Enhanced Sensitivity of CO on Two-Dimensional, Strained, and Defective GaSe
Abstract
1. Introduction
2. Results
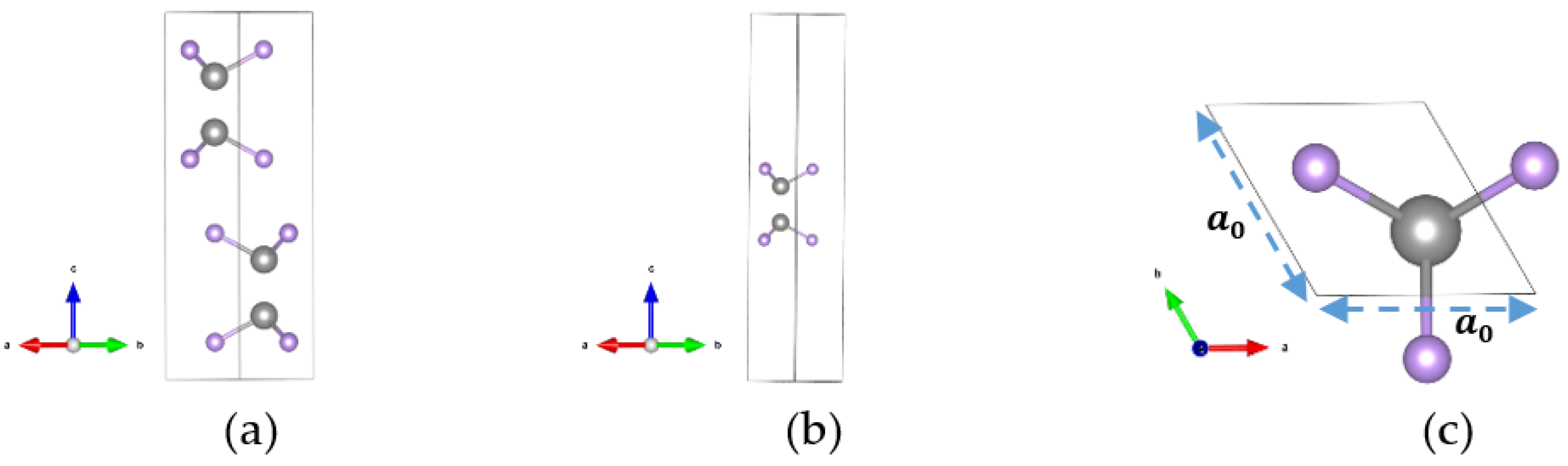
2.1. Pristine GaSe Crystal Structure
2.2. The Structure and Adsorption Energy of CO Adsorbed on a Monolayer GaSe Supercell
2.2.1. Crystal Structure of CO Adsorbed on the GaSe Monolayer
2.2.2. CO Adsorption without Strain Effect
2.2.3. CO Adsorption with Strain Effect
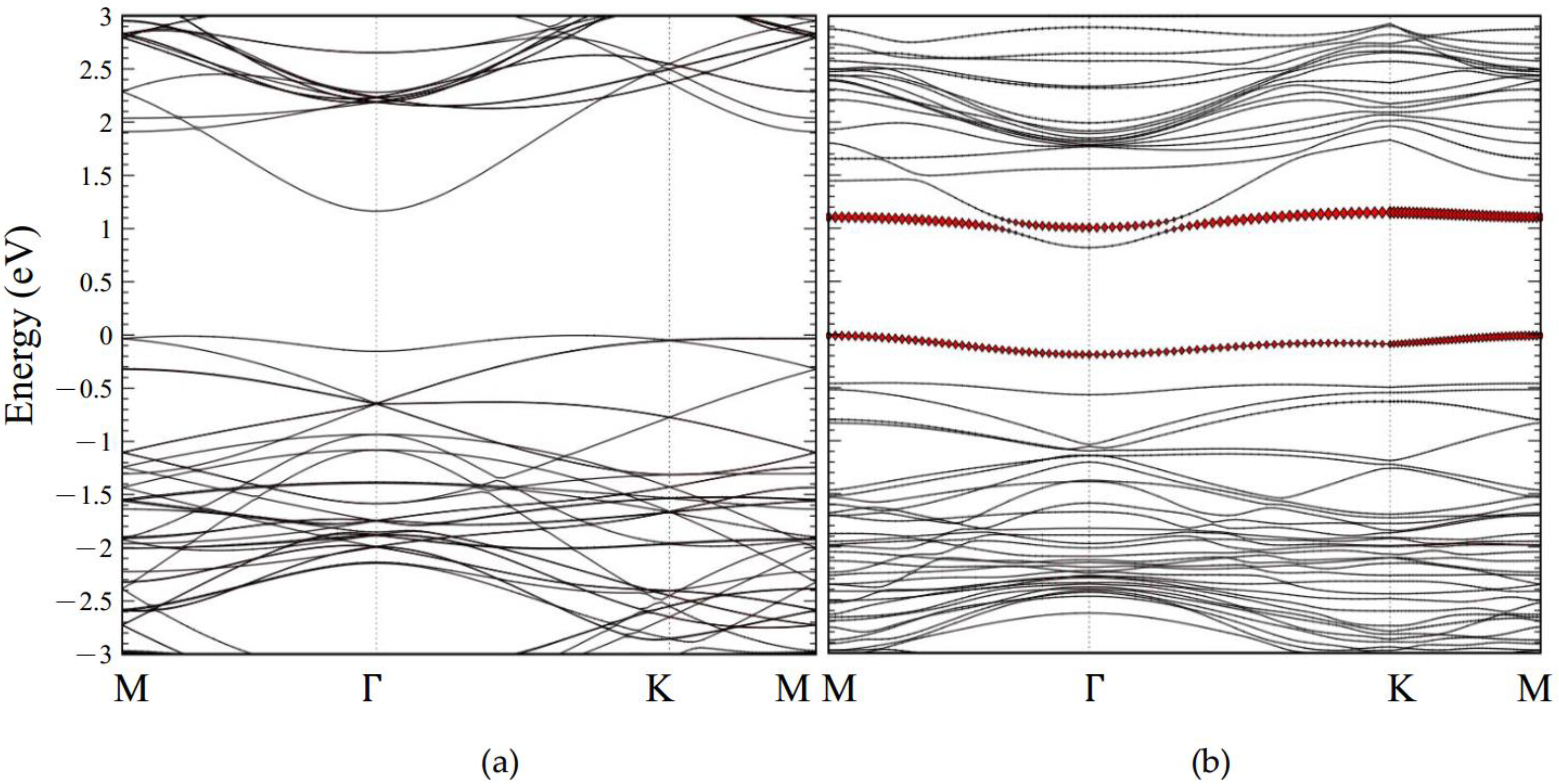
2.3. Electronic Structure Analysis
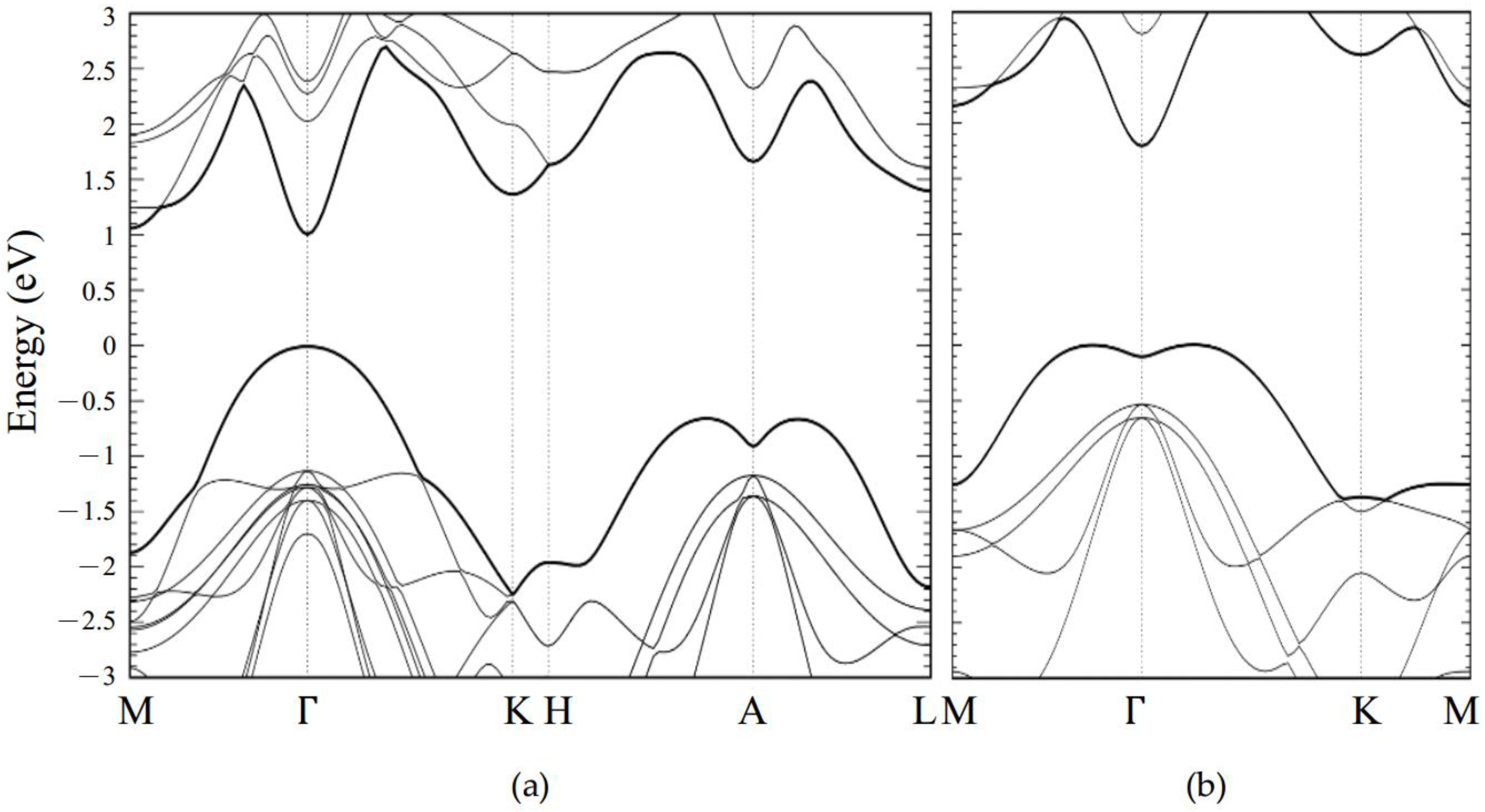
2.3.1. Pristine GaSe
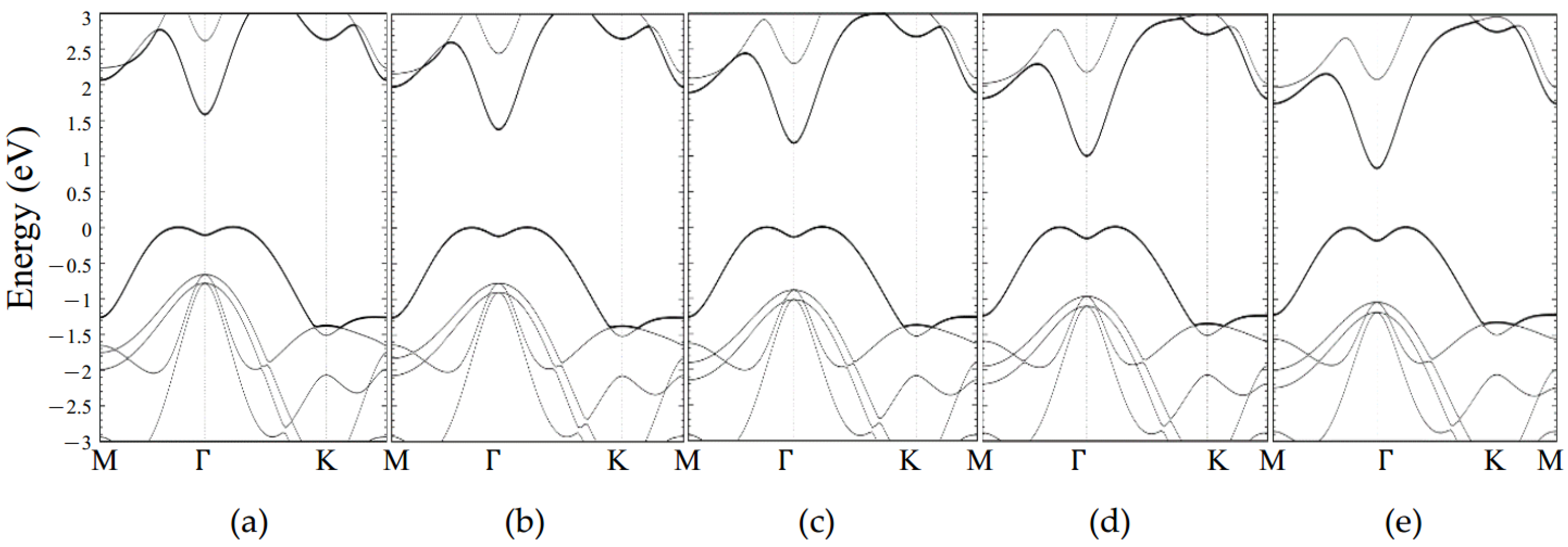
2.3.2. Pristine Monolayer GaSe with Strain Effect
2.3.3. CO Adsorption with Monolayer GaSe
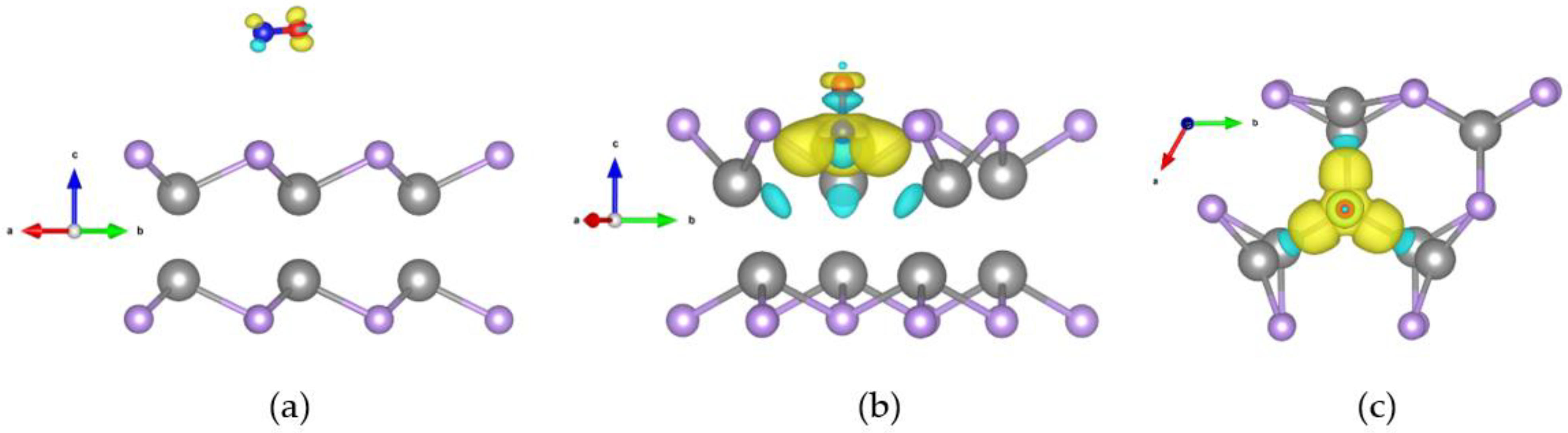
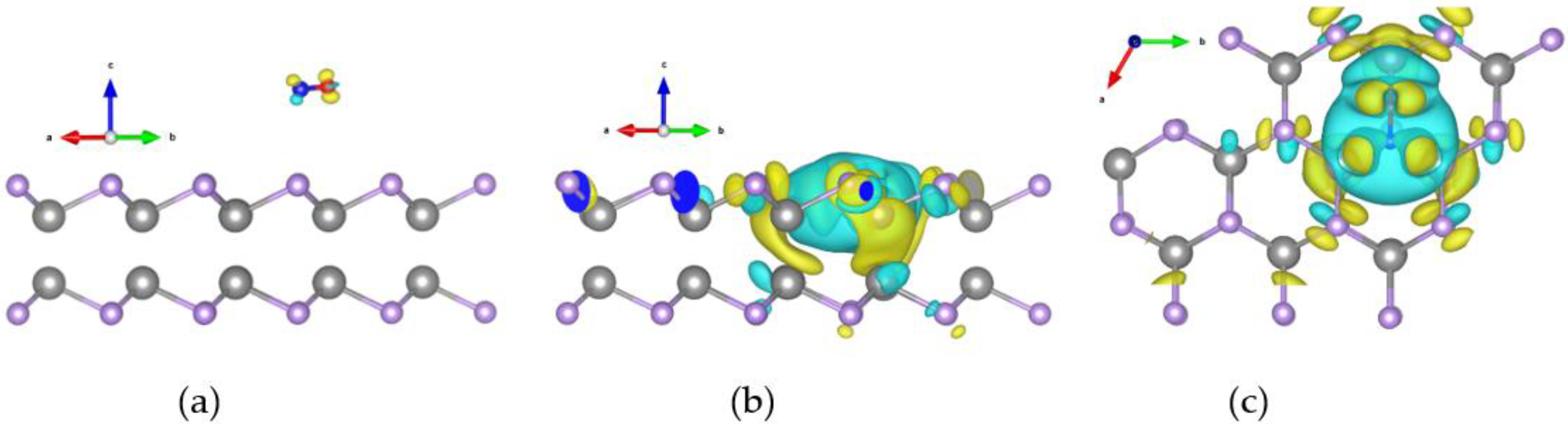
2.4. Differential Charge Densities Pattern Analysis
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Electronic Structure for Pristine GaSe
Appendix B. Electronic Structure for Monolayer GaSe with Strain Effect
| Strain | −5% | −4% | −3% | −2% | −1% | 0% | 1% | 2% | 3% | 4% | 5% |
| 2.29 | 2.43 | 2.45 | 2.24 | 2.02 | 1.79 | 1.58 | 1.37 | 1.18 | 1.00 | 0.83 |

Appendix C. Calculation Results of Monolayer GaSe Adsorbed with CO
| Supercell | GaSe Structure | Strain (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 × 2 | Pristine | 0 | 0 | −64.89 | −0.017 | 3.50 | 3.53 | - |
| 1 | 0.13 | −64.79 | −0.015 | 3.60 | 3.62 | - | ||
| 2 | 0.32 | −64.41 | −0.016 | 3.54 | 3.56 | - | ||
| 3 | 0.55 | −62.64 | −0.014 | 3.48 | 3.51 | - | ||
| 4 | 0.82 | −62.00 | −0.015 | 3.49 | 3.52 | - | ||
| 5 | 1.13 | −63.72 | −0.018 | 3.52 | 3.55 | - | ||
| Se-defective | 0 | 0 | −238.49 | −0.143 | 0.37 | 3.86 | 2.79 | |
| 1 | 0.1 | −428.67 | −0.188 | −0.20 | 3.81 | 2.44 | ||
| 2 | 0.24 | −524.80 | −0.160 | −0.23 | 3.80 | 2.49 | ||
| 3 | 0.35 | −684.13 | −0.096 | −0.16 | 3.94 | 3.24 | ||
| 4 | 0.53 | −771.41 | −0.081 | −0.06 | 3.98 | 3.31 | ||
| 5 | 0.74 | −844.39 | −0.079 | −0.13 | 4.01 | 3.30 | ||
| 3 × 3 | Pristine | 0 | 0 | −65.59 | −0.015 | 3.53 | 3.53 | - |
| 1 | 0.32 | −64.54 | −0.017 | 3.54 | 3.54 | - | ||
| 2 | 0.74 | −63.58 | −0.013 | 3.53 | 3.53 | - | ||
| 3 | 1.26 | −62.56 | −0.017 | 3.51 | 3.51 | - | ||
| 4 | 1.87 | −62.64 | −0.017 | 3.54 | 3.54 | - | ||
| 5 | 2.55 | −61.31 | −0.014 | 3.54 | 3.54 | - | ||
| Se-defective | 0 | 0 | −146.07 | −0.055 | 1.93 | 3.86 | 3.44 | |
| 1 | 0.4 | −141.65 | −0.052 | 1.97 | 3.89 | 3.47 | ||
| 2 | -0.25 | −1275.20 | −0.784 | −0.66 | 3.65 | 2.09 | ||
| 3 | 0.22 | −1374.28 | −0.776 | −0.60 | 3.71 | 2.11 | ||
| 4 | 0.78 | −1456.81 | −0.763 | −0.59 | 3.73 | 2.12 | ||
| 5 | 1.42 | −1515.68 | −0.750 | −0.55 | 3.78 | 2.13 |
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Druffel, D.L.; Woomer, A.H.; Kuntz, K.L.; Pawlik, J.T.; Warren, S.C. Electrons on the surface of 2D materials: From layered electrides to 2D electrenes. J. Mater. Chem. C 2017, 5, 11196–11213. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Liu, K. Chemical and structural stability of 2D layered materials. 2D Mater. 2019, 6. [Google Scholar] [CrossRef]
- Zhang, C.-W. First-principles study on electronic structures and magnetic properties of AlN nanosheets and nanoribbons. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Varghese, S.; Varghese, S.; Swaminathan, S.; Singh, K.; Mittal, V. Two-Dimensional Materials for Sensing: Graphene and Beyond. Electronics 2015, 4, 651–687. [Google Scholar] [CrossRef]
- Ouyang, T.; Qian, Z.; Hao, X.; Ahuja, R.; Liu, X. Effect of defects on adsorption characteristics of AlN monolayer towards SO2 and NO2: Ab initio exposure. Appl. Surf. Sci. 2018, 462, 615–622. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-Dimensional Nanomaterials for Gas Sensing Applications: The Role of Theoretical Calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, N.; Ren, H.; Tang, C.; Yang, L.; Zhao, X. Gas adsorption on MoS2/WS2 in-plane heterojunctions and the I–V response: A first principles study. RSC Adv. 2016, 6, 17494–17503. [Google Scholar] [CrossRef]
- Ao, Z.M.; Li, S.; Jiang, Q. Correlation of the applied electrical field and CO adsorption/desorption behavior on Al-doped graphene. Solid State Commun. 2010, 150, 680–683. [Google Scholar] [CrossRef]
- Raub, J.A.; Mathieu-Nolf, M.; Hampson, N.B.; Thom, S.R. Carbon monoxide poisoning—A public health perspective. Toxicology 2000, 145, 1–14. [Google Scholar] [CrossRef]
- Nandy, T.; Coutu, R.A., Jr.; Ababei, C. Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems. Sensors 2018, 18, 3443. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef]
- Kumar, J.; Nemade, H.B.; Giri, P.K. Adsorption of Small Molecules on Niobium Doped Graphene: A Study Based on Density Functional Theory. IEEE Electron. Device Lett. 2018, 39, 296–299. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Wang, L.; Yan, T.; Sun, Y.-Y.; Zhang, S.B. Titanium-decorated graphene oxide for carbon monoxide capture and separation. Phys. Chem. Chem. Phys. 2011, 13, 21126–21131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ning, F.; Rao, F.; Lei, X.; Wu, M.; Zhou, L. First-principles study of nitrogen and carbon monoxide adsorptions on silicene. Int. J. Mod. Phys. B 2016, 30, 1650176. [Google Scholar] [CrossRef]
- Bui, V.Q.; Pham, T.T.; Le, D.A.; Thi, C.M.; Le, H.M. A first-principles investigation of various gas (CO, H2O, NO, and O2) absorptions on a WS2 monolayer: Stability and electronic properties. J. Phys. Condens. Matter 2015, 27, 305005. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, R.; Zhao, X.; An, Y.; Dai, X.; Xia, C. Tunable donor and acceptor impurity states in a WSe2 monolayer by adsorption of common gas molecules. RSC Adv. 2016, 6, 82793–82800. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT study of transition metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-embedded monolayer MoS2 for gas adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Tian, J.; Ma, D.; Wang, Y. First-principles study on the gas sensing property of the Ge, As, and Br doped PtSe2. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Fuh, H.-R.; Chang, C.-R. First-Principles Study for Gas Sensing of Defective SnSe2 Monolayers. Appl. Sci. 2020, 10, 1623. [Google Scholar] [CrossRef]
- Beheshtian, J.; Baei, M.T.; Peyghan, A.A. Theoretical study of CO adsorption on the surface of BN, AlN, BP and AlP nanotubes. Surf. Sci. 2012, 606, 981–985. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, K. Strain engineering in functional 2-dimensional materials. J. Appl. Phys. 2019, 125. [Google Scholar] [CrossRef]
- Conley, H.J.; Wang, B.; Ziegler, J.I.; Haglund, R.F., Jr.; Pantelides, S.T.; Bolotin, K.I. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 2013, 13, 3626–3630. [Google Scholar] [CrossRef]
- Fei, R.; Yang, L. Strain-engineering the anisotropic electrical conductance of few-layer black phosphorus. Nano Lett. 2014, 14, 2884–2889. [Google Scholar] [CrossRef]
- Guinea, F.; Katsnelson, M.I.; Geim, A.K. Energy gaps and a zero-field quantum Hall effect in graphene by strain engineering. Nat. Phys. 2009, 6, 30–33. [Google Scholar] [CrossRef]
- Li, H.; Contryman, A.W.; Qian, X.; Ardakani, S.M.; Gong, Y.; Wang, X.; Weisse, J.M.; Lee, C.H.; Zhao, J.; Ajayan, P.M.; et al. Optoelectronic crystal of artificial atoms in strain-textured molybdenum disulphide. Nat. Commut. 2015, 6, 7381. [Google Scholar] [CrossRef]
- Kuang, Y.; Lindsay, L.; Huang, B. Unusual Enhancement in Intrinsic Thermal Conductivity of Multilayer Graphene by Tensile Strains. Nano Lett. 2015, 15, 6121–6127. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Apte, A.; Kochat, V.; Rajak, P.; Krishnamoorthy, A.; Manimunda, P.; Hachtel, J.A.; Idrobo, J.C.; Syed Amanulla, S.A.; Vashishta, P.; Nakano, A.; et al. Structural Phase Transformation in Strained Monolayer MoWSe2 Alloy. ACS Nano 2018, 12, 3468–3476. [Google Scholar] [CrossRef]
- Pak, S.; Lee, J.; Lee, Y.W.; Jang, A.R.; Ahn, S.; Ma, K.Y.; Cho, Y.; Hong, J.; Lee, S.; Jeong, H.Y.; et al. Strain-Mediated Interlayer Coupling Effects on the Excitonic Behaviors in an Epitaxially Grown MoS2/WS2 van der Waals Heterobilayer. Nano Lett. 2017, 17, 5634–5640. [Google Scholar] [CrossRef]
- Li, W.; Li, J. Piezoelectricity in two-dimensional group-III monochalcogenides. Nano Res. 2015, 8, 3796–3802. [Google Scholar] [CrossRef]
- Plucinski, L.; Johnson, R.L.; Kowalski, B.J.; Kopalko, K.; Orlowski, B.A.; Kovalyuk, Z.D.; Lashkarev, G.V. Electronic band structure of GaSe(0001): Angle-resolved photoemission andab initiotheory. Phys. Rev. B 2003, 68. [Google Scholar] [CrossRef]
- Rybkovskiy, D.V.; Arutyunyan, N.R.; Orekhov, A.S.; Gromchenko, I.A.; Vorobiev, I.V.; Osadchy, A.V.; Salaev, E.Y.; Baykara, T.K.; Allakhverdiev, K.R.; Obraztsova, E.D. Size-induced effects in gallium selenide electronic structure: The influence of interlayer interactions. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Yu, L.; Huang, B. Tunable electronic and dielectric behavior of GaS and GaSe monolayers. Phys. Chem. Chem. Phys. 2013, 15, 7098–7105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fuh, H.-R.; Zhang, D.; Coileáin, C.Ó.; Xu, H.; Cho, J.; Choi, M.; Chun, B.S.; Jiang, X.; Abid, M.; et al. Simultaneous large continuous band gap tunability and photoluminescence enhancement in GaSe nanosheets via elastic strain engineering. Nano Energy 2017, 32, 157–164. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Luo, J.; Yan, A.; Matte, H.S.; Grayson, M.; Rao, C.N.; Dravid, V.P. GaS and GaSe ultrathin layer transistors. Adv. Mater. 2012, 24, 3549–3554. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.-X.; Zhang, H.-R.; Du, L.-N.; Wang, L.; Yang, F.-Y.; Zhang, X.-Z.; Liu, Q. Synthesis of atomically thin GaSe wrinkles for strain sensors. Front. Phys. 2016, 11, 116802. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, H.; Wu, Y.; Lin, W.; Yang, W.; Dong, L. Effect of external strain on the charge transfer: Adsorption of gas molecules on monolayer GaSe. Mater. Chem. Phys. 2017, 198, 49–56. [Google Scholar] [CrossRef]
- Ueno, K.; Takeda, N.; Sasaki, K.; Koma, A. Investigation of the growth mechanism of layered semiconductor GaSe. Appl. Surf. Sci. 1997, 113, 38–42. [Google Scholar] [CrossRef]
- Fan, Y.; Schittkowski, T.; Bauer, M.; Kador, L.; Allakhverdiev, K.R.; Salaev, E.Y. Confocal photoluminescence studies on GaSe single crystals. J. Lumin. 2002, 98, 7–13. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A.; et al. Understanding band gaps of solids in generalized Kohn-Sham theory. Proc. Natl. Acad. Sci. USA 2017, 114, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Yagmurcukardes, M.; Senger, R.T.; Peeters, F.M.; Sahin, H. Mechanical properties of monolayer GaS and GaSe crystals. Phys. Rev. B 2016, 94. [Google Scholar] [CrossRef]
- Camargo Moreira, O.L.; Cheng, W.Y.; Fuh, H.R.; Chien, W.C.; Yan, W.; Fei, H.; Xu, H.; Zhang, D.; Chen, Y.; Zhao, Y.; et al. High Selectivity Gas Sensing and Charge Transfer of SnSe2. ACS Sens. 2019, 4, 2546–2552. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014, 595-596, 35–42. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Smith, S.C.; Du, A.; Zhu, Z. Electrocatalytically switchable CO2 capture: First principle computational exploration of carbon nanotubes with pyridinic nitrogen. ChemSusChem 2014, 7, 435–441. [Google Scholar] [CrossRef]
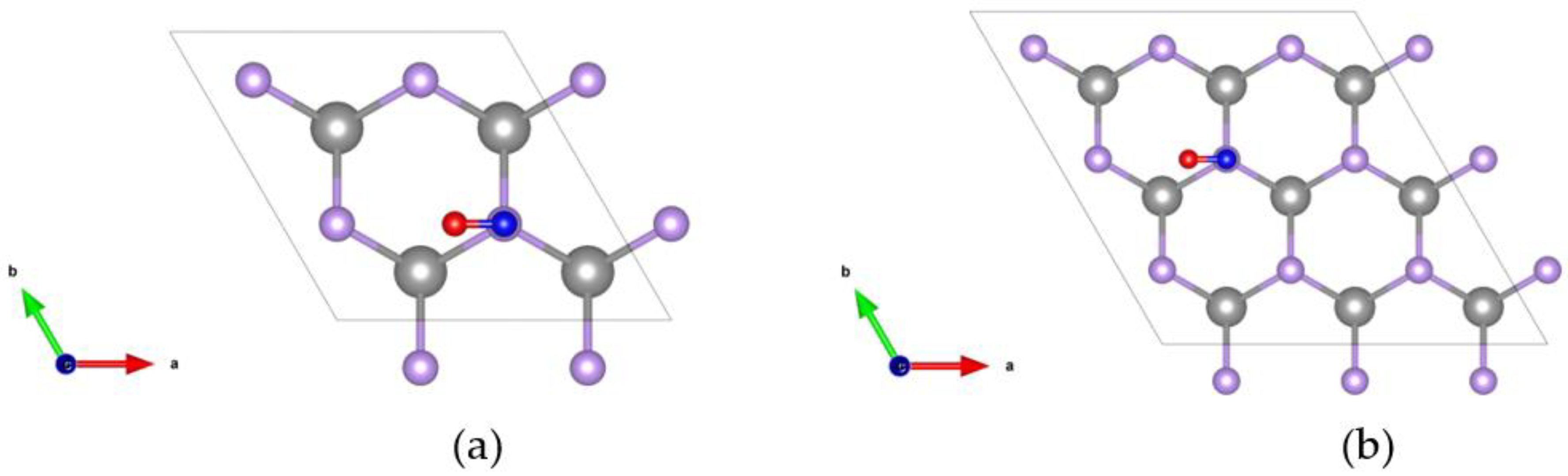
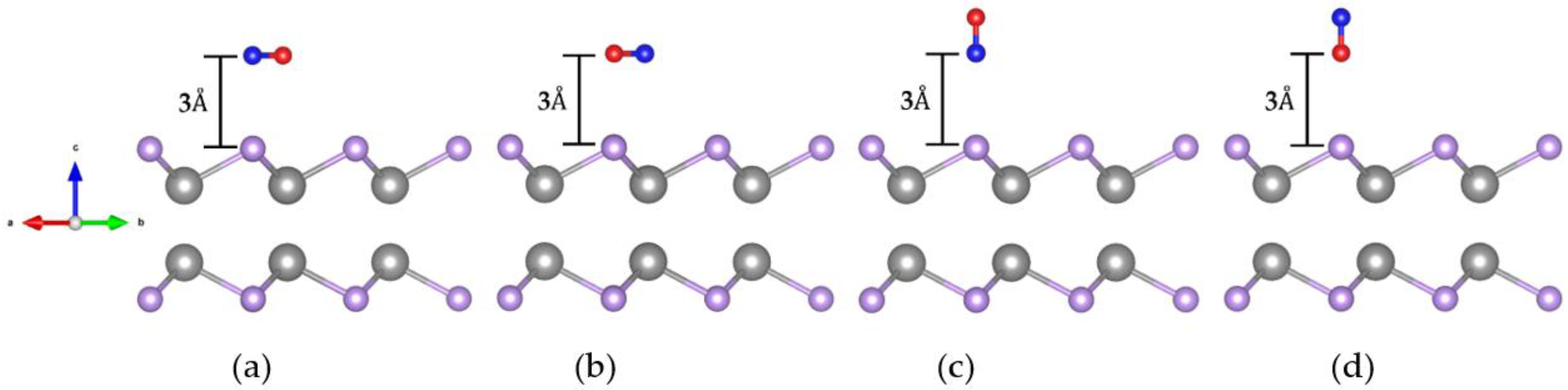
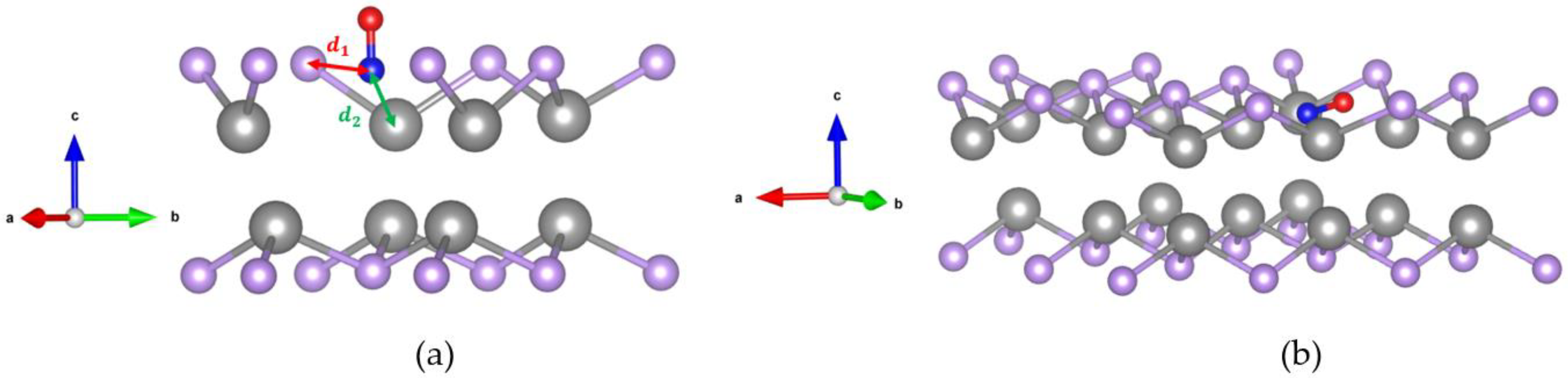

| GaSe | Orientation | ||||||
|---|---|---|---|---|---|---|---|
| Pristine | A | 0.01 | −64.89 | −0.017 | 3.50 (C-Se) 1 | 3.53 (C-Se) | - |
| B | 0 | −70.13 | −0.013 | 3.13 (C-Se) | 3.54 (C-Se) | - | |
| C | 0.04 | −33.85 | −0.005 | 3.85 (C-Se) | 3.85 (C-Se) | - | |
| D | 0.03 | −36.42 | −0.007 | 3.66 (O-Se) | 3.67 (O-Se) | - | |
| Se-defective | A | 0 | −238.49 | −0.143 | 0.37 (C-Se) | 3.86 (C-Se) | 2.79 (C-Ga) |
| B | 0.01 | −321.60 | −0.119 | 0.58 (C-Se) | 3.92 (C-Se) | 3.93 (C-Ga) | |
| C | 0.24 | −91.38 | −0.012 | 2.54 (C-Se) | 4.59 (C-Se) | 4.19 (C-Ga) | |
| D | 0.25 | −79.81 | −0.015 | 2.39 (O-Se) | 4.50 (O-Se) | 4.05 (O-Ga) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-P.; Fuh, H.-R.; Chang, C.-R. Enhanced Sensitivity of CO on Two-Dimensional, Strained, and Defective GaSe. Molecules 2021, 26, 812. https://doi.org/10.3390/molecules26040812
Huang H-P, Fuh H-R, Chang C-R. Enhanced Sensitivity of CO on Two-Dimensional, Strained, and Defective GaSe. Molecules. 2021; 26(4):812. https://doi.org/10.3390/molecules26040812
Chicago/Turabian StyleHuang, Hsin-Pan, Huei-Ru Fuh, and Ching-Ray Chang. 2021. "Enhanced Sensitivity of CO on Two-Dimensional, Strained, and Defective GaSe" Molecules 26, no. 4: 812. https://doi.org/10.3390/molecules26040812
APA StyleHuang, H.-P., Fuh, H.-R., & Chang, C.-R. (2021). Enhanced Sensitivity of CO on Two-Dimensional, Strained, and Defective GaSe. Molecules, 26(4), 812. https://doi.org/10.3390/molecules26040812





