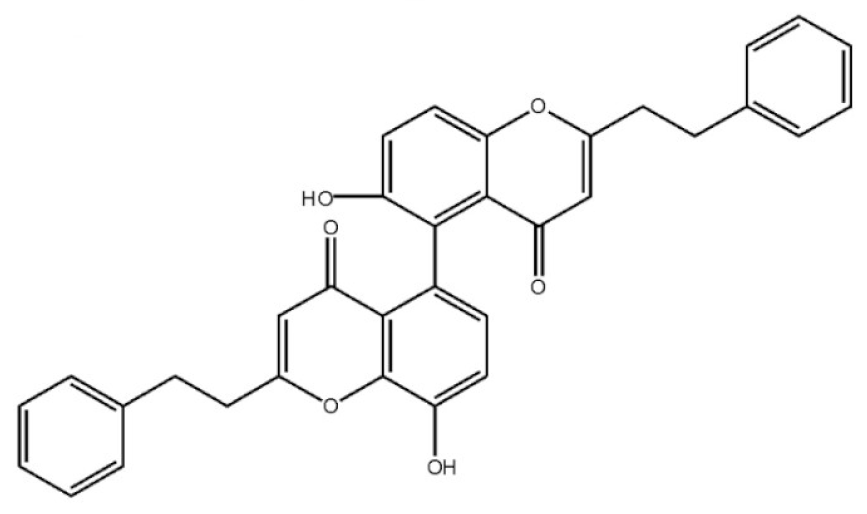
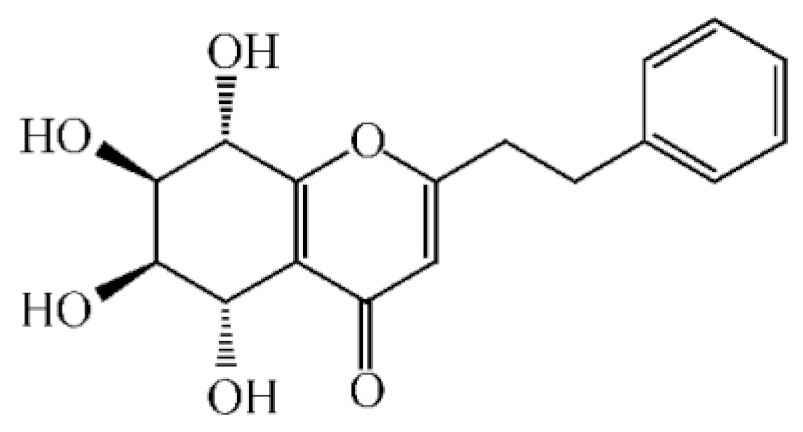
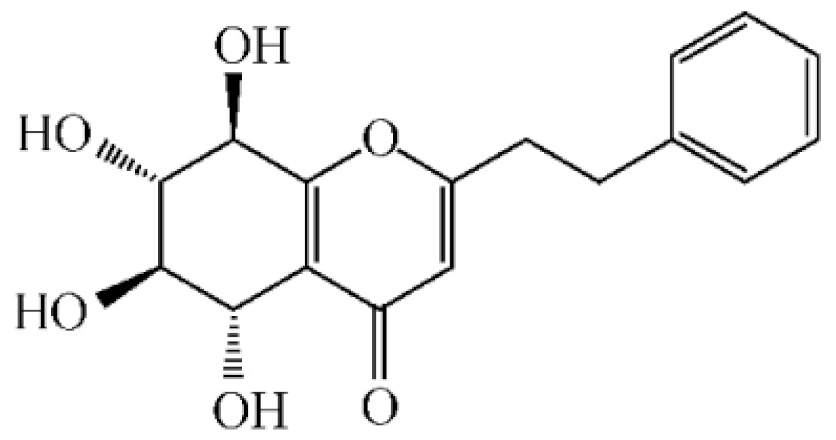
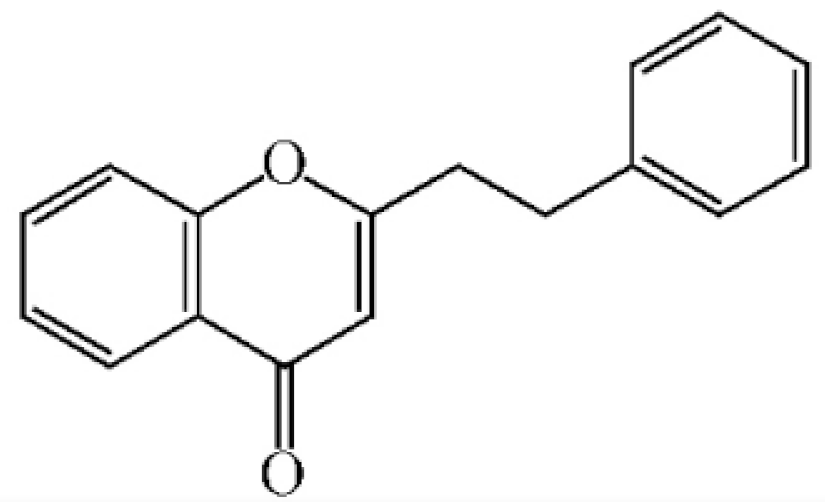
Abstract
Agarwood is a highly valuable fragrant wood of Aquilaria spp. (Thymelaeaceae) which has been widely utilized in traditional medicine, religious rites, and cultural activities. This study summarizes a review on the identification of Aquilaria cultivars, volatile and non-volatile phytochemicals, pharmacological uses, and agarwood grading system to determine its quality, and different agarwood induction methods. Due to the highly demanding and depleted natural resources, the research on agarwood is still insufficient, and it has broad research and development prospects in many industries. However, due to the significant scientific nature of agarwood application, developing high-quality products and drugs from agarwood have become highly important, while no one has discussed in detail the phytochemicals uses and provided a summary until now. The main phytochemicals of agarwood include terpenoids, dominated by sesquiterpenes. For centuries, terpenoids have been used in traditional Chinese medicine and have been shown to possess various pharmacological properties, including bacteriostatic, antibacterial, sedation, analgesia, anti-inflammation, anti-asthmatic, hypoglycemic, antidepressant, and many others. Alongside biological activity screening, phytochemical advances and pharmacological research have also made certain progress. Therefore, this review discusses the research progress of agarwood in recent years and provides a reference basis for further study of Aquilaria plants and agarwood.
1. Introduction
Aquilaria sinensis (Lour.) Gilg is the resin-containing wood of the Aquilaria. Agarwood is a traditional Chinese medicine included in the 2020 edition of Chinese Pharmacopoeia [1]. There are about 17 species of agarwood in the world, most of which are distributed in China, India, and Southeast Asian countries. In recent years, the majority of agarwood, headed by Aquilaria sinensis (Lour.) Spreng, has continuously attracted attention. Agarwood plants are likely to secrete a variety of secondary metabolites when they are damaged, including by lightning, chopping, burning, moth-eating, or microbial invasion of natural factors [2]. The traditional artificial agarwood formation techniques are based on physical methods such as cutting and digging holes. Modern artificial agarwood formation techniques are mainly biochemical methods, such as chemical reagent invasion and bacteria inoculation, which are deposited in the xylem after a complex agarwood formation process after wound formation [3,4]. There are few reports about artificial agarwood, mostly focused on drilling and chemical reagent dripping, but insect infestation is the best method for producing natural agarwood (Figure 1). In recent years, there are many kinds of agarwood commodities and crafts in the market, and the phenomenon of counterfeit mixing is serious. At present, there are many ITS (Internal Transcribed Spacer) intergenic regions for the identification of agarwood species, and the research on agarwood has made some progress, but its identification method is not yet mature. At present, a variety of methods have been established to control the quality of medicinal materials, including the coloration of chemical reagents, the content of alcohol extracts, the content of agarwood tetraol, the content of chromone, the HPLC fingerprint of alcohol extract, etc. Most of the effective components of agarwood are focused on the study of volatile oil, chromone, and sesquiterpene. Agarwood is a valuable traditional Chinese medicine and natural high-grade spice in the world. It has the effects of relieving pain, asthma, and vomiting. It has significant effects when combined with other traditional Chinese medicines such as Bawei Chenxiang Powder and Chenxiang Tongbian Powder. This study focused on pharmacological and clinical research of antibacterial, bacteriostatic, anti-tumor, antidepressant, and antioxidation, as well as the application for cardiovascular and cerebrovascular diseases [5]. However, there is a lack of further research on the mechanism of pharmacological action. In this paper, a comprehensive review was made on the species and identification, quality control, chemical composition, pharmacological action, compatibility, and artificial agarwood to provide a reference for further research and development. It also provides a scientific basis for the development and application of precious medicinal material. There are 17 Aquilaria spp distributed around the world, as shown in Table 1.
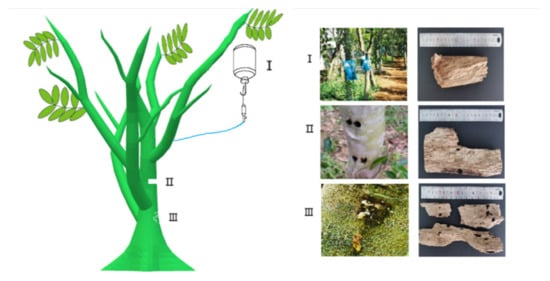
Figure 1.
Wound tissues and agarwood sample collection from Aquilaria sinensis.

Table 1.
The species of Aquiliria and its distribution.
Type I: agarwood formed by chemical reagent dripping. Type II: agarwood formed by using a nail to dig a hole in the agarwood. Type III: agarwood induced by insects forming irregular wormholes for agarwood production.
2. Economic Value and Aquilaria Species Distribution
Economic Value
High-quality agarwood can be sold at US $100,000 per kg, while those of poor quality can only be sold at US $100 per kg. As a high-quality and high-grade agarwood essential oil, it can even be sold for US $1500 per 11.7 g [11,12,13]. The higher the grade of agarwood, the richer the layers of aroma. The best agarwood fragrance is mellow and sweet, full of penetration and persistence, and the powdery waxy material on the surface can be scraped off and kneaded it into a ball. Its aroma is regarded as a symbol of high quality. There are various kinds of volatile components, each with a unique aroma [12,14,15]. Aquiliria spp. Asiatic distribution map is shown in Figure 2.
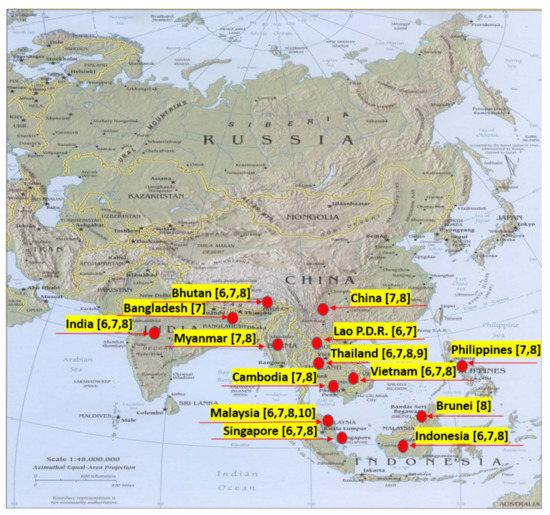
Figure 2.
Aquiliria spp. Asiatic distribution map. Red spot showing the distribution in different countries.
3. Aquilaria Identification Methods and Phytochemicals
3.1. Identification Methods
There are many kinds of agarwood trees, and their morphological characteristics are very similar, which increases the difficulty in the identification of agarwood. Even if there are different batches of agarwood from the same producing area and different producing areas, the chemical constituents measured by the same experimental method are very different [16]. It is necessary to establish a set of systematic and accurate identification methods. At present, the differences in intragenic regions of Internal Transcribed Spacer (ITS), ITS1, and ITS2 are often used to distinguish different species and genera. For example, Zou et al. [17] used a variety of different extraction methods for identifying different agarwood species by the ITS2 sequence analysis method, using its DNA. The results showed that the DNA barcode sequence based on ITS2 can accurately identify the real and fake species, and the species can be identified by sequence comparison. The similarity of the comparison result with the original agarwood is up to 100%, which proves that this method can effectively distinguish true and fake products and the relationship between species and genera. Meanwhile, Niu et al. [18] identified the sequence-specific regions (ITS1 and ITS2) using PCR amplification. The results showed that there were various sequence-specific regions in the rDNA sequence of agarwood. Shen et al. [19] analyzed the sequence-specific regions (ITS sequence of DNA), and the different regions were distinguished by sequence alignment.
3.2. Phytochemicals
The major chemical constituents from Aquilaria plants are sesquiterpenoids and chromones (Table 2, Table 3 and Table 4). These are divided into two categories: (A) Volatile compounds of agarwood and (B) Non-volatile compounds of agarwood.

Table 2.
Aromatic compounds from agarwood.

Table 3.
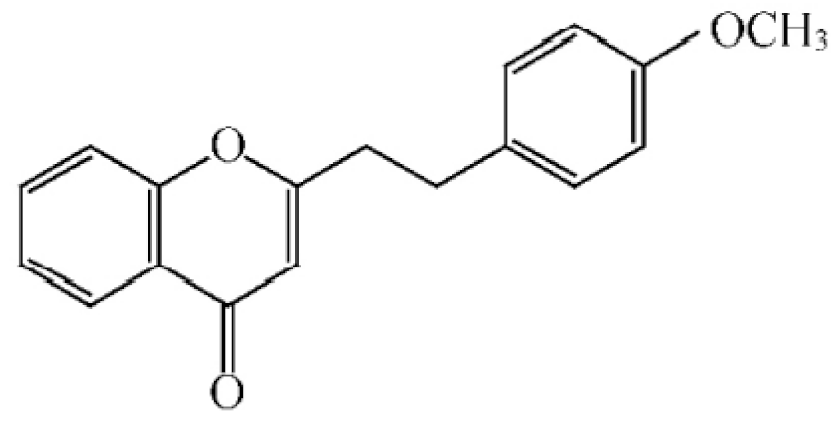
Chemical structure of 2-(2-phenylethyl) chromones in agarwood.

Table 4.
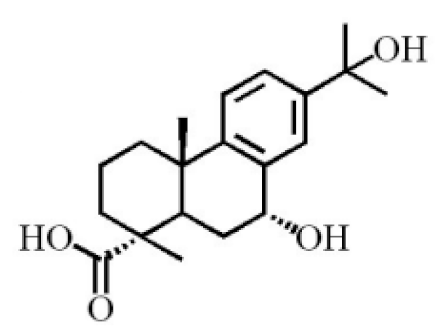
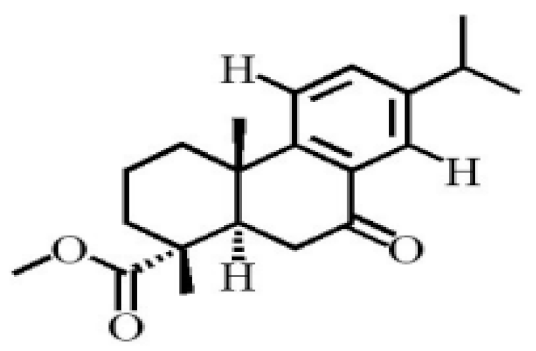
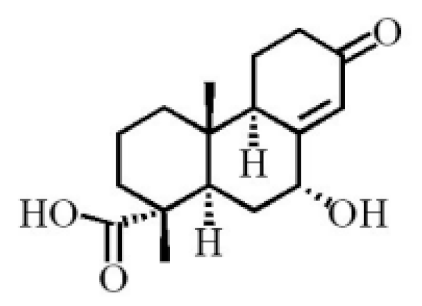
Chemical structure of terpenoids from agarwood.
3.2.1. (A) Volatile Compounds of Agarwood
- Aromatic group
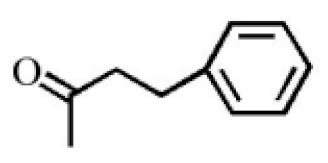
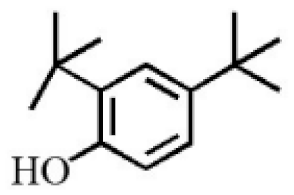
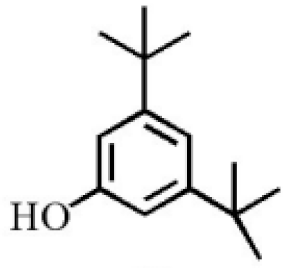
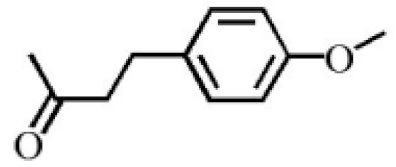
Aromatic volatile compounds are another important component in agarwood. For example, benzylacetone, which has been widely studied, is regarded as a landmark substance in aromatic components. Chen et al. [20] used the GC-MS and found that chloroform could effectively extract and separate aromatic compounds. Zhang et al. [21] isolated a large number of aromatic compounds from the volatile oil, in which the mass fraction of benzylacetone was the highest, reaching 19.51%. There are great differences in the mass fraction of aromatic compounds in different species. Tajuddin and Yusoff [22] found that the proportion of aromatic compounds in Malaysia agarwood volatile oil is larger than that of other species, of which 4-phenyl-2-butanone accounts for 32.1% of the volatile oil. Mei et al. [23] also isolated some aromatic compounds from agarwood, such as benzylacetone, 2,4-di-tert-butylphenol, 3,5-di-tert-butylphenol, and 4-methoxyphenylacetone (Table 2). These chemical structures were isolated on the basis of spectroscopic evidence with no cytotoxic activity against different cell lines [23].
3.2.2. (B) Non-Volatile Compounds of Agarwood
- Fatty acids
Lin et al. [24] used GC-MS to extract the agarwood produced by the nailing method, burrowing method, and cutting method with ether, and it was concluded that the agarwood in the cutting method was mainly fatty acid. Bhardwaj [25] found stearic acid, oleic acid, linoleic acid, and palmitic acid in the agarwood volatile oil by GC-MS. Mei et al. [26] isolated many kinds of fatty acids, such as hexadecenoic acid, tridecanoic acid, octadecenoic acid, and so on.
- Chromones
These compounds mainly exist in the Aquilaria, and they are volatile constituents of agarwood (Table 3). The dimeric 2-(2-phenylethyl) chromones of agarwood lead to the identification by structure. Liu et al. [27] isolated different kinds of 2-(2-phenylethyl) chromones from agarwood. Mei et al. [28] analyzed the extracts by GC-MS after extracting high-quality agarwood with ether, and found that the relative content of 2-(2-phenylethyl) chromone compounds was 60% in high-quality agarwood. Xia et al. [29] and Yang et al. [30] used a spectrophotometer to evaluate the quality of agarwood and determine the absorbance value of 2-(2-phenylethyl) chromones at the wavelengths of 230 nm and 250 nm. Zhang et al. [31] obtained various derivatives of 2-(2-phenylethyl) chromones by co-fermentation of endophytic strains with agarwood. Thirteen 2-(2-phenylethyl) chromone derivatives were obtained by ethanol extraction, elution, and purification, and then bioassay for antibacterial activity. The results showed that some of 2-(2-phenylethyl) chromone derivatives had good inhibitory effect on Staphylococcus aureus [32]. Yang Lin also used a spectrophotometer to isolate different chromones, such as 2-[2-(4-hydroxyphenyl)ethyl]chromone, 5,6,7,8,-tetrahydroxy-5,6,7,8-tetrahydro-2-[2-(4-methoxyphenyl)ethy]-chromone, Rel-(1AR,2R,3R,7bS)-1a,2,3,7b-tetrahydro-2,3-hidydroxy-5[2-(4-methoxyphenyl)ethy]-7H-oxireno[f][1]benzophran-7-one and oxidoagarchromones (Table 3), which showed the mechanism of analgesia and sedation related to the regulation of gene expression of the GABAA receptor, GABAA receptor function, and promotion of Cl−1 influx.
- Terpenoids
Terpenoids are compounds derived from mevalonic acid that have two or more isoprene units in their basic carbon frame. The major components of agarwood are terpenoids, which include sesquiterpenes and diterpenes. One of the criteria used to evaluate the quality of agarwood is the content of sesquiterpenes and triterpenes (Table 4). Sukkaew et al. [36] isolated and identified volatile compounds from agarwood by GC-MS. Yang et al. [37] isolated and identified a monoterpene derivative [(-)-bornyl ferulate]. According to the chemical structure, sesquiterpenes isolated from agarwood are divided into different categories including agarofurans, eudesmanes, eremophilanes, guaianes, agarospirols, and cadinanes [38,39]. Several kinds of components with a high content (white caryophyllus, white caryophyllenol, and α-white fragrant alkene, etc.) were calculated by the normalization method [40], extracting sesquiterpenes from the “whole-tree agarwood-inducing“ technology in artificial agarwood. Jin et al. [41] have found that the structures of sesquiterpenes are similar, so it is difficult to separate and purify sesquiterpenes by conventional separation methods, such as chromatographic column separation and alcohol extraction. Ismail et al. [42] also found that all the volatile oil chemical structures were mainly composed of sesquiterpenes, such as α-agarofuran, (5S,7S,10S)-(-)selina-3, (+)-(4S,5R)-dihydrokaranone, α-guaiene, agarospirol, and 8-β-H-dihydrogmelofuran (Table 4).
By using silica gel column chromatography to isolate a variety of compounds from traditional Chinese medicinal agarwood, triterpenes (e.g., Ivy sapogenin and 3-oxo-22-hydroxyhopane, etc.) were isolated by recrystallization [43]. Various triterpenoids (approximately 14) were separated and purified from agarwood, including the first discovery of hydroxy-domperidone [44]. Tian and Haigang [45] used petroleum ether and ethanol to extract 10 diterpenoids from agarwood.
- Steriods
Some steroids, such as β-sitosterol and 12 flavonoids, were isolated [48].
- Flavonoids
Qi et al. [49] isolated 31 flavonoids, including flavonoid glycosides with anti-inflammatory activity. Chen et al. [50] isolated lignans and phenylpropanoids from agarwood, while Hendra et al. [51] and Li et al. [52] isolated several types of phenolic acids including p-methoxyphenylpropionic acid, 4-hydroxyphenylpropionic acid, and others.
- Alkaloids
Chen et al. [53] isolated alkaloids from the wood of agarwood. Qi et al. [49] isolated benzophenones from agarwood and found the compound iriflophene, which can inhibit the respiratory burst of neutrophils.
3.3. Pharmacological Uses
A variety of phytochemicals from agarwood has obvious pharmacological uses and has always been an interesting topic among researchers and scientists (Table 5). The majority of phytochemicals from Aquilaria plants are sesquiterpenoids and chromones, which have been widely used in the pharmacological industry. Some of the pharmacological uses of these compounds are described below.

Table 5.
A summary of pharmacological effects of agarwood.
3.3.1. Antibacterial
Dahham et al. [54] used the sesquiterpene component (β-caryophyllene) against six kinds of human pathogenic bacteria and two kinds of fungi. It was found that β-caryophyllene had an inhibitory effect on the growth rate of bacteria, while the antibacterial activity of Gram-positive bacteria was higher than that of Gram-negative bacteria. The volatile oil extracted by Mei et al. [32] was determined by the agar diffusion method. The results showed that the volatile oil had antibacterial activity, especially against methicillin-resistant Staphylococcus aureus. Wang et al. [55] carried out antibacterial experiments with 5-deoxylongiferol isolated from agarwood. The results showed that the compound had an antibacterial effect on Staphylococcus aureus and Ralstonia solanacearum.
3.3.2. Anti-Tumor
The volatile oil of agarwood contains a variety of anti-tumor components, which have inhibitory effects on many kinds of cancer cells. For example, 2-(2-phenylethyl) chromone compounds were isolated from agarwood for the cytotoxic activity test. It was found that these compounds had inhibitory activity on five kinds of human tumor cell lines [56]. Chen et al. [57] used chloroform to extract the active substances from agarwood and found that the extract had anti-tumor activity against four kinds of cells (IC50 = 11.11−58.55 μg mL−1). Durham et al. [58] used the essential oil to carry out anti-tumor experiments in nude mice and found that the essential oil had an inhibitory effect on colon cancer cells.
3.3.3. Analgesia, Sedation, Anti-Inflammation
As a breach power medicine, agarwood has the effect of activating breach power and relieving pain. Wang et al. [59] conducted hypnotic experiments with alcohol extract of agarwood and the volatile oil combined with pentobarbital sodium. It was found that both of them could significantly increase the rate of falling asleep and prolong the sleeping time in mice. In addition, it was also found that the volatile oil in agarwood could significantly shorten the falling asleep latency. Wang et al. [60] explored the potential mechanism of essential oil on the γ-aminobutyric acid (GABA) system and found that the essential oil has a sedative and hypnotic effect; its mechanism is related to the regulation of GABAA receptor gene expression, mainly by enhancing the expression function of GABAA receptor and promoting Cl−1 influx. Gao [61] studied the anti-inflammatory activity of the extracted essential oil. After repeated intragastric administration, it was found that the essential oil could alleviate the inflammation induced by lipopolysaccharide.
3.3.4. Relieving against Cough and Asthma
Agarwood has some significant effects in relieving cough and asthma. Wu et al. [62] treated cough with agarwood Bawei Powder and found that it cannot only relieve the symptoms of cough, but also treat asthma. Zhou et al. [63] found that alcohol extract could effectively prolong the latent period of a cough caused by concentrated ammonia, and reduce coughing.
3.3.5. Antidepression and Anxiety
Agarwood has an effect in the treatment of anti-depression and anxiety. Wang et al. [64] found that the extracted essential oil from agarwood can effectively inhibit depression and anxiety, indicating that its mechanism may be related to the inhibition of some gene expression. Yang et al. [47] found that diterpenoids isolated from agarwood can effectively inhibit synaptic reuptake of serotonin and norepinephrine, showing obvious antidepressant activity.
3.3.6. Anti-Oxidation and Anti-Aging
Agarwood is a world-famous spice, known as the “king of incense “, and is an important raw material for the agarwood and cosmetics industry. Lin et al. [65] found that agarwood’s alcoholic extract from agarwood leaves had antioxidant and anti-aging activity. Lee et al. [66] found that the alcohol extract has a neuroprotective effect on stress-induced oxidative damage in the hippocampus. Xie et al. [67] found that agarwood volatile oil, at a certain concentration, can significantly reduce the oxidative damage caused by hydrogen peroxide on mouse adrenal pheochromocytoma monoclonal cells, and has a good antioxidant effect.
3.3.7. Effect on the Cardiovascular System
Yang et al. [68] found that Bawei Chenxiang Powder can enhance the hypoxia tolerance of cardiomyocytes, which has a good preventive and therapeutic effect on some common heart diseases. Sugiyama et al. [69] found that several 2-(2-phenylethyl) chromones isolated from incense could inhibit PED 3A in phosphodiesterase (PDEs). Chunyan et al. [70] found that Bawei Chenxiang Powder has a protective effect on the rat model of myocardial ischemia.
3.3.8. Clinical Application
Agarwood is mostly used in the form of a clinical drug, and it has obvious curative effect in the cardio-cerebrovascular system, urinary system, respiratory system, and against other diseases. After continuous treatment of patients with bronchial asthma with Bawei Chenxiang Powder (agarwood, nutmeg, jujube, travertine, frankincense, radix aucklandiae, chebula, kapok), it was found that the total effective rate of patients with Bawei Chenxiang Powder was higher than other medicines, and this condition was better relieved and controlled [71]. Gao [72] described that the patients with angina pectoris (a coronary heart disease) were treated with conventional medicine and Bawei Chenxiang Powder. In the experimental group of 50 patients, the effective rate with Bawei Chenxiang Powder was higher (26.0%) than that of patients treated with routine western medicine, after continuous administration for four weeks. This indicates that Bawei Chenxiang Powder has a significant effect in the treatment of angina pectoris caused by coronary heart disease. Wang el al. [73] treated constipation by grinding Chenxiang Tongbian Powder (agarwood, atractylodes macrocephala, semen raphani, rhubarb, mirabilite, fructus aurantii, peach kernel, radixpaeoniae alba, astragalus membranaceus, magnolia officinalis) and applying it on the navel, combined with acupuncture. It was found that the total effective rate of 30 patients treated with Chenxiang Tongbian Powder was higher (13.33%) than that of patients treated with polyethylene glycol electrolyte orally, and the symptoms of the patients were significantly relieved after two weeks of treatment.
3.4. Grading System for Agarwood Identification
The morphological grading system of agarwood is shown in Table 6. At present, the incense sold in the market comes from a wide range of sources, which needs to be processed before it can be used in medicine. It is difficult to distinguish its quality by observing its morphological characteristics, and the handicrafts of incense are even more difficult to identify. It is almost impossible to identify through the naked eye, the circulation in the market is more chaotic, and the phenomenon of adulteration and fraud is more common. The Chinese Pharmacopoeia (2020 edition) indicates that the content of the ethanol extract of resin shall not be less than 10%, and the content of agarotetrol (C17H18O6) shall not be less than 0.1% [1]. Xie et al. [74] digitized the chemical composition extracted from HPLC-Q-TOF-MS fingerprints, and the quality was evaluated according to the proportion of components. Ismail et al. [75] extracted and separated the volatile oil of Malaysia agarwood. After analysis and screening, the content of γ-eucalyptus oleol was determined as the quality standard of agarwood volatile oil. Chen et al. [57] detected the incense in different fragrance processes by GC-MS, analyzed the different chemical components, distinguished the incense in different ways by the method of grey relational analysis, and, finally, reflected the quality of incense by ranking. The several chemical components of agarwood were isolated by high-performance liquid chromatography (HPLC). After evaluation and analysis, the content of agaropiric acid in incense was selected as the index to establish a standard method for evaluating the quality of artificial incense, with the content of agaropiric acid as the evaluation standard [76].

Table 6.
Quality grading system of agarwood.
3.5. Agarwood Induction Technique
3.5.1. Natural Induction
With the rapid development of medicine, the demand for incense resources is increasing day by day, and it is even more valuable as medicinal material. Most of the agarwood resources mainly come from the wild, and agarwood needs to be damaged and can be harvested for a long time, and the number of agarwood resources that can be harvested is decreasing day by day.
3.5.2. Artificial Induction
The molecular mechanism of agarwood induction is shown in Figure 3. Artificial incense will replace wild incense to become the main source. The artificial incense-forming technology mainly includes the physical injury method, chemical reagent method, biological planting method, and many more [2]. With the development of incense forming technology, most of the artificial incense is mainly composed of chemical techniques. Zhao and Fang [77] evaluated the quality of agarwood produced by the whole-tree agarwood-inducing technology, and preliminarily considered that the quality of incense produced by this technology conformed to the standard of the Chinese Pharmacopoeia. However, the quality of artificial incense is still different from that of natural incense, it is far from the ratio of high-quality insects in natural incense, and there are few reports on how to use insect microorganisms to cause damage to the incense to form high-quality insect incense. The method of using artificial intervention to make the fragrance quality close to that achieved using natural insects is still a blank, so it is necessary to combine incense with insect microorganisms. To produce the incense using high-quality insect leakage is an area that still requires considerable effort. At present, the studies on artificial incense technology are limited, a problem needs to be solved. Therefore, there is a need to establish a standardized incense base and explore the method of combining animals and plants to improve incense technology and provide high-quality medicinal resources.
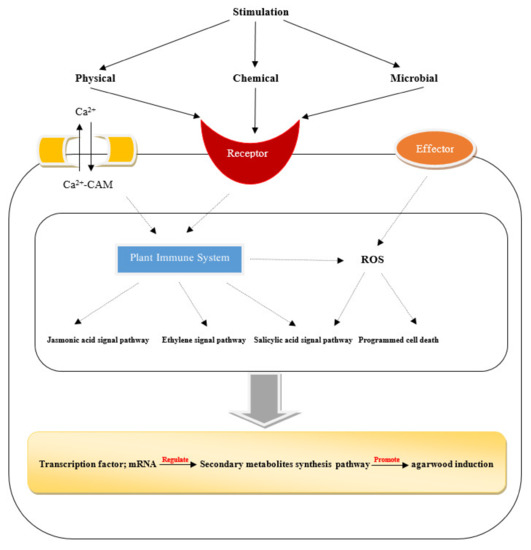
Figure 3.
A schematic diagram showing the molecular mechanism of agarwood induction.
3.6. Future Perspectives and Limitations
There are still many problems to be solved: (1) Because agarwood is a valuable medicinal material, the commodity price is expensive, and the mixing of fake and inferior products in the market increases. It is difficult for people to identify these inferior products with the naked eye. It is even more difficult to identify when processed into handicrafts, and the mixing of adulterated products seriously affects the safety of clinical drug use of agarwood. How to strengthen market supervision and how to establish an accurate and rapid identification of fake and shoddy products is an urgent problem to be solved. (2) Agarwood ranks first among the four famous incenses of agarwood, sandalwood, ambergris, and musk. It has many effects, such as relieving pain, vomiting, asthma, etc., and is widely used in the clinic, such as for anti-bacteria and anti-inflammation, sedation and analgesia, and relieving cough and asthma, to name but a few. However, the specific active substances and pharmacological mechanisms of various pharmacological actions have not been confirmed, which greatly limits the development and utilization of incense resources. Therefore, it is necessary to further study the effective components of agarwood and explore the action mechanism of its active substances. (3) With the massive felling of natural incense, and it will take several years, or even more than ten years, to replace. As the availability of natural incense gradually decreases, artificial incense will become the main source, but the technology of artificial incense is still unknown. Compared with insect incense, there is still a large gap between normal incense and insect incense, and methods to produce high-quality agarwood incense still need to be further explored. At the same time, large-scale planting and scientific cultivation is also an urgent technical problem to be solved.
4. Conclusions
Agarwood has great development potential, and its application has a broad prospect. In this article, we talked about the varieties, regional distribution, and current main identification methods of agarwood, discussed some chemical components contained in agarwood, and enumerated the biological pharmacological effects of its components. This report also combined the clinical application of other medicinal materials and classified the advantages and disadvantages of the incense sold in the market. Finally, we provided an example of the agarwood induction mechanism to yield high quality incense.
Currently, the research and development of agarwood are still insufficient, especially in areas such as the rapid identification of mixed and inferior products, the mechanism of pharmacological action, the development of new products, the optimization and development of agarwood-inducing technology, etc. New techniques have been tried, and some progress has been made, in artificial planting and the improvement of agarwood-inducing technology, but ways to improve the scientific utilization value of incense and how to exert its greater value in combination with medicine, agronomy, and insect microbiology still need to be discussed.
Author Contributions
Conceptualization, Y.W., M.H., H.L. and R.M.; methodology, Y.W., M.H., Z.J., Z.W., J.G, F.Y., H.L. and R.M.; investigation, Y.W., M.H., Z.J., Z.W., J.G. and F.Y.; data curation, Y.W., M.H., Z.J., H.L., Z.W., J.G., F.Y. and R.M.; writing—original draft preparation, Y.W., M.H., H.L., Z.W., J.G., F.Y. and R.M.; writing—review and editing, Y.W., M.H., Z.J., H.L., Z.W., J.G., F.Y. and R.M; supervision, M.H., H.L. and R.M.; project administration, M.H., H.L. and R.M.; funding acquisition, M.H., H.L. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the GDAS Special Project of Science and Technology Development (2020GDASYL-20200103096, 2021GDASYL-20210103051); Science and Technology Planning Project of Guangdong Province (2017B020202005), and the Research Programs of Guangzhou (202103000065).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to the researchers who have contributed to this field.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Chinese Pharmacopoeia. National Pharmacopoeia Committee. 2020. Available online: http://wp.chp.org.cn/front/chpint/en/ (accessed on 19 December 2021).
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Kumeta, Y.; Ito, M. Characterization of α-humulene synthases responsible for the production of sesquiterpenes induced by methyl jasmonate in Aquilaria cell culture. J. Nat. Med. 2016, 70, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chen, D.L.; Wei, J.H.; Feng, J.; Zhang, Z.; Yang, Y.; Zheng, W. Four new 2-(2-phenylethyl) chromone derivatives from Chinese agarwood produced via the whole-tree agarwood-inducing technique. Molecules 2016, 21, 1433. [Google Scholar] [CrossRef] [PubMed]
- Yumi, Z.; Has-Yun, H.; Kerr, P.G.; Phirdaous, A.; Hamzah, M.S. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189. [Google Scholar] [CrossRef]
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the matter: Agarwood use and trade and CITES implementation for Aquilaria malaccensis. Traffic Network Rep. 2000, 46, 17–18. [Google Scholar]
- Gärdenfors, U.; Hilton Taylor, C.; Mace, G.M.; Rodriguez, J. The application of IUCN red list criteria at regional levels. Conserv. Biol. 2001, 15, 1206–1212. [Google Scholar] [CrossRef]
- Germplasm Resources Information Network Taxonomy for Plant. Available online: https://trade.cites.org/# (accessed on 12 December 2021).
- Hsuan, K. The Concise Flora of Singapore. Gymnosperms and Dicotyledons; University Press: Singapore, 1990. [Google Scholar] [CrossRef]
- Parris, B.S. Flora of Peninsular Malaysia; Forest Research Institute: Kuala Lumpur, Malaysia, 2010. [Google Scholar]
- Wong, Y.F.; Chin, S.T.; Perlmutter, P.; Marriott, P.J. Evaluation of comprehensive two-dimensional gas chromatography with accurate mass time-of-flight mass spectrometry for the metabolic profiling of plant–fungus interaction in Aquilaria malaccensis. J. Chromatogr. 2015, 1387, 104–115. [Google Scholar] [CrossRef]
- Ismail, N.; Ali, N.A.M.; Jamil, M.; Rahiman, M.H.F.; Tajuddin, S.N.; Taib, M.N. A review study of agarwood oil and its quality analysis. J. Teknol. 2014, 68, 37–42. [Google Scholar] [CrossRef]
- Sen, S.; Talukdar, N.C.; Khan, M. A simple metabolite profiling approach reveals critical biomolecular linkages in fragrant agarwood oil production from Aquilaria malaccensis–a traditional agro-based industry in North East India. Curr. Sci. 2015, 108, 63–71. [Google Scholar]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Components of the agarwood smoke on heating. J Essent. Oil Res. 1993, 5, 419–423. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Components of the volatile concentrate of agarwood. J. Essent. Oil Res. 1993, 5, 283–289. [Google Scholar] [CrossRef]
- Du, H.f.; Xiong, L.y.; Lin, L.; Sshuai, O.; Qi, L.k. Simultaneous determination of five kinds of chromone in Aguilariae lignum resinatum by HPLC. CTMFJ 2015, 20, 87–90. [Google Scholar]
- Zou, M.M.; Xia, Z.Q.; Lu, C.; Wang, H.; Ji, J.; Wang, W.J. Genetic diversity and differentiation of Aquilaria sinensis (Lour.) Gilg revealed by ISSR and SRAP markers. Crop Sci. 2012, 52, 2304–2313. [Google Scholar] [CrossRef]
- Niu, X.; Ji, K.; Lu, G. Preliminary studies on identification of Aquilaria sinensis (L.) Gilg by the PCR product of rDNA ITS sequencing. Gd. Agric. Sci. 2010, 37, 167–169. [Google Scholar]
- Shen, Y.; Tan, X.; Zhao, X.; Pang, Q.; Zhao, S.J. Pharmacy, Ribosomal DNA ITS sequence analysis of Aquilaria sinensis from different geographical origin in China. Chin. J. Trad. Chin. Med. Pharm. 2009, 24, 539–541. [Google Scholar]
- Chen, X.; Gao, Y.; Li, W.J.C.P. Study on the correlation between the volatile constituents of Aquilaria sinensis and the inducing methods. Chin. Pharm. 2012, 23, 1017–1020. [Google Scholar]
- Zhang, Z.; Han, X.-M.; Wei, J.-H.; Xue, J.; Yang, Y.; Liang, L.; Li, X.J.; Guo, Q.M.; Xu, Y.H.; Gao, Z.H. Compositions and antifungal activities of essential oils from agarwood of Aquilaria sinensis (Lour.) Gilg induced by Lasiodiplodia theobromae (Pat.) Griffon. & Maubl. J. Brazil. Chem. Soc. 2014, 25, 20–26. [Google Scholar]
- Tajuddin, S.N.; Yusoff, M.M. Chemical composition of volatile oils of Aquilaria malaccensis (Thymelaeaceae) from Malaysia. Nat. Prod. Commun. 2010, 5, 1965–1968. [Google Scholar] [CrossRef]
- Mei, W.L.; Zeng, Y.B.; Liu, J.; Dai, F. GC-MS analysis of volatile constituents from five different kinds of Chinese eaglewood. J. Chin. Med. Mat. 2007, 30, 551–555. [Google Scholar]
- Lin, F.; Mei, W.L.; Wu, J.; Dai, F. GC-MS analysis of volatile constituents from Chinese agarwood produced by artificial methods. Chin. Herb. Med. 2010, 33, 222–225. [Google Scholar]
- Bhardwaj, I.; Sharma, M.J. Pharmacology, Herbal anesthetic agents: An overview on sources, uses and future perspectives. AJPS 2019, 5, 21–27. [Google Scholar]
- Mei, W.L.; Zeng, Y.B.; Wu, J.; Cui, H.B.; Dai, F. Chemical composition and anti-MRSA activity of the essential oil from Chinese eaglewood. AJPS 2008, 17, 225–229. [Google Scholar]
- Liu, J.; Zhang, X.; Yang, J.; Zhou, J.; Yuan, Y.; Jiang, C.; Chi, X.; Huang, L.J. Agarwood wound locations provide insight into the association between fungal diversity and volatile compounds in Aquilaria sinensis. R. Soc. Open. Sci. 2019, 6, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.-L.; Yang, D.-L.; Wang, H.; Yang, J.-L.; Zeng, Y.-B.; Guo, Z.-K.; Dong, W.-H.; Li, W.; Dai, F. Characterization and determination of 2-(2-phenylethyl) chromones in agarwood by GC-MS. J. Med. 2013, 18, 12324–12345. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, W.; Wang, H.; Chen, H.; Cai, C.; Yang, L.; Jiang, B.; Yang, Y.; Mei, W.; Dai, F. LC-MS guided identification of dimeric 2-(2-phenylethyl) chromones and sesquiterpene-2-(2-phenylethyl) chromone conjugates from agarwood of Aquilaria crassna and their cytotoxicity. Fitoterapia 2019, 138. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mei, W.L.; Kong, F.D.; Chen, H.Q.; Li, W.; Chen, Z.B.; Dai, F. Four new bi-2-(2-phenylethyl) chromone derivatives of agarwood from Aquilaria crassna. Fitoterapia 2017, 119, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.X.; Li, W.S.; Tao, M.H.; Pan, Q.L.; Sun, Z.H.; Ye, W.; Li, H.H.; Zhang, W.M. 2-(2-phenylethyl) chromones from endophytic fungal strain Botryosphaeria rhodina A13 from Aquilaria sinensis. Chin. Herb. Med. 2017, 9, 58–62. [Google Scholar] [CrossRef]
- Mei, W.L.; Cai, H.C.; Dong, W.H.; Guo, Z.K.; Wang, H.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar]
- Yang, J.J. Study on quality evaluation method of agarwood from China. Master’s Thesis, Hainan University, Haikou, China, 2015. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201702&filename=1015592278.nh&uniplatform=NZKPT&v=UxkWd_b6Efird7uOwyv1LxhWn2np-jaCL5kGrHJG0gB9s3ToeGtfVufTgjYZpRhf (accessed on 19 December 2021).
- Shimada, Y.; Tominaga, T.; Konishi, T.; Kiyosawa, S. Studies on the agarwood (Jinko). I. Structures of 2-(2-phenylethyl) chromone derivatives. Chem. Pharm. Bull. 1982, 30, 3791–3795. [Google Scholar] [CrossRef]
- Shimada, Y.; Konishi, T.; Kiyosawa, S.; Nishi, M.; Miyahara, K.; Kawasaki, T. Studies on the agalwood (Jinko). IV.: Structures of 2-(2-Phenylethyl) chromone derivatives, agarotetrol and isoagarotetrol. Chem. Pharm. Bull. 1986, 34, 2766–2773. [Google Scholar] [CrossRef][Green Version]
- Sukkaew, S.; Pripdeevech, P.; Thongpoon, C.; Machan, T.; Wongchuphan, R. Volatile constituents of murraya koenigii fresh leaves using headspace solid phase microextraction–gas chromatography–mass spectrometry. Nat. Prod. Commun. 2014, 9, 1783–1786. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour. Frag. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.J. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, W.; Hu, Z.; Jin, M.; Yu, X.J. Recent advances in chromatography coupled bioassay for screening bioactive natural products. Chin. J. Chromatogr. 2004, 22, 616–619. [Google Scholar]
- Ismail, N.; Rahiman, M.H.F.; Taib, N.; Ibrahim, M.; Zareen, S.; Tajuddin, S.N. Direct thermal desorption (DTD) extraction for different qualities of agarwood incense analysis. CSPA 2016, 12, 291–294. [Google Scholar]
- Yang, L.; Qiao, L.R.; Xie, D.; Dai, F.; Guo, S.X. Sesquiterpenes and monoterpene from Aquilaria sinensis. Chin. J. Chin. Mater. Med. 2012, 13, 1973–1976. [Google Scholar]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Haigang, P. Study on the chemical constituents of volatile oil from tagara agarwood. Gd. Chem. Ind. 2012, 39, 257–258. [Google Scholar]
- Yang, L.; Qiao, L.R.; Xie, D.; Dai, J.G.; Guo, S.X. Diterpenoids from Chinese eaglewood. Chin. J. Chin. Mat. Med. 2011, 36, 2088–2091. [Google Scholar]
- Yang, L.; Qiao, L.R.; Ji, C.X.; Xie, D.; Gong, N.B.; Lu, Y.; Zhang, J.J.; Dai, J.G.; Guo, S.X. Antidepressant abietane diterpenoids from Chinese eaglewood. J. Nat. Prod. 2013, 76, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Dan, B.; Yue, L.S.; Peng, F.T. Flavanoids from the stems of Aquilaria sinensis. Chin. J. Nat. Med. 2012, 10, 87–291. [Google Scholar]
- Qi, J.; Lu, J.J.; Liu, J.H.; Yu, B.Y. Flavonoid and a rare benzophenone glycoside from the leaves of Aquilaria sinensis. Chem. Pharm. Bull. 2009, 57, 134–137. [Google Scholar] [CrossRef]
- Chen, D.; Song, Y.L.; Nie, C.X.; Ma, X.; Tu, P.F. Chemical constituents from Aquilaria sinensis (Lour.) Gilg. J. Chin. Pham. Sci. 2012, 21, 88–92. [Google Scholar]
- Hendra, H.; Moeljopawiro, S.; Nuringtyas, T.R. Antioxidant and antibacterial activities of agarwood (Aquilaria malaccensis Lamk.) leaves. AIP Conf. Proc. 2016, 1755. [Google Scholar] [CrossRef]
- Li, W.; Mei, W.; Dong, W.; Cai, C.; Gai, C.J. Study on chemical constituents of Chinese agarwood “Qi-Nan”. J. Trop. Subtrop. Bot. 2019, 27, 196–202. [Google Scholar]
- Chen, C.T.; Yeh, Y.T.; Chao, D.; Chen, C.Y. Chemical constituents from the wood of Aquilaria sinensis. Chem. Nat. Compd. 2013, 49, 113–114. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M.J.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.L.; Dai, F. Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef]
- Suzuki, A.; Miyake, K.; Saito, Y.; Rasyid, F.A.; Tokuda, H.; Takeuchi, M.; Suzuki, N.; Ichiishi, E.; Fujie, T.; Goto, M. Phenylethylchromones with in vitro antitumor promoting activity from Aquilaria filaria. Planta. Med. 2017, 83, 300–305. [Google Scholar] [CrossRef]
- Chen, X.Y.; Huang, Y.Q. Grey relational analysis of volatile components and anti-tumor activity of agarwood. Proprietary Chin. Med. 2018, 40, 224–227. [Google Scholar]
- Dahham, S.S.; Hassan, L.E.A.; Ahamed, M.B.K.; Majid, A.S.A.; Majid, A.M.S.A.; Zulkepli, N.N. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna). BMC Complem. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Fuchao, M.; Zhang, Q.; Liu, Y.; Gong, B.; Guo, P.; Wei, J.H. Effect of agarwood produced by whole-tree agarwood-inducing technique on hypnotic and spontaneous activity inhibition of mice. J. Int. Pharm. Res. 2016, 43, 1082–1087. [Google Scholar]
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J. Agarwood essential oil displays sedative-hypnotic effects through the GABAergic system. Molecules 2017, 22, 2190. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L. Anti-inflammatory effect of Chinese agarwood essential oil via inhibiting p-STAT3 and IL-1β /IL-6. Chin. Pharm. J. 2019, 54, 1951–1957. [Google Scholar]
- Wu, G.L. A brief discussion on the clinical effect of anesthetic medicine Ba Wei Chen Xiang in the treatment of bronchial asthma. Latest Med. Inf. Abs. 2017, 17, 142–144. [Google Scholar]
- Zhou, F.; Tian, S.; Pan, J.; Mei, Q. Contrastive study on the pharmacological action of alcohol extract caulis cinnamomi camphorae and lignum Aquilariae Sinensis. J. Trad. Chin. Med. 2013, 28, 1849–1850. [Google Scholar]
- Wang, S.; Wang, C.; Yu, Z.; Wu, C.; Peng, D.; Liu, X.; Liu, Y.; Yang, Y.; Guo, P.; Wei, J.H. Agarwood essential oil ameliorates restrain stress-induced anxiety and depression by inhibiting HPA axis hyperactivity. Molecules 2018, 19, 3468. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.h.; Peng, Y.h.; Ke, F.f.; Deng, Y.L. Experimental study on the content, antioxidant activity in vitro, and delaying aging effect of tannins from the leaf of Aquilaria sinensis (Lour.) Gilg. J. Gd. Pham. Univer. 2012, 3, 259–262. [Google Scholar]
- Lee, H.Y.; Lee, J.S.; Kim, H.G.; Kim, W.Y.; Lee, S.B.; Choi, Y.H.; Son, C.G. The ethanol extract of Aquilariae Lignum ameliorates hippocampal oxidative stress in a repeated restraint stress mouse model. BMC Complem. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.X.; Tang, S.H.; Zheng, Y.C. Development, progress of studies on bioactive peptides derived from meat and its by-product. Food R&D 2017, 6, 206–212. [Google Scholar]
- Yang, M.; Li, Y.F.; Ma, Q.S.; Ge, R.L. Effect of 50% ethanol-extract from Bawei Chenxiang Powder on hemodynamics in myocardial ischemia rats. Chin. J. Trad. Chin. Med. 2011, 11, 2740–2742. [Google Scholar]
- Sugiyama, T.; Narukawa, Y.; Shibata, S.; Masui, R.; Kiuchi, F. Three new 5, 6, 7, 8-tetrahydroxy-5, 6, 7, 8-tetrahydrochromone derivatives enantiomeric to agarotetrol from agarwood. J. Nat. Med. 2018, 72, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Chunyan, Y.; ZhuYanmei, W.L.; Qiong, W. Effect of tibetan BaWei ChenXiang Powder on cerebral ischemia reperfusion injury in rats with apoptosis neurons at hippocampal CA1 area. J. QH. Med. Colle. 2011, 4, 243–246. [Google Scholar]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of tibetan medicine and the pharmacological research of tibetan materia medica. Pham. Res. 2020, 155, 104688. [Google Scholar] [CrossRef]
- Gao, A.R. Clinical observation of BaWwei Chenxiang Powder in the treatment of coronary heart disease and angina pectoris. Latest Med. Inf. Abs. 2018, 18, 246–254. [Google Scholar]
- Wang, Y.Q.; Liu, F. Clinical therapeutic effects on functional constipation treated with the external application of Chenxiang Tongbian Powder on the Umbilicus and Moxibustion. World J. Integr. Tradit. West Med. 2017, 12, 1546–1549. [Google Scholar]
- Xie, B.; Luo, H.; Huang, X.; Huang, F.; Zhang, Q.; Wu, X.; Zhou, X.; Wu, H. Pharmacokinetic studies of six major 2-(2-phenylethyl) chromones in rat plasma using ultra high performance liquid chromatography with tandem mass spectrometry after oral administration of agarwood ethanol extract. J. Separ. Sci. 2021, 44, 2418–2426. [Google Scholar] [CrossRef]
- Ismail, N.; Ali, N.A.M.; Jamil, M.; Rahiman, M.H.F.; Taib, M.N.; Tajuddin, S.N. Major volatile chemical compounds of agarwood oils from Malaysia based on z-score technique. J. Chem. Nat. Compd. 2015, 51, 776–779. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, Y.; Lei, Z.; Pan, Q.; Zhong, Z.; Liu, D.; Zhang, W.; Gao, X. Correlation between agaropiric acid and extract contents in artificial Aquilariae lignum resinatum. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 55–59. [Google Scholar]
- Zhao, Y.; Fang, Z.J. Quality assessment of Chinese eaglewood by the technique of Aquilaria sinensis quintana making incense and the determination of benzyl acetone. J. Gd. Pham. Univ. 2013, 29, 253–257. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).