, providing a direct and effective access to 2H-chromene derivatives 139 in generally good to high yields. Mechanistic studies indicated that the 3H-pyrazole 138 is the key intermediate in this cascade transformation, and this critical intermediate was isolated and confirmed by single-crystal X-ray diffraction and spectroscopy analysis for the first time (Scheme 35).
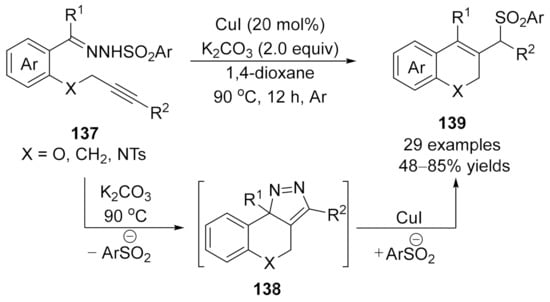
Scheme 35.
Copper-catalyzed formal carbene/alkyne metathesis for the synthesis of 2H-chromene derivatives.
Based on a similar protocol, a copper-catalyzed formal [1+2+2]-annulation of alkyne-tethered diazo compounds 140 with pyridines 141 has been reported by Xu’s group recently [174]. In contrast to the previously reported cascade reaction that terminated the copper carbene intermediate on the carbonic center, a vinylogous addition of vinyl carbene intermediate with pyridine derivatives occurred in this reaction, followed by an intramolecular annulation to form cycloadducts 146, which underwent a decarboxylative aromatization process to form the desired polycyclic fused indolizine derivatives 147 in good to high yields (Scheme 36, path a); although, direct formal [3+2]-cycloaddition via pyridinium ylide pathway could not be ruled out so far (Scheme 36, path b).
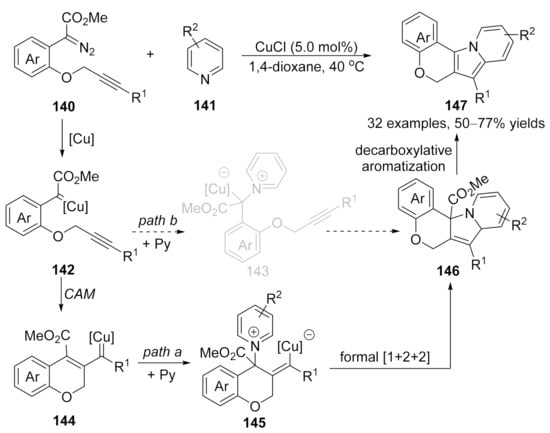
Scheme 36.
Copper-catalyzed formal carbene/alkyne metathesis for the synthesis of polycyclic indolizines.
4. Conclusions
This review has summarized the recent progress of catalytic alkyne functionalization involving copper carbene intermediates. Copper carbene species derived from different carbene precursors have been introduced to react with alkynes through two distinguished reaction models: the cross-coupling reaction of copper carbene intermediates with terminal alkynes and the addition of copper carbene intermediates onto the C–C triple bond. The former reaction involves alkynoate or allenoate copper intermediates, followed by protonation, nucleophilic substitution, electrophilic addition, or elimination process, yielding functionalized alkynes and allenes, respectively. The latter version includes cyclopropenation and cascade reaction via carbene/alkyne metathesis process. Although substantial progress has been realized in this field, challenges remain, e.g., the carbene precursors are still mainly limited to the diazo compounds; the catalytic efficiency could be further improved, especially in the asymmetric catalysis; and synthetic applications of this chemistry are still under exploration. Therefore, the synthetic potential of this chemistry could be envisioned through the introduction of a variety of readily accessible carbene precursors and with the development of robust copper catalysts/ligands, including novel methodology discovery for the catalytic alkyne functionalization, and expeditious assembly of molecules with structural complexity and diversity for the leading compound development.
Author Contributions
Conceptualization, K.D. and X.X.; writing—original draft preparation, K.D. and X.X.; writing—review and editing, K.D., M.L. and X.X.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21971262, 92056201), Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (No. 2016ZT06Y337).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The following abbreviations are used in this text.
Table A1.
The following abbreviations are used in this text.
| Cu(acac)2 | Copper(II) acetylacetonate |
| Cu(hfacac)2 | Copper(II) hexafluoroacetylacetonate |
| CuOTf | Copper(I) trifluoromethanesulfonate |
| Cu(OTf)2 | Copper(II) trifluoromethanesulfonate |
| CuPF6 | Copper(I) hexafluorophosphate |
| Cu(MeCN)4PF6 | Tetrakis(acetonitrile)copper(I) hexafluorophosphate |
| DAFO | 4,5-Diazafluoren-9-one |
| TBAI | Tetrabutylammonium iodide |
| TBAB | Tetrabutylammonium bromide |
| Xantphos | 9,9-Dimethyl-4,5-bis(diphenylphosphino)xanthene |
| TMS | Trimethylsilyl |
| TIPS | Triisopropylsilyl |
| TBDPS | tert-Butyldiphenylsiyl |
| Boc | tert-Butoxycarbonyl |
| Ts | 4-Toluolsulfonyl |
| Bpin | Boron pinacol ester |
| EDA | Ethyl diazoacetate |
| PG | Protecting group |
References
- Trost, B.M.; Masters, J.T. Transition Metal-Catalyzed Couplings of Alkynes to 1,3-Enynes: Modern Methods and Synthetic Applications. Chem. Soc. Rev. 2016, 45, 2212–2238. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.L.; Wang, J. Cu(I)-Catalyzed Cross-Coupling of Diazo Compounds with Terminal Alkynes: An Efficient Access to Allenes. Chem. Rec. 2018, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kong, W. Ni-Catalyzed Stereoselective Difunctionalization of Alkynes. Org. Chem. Front. 2020, 7, 3941–3955. [Google Scholar] [CrossRef]
- Sun, K.; Wang, X.; Li, C.; Wang, H.; Li, L. Recent Advances in Tandem Selenocyclization and Tellurocyclization with Alkenes and Alkynes. Org. Chem. Front. 2020, 7, 3100–3119. [Google Scholar] [CrossRef]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef] [PubMed]
- Trotus, I.T.; Zimmermann, T.; Schueth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef]
- Campeau, D.; León Rayo, D.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. [Google Scholar] [CrossRef]
- Patil, N.T.; Yamamoto, Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Yamamoto, Y. Introduction: Coinage Metals in Organic Synthesis. Chem. Rev. 2008, 108, 2793–2795. [Google Scholar] [CrossRef]
- Ye, T.; McKervey, M.A. Organic Synthesis with α-Diazo Carbonyl Compounds. Chem. Rev. 1994, 94, 1091–1160. [Google Scholar] [CrossRef]
- Doyle, M.P.; McKervey, M.A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Doyle, M.P.; Forbes, D.C. Recent Advances in Asymmetric Catalytic Metal Carbene Transformations. Chem. Rev. 1998, 98, 911–935. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Chen, K.; Zhu, S. Transition-Metal-Catalyzed Intramolecular Nucleophilic Addition of Carbonyl Groups to Alkynes. Chem 2018, 4, 1208–1262. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Ma, X.; Cheng, X.; Zhao, K.; Gutman, K.; Li, T.; Zhang, L. Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev. 2021, 121, 8979–9038. [Google Scholar] [CrossRef]
- Hong, F.L.; Ye, L.W. Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis. Acc. Chem. Res. 2020, 53, 9, 2003–2019. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Wang, J. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc. Chem. Res. 2013, 46, 236–247. [Google Scholar] [CrossRef]
- Wang, T.; Hashmi, A.S.K. 1,2-Migrations onto Gold Carbene Centers. Chem. Rev. 2021, 121, 8948–8978. [Google Scholar] [CrossRef]
- Fructos, M.R.; Díaz-Requejo, M.M.; Pérez, P.J. Gold and Diazo Reagents: A Fruitful Tool for Developing Molecular Complexity. Chem. Commun. 2016, 52, 7326–7335. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, Z.; Wang, Y.; Zhang, L. Au-Catalysed Oxidative Cyclisation. Chem. Soc. Rev. 2016, 45, 4448–4458. [Google Scholar] [CrossRef]
- Zhang, L. A Non-Diazo Approach to α-Oxo Gold Carbenes via Gold-Catalyzed Alkyne Oxidation. Acc. Chem. Res. 2014, 47, 877–888. [Google Scholar] [CrossRef]
- Asiria, A.M.; Hashmi, A.S.K. Gold-Catalysed Reactions of Diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. [Google Scholar] [CrossRef] [PubMed]
- Obradors, C.; Echavarren, A.M. Gold-Catalyzed Rearrangements and Beyond. Acc. Chem. Res. 2014, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Zia, W.; Toste, F.D. Recent Advances in Enantioselective Gold Catalysis. Chem. Soc. Rev. 2016, 45, 4567–4589. [Google Scholar] [CrossRef] [PubMed]
- Rokade, B.V.; Barkera, J.; Guiry, P.J. Development of and Recent Advances in Asymmetric A3 Coupling. Chem. Soc. Rev. 2019, 48, 4766–4790. [Google Scholar] [CrossRef]
- Mo, J.N.; Su, J.; Zhao, J. The Asymmetric A3(Aldehyde–Alkyne–Amine) Coupling: Highly Enantioselective Access to Propargylamines. Molecules 2019, 24, 1216. [Google Scholar] [CrossRef] [Green Version]
- Bisai, V.; Suneja, A.; Singh, V.K. Asymmetric Alkynylation/Lactamization Cascade: An Expeditious Entry to Enantiomerically Enriched Isoindolinones. Angew. Chem. Int. Ed. 2014, 53, 10737–10741. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Y.; Huang, T.; Liu, X.; Lin, L.; Feng, X. Copper/Guanidine-Catalyzed Asymmetric Alkynylation of Isatins. Angew. Chem. Int. Ed. 2016, 55, 5286–5289. [Google Scholar] [CrossRef]
- Maity, P.; Srinivas, H.D.; Watson, M.P. Copper-Catalyzed Enantioselective Additions to Oxocarbenium Ions: Alkynylation of Isochroman Acetals. J. Am. Chem. Soc. 2011, 133, 17142–17145. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Tan, C.; Tang, J.; Zhang, Y.; Gao, W.; Wu, H.; Yu, Y.H.; Zhou, J. Asymmetric Copper(I)-Catalyzed Azide–Alkyne Cycloaddition to Quaternary Oxindoles. J. Am. Chem. Soc. 2013, 135, 10994–10997. [Google Scholar] [CrossRef]
- Guo, S.; Dong, P.; Chen, Y.; Feng, X.; Liu, X. Chiral Guanidine/Copper Catalyzed Asymmetric Azide-Alkyne Cycloaddition/[2 + 2] Cascade Reaction. Angew. Chem. Int. Ed. 2018, 57, 16852–16856. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.H.; Wang, Y.H.; Zheng, Z.; Xu, J.; Hu, X.P. Highly Diastereo- and Enantioselective Cu-Catalyzed [3 + 3] Cycloaddition of Propargyl Esters with Cyclic Enamines toward Chiral Bicyclo[n. 3. 1] Frameworks. J. Am. Chem. Soc. 2012, 134, 9585–9588. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Takiguchi, Y.; Maruoka, K. Catalytic Asymmetric Three-Component 1,3-Dipolar Cycloaddition of Aldehydes, Hydrazides, and Alkynes. J. Am. Chem. Soc. 2013, 135, 11473–11476. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.L.; Wang, Z.S.; Wei, D.D.; Zhai, T.Y.; Deng, G.C.; Lu, X.; Liu, R.S.; Ye, L.W. Generation of Donor/Donor Copper Carbenes through Copper-Catalyzed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles. J. Am. Chem. Soc. 2019, 141, 16961–16970. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Yang, C.; Xue, Q.Y.; Zhao, M.; Shan, C.C.; Xu, Y.H.; Loh, T.P. Copper-Catalyzed Asymmetric Silylation of Propargyl Dichlorides: Access to Enantioenriched Functionalized Allenylsilanes. Angew. Chem. Int. Ed. 2019, 58, 16538–16542. [Google Scholar] [CrossRef]
- Kondo, Y.; Nagao, K.; Ohmiya, H. Reductive Umpolung for Asymmetric Synthesis of Chiral α-Allenic Alcohols. Chem. Commun. 2020, 56, 7471–7474. [Google Scholar] [CrossRef]
- Zhong, F.; Xue, Q.Y.; Yin, L. Construction of Chiral 2,3-Allenols through a Copper(I)-Catalyzed Asymmetric Direct Alkynylogous aldol Reaction. Angew. Chem. Int. Ed. 2020, 59, 1562–1566. [Google Scholar] [CrossRef]
- Huang, Y.; Pozo, J.; Torker, S.; Hoveyda, A. Enantioselective Synthesis of Trisubstituted Allenyl–B(pin) Compounds by Phosphine–Cu-Catalyzed 1,3-Enyne Hydroboration. Insights Regarding Stereochemical Integrity of Cu–Allenyl Intermediates. J. Am. Chem. Soc. 2018, 140, 2643–2655. [Google Scholar] [CrossRef]
- Gao, D.W.; Xiao, Y.; Liu, M.; Liu, Z.; Karunananda, M.; Chen, J.; Engle, K. Catalytic, Enantioselective Synthesis of Allenyl Boronates. ACS Catal. 2018, 8, 3650–3654. [Google Scholar] [CrossRef]
- Jung, H.Y.; Feng, X.; Kim, H.; Yun, J. Copper-Catalyzed Boration of Activated Alkynes. Chiral Boranes via A One-pot Copper-Catalyzed Boration and Reduction Protocol. Tetrahedron 2012, 68, 3444–3449. [Google Scholar] [CrossRef]
- Jung, H.Y.; Yun, J. Copper-Catalyzed Double Borylation of Silylacetylenes: Highly Regio- and Stereoselective Synthesis of Syn-Vicinal Diboronates. Org. Lett. 2012, 14, 2606–2609. [Google Scholar] [CrossRef]
- Liu, P.; Fukui, Y.; Tian, P.; He, Z.T.; Sun, C.Y.; Wu, N.Y.; Lin, G.Q. Cu-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Enynes. J. Am. Chem. Soc. 2013, 135, 11700–11703. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.W.; Gao, Y.; Shao, H.; Qiao, T.Z.; Wang, X.; Sanchez, B.B.; Chen, J.S.; Liu, P.; Engle, K.M. Cascade CuH-Catalysed Conversion of Alkynes into Enantioenriched 1,1-Disubstituted Products. Nat. Catal. 2020, 3, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.L.; Buchwald, S.L. Copper-Catalysed Selective Hydroamination Reactions of Alkynes. Nat. Chem. 2015, 7, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silberrad, O.; Roy, C.S. Gradual Decomposition of Ethyl Diazoacetate. J. Chem. Soc. Trans. 1906, 89, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, H.; Moriuti, S.; Yamabe, M.; Noyori, R. Reactions of Diphenyldiazomethane in the Presence of Bis(acetylacetonato) Copper (II). Modified Diphenylmethylene Reactions. Tetrahedron Lett. 1966, 7, 59–63. [Google Scholar] [CrossRef]
- Nozaki, H.; Takaya, H.; Moriuti, S.; Noyori, R. Homogeneous Catalysis in the Decomposition of Diazo Compounds by Copper Chelates: Asymmetric Carbenoid Reactions. Tetrahedron 1968, 24, 3655–3669. [Google Scholar] [CrossRef]
- Dauben, W.G.; Hendricks, R.T.; Luzzio, M.J.; Ng, H.P. Enantioselectively Catalyzed Intramolecular Cyclopropanations of Unsaturated Diazo Carbonyl Compounds. Tetrahedron Lett. 1990, 31, 6969–6972. [Google Scholar] [CrossRef]
- Lowenthal, R.E.; Abiko, A.; Masamune, S. Asymmetric Catalytic Cyclopropanation of Olefins: Bis-Oxazoline Copper Complexes. Tetrahedron Lett. 1990, 31, 6005–6008. [Google Scholar] [CrossRef]
- Evans, D.A.; Woerpel, K.A.; Hinman, M.M.; Faul, M.M. Bis(oxazolines) as Chiral Ligands in Metal-Catalyzed Asymmetric Reactions. Catalytic, Asymmetric Cyclopropanation of Olefins. J. Am. Chem. Soc. 1991, 113, 726–728. [Google Scholar] [CrossRef]
- Evans, D.A.; Woerpel, K.A.; Scott, M.J. Bis(oxazolines)’ as Ligands for Self-Assembling Chiral Coordination Polymers—Structure of a Copper(I) Catalyst for the Enantioselective Cyclopropanation of Olefins. Angew. Chem. Int. Ed. 1992, 31, 430–432. [Google Scholar] [CrossRef]
- Pfaltz, A. Chiral Semicorrins and Related Nitrogen Heterocycles as Ligands in Asymmetric Catalysis. Acc. Chem. Res. 1993, 26, 339–345. [Google Scholar] [CrossRef]
- Díaz-Requejo, M.M.; Pérez, P.J. The Use of Polypyrazolylborate Copper(I) Complexes as Catalysts in the Conversion of Olefins into Cyclopropanes, Aziridines and Epoxides and Alkynes into Cyclopropenes. J. Organomet. Chem. 2001, 617, 110–118. [Google Scholar] [CrossRef]
- Straub, B.F.; Hofmann, P. Copper(I) Carbenes: The Synthesis of Active Intermediates in Copper-Catalyzed Cyclopropanation B.F.S. thanks the Fonds der Chemischen Industrie for a doctoral fellowship. Angew. Chem. Int. Ed. 2001, 40, 1288–1290. [Google Scholar] [CrossRef]
- Fraile, J.M.; GarcTa, J.I.; MartTnez-Merino, V.; Mayoral, J.A.; Salvatella, L. Theoretical (DFT) Insights into the Mechanism of Copper-Catalyzed Cyclopropanation Reactions. Implications for Enantioselective Catalysis. J. Am. Chem. Soc. 2001, 123, 7616–7625. [Google Scholar] [CrossRef]
- Liao, S.; Sun, X.L.; Tang, Y. Side Arm Strategy for Catalyst Design: Modifying Bisoxazolines for Remote Control of Enantioselection and Related. Acc. Chem. Res. 2014, 47, 2260–2272. [Google Scholar] [CrossRef]
- Xie, J.H.; Zhu, Q.L. Chiral Diphosphine and Monodentate Phosphorus Ligands on a Spiro Scaffold for Transition-Metal-Catalyzed Asymmetric Reactions. Acc. Chem. Res. 2008, 41, 581–593. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Diaz-Requejo, M.M.; Belderrain, T.R.; Nicasio, M.C.; Trofimenko, S.; Pérez, P.J. Intermolecular Copper-Catalyzed Carbon-Hydrogen Bond Activation via Carbene insertion. J. Am. Chem. Soc. 2002, 124, 896–897. [Google Scholar] [CrossRef]
- Flynn, C.J.; Elcoate, C.J.; Lawrence, S.E.; Maguire, A.R. Highly Enantioselective Intramolecular Copper Catalyzed C-H Insertion Reactions of α-Diazosulfones. J. Am. Chem. Soc. 2010, 132, 1184–1185. [Google Scholar] [CrossRef]
- Carreras, V.; Besnard, C.; Gandon, V.; Ollevier, T. Asymmetric CuI-Catalyzed Insertion Reaction of 1-Aryl-2,2,2-trifluoro-1-diazoethanes into Si–H Bonds. Org. Lett. 2019, 21, 9094–9098. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhu, S.F.; Wang, L.X.; Zhou, Q.L. Copper-Catalyzed Highly Enantioselective Carbenoid Insertion into Si-H Bonds. Angew. Chem. Int. Ed. 2008, 47, 8496–8498. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.C.; Fu, G.C. Catalytic Enantioselective O-H Insertion Reactions. J. Am. Chem. Soc. 2006, 128, 4594–4595. [Google Scholar] [CrossRef] [PubMed]
- Morilla, M.E.; Molina, M.J.; Diaz-Requejo, M.M.; Belderrain, T.R.; Nicasio, M.C.; Trofimenko, S.; Pérez, P.J. Copper-Catalyzed Carbene Insertion into O−H Bonds: High Selective Conversion of Alcohols into Ethers. Organometallics 2003, 22, 2914–2918. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Cai, Y.; Wang, G.; Zhu, S.F.; Zhou, Q.L. Highly Enantioselective Copper- and Iron-Catalyzed Intramolecular Cyclopropanation of Indoles. J. Am. Chem. Soc. 2017, 139, 7697–7700. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Takaya, H.; Nakanisi, Y.; Nozaki, H. Partial Asymmetric Synthesis of Methylenecyclopropanes and Spiropentanes. Can. J. Chem. 1969, 47, 1242–1245. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Wang, X.; Yu, Y.; Zheng, J.; Wu, W.; Jiang, H. Copper-Catalyzed Cyanothiolation to Incorporate a Sulfur-Substituted Quaternary Carbon Center. Chem. Sci. 2017, 8, 7047–7051. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Pei, C.; Zeng, Q.; Qiu, L.; Hu, W.; Qian, Y.; Xu, X. Copper-Catalyzed [4 + 1]-Annulation of 2-Alkenylindoles with Diazoacetates: A Facile Access to Dihydrocyclopenta[b]indoles. Chem. Commun. 2019, 55, 6393–6396. [Google Scholar] [CrossRef]
- Luo, H.; He, C.; Jiang, H.; Zhu, S. Rapid Access to Oxabicyclo[2.2.2]octane Skeleton through Cu(I)-Catalyzed Generation and Trapping of Vinyl-o-quinodimethanes (Vinyl-o-QDMs). Chin. J. Chem. 2020, 38, 1052–1056. [Google Scholar] [CrossRef]
- Qu, J.; Xu, Z.; Zhou, J.; Cao, C.; Sun, X.; Dai, L.; Tang, Y. Ligand-Accelerated Asymmetric [1, 2]-Stevens Rearrangment of Sulfur Ylides via Decomposition of Diazomalonates Catalyzed by Chiral Bisoxazoline/Copper Complex. Adv. Synth. Catal. 2009, 351, 308–312. [Google Scholar] [CrossRef]
- Alavala, G.K.; Sajjad, F.; Shi, T.; Kang, Z.; Ma, M.; Xing, D.; Hu, W. Diastereoselective Synthesis of Isochromans via the Cu(II)-Catalysed Intramolecular Michael-type Trapping of Oxonium Ylides. Chem. Commun. 2018, 54, 12650–12653. [Google Scholar] [CrossRef]
- Nair, V.N.; Kojasoy, V.; Laconsay, C.J.; Kong, W.Y.; Tantillo, D.J.; Tambar, U.K. Catalyst-Controlled Regiodivergence in Rearrangements of Indole-Based Onium Ylides. J. Am. Chem. Soc. 2021, 143, 9016–9025. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Dong, K.; Pei, C.; Dong, S.; Hu, W.; Qiu, L.; Xu, X. Divergent Construction of Macrocyclic Alkynes via Catalytic Metal Carbene C(sp2)–H Insertion and the Buchner Reaction. ACS Catal. 2019, 9, 10773–10779. [Google Scholar] [CrossRef]
- Pei, C.; Rong, G.W.; Yu, Z.X.; Xu, X. Copper-Catalyzed Intramolecular Annulation of Conjugated Enynones to Substituted 1H-Indenes and Mechanistic Studies. J. Org. Chem. 2018, 83, 13243–13255. [Google Scholar] [CrossRef] [PubMed]
- Marichev, K.O.; Wang, K.; Dong, K.; Greco, N.; Massey, L.A.; Deng, Y.; Arman, H.; Doyle, M.P. Synthesis of Chiral Tetrasubstituted Azetidines from Donor-acceptor Azetines via Asymmetric Copper(I)-catalyzed Imido-ylide [3 + 1]-cycloaddition with Metallo-enolcarbenes. Angew. Chem. Int. Ed. 2019, 58, 16188–16192. [Google Scholar] [CrossRef] [PubMed]
- Marichev, K.O.; Dong, K.; Massey, L.A.; Deng, Y.; Angelis, L.; Wang, K.; Arman, H.; Doyle, M.P. Chiral Donor-acceptor Azetines as Powerful Reactants for Synthesis of Amino Acid Derivatives. Nat. Commun. 2019, 10, 5328. [Google Scholar] [CrossRef] [Green Version]
- Marichev, K.O.; Doyle, M.P. Catalytic Asymmetric Cycloaddition Reactions of Enoldiazo Compounds. Org. Biomol. Chem. 2019, 17, 4183–4195. [Google Scholar] [CrossRef]
- Yang, J.M.; Li, Z.Q.; Li, M.L.; He, Q.; Zhu, S.F.; Zhou, Q.L. Catalytic B−H Bond Insertion Reactions Using Alkynes as Carbene Precursors. J. Am. Chem. Soc. 2017, 139, 3784–3789. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, Q.L. Transition-Metal-Catalyzed Enantioselective Heteroatom–Hydrogen Bond Insertion Reactions. Acc. Chem. Res. 2012, 45, 1365–1377. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J. Transition-Metal-Catalyzed Cross-Coupling with Ketones or Aldehydes via N-Tosylhydrazones. J. Am. Chem. Soc. 2020, 142, 10592–10605. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, D.; Wang, J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J. N-Tosylhydrazones: Versatile Synthons in the Construction of Cyclic Compounds. Chem. Soc. Rev. 2017, 46, 2306–2362. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Besora, M.; Molina, F.; Maseras, F.; Belderrain, T.R.; Pérez, P.J. Two Copper-Carbenes from One Diazo Compound. J. Am. Chem. Soc. 2021, 143, 4837–4843. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.M.; Molina, F.; Díaz-Requejo, M.M.; Pérez, P.J. Copper-Catalyzed Selective Pyrrole Functionalization by Carbene Transfer Reaction. Adv. Synth. Catal. 2020, 362, 1998–2004. [Google Scholar] [CrossRef]
- Hedleya, S.J.; Davies, H.M.L. Intermolecular Reactions of Electron-rich Heterocycles with Copper and Rhodium Carbenoids. Chem. Soc. Rev. 2007, 36, 1109–1119. [Google Scholar]
- Alford, J.S.; Davies, H.M.L. Reactions of Metallocarbenes Derived from N-sulfonyl-1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 5151–5162. [Google Scholar]
- Che, J.; Xing, D.; Hu, W. Metal-Catalyzed Cross-Coupling of Terminal Alkynes with Different Carbene Precursors. Curr. Org. Chem. 2015, 20, 41–60. [Google Scholar] [CrossRef]
- Jones, V.K.; Deutschman, A.J., Jr. The Copper Sulfate Catalyzed Reaction of Ethyl Diazoacetate and 1-Octyne1. J. Org. Chem. 1965, 30, 3978–3980. [Google Scholar] [CrossRef]
- Suarez, A.; Fu, G.C. A Straightforward and Mild Synthesis of Functionalized 3-Alkynoates. Angew. Chem. Int. Ed. 2004, 43, 3580–3582. [Google Scholar] [CrossRef]
- Ye, F.; Ma, X.; Xiao, Q.; Li, H.; Zhang, Y.; Wang, J. C(sp)–C(sp3) Bond Formation through Cu-Catalyzed Cross-Coupling of N-Tosylhydrazones and Trialkylsilylethynes. J. Am. Chem. Soc. 2012, 134, 5742–5745. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, P.; Xie, Y.; Wang, J.; Zhou, L. CuI-catalyzed Cross-coupling of Terminal Alkynes with Dialkoxycarbenes: A General Method for the Synthesis of Unsymmetrical Propargylic Acetals. Org. Biomol. Chem. 2014, 12, 6215–6222. [Google Scholar] [CrossRef] [Green Version]
- Osako, T.; Nagaosa, M.; Hamasaka, G.; Uozumi, Y. Asymmetric Copper-Catalyzed C(sp)–H Bond Insertion of Carbenoids Derived from N-Tosylhydrazones. Synlett 2018, 29, 2251–2256. [Google Scholar]
- Wang, C.; Ye, F.; Wu, C.; Zhang, Y.; Wang, J. Construction of All-Carbon Quaternary Centers through Cu-Catalyzed Sequential Carbene Migratory Insertion and Nucleophilic Substitution/Michael Addition. J. Org. Chem. 2015, 80, 8748–8757. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Kang, Z.; Xing, D.; Hu, W. Cu(I)-Catalyzed Three-Component Reaction of Diazo Compound with Terminal Alkyne and Nitrosobenzene for the Synthesis of Trifluoromethyl Dihydroisoxazoles. Org. Lett. 2018, 20, 4843–4847. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Reddy, A.; Niu, L.; Xing, D.; Hu, W. Cu(I)-Catalyzed Three-Component Reaction of α-Diazo Amide with Terminal Alkyne and Isatin Ketimine via Electrophilic Trapping of Active Alkynoate-Copper Intermediate. Org. Lett. 2019, 21, 4571–4574. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Wu, G.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Cross-Coupling of Terminal Alkynes with Trifluoromethyl Ketone N-Tosylhydrazones: Access to 1,1-Difluoro-1,3-enynes. Org. Lett. 2015, 17, 2474–2477. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Z.; Zhang, Z.; Ye, F.; Deng, G.; Zhang, Y.; Wang, J. Copper(I)-Catalyzed Stereoselective Synthesis of (E)-α-Alkynyl α, β-Unsaturated Esters from a Terminal Alkyne, Diazoesters and Aldehydes. Adv. Synth. Catal. 2016, 358, 2480–2488. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, F.; Zhou, Q.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Tandem Reaction of Carbene Coupling and Horner–Wadsworth–Emmons Type Olefination: Access Toward Enynes. Org. Lett. 2016, 18, 2024–2027. [Google Scholar] [CrossRef]
- Hassink, M.; Liu, X.; Fox, J.M. Copper-Catalyzed Synthesis of 2,4-Disubstituted Allenoates from α-Diazoesters. Org. Lett. 2011, 13, 2388–2391. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ding, D.; Liu, L.; Sun, J. CuI-Catalyzed Cross-Coupling of Diazoacetamide with Terminal Alkynes: An Approach to Synthesizing Substituted Dienamides and 3-Butynamides. RSC Adv. 2013, 3, 21260–21266. [Google Scholar] [CrossRef]
- Hossain, M.L.; Ye, F.; Zhang, Y.; Wang, J. CuI-Catalyzed Cross-Coupling of N-Tosylhydrazones with Terminal Alkynes: Synthesis of 1,3-Disubstituted Allenes. J. Org. Chem. 2013, 78, 1236–1241. [Google Scholar] [CrossRef]
- Wu, C.; Hu, F.; Liu, Z.; Deng, G.; Ye, F.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Coupling of Diaryldiazomethanes with Terminal Alkynes: An Efficient Synthesis of Tri-aryl-substituted Allenes. Tetrahedron 2015, 71, 9196–9201. [Google Scholar] [CrossRef]
- Ye, F.; Wang, C.; Ma, X.; Hossain, M.L.; Xia, Y.; Zhang, Y.; Wang, J. Synthesis of Terminal Allenes through Copper-Mediated Cross-Coupling of Ethyne with N-Tosylhydrazones or α-Diazoesters. J. Org. Chem. 2015, 80, 647–652. [Google Scholar] [CrossRef]
- Xu, S.; Chen, R.; Fu, Z.; Gao, Y.; Wang, J. Cu(I)-Catalyzed Coupling of Bis(trimethylsilyl)diazomethane with Terminal Alkynes: A Synthesis of 1,1-Disilyl Allenes. J. Org. Chem. 2018, 83, 6186–6192. [Google Scholar] [CrossRef] [PubMed]
- Poh, J.S.; Tran, D.N.; Battilocchio, C.; Hawkins, J.M.; Ley, S.V. A Versatile Room-Temperature Route to Di- and TrisubstitutedAllenes Using Flow-Generated Diazo Compounds. Angew. Chem. Int. Ed. 2015, 54, 7920–7923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Xia, Y.; Ma, C.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Synthesis of Furan-Substituted Allenes by Use of Conjugated Ene-yne Ketones as Carbene Precursors. J. Org. Chem. 2016, 81, 3275–3285. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Q.; Liu, X.; Wang, G.; Lin, L.; Feng, X. Direct Synthesis of Chiral Allenoates from the Asymmetric C-H bond Insertion of α-Diazoesters into Terminal Alkynes. Angew. Chem. Int. Ed. 2015, 54, 9512–9516. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Zhang, L.; Zhang, Z.; Zhou, Q.; Mo, F.; Zhang, Y.; Wang, J. Enantioselective Synthesis of Trisubstituted Allenes via Cu(I)-Catalyzed Coupling of Diazoalkanes with Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 14558–14561. [Google Scholar] [CrossRef]
- Poh, J.S.; Makai, S.; Keutz, T.; Tran, D.N.; Battilocchio, C.; Pasau, P.; Ley, S.V. Rapid Asymmetric Synthesis of Disubstituted Allenes by Coupling of Flow-Generated Diazo Compounds and Propargylated Amines. Angew. Chem. Int. Ed. 2017, 56, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Xu, J.; Yang, J.; Lin, L.; Feng, X.; Liu, X. Asymmetric Three-Component Reaction for the Synthesis of Tetrasubstituted Allenoates via Allenoate-Copper Intermediates. Chem 2018, 4, 1658–1672. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Wang, Z.; Shao, Y.; Sun, J. Copper-Catalyzed Tandem Cross-Coupling and Alkynylogous aldol Reaction: Access to Chiral Exocyclic α-Allenols. Org. Lett. 2021, 23, 5175–5179. [Google Scholar] [CrossRef]
- Ma, S. Electrophilic Addition and Cyclization Reactions of Allenes. Acc. Chem. Res. 2009, 42, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Tius, M.A. Cationic Cyclopentannelation of Allene Ethers. Acc. Chem. Res. 2003, 36, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Transition Metal-Catalyzed/Mediated Reaction of Allenes with a Nucleophilic Functionality Connected to the α-Carbon Atom. Acc. Chem. Res. 2003, 36, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mascareñas, J.L.; Varela, I.; López, F. Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479. [Google Scholar] [CrossRef] [Green Version]
- Blieck, R.; Taillefer, M.; Monnier, F. Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds. Chem. Rev. 2020, 120, 13545–13598. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Some Typical Advances in the Synthetic Applications of Allenes. Chem. Rev. 2005, 105, 2829–2872. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Almendros, P. Deciphering the Chameleonic Chemistry of Allenols: Breaking the Taboo of a Onetime Esoteric Functionality. Chem. Rev. 2021, 121, 4193–4252. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Kwon, O. Phosphine Catalysis of Allenes with Electrophiles. Chem. Soc. Rev. 2014, 43, 2927–2940. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.S.; Weatherly, C.D.; Burkea, E.G.; Schomaker, J.M. The Conversion of Allenes to Strained Three-Membered Heterocycles. Chem. Soc. Rev. 2014, 43, 3136–3163. [Google Scholar] [CrossRef]
- Ye, F.; Shi, Y.; Zhou, L.; Xiao, Q.; Zhang, Y.; Wang, J. Expeditious Synthesis of Phenanthrenes via CuBr2-Catalyzed Coupling of Terminal Alkynes and N-Tosylhydrazones Derived from O-Formyl Biphenyls. Org. Lett. 2011, 13, 5020–5023. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y.; Ye, F.; Zhang, Y.; Wang, J. CuBr-Catalyzed Coupling of N-Tosylhydrazones and Terminal Alkynes: Synthesis of Benzofurans and Indoles. Org. Lett. 2011, 13, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, G.; Jayaprakash, N.; Balakishan, G. Cu(I)-Catalyzed Tandem Benzyldiazoester Coupling with Terminal Alkyne–Allene Formation–Michael Reaction: Application to the Syntheses of Oxa and Azacycles. Org. Biomol. Chem. 2011, 9, 7913–7920. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhu, C.; Min, J.; Peng, S.; Xu, G.; Sun, J. Stereodivergent Synthesis of N-H bondeterocycles by Catalyst-Controlled, Activity-Directed Tandem Annulation of Diazo Compounds with Amino Alkynes. Angew. Chem. Int. Ed. 2015, 54, 12962–12967. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Liu, K.; Sun, J. Tandem Reaction of Allenoate Formation and Cyclization: Divergent Synthesis of Four- to Six-Membered Heterocycles. Org. Lett. 2018, 20, 7708–7711. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Song, R.; Guo, S.; Wang, Z.; Li, J. Copper/Silver-Cocatalyzed Conia-Ene Reaction of Linear β-Alkynic β-Ketoesters. Org. Lett. 2007, 9, 5111–5114. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ferrali, A.; Sladojevich, F.; Campbell, L.; Dixon, D.J. Brønsted Base/Lewis Acid Cooperative Catalysis in the Enantioselective Conia-Ene Reaction. J. Am. Chem. Soc. 2009, 131, 9140–9141. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, G.; Balakishan, G. Copper(I)-Catalysed Domino Coupling and Cyclisation Reaction: A Mild, Expedient Route for the Synthesis of Indene and Dihydronaphthalene Derivatives. Eur. J. Org. Chem. 2015, 2015, 3141–3146. [Google Scholar] [CrossRef]
- Min, J.; Xu, G.; Sun, J. Synthesis of Six-Membered Carbo-/Heterocycles via Cascade Reaction of Alkynes and Diazo Compounds. J. Org. Chem. 2017, 82, 5492–5498. [Google Scholar] [CrossRef]
- Helan, V.; Gulevich, A.V.; Gevorgyan, V. Cu-Catalyzed Trans-Annulation Reaction of Pyridotriazoles with Terminal Alkynes under Aerobic Conditions: Efficient Synthesis of Indolizines. Chem. Sci. 2015, 6, 1928–1931. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Li, L.; Liu, L.; Guan, Q.; Yang, Y.; Zha, Z.; Wang, Z. Copper-Catalyzed Geminal Difunctionalization of Terminal Alkynes by Splitting Sulfonyl Hydrazones into Two Parts. Org. Lett. 2018, 20, 5592–5596. [Google Scholar] [CrossRef]
- Li, Z.; Sun, J. Copper-Catalyzed 1,1-Boroalkylation of Terminal Alkynes: Access to Alkenylboronates via a Three-Component Reaction. Org. Lett. 2021, 23, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, V.N.G.; Fiset, D.; Gritsch, P.J.; Azzi, S.; Charette, A.B. Stereoselective Rh2(S-IBAZ)4-Catalyzed Cyclopropanation of Alkenes, Alkynes, and Allenes: Asymmetric Synthesis of Diacceptor Cyclopropylphosphonates and Alkylidenecyclopropanes. J. Am. Chem. Soc. 2013, 135, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Takeda, K.; Shimada, N.; Nambu, H.; Anada, M.; Shiro, M.; Ando, K.; Hashimoto, S. Highly Enantioselective Cyclopropenation Reaction of 1-Alkynes with α-Alkyl-α-Diazoesters Catalyzed by Dirhodium(II) Carboxylates. Angew. Chem. Int. Ed. 2011, 50, 6803–6808. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhao, Z.; Zhang, F.; Wu, S.; Yan, G.; Quan, Y.; Ma, B. One-Pot Novel Regioselective Cycloisomerization Synthesis of 2-Substituted or 3-Substituted 4H-Furo[3, 2-c]chromene through the Intermediate Cyclopropenes of 3-Diazochroman-4-one and Phenylacetylene. Org. Lett. 2014, 16, 5524–5527. [Google Scholar] [CrossRef]
- Briones, J.F.; Davies, H.M.L. Rh2(S-PTAD)4-Catalyzed Asymmetric Cyclopropenation of Aryl Alkynes. Tetrahedron 2011, 67, 4313–4317. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, M.; Xue, X.; Marek, I.; Zhang, F.; Ma, J. Catalytic Enantioselective Cyclopropenation of Internal Alkynes: Access to Difluoromethylated Three-Membered Carbocycles. Angew. Chem. Int. Ed. 2019, 58, 618191–618196. [Google Scholar]
- Cui, X.; Xu, X.; Lu, H.; Zhu, S.; Wojtas, L.; Zhang, X.P. Enantioselective Cyclopropenation of Alkynes with Acceptor/Acceptor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc. 2011, 133, 3304–3307. [Google Scholar] [CrossRef]
- Briones, J.F.; Davies, H.M.L. Gold(I)-Catalyzed Asymmetric Cyclopropenation of Internal Alkynes. J. Am. Chem. Soc. 2012, 134, 11916–11919. [Google Scholar] [CrossRef]
- Briones, J.F.; Davies, H.M.L. Silver Triflate-Catalyzed Cyclopropenation of Internal Alkynes with Donor-/Acceptor-Substituted Diazo Compounds. Org. Lett. 2011, 13, 3984–3987. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Liao, P.; Bi, X. Silver-Catalyzed [2 + 1] Cyclopropenation of Alkynes with Unstable Diazoalkanes: N-Nosylhydrazones as Room-Temperature Decomposable Diazo Surrogates. Chem. Eur. J. 2017, 23, 4756–4760. [Google Scholar] [CrossRef]
- Chen, L.; Leslie, D.; Coleman, M.G.; Mack, J. Recyclable Heterogeneous Metal Foil-Catalyzed Cyclopropenation of Alkynes and Diazoacetates under Solvent-Free Mechanochemical Reaction Condition. Chem. Sci. 2018, 9, 4650–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, M.; Suematsu, H.; Yasutomi, Y.; Katsuki, T. Enantioenriched Synthesis of Cyclopropenes with a Quaternary Stereocenter, Versatile Building Blocks. J. Am. Chem. Soc. 2011, 133, 170–171. [Google Scholar] [CrossRef] [PubMed]
- González, M.J.; López, L.A.; Vicente, R. Zinc-Catalyzed Cyclopropenation of Alkynes via 2-Furylcarbenoids. Org. Lett. 2014, 16, 5780–5783. [Google Scholar] [CrossRef] [PubMed]
- Martίnez-Garcί, H.; Morales, D.; Perez, J.; Puerto, M.; Miguel, D. 1,3,5-Tris(thiocyanatomethyl)mesitylene as a Ligand. Pseudooctahedral Molybdenum, Manganese, and Rhenium Carbonyl Complexes and Copper and Silver Dimers. Copper-Catalyzed Carbene- and Nitrene-Transfer Reactions. Inorg. Chem. 2010, 49, 6974–6985. [Google Scholar] [CrossRef] [PubMed]
- Noonikara-Poyil, A.; Ridlen, S.G.; Rasika Dias, H.V. Isolable Copper(I) η2-Cyclopropene Complexes. Inorg. Chem. 2020, 59, 17860–17865. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Merritt, B.A.; Lemma, B.E.; McKoy, A.M.; Nguyen, T.; Swenson, A.K.; Mills, J.L.; Coleman, M.G. Cyclopropenation of Internal Alkynylsilanes and Diazoacetates Catalyzed by Copper(I) N-H Bondeterocyclic Carbene Complexes. Org. Biomol. Chem. 2016, 14, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Padin, D.; Varela, J.A.; Saá, C. Recent Advances in RutheniumCatalyzed Carbene/Alkyne Metathesis (CAM) Transformations. Synlett 2020, 31, 1147–1157. [Google Scholar]
- Dey, S.; De Sarkar, S. Synthetic Applications of Vinyl Ruthenium Carbenes Derived from Diazoalkanes and Alkynes. Adv. Synth. Catal. 2017, 359, 2709–2722. [Google Scholar] [CrossRef]
- Pei, C.; Zhang, C.; Qian, Y.; Xu, X. Catalytic Carbene/Alkyne Metathesis (CAM): A Versatile Strategy from Alkyne Bifunctionalization. Org. Biomol. Chem. 2018, 16, 8677–8685. [Google Scholar] [CrossRef]
- Qian, Y.; Shanahan, C.S.; Doyle, M.P. Templated Carbene Metathesis Reactions from the Modular Assembly of Enol-diazo Compounds and Propargyl Acetates. Eur. J. Org. Chem. 2013, 27, 6032–6037. [Google Scholar] [CrossRef]
- Archambeau, A.; Miege, F.; Meyer, C.; Cossy, J. Intramolecular Cyclopropanation and C–H Insertion Reactions with Metal Carbenoids Generated from Cyclopropenes. Acc. Chem. Res. 2015, 48, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Blacklock, T.J.; Loza, R. Silver-Promoted Isomerizations of Some Cyclopropene Derivatives. J. Am. Chem. Soc. 1981, 103, 2404–2405. [Google Scholar] [CrossRef]
- Padwa, A.; Xu, S.L. A New Phenol Synthesis from the Rhodium (I) Catalyzed Reaction of Cyclopropenes and Alkynes. J. Am. Chem. Soc. 1992, 114, 5881–5882. [Google Scholar] [CrossRef]
- Cambeiro, F.; López, S.; Varela, J.A.; Saá, C. Cyclization by Catalytic Ruthenium Carbene Insertion into C-H bond Bonds. Angew. Chem. Int. Ed. 2012, 51, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dinsmore, C.J. Rhodium(II) Acetate Catalyzed Alkyne Insertion Reactions of Alpha.-Diazo Ketones: Mechanistic Inferences. J. Am. Chem. Soc. 1991, 113, 4343–4345. [Google Scholar] [CrossRef]
- Dong, K.; Pei, C.; Zeng, Q.; Wei, H.; Doyle, M.P.; Xu, X. Selective C(sp3)–H Bond Insertion in Carbene/Alkyne Metathesis Reactions. Enantioselective Construction of Dihydroindoles. ACS Catal. 2018, 8, 9543–9549. [Google Scholar] [CrossRef]
- Dong, K.; Fan, X.; Pei, C.; Zheng, Y.; Chang, S.; Cai, J.; Qiu, L.; Yu, Z.; Xu, X. Transient-Axial-Chirality Controlled Asymmetric Rhodium-Carbene C(sp2)-H bond Functionalization for the Synthesis of Chiral Fluorenes. Nat. Commun. 2020, 11, 2363. [Google Scholar] [CrossRef]
- Panne, P.; Fox, J.M. Rh-Catalyzed Intermolecular Reactions of Alkynes with α-Diazoesters That Possess β-H bondydrogens: Ligand-Based Control over Divergent Pathways. J. Am. Chem. Soc. 2007, 129, 22–23. [Google Scholar] [CrossRef]
- Ni, Y.; Montgomery, J. Synthetic Studies and Mechanistic Insight in Nickel-Catalyzed [4 + 2 + 1] Cycloadditions. J. Am. Chem. Soc. 2006, 128, 2609–2614. [Google Scholar] [CrossRef] [Green Version]
- Cambeiro, F.; López, S.; Varela, J.A.; Saá, C. Vinyl Dihydropyrans and Dihydrooxazines: Cyclizations of Catalytic Ruthenium Carbenes Derived from Alkynals and Alkynones. Angew. Chem. Int. Ed. 2014, 53, 5959–5963. [Google Scholar] [CrossRef]
- Xia, L.; Lee, Y.R. Regioselective Synthesis of Highly Functionalized Furans through the RuII-Catalyzed [3 + 2] Cycloaddition of Diazodicarbonyl Compounds. Eur. J. Org. Chem. 2014, 2014, 3430–3442. [Google Scholar] [CrossRef]
- Kurandina, D.; Gevorgyan, V. Rhodium Thiavinyl Carbenes from 1,2,3-Thiadiazoles Enable Modular Synthesis of Multisubstituted Thiophenes. Org. Lett. 2016, 18, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, X.; Wojtas, L.; Kim, M.; Zhang, X.P. Regioselective Synthesis of Multisubstituted Furans via Metalloradical Cyclization of Alkynes with α-Diazocarbonyls: Construction of Functionalized α-Oligofurans. J. Am. Chem. Soc. 2012, 134, 19981–19984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulevich, A.V.; Dudnik, A.S.; Chernyak, N.; Gevorgyan, V. Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem. Rev. 2013, 113, 3084–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.L.; Ye, J.; Zhang, Y.; Wang, J. Cu(I)-Catalyzed Reaction of Diazo Compounds with Terminal Alkynes: A Direct Synthesis of Trisubstituted Furans. Tetrahedron 2014, 70, 6957–6962. [Google Scholar] [CrossRef]
- Thomas, T.J.; Merritt, B.A.; Lemma, B.E.; McKoy, A.M.; Nguyen, T.; Swenson, K.; Mills, J.L.; Coleman, M.G. Highly Selective Synthesis of Tetra-Substituted Furans and Cyclopropenes: Copper(I)-Catalyzed formal Cycloadditions of Internal Aryl Alkynes and Diazoacetates. Org. Biomol. Chem. 2016, 14, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chang, S.; Qiu, L.; Xu, X. Chemodivergent Synthesis of Multi-Substituted/Fused Pyrroles via Copper-Catalyzed Carbene Cascade Reaction of Propargyl α-Iminodiazoacetates. Chem. Commun. 2016, 52, 12470–12473. [Google Scholar] [CrossRef]
- Yao, R.; Rong, G.; Yan, B.; Qiu, L.; Xu, X. Dual-Functionalization of Alkynes via Copper-Catalyzed Carbene/Alkyne Metathesis: A direct Access to the 4-Carboxyl Quinolines. ACS Catal. 2016, 6, 1024–1027. [Google Scholar] [CrossRef]
- Shen, W.B.; Sun, Q.; Li, L.; Liu, X.; Zhou, B.; Yan, J.Z.; Lu, X.; Ye, L.W. Divergent Synthesis of N-H bondeterocycles via Controllable Cyclization of Azido-Diynes Catalyzed by Copper and Gold. Nat. Commun. 2017, 8, 1748. [Google Scholar] [CrossRef]
- Qiu, H.; Deng, Y.; Marichev, K.O.; Doyle, M.P. Diverse Pathways in Catalytic Reactions of Propargyl Aryldiazoacetates. Selectivity Between Three Reaction Sites. J. Org. Chem. 2017, 82, 1584–1590. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, K.; Huang, J.; Qiu, L.; Xu, X. Copper-Catalyzed Carbene/Alkyne Metathesis Terminated with the Buchner reaction: Synthesis of Dihydrocyclohepta[b]indoles. Org. Biomol. Chem. 2019, 17, 2326–2330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qiu, L.; Hong, K.; Dong, S.; Xu, X. Copper- or Thermally Induced Divergent Outcomes: Synthesis of 4-Methyl 2H-Chromenes and Spiro-4H-Pyrazoles. Chem.–A Eur. J. 2018, 24, 6705–6711. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, J.; Sha, H.; Qiu, L.; Hu, W.; Xu, X. Copper-Catalyzed Formal [1 + 2 + 2]-Annulation of Alkyne-Tethered Diazoacetates and Pyridines: Access to Polycyclic Indolizines. Org. Biomol. Chem. 2020, 18, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).