Abstract
Hypervalent iodine heterocycles represent one of the important classes of hypervalent iodine reagents with many applications in organic synthesis. This paper reports a simple and convenient synthesis of benziodazolones by the reaction of readily available iodobenzamides with m-chloroperoxybenzoic acid in acetonitrile at room temperature. The structure of one of these new iodine heterocycles was confirmed by X-ray analysis. In combination with PPh3 and pyridine, these benziodazolones can smoothly react with alcohols or amines to produce the corresponding esters or amides of 3-chlorobenzoic acid, respectively. It was found that the novel benziodazolone reagent reacts more efficiently than the analogous benziodoxolone reagent in this esterification.
1. Introduction
Iodine(III)-containing heterocycles represent one of the most important classes of hypervalent iodine reagents [1,2,3,4,5,6,7,8]. Among them, the five-membered iodine-oxygen heterocycles, which are known by the general name of benziodoxolones, have found wide application in organic synthesis [9,10,11,12,13,14]. In particular, benziodoxolone derivatives 1 (Figure 1) have attracted significant interest as atom-transfer reagents and oxidants [5,10,11,12,13,14]. Recently, benziodoxolone derivatives have been used as effective oxidants for coupling of carboxylic acids with alcohols or amines leading to the corresponding esters or amides, respectively [15,16,17,18,19]. Benziodazolone compounds 2, nitrogen analogs of benziodoxolones, are also known and offer the possibility of fine-tuning the reactivity by modifying the substituent on the nitrogen atom [20,21,22,23]. Thus, a number of reagents of 2 with various nitrogen-containing ligands and functional groups have been developed [24,25,26,27,28]. For example, benziodazolone compounds 2 supported by various ligands are known as atom-transfer reagents and can act as very effective electrophilic reagents for a variety of substrates, including azidation [20], alkynylation [24], and trifluorothiomethylation reagents [25]. Very recently, Zhang and coworkers investigated the synthesis and structural characterization of a stable fluorobenziodazolone compound and demonstrated that the novel reagent can efficiently perform ring-extended fluorination reactions for various three-membered ring compounds [26]. In addition, benziodazolones 3 are also capable of working as oxidants in dehydrogenative coupling reactions between various two-component molecules [27]. To the best of our knowledge, however, this is the first report where benziodazolones serve as the coupling assistant reagents, and their ligands serve as the coupling partners for alcohols and amines. Our group previously reported that bicyclic benziodazolone 4 could be employed as an efficient reagent for oxidatively assisted coupling of carboxylic acids with alcohols or amines in the presence of phosphines [28]. Therefore, in view of the growing interest in cyclic hypervalent iodine reagents, we focused on benzoidazolone 3, whose acyloxy ligand is also a potential coupling partner for alcohols and amines.
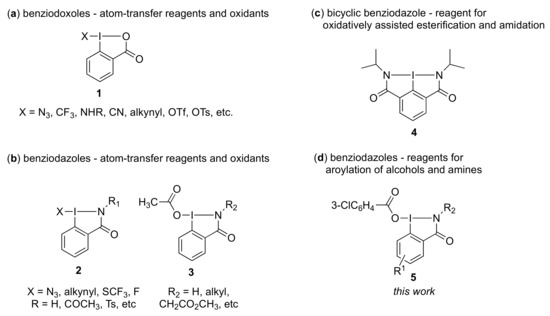
Figure 1.
Important iodine(III)-containing heterocycles, benziodoxolones and benziodazolones. (a): benziodoxoles-atom transfer reagents and oxidants; (b): bicyclic benziodazole-reagent for oxidatively assisted esterification and amidation; (c): benziodazoles-atom-transfer reagent and oxidants; (d): benziodazoles-reagent for aroylation of alcohols and amides; 1-benziodoxoles; 2-bicyclic benziodazole; 3, 4-beniodazoles; 5-benziodazoles.
In the present paper, we report a convenient and one-step procedure for the preparation of various 3-chlorobenzoyloxy-substituted benziodazolone derivatives 5 from the respective benzamides. In combination with PPh3 and pyridine, these new benziodazolones can smoothly react with alcohols or amines producing the corresponding esters or amides of 3-chlorobenzoic acid, respectively.
2. Results and Discussion
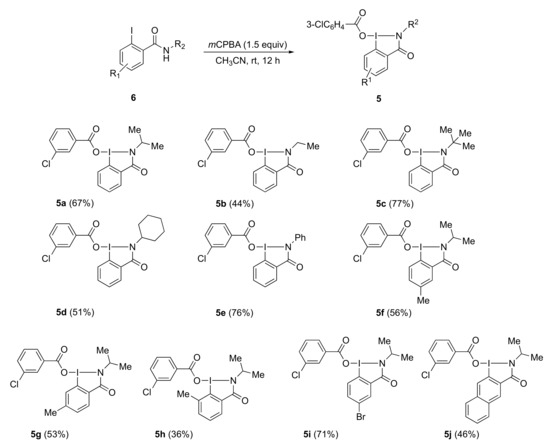
A novel series of 3-chlorobenzoyloxy-substituted benziodazolone derivatives 5a–j were prepared in one step by the reaction of readily available benzamides 6 with m-chloroperoxybenzoic acid (mCPBA) in acetonitrile at room temperature. In this method, by evaporation of the solvent and simply washing with ether, analytically pure benziodazolones 5 were obtained as stable, white solids in moderate to good yields. The structures of the products of 5 were confirmed by NMR spectroscopy, high-resolution mass spectrometry, and single-crystal X-ray crystallography of benziodazolone 5a (Scheme 1 and Figure 2).
Scheme 1.
Preparation of benziodazolones 5. 5-benziodazoles; 6-iodobenzamides.
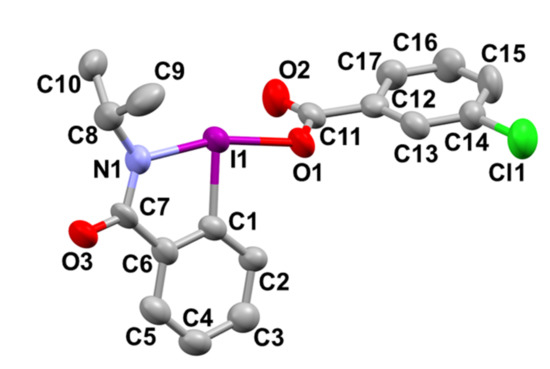
Figure 2.
X-ray crystal structure of compound 5a. Typical bond distances in Å: I1–N1 = 2.126(5), I1–C1 = 2.085(5), I1–O1 = 2.174(4). C1–C17; carbon atom, O1–O3; oxygen atom; Cl1; chlorine atom; I1; iodine atom.
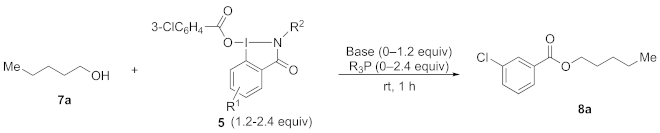
Next, we investigated the use of benziodazolone 5a as a reagent for oxidatively assisted cross-coupling of alcohols with specially added carboxylic acids in the presence of a base (4-dimethylaminopyridine, DMAP) and PPh3 based on the previously reported procedures [15,16,17,18,19,27]. However, in contrast to previously reported reactions, yields of the desired carboxylic esters were low, and the main isolated products were m-chlorobenzoates formed by direct aroylation of alcohols with reagent 5a. Therefore, considering the importance of substituted benzoates in organic chemistry, we focused on the reactions of the 3-chlorobenzoyloxy ligand in benziodazolone 5a with alcohols and amines.
Optimization studies of this new reaction using alcohol 7a as a model substrate have been performed in the absence of solvent with varying bases, phosphines, and ratios of reactants (Table 1). The reaction of 7a in the presence of excessive benziodazolone 5a (2.4 equiv.), Ph3P (2.4 equiv.) and DMAP (2.4 equiv.) afforded ester 8a in a 71% yield (entry 1). The further addition of m-chlorobenzoic acid to the reaction mixture did not improve the yield (entry 2). However, lowering the amounts of 5a, DMAP, and Ph3P to 1.8 equivalents did not significantly change the yield (entry 3), and when the amounts of 5a, DMAP, and Ph3P were lowered to 1.2 equivalents, the yield increased to 84% (entry 4). Whereas, the further reduction of the amount of DMAP (0.6 equiv.) resulted in a significantly reduced yield (entry 5). Then, we tested several other bases (entries 6–11), and pyridine showed superior results with yields up to 91% (entry 9). When Bu3P was used instead of Ph3P, the yield of the product was lower (entry 11). In addition, no product was formed in the absence of a phosphine (entry 12), and a low yield was observed in the absence of a base (entry 13). Next, we investigated the reactivity of the prepared benziodazolones 5 under the similar condition of entry 9 (entries 14–22). Likely due to the decomposition of the reagents during the reaction with 5b, c, e, and j, the reaction did not proceed efficiently, and 7a was detected in the reaction mixture (entries 14, 15, 17, and 22). In contrast, when other benziodazolones 5d, f–i, were employed, the reactions proceeded effectively to give the desired ester compound 8a in moderate to good yields (entries 16, 18–21).

Table 1.
Optimization of the condensation of n-pentanol 7a using benziodazolone 5 conditions 1.
Using optimized reaction conditions, we investigated the scope of the esterification reaction of alcohols 7 with benziodazolone 5a (Scheme 2). In general, the reactions of primary and secondary alcohols 7a–h afforded esters 8a–h in moderate to high yields. In the reactions with alcohols 7i–l having unsaturated bonds, the respective ester compounds 8i–k were obtained in low to good yields without any loss of unsaturated bonds, albeit with a low yield of 8l. Meanwhile, the reaction with sterically hindered tert-butanol gave only trace amounts of product 8m.
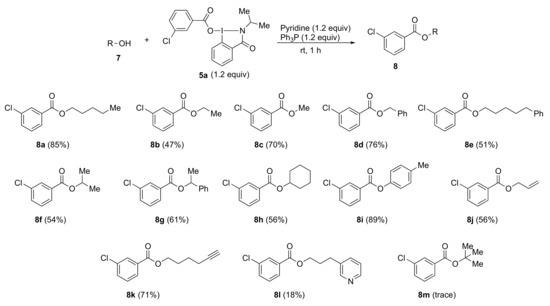
Scheme 2.
Esterification of alcohols using reagent 5a; 7-alcohols; 5a-benziodazolone; 8a–m-esters.
Under similar reaction conditions, primary amines 9a–d reacted with reagent 5a to form amides 10a–d in moderate to high yields (Scheme 3). In the case of the reaction with sterically hindered tert-butylamine, the desired amide compound 10d was obtained in 43%. This is likely because of the high nucleophilicity of amines.
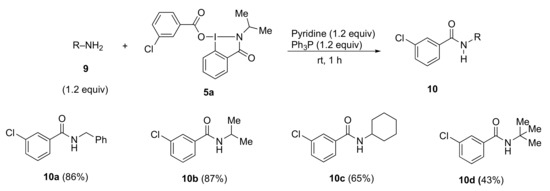
Scheme 3.
Amidation of amines using reagent 5a; 9-amines; 5a-benziodazolone; 10a–d-amides.
In the next study, the reactivity between benziodazolone 5a and benziodoxolone 11, which could be easily synthesized from acetoxybenziodoxolone and 3-chlorobenzoic acid via a ligand exchange procedure, was compared. The condensation reaction of alcohol 7a and the prepared reagent 11 gave the desired ester 8a in only a 21% yield (Scheme 4). This is because 11 was hardly miscible in the mixture. From this result, it was found that the efficiency of benziodazolone 5a was better than that of benziodoxolone 11 in this esterification reaction system.
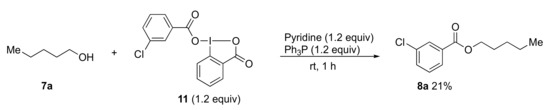
Scheme 4.
Esterification of pentanol 7a using reagent 11; 7a-n-pentanol; 11-benziodoxolone; 8a-pentyl 3-chlorobenzoate.
In order to clarify the reaction mechanism of esterification, we carried out several control experiments (Scheme 5). Firstly, when the reaction of alcohol 7a with benziodazolone 5a did not proceed with the ligand exchange reaction, 5a was recovered from the reaction mixture in a quantitative amount (reaction 1). Then, we performed mass spectrometry experiments (see Supplementary Materials for detail). When 5a was treated with pyridine, the peak of the ligand exchange product 12 was not detected, but when 5a was treated with DMAP, the peak of the ligand exchange product 13 was detected. This may be due to DMAP being a stronger nucleophile than pyridine (reactions 2 and 3) [29]. Next, when the reaction of benziodazolone 5a with alcohol 7a and Ph3P was attempted, the peaks of Ph3PO and the estimated benziodazolone derivative structures such as 14 and 15 could be detected, while unfortunately, the mass peak of the expected ligand exchange intermediate 16 could not be observed directly (reaction 4 and Figure 3). The observed peaks were probably generated by the reaction of intermediate 16 from the ligand exchange reaction between Ph3P and 5a with moisture in the air during the mass experiment. Thus, these results may indicate that benziodazolone 5a reacts with Ph3P before pyridine or alcohol 7a. Notably, the reaction using benziodazolone 5a in the absence of Ph3P has been found to not proceed with the desired esterification at all (Table 1, entry 12).
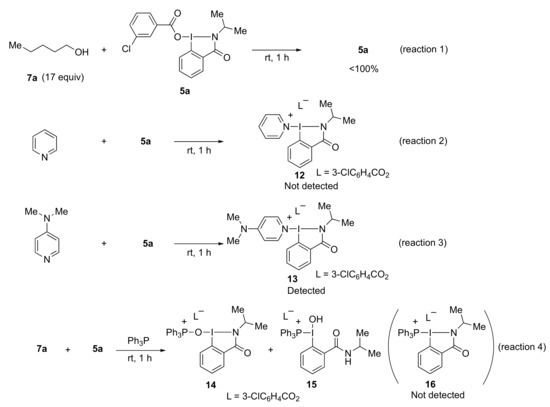
Scheme 5.
Control experiment; 5a-benziodazolone; 7a-n-pentanol; 12,13, and 16-ligand exchange products; 14,15-estimated benziodazolone derivative structures.
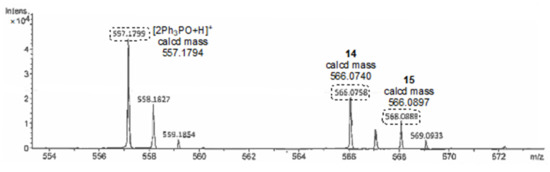
Figure 3.
Mass result for Scheme 5, reaction 4; 14,15-estimated benziodazolone derivative structures.
Based on the results of these blank experiments and considering previously published mechanistic rationalizations [15,17,19], we propose the following mechanistic scheme for the esterification reaction (Scheme 6). The reaction initially involves the ligand exchange between benziodazolone 5a and Ph3P via TS1 to generate the zwitterion intermediate 16. Then the carboxylate anion attacks the phosphorus center to produce intermediate 17. Next, 17 is converted to the phosphonium salt 18, which then reacts with pyridine and alcohol 7, respectively, to afford N-acyl pyridinium salt 19. Finally, 19 undergoes nucleophilic acyl substitution from the alkoxide of the counterion giving the desired ester 8. Although benziodoxolone requires a base such as DMAP in the ligand exchange with phosphine, it can smoothly proceed without a base in the case of benziodazolone 5a due to the significantly greater trans influence of the benziodazolone ring compared to the benziodoxolone ring [30]. Therefore, pyridine may mainly play a role in accelerating the nucleophilic acyl substitution for the formation of N-acyl pyridinium salts 19.
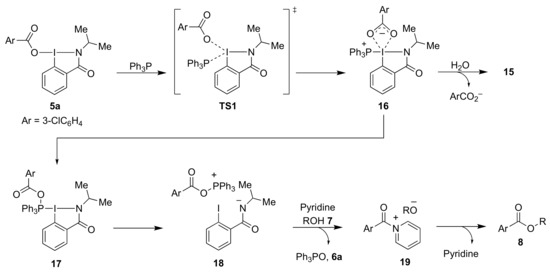
Scheme 6.
Proposed mechanism of esterification reaction using reagent 5a; 5a-benziodazolone; 6a-iodobezamide; Ts1-transition state 1; 15-estimated benziodazolone structure; 16,17-intermediates; 18-phosphonium salt; 19-N-acyl pyridinium salt.
3. Materials and Methods
3.1. General Experimental Remarks
All reactions were performed in open air with a stopper and oven-dried glassware. All commercial reagents were ACS grade and were used without further purification. NMR spectra were recorded on a Varian Inova 500 MHz NMR spectrophotometer (1H NMR and 13C NMR; Palo Alto, CA, USA) and Bruker 400 MHz NMR spectrophotometer (1H NMR and 13C NMR; Billerica, MA, USA). Melting points were determined in an open capillary tube with a Mel-temp II melting point apparatus. Infrared spectra were recorded on PerkinElmer Spectrum 1600 series FT-IR spectrometer (Waltham, MA, USA).
3.2. General Procedure for Preparation of Iodobenzamide 6
Amine was added dropwise at 0 °C to a stirred mixture of iodobenzoyl chloride and triethylamine in dichloromethane. The reaction was stirred for 1 h at 0 °C. After completion of the reaction, water (10–20 mL) was added, and the mixture was extracted with dichloromethane. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by recrystallization (CH2Cl2-hexane) afforded the analytically pure iodobenzamide 6.
2-Iodo-N-isopropylbenzamide 6a [31]. The reaction with 2-iodobenzoyl chloride (3171 mg, 11.9 mmol), triethylamine (2649 mg, 26.2 mmol), and isopropylamine (774 mg, 13.1 mmol) in dichloromethane (15 mL) according to the general procedure afforded 3325 mg (97%) of product 6a, isolated as a white solid: mp 136.4–137.7 °C (lit. [31], mp 135–136 °C); 1H NMR (500 MHz, CDCl3): δ 7.84 (d, J = 8.0 Hz, 1H), 7.41–7.34 (m, 2H), 7.11–7.05 (m, 1H), 5.57 (brs, 1H), 4.38–4.22 (m, 1H), and 1.29 (d, J = 7.0 Hz, 1H).
N-Ethyl-2-iodobenzamide 6b [32]. The reaction with 2-iodobenzoyl chloride (1062 mg, 3.99 mmol), triethylamine (887 mg, 8.77 mmol), and ethylamine (198 mg, 4.38 mmol) in dichloromethane (15 mL) according to the general procedure afforded 1032 mg (94%) of product 6b, isolated as a white solid: mp 114.3–115.5 °C (lit. [32], 114–116 °C); 1H NMR (500 MHz, CDCl3): δ 7.85 (d, J = 8.0 Hz, 1H), 7.41–7.33 (m, 2H), 7.11–7.05 (m, 1H), 5.78 (brs, 1H), 3.62–3.40 (m, 2H), and 1.27 (t, J = 7.3 Hz, 3H).
N-(tert-Butyl)-2-iodobenzamide 6c [33]. The reaction with 2-iodobenzoyl chloride (1063 mg, 3.99 mmol), triethylamine (888 mg, 8.78 mmol), and tert-butylamine (321 mg, 4.39 mmol) in dichloromethane (15 mL) according to the general procedure afforded 1140 mg (94%) of product 6c, isolated as a white solid: mp 122.2–124.1 °C (Lit. [33], 120–122 °C); 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 8.0 Hz, 1H), 7.41–7.32 (m, 2H), 7.09–7.03 (m, 1H), 5.55 (brs, 1H), and 1.49 (s, 9H).
N-Cyclohexyl-2-iodobenzamide 6d [33]. The reaction with 2-iodobenzoyl chloride (1025 mg, 3.85 mmol), triethylamine (857 mg, 8.46 mmol), and cyclohexylamine (420 mg, 4.23 mmol) in dichloromethane (15 mL) according to the general procedure afforded 1201 mg (95%) of product 6d, isolated as a white solid: mp 141.2–142.3 °C (lit. [33], mp 141–143 °C); 1H NMR (500 MHz, CDCl3): δ 7.85 (d, J = 8.0 Hz, 1H), 7.41–7.34 (m, 2H), 7.11–7.05 (m, 1H), 5.62 (brs, 1H), 4.07–3.94 (m, 1H), 2.12–2.04 (m, 2H), 1.80–1.72 (m, 2H), 1.69–1.61 (m, 1H), 1.49–1.38 (m, 2H), and 1.32–1.16 (m, 3H).
2-Iodo-N-phenylbenzamide 6e [33]. The reaction with 2-iodobenzoyl chloride (1089 mg, 4.87 mmol), triethylamine (908 mg, 9.00 mmol), and aniline (419 mg, 4.50 mmol) in dichloromethane (15 mL) according to the general procedure afforded 1085 mg (82%) of product 6e, isolated as a white solid: mp 144.2–145.0 °C (Lit. [33], 144–146 °C); 1H NMR (500 MHz, CDCl3): δ 7.92 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 8.0 Hz, 1H), 7.47–7.42 (m, 2H), 7.42–7.35 (m, 2H), and 7.21–7.13 (m, 2H).
2-Iodo-N-isopropyl-5-methylbenzamide 6f [34]. The reaction with 2-iodo-5-methylbenzoyl chloride (2140 mg, 7.63 mmol), triethylamine (1698 mg, 16.8 mmol), and isopropylamine (496 mg, 8.39 mmol) in dichloromethane (10 mL) according to the general procedure afforded 2220 mg (96%) of product 5f, isolated as a white solid: mp 147.8–148.7 °C (Lit. [34], 147–149 °C); 1H NMR (500 MHz, CDCl3): δ 7.68 (d, J = 8.0 Hz, 1H), 7.20 (s, 1H), 6.90 (d, J = 8.0 Hz, 1H), 5.62 (brs, 1H), 4.33–4.233 (m, 1H), 2.30 (s, 3H), and 1.28 (d, J = 7.0 Hz, 6H); HRMS (ESI-positive): calcd for C11H15INO ([M + H])+: 304.0198, found: 304.0196.
2-Iodo-N-isopropyl-4-methylbenzamide 6g. The reaction with 2-iodo-4-methylbenzoyl chloride (1070 mg, 3.81 mmol), triethylamine (849 mg, 8.38 mmol), and isopropylamine (248 mg, 4.19 mmol) in dichloromethane (10 mL) according to the general procedure afforded 1118 mg (97%) of product 5g, isolated as a white solid: mp 117.0–119.0 °C; IR (neat) cm−1: 3305, 3062, 2973, 2927, 1874, 1637, 1598. 1531, 1484, 1464, 1450, 1389, 1369, 1351, 1329, 1289, 1264, 1178, 1127, 1036, 883, 850, 831, 821, 805, 601, and 570; 1H NMR (500 MHz, CDCl3): δ 7.69 (s, 1H), 7.27 (d, J = 8.0 Hz, 1H), 7.15 (d, J = 8.0 Hz, 1H), 5.61 (brs, 1H), 4.33–4.22 (m, 1H), 2.31 (s, 3H), and 1.27 (d, J = 7.0 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 168.5, 141.4, 140.2, 139.5, 128.9, 128.1, 92.4, 42.2, 22.6, and 20.7; HRMS (ESI-positive): calcd for C11H15INO ([M + H])+: 304.0198, found: 304.0193.
2-Iodo-N-isopropyl-3-methylbenzamide 6h. The reaction with 2-iodo-3-methylbenzoyl chloride (1063 mg, 3.79 mmol), triethylamine (844 mg, 8.34 mmol), and isopropylamine (224 mg, 4.17 mmol) in dichloromethane (10 mL) according to the general procedure afforded 1084 mg (94%) of product 6h, isolated as a white solid: mp 135.6–137.2 °C.; IR (neat) cm−1: 3248, 3069, 2970, 2937, 2875, 1657, 1551, 1459, 1366, 1352, 1331, 1303, 1266, 1204, 1156, 1129, 1012, 919, 858, 805, 780, 738, 718, and 685; 1H NMR (500 MHz, CDCl3): δ 7.33–7.19 (m, 2H), 7.16–7.05 (m, 1H), 5.50 (brs, 1H), 4.41–4.21 (m, 1H), 2.45 (s, 3H), and 1.29 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 169.9, 144.2, 142.8, 130.2, 128.1, 124.9, 99.3, 42.1, 29.1, and 22.6; HRMS (ESI-positive): calcd for C11H15INO ([M + H])+: 304.0198, found: 304.0190.
5-Bromo-2-iodo-N-isopropylbenzamide 6i. The reaction with 5-bromo-2-iodobenzoyl chloride (1317 mg, 3.81 mmol), triethylamine (848 mg, 8.38 mmol), and isopropylamine (248 mg, 4.19 mmol) in dichloromethane (10 mL) according to the general procedure afforded 1347 mg (96%) of product 6i, isolated as a white solid: mp 187.8–188.5 °C; IR (neat) cm−1: 3255, 3073, 2971, 1642, 1541, 1453, 1083, 1017, 900, 810, and 727; 1H NMR (500 MHz, CDCl3): δ 7.69 (d, J = 8.0 Hz, 1H), 7.52–7.48 (m, 1H), 7.24–7.19 (m, 1H), 5.58 (brs, 1H), 4.36–4.16 (m, 1H), and 1.29 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 167.0, 144.1, 141.1, 134.0, 131.2, 122.6, 90.4, 42.4, and 22.6; HRMS (APCI-positive): calcd for C10H1279BrINO ([M + H])+: 367.9147, found: 367.9122.
3-Iodo-N-isopropyl-2-naphthamide 6j. The reaction with 3-iodo-2-naphthoyl chloride (158 mg, 0.50 mmol), triethylamine (111 mg, 1.1 mmol), and isopropylamine (32.5 mg, 0.55 mmol) in dichloromethane (5.0 mL) according to the general procedure afforded 105 mg (62%) of product 6j, isolated as a white solid: mp 191.6–193.3 °C; IR (neat) cm−1: 3279, 2970, 1642, 1541, 1124, 954, 908, 895, 880, 805, and 743; 1H NMR (500 MHz, CDCl3): δ 8.38 (s, 1H), 7.86 (s, 1H), 7.84–7.79 (m, 1H), 7.75–7.71 (m, 1H), 7.57–7.50 (m, 2H), 5.68 (brs, 1H), 4.49–4.28 (m, 1H), and 1.33 (d, J = 6.5 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 168.5, 139.3, 139.0, 134.9, 131.9, 128.1, 127.8, 127.4, 127.3, 126.7, 88.8, 42.3, and 22.7; HRMS (ESI-positive): calcd for C14H15INO ([M + H])+: 340.0198, found: 340.0188.
3.3. General Procedure for Preparation of 3-Chlorobenzoyloxybenziodazolone 5
Iodobenzamide 6 was added at 0 °C to a stirred mixture of m-CPBA in acetonitrile. The reaction was stirred for 12 h at room temperature. After completion of the reaction, the solvent was removed under reduced pressure to give solid residue. Then diethyl ether was added to the solid residue to prepare the suspended solution, which was filtered, and dried in a vacuum to give the desired 3-chlorobenzoyloxybenziodazolone 5.
2-Isopropyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5a. The reaction with 2-iodo-N-isopropylbenzamide 6a (289 mg, 1.00 mmol) and m-CPBA (259 mg, 1.50 mmol) in acetonitrile (10 mL) according to the general procedure afforded 296 mg (67%) of product 5a, isolated as a white solid: mp 142.0–142.8 °C; IR (neat) cm−1: 3067, 2967, 2932, 2875, 1627, 1588, 1570, 1296, 1257, and 739; 1H NMR (500 MHz, CDCl3): δ 8.28 (d, J = 8.0 Hz, 1H), 8.22 (dd, J = 7.8, 1.8 Hz, 1H), 8.04 (s, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.88–7.80 (m, 1H), 7.76–7.69 (m, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.45–7.37 (m, 1H), 4.52 (sept., J = 6.5 Hz, 1H), and 1.47 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 171.3, 166.0, 134.6, 133.7, 133.6, 132.3, 131.8, 131.0, 130.0, 129.8, 129.6, 127.9, 116.6, 46.7, and 24.4; HRMS (APCI-positive): calcd for C17H16ClINO3 ([M + H])+: 443.9863, found: 443.9877.
Single crystals of product 5a suitable for X-ray crystallographic analysis were obtained by slow crystallization from the acetonitrile solution. X-ray diffraction data for 5a were collected on Rigaku RAPID II Image Plate system using graphite-monochromated CuKα radiation (λ = 1.54187 Å) at 173 K. The structure was solved by Sir 2011 and refined on F2 using ShelXle. Crystal data for 5a C17H15ClINO3 are as follows: monoclinic, space group P21/c, a = 12.6913(3), b = 14.8285(4), c = 18.3997(13) Å, α =90°, β = 104.695(7)°, γ = 90°, V = 3349.4(3) Å3, Z = 8, 22,784 reflections measured, and 5815 unique (4560 I > 2σ/(I)); Rint = 0.0780, Rsigma = 0.0834, R1 (I > 2σ/(I)) = 0.0595, R1 = 0.0696, wR2all = 0.1700, and S = 1.086; please see the cif for more detailed information: CCDC-2122170.
2-Ethyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5b. The reaction with N-ethyl-2-iodobenzamide 6b (550 mg, 2.00 mmol) and m-CPBA (518 mg, 3.00 mmol) in acetonitrile (15 mL) according to the general procedure afforded 380 mg (44%) of product 5b, isolated as a white solid: mp 76.0–78.0 °C; IR (neat) cm−1: 3074, 2969, 2935, 2876, 1627, 1590, 1571, 1442, 1319, 1263, 756, and 739; 1H NMR (500 MHz, CDCl3): δ 8.29 (d, J = 8.0 Hz, 1H), 8.23 (d, J = 7.5 Hz, 1H), 8.04 (s, 1H), 7.96 (d, J = 8.0 Hz, 1H), 7.90–7.83 (m, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.44–7.37 (m, 1H), 3.76 (q, J = 7.3 Hz, 2H), and 1.37 (t, J = 7.3 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 171.3, 166.3, 134.9, 134.4, 133.6, 132.5, 132.4, 131.9, 131.0, 130.0, 129.8, 129.6, 127.9, 116.9, 38.3, and 16.2; HRMS (ESI-positive): calcd for C9H11INO2 ([M-3ClC6H4CO2+H])+: 291.9834, found: 291.9817.
2-(tert-Butyl)-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5c. The reaction with N-(tert-butyl)-2-iodobenzamide 6c (606 mg, 2.00 mmol) and m-CPBA (518 mg, 3.00 mmol) in acetonitrile (15 mL) according to the general procedure afforded 705 mg (77%) of product 5c, isolated as a white solid: mp 170.8–172.0 °C; IR (neat) cm−1: 3070, 2966, 1628, 1585, 1570, 1438, 1317, 1262, 930, 756, and 740; 1H NMR (500 MHz, CDCl3): δ 8.26 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 7.5 Hz, 1H), 8.03 (s, 1H), 7.95 (d, J = 7.5 Hz, 1H), 7.84 (t, J = 7.5 Hz, 1H), 7.73–7.67 (m, 1H), 7.52 (d, J = 7.5 Hz, 1H), 7.42–7.37 (m, 1H), and 1.71 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 171.3, 165.8, 135.0, 134.5, 134.4, 133.7, 132.3, 131.8, 130.9, 129.6, 129.5, 127.9, 114.8, 58.2, and 30.2; HRMS (ESI-positive): calcd for C11H15INO2 ([M-3ClC6H4CO2+H])+: 320.0147, found: 320.0137.
2-Cyclohexyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5d. The reaction with N-cyclohexyl-2-iodobenzamide 6d (750 mg, 2.28 mmol) and m-CPBA (590 mg, 3.42 mmol) in acetonitrile (17.1 mL) according to the general procedure afforded 633 mg (51%) of product 5d, isolated as a white solid: mp 159.0–160.3 °C; IR (neat) cm−1: 3071, 2929, 2854, 1629, 1589, 1566, 1439, 1323, 1294, 1621, 1067, 967, 756, 739, and 659; 1H NMR (500 MHz, CDCl3): δ 8.29 (d, J = 8.5 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.04 (s, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.83 (t, J = 7.8 Hz, 1H), 7.75–7.69 (m, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.43–7.38 (m, 1H), 4.30–4.06 (m, 1H), 2.34–2.13 (m, 2H), 1.95–1.85 (m, 2H), 1.78–1.71 (m, 1H), 1.54–1.43 (m, 4H), and 1.30–1.20 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 171.4, 165.9, 134.5, 134.4, 133.7, 133.6, 132.3, 131.8, 131.0, 130.0, 129.8, 129.6, 127.8, 116.9, 54.2, 35.5, 25.7, and 25.3; HRMS (ESI-positive): calcd for C13H17INO2 ([M-3ClC6H4CO2+H])+: 346.0304, found: 346.0286.
3-Oxo-2-phenyl-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5e. The reaction with 2-iodo-N-phenylbenzamide 6e (750 mg, 2.32 mmol) and m-CPBA (601 mg, 3.48 mmol) in acetonitrile (17.4 mL) according to the general procedure afforded 841 mg (76%) of product 5e, isolated as a white solid: mp 154.8 °C (decomp.); IR (neat) cm−1: 3067, 3033, 1637, 1586, 1569, 1488, 1441, 1507, 1262, 1125, 754, 741, and 659; 1H NMR (500 MHz, CDCl3): δ 8.34 (d, J = 6.0 Hz, 1H), 8.27 (d, J = 8.5 Hz, 1H), 8.03 (s, 1H), 7.97–7.90 (m, 2H), 7.81–7.74 (m, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.50–7.39 (m, 5H), and 7.35–7.28 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 170.9, 164.6, 138.1, 135.3, 134.5, 132.9, 132.8, 132.6, 132.6, 131.2, 129.9, 129.8, 129.7, 129.7, 128.0, 127.2, 126.4, and 117.2; HRMS (ESI-positive): calcd for C13H11INO2 ([M-3ClC6H4CO2+H])+: 339.9834, found: 339.9807.
2-Isopropyl-5-methyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5f. The reaction with 2-iodo-N-isopropyl-5-methylbenzamide 6f (606 mg, 2.00 mmol) and m-CPBA (518 mg, 3.00 mmol) in acetonitrile (15 mL) according to the general procedure afforded 513 mg (56%) of product 5f, isolated as a white solid: mp 148.0–15.0 °C; IR (neat) cm−1: 3067, 2965, 2927, 2874, 1630, 1574, 1456, 1307, 1260, 1143, 756, and 740; 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 9.5 Hz, 1H), 8.02 (s, 2H), 7.95 (d, J = 7.5 Hz, 1H), 7.66–7.61 (m, 1H), 7.54–7.50 (m, 1H), 7.43–7.37 (m, 1H), 4.61–4.42 (m, 1H), 2.54 (s, 3H), and 1.45 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 171.2, 166.1, 141.9, 135.6, 134.3, 133.8, 133.4, 132.3, 132.2, 129.8, 129.6, 129.6, 127.9, 112.8, 46.7, 24.3, and 20.9; HRMS (ESI-positive): calcd for C11H15INO2 ([M-3ClC6H4CO2+H])+: 320.0147, found: 320.0141.
2-Isopropyl-6-methyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5g. The reaction with 2-iodo-N-isopropyl-4-methylbenzamide 6g (606 mg, 2.00 mmol) and m-CPBA (518 mg, 3.00 mmol) in acetonitrile (15 mL) according to the general procedure afforded 486 mg (53%) of product 6g, isolated as a white solid: mp 150.2–151.5 °C; IR (neat) cm−1: 3073, 2964, 2929, 1618, 1569, 1463, 1313, 1295, 1260, 1143, 757, 740, and 667; 1H NMR (500 MHz, CDCl3): δ 8.07 (d, J = 7.5 Hz, 1H), 8.06–8.02 (m, 2H), 7.95 (d, J = 7.8 Hz, 1H), 7.55–7.49 (m, 2H), 7.41 (t, J = 7.8 Hz, 1H), 4.60–4.41 (m, 1H), 2.56 (s, 3H), and 1.45 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 171.3, 166.1, 146.0, 134.4, 133.8, 132.3, 132.1, 131.5, 131.0, 129.9, 129.8, 129.6, 127.8, 116.8, 46.6, 24.4, and 22.3; HRMS (ESI-positive): calcd for C11H15INO2 ([M-3ClC6H4CO2+H])+: 320.0147, found: 320.0140.
2-Isopropyl-7-methyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5h. The reaction with 2-iodo-N-isopropyl-3-methylbenzamide 6h (736 mg, 2.00 mmol) and m-CPBA (518 mg, 1.50 mmol) in acetonitrile (15 mL) according to the general procedure afforded 738 mg (71%) of product 5h, isolated as a white solid: mp 117.2–117.8 °C; IR (KBr) cm−1: 3127, 2960, 2909, 1648, 1608, 1569, 1384, 1317, 1139, and 745; 1H NMR (400 MHz, CDCl3): δ 8.08–8.02 (m, 1H), 7.97 (s, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.64–7.56 (m, 2H), 7.49 (t, J = 8.0 Hz, 1H), 7.47 (t, J = 7.8 Hz, 1H), 4.57–4.44 (m, 1H), 2.80 (s, 3H), and 1.49 (d, J = 6.4 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 172.1, 166.7, 140.2, 138.2, 134.9, 134.4, 134.4, 132.1, 130.9, 129.8, 129.6, 127.7, 118.9, 47.1, 24.4, and 23.6; HRMS (ESI-positive): calcd for C11H15INO2 ([M-3ClC6H4CO2+H])+: 320.0147, found: 320.0146.
5-Bromo-2-isopropyl-3-oxo-2,3-dihydro-1H-1λ3-benzo[d][1,2]iodazol-1-yl 3-chlorobenzoate 5i. The reaction with 5-bromo-2-iodo-N-isopropylbenzamide 6i (736 mg, 2.00 mmol) and m-CPBA (518 mg, 1.50 mmol) in acetonitrile (15 mL) according to the general procedure afforded 738 mg (71%) of product 5i, isolated as a white solid: mp 151.6–152.6 °C; IR (neat) cm−1: 3103, 3066, 2968, 2931, 1636, 1617, 1568, 1557, 1464, 1443, 1405, 1305, 1260, 1141, 1070, 959, 903, 813, 755, and 742; 1H NMR (500 MHz, CDCl3): δ 8.35–8.33 (m, 1H), 8.13 (d, J = 9.0 Hz, 2H), 8.02–8.00 (m, 1H), 7.95–7.91 (m, 2H), 7.56–7.52 (m, 1H), 7.43–7.39 (m, 1H), 4.56–4.41 (m, 1H), and 1.46 (d, J = 6.5 Hz, 6H); 13C NMR (125 MHz, CDCl3): δ 171.3, 164.5, 137.4, 135.4, 134.7, 134.4, 133.3, 132.5, 131.4, 129.8, 129.7, 127.8, 126.4, 114.5, 47.0, and 24.3; HRMS (ESI-positive): calcd for C10H1279BrINO2 ([M-3ClC6H4CO2+H])+: 383.9096, found: 383.9101.
2-Isopropyl-3-oxo-2,3-dihydro-1H-1λ3-naphtho[2,3-d][1,2]iodazol-1-yl 3-chlorobenzoate 5j. The reaction with 3-iodo-N-isopropyl-2-naphthamide 6j (85 mg, 0.250 mmol) and m-CPBA (65 mg, 0.375 mmol) in acetonitrile (1.9 mL) according to the general procedure afforded 56 mg (46%) of product 5j, isolated as a white solid: mp 153.3–155.0 °C; IR (neat) cm−1: 3058, 2966, 2922, 1636, 1619, 1569, 1296, 1144, 758, and 740; 1H NMR (500 MHz, CDCl3): δ 8.74 (s, 2H), 8.14–8.08 (m, 2H), 8.04–7.98 (m, 2H), 7.77–7.69 (m, 2H), 7.58–7.53 (m, 1H), 7.44 (t, J = 7.8 Hz, 1H), 4.66–4.47 (m, 1H), and 1.49 (d, J = 6.5 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 171.3, 165.9, 136.7, 134.4, 133.8, 133.4, 132.4, 130.6, 129.9, 129.7, 129.3, 129.2, 128.9, 128.7, 128.3, 127.9, 111.1, 46.7, and 24.2; HRMS (ESI-positive): calcd for C14H14INO2 ([M-3ClC6H4CO2+H])+: 356.0147, found: 356.0119.
3.4. Preparation of 3-Chlorobenzoyloxybenziodoxole 11
A mixture of 3,3-dimethyl-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (1530 mg, 5.00 mmol) in CHCl3 (15 mL) was added under stirring. The reaction was stirred at reflux for 18 h. After completion of the reaction, the solvent was removed under reduced pressure to give solid residue. The solid residue was filtrated and washed with diethyl ether and hexane several times and then dried under a vacuum to give 1712 mg (85%) of product 11, isolated as white solid; IR (KBr) cm−1 3059, 1695, 1623, 1569, 1309, 1266, 1235, 1116, and 747; 1H NMR (400 MHz, CDCl3): δ 8.30 (d, J = 7.6 Hz, 1H), 8.13–7.95 (m, 4H), 7.81–7.73 (m, 1H), 7.65–7.57 (m, 1H), and 7.45 (d, J = 8.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 170.1, 168.0, 136.5, 134.8, 133.5, 131.5, 130.7, 130.1, 130.0, 129.1, 128.8, 128.3, and 118.7; HRMS (ESI-positive): calcd for C14H835ClIO4 ([M + H])+: 402.9229, found: 402.9214.
3.5. Esterification of 1-Pentanol with 3-Cholorbenzoyloxybenziodazolones
Pentyl 3-chlorobenzoate 8a [35]. Triphenylphosphine (47 mg, 0.180 mmol), pyridine (14 mg, 0.180 mmol), and 1-pentanol 7a (13 mg, 0.150 mmol) were added to a test tube containing benziodazolones 5 (0.180 mmol). The mixture was then stirred at room temperature for 1 h. After the reaction was completed, dichloromethane (3.0 mL) was used to transfer the reaction mixture to a separatory funnel. Then saturated NaHCO3 (3.0 mL) was added, and the reaction mixture was extracted with dichloromethane. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by preparative TLC (hexane:ethyl acetate = 3:1) afforded the analytically pentyl 3-chlorobenzoate 8a, isolated as a colorless oil; IR (neat) cm−1: 2961, 1724, 1576, 1469, 1293, 1256, 1127, 967, and 675; 1H NMR (500 MHz, CDCl3): δ 8.01 (s, 1H), 7.93 (d, J = 7.8 Hz, 1H), 7.54–7.49 (m, 1H), 7.38 (d, J = 7.8 Hz, 1H), 4.32 (t, J = 6.9 Hz, 2H), 1.77 (quint, J = 6.9 Hz, 1H), 1.48–1.43 (m, 4H), and 0.93 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 165.4, 134.5, 132.8, 132.3, 129.7, 129.6, 127.7, 65.6, 28.4, 28.2, 22.4, and 14.0; HRMS (APCI-positive): calcd for C12H17ClO2 ([M + H])+: 227.0839, found: 227.0846.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5a (80 mg, 0.180 mmol) according to the general procedure afforded 29 mg (85%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5b (99 mg, 0.180 mmol) according to the general procedure afforded 2 mg (6%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5c (82 mg, 0.180 mmol) according to the general procedure afforded 3 mg (10%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5d (88 mg, 0.180 mmol) according to the general procedure afforded 21 mg (61%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5e (86 mg, 0.180 mmol) according to the general procedure afforded 9 mg (25%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5f (82 mg, 0.180 mmol) according to the general procedure afforded 22 mg (64%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5g (82 mg, 0.180 mmol) according to the general procedure afforded 21 mg (62%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5h (82 mg, 0.180 mmol) according to the general procedure afforded 19 mg (56%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (13 mg, 0.150 mmol) using benziodazolone 5i (94 mg, 0.180 mmol) according to the general procedure afforded 19 mg (54%) of product 8a, isolated as a colorless oil.
The reaction of 1-pentanol 7a (5.1 mg, 0.057 mmol) using benziodazolone 5j (34 mg, 0.069 mmol) according to the general procedure afforded 3.2 mg (25%) of product 8a, isolated as a colorless oil.
3.6. Typical Procedure for Esterification and Amidation Using Benziodazolones
Triphenylphosphine (47 mg, 0.18 mmol), pyridine (14 mg, 0.18 mmol), and alcohol 7 (0.15 mmol) or amine 9 (0.15 mmol) were added to a test tube containing benziodazolones 5 (0.180 mmol). The mixture was then stirred at room temperature for 1 h. After the reaction was completed, dichloromethane (3.0 mL) was used to transfer the reaction mixture to a separatory funnel. Then saturated NaHCO3 (3.0 mL) was added, and the reaction mixture was extracted with dichloromethane. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by preparative TLC (hexane:ethyl acetate = 3:1) afforded the analytically pure 8 or 10.
Ethyl 3-chlorobenzoate 8b [36]. The reaction of ethanol 7b (7 mg, 0.150 mmol) according to the general procedure afforded 13 mg (47%) of product 8b, isolated as a colorless oil; IR (neat) cm−1: 2924, 1727, 1573, 1466, 1370, 1293, 1281, 1256, 915, and 749; 1H NMR (500 MHz, CDCl3): δ 8.03 (s, 1H), 7.94 (d, J = 7.5 Hz, 1H), 7.56–7.52 (m, 1H), 7.39 (t, J = 7.5 Hz, 1H), 4.39 (q, J = 7.0 Hz, 2H), and 1.41 (t, J = 7.0 Hz, 3H); HRMS (APCI-positive): calcd for C9H10ClO2 ([M + H])+: 185.0369, found: 185.0389.
Methyl 3-chlorobenzoate 8c [37]. The reaction of methanol 7c (5 mg, 0.150 mmol) according to the general procedure afforded 18 mg (70%) of product 8c, isolated as a colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.02 (s, 1H), 7.93 (d, J = 7.8 Hz, 1H), 7.56–7.51 (m, 1H), 7.39 (t, J = 7.8 Hz, 1H), and 3.93 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 165.9, 134.5, 133.0, 131.9, 130.0, 129.7, 127.7, and 52.4.
Benzyl 3-chlorobenzoate 8d [38]. The reaction of benzyl alcohol 7d (16 mg, 0.150 mmol) according to the general procedure afforded 28 mg (76%) of product 8d, isolated as a colorless oil; IR (neat) cm−1: 2955, 1721, 1576, 1429, 1290, 1278, 1124, 955, and 659; 1H NMR (500 MHz, CDCl3): δ 8.04 (s, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.54–7.50 (m, 1H), 7.47–7.42 (m, 2H), 7.42–7.33 (m, 4H), and 5.36 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 165.2, 135.6, 134.5, 133.1, 131.9, 129.7, 129.7, 128.7, 128.4, 128.3, 127.8, and 67.1; HRMS (ESI-positive): calcd for C14H11ClO2Na ([M + Na])+: 269.0345, found: 269.0341.
5-Phenylpentyl 3-chlorobenzoate 8e. The reaction of 5-phenyl-1-pentyl alcohol 7e (25 mg, 0.150 mmol) according to the general procedure afforded 23 mg (51%) of product 8e, isolated as a colorless oil; IR (neat) cm−1: 2937, 1724, 1576, 1429, 1293, 1256, 1124, 958, 699, 675, and 659; 1H NMR (500 MHz, CDCl3): δ 7.99 (s, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.39–7.33 (m, 1H), 7.30–7.22 (m, 2H), 7.20–7.13 (m, 3H), 4.31 (t, J = 6.5 Hz, 2H), 2.75–2.53 (m, 2H), 1.83–1.75 (m, 2H), 1.73–1.65 (m, 2H), and 1.52–1.43 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 165.4, 142.3, 134.5, 132.8, 132.2, 129.7, 129.6, 128.4, 128.3, 127.7, 125.7, 65.4, 35.8, 31.0, 28.5, and 25.6; HRMS (APCI-positive): calcd for C18H20ClO2 ([M + H])+: 303.1152, found: 303.1173.
Isopropyl 3-chlorobenzoate 8f [39]. The reaction of isopropanol 7f (9 mg, 0.150 mmol) according to the general procedure afforded 16 mg (54%) of product 8f, isolated as a colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.01 (s, 1H), 7.92 (d, J = 7.9 Hz, 1H), 7.54–7.50 (m, 1H), 7.37 (d, J = 7.9 Hz, 1H), 5.25 (sept, J = 6.5 Hz, 1H), and 1.37 (d, J = 6.5 Hz, 6H).
1-Phenylethyl 3-chlorobenzoate 8g [40]. The reaction of 1-phenylethyl alcohol 7g (18 mg, 0.150 mmol) according to the general procedure afforded 24 mg (61%) of product 8g, isolated as a colorless oil; IR (neat) cm−1: 2985, 1723, 1574, 1496, 1456, 1428, 1256, 1129, and 697; 1H NMR (500 MHz, CDCl3): δ 8.05 (s, 1H), 7.97 (d, J = 8.0 Hz, 1H), 7.56–7.52 (m, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.42–7.36 (m, 3H), 7.36–7.30 (m, 1H), 6.14 (q, J = 6.9 Hz, 1H), and 1.69 (d, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 164.6, 141.4, 134.5, 133.0, 132.3, 129.7, 128.7, 128.1, 127.8, 126.1, 73.5, and 22.3; HRMS (ESI-positive): calcd for C15H13ClO2Na ([M + Na])+: 283.0502, found: 283.0489.
Cyclohexyl 3-chlorobenzoate 8h [40]. The reaction of cyclohexanol 7h (15 mg, 0.150 mmol) according to the general procedure afforded 20 mg (56%) of product 8h, isolated as a colorless oil; IR (neat) cm−1: 2937, 1721, 1576, 1469, 1290, 1253, 1124, 945, 749, and 678; 1H NMR (500 MHz, CDCl3): δ 8.03–8.00 (m, 1H), 7.95–7.91 (m, 1H), 7.54–7.50 (m, 1H), 7.38 (t, J = 8.0 Hz, 1H), 5.09–4.94 (m, 1H), 1.99–1.91 (m, 2H), 1.84–1.75 (m, 2H), 1.64–1.53 (m, 3H), 1.50–1.40 (m, 2H), and 1.40–1.30 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 164.8, 134.4, 132.8, 132.7, 129.6, 127.7, 73.7, 31.6, 25.4, and 23.7; HRMS (APCI-positive): calcd for C13H16ClO2 ([M + H])+: 239.0839, found: 239.0843.
p-Tolyl 3-chlorobenzoate 8i [41]. The reaction of p-cresol 7i (16 mg, 0.150 mmol) according to the general procedure afforded 33 mg (89%) of product 8i, isolated as a white solid, mp 75.4–76.0 °C; IR (neat) cm−1: 2925, 1742, 1578, 1474, 1288, 1251, 1197, 1165, 1106, 811, and 743; 1H NMR (500 MHz, CDCl3): δ 8.17 (s, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.61–7.56 (m, 1H), 7.43 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 8.5 Hz, 2H), 7.08 (d, J = 8.5 Hz, 2H), and 2.37 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.2, 148.5, 135.8, 134.7, 133.5, 131.5, 130.2, 130.1, 129.9, 128.3, 121.2, and 20.9; HRMS (ESI-positive): calcd for C14H12ClO2 ([M + H])+: 247.0526, found: 247.0528.
Allyl 3-chlorobenzoate 8j [42]. The reaction of allyl alcohol 7j (13 mg, 0.150 mmol) according to the general procedure afforded 16 mg (56%) of product 8j, isolated as a colorless oil; IR (neat) cm−1: 3077, 2945, 2883, 1726, 1576, 1426, 1289, 1256, 1128, and 748; 1H NMR (400 MHz, CDCl3): δ 8.07–8.02 (m, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.60–7.50 (m, 1H), 7.39 (t, J = 7.8 Hz, 1H), 5.42 (dd, J = 17.2, 1.6 Hz, 1H), 5.31 (dd, J = 10.4, 1.6 Hz, 1H), and 4.83 (d, J = 5.6 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 165.1, 134.6, 133.0, 131.9, 129.7, 127.8, 118.7, 74.2, and 66.0; HRMS (EI): calcd for C10H935ClO2 ([M + H])+: 196.0291, found: 196.0285.
Hex-5-yn-1-yl 3-chlorobenzoate 8k. The reaction of 5-hexyn-1-ol 7k (15 mg, 0.150 mmol) according to the general procedure afforded 25 mg (71%) of product 8k, isolated as a colorless oil; IR (neat) cm−1: 3298, 3074, 2951, 2870, 2117, 1722, 1575, 1428, 1285, 1260, 1130, 747, and 641; 1H NMR (400 MHz, CDCl3): δ 8.01 (s, 1H), 7.93 (d, J = 7.8 Hz, 1H), 7.56–7.50 (m, 1H), 7.38 (d, J = 7.8 Hz, 1H), 4.36 (t, J =6.4 Hz, 2H), 2.36–2.16 (m, 2H), 1.99 (t, J = 2.8 Hz, 1H), 1.96–1.87 (m, 2H), 1.75–and 1.64 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 165.4, 134.5, 132.9, 132.1, 129.7, 129.6, 127.7, 83.8, 68.9, 64.9, 27.7, 25.0, and 18.1; HRMS (CI): calcd for C13H1335ClO2 ([M + H])+: 254.0942, found:254.0934.
3-(Pyridin-3-yl)propyl 3-chlorobenzoate 8l. The reaction of 3-pyridinepropanol 7l (21 mg, 0.150 mmol) according to the general procedure afforded 8 mg (18%) of product 8l, isolated as a colorless oil; IR (neat) cm−1: 3031, 2958, 2909, 2818, 1721, 1575, 1424, 1288, 1257, 1129, 1074, and 749; 1H NMR (400 MHz, CDCl3): δ 8.50 (s, 1H), 8.47 (d, J = 4.8 Hz, 1H), 8.00–7.95 (m, 1H), 7.93–7.87 (m, 1H), 7.54 (d, J = 7.6 Hz, 2H), 7.40 (t, J = 7.6 Hz, 1H), 7.26–7.20 (m, 1H), 4.37 (t, J = 6.4 Hz, 2H), 2.85–2.75 (m, 2H), and 2.18–2.07 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 165.3, 149.9, 147.8, 136.3, 135.8, 134.6, 133.1, 131.9, 129.7, 129.6, 127.7, 123.4, 64.4, 29.9, and 29.6; HRMS (ESI-positive): calcd for C15H1435ClNO2 ([M + H])+: 276.0786, found: 276.0786.
N-Benzyl 3-chlorobenzamide 10a [43]. The reaction of benzylamine 9a (16 mg, 0.150 mmol) according to the general procedure afforded 32 mg (86%) of product 10a, isolated as a white solid; 1H NMR (500 MHz, CDCl3): δ 7.77 (s, 1H), 7.65 (d, J = 7.5 Hz, 1H), 7.51–7.43 (m, 1H), 7.41–7.28 (m, 6H), 6.44 (brs, 1H), and 4.63 (d, J = 5.5 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 166.0, 137.8, 136.2, 134.8, 131.6, 129.9, 128.8, 128.0, 127.8, 127.3, 125.0, and 44.3; HRMS (APCI-positive): calcd for C14H13ClNO ([M + H])+: 246.0686, found: 246.0706.
3-Chloro-N-isopropylbenzamide 10b [44]. The reaction of isopropylamine 9b (9 mg, 0.150 mmol) according to the general procedure afforded 26 mg (87%) of product 10b, isolated as a white solid; 1H NMR (400 MHz, CDCl3): δ 7.73 (t, J = 1.8 Hz, 1H), 7.69–7.59 (m, 1H), 7.49–7.41 (m, 1H), 7.35 (t, J = 7.8 Hz, 1H), 6.03 (br. s, 1H), 4.44–4.13 (m, 1H), and 1.26 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 165.4, 136.8, 134.7, 131.3, 129.8, 127.2, 125.0, 42.1, and 22.8; HRMS (ESI-positive): calcd for C12H1235ClNO ([M + H])+: 198.0680, found: 198.0704.
3-Chloro-N-cyclohexylbenzamide 10c [45]. The reaction of cyclohexylamine 9c (15 mg, 0.150 mmol) according to the general procedure afforded 22 mg (65%) of product 10c, isolated as a white solid; IR (neat) cm−1: 3322, 3070, 2929, 2853, 1633, 1539, 1327, 1081, 753; 1H NMR (400 MHz, CDCl3): δ 7.75–7.71 (m, 1H), 7.64–7.60 (m, 1H), 7.47–7.42 (m, 1H), 7.35 (t, J = 7.8 Hz, 1H), 6.06 (brs, 1H), 4.03–3.89 (m, 1H), 2.07–1.98 (m, 2H), 1.81–1.71 (m, 2H), 1.70–1.62 (m, 1H), 1.49–1.35 (m, 2H), and 1.30–1.17 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 165.3, 136.9, 134.7, 131.3, 129.8, 127.2, 125.0, 48.9, 33.2, 25.5, and 24.9; HRMS (ESI-positive): calcd for C13H16ClNO ([M + H])+: 238.0993, found: 238.1009.
N-(tert-butyl)-3-chlorobenzamide 10d [46]. The reaction of tert-butyl amine 9d (11 mg, 0.150 mmol) according to the general procedure afforded 14 mg (43%) of product 10d, isolated a white solid; 1H NMR (400 MHz, CDCl3): δ 7.69 (t, J = 2.0 Hz, 1H), 7.61–7.56 (m, 1H), 7.46–7.42 (m, 1H), 7.35 (t, J = 7.8 Hz, 1H), 5.92 (brs, 1H), and 1.47 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 165.5, 137.8, 134.6, 131.1, 129.8, 127.1, 124.9, 51.9, and 28.8; HRMS (ESI-positive): calcd for C11H14ClNO ([M + H])+: 212.0837, found: 212.0853.
4. Conclusions
In conclusion, we have developed novel benziodazolone compounds readily prepared from iodobenzamides using mCPBA, and the solid structure was confirmed by X-ray crystallography. These new benziodazolones can act as coupling assistant reagents to alcohols and amines, and their ligands can act as a coupling partner to give the corresponding ester and amides in moderate to good yields. In addition, it was found that the newly synthesized benzoiodazolone demonstrated better efficiency than the corresponding benziodoxolone in the esterification reaction.
Supplementary Materials
The following are available online, the 1H and 13C NMR spectra of 5a–j, 6a–j, 8a–l, 10a–d, 11, Figure S1: X-ray crystallography data of 5a, Figure S2: Mass study.
Author Contributions
Conceptualization, A.Y.; methodology, A.Y. and M.T.S.; investigation, M.T.S.; resources, M.T.S., Y.A.V., P.S.P. and M.S.Y.; data analysis, A.Y., M.T.S. and A.S.; Data Discussion, A.Y., M.T.S., Y.A.V., P.S.P. and A.S., X-ray analysis, G.T.R.; writing—original draft preparation, A.Y., V.V.Z. and A.S.; writing—review and editing, A.Y., M.T.S., A.S. and G.T.R.; supervision, A.Y.; project administration, A.Y. and A.S.; funding acquisition, M.S.Y., V.V.Z. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation, grant number CHE-1759798, JSPS Fund for the Promotion of Joint International Research (Grant No 16KK0199), JST CREST (No. JRMJCR19R2), and a research grant from the Russian Science Foundation (RSF-21-73-20031).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data for the compounds are included in the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olofsson, B.; Ilan, M.; Rappoport, Z. Patai’s The Chemistry of Hypervalent Halogen Compounds; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Wirth, T. (Ed.) Hypervalent Iodine Chemistry; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Zhdankin, V.V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; Wiley: Chichester, UK, 2013. [Google Scholar]
- Hari, D.P.; Caramenti, P.; Waser, J. Cyclic Hypervalent Iodine Reagents: Enabling Tools for Bond Disconnection via Reactivity Umpolung. Acc. Chem. Res. 2018, 51, 3212–3225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hari, D.P.; Vita, M.V.; Waser, J. Cyclic Hypervalent Iodine Reagents for Atom-Transfer Reactions: Beyond Trifluoromethylation. Angew. Chem. Int. Ed. 2016, 55, 4436–4454. [Google Scholar] [CrossRef] [Green Version]
- Robidas, R.; Legault, C.Y. Cyclic Haloiodanes: Syntheses, Applications and Fundamental Studies. Helv. Chim. Acta 2021, 104, e2100111. [Google Scholar] [CrossRef]
- Vlasenko, Y.; Yusubov, M.S.; Shafir, A.; Postnikov, P.S. Hypervalent iodine in the structure of N-heterocycles: Synthesis, structure, and application in organic synthesis. Chem. Heterocycl. Compd. 2020, 56, 854–866. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.-G.; Hu, Z.-N.; Jia, M.-C.; Du, F.-H. Recent Advances and the Prospect of Hypervalent Iodine Chemistry. Synlett 2021, 32, 1289–1296. [Google Scholar] [CrossRef]
- Declas, N.; Pisella, G.; Waser, J. Vinylbenziodoxol(on)es: Synthetic Methods and Applications. Helv. Chim. Acta 2020, 103, e2000191. [Google Scholar] [CrossRef]
- Waser, J. Benziodoxol(on)e Reagents as Tools in Organic Synthesis: The Background behind the Discovery at the Laboratory of Catalysis and Organic Synthesis. Synlett 2016, 27, 2761–2773. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, J.; Früh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2014, 115, 650–682. [Google Scholar] [CrossRef] [PubMed]
- Le Vaillant, F.; Waser, J. Alkynylation of radicals: Spotlight on the “Third Way” to transfer triple bonds. Chem. Sci. 2019, 10, 8909–8923. [Google Scholar] [CrossRef]
- Zhdankin, V.V. Benziodoxole-Based Hypervalent Iodine Reagents in Organic Synthesis. Curr. Org. Synth. 2005, 2, 121–145. [Google Scholar] [CrossRef]
- Tian, J.; Gao, W.-C.; Zhou, D.-M.; Zhang, C. Recyclable Hypervalent Iodine(III) Reagent Iodosodilactone as an Efficient Coupling Reagent for Direct Esterification, Amidation, and Peptide Coupling. Org. Lett. 2012, 14, 3020–3023. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-C.; Zhang, C. Synthesis, characterization, and initial reaction study of two new bicyclic hypervalent iodine(III) reagents. Tetrahedron Lett. 2014, 55, 2687–2690. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.-S.; Sun, B.; Tian, J. Practical Peptide Synthesis Mediated by a Recyclable Hypervalent Iodine Reagent and Tris(4-methoxyphenyl)phosphine. Org. Lett. 2015, 17, 4106–4109. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.-L.; Qu, J.; Zhang, C. Recyclable hypervalent-iodine-mediated solid-phase peptide synthesis and cyclic peptide synthesis. Beilstein J. Org. Chem. 2018, 14, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-J.; Liu, D.; Zheng, K.; Zhang, M.; Zhang, C. A Benziodoxole-Based Hypervalent Iodine(III) Compound Functioning as a Peptide Coupling Reagent. Front. Chem. 2020, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhdankin, V.V.; Arbit, R.M.; McSherry, M.; Mismash, B.; Young, V.G. Structure and chemistry of acetoxybenziodazole. Ac-id-catalyzed rearrangement of benziodazoles to 3-iminobenziodoxoles. J. Am. Chem. Soc. 1997, 119, 7408–7409. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Koposov, A.E.; Smart, J.T.; Tykwinski, R.R.; McDonald, R.; Morales-Izquierdo, A. Secondary Bonding-Directed Self-Assembly of Amino Acid Derived Benziodazoles: Synthesis and Structure of Novel Hypervalent Iodine Macrocycles. J. Am. Chem. Soc. 2001, 123, 4095–4096. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Arbit, R.M.; Lynch, B.; Kiprof, P.; Young, J.V.G. Structure and Chemistry of Hypervalent Iodine Heterocycles: Acid-Catalyzed Rearrangement of Benziodazol-3-ones to 3-Iminiumbenziodoxoles. J. Org. Chem. 1998, 63, 6590–6596. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Koposov, A.Y.; Su, L.S.; Boyarskikh, V.V.; Netzel, B.C.; Young, V.G. Synthesis and structure of amino ac-id-derived benziodazoles: New hypervalent iodine heterocycles. Org. Lett. 2003, 5, 1583–1586. [Google Scholar] [CrossRef]
- Hari, D.P.; Schouwey, L.; Barber, V.; Scopelliti, R.; Fadaei-Tirani, F.; Waser, J. Ethynylbenziodazolones (EBZ) as Electrophilic Al-kynylation Reagents for the Highly Enantioselective Copper-Catalyzed Oxyalkynylation of Diazo Compounds. Chem. Eur. J. 2019, 25, 9522–9528. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Zheng, K.; Zhang, C. Electrophilic Hypervalent Trifluoromethylthio-Iodine(III) Reagent. Org. Lett. 2020, 22, 2026–2031. [Google Scholar] [CrossRef]
- Ren, J.; Du, F.; Jia, M.; Hu, Z.; Chen, Z.; Zhang, C. Ring Expansion Fluorination of Unactivated Cyclopropanes Mediated by a New Monofluoroiodane(III) Reagent. Angew. Chem. Int. Ed. 2021, 60, 24171–24178. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, C. Rhenium-catalyzed dehydrogenative olefination of C(sp3)–H bonds with hypervalent iodine(iii) reagents. Org. Biomol. Chem. 2015, 13, 5880–5884. [Google Scholar] [CrossRef]
- Yoshimura, A.; Shea, M.T.; Makitalo, C.L.; Jarvi, M.E.; Rohde, G.T.; Saito, A.; Yusubov, M.S.; Zhdankin, V.V. Preparation, structure, and reactivity of bicyclic benziodazole: A new hypervalent iodine heterocycle. Beilstein J. Org. Chem. 2018, 14, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Brotzel, F.; Kempf, B.; Singer, T.; Zipse, H.; Mayr, H. Nucleophilicities and Carbon Basicities of Pyridines. Chem. Eur. J. 2006, 13, 336–345. [Google Scholar] [CrossRef]
- Ochiai, M.; Sueda, T.; Miyamoto, K.; Kiprof, P.; Zhdankin, V.V. trans influence on hypervalent bonding of aryl λ3-iodanes: Their stabilities and isodesmic reactions of benziodoxolones and benziodazolones. Angew. Chem. Int. Ed. 2006, 45, 8203–8206. [Google Scholar] [CrossRef]
- Qiu, F.-C.; Yang, W.-C.; Chang, Y.-Z.; Guan, B. Palladium-Catalyzed ortho -Halogenation of Tertiary Benzamides. Asian J. Org. Chem. 2017, 6, 1361–1364. [Google Scholar] [CrossRef]
- Sperger, C.A.; Fiksdahl, A. Gold-Catalyzed Tandem Cyclizations of 1,6-Diynes Triggered by Internal N- and O-Nucleophiles. J. Org. Chem. 2010, 75, 4542–4553. [Google Scholar] [CrossRef] [PubMed]
- Pacula, A.J.; Scianowski, J.; Aleksandrzak, K.B. Highly efficient synthesis and antioxidant capacity of N-substituted ben-zisoselenazol-3(2H)-ones. RSC Adv. 2014, 4, 48959–48962. [Google Scholar] [CrossRef]
- Yakura, T.; Fujiwara, T.; Yamada, A.; Nambu, H. 2-Iodo-N-isopropyl-5-methoxybenzamide as a highly reactive and environmen-tally benign catalyst for alcohol oxidation. Beilstein J. Org. Chem. 2018, 14, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.B.; Haken, J.K. Dispersion and selectivity indexes in gas chromatography. III. Alkyl, ω-chloroethyl and alkenyl benzoate and chlorobenzoate esters. J. Chromatogr. 1989, 462, 31–37. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Batra, S. Direct Transformation of Arylamines to Aryl Halides via Sodium Nitrite and N -Halosuccinimide. Chem. Eur. J. 2018, 24, 14622–14626. [Google Scholar] [CrossRef]
- Molander, G.A.; Cavalcanti, L.N. Metal-Free Chlorodeboronation of Organotrifluoroborates. J. Org. Chem. 2011, 76, 7195–7203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sarkar, S.; Grimme, S.; Studer, A. NHC Catalyzed Oxidations of Aldehydes to Esters: Chemoselective Acylation of Alcohols in Presence of Amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef]
- Wybon, C.C.D.; Mensch, C.; Hollanders, C.; Gadais, C.; Herrebout, W.A.; Ballet, S.; Maes, B.U.W. Zn-Catalyzed tert-Butyl Nic-otinate-Directed Amide Cleavage as a Biomimic of Metallo-Exopeptidase Activity. ACS Catal. 2018, 8, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Simeonov, S.; Simeonov, A.P.; Todorov, A.R.; Kurteva, V.B. Enantioresolution of a Series of Chiral Benzyl Alcohols by HPLC on a Dinitrobenzoylphenylglycine Stationary Phase after Achiral Pre-Column Derivatization. Am. J. Anal. Chem. 2010, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Huston, R.C.; Robinson, K.R. Chloro-substituted diphenylmethanes, phenyl benzyl ethers, and benzophenones prepared from o- or p-cresol. J. Am. Chem. Soc. 1951, 73, 2483–2486. [Google Scholar] [CrossRef]
- Muñiz, K.; García, B.; Martínez, C.; Piccinelli, A. Dioxoiodane Compounds as Versatile Sources for Iodine(I) Chemistry. Chem. Eur. J. 2016, 23, 1539–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanjari, R.; Guntreddi, T.; Singh, K.N. MnO2 Promoted Sequential C–O and C–N Bond Formation via C–H Activation of Methylarenes: A New Approach to Amides. Org. Lett. 2013, 15, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Park, E.; Park, J.; Kim, I.S. Tandem Rh(III)-Catalyzed Oxidative Acylation of Secondary Benzamides with Aldehydes and Intramolecular Cyclization: The Direct Synthesis of 3-Hydroxyisoindolin-1-ones. Org. Lett. 2012, 14, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Nandi, J.; Vaughan, M.Z.; Sandoval, A.L.; Paolillo, J.M.; Leadbeater, N.E. Oxidative Amidation of Amines in Tandem with Transamidation: A Route to Amides Using Visible-Light Energy. J. Org. Chem. 2020, 85, 9219–9229. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Jang, G.S.; Kim, R.H.; Jung, J.; Woo, S.K. Weak base-promoted selective rearrangement of oxaziridines to amides via visible-light photoredox catalysis. Chem. Commun. 2021, 57, 9995–9998. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).