Development and Effects of Influenza Antiviral Drugs
Abstract
1. Background
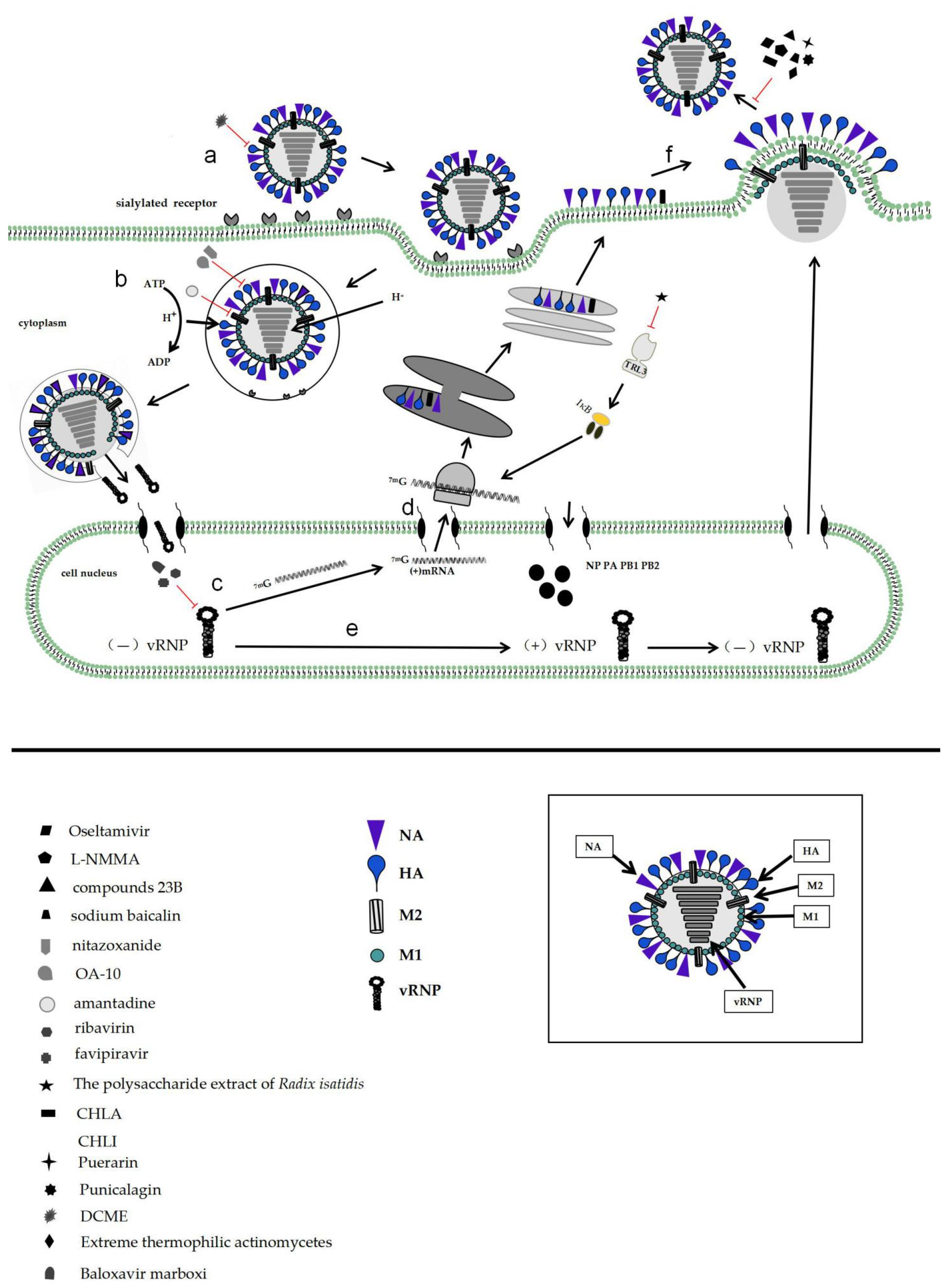
2. Influenza Virus Invades Host Mechanisms
3. Development and Effects of Influenza Antiviral Drugs
3.1. Development and Effects of Chemical Synthesis Drugs on Influenza Virus Resistance
3.2. Development and Effects of Plant Extracts on Influenza Virus Resistance
3.3. Development and Role of Microbial Metabolites in Influenza Virus Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durães-Carvalho, R.; Salemi, M. In-depth phylodynamics, evolutionary analysis and in silico predictions of universal epitopes of Influenza A subtypes and Influenza B viruses. Mol. Phylogenet. Evol. 2018, 121, 174–182. [Google Scholar] [CrossRef]
- Huang, S.S.H.; Banner, D.; Paquette, S.G.; Leon, A.J.; Kelvin, A.A.; Kelvin, D.J. Pathogenic influenza B virus in the ferret model establishes lower respiratory tract infection. J. Gen. Virol. 2014, 95, 2127–2139. [Google Scholar] [CrossRef]
- Bodewes, R.; Morick, D.; de Mutsert, G.; Osinga, N.; Bestebroer, T.; van der Vliet, S.; Smits, S.L.; Kuiken, T.; Rimmelzwaan, G.F.; Fouchier, R.A.; et al. Recurring influenza B virus infections in seals. Emerg. Infect. Dis. 2013, 19, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, S.M.; Österlund, P.; Westenius, V.; Latvala, S.; Diamond, M.S.; Gale, M., Jr.; Julkunen, I. RIG-I Signaling Is Essential for Influenza B Virus-Induced Rapid Interferon Gene Expression. J. Virol. 2015, 89, 12014–12025. [Google Scholar] [CrossRef]
- Sharabi, S.; Drori, Y.; Micheli, M.; Friedman, N.; Orzitzer, S.; Bassal, R.; Glatman-Freedman, A.; Shohat, T.; Mendelson, E.; Hindiyeh, M.; et al. Epidemiological and Virological Characterization of Influenza B Virus Infections. PLoS ONE 2016, 11, e0161195. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Lopes, T.J.; Neumann, G.; Kawaoka, Y. Development of high-yield influenza B virus vaccine viruses. Proc. Natl. Acad. Sci. USA 2016, 113, E8296–E8305. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, H.; Huang, J.; Zheng, Y.; Liu, C.; Su, J.; Ping, J. Features of Nuclear Export Signals of NS2 Protein of Influenza D Virus. Viruses 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Veit, M. Hemagglutinin-esterase-fusion (HEF) protein of influenza C virus. Protein Cell 2016, 7, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Cobey, S. Immune History and Influenza Vaccine Effectiveness. Vaccines 2018, 6, 28. [Google Scholar] [CrossRef]
- White, M.C.; Lowen, A.C. Implications of segment mismatch for influenza A virus evolution. J. Gen. Virol. 2018, 99, 3–16. [Google Scholar] [CrossRef]
- Brooke, C.B. Population Diversity and Collective Interactions during Influenza Virus Infection. J. Virol. 2017, 91, e01164-17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Szymański, K.; Łuniewska, K.; Hallmann-Szelińska, E.; Sałamatin, R.; Masny, A.; Brydak, L.B. Regional Activity and Spread of Influenza Viruses in Poland in the Context of Neighboring Countries in the Epidemic Season 2017-2018: An Epidemiological Review. Adv. Exp. Med. Biol. 2020, 1271, 11–19. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, J.; Ye, C.; Yu, J.; Peng, Z.; Feng, L.; Wang, L.; Qin, Y.; Zheng, Y.; Li, Z. Characteristics of Seasonal Influenza Virus Activity in a Subtropical City in China, 2013–2019. Vaccines 2020, 8, 108. [Google Scholar] [CrossRef]
- Zambon, M. Influenza and other emerging respiratory viruses. Medicine 2014, 42, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, B.; Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015, 33, 459–464. [Google Scholar] [CrossRef]
- Elbahesh, H.; Gerlach, T.; Saletti, G.; Rimmelzwaan, G.F. Response Modifiers: Tweaking the Immune Response Against Influenza A Virus. Front. Immunol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Yoo, S.J.; Kwon, T.; Lyoo, Y.S. Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure. Clin. Exp. Vaccine Res. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Meineke, R.; Rimmelzwaan, G.F.; Elbahesh, H. Influenza Virus Infections and Cellular Kinases. Viruses 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Moreno, R.; Martínez-Romero, C.; García-Sastre, A. Induction and Evasion of Type-I Interferon Responses during Influenza A Virus Infection. Cold Spring Harb. Perspect. Med. 2020. [Google Scholar] [CrossRef]
- Luo, M. Influenza virus entry. Adv. Exp. Med. Biol. 2012, 726, 201–221. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Entry of influenza virus. Adv. Exp. Med. Biol. 2013, 790, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, N.; Lane, A.P.; Pekosz, A. Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication. J. Virol. 2018, 92, e01425-18. [Google Scholar] [CrossRef]
- Vanderlinden, E.; Naesens, L. Emerging antiviral strategies to interfere with influenza virus entry. Med. Res. Rev. 2014, 34, 301–339. [Google Scholar] [CrossRef] [PubMed]
- Jakubcová, L.; Vozárová, M.; Hollý, J.; Tomčíková, K.; Fogelová, M.; Polčicová, K.; Kostolanský, F.; Fodor, E.; Varečková, E. Biological properties of influenza A virus mutants with amino acid substitutions in the HA2 glycoprotein of the HA1/HA2 interaction region. J. Gen. Virol. 2019, 100, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Righetto, I.; Milani, A.; Cattoli, G.; Filippini, F. Comparative structural analysis of haemagglutinin proteins from type A influenza viruses: Conserved and variable features. BMC Bioinform. 2014, 15, 363. [Google Scholar] [CrossRef]
- Jiang, S.; Li, R.; Du, L.; Liu, S. Roles of the hemagglutinin of influenza A virus in viral entry and development of antiviral therapeutics and vaccines. Protein Cell 2010, 1, 342–354. [Google Scholar] [CrossRef]
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef]
- Galloway, S.E.; Reed, M.L.; Russell, C.J.; Steinhauer, D.A. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: Implications for host range and adaptation. PLoS Pathog. 2013, 9, e1003151. [Google Scholar] [CrossRef]
- Kubiszewski-Jakubiak, S.; Worch, R. Influenza A H1 and H3 Transmembrane Domains Interact Differently with Each Other and with Surrounding Membrane Lipids. Viruses 2020, 12, 1461. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Yang, Z.; Chi, X.; Zhang, J.; Chen, J.L. Targeted disruption of influenza A virus hemagglutinin in genetically modified mice reduces viral replication and improves disease outcome. Sci. Rep. 2016, 6, 23746. [Google Scholar] [CrossRef]
- Hamilton, B.S.; Whittaker, G.R.; Daniel, S. Influenza virus-mediated membrane fusion: Determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses 2012, 4, 1144–1168. [Google Scholar] [CrossRef]
- Jakubcová, L.; Hollý, J.; Varečková, E. The role of fusion activity of influenza A viruses in their biological properties. Acta Virol. 2016, 60, 121–135. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Shen, X.; Liu, S. Influenza A virus entry inhibitors targeting the hemagglutinin. Viruses 2013, 5, 352–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Rabouw, H.; Slomp, A.; Dai, M.; van der Vegt, F.; van Lent, J.W.M.; McBride, R.; Paulson, J.C.; de Groot, R.J.; van Kuppeveld, F.J.M.; et al. Kinetic analysis of the influenza A virus HA/NA balance reveals contribution of NA to virus-receptor binding and NA-dependent rolling on receptor-containing surfaces. PLoS Pathog. 2018, 14, e1007233. [Google Scholar] [CrossRef]
- Kwak, D.K.; Kim, J.S.; Lee, M.K.; Ryu, K.S.; Chi, S.W. Probing the Neuraminidase Activity of Influenza Virus Using a Cytolysin A Protein Nanopore. Anal. Chem. 2020, 92, 14303–14308. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Wolff, T.; Herwig, A.; Pleschka, S.; Klenk, H.D. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: A study by reverse genetics. J. Virol. 2000, 74, 6316–6323. [Google Scholar] [CrossRef]
- Du, W.; de Vries, E.; van Kuppeveld, F.J.M.; Matrosovich, M.; de Haan, C.A.M. Second sialic acid-binding site of influenza A virus neuraminidase: Binding receptors for efficient release. FEBS J. 2020. [Google Scholar] [CrossRef]
- Vahey, M.D.; Fletcher, D.A. Influenza A virus surface proteins are organized to help penetrate host mucus. Elife 2019, 8, e43764. [Google Scholar] [CrossRef] [PubMed]
- Han, C.W.; Jeong, M.S.; Jang, S.B. Structure and Function of the Influenza A Virus Non-Structural Protein 1. J. Microbiol. Biotechnol. 2019, 29, 1184–1192. [Google Scholar] [CrossRef]
- Makau, J.N.; Watanabe, K.; Otaki, H.; Mizuta, S.; Ishikawa, T.; Kamatari, Y.O.; Nishida, N. A Quinolinone Compound Inhibiting the Oligomerization of Nucleoprotein of Influenza A Virus Prevents the Selection of Escape Mutants. Viruses 2020, 12, 337. [Google Scholar] [CrossRef]
- Massari, S.; Desantis, J.; Nizi, M.G.; Cecchetti, V.; Tabarrini, O. Inhibition of Influenza Virus Polymerase by Interfering with Its Protein-Protein Interactions. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, X.; Yan, D.; Chen, C.; Liu, X.; Huang, C.; Fu, X.; Tian, G.; Ding, C.; Wu, J.; et al. V292I mutation in PB2 polymerase induces increased effects of E627K on influenza H7N9 virus replication in cells. Virus Res. 2021, 291, 198186. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, P.; Mok, B.W.; Lau, S.Y.; Huang, X.; Wu, W.L.; Zheng, M.; Wen, X.; Yang, S.; Chen, Y.; et al. The K526R substitution in viral protein PB2 enhances the effects of E627K on influenza virus replication. Nat. Commun. 2014, 5, 5509. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 2016, 14, 479–493. [Google Scholar] [CrossRef]
- Davis, A.M.; Chabolla, B.J.; Newcomb, L.L. Emerging antiviral resistant strains of influenza A and the potential therapeutic targets within the viral ribonucleoprotein (vRNP) complex. Virol. J. 2014, 11, 167. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Zhang, L.; Zhang, R.; Chi, X.; Yang, Z.; Xie, Y.; Shu, S.; Liao, Y.; Chen, J.L. Identification of two residues within the NS1 of H7N9 influenza A virus that critically affect the protein stability and function. Vet. Res. 2018, 49, 98. [Google Scholar] [CrossRef]
- Ai, H.; Zhang, L.; Chang, A.K.; Wei, H.; Che, Y.; Liu, H. Virtual screening of potential inhibitors from TCM for the CPSF30 binding site on the NS1A protein of influenza A virus. J. Mol. Model. 2014, 20, 2142. [Google Scholar] [CrossRef]
- Twu, K.Y.; Noah, D.L.; Rao, P.; Kuo, R.L.; Krug, R.M. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 2006, 80, 3957–3965. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. Curr. Top. Microbiol. Immunol. 2014, 385, 359–375. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Lyons, D.M.; Lauring, A.S. Mutation and Epistasis in Influenza Virus Evolution. Viruses 2018, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, Q.; Bai, Z.; Qi, H.; Ma, J.; Liu, W.; Ding, F.; Li, J. Could Environment Affect the Mutation of H1N1 Influenza Virus? Int. J. Environ. Res. Public Health 2020, 17, 3092. [Google Scholar] [CrossRef]
- Shen, Z.; Lou, K.; Wang, W. New small-molecule drug design strategies for fighting resistant influenza A. Acta Pharm. Sin. B 2015, 5, 419–430. [Google Scholar] [CrossRef]
- Yen, H.L. Current and novel antiviral strategies for influenza infection. Curr. Opin. Virol. 2016, 18, 126–134. [Google Scholar] [CrossRef]
- Bassetti, M.; Castaldo, N.; Carnelutti, A. Neuraminidase inhibitors as a strategy for influenza treatment: Pros, cons and future perspectives. Expert Opin. Pharmacother. 2019, 20, 1711–1718. [Google Scholar] [CrossRef]
- Cohen, M.; Zhang, X.Q.; Senaati, H.P.; Chen, H.W.; Varki, N.M.; Schooley, R.T.; Gagneux, P. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol. J. 2013, 10, 321. [Google Scholar] [CrossRef]
- Vorobjev, Y.N. An effective molecular blocker of ion channel of M2 protein as anti-influenza a drug. J. Biomol. Struct. Dyn. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.H.; Kakkola, L.; Nagaraj, A.S.; Cheltsov, A.V.; Anastasina, M.; Kainov, D.E. Emerging cellular targets for influenza antiviral agents. Trends Pharmacol. Sci. 2012, 33, 89–99. [Google Scholar] [CrossRef]
- Pagadala, N.S. AZT acts as an anti-influenza nucleotide triphosphate targeting the catalytic site of A/PR/8/34/H1N1 RNA dependent RNA polymerase. J. Comput. Aided Mol. Des. 2019, 33, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Georgel, A.F.; Cayet, D.; Pizzorno, A.; Rosa-Calatrava, M.; Paget, C.; Sencio, V.; Dubuisson, J.; Trottein, F.; Sirard, J.C.; Carnoy, C. Toll-like receptor 5 agonist flagellin reduces influenza A virus replication independently of type I interferon and interleukin 22 and improves antiviral efficacy of oseltamivir. Antivir. Res. 2019, 168, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Caffrey, D.R.; Zeldovich, K.B.; Liu, P.; Gallagher, G.R.; Aiello, D.; Porter, A.J.; Kurt-Jones, E.A.; Bolon, D.N.; Poh, Y.P.; et al. Evolution of the influenza A virus genome during development of oseltamivir resistance in vitro. J. Virol. 2014, 88, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Boianelli, A.; Sharma-Chawla, N.; Bruder, D.; Hernandez-Vargas, E.A. Oseltamivir PK/PD Modeling and Simulation to Evaluate Treatment Strategies against Influenza-Pneumococcus Coinfection. Front. Cell. Infect. Microbiol. 2016, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Ait-Aissa, A.; Derrar, F.; Hannoun, D.; Gradi, E.A.; Scaravelli, D.; Bouslama, Z. Surveillance for antiviral resistance among influenza viruses circulating in Algeria during five consecutive influenza seasons (2009–2014). J. Med. Virol. 2018, 90, 844–853. [Google Scholar] [CrossRef]
- Takahashi, E.; Sawabuchi, T.; Kimoto, T.; Sakai, S.; Kido, H. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 feeding enhances humoral immune responses, which are suppressed by the antiviral neuraminidase inhibitor oseltamivir in influenza A virus-infected mice. J. Dairy Sci. 2019, 102, 9559–9569. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Zhang, R.; Chan, C.C.; Xi, B.; Zhu, Z.; Yang, J.; Poon, V.K.; Zhou, J.; Chen, M.; et al. CL-385319 inhibits H5N1 avian influenza A virus infection by blocking viral entry. Eur. J. Pharmacol. 2011, 660, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Dagley, A.; Tarbet, E.B. Combinations of L-N(G)-monomethyl-arginine and oseltamivir against pandemic influenza A virus infections in mice. Antivir. Chem. Chemother. 2017, 25, 11–17. [Google Scholar] [CrossRef][Green Version]
- Belardo, G.; Cenciarelli, O.; La Frazia, S.; Rossignol, J.F.; Santoro, M.G. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob. Agents Chemother. 2015, 59, 1061–1069. [Google Scholar] [CrossRef]
- Fernandes, A.C.L.; Vale, A.J.M.; Guzen, F.P.; Pinheiro, F.I.; Cobucci, R.N.; de Azevedo, E.P. Therapeutic Options Against the New Coronavirus: Updated Clinical and Laboratory Evidences. Front. Med. 2020, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lei, Z.; Zhao, L.; Chen, B.; Yang, F.; Liu, K.; Zhu, H.; Zhao, H.; Cao, R.; Zhang, K.; et al. Design, synthesis and biological evaluation of oseltamivir derivatives containing pyridyl group as potent inhibitors of neuraminidase for influenza A. Eur. J. Med. Chem. 2020, 185, 111841. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Y.; Wang, D.; Ma, L.; Guo, M.; Zhou, C.; Dou, J. The inhibitory effect of sodium baicalin on oseltamivir-resistant influenza A virus via reduction of neuraminidase activity. Arch. Pharm. Res. 2018, 41, 664–676. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Tao, H.; Steel, J.; Lowen, A.C. H5N8 and H7N9 packaging signals constrain HA reassortment with a seasonal H3N2 influenza A virus. Proc. Natl. Acad. Sci. USA 2019, 116, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Limburg, H.; Harbig, A.; Bestle, D.; Stein, D.A.; Moulton, H.M.; Jaeger, J.; Janga, H.; Hardes, K.; Koepke, J.; Schulte, L.; et al. TMPRSS2 Is the Major Activating Protease of Influenza A Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes. J. Virol. 2019, 93, e00649-19. [Google Scholar] [CrossRef]
- Ye, M.; Liao, Y.; Wu, L.; Qi, W.; Choudhry, N.; Liu, Y.; Chen, W.; Song, G.; Chen, J. An Oleanolic Acid Derivative Inhibits Hemagglutinin-Mediated Entry of Influenza A Virus. Viruses 2020, 12, 225. [Google Scholar] [CrossRef]
- Scott, C.; Kankanala, J.; Foster, T.L.; Goldhill, D.H.; Bao, P.; Simmons, K.; Pingen, M.; Bentham, M.; Atkins, E.; Loundras, E.; et al. Site-directed M2 proton channel inhibitors enable synergistic combination therapy for rimantadine-resistant pandemic influenza. PLoS Pathog. 2020, 16, e1008716. [Google Scholar] [CrossRef]
- Manzoor, R.; Igarashi, M.; Takada, A. Influenza A Virus M2 Protein: Roles from Ingress to Egress. Int. J. Mol. Sci. 2017, 18, 2649. [Google Scholar] [CrossRef]
- Jalily, P.H.; Duncan, M.C.; Fedida, D.; Wang, J.; Tietjen, I. Put a cork in it: Plugging the M2 viral ion channel to sink influenza. Antivir. Res. 2020, 178, 104780. [Google Scholar] [CrossRef]
- Radosevic, D.; Sencanski, M.; Perovic, V.; Veljkovic, N.; Prljic, J.; Veljkovic, V.; Mantlo, E.; Bukreyeva, N.; Paessler, S.; Glisic, S. Virtual Screen for Repurposing of Drugs for Candidate Influenza a M2 Ion-Channel Inhibitors. Front. Cell. Infect. Microbiol. 2019, 9, 67. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Wu, S.; Xu, R.; Li, Z.; Zhu, J.; Wang, H.; Li, X.; Tian, M.; Lu, H.; et al. Tauroursodeoxycholic acid (TUDCA) inhibits influenza A viral infection by disrupting viral proton channel M2. Sci. Bull. 2019, 64, 180–188. [Google Scholar] [CrossRef]
- Vanderlinden, E.; Vrancken, B.; Van Houdt, J.; Rajwanshi, V.K.; Gillemot, S.; Andrei, G.; Lemey, P.; Naesens, L. Distinct Effects of T-705 (Favipiravir) and Ribavirin on Influenza Virus Replication and Viral RNA Synthesis. Antimicrob. Agents Chemother. 2016, 60, 6679–6691. [Google Scholar] [CrossRef]
- Ilyushina, N.A.; Hay, A.; Yilmaz, N.; Boon, A.C.; Webster, R.G.; Govorkova, E.A. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob. Agents Chemother. 2008, 52, 3889–3897. [Google Scholar] [CrossRef]
- Jordan, P.C.; Stevens, S.K.; Deval, J. Nucleosides for the treatment of respiratory RNA virus infections. Antivir. Chem. Chemother. 2018, 26. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef] [PubMed]
- Brendish, N.J.; Clark, T.W. Antiviral treatment of severe non-influenza respiratory virus infection. Curr. Opin. Infect. Dis. 2017, 30, 573–578. [Google Scholar] [CrossRef]
- Bank, C.; Renzette, N.; Liu, P.; Matuszewski, S.; Shim, H.; Foll, M.; Bolon, D.N.; Zeldovich, K.B.; Kowalik, T.F.; Finberg, R.W.; et al. An experimental evaluation of drug-induced mutational meltdown as an antiviral treatment strategy. Evolution 2016, 70, 2470–2484. [Google Scholar] [CrossRef] [PubMed]
- Ormond, L.; Liu, P.; Matuszewski, S.; Renzette, N.; Bank, C.; Zeldovich, K.; Bolon, D.N.; Kowalik, T.F.; Finberg, R.W.; Jensen, J.D.; et al. The Combined Effect of Oseltamivir and Favipiravir on Influenza A Virus Evolution. Genome Biol. Evol. 2017, 9, 1913–1924. [Google Scholar] [CrossRef]
- Hashimoto, T.; Baba, K.; Inoue, K.; Okane, M.; Hata, S.; Shishido, T.; Naito, A.; Wildum, S.; Omoto, S. Comprehensive assessment of amino acid substitutions in the trimeric RNA polymerase complex of influenza A virus detected in clinical trials of baloxavir marboxil. Influenza Other Respir. Viruses 2020. [Google Scholar] [CrossRef]
- Ison, M.G.; Portsmouth, S.; Yoshida, Y.; Shishido, T.; Mitchener, M.; Tsuchiya, K.; Uehara, T.; Hayden, F.G. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): A randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2020, 20, 1204–1214. [Google Scholar] [CrossRef]
- Ando, Y.; Noshi, T.; Sato, K.; Ishibashi, T.; Yoshida, Y.; Hasegawa, T.; Onishi, M.; Kitano, M.; Oka, R.; Kawai, M.; et al. Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection. J. Antimicrob. Chemother. 2021, 76, 189–198. [Google Scholar] [CrossRef]
- Mishin, V.P.; Patel, M.C.; Chesnokov, A.; De La Cruz, J.; Nguyen, H.T.; Lollis, L.; Hodges, E.; Jang, Y.; Barnes, J.; Uyeki, T.; et al. Susceptibility of Influenza A, B, C, and D Viruses to Baloxavir(1). Emerg. Infect. Dis. 2019, 25, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Noshi, T.; Kitano, M.; Taniguchi, K.; Yamamoto, A.; Omoto, S.; Baba, K.; Hashimoto, T.; Ishida, K.; Kushima, Y.; Hattori, K.; et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antivir. Res. 2018, 160, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Hu, J.; Wang, C.; Prinz, R.A.; Peng, D.; Liu, X.; Xu, X. A77 1726, the active metabolite of the anti-rheumatoid arthritis drug leflunomide, inhibits influenza A virus replication in vitro and in vivo by inhibiting the activity of Janus kinases. FASEB J. 2020, 34, 10132–10145. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Palombo, E.A.; Chia Yeo, T.; Lim Siok Ley, D.; Lee Tu, C.; Malherbe, F.; Grollo, L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE 2013, 8, e79293. [Google Scholar] [CrossRef]
- Ti, H. Phytochemical profiles and their anti-inflammatory responses against influenza from Traditional Chinese medicine or herbs. Mini-Rev. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Jin, J.; Dou, J.; Guo, Q.; Ke, X.; Zhou, C.; Guo, M. Inhibitory Activity of Honeysuckle Extracts against Influenza A Virus In Vitro and In Vivo. Virol. Sin. 2020. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix isatidis Polysaccharides Inhibit Influenza a Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules 2017, 22, 116. [Google Scholar] [CrossRef]
- Wong, J.P.; Christopher, M.E.; Viswanathan, S.; Dai, X.; Salazar, A.M.; Sun, L.Q.; Wang, M. Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr. Pharm. Des. 2009, 15, 1269–1274. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Wang, Y.; Hou, X.; Wang, L.; Zhao, X.; Zhan, P.; Liu, X.; Rong, L.; Cui, Q. Identification of Chebulinic Acid and Chebulagic Acid as Novel Influenza Viral Neuraminidase Inhibitors. Front. Microbiol. 2020, 11, 182. [Google Scholar] [CrossRef]
- Wang, H.X.; Zeng, M.S.; Ye, Y.; Liu, J.Y.; Xu, P.P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Chen, Z.; Wang, Y.; Zhan, P.; Liu, X.; Kang, D.; Chen, Z.; Zhao, X.; Wang, L.; et al. Punicalagin is a neuraminidase inhibitor of influenza viruses. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Prinz, R.A.; Liu, X.; Xu, X. In Vitro and In Vivo Antiviral Activity of Gingerenone A on Influenza A Virus Is Mediated by Targeting Janus Kinase 2. Viruses 2020, 12, 1141. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, N.X.; Duan, N.; Liu, B.; Zhu, H.; Zhang, C.; Li, L.; Lu, C.; Huang, L. Traditional Chinese Medicine in Treating Influenza: From Basic Science to Clinical Applications. Front. Pharmacol. 2020, 11, 575803. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, Y.; Cui, Q.; Gong, L.; Yang, Y.; Jiang, H.; Rong, L.; Rong, R.; Du, R. Screening for Anti-Influenza Actives of Prefractionated Traditional Chinese Medicines. Evid. Based Complement. Altern. Med. 2020, 2020, 4979850. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Feng, X.L.; Zhang, L.L.; Xu, S.P.; Wu, C.Y.; Wei, W. Antiviral effects of an effective section of a prescription of traditional Chinese medicine on influenza virus A in vitro. Zhong Yao Cai 2009, 32, 391–394. [Google Scholar]
- Shi, Y.; Xu, H.; Xiao, Y.; Liu, P.; Pang, P.; Wu, S.; Deng, L.; Chen, X. Gegen Qinlian Decoction Downregulates the TLR7 Signalling Pathway to Control Influenza A Virus Infection. Biomed. Pharmacother. 2020, 121, 109471. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Xiao, J.; Wu, C.; Zhang, H.; Chen, Y.; Hu, Z.; Lin, W.; Xie, Q.; Li, H. Effect of feeding Chinese herb medicine ageratum-liquid on intestinal bacterial translocations induced by H9N2 AIV in mice. Virol. J. 2019, 16, 24. [Google Scholar] [CrossRef]
- Ding, Y.; Zeng, L.; Li, R.; Chen, Q.; Zhou, B.; Chen, Q.; Cheng, P.L.; Yutao, W.; Zheng, J.; Yang, Z.; et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017, 17, 130. [Google Scholar] [CrossRef]
- Fu, Y.J.; Yan, Y.Q.; Qin, H.Q.; Wu, S.; Shi, S.S.; Zheng, X.; Wang, P.C.; Chen, X.Y.; Tang, X.L.; Jiang, Z.Y. Effects of different principles of Traditional Chinese Medicine treatment on TLR7/NF-κB signaling pathway in influenza virus infected mice. Chin. Med. 2018, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, X.; Zhong, J.; Ye, H.; Zhang, S.; Ge, D.; Wang, X.; Wu, Y. The effects of the Xijiao Dihuang decoction combined with Yinqiao powder on miRNA-mRNA profiles in mice infected with influenza a virus. BMC Complement. Med. Ther. 2020, 20, 286. [Google Scholar] [CrossRef]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary Metabolites of Actinomycetes and their Antibacterial, Antifungal and Antiviral Properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, S.; Xie, X.; Fan, S.; Chen, D.; Wu, S.; He, J. Antiviral properties of extracts of Streptomyces sp. SMU 03 isolated from the feces of Elephas maximus. Fitoterapia 2020, 143, 104600. [Google Scholar] [CrossRef] [PubMed]
- Berezin, V.; Abdukhakimova, D.; Trenozhnikova, L.; Bogoyavlenskiy, A.; Turmagambetova, A.; Issanov, A.; Azizan, A. Antiviral activities of extremophilic actinomycetes extracts from Kazakhstan’s unique ecosystems against influenza viruses and paramyxoviruses. Virol. J. 2019, 16, 150. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Qin, Y.; Karthik, L.; Zhu, G.; Hou, C.; Jiang, L.; Liu, M.; Ye, X.; Liu, M.; et al. A new abyssomicin polyketide with anti-influenza A virus activity from a marine-derived Verrucosispora sp. MS100137. Appl. Microbiol. Biotechnol. 2020, 104, 1533–1543. [Google Scholar] [CrossRef]
- Wang, S.; Luo, X.; Yan, R.; Wang, Q.; Qi, Q.; Chi, X.; Zhang, L.; Yu, Z.; Cai, B.; Chen, J.L.; et al. 3-Anhydro-6-hydroxy-ophiobolin A displays high in vitro and in vivo efficacy against influenza A virus infection. Protein Cell 2016, 7, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Yang, J.L.; Qi, Q.Y.; Bao, L.; Yang, X.L.; Liu, M.M.; Huang, P.; Zhang, L.X.; Chen, J.L.; Cai, L.; et al. 3-Anhydro-6-hydroxy-ophiobolin A, a new sesterterpene inhibiting the growth of methicillin-resistant Staphylococcus aureus and inducing the cell death by apoptosis on K562, from the phytopathogenic fungus Bipolaris oryzae. Bioorg. Med. Chem. Lett. 2013, 23, 3547–3550. [Google Scholar] [CrossRef]
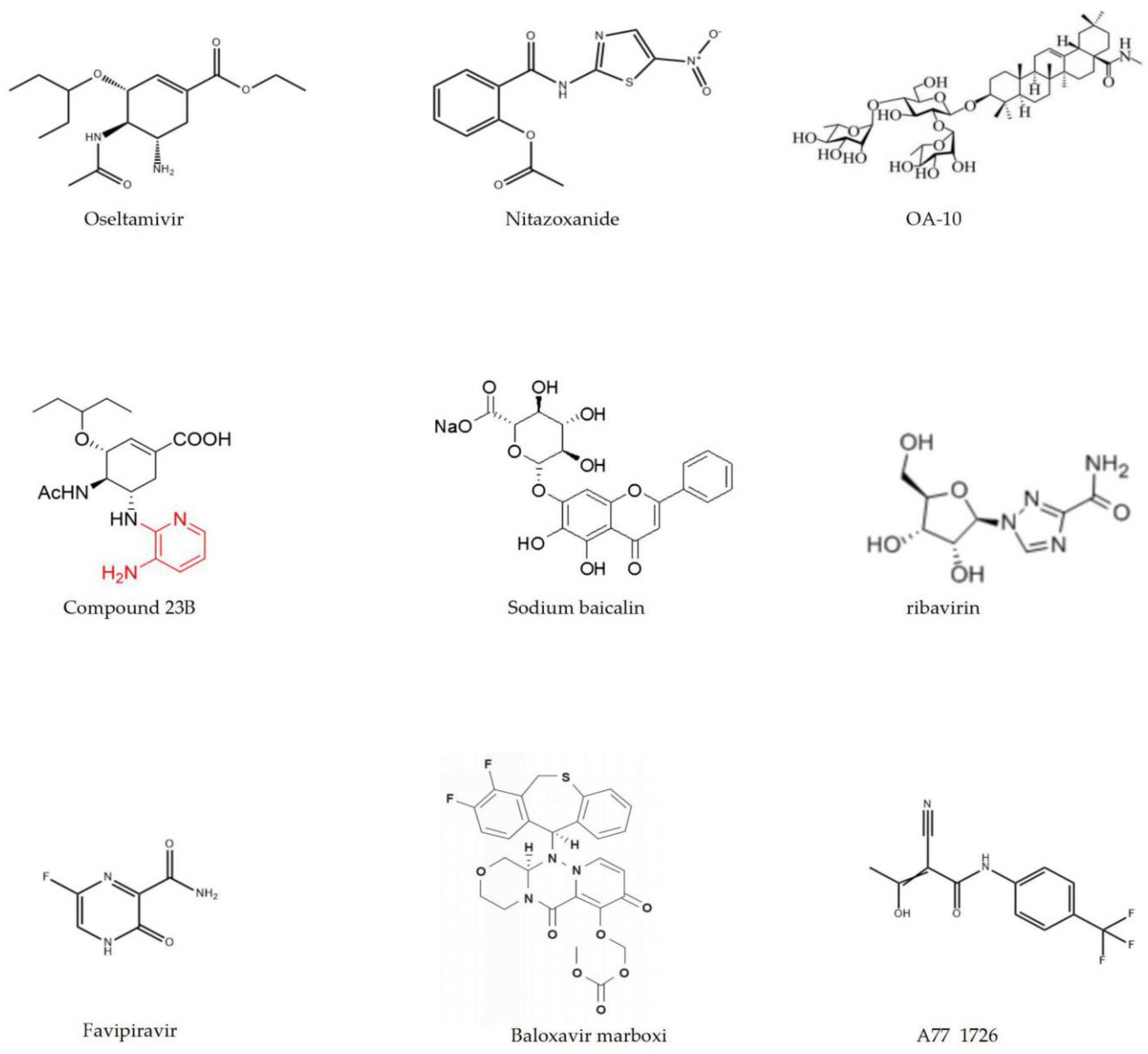
| Compounds | Source | Function | IC50/EC50 |
|---|---|---|---|
| Oseltamivir [64] | Shikimic acid | A/Brisbane/59/2007 (H1N1) | IC50 = 49.8 ± 6.8 nM |
| Nitazoxanide [70] | — | A/Puerto Rico/8/1934 (H1N1) | IC50 = 3.2 ± 0.0 μM |
| A/WSN/1933 (H1N1) | IC50 = 1.6 ± 0.2 μM | ||
| A/California/7/2009 (H1N1) | IC50 = 3.2 ± 0.0 μM | ||
| A/Parma/24/2009 (H1N1) | IC50 = 1.9 ± 0.0 μM | ||
| A/Parma/06/2007 (H3N2) | IC50 = 1.0 ± 0.0 μM | ||
| A/Chicken/Italy/9097/1997 (H5N9) | IC50 = 3.2 ± 0.5 μM | ||
| A/Goose/Italy/296,246/2003 (H1N1) | IC50 = 3.2 ± 0.2 μM | ||
| A/Turkey/Italy/RA5563/1999 (H7N1) | IC50 = 1.6 ± 0.2 μM | ||
| Compound 23B [72] | — | A/LiaoNing-ZhenXing/1109/2010 (H1N1) | EC50 = 14.31 ± 2.59 µM |
| A/Puerto Rico/8/1934 (H1N1) | EC50 = 12.68 ± 8.96 µM | ||
| Sodium baicalin [73] | baicalin | A/FM/1/47 (H1N1-H275Y) | EC50 = 20.1 ± 2.3 µM |
| A/FM/1/47 (H1N1) | EC50 = 4.0 ± 1.1 µM | ||
| A/Beijing/32/92 (H3N2) | EC50 = 2.7 ± 1.2 µM | ||
| OA-10 [76] | Oleanolic Acid | H5N1 | EC50 = 14.0 ± 2.3 μM |
| PR8 (H1N1) | EC50 = 6.7 ± 1.4 μM | ||
| H9N2 | EC50 = 15.3 ± 2.5 μM | ||
| H3N2 | EC50 = 19.6 ± 3.7 μM | ||
| Ribavirin [82] | — | influenza virus A/X-31 strain | EC50 = 8.1 ± 1.3 µM |
| Favipiravir [85] | — | all influenza virus tested | EC50 = 0.014~0.55 µg/mL |
| Baloxavir marboxi [93] | — | influenza A virus | IC50 = 1.4~3.1 nM |
| influenza B virus | IC50 = 4.5~8.9 nM | ||
| A77 1726 [94] | leflunomide | H1N1, H5N1, H9N2 | IC50 a < 50 µM |
| Original Plant | Active Fraction | Function | IC50/EC50 |
|---|---|---|---|
| Honeysuckle [97] | Acids extract | H1N1 | IC50 = 112.3 ± 17.7 μg/mL |
| H3N2 | IC50 = 332.6 ± 34.5 μg/mL | ||
| H7N9 | IC50 = 55.9 ± 5.1 μg/mL | ||
| H1N1-H275Y | IC50 = 150.4 ± 13.6 μg/mL | ||
| flavonoids extract | H1N1 | IC50 = 90.9 ± 8.6 μg/mL | |
| H3N2 | IC50 = 196.0 ± 23.4 μg/mL | ||
| H7N9 | IC50 = 24.7 ± 2.3 μg/mL | ||
| H1N1-H275Y | IC50 = 108.4 ± 17.0 μg/mL | ||
| acids-flavonoids mixture | H1N1 | IC50 = 100.1 ± 11.4 μg/mL | |
| H3N2 | IC50 = 203.8 ± 9.9 μg/mL | ||
| H7N9 | IC50 = 35.2 ± 3.1 μg/mL | ||
| H1N1-H275Y | IC50 = 125.7 ± 14.7 μg/mL | ||
| Radix isatidis [98] | Polysaccharide | A/Chicken/Guangdong/1996 (H9N2) | IC50 = 20.57 ± 0.25 mg/mL |
| A/PR/8/34 (H1N1) | IC50 = 20.48 ± 0.31 mg/mL | ||
| A/Guangzhou/GIRD07/09 (H1N1) | IC50 = 8.47 ± 0.07 mg/mL | ||
| A/Aichi/2/68 (H3N2) | IC50 = 4.35 ± 0.05 mg/mL | ||
| A/Duck/Guangdong (H6N2) | IC50 = 28.20 ± 0.49 mg/mL | ||
| Terminalia chebula Retz [100] | CHLA | reporter virus PR8-PB2-Gluc | IC50 = 1.36 ± 0.36 µM |
| CHLI | reporter virus PR8-PB2-Gluc | IC50 = 1.86 ± 0.98 µM | |
| Pueraria lobata [101] | Puerarin | A/FM/1/1947 (H1N1) | EC50 = 52.06 μM |
| Pomegranate [102] | Punicalagin | PR8-PB2-Gluc (H1N1) | IC50 = 1.25 ± 0.06 μM |
| Ginger [103] | Gingerenone A | H5N1 | IC50 = 10.2~24.5 µM |
| H9N2 | IC50 = 12 µM | ||
| H1N1 | IC50 = 10.2 µM |
| Prescriptions | Relieve Symptoms | Mechanism | Composition |
|---|---|---|---|
| GQD [107] | Cough, fever, anti-inflammatory | Down-regulating the expression of TLR signaling pathway factors, and affecting the differentiation of CD4+ T cells, thus limiting immune pathological injury caused by virus infection | Radix puerariae, Radix scutellariae, Rhizoma coptidis, Radix glycyrrhizae |
| LH-C [109] | Fever, cough, sore throat, fatigue | Inhibiting the activity of NF-κB and blocking the nuclear export of the viral RNP | Forsythia suspensa, Ephedra sinica Stapf, Lonicera japonica Thunb, Isatis indigotica Fortune, Mentha haplocalyx Briq, Dryopteris crassirhizoma Nakai, Rhodiola rosea L ., Gypsum Fibrosum , Pogostemon cablin (Blanco) Benth, Rheum palmatum L ., Houttuynia cordata Thunb, Glycyrrhiza uralensis Fisch, Armeniaca |
| Yinqiao Powder [110] | Cough, headache, fever | Playing an important anti-influenza role by regulating the TLR7/NF-κB signaling pathway | honeysuckle, Forsythiae fructus, Balloon flower root, Mint, Licorice root, Herba lophatheri, Fermented soybean, Schizonepeta spike, Great Burdock Achene |
| Microbial | Active Fraction | Function | IC50/EC50 |
|---|---|---|---|
| SMU-03 [113] | DCME | A/PR/8/34 (H1N1) | IC50 = 0.37 ± 0.22 μg/mL |
| A/FM/1/47 (H1N1) | IC50 = 0.81 ± 0.09 μg/mL | ||
| A/Aichi/2/68 (H3N2) | IC50 = 14.44 ± 0.79 μg/mL | ||
| influenza B virus | IC50 = 0.66 ± 0.03 μg/mL | ||
| Extreme thermophilic Actinomycetes [114] | K-192-2S | H1N1, H3N2, H5N3, H7N1 | EC50 = 0.80~0.14 mg/mL |
| K-340-2S | H1N1, H3N2, H5N3, H7N1 | EC50 = 0.05~0.15 mg/mL | |
| K-362-2N | H1N1, H3N2, H5N3, H7N1 | EC50 = 0.05~0.20 mg/mL | |
| MS100137 [115] | Compound 1 | H1N1 | EC50 < 10 μM |
| Compound 2 | H1N1 | EC50 < 10 μM | |
| Compound 3 | H1N1 | EC50 < 10 μM | |
| Bipolaris oryzae [116] | L435-3 | A/WSN/1933 (H1N1) | IC50 = 0.365 μM |
| A/PR/8/34 (H1N1) | IC50 = 0.391 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.-L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. https://doi.org/10.3390/molecules26040810
Yin H, Jiang N, Shi W, Chi X, Liu S, Chen J-L, Wang S. Development and Effects of Influenza Antiviral Drugs. Molecules. 2021; 26(4):810. https://doi.org/10.3390/molecules26040810
Chicago/Turabian StyleYin, Hang, Ning Jiang, Wenhao Shi, Xiaojuan Chi, Sairu Liu, Ji-Long Chen, and Song Wang. 2021. "Development and Effects of Influenza Antiviral Drugs" Molecules 26, no. 4: 810. https://doi.org/10.3390/molecules26040810
APA StyleYin, H., Jiang, N., Shi, W., Chi, X., Liu, S., Chen, J.-L., & Wang, S. (2021). Development and Effects of Influenza Antiviral Drugs. Molecules, 26(4), 810. https://doi.org/10.3390/molecules26040810






