Abstract
Ascorbic acid (AA) has antioxidant properties. However, in the presence of Fe2+/Fe3+ ions and H2O2, it may behave as a pro-oxidant by accelerating and enhancing the formation of hydroxyl radicals (•OH). Therefore, in this study we evaluated the effect of AA at concentrations of 1 to 200 µmol/L on •OH-induced light emission (at a pH of 7.4 and temperature of 37 °C) from 92.6 µmol/L Fe2+—185.2 µmol/L EGTA (ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid)—2.6 mmol/L H2O2, and 92.6 µmol/L Fe3+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 systems. Dehydroascorbic acid (DHAA) at the same range of concentrations served as the reference compound. Light emission was measured with multitube luminometer (AutoLumat Plus LB 953) for 120 s after automatic injection of H2O2. AA at concentrations of 1 to 50 µmol/L and of 1 to 75 µmol/L completely inhibited light emission from Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2, respectively. Concentrations of 100 and 200 µmol/L did not affect chemiluminescence of Fe3+-EGTA-H2O2 but tended to increase light emission from Fe2+-EGTA-H2O2. DHAA at concentrations of 1 to 100 µmol/L had no effect on chemiluminescence of both systems. These results indicate that AA at physiological concentrations exhibits strong antioxidant activity in the presence of chelated iron and H2O2.
1. Introduction
Reactive oxygen species (ROS) are involved in numerous physiological and pathological processes in the human body [1,2]. ROS include various radicals and oxidants and among them the most reactive species are hydroxyl radicals (•OH) [1]. Iron (Fe2+) dependent reduction of H2O2 (Fenton chemistry) is the major source of •OH radicals [1,3]. However, other transition metals such as copper, manganese, vanadium [4,5], as well as ionizing radiation [6,7] and peroxynitrite [8,9] could also contribute to generation of •OH radicals in vivo. The reaction of Fe2+ with H2O2 initiates numerous radical and non-radical processes, leading to the formation of •OH radicals, Fe3+, superoxide radicals (O2•−), and singlet oxygen [10,11,12]. O2•− can reduce Fe3+ to Fe2+ and this in turn accelerates the formation of •OH radicals [10,11]. Moreover, H2O2 can directly react with Fe3+ with subsequent creation of Fe2+, hydroperoxyl radical (HO2•), and H+ [11]. Thus, one may say that Fe2+ ions are regenerated and catalyze the reduction of H2O2 into •OH radicals. It is well known that chemical compounds which can effectively reduce Fe3+ to Fe2+ added to Fenton’s reagent (aqueous solution containing Fe2+ and H2O2) strongly enhanced •OH radicals generation. This was noted for hydroxylamine, ascorbic acid (AA), cysteine, 3-hydroxyanthranilic acid [13,14], and plant phenolics such as gallic acid, phloroglucinol, 3,4-dihydroxyphenylacetic acid, and phloretin [15]. Moreover, AA can undergo autooxidation, generating additional amounts of H2O2 [16,17]. Application of Fe2+-regenerating compounds appears to be a promising approach for Fenton chemistry in order to develop the effective methods of degradation of hazardous pollutants [13,14]. However, in the case of ascorbic acid (AA), this activity seems to be a double-edged sword. This is because H2O2 and free Fe2+ and Fe3+ ions (labile plasma iron) when present in circulating blood [18,19,20] AA may promote the generation of •OH radicals and induction of the peroxidative damage to a variety of biomolecules [20]. It should be pointed out that combinations of iron ions with AA and/or H2O2 were widely used for the induction of DNA damage in in vitro studies [21,22,23,24]. On the other hand, this mechanism was proposed as one of those responsible for the killing of cancer cells by high AA concentrations [16,17,25]. Recently, we developed a system composed of Fe2+, EGTA (ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid) and H2O2, which is a source of •OH radicals-induced ultra-weak photon emission (UPE) [26]. •OH radicals generated in the reaction of Fe2+ with H2O2 can attack and cleave the ether bond in an EGTA backbone structure. This leads to the formation of products containing triplet excited carbonyl groups and photons emissions [26]. Some studies indicate that the effect of AA on •OH generation by Fenton’s reagent may depend on its concentration and time of addition from the onset of reaction of Fe2+ with H2O2 [13]. Furthermore, AA itself can react with •OH radicals [27,28] while making the chemistry aspects of this process more complicated. Therefore, in this study we examined the effect of AA at a wide range of concentrations (including those present in human plasma) on the UPE of an Fe2+-EGTA-H2O2 system, as well as the UPE of medium containing Fe3+-EGTA-H2O2. The activity of AA was compared with that revealed by dehydroascorbic acid (DHAA), a relatively stable product of AA oxidation [13].
2. Results
The light emission (UPE-ultra weak photon emission) from 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system was 2306 ± 910 (2052; 71) RLU (n = 11). The UPE from incomplete control systems Fe2+-H2O2 and Fe2+-EGTA was significantly lower (p < 0.05, n = 11) and reached 1022 ± 295 (958; 339) RLU and 675 ± 111 (678; 137) RLU, respectively. These results are in agreement with those previously described [26], which also showed no light emissions from EGTA-H2O2 and H2O2 alone in comparison to the medium alone.
2.1. Effect of Ascorbic Acid and Dehydroascorbic Acid on Light Emission from Fe2+-EGTA-H2O2
The addition of AA (final concentrations of 1, 5, 10, 25, and 50 µmol/L) to the Fe2+-EGTA-H2O2 system completely abolished light emission (Figure 1A). The percent inhibition of UPE for concentrations of AA of 1, 5, 10, 25, and 50 µmol/L reached 102.3 ± 5.9 (99.7; 4.8), 101.7 ± 4.6 (101.7; 5.6), 100.2 ± 5.1 (100.0; 5.3), 100.7 ± 3.1 (99.3; 2.8), and 96.6 ± 2.4 (96.9; 1.0) (p < 0.05), respectively. The inhibition percentage slightly higher than 100 was most probably due to fluctuation of the baseline signal from medium alone. Higher concentrations of AA did not suppress the UPE of Fe2+-EGTA-H2O2 (Figure 1A). Even at concentrations of 100 µmol/L and 200 µmol/L, a moderate tendency (but not significant) of the enhancement of light emission was noted (Figure 1A). Control experiments showed no effect of AA (final concentrations of 1 and 200 µmol/L) on the UPE of incomplete systems (Fe2+-H2O2 and Fe2+-EGTA-H2O) and medium alone (Figure 1B). In these cases, the light emissions did not differ from the signal noted for medium alone.
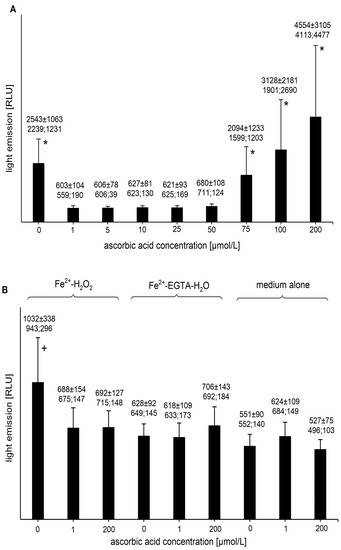
Figure 1.
(A)—Effect of ascorbic acid on light emission from 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system (B)—Effect of ascorbic acid on light emission from control systems with 92.6 µmol/L Fe2+—2.6 mmol/L H2O2, 92.6 µmol/L Fe2+—185.2 µmol/L EGTA-H2O, and medium alone with H2O. Total light emission was measured for 2 min just after automatic injection of 100 µL of H2O2 solution or distilled water. The final sample volume was 1080 µL. Results obtained from seven series of experiments are expressed as mean and standard deviation and (median; interquartile range). * vs. ascorbic acid concentrations of 1, 5, 10, 25, and 50 µmol/L, p < 0.05. † vs. Fe2+-H2O2 with the addition of ascorbic acid at concentrations of 1 and 200 µmol/L, Fe2+-EGTA and medium alone with or without addition of ascorbic acid at concentration of 1 and 200 µmol/L, p < 0.05. EGTA–ethylene glycol-bis (β-amino ethyl ether)-N,N,N′,N′-tetra acetic acid, RLU—relative light units.
All tested concentrations of DHAA had no effect on the UPE of the Fe2+-EGTA-H2O2 system (Figure 2A), while the DHAA at concentrations of 1 and 200 µmol/L did not alter the light emission from the control systems, Fe2+-H2O2, Fe2+-EGTA-H2O, or the medium alone (Figure 2B).
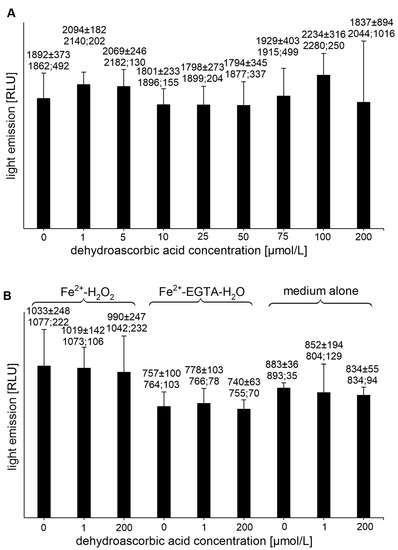
Figure 2.
(A)—Effect of dehydroascorbic acid on light emission from 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system (B)—Effect of dehydroascorbic acid on light emission from control systems with 92.6 µmol/L Fe2+—2.6 mmol/L H2O2, 92.6 µmol/L Fe2+—185.2 µmol/L EGTA-H2O, and medium alone with H2O. Total light emission was measured for 2 min just after automatic injection of 100 µL of H2O2 solution or distilled water. Final sample volume: 1080 µL. Results obtained from four series of experiments expressed as mean and standard deviation and (median; interquartile range). EGTA—ethylene glycol-bis (β-amino ethyl ether)-N,N,N′,N′-tetra acetic acid, RLU—relative light units.
2.2. Effect of Ascorbic Acid and Dehydroascorbic Acid on Light Emission from Fe3+-EGTA-H2O2
The Fe3+-EGTA-H2O2 system was a weaker light emitter than the Fe2+-EGTA-H2O2 system [1531 ± 292, (1493; 443) RLU, n = 8 vs. 2306 ± 910 (2052; 71) RLU, n = 11, p < 0.05]. Addition of AA to the Fe3+-EGTA-H2O2 system to final concentrations of 1, 5, 10, 25, 50, and 75 µmol/L completely abolished the UPE (Figure 3A) and the percent inhibition of the UPE was 103.1 ± 5.8 (105.1; 3.9), 92.5 ± 22.0 (102.3; 13.2), 102.4 ± 1.7 (102.8; 2.2), 106.5 ± 2.7 (105.3; 1.6), 106.3 ± 3.5 (106.5; 3.2), and 100.5 ± 3.6 (101.4; 4.0), respectively. AA at concentrations of 100 and 200 µmol/L did not significantly change the UPE of the Fe3+-EGTA-H2O2 system (p > 0.05, Figure 3A). AA at concentrations of 1 and 200 µmol/L decreased the light emission from the Fe3+-H2O2 (p < 0.05) but had no effect on Fe3+-EGTA-H2O and medium alone (Figure 3B).
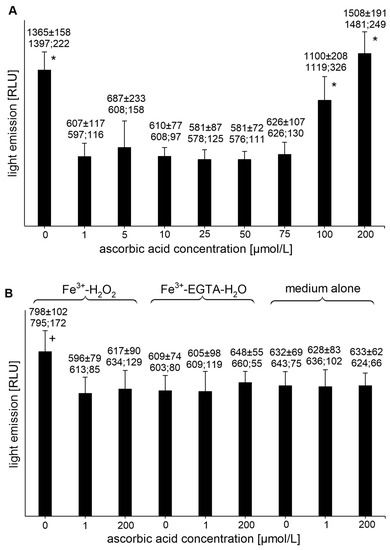
Figure 3.
(A)—Effect of ascorbic acid on light emission from 92.6 µmol/L Fe3+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system (B)—Effect of ascorbic acid on light emission from control systems with 92.6 µmol/L Fe3+—2.6 mmol/L H2O2, 92.6 µmol/L Fe3+—185.2 µmol/L EGTA-H2O, and medium alone with H2O. Total light emission was measured for 2 min just after automatic injection of 100 µL of H2O2 solution or distilled water. Final sample volume: 1080 µL. Results obtained from four series of experiments expressed as mean and standard deviation and (median; interquartile range). * vs. ascorbic acid concentrations of 1, 5, 10, 25, 50, and 75 µmol/L, p < 0.05. † vs. Fe3+-H2O2 with addition of ascorbic acid at concentrations of 1 and 200 µmol/L, Fe3+-EGTA, and medium alone with or without the addition of ascorbic acid at concentrations of 1 and 200 µmol/L, p < 0.05. EGTA—ethylene glycol-bis (β-amino ethyl ether)-N,N,N′,N′-tetra acetic acid, RLU—relative light units.
DHAA at concentrations of 1, 5, 10, 25, and 50 µmol/L had no effect on the UPE of the Fe3+-EGTA-H2O2 system (Figure 4A). For the concentrations of 75 and 100 µmol/L, a slight tendency to increase the light emission was noted, while at the DHAA concentration of 200 µmol/L, a tremendous increase in mean UPE (almost 70-times) was found (Figure 4A). DHAA at concentrations of 1 and 200 µmol/L also increased the light emission from Fe3+-H2O2 (p < 0.05) but had no effect on other controls (Fe3+-EGTA or medium alone) (Figure 4B).
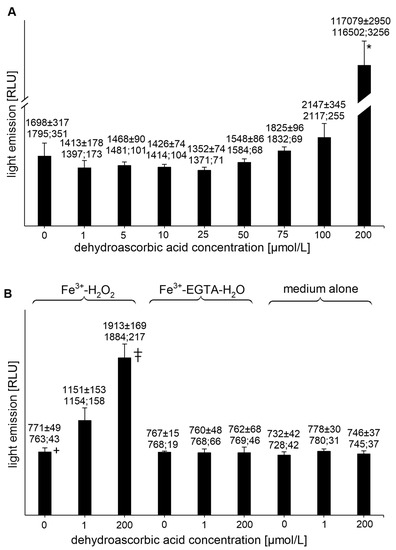
Figure 4.
(A)—Effect of dehydroascorbic acid on light emission from 92.6 µmol/L Fe3+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system (B)—Effect of dehydroascorbic acid on light emission from control systems with 92.6 µmol/L Fe3+—2.6 mmol/L H2O2, 92.6 µmol/L Fe3+—185.2 µmol/L EGTA-H2O, and medium alone with H2O. Total light emission was measured for 2 min just after automatic injection of 100 µL of H2O2 solution or distilled water. Final sample volume: 1080 µL. Results obtained from four series of experiments expressed as mean and standard deviation and (median; interquartile range). * vs. dehydroascorbic acid concentrations of 0, 1, 5, 10, 25, 50, 75, and 100 µmol/L, p < 0.05. † vs. Fe3+—H2O2 with the addition of dehydroascorbic acid at concentrations of 1 and 200 µmol/L. ‡ vs. dehydroascorbic acid concentration of 1 µmol/L, p < 0.05. EGTA—ethylene glycol-bis (β-amino ethyl ether)-N,N,N′,N′-tetra acetic acid, RLU—relative light units.
3. Discussion
Numerous reactions can occur simultaneously in an Fe2+-EGTA-H2O2 system [12,13,14,29,30]. Those leading to the generation of •OH radicals, superoxide radicals (O2•−), hydroperoxyl radicals (HO2•), singlet oxygen (O2(1∆g)), and the reduction of Fe3+ to Fe2+ are presented below:
Fe2+-EGTA + H2O2 → Fe3+-EGTA + OH− + •OH (formation of hydroxyl radicals)
Fe3+-EGTA + H2O2 → Fe3+OOH−-EGTA + H+
Fe3+OOH−-EGTA + H2O2 → FeO2+-EGTA + HO2• + H2O
FeO2+-EGTA + H2O2 → Fe3+-EGTA + HO2• + OH−
HO2• → H+ + O2•− (formation of superoxide radicals)
Fe3+-EGTA + O2•− → Fe2+-EGTA + O2
Fe3+-EGTA + H2O2 → Fe2+-EGTA + HO2•
Fe3+-EGTA + HO2• → Fe2+-EGTA + O2 + H+.
Hydroperoxyl radicals (HO2•) are generated in reactions (3), (4), and (7).
Reduced iron formed in reactions (6), (7), and (8) can enter reaction 1 to enhance the generation of hydroxyl radicals
O2•− + •OH + H+ → H2O2 + O2(1∆g) formation of singlet oxygen
2O2•− + 2H+ → H2O2 + O2(1∆g) formation of singlet oxygen.
Hydroxyl radicals generated in an Fe2+-EGTA-H2O2 system can cleave one of the ether bonds in the backbone structure of EGTA, leading to formation of products with a triplet excited carbonyl group responsible for light emission [26]. O2•− radicals can reduce Fe3+ into Fe2+ ions, which again react with H2O2 and generate •OH radicals. Therefore, the UPE of the Fe2+-EGTA-H2O2 system was strongly inhibited by scavengers of •OH radicals (dimethylsulfoxide and mannitol) and partially by superoxide dismutase, which very rapidly catalyzes the dismutation of O2•− radicals into O2 and H2O2 [26]. The rates of reactions (7) and (8) are much slower than that of the reaction (1) [13,14]. Therefore, the UPE of Fe2+-EGTA-H2O2 was higher than the light emissions from Fe3+-EGTA-H2O2. In both Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 systems, the iron concentration was 28-times lower than the concentration of H2O2. Therefore, one may expect that the addition of reducing agent to both systems would increase the light emissions via the regeneration of Fe2+ ions and enhanced •OH radicals formation.
3.1. Effect of Ascorbic Acid and Dehydroascorbic Acid on the Light Emission from Fe2+-EGTA-H2O2
AA is a powerful reducer of Fe3+ ions [13,14]. Its ability to reduce Fe3+ ions into Fe2+ ions is stronger than that of uric acid, bilirubin, Trolox, and numerous plant phenolics such as ferulic acid, catechin, gallic acid, and quercetin [31,32]. Therefore, we expected that the addition of ascorbic acid to an Fe2+-EGTA-H2O2 system would enhance its UPE. Surprisingly, AA at concentrations of 1 to 50 µmol/L completely abolished the light emissions. Only higher AA concentrations of 100 and 200 µmol/L tended to increase the UPE of Fe2+-EGTA-H2O2 but this effect was not significant. AA can react with various reactive oxygen species such as H2O2, O2•−, O2(1∆g), and especially •OH radicals [33,34,35]. In our previous experiments, sodium azide as a scavenger of O2(1∆g) did not suppress the light emission from Fe2+-EGTA-H2O2 and the contribution of O2•− to this phenomenon was relatively low [26]. Moreover, the concentration of H2O2 was many times higher than that of AA, Fe2+, and EGTA in the Fe2+-EGTA-H2O2 system. Therefore, the plausible reaction of AA with H2O2 and O2•− seems to not be responsible for the quenching of the UPE. Thus, the reaction of AA with •OH radicals could have a crucial effect on the UPE of the Fe2+-EGTA-H2O2 system. The reaction of AA with •OH radical leads to the formation of ascorbate radical and H2O. At a physiological pH, the reaction of disproportionation of two molecules of ascorbate radicals is thermodynamically favored. This is a complex process and involves dimerization of an ascorbate radical, internal electron transfer, and hydrolysis of temporal dimer, and results in the formation of one molecule of DHAA and AA [35,36] which can again react with an •OH radical. The rate of the reaction of AA with Fe3+, which promotes •OH radicals generation, was affected by the pH of the solution and at the pH higher than 6, the rate was slow [37]. Therefore, under conditions of our experiments (pH = 7.4), the reaction of AA with •OH radicals dominates and protects molecules of EGTA from oxidative attack and generation of end-products, with triplet excited carbonyl groups responsible for light emission. These may explain the strong inhibitory effect of AA at concentrations of 1 to 50 µmol/L on the UPE of Fe2+-EGTA-H2O2. However, AA at higher concentrations of 75 to 200 µmol/L did not inhibit the UPE of Fe2+-EGTA-H2O2. This suggests that under those conditions, there is a relative balance between •OH radicals generation and their scavenging by AA and thus the activity of •OH radicals is similar in Fe2+-EGTA-H2O2 with and without high concentrations of AA. AA has chelating activity and was reported to form complexes with Fe2+ and Fe3+ ions [33,38]. Moreover, the formation of AA-Fe3+ complexes is necessary for AA- induced reduction of Fe3+ to Fe2+ [37,38]. EGTA is a chelating agent which complexes Fe2+ and Fe3+ ions [39]. In experiments with concentrations of AA of 75 to 200 µmol/L (close to concentration of EGTA of 185.2 µmol/L), there is a substantial possibility of formation of AA-Fe3+ complexes. Moreover, it cannot be excluded that under these conditions, mixed chelate complexes of EGTA-AA-Fe3+ could be formed. This is supported by the description of Fe3+- deferiprone-AA complexes (deferiprone is an iron chelator indicated for the treatment of iron overload) in medium of pH = 7.4 in vitro [33]. Thus, at concentrations of AA of 75 to 200 µmol/L, considerable augmentation of Fe2+ ions regeneration can occur. Therefore, the intensities of two reactions: AA- induced scavenging of •OH radicals and AA- induced Fe2+ regeneration, are comparable and these explain why higher AA concentrations did not inhibit the UPE of the Fe2+-EGTA-H2O2 system. On the other hand, low concentrations of AA (1 to 50 µmol/L) could not form sufficient amounts of redox active complexes with Fe3+ due to an excess of EGTA. These outcomes additionally explain the strong inhibitory effect of low AA concentrations on light emission from the Fe2+-EGTA-H2O2 system. Figure 5 summarizes the mechanism of inhibitory effect of AA (concentrations of 1 to 50 µmol/L) on the •OH radicals-induced UPE of the Fe2+-EGTA-H2O2 system. Although DHAA can react with H2O2 and •OH radicals [40], no effect of DHAA on UPE of Fe2+-EGTA-H2O2 was noted. DHAA is the product of two-electron oxidation of AA [36]. Therefore, it is a much weaker electron donor than AA and the involvement of DHAA in redox reactions after an addition to Fe2+-EGTA-H2O2 was many times lower than that in the case of AA. Hence, DHA did not alter the light emission from the Fe2+-EGTA-H2O2 system.
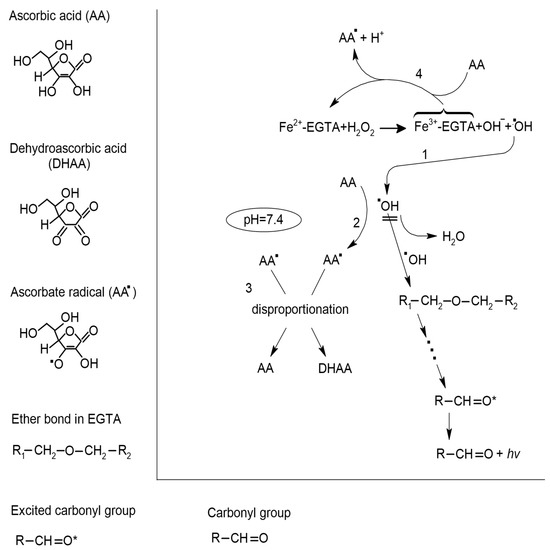
Figure 5.
Postulated mechanism of inhibitory effect of ascorbic acid (AA) on the •OH radical-induced ultraweak photon emission (UPE) of the Fe2+-EGTA-H2O2 system in medium of pH = 7.4. •OH radicals generated in the reaction of Fe2+ with H2O2 (1) attack one of the two ether bonds in the backbone structure of EGTA, leading to its cleavage and formation of other radicals that results in the creation of one product with a triplet excited carbonyl group (R-CH = O *). Electronic transitions from the triplet excited state to the ground state are accompanied by the photon emission (λν). AA can effectively react with •OH radicals (2) through the formation of an ascorbate radical (AA•). This protects the ether bonds of EGTA from oxidative attack and completely inhibits light emission from the Fe2+-EGTA-H2O2 system. Two molecules of AA• undergo disproportionation reaction (3) with the formation of one molecule of dehydroascorbic acid (DHAA) and one of AA, which again can react with •OH radicals. AA can reduce Fe3+ to Fe2+ (4) and therefore, enhances •OH radicals generation. However, under conditions of pH = 7.4 and excess of EGTA as chelating agent, this process has low intensity. This pathway of Fe2+ regeneration is enhanced for higher concentrations of AA (75 to 200 µmol/L) and therefore, they do not inhibit the UPE of the Fe2+-EGTA-H2O2 system. For more details, please refer to [26,35,36].
3.2. Effect of Ascorbic Acid and Dehydroascorbic Acid on the Light Emission from Fe3+-EGTA-H2O2
As was stated before, the Fe3+-EGTA-H2O2 system was a weaker light emitter than the Fe2+-EGTA-H2O2 one. •OH radicals initiating the light emission from Fe3+-EGTA-H2O2 system are formed in the reaction (1), which occurs as a result of the reaction (7). AA at concentrations of 1 to 75 µmol/L inhibited the UPE of the Fe3+-EGTA-H2O2 system through direct scavenging of •OH radicals. Due to a medium pH of 7.4 and excess of EGTA, these low concentrations of AA could not effectively reduce Fe3+ ions to Fe2+ ions, therefore the inhibition of light emission was complete. However, at higher concentrations of AA (100 and 200 µmol/L), the process of Fe2+ ions formation was enhanced and resulted in higher generation of •OH radicals. Thus, AA at concentrations of 100 and 200 µmol/L did not alter the light emission from the Fe3+ EGTA-H2O2 system due to a dynamic balance between •OH radicals scavenging and the promotion of •OH radicals generation caused by this vitamin. Because DHAA is a weaker electron donor than AA [36], this compound at concentrations of 1 to 100 µmol/L had no significant effect on the UPE of the Fe3+-H2O2-EGTA system. However, the concentration of DHAA of 200 µmol/L tremendously (by about 70-times) increased photons emission from the Fe3+-H2O2-EGTA system. DHAA was reported to react with H2O2 through the formation of 4-O-oxalyl-threonate and 3-O-oxalyl-threonate as the main products, small amounts of cyclic oxalyl-threonate, 2-keto-L-xylonate, and threonic acid, and trace amounts of oxalic acid while oxidation of DHAA by •OH radicals generated by Fenton’s reagent (Fe2+-EDTA-H2O2) produced mainly oxalic acid and both isomers of oxalyl threonate and small amounts of threonic acid [40]. Because DHAA at a concentration of 200 µmol/L had no effect on light emission from Fe2+-EGTA-H2O2 and Fe2+-H2O2 as well as Fe3+-EGTA-H2O2 generated substantially less •OH radicals than Fe2+-EGTA-H2O2, one may conclude that reactions leading to formation of cyclic oxalyl-threonate and 2-keto-L-xylonate may be involved in very strong augmentation of the UPE of the Fe3+-H2O2-EGTA system. DHAA also augmented the light emission from Fe3+-H2O2 by about 2.5-times, having no effect on this process in medium containing Fe3+ and EGTA. This suggests that EGTA is not necessary for moderate augmentation of UPE by DHAA oxidized in the presence of Fe3+ and H2O2. On the other hand, the combination of EGTA or its derivatives formed after •OH- induced oxidative attack with cyclic oxalyl-threonate or 2-keto-L-xylonate may result in chemical reactions which efficiently generate light and strongly augment the UPE of the Fe3+-H2O2-EGTA system. However, confirmation of these hypothetical mechanisms requires further studies.
3.3. Relevance to Human Physiology
It is believed that plasma concentrations of H2O2 range from 1 to 5 µmol/L in healthy subjects. However, in the course of certain diseases, the levels of H2O2 in plasma can increase up to 50 µmol/L [18]. The plasma concentration of iron complexed with low molecular weight compounds is about 1 µmol/L in healthy subjects while in patients with hemochromatosis, this can reach 10 µmol/L [41]. The median concentrations of AA and DHAA in plasma of healthy subjects were around 61.4 µmol/L and 2.3 µmol/L [42], however in critically ill patients (sepsis, major-organ failure, severe accidental injury), they decreased to 9.0 µmol/L and 1.4 µmol/L, respectively [42]. Therefore, the studied concentrations of AA and DHAA included the concentration ranges which can occur in healthy subjects and those with a strong inflammatory response. Because oxygen pressure in arterial blood ranges from 75 mmHg to 100 mmHg in healthy subjects [43] and O2 is involved in final reactions, leading to the formation of a product with triplet excited carbonyl groups [26], we did not use deaerated solutions in our experiments. In addition, AA was stable in undeaerated phosphate buffers of pH = 7.2 and 7.8 for at least 50 min [44]. Thus, unspecific decompositions of AA could not have had any influence on the results of our experiments.
The most important finding was that AA at concentrations of 5 to 50 µmol/L which can occur in human plasma suppressed the •OH radicals-induced light emission from both systems: Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2. Moreover, the concentration of AA of 75 µmol/L inhibited the UPE of the Fe3+-EGTA-H2O2 but had no significant effect on that of Fe2+-EGTA-H2O2.These suggest that under physiological conditions, the antioxidant activity (scavenging of •OH radicals) of AA prevails over its plausible pro-oxidant activity related to the reduction of Fe3+ to Fe2+ ions. It should be pointed out that even higher concentrations of AA of 100 and 200 µmol/L did not significantly alter the UPE of Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 systems. However, circulating blood plasma containing H2O2 and iron complexed with low molecular weight chelating compounds is much more complex medium than our in vitro model. Recent clinical studies showing that intravenous administration of AA in a single dose of 750 mg or 7500 mg for six days did not increase oxidative stress markers (plasma concentrations of thiobarbituric acid reactive substances and urinary 8-oxoguanosine) in healthy subjects [45] support our observations.
Circulating blood has a pH of around 7.4 and a temperature of 37 °C. However, locally at the place of inflammation and also in certain solid tumors, the tissue environment could be acidic with a pH ranging from 5.7 to 7.0 [46]. This may predispose towards the reduction of Fe3+ to Fe2+ by AA and the enhanced generation of •OH radicals. Therefore, pro-oxidant activity of ascorbate cannot be excluded under such circumstances.
3.4. Limitations of the Study
The UPE was measured with a luminometer equipped with a photon counter sensitive to photons emitted in the 380 nm–630 nm range. We proposed a mechanism of light emission by Fe2+-EGTA-H2O2 which involves an •OH- induced cleavage of the ether bond in the backbone chain of EGTA molecule, its further degradation and formation of another radical, and triplet excited carbonyl groups [26]. Triplet excited carbonyl groups emit photons with a spectral range of 350 nm to 550 nm [47]. The human body spontaneously emits light, mostly within the wavelength range of 420 nm to 570 nm [48]. This suggests the occurrence of other sources of UPE in body fluids than triplet excited carbonyl groups. Therefore, a lack of spectral analysis of the UPE of Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 with and without studied compounds could be recognized as the limitation of our study. Unfortunately, there were no technical capabilities to use any cut-off filters for spectral analysis in AutoLumat Plus LB 953. Spectral analysis of the UPE would be especially helpful for an explanation of 200 µmol/L DHAA-induced enhancement of light emission form Fe3+-EGTA-H2O2. If the emission spectra of Fe3+-EGTA-H2O2 and Fe3+-EGTA-DHAA-H2O2 would be similar, one may conclude that this enhancement of the UPE is the consequence of increased formation of triplet excited carbonyl groups. However, from the physiological and clinical point of view, this is not important because concentrations of DHAA of 200 µmol/L could not occur in human plasma, even after intravenous administration of high doses of AA [45]. On the other hand, it cannot be excluded that strong light emission from Fe3+-EGTA-DHAA-H2O2 may be used for the determination of anti-oxidant properties of other compounds. Therefore, further experiments to elucidate the mechanism of DHAA-induced augmentation of the UPE of Fe3+-EGTA-H2O2 are worth conducting.
4. Materials and Methods
4.1. Reagents
All chemicals were of analytical grade. Iron (II) sulfate heptahydrate (FeSO4 × 7H2O), iron (III) chloride hexahydrate (FeCl3 × 6H2O), sodium L-ascorbate (AA), L-dehydroascorbic acid (DHAA), and ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA.) H2O2 30% solution (w/w) was from Chempur (Piekary Slaskie, Poland). Sterile phosphate buffered saline (PBS, pH 7.4, without Ca2+ and Mg2+) was obtained from Biomed (Lublin, Poland). Sterile deionized pyrogen-free water (freshly prepared, resistance >18 MW/cm, HPLC H2O Purification System, USF Elga, Buckinghamshire, UK) was used throughout the study. Working aqueous solutions of 5 mmol/L of FeSO4 and 5 mmol/L of FeCl3 were prepared before the assay. A working solution of 28 mmol/L of H2O2 was also prepared before the assay by dilution of 30% of H2O2 solution and the concentration was confirmed by the measurement of absorbance at 240 nm using a molar extinction coefficient of 43.6 mol−1cm−1 [49]. A stock solution of EGTA (100 mmol/L) was prepared in PBS with pH adjusted to 8.0 with 5 mol/L of NaOH and was stored at room temperature in the dark for no longer than 3 months. Ten mmol/L of EGTA working solution was obtained by appropriate dilution of EGTA stock solution with water before the assay. AA and DHAA solutions in PBS (7.2 mmol/L) and their 2-, 2.7-, 4-, 8-, 20-, 40-, and 200-times dilutions were prepared freshly before the assay.
4.2. System Generating Light and Measurement of Light Emission
For light generation, we used 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system, as previously described [26]. This system generates the UPE, which depends mainly on •OH radicals. •OH generated in the course of reaction of Fe2+ with H2O2 can oxidatively attack and cleave ether bonds in EGTA molecule, which leads to formation of triplet excited carbonyl groups and light emission [26]. Briefly, 20 µL of 10 mmol/L EGTA solution was added to the tube (Lumi Vial Tube, 5 mL, 12 × 75 mm, Berthold Technologies, Bad Wildbad, Germany) containing 940 µL of PBS. Afterwards, 20 µL of 5 mmol/L solution of FeSO4 was added and after gentle mixing, the tube was placed in the luminometer chain and incubated for 10 min in the dark at 37 °C. Then, 100 µL of 28 mmol H2O2 solution was added by an automatic dispenser and the total light emission (expressed in RLU—relative light units) was measured for 120 s with a multitube luminometer (AutoLumat Plus LB 953, Berthold, Germany) equipped with a Peltier-cooled photon counter (spectral range from 380 to 630 nm) to ensure high sensitivity and low and stable background noise signals.
4.3. Effect of Ascorbic Acid and Dehydroascorbic Acid on Light Emission from Fe2+-EGTA-H2O2 System
In order to determine the effect of AA on the UPE of 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 system, 30 µL of working solution of AA in PBS or its appropriate dilutions were added to the luminometer tube containing EGTA and FeSO4 in PBS and incubated for 10 min at 37 °C in the dark and then 100 µL of H2O2 solution was injected and the total light emission was measured for 2 min. The final concentrations of AA in the reaction mixture were 1, 5, 10, 25, 50, 75, 100, and 200 µmol/L, respectively. Controls included: Fe2+-EGTA-H2O2 in PBS without AA, incomplete system Fe2+-H2O2 with and without AA, Fe2+-EGTA with and without AA, AA alone in PBS, and medium alone. The final concentrations of AA in the controls were 1 and 200 µmol/L. The same procedures were executed when the effect of DHAA on the UPE of 92.6 µmol/L Fe2+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 was studied. The design of these experiments is shown in Table 1. In each series of experiments (repeated at least 4 times), eight concentrations of AA or DHAA were tested. The inhibitory effect of AA or DHAA on the light emission was expressed as a percent inhibition (%I) calculated according to the formula: %I = [(A − B)/(A − C)] × 100% where A, B, and C are the total light emission from Fe2+-EGTA-H2O2, Fe2+-EGTA-studied compound (AA or DHAA)-H2O2, and medium (H2O injected into PBS), respectively. In the case of augmentation of the UPE, the percent enhancement (%E) was calculated as follows: %E = [(B − A)/(A − C)] × 100%. In additional experiments, the effect of AA and DHAA on light emission from 92.6 µmol/L Fe3+—185.2 µmol/L EGTA—2.6 mmol/L H2O2 was examined. The design of these experiments was the same as in Table 1, except for the addition of 20 µL of working solution of FeCl3 instead of 20 µL of working solution of Fe2SO4.

Table 1.
Design of experiments on the effect of ascorbic acid and dehydroascorbic acid on light emissions from the Fe2+-EGTA-H2O2 system.
4.4. Statistical Analysis
Results (total light emission, % inhibition or % enhancement of light emission) were expressed as mean (standard deviation) and median and interquartile range (IQR). The comparisons between the UPE of the Fe2+-EGTA-H2O2 system and the light emission from corresponding samples of a modified system (e.g., an incomplete system, system with the addition of AA or DHAA, Fe3+-EGTA-H2O2 with and without addition of AA or DHAA, and medium alone) were analyzed with the independent-samples (unpaired) t-test or Mann-Whitney U test depending on the data distribution, which was tested with the Kolmogorov–Smirnov–Liliefors test. The Brown–Forsythe test for analysis of the equality of the group variances was used prior to the application of the unpaired t-test and if variances were unequal, then the Welch’s t-test was used instead of the standard t-test. The comparisons of % inhibition or % enhancement of light emission caused by AA and DHAA were analyzed in the same way. A p-value < 0.05 was considered significant.
5. Conclusions
Ascorbic acid within the concentration range of 1 to 50 µmol/L very effectively inhibited •OH-induced light emission from Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 systems in vitro. Higher concentrations of 75 to 200 µmol/L did not significantly enhance the UPE of both modified Fenton systems. Because studied concentrations of AA involved those present in human plasma, one may conclude that AA can act as an antioxidant in the presence of iron complexed with low molecular weight compounds in circulating blood. Dehydroascorbic acid within the range of physiological concentrations of 1 to 5 µmol/L had no effect on the intensity of •OH- induced reaction, resulting in the light emission. Although these results were obtained from in vitro experiments, they strongly suggest the low risk of pro-oxidant activity of AA in healthy subjects.
Author Contributions
M.N., W.T., and D.N. conceived and designed the experiments. M.N., A.S., and A.W. performed the experiments. M.N. and P.J.N. analyzed the data. M.N., A.S., A.W., and D.N. contributed reagents, materials, and analysis tools. M.N., W.T., and D.N. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 503/1-079-01/503-11-001 Medical University Lodz.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in study design, data collection, and analysis, the decision to publish, or preparation of the manuscript.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Jomova, K.; Valko, M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr. Pharm. Des. 2011, 17, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V.; Shaw, S.; Jayatilleke, E.; Stopler-Kasdan, T. Most free-radical injury is iron-related: It is promoted by iron, hemin, holoferritin and vitamin C, and inhibited by desferoxamine and apoferritin. Stem Cells 1994, 12, 289–303. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos. Environ. 2011, 45, 7555–7562. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Moreno, A.; Poschenrieder, C.; Shabala, S. Transition metals: A double edge sward in ROS generation and signaling. Plant Signal. Behav. 2013, 8, e23425. [Google Scholar] [CrossRef]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction-oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, H.; Li, Y.; Misra, H.P. Potent inhibition of peroxynitrite-induced DNA strand breakage and hydroxyl radical formation by dimethyl sulfoxide at very low concentrations. Exp. Biol. Med. 2010, 235, 614–622. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Barbusinski, K. Fenton reaction-controversy concerning the chemistry. Ecol. Chem. Eng. S 2009, 16, 347–358. [Google Scholar]
- Liu, X.; Sang, Y.; Yin, H.; Lin, A.; Guo, Z.; Liu, Z. Progress in the mechanism and kinetics of Fenton reaction. MOJ Eco. Environ. Sci. 2018, 3, 00060. [Google Scholar] [CrossRef]
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M.; Aristova, N.A.; Burhina, O.E.; Soshnikova, O.O. Mechanism of chemilumines-cence in Fenton reaction. J. Biophys. Chem. 2012, 3, 88–100. [Google Scholar] [CrossRef]
- He, D.Q.; Zhang, Y.J.; Pei, D.N.; Huang, G.X.; Liu, C.; Li, J.; Yu, H.Q. Degradation of benzoic acid in an advanced oxidation process: The effects of reducing agents. J. Hazard. Mater. 2020, 382, 121090. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.S.; Nicodemos-Ramos, M.D.; Vieira-Velloso, C.C.; Aguiar, A. Kinetic evaluation of dye decolorization by Fenton processes in the presence of 3-hydroxyanthranilic acid. Int. J. Environ. Res. Public. Health 2019, 16, 1602. [Google Scholar] [CrossRef] [PubMed]
- de Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H₂O₂ system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Esposito, B.P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z.I. Labile plasma iron in iron overload: Redox activity and susceptibility to chelation. Blood 2003, 102, 2670–2677. [Google Scholar] [CrossRef]
- Cao, H.; Wang, Y. Quantification of oxidative single-base and intrastrand cross-link lesions in unmethylated and CpG-methylated DNA induced by Fenton-type reagents. Nucleic Acids Res. 2007, 35, 4833–4844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Hu, N. Electrochemical detection of natural DNA damage induced by ferritin/ascorbic acid/H2O2 system and amplification of DNA damage by endonuclease Fpg. Biosens. Bioelectron. 2009, 25, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Ferlazzo, A.M.; Calatroni, A. Reduction of DNA fragmentation and hydroxyl radical production by hyaluronic acid and chondroitin-4-sulphate in iron plus ascorbate-induced oxidative stress in fibroblast cultures. Free Radic. Res. 2004, 38, 601–611. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Ponka, P.; Schulman, H.M. The iron chelator pyridoxal isonicotinoyl hydrazone (PIH) and its analogues prevent damage to 2-deoxyribose mediated by ferric iron plus ascorbate. Biochim. Biophys. Acta 2000, 1523, 154–160. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Abu-Hejleh, T.; et al. O2- and H2O-mediated dis-ruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017, 31, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Light emission from the Fe2+-EGTA-H₂O₂ system: Possible application for the determination of antioxidant activity of plant phenolics. Molecules 2018, 23, 866. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.C.; Bode, A.M. Biology of free radical scavengers: An evaluation of ascorbate. FASEB J. 1993, 7, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, H.; Zhou, Y.; Ogawa, N.; Lin, J.M. Self-catalytic degradation of ortho-chlorophenol with Fenton’s reagent studied by chemiluminescence. J. Environ. Sci. 2012, 24, 550–557. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Maidt, L.; Poyer, L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and fer-ric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron (II). Biochem. J. 1990, 269, 169–174. [Google Scholar] [CrossRef] [PubMed]
- DeGraft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric- reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox interactions of vitamin C and iron: Inhibition of the pro-oxidant activity by deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 075021. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free. Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO/O2- radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Keypour, H.; Silver, J.; Wilson, M.T.; Hamed, M.Y. Studies on the reactions of ferric iron with ascorbic acid. A study of solution chemistry using Mössbauer spectroscopy and stopped-flow techniques. Inorganica Chim. Acta 1986, 97, 97–106. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to solve the puzzle of the interaction of ascorbic acid and iron: Redox, chelation and therapeutic implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef]
- Martell, A.E.; Motekaitis, R.J.; Chen, D.; Hancock, R.D.; McManus, D. Selection of new Fe(lll)/Fe(ll) chelating agents as catalysts for the oxidation of hydrogen sulfide to sulfur by air. Can. J. Chem. 1996, 74, 1872–1879. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef]
- Dziuba, N.; Hardy, J.; Lindahl, P.A. Low-molecular-mass iron complexes in blood plasma of iron-deficient pigs do not originate directly from nutrient iron. Metallomics 2019, 11, 1900–1911. [Google Scholar] [CrossRef]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood. Res. 2019, 9, 1–14. [Google Scholar] [PubMed]
- Golubitskii, G.B.; Budko, E.V.; Basova, E.M.; Kostarnoi, A.V.; Ivanov, V.M. Stability of ascorbic acid in aqueous and aqueous–organic solutions for quantitative determination. J. Anal. Chem. 2007, 62, 742–747. [Google Scholar] [CrossRef]
- Mühlhöfer, A.; Mrosek, S.; Schlegel, B.; Trommer, W.; Rozario, F.; Böhles, H.; Schremmer, D.; Zoller, W.G.; Biesalski, H.K. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur. J. Clin. Nutr. 2004, 58, 1151–1158. [Google Scholar] [CrossRef]
- Erra, D.F.; Dantas, E.; Geffner, J. Unravelling the interplay between extracellular acidosis and immune cells. Mediat. Inflamm. 2018, 2018, 1218297. [Google Scholar]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Wijk, E.P.; Wijk, R.V. Multi-site recording and spectral analysis of spontaneous photon emission from human body. Forsch. Komplement. Klass. Nat. 2005, 12, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.W.; Gibson, Q.H. The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J. Biol. Chem. 1970, 245, 2409–2413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).