Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach
Abstract
1. Introduction
2. Results and Discussions
2.1. Chemical Composition
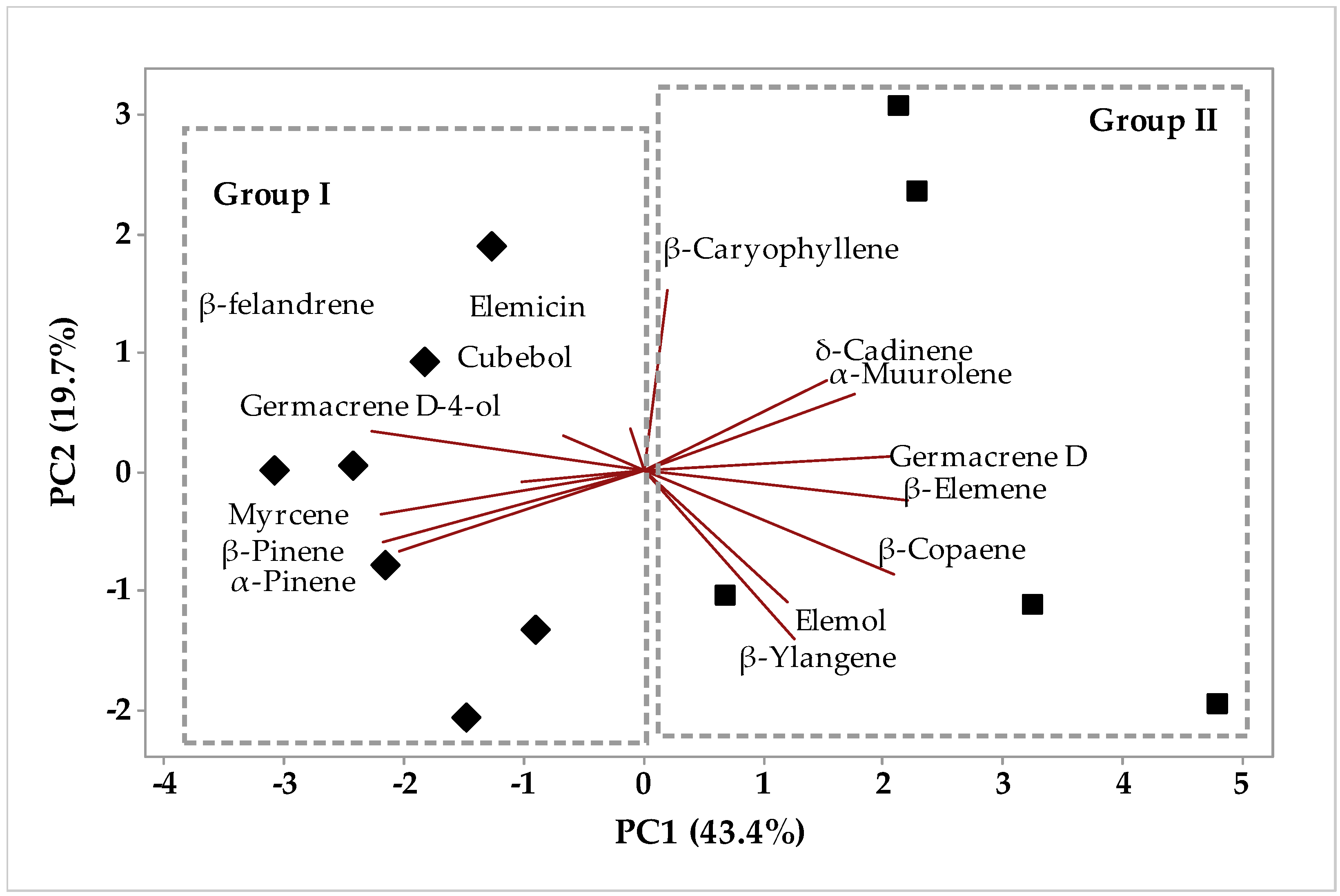
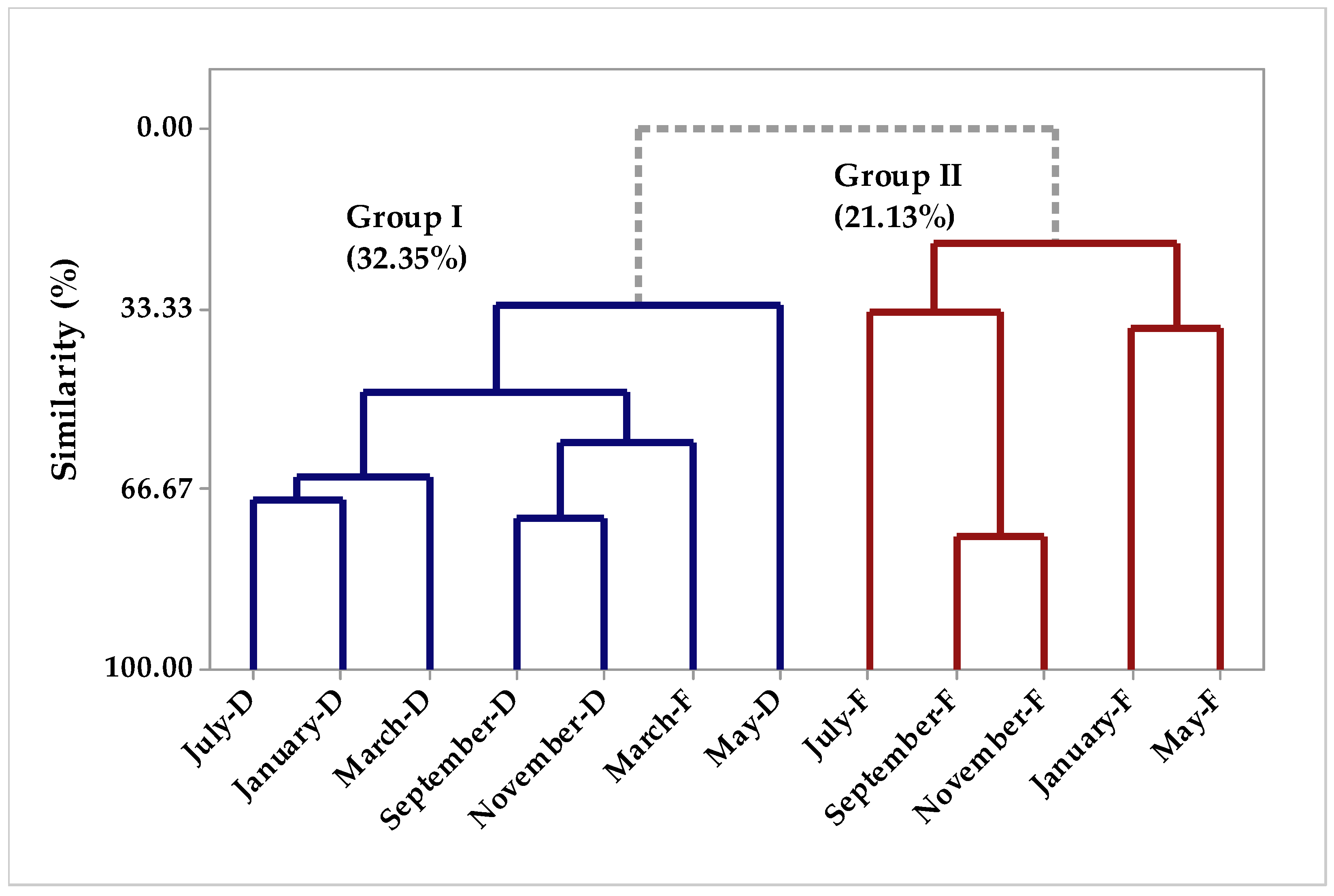
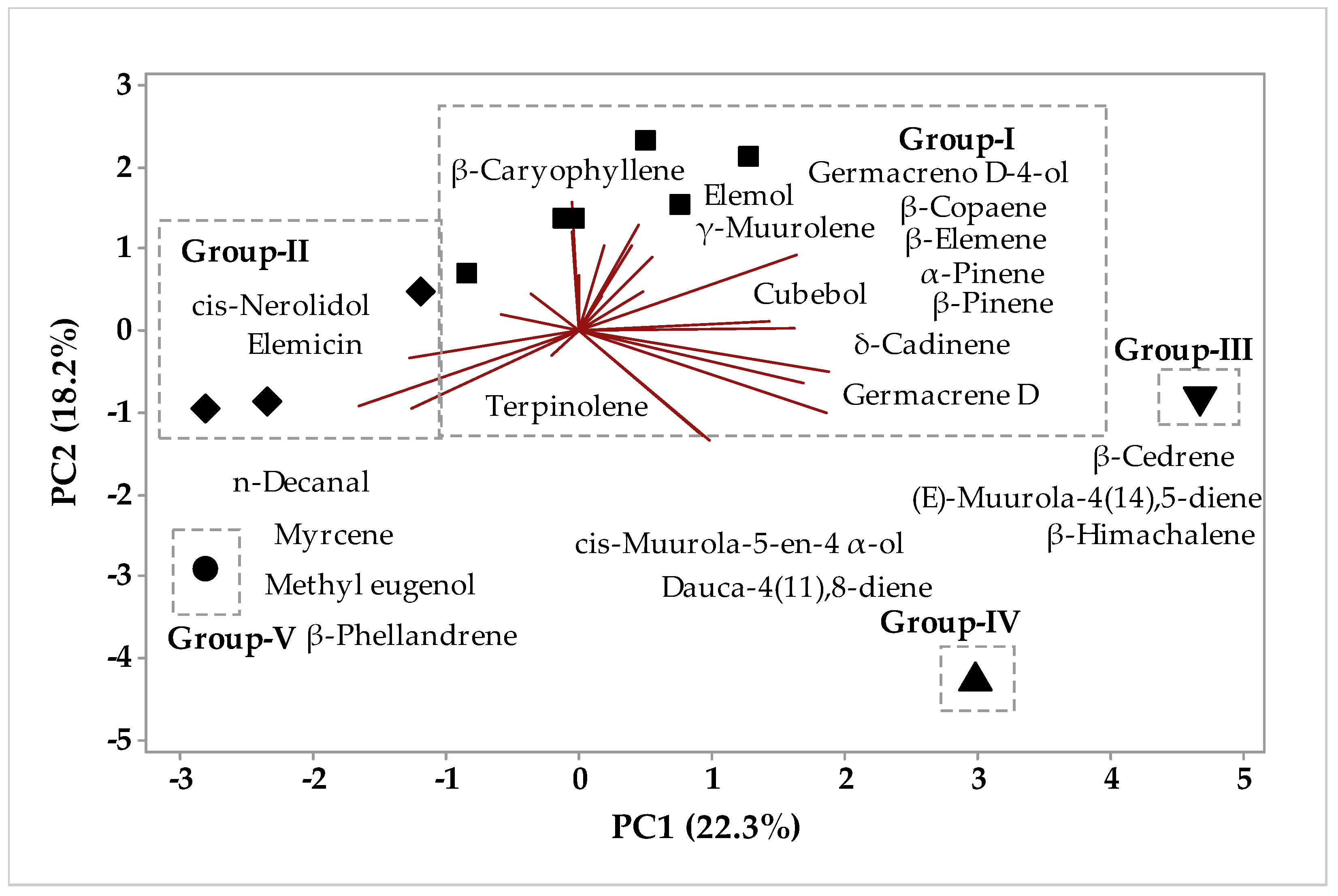
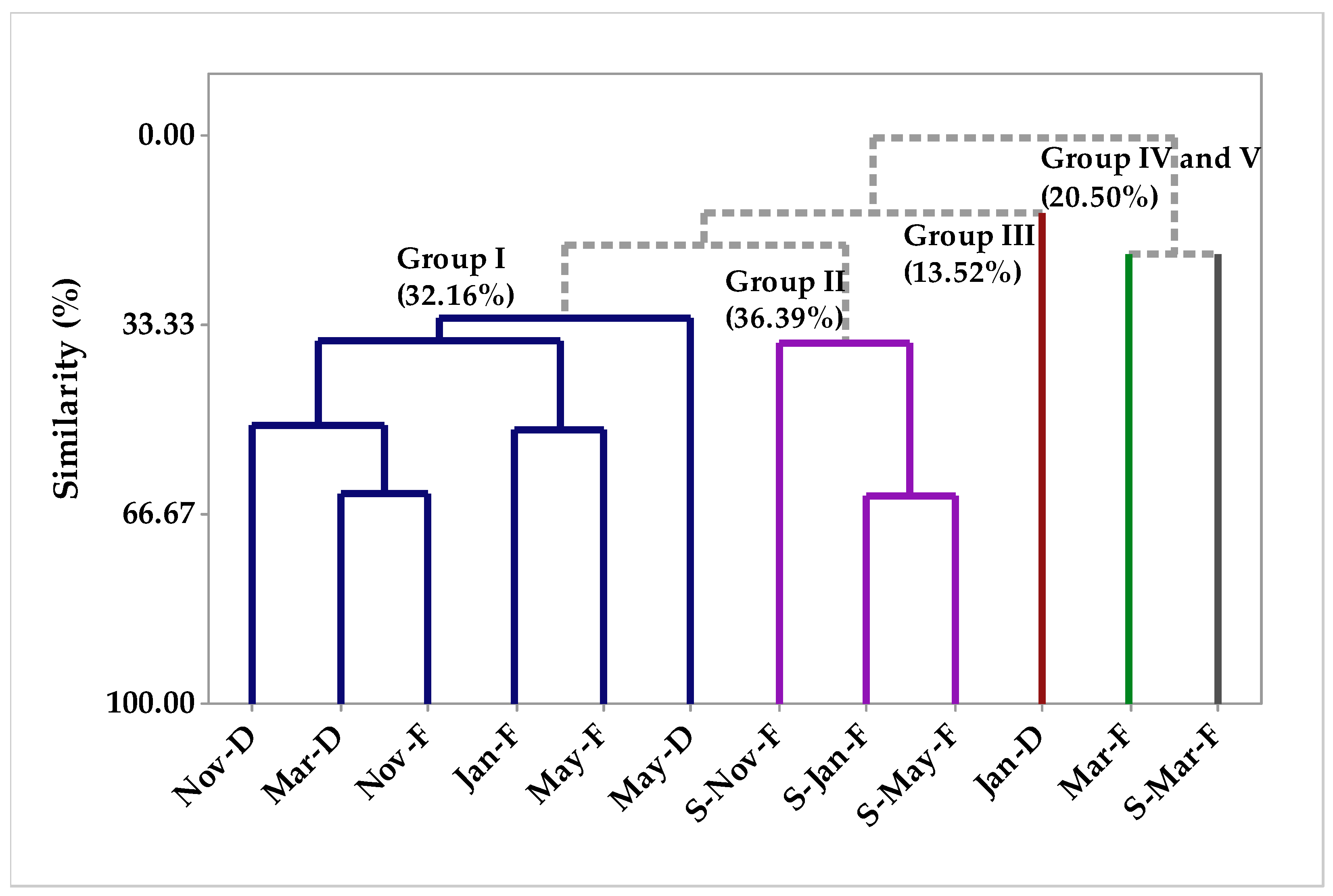
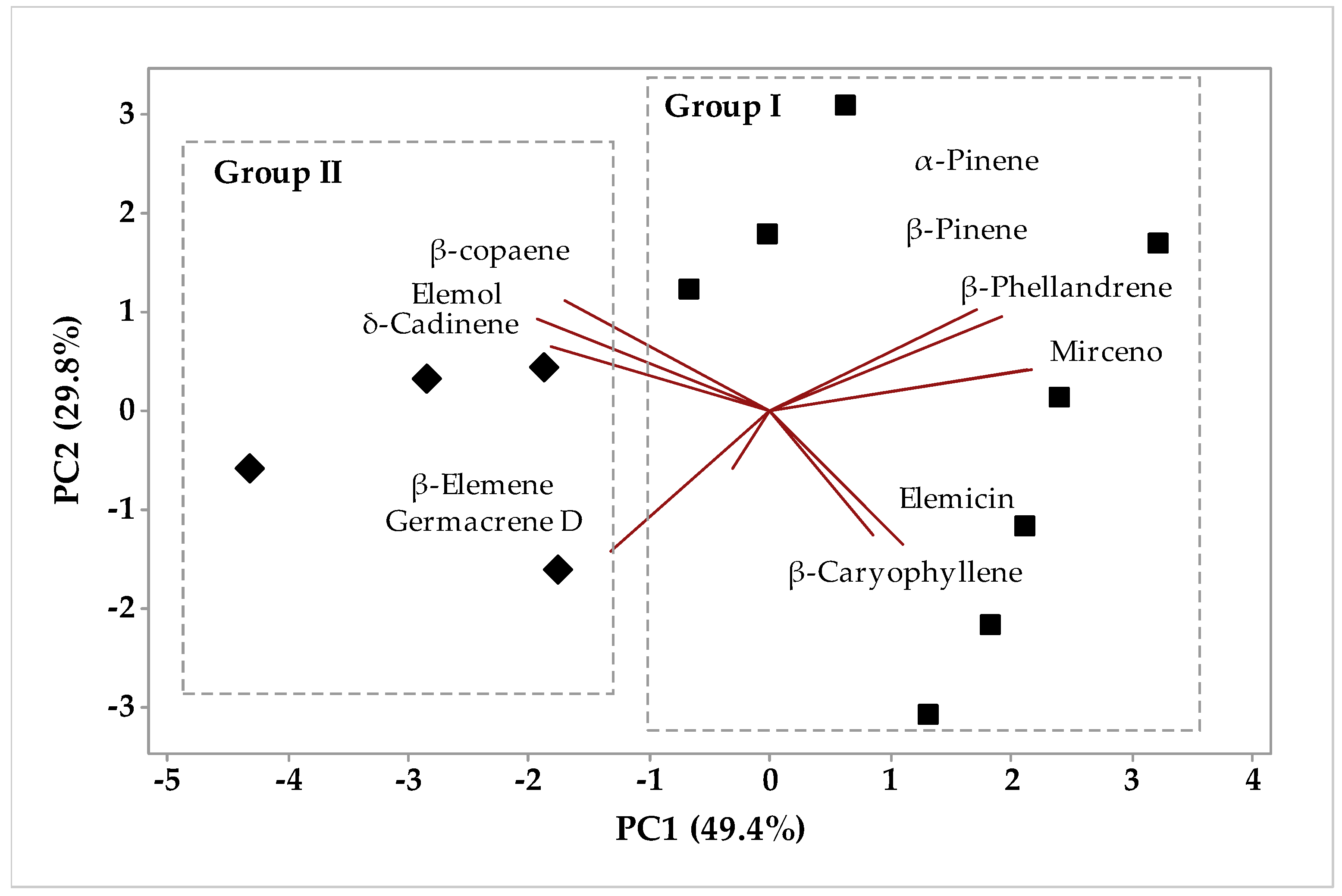
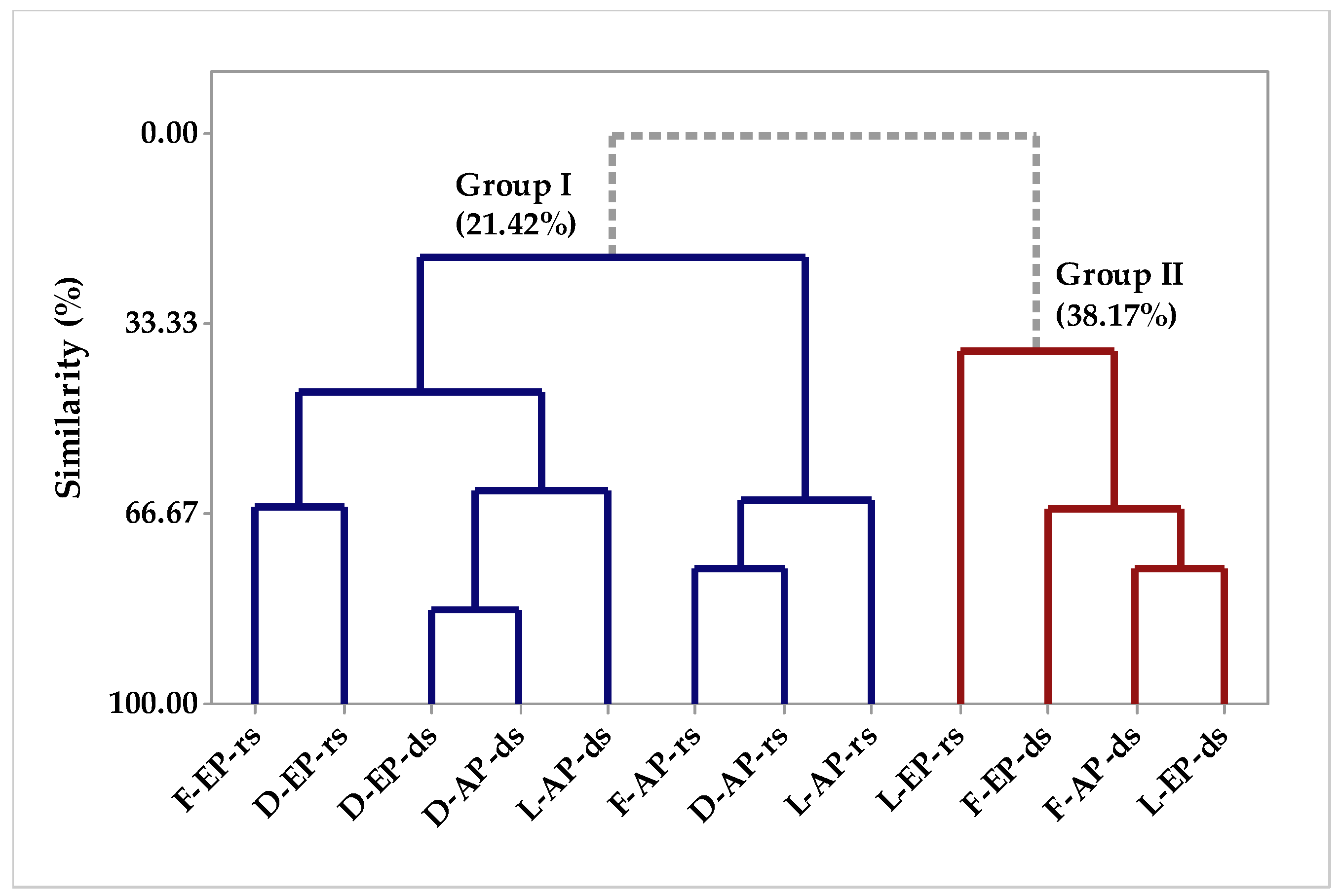
2.1.1. Multivariate Analysis
Chemical Composition of Seasonal Study
Multivariate Analysis of the Aroma Chemical Composition
Multivariate Analysis of the Circadian Study Chemical Composition
2.2. Cytotoxicity Bioassay in Artemia Salina
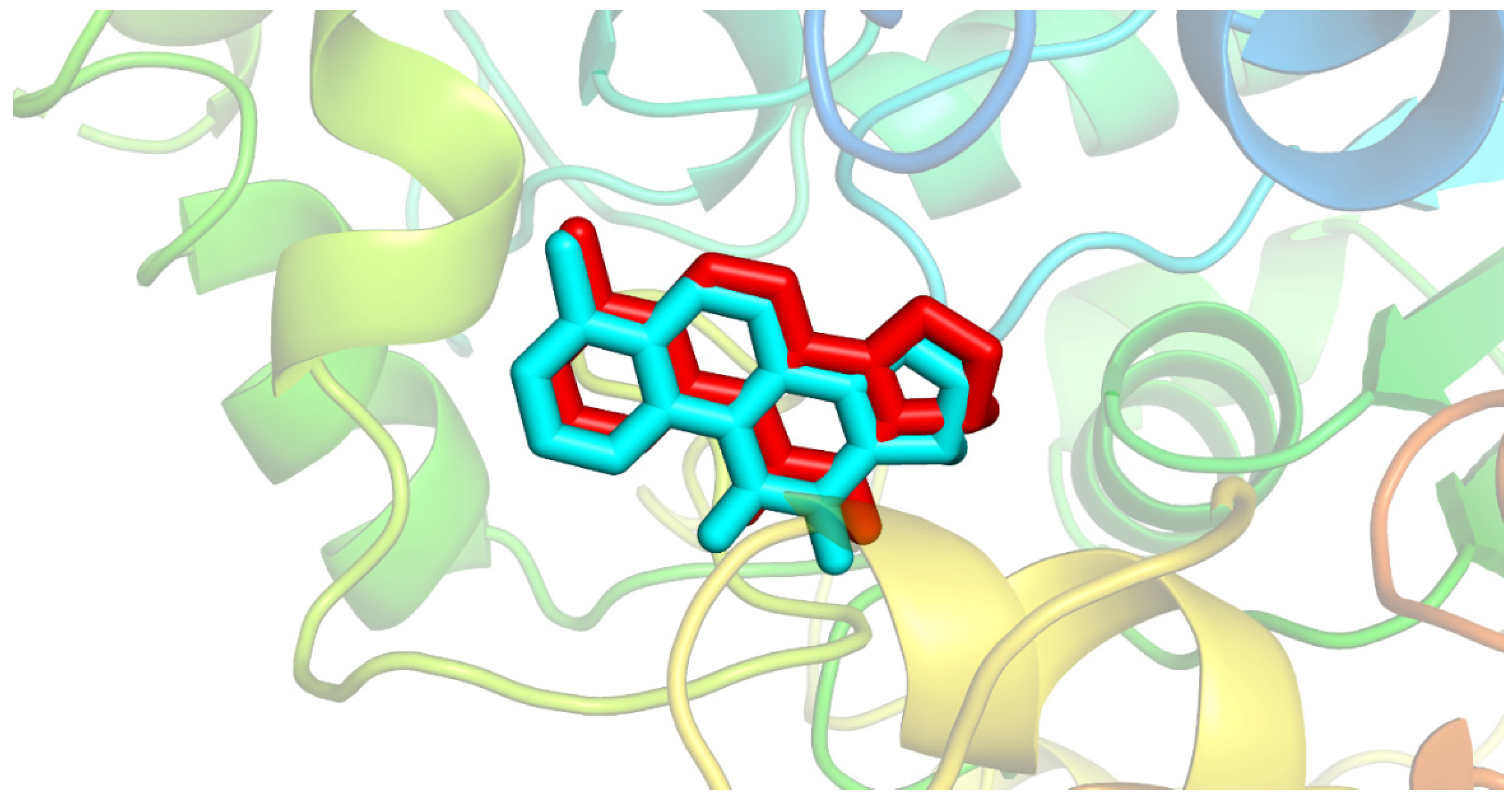
2.3. In Silico Evaluation of Interaction with AChE
3. Materials and Methods
3.1. Collection of Botanical Material for Seasonal and Circadian Study
3.2. Processing of Botanical Material
3.2.1. Extraction Methods
Hydrodistillation
Simultaneous Distillation–Extraction
3.3. Identification of Chemical Constituents
3.4. Preliminary Toxicity Bioassay with Artemia Salina Leach
3.5. Molecular Docking
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaramillo, M.A.; Manos, P.S.; Zimmer, E.A. Phylogenetic Relationships of the Perianthless Piperales: Reconstructing the Evolution of Floral Development. Int. J. Plant Sci. 2004, 165, 403–416. [Google Scholar] [CrossRef]
- Melo, A.; Guimarães, E.F.; Alves, M. Synopsis of the genus Peperomia Ruiz & Pav. (Piperaceae) in Roraima State, Brazil. Hoehnea 2016, 43, 119–134. [Google Scholar] [CrossRef][Green Version]
- Wanke, S.; Samain, M.-S.; Vanderschaeve, L.; Mathieu, G.; Goetghebeur, P.; Neinhuis, C. Phylogeny of the Genus Peperomia (Piperaceae) Inferred from the trnK/matK Region (cpDNA). Plant Biol. 2006, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Paz, R.F.; Guimarães, E.F.; Ramos, C.S. The occurrence of phenylpropanoids in the saps of six Piper species (Piperaceae) from Brazil. Gayana Bot. 2017, 74, 236–239. [Google Scholar] [CrossRef]
- Vergara-Rodrígue, D.; Mathieu, G.; Samain, M.S.; Armenta-Montero, S.; Krömer, T. Diversity, distribution, and conservation status of Peperomia (Piperaceae) in the state of Veracruz, Mexico. Trop. Conserv. Sci. 2017, 10, 1940082917702383. [Google Scholar] [CrossRef]
- Frenzke, L.; Scheiris, E.; Pino, G.; Symmank, L.; Goetghebeur, P.; Neinhuis, C.; Wanke, S.; Samain, M.S. A revised infrageneric classification of the genus Peperomia (Piperaceae). Taxon 2015, 64, 424–444. [Google Scholar] [CrossRef]
- Sarnaglia Junior, V.B.; De Lírio, E.J.; Freitas, J.; Guimarães, E.F. New records of Peperomia armondii Yunck, Peperomia hispidula (Sw.) A. Dietr., and Peperomia mandioccana Miq. for the state of Espírito Santo, southeastern Brazil. Check List 2015, 11, 1580. [Google Scholar] [CrossRef][Green Version]
- Carvalho-Silva, M.; Guimarães, E.F.; Sarnaglia, V.B. Two new species of Peperomia Ruiz & Pavon (Piperaceae) from southeastern Brazil and four new synonymies. Phytotaxa 2019, 422, 225–232. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Sauter, I.P.; Rossa, G.E.; Lucas, A.M.; Cibulski, S.P.; Roehe, P.M.; da Silva, L.A.A.; Rott, M.B.; Vargas, R.M.F.; Cassel, E.; von Poser, G.L. Chemical composition and amoebicidal activity of Piper hispidinervum (Piperaceae) essential oil. Ind. Crops Prod. 2012, 40, 292–295. [Google Scholar] [CrossRef]
- Da Silva, M.F.R.; Bezerra-Silva, P.C.; de Lira, C.S.; de Lima Albuquerque, B.N.; Agra Neto, A.C.; Pontual, E.V.; Maciel, J.R.; Paiva, P.M.G.; Navarro, D.M.d.A.F. Composition and biological activities of the essential oil of Piper corcovadensis (Miq.) C. DC (Piperaceae). Exp. Parasitol. 2016, 165, 64–70. [Google Scholar] [CrossRef]
- Oyemitan, I.A.; Olayera, O.A.; Alabi, A.; Abass, L.A.; Elusiyan, C.A.; Oyedeji, A.O.; Akanmu, M.A. Psychoneuropharmacological activities and chemical composition of essential oil of fresh fruits of Piper guineense (Piperaceae) in mice. J. Ethnopharmacol. 2015, 166, 240–249. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Rodrigues, F.C.; Pereira da Cruz, R.; da Silva, L.E.; do Amaral, W.; Andrade Rebelo, R.; Begnini, I.M.; Fonseca Bezerra, C.; Iriti, M.; Varoni, E.M.; et al. Antibiotic Potential and Chemical Composition of the Essential Oil of Piper caldense C. DC. (Piperaceae). Appl. Sci. 2020, 10, 631. [Google Scholar] [CrossRef]
- Andrés, M.F.; Rossa, G.E.; Cassel, E.; Vargas, R.M.F.; Santana, O.; Díaz, C.E.; González-Coloma, A. Biocidal effects of Piper hispidinervum (Piperaceae) essential oil and synergism among its main components. Food Chem. Toxicol. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Salleha, W.M.N.H.W.; Ahmada, F.; Yenb, K.H. Chemical compositions and antimicrobial activity of the essential oils of piper abbreviatum, P. Erecticaule and P. Lanatum (Piperaceae). Nat. Prod. Commun. 2014, 9, 1795–1798. [Google Scholar] [CrossRef]
- Turchen, L.M.; Piton, L.P.; Dall’Oglio, E.L.; Butnariu, A.R.; Pereira, M.J.B. Toxicity of Piper aduncum (Piperaceae) Essential Oil Against Euschistus heros (F.) (Hemiptera: Pentatomidae) and Non-Effect on Egg Parasitoids. Neotrop. Entomol. 2016, 45, 604–611. [Google Scholar] [CrossRef]
- Kpadonou Kpoviessi, B.G.H.; Ladekan, E.Y.; Kpoviessi, D.S.S.; Gbaguidi, F.; Yehouenou, B.; Quetin-Leclercq, J.; Figueredo, G.; Moudachirou, M.; Accrombessi, G.C. Chemical Variation of Essential Oil Constituents of Ocimum gratissimum L. from Benin, and Impact on Antimicrobial Properties and Toxicity against Artemia salinaLeach. Chem. Biodivers. 2012, 9, 139–150. [Google Scholar] [CrossRef]
- Oliva, M.D.L.M.; Gallucci, N.; Zygadlo, J.A.; Demo, M.S. Cytotoxic activity of Argentinean essential oils on Artemia salina. Pharm. Biol. 2007, 45, 259–262. [Google Scholar] [CrossRef]
- Soares, B.V.; Morais, S.M.; dos Santos Fontenelle, R.O.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.C.; Brito, E.S.; Neto, M.A.S.; Brito, E.H.S.; Cavalcante, C.S.P.; et al. Antifungal Activity, Toxicity and Chemical Composition of the Essential Oil of Coriandrum sativum L. Fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Lima, L.R.; Andrade, F.K.; Alves, D.R.; de Morais, S.M.; Vieira, R.S. Anti-acetylcholinesterase and toxicity against Artemia salina of chitosan microparticles loaded with essential oils of Cymbopogon flexuosus, Pelargonium x ssp and Copaifera officinalis. Int. J. Biol. Macromol. 2021, 167, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Lobato, R.C.L.; Tavares, A.C.C.; Souza, A.P.S.; Conceição, C.C.C.; Guimarães, E.F. Peperomia circinnata Link and Peperomia rotundifolia (L.) Kunth growing on different host-trees in Amazon: Volatiles and relationship with bryophytes. Biochem. Syst. Ecol. 2005, 33, 269–274. [Google Scholar] [CrossRef]
- Da Silva, M.H.L.; Zoghbi, M.D.G.B.; Andrade, E.H.A.; Maia, J.G.S. The essential oils ofPeperomia pellucida Kunth andP. circinnata Link var.circinnata. Flavour Fragr. J. 1999, 14, 312–314. [Google Scholar] [CrossRef]
- Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. The chemistry and biological activities of Peperomia pellucida (Piperaceae): A critical review. J. Ethnopharmacol. 2019, 232, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.G.; Silva, A.S.B.; Souza, G.E.P.; Figueiredo, J.G.; Cunha, F.Q.; Lahlou, S.; Da Silva, J.K.R.; Maia, J.G.S.; Sousa, P.J.C. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (Sw.) Loud. J. Ethnopharmacol. 2011, 138, 479–486. [Google Scholar] [CrossRef]
- Noriega Rivera, P.; Mosquera, T.; Baldisserotto, A.; Abad, J.; Aillon, C.; Cabezas, D.; Piedra, J.; Coronel, I.; Manfredini, S. Chemical Composition and in-vitro biological activities of the essential oil from leaves of Peperomia inaequalifolia Ruiz & Pav. Am. J. Essent. Oil Nat. Prod. 2015, 2, 29–31. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Chauhan, A. Essential oil composition of Peperomia pellucida (L.) Kunth from India. J. Essent. Oil Res. 2015, 27, 89–95. [Google Scholar] [CrossRef]
- Usman, L.A.; Ismaeel, R.O. Chemical Composition of Root Essential oil of Peperomia pellucida (L.) Kunth. Grown in Nigeria. J. Essent. Oil Bear. Plants 2020, 23, 628–632. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Figueredo, F.G.; Ferreira, J.V.A.; Coutinho, H.D.M.; Silva, J.M.F.L.; Ribeiro-Filho, J.; Costa, J.G.M. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Ribeiro, P.H.S.; dos Santos, M.L.; da Camara, C.A.G.; Born, F.S.; Fagg, C.W. Seasonal chemical compositions of the essential oils of two eugenia species and their acaricidal proPERTIES. Quim. Nova 2015, 39, 38–43. [Google Scholar] [CrossRef]
- Sarrazin, S.; da Silva, L.; de Assunção, A.; Oliveira, R.; Calao, V.; da Silva, R.; Stashenko, E.; Maia, J.; Mourão, R. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Hazarika, U.; Gosztola, B. Lyophilization and its effects on the essential oil content and composition of herbs and spices—A review. Acta Sci. Pol. Technol. Aliment. 2020, 19, 467–473. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Cabezudo, M.D. Effect of Drying Method on the Volatiles in Bay Leaf (Laurus nobilis L.). J. Agric. Food Chem. 2002, 50, 4520–4524. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, M.C.; González Viñas, M.A.; Cabezudo, M.D. Evaluation of the effect of drying on aroma of parsley by free choice profiling. Eur. Food Res. Technol. 2003, 216, 227–232. [Google Scholar] [CrossRef]
- Yousif, A.N.; Scaman, C.H.; Durance, T.D.; Girard, B. Flavor Volatiles and Physical Properties of Vacuum-Microwave- and Air-Dried Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 1999, 47, 4777–4781. [Google Scholar] [CrossRef]
- De Lira, P.N.B.; da Silva, J.K.R.; Andrade, E.H.A.; Sousa, P.J.C.; Silva, N.N.S.; Maia, J.G.S. Essential Oil Composition of Three Peperomia Species from the Amazon, Brazil. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Adams, R.P., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Ghasemi Pirbalouti, A.; Mahdad, E.; Craker, L. Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem. 2013, 141, 2440–2449. [Google Scholar] [CrossRef]
- Lagartoparra, A. Comparative study of the assay of and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 133, 110794. [Google Scholar] [CrossRef]
- Nguta, J.M.; Mbaria, J.M.; Gakuya, D.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Cytotoxicity of antimalarial plant extracts from Kenyan biodiversity to the brine shrimp, Artemia salina L. (Artemiidae). Drugs Ther. Stud. 2012, 2, 12. [Google Scholar] [CrossRef]
- Costa, W.K.; de Oliveira, J.R.S.; de Oliveira, A.M.; Santos, I.B.D.S.; da Cunha, R.X.; de Freitas, A.F.S.; da Silva, J.W.L.M.; Silva, V.B.G.; Aguiar, J.C.R.D.O.F.D.; da Silva, A.G.; et al. Essential oil from Eugenia stipitata McVaugh leaves has antinociceptive, anti-inflammatory and antipyretic activities without showing toxicity in mice. Ind. Crops Prod. 2020, 144, 112059. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; da Silva, M.A.P.; Rocha, M.I.; Boligon, A.A.; da Rocha, J.B.T.; Barros, L.M.; Kamdem, J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 437–442. [Google Scholar] [CrossRef]
- Aboaba, S.; Akande, A.; Flamini, G. Chemical Composition, Toxicity and Antibacterial activity of the Essential Oils of Garcinia mangostana from Nigeria. J. Essent. Oil Bear. Plants 2014, 17, 78–86. [Google Scholar] [CrossRef]
- Tavakoli, S.; Vatandoost, H.; Zeidabadinezhad, R.; Hajiaghaee, R.; Hadjiakhoondi, A.; Abai, M.R.; Yassa, N. Gas Chromatography, GC/Mass analysis and bioactivity of essential oil from aerial parts of Ferulago trifida: Antimicrobial, antioxidant, AChE inhibitory, general toxicity, MTT assay and larvicidal activities. J. Arthropod-Borne Dis. 2017, 11, 414–426. [Google Scholar]
- Cansian, R.L.; Vanin, A.B.; Orlando, T.; Piazza, S.P.; Puton, B.M.S.; Cardoso, R.I.; Gonçalves, I.L.; Honaiser, T.C.; Paroul, N.; Oliveira, D. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina. Braz. J. Biol. 2017, 77, 155–161. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity of some essential oil formulations against the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae). Crop Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- Betim, F.C.M.; de Oliveira, C.F.; de Souza, A.M.; Szabo, E.M.; Zanin, S.M.W.; Miguel, O.G.; Miguel, M.D.; Dias, J.D.F.G. Ocotea nutans (Nees) Mez (Lauraceae): Chemical composition, antioxidant capacity and biological properties of essential oil. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef]
- Retnowati, R.; Rahman, M.F.; Yulia, D. Chemical Constituents of the Essential Oils of White Turmeric (Curcuma zedoaria (Christm.) Roscoe) from Indonesia and its Toxicity toward Artemia salina Leach. J. Essent. Oil Bear. Plants 2014, 17, 393–396. [Google Scholar] [CrossRef]
- Radulović, N.S.; Genčić, M.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Stojiljković, N.I. Toxic essential oils. Part V: Behaviour modulating and toxic properties of thujones and thujone-containing essential oils of Salvia officinalis L., Artemisia absinthium L., Thuja occidentalis L. and Tanacetum vulgare L. Food Chem. Toxicol. 2017, 105, 355–369. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.S.; Da Cruz, J.N.; Da Costa, W.A.; Silva, S.G.; Brito, M.D.P.; De Menezes, S.A.F.; Neto, A.M.D.J.C.; Andrade, E.H.D.A.; Junior, R.N.D.C. Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules 2020, 25, 3852. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.L.G.; Cruz, J.N.; Sodré, D.F.; Correa-Barbosa, J.; Azonsivo, R.; de Oliveira, M.S.; de Sousa Siqueira, J.E.; da Rocha Galucio, N.C.; de Oliveira Bahia, M.; Burbano, R.M.R.; et al. Evaluation of the genotoxicity and mutagenicity of isoeleutherin and eleutherin isolated from Eleutherine plicata herb. using bioassays and in silico approaches. Arab. J. Chem. 2021, 14, 103084. [Google Scholar] [CrossRef]
- Souza da Silva Júnior, O.; de Jesus Pereira Franco, C.; Barbosa de Moraes, A.A.; Cruz, J.N.; Santana da Costa, K.; Diniz do Nascimento, L.; Helena de Aguiar Andrade, E. In silico analyses of toxicity of the major constituents of essential oils from two Ipomoea L. species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Silva, S.G.; Cruz, J.N.; de Oliveira, M.S.; Oliveira, J.; de Moraes, A.A.B.; da Costa, F.A.M.; da Costa, K.S.; Nascimento, L.D.D.; Andrade, E.H.D.A. First report on the Annona exsucca DC. Essential oil and in silico identification of potential biological targets of its major compounds. Nat. Prod. Res. 2021, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Santana de Oliveira, M.; Pereira da Silva, V.M.; Cantão Freitas, L.; Gomes Silva, S.; Nevez Cruz, J.; de Aguiar Andrade, E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021, 15, e2000982. [Google Scholar] [CrossRef]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of human acetylcholinesterase bound to dihydrotanshinone i and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef]
- Leão, R.P.; Cruz, J.V.; da Costa, G.V.; Cruz, J.N.; Ferreira, E.F.B.; Silva, R.C.; de Lima, L.R.; Borges, R.S.; dos Santos, G.B.; Santos, C.B.R. Identification of New Rofecoxib-Based Cyclooxygenase-2 Inhibitors: A Bioinformatics Approach. Pharmaceuticals 2020, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.H.F.; Ramos, R.S.; da Cruz, J.N.; Silva, S.G.; Ferreira, E.F.B.; de Lima, L.R.; Macêdo, W.J.C.; Espejo-Román, J.M.; Campos, J.M.; Santos, C.B.R. Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules 2020, 25, 4183. [Google Scholar] [CrossRef]
- Costa, E.; Silva, R.; Espejo-Román, J.; Neto, M.D.A.; Cruz, J.; Leite, F.; Silva, C.; Pinheiro, J.; Macêdo, W.; Santos, C. Chemometric methods in antimalarial drug design from 1,2,4,5-tetraoxanes analogues. SAR QSAR Environ. Res. 2020, 31, 677–695. [Google Scholar] [CrossRef]
- Mascarenhas, A.M.S.; de Almeida, R.B.M.; de Araujo Neto, M.F.; Mendes, G.O.; da Cruz, J.N.; dos Santos, C.B.R.; Botura, M.B.; Leite, F.H.A. Pharmacophore-based virtual screening and molecular docking to identify promising dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. J. Biomol. Struct. Dyn. 2020, 39, 6021–6030. [Google Scholar] [CrossRef]
- Ordentlich, A.; Barak, D.; Kronman, C.; Flashner, Y.; Leitner, M.; Segall, Y.; Ariel, N.; Cohen, S.; Velan, B.; Shafferman, A. Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J. Biol. Chem. 1993, 268, 17083–17095. [Google Scholar] [CrossRef]
- Xu, Y.; Colletier, J.P.; Weik, M.; Jiang, H.; Moult, J.; Silman, I.; Sussman, J.L. Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray versus molecular dynamics. Biophys. J. 2008, 95, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.O.; Da Cruz, J.N.; Franco, C.D.J.P.; Silva, S.G.; Da Costa, W.A.; De Oliveira, M.S.; Andrade, E.H.D.A. First report on yield and chemical composition of essential oil extracted from myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules 2020, 25, 783. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; Andrade, E.H.A.; da Silva, M.H.L. Aroma volatiles of pequi fruit (Caryocar brasiliense Camb.). J. Food Compos. Anal. 2008, 21, 574–576. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. The NIST mass spectral search program for the nist/epa/nih mass spectra library. In Standard Reference Data Program of the National Institute of Standards and Technology; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Silva, S.G.; Figueiredo, P.L.B.; Nascimento, L.D.; da Costa, W.A.; Maia, J.G.S.; Andrade, E.H.A. Planting and seasonal and circadian evaluation of a thymol-type oil from Lippia thymoides Mart. & Schauer. Chem. Cent. J. 2018, 12, 113. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis. J. Pharm. Sci. 1971, 60, 1432. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. DJ Gaussian 09; Revision E.01; Contacting Gaussian, Inc.: Wallingford, CT, USA, 2009; pp. 2–3. [Google Scholar]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.; Cruz, J.; Ferreira, O.; Pereira, D.; Pereira, N.; Oliveira, M.; Venturieri, G.; Guilhon, G.; Filho, A.S.; Andrade, E. Chemical Composition of Volatile Compounds in Apis mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef] [PubMed]

| July | September | November | January | March | May | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | F | D | F | D | F | D | F | D | F | D | F | |
| % EOs | 2.40 | 7.90 | 2.00 | 4.30 | 2.40 | 5.60 | 1.70 | 3.60 | 1.50 | 3.50 | 1.20 | 4.20 |
| March | November | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evening | Afternoon | Evening | Afternoon | |||||||||
| F | D | L | F | D | L | F | D | L | F | D | L | |
| % oil | 3.5 | 1.5 | 1.8 | 2.9 | 1.5 | 1.2 | 5.6 | 2.5 | 1.6 | 5.1 | 2.3 | 1.6 |
| Moisture | 92.4 | 22.0 | 43.5 | 90.9 | 30.6 | 49.2 | 91.1 | 37.4 | 45.0 | 88.9 | 12.7 | 33.6 |
| Constituents | RIL | RIC | Jul-D | Jul-F | Set-D | Set-F | Nov-D | Nov-F | Jan-D | Jan-F | Mar-D | Mar-F | May-D | May-F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-pinene | 932 | 932 | 2.9 | 1.4 | 3 | 0.5 | 2.5 | 0.8 | 1.7 | 1.1 | 2.4 | 3.5 | 0.7 | 0.2 |
| β-pinene | 974 | 978 | 3.2 | 2.2 | 3.3 | 0.8 | 2.9 | 1.2 | 2.3 | 1.6 | 3 | 3.2 | 1.4 | 1 |
| mycrene | 988 | 987 | 10.8 | 7 | 15.3 | 6.2 | 10.9 | 5.5 | 12.1 | 7.9 | 13.9 | 16.4 | 9.2 | 4.7 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 0.3 | 0.2 | 0.6 | 0.8 | 0.3 | 0.6 | 0.5 | 0.7 | 1.1 | 1.2 | 0.9 | |
| β-phellandrene | 1025 | 1029 | 21.5 | 14 | 16.5 | 4.3 | 20.6 | 8 | 16.1 | 11.1 | 23.3 | 28.1 | 24.9 | 16.5 |
| terpinolene | 1086 | 1083 | 1.2 | 1 | 1.4 | 1 | 1.8 | 1 | 1.4 | 1.4 | 1.1 | 2.5 | 2.1 | 1 |
| n-decanal | 1201 | 1205 | 1.2 | 1.8 | 1.7 | 2 | 0.7 | 1.4 | 2.2 | 0.9 | 1.7 | 0.7 | ||
| α-ylangene | 1373 | 1366 | 0.4 | 1.9 | 0.3 | 0.4 | 0.2 | 0.4 | 0.2 | 0.2 | 0.5 | 0.7 | 0.5 | 0.8 |
| α-copaene | 1374 | 1373 | 1.6 | 1.5 | 2.8 | 1.3 | 2.3 | 1.5 | 1.9 | 1.6 | 2 | 1.2 | 2.7 | |
| β-elemene | 1389 | 1387 | 5.9 | 7.3 | 6 | 10 | 4.3 | 8.3 | 5.1 | 6.4 | 5.9 | 2.7 | 5.9 | 7.7 |
| methyl eugenol | 1403 | 1401 | 0.2 | 0.3 | 0.3 | 0.4 | 0.1 | |||||||
| dodecanal | 1408 | 1409 | 0.7 | 1.2 | 0.7 | 1.3 | 0.4 | 0.9 | 0.7 | 1.3 | 0.5 | 0.6 | 0.6 | 0.7 |
| β-ylangene | 1419 | 1417 | 1.5 | 2 | 1.5 | 3 | 1.8 | 2 | ||||||
| β-caryophyllene | 1417 | 1418 | 1 | 0.6 | 1 | 1 | 1 | 1.6 | 2.3 | 3.1 | 2.2 | 2.2 | 2.5 | 4.1 |
| β-cedrene | 1419 | 1420 | 0.6 | 0.4 | 0.3 | 0.2 | 0.4 | 0.5 | 0.3 | 0.5 | 0.8 | 0.5 | 1.1 | |
| β-copaene | 1430 | 1426 | 1.8 | 2.6 | 2.3 | 3.7 | 2.3 | 3.2 | 1.7 | 2.2 | 1.6 | 2.1 | 1.4 | 2.3 |
| α-neo-clovene | 1452 | 1447 | 0.6 | 0.7 | 1.1 | 1 | 1 | 0.5 | 1.3 | 0.6 | 0.6 | 0.5 | 1.1 | |
| α-humulene | 1452 | 1451 | 0.6 | 0.4 | 0.5 | 0.8 | 0.6 | 0.9 | 0.5 | 0.7 | 0.5 | 0.3 | 0.6 | 1 |
| Alloaromadendrene | 1458 | 1455 | 0.4 | 0.8 | 0.5 | 0.8 | 0.5 | 0.7 | 0.5 | 0.5 | 1.1 | 0.5 | 0.4 | 0.8 |
| E-β-farnesene | 1454 | 1464 | 0.6 | 0.3 | 0.2 | 0.1 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 1 | |
| γ-muurolene | 1478 | 1471 | 1 | 1.1 | 1.4 | 1.5 | 0.8 | 1.6 | 1.3 | 0.8 | 1.4 | 1.5 | 1.4 | 2.4 |
| germacrene D | 1484 | 1478 | 5.8 | 7.3 | 6.8 | 11.3 | 6.8 | 8.8 | 5.8 | 7.8 | 6.6 | 6.2 | 5.3 | 13 |
| trans-muurola-4 (14),5-diene | 1493 | 1487 | 0.4 | 0.5 | 0.6 | 0.9 | 0.7 | 1.5 | 0.6 | 0.5 | 0.6 | 0.5 | 0.9 | |
| epi-cubebol | 1493 | 1492 | 1 | 1 | 1.2 | 1.2 | 1.1 | 1.2 | 1.4 | 1.8 | 0.8 | 1.5 | 1.8 | |
| α-muurolene | 1500 | 1495 | 1 | 1.3 | 1.2 | 2.5 | 1.4 | 2.2 | 1.1 | 4.1 | 1.2 | 1.4 | 1.1 | 1.9 |
| cubebol | 1514 | 1512 | 5.1 | 0.4 | 4.6 | 3.7 | 3.7 | 4 | 6.4 | 7.5 | 2.8 | 5.6 | 4.9 | |
| δ-cadinene | 1522 | 1515 | 2.3 | 1.8 | 2.3 | 5.7 | 5.2 | 5.5 | 2.1 | 9.4 | 2.1 | 3.6 | 5 | 4.6 |
| zonarene | 1528 | 1518 | 0.4 | 1.3 | 0.3 | |||||||||
| cis-nerolidol | 1531 | 1521 | 0.6 | 0.3 | 1.7 | 0.8 | ||||||||
| elemol | 1548 | 1542 | 0.1 | 2.9 | 10 | 15.1 | 11.2 | 15 | 0.5 | 6 | 4.6 | 4.4 | 4.2 | |
| elemicin | 1555 | 1547 | 13.9 | 22 | 1.1 | 18.3 | 18.1 | |||||||
| germacrene D-4-ol | 1574 | 1573 | 0.6 | 0.7 | 0.4 | 0.2 | 1.4 | 0.2 | 0.4 | 0.1 | 0.6 | 3.9 | ||
| junenol | 1618 | 1603 | 0.7 | 0.7 | 0.7 | 0.9 | 0.6 | 1.2 | 0.8 | 1 | 0.9 | 0.6 | 0.5 | 1.1 |
| 1.10-di-epi-cubenol | 1618 | 1623 | 0.4 | 0.4 | 0.7 | 1.3 | 0.7 | 1.6 | 0.8 | 1.4 | 0.7 | 0.8 | 0.5 | 1.1 |
| epi-α-cadinol | 1638 | 1633 | 0.1 | 0.6 | 0.9 | 0.9 | 0.4 | 0.6 | ||||||
| epi-α-muurolol | 1640 | 1639 | 0.6 | 0.5 | 0.8 | 1.8 | 0.6 | 1.9 | 0.7 | 0.7 | 1 | 0.8 | 0.7 | |
| α-muurolol | 1644 | 1642 | 0.4 | 0.6 | 0.7 | 1 | 0.7 | 1 | 0.2 | 0.8 | 0.5 | 0.5 | ||
| α-cadinol | 1652 | 1651 | 0.8 | 0.7 | 1.2 | 1.5 | 2 | 1.9 | 1.5 | 2 | 0.8 | 0.4 | 1.9 | 1 |
| Monoterpene hydrocarbons | 39.9 | 25.8 | 40.1 | 12.8 | 39.5 | 16.8 | 34.2 | 23.6 | 44.4 | 54.8 | 39.5 | 24.3 | ||
| Oxygenated monoterpenes | 1.2 | 1.8 | 1.7 | 2 | 0.7 | 1.4 | 2.2 | 0.9 | 1.7 | 0.7 | ||||
| Sesquiterpenes Hydrocarbons | 26.1 | 28.6 | 27.1 | 45.6 | 30.2 | 41.6 | 24.1 | 39.1 | 26.3 | 25.9 | 28.5 | 45.7 | ||
| Oxygenated sesquiterpenes | 9.7 | 7.9 | 20.4 | 26.7 | 22.6 | 28 | 13.6 | 6.9 | 18.3 | 11.5 | 20 | 15.4 | ||
| Phenylpropanoids | 13.9 | 22 | 1.1 | 18.5 | 18.4 | 0.3 | 0.4 | 0.1 | ||||||
| Others | 0.7 | 1.2 | 0.7 | 1.3 | 0.4 | 0.9 | 0.7 | 1.3 | 0.5 | 0.6 | 0.6 | 0.7 | ||
| Totals | 91.5 | 87.3 | 91.1 | 88.4 | 93.4 | 87.3 | 92.5 | 91.5 | 90.7 | 93.2 | 90.4 | 86.8 |
| Whole Dry Plant | Whole Fresh Plant | Fresh Spike | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituents | RIL | RIC | Nov-D | Jan-D | Mar-D | May-D | Nov-F | Jan-F | Mar-F | May-F | Nov-F | S-Jan-F | S-Mar-F | S-May-F |
| α-pinene | 932 | 932 | 5.2 | 3.9 | 5.2 | 1.6 | 4.8 | 3 | 5.2 | 1.3 | 3.3 | 1.4 | 1.6 | 1.3 |
| β-pinene | 974 | 978 | 4.3 | 3 | 4.3 | 1.8 | 2.1 | 4.1 | 1.9 | 0.7 | 0.5 | 1 | 0.4 | |
| mycrene | 988 | 987 | 15.4 | 9.5 | 13.8 | 11.8 | 20 | 13.5 | 16.9 | 8.3 | 20.3 | 22.9 | 31.7 | 12 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 1.3 | 1.2 | 2.1 | 1.6 | 1.3 | 0.7 | 1.4 | 1.5 | 0.4 | 0.6 | 1.1 | |
| β-felandrene | 1025 | 1029 | 17.2 | 8.6 | 13.2 | 19.1 | 19 | 16.6 | 18.7 | 14 | 11.5 | 7.3 | 24.4 | 11.2 |
| terpinolene | 1086 | 1083 | 2.4 | 2 | 2.1 | 3.1 | 1.9 | 1.9 | 2.6 | 2 | 4.2 | 2.1 | 0.8 | 2.6 |
| Octen-3-yl acetate | 1110 | 1106 | 0.6 | 1.1 | 0.8 | 1.4 | 0.3 | 0.3 | 0.8 | 0.8 | 0.5 | 1.1 | 0.6 | |
| n-decanal | 1201 | 1205 | 0.7 | 2 | 1.7 | 2.3 | 0.7 | 2.4 | 1.1 | 1 | 4.4 | 6.5 | 1.6 | 4.5 |
| α-copaene | 1374 | 1373 | 0.8 | 1.5 | 1.5 | 0.7 | 1.4 | 1.1 | 1.4 | 1.8 | 0.4 | 0.6 | 0.1 | 0.3 |
| β-bourbonene | 1387 | 1379 | 0.3 | 1 | 0.4 | 0.3 | 0.4 | 0.4 | 0.3 | 0.6 | 0.3 | 0.5 | 0.1 | 0.2 |
| β-elemene | 1389 | 1387 | 2.9 | 5.6 | 4.9 | 4.1 | 3.6 | 4.5 | 2.5 | 5.9 | 3.6 | 7.6 | 1.3 | 4.4 |
| methyl eugenol | 1403 | 1399 | 0.1 | 0.3 | 0.1 | 0.2 | 5.4 | 28.8 | 27.4 | 31.6 | ||||
| dodecanal | 1408 | 1409 | 0.4 | 0.9 | 0.8 | 0.4 | 0.5 | 1.2 | 0.1 | 0.7 | 1.2 | 1.8 | 0.2 | 0.9 |
| β-caryophyllene | 1417 | 1415 | 2 | 2.5 | 1.6 | 2.1 | 2.3 | 0.2 | 3.6 | 1.1 | 0.2 | 0.7 | ||
| β-cedrene | 1419 | 1419 | 0.2 | 5.2 | 0.5 | 0.3 | 0.4 | 0.4 | 1.9 | 0.8 | ||||
| β-copaene | 1430 | 1426 | 2.2 | 3.6 | 1.3 | 0.8 | 1.8 | 1.2 | 0.7 | 2.4 | 1.4 | 0.6 | 0.1 | 0.6 |
| α-neo-clovene | 1452 | 1447 | 0.8 | 0.6 | 0.8 | 1 | 0.3 | 1.5 | 0.2 | 0.1 | ||||
| α-humulene | 1452 | 1450 | 0.5 | 1.4 | 1 | 0.4 | 0.4 | 0.5 | 0.8 | 0.2 | 0.1 | 0.1 | ||
| γ-muurolene | 1478 | 1474 | 5 | 1 | 1.3 | 0.6 | 0.4 | 1.9 | 0.3 | 0.1 | 0.2 | |||
| trans-4,10-epoxy-amorphane | 1478 | 1473 | 0.5 | 1.8 | 0.1 | 0.1 | 0.1 | |||||||
| germacrene D | 1484 | 1481 | 0.4 | 4.9 | 3.5 | 5.7 | 5 | 1.7 | 8.6 | 2 | 1.5 | 0.2 | 1.2 | |
| trans-muurola-4 (14),5-diene | 1493 | 1489 | 6.5 | 0.4 | 0.4 | 0.6 | 0.6 | 5.4 | 0.9 | 0.2 | 0.1 | 0.3 | ||
| epi-cubebol | 1493 | 1494 | 1 | 0.6 | 2.5 | 1 | 1.6 | 0.4 | 1.8 | 0.5 | 0.7 | 0.3 | ||
| α-muurolene | 1500 | 1496 | 0.1 | 0.8 | 0.7 | 1 | 1.5 | 1.5 | 0.5 | 0.4 | 0.1 | 0.4 | ||
| trans-β-guaiene | 1502 | 1501 | 0.2 | 1.6 | 0.3 | |||||||||
| β-himachalene | 1500 | 1500 | 3.4 | 0.1 | 1 | 0.1 | 0.1 | |||||||
| cubebol | 1514 | 1518 | 8 | 0.9 | 10.6 | 10.7 | 2.9 | 2.6 | 1.4 | 1.3 | ||||
| δ-cadinene | 1522 | 1515 | 8 | 15.7 | 0.3 | 6.8 | 2.8 | 0.8 | 0.6 | 3.4 | ||||
| zonarene | 1528 | 1518 | 0.2 | 0.4 | 0.1 | 1.5 | ||||||||
| cis-nerolidol | 1531 | 1521 | 3 | 5.4 | 9.3 | 0.1 | ||||||||
| dauca-4 (11),8-diene | 1530 | 1524 | 0.2 | 0.2 | 9.2 | 0.7 | 0.1 | 0.2 | ||||||
| elemol | 1548 | 1547 | 9.3 | 8.4 | 6.2 | 5.4 | 7.6 | 3.2 | 0.2 | 5 | 12.7 | 3.7 | 0.3 | 4.5 |
| elemicin | 1555 | 1555 | 12.7 | 2.4 | 7 | |||||||||
| cis-muurol-5-en-4 α-ol | 1559 | 1572 | 0.4 | 7.7 | 0.7 | 0.2 | 0.1 | 0.2 | ||||||
| germacrene D-4-ol | 1574 | 1575 | 3 | 2.5 | 2.2 | 7.8 | 0.7 | 0.9 | 0.6 | 1.4 | 0.1 | 0.5 | ||
| junenol | 1618 | 1603 | 0.4 | 1.4 | 1.4 | 0.6 | 0.9 | 0.7 | 0.2 | 1.1 | 0.1 | 0.2 | 0.2 | |
| α-cadinol | 1652 | 1651 | 1.2 | 1 | 0.5 | 1 | 1 | 1.5 | 0.6 | 1.8 | 2.9 | 1.3 | 1.6 | |
| Monoterpene hydrocarbons | 45.8 | 28.2 | 40.7 | 39 | 47 | 37.8 | 48.9 | 29 | 40.4 | 34.8 | 60.6 | 27.5 | ||
| Oxygenated monoterpenes | 1.3 | 3.1 | 2.5 | 3.7 | 1 | 2.7 | 1.1 | 1.8 | 5.2 | 7 | 2.7 | 5.1 | ||
| Sesquiterpenes Hydrocarbons | 15.2 | 36.2 | 18.6 | 30.1 | 19.5 | 19.4 | 27.1 | 38.5 | 11.4 | 14 | 3 | 13.6 | ||
| Oxygenated sesquiterpenes | 26.4 | 15.2 | 25.2 | 15.8 | 22.5 | 11.7 | 9.1 | 13.9 | 26.8 | 8.9 | 2.4 | 8.4 | ||
| Phenylpropanoids | 0.1 | 0.3 | 12.8 | 0 | 2.6 | 5.4 | 28.8 | 27.4 | 38.6 | |||||
| Others | 0.4 | 0.9 | 0.8 | 0.4 | 0.5 | 1.2 | 0.1 | 0.7 | 1.2 | 1.8 | 0.2 | 0.9 | ||
| Totals | 89.1 | 83.6 | 87.8 | 89.1 | 90.8 | 85.6 | 86.3 | 86.5 | 90.4 | 95.3 | 96.3 | 94.1 | ||
| Constituents | RIL | RIC | FEP-rs | FAP-rs | D-EP-rs | D-AP-rs | L-EP-rs | L-AP-rs | FEP-ds | FAP-ds | D-EP-ds | D-AP-ds | L-EP-ds | L-AP-ds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-pinene | 932 | 932 | 3.5 | 1.8 | 2.4 | 2.4 | 1.1 | 1.8 | 0.8 | 1.1 | 2.5 | 2.1 | 1.7 | 2.7 |
| β-pinene | 974 | 978 | 3.2 | 2.1 | 3 | 2.7 | 1.3 | 2.6 | 1.2 | 1.9 | 2.9 | 2.5 | 2.2 | 3.7 |
| myrcene | 988 | 987 | 16.4 | 13.8 | 13.9 | 11.4 | 6.7 | 9.4 | 5.5 | 6.3 | 10.9 | 9.8 | 9 | 11.3 |
| ρ-mentha-1 (7),8-diene | 1003 | 1006 | 1.1 | 1 | 0.7 | 1 | 0.5 | 0.8 | 0.3 | 0.61 | 0.8 | 0.8 | 0.6 | 1.2 |
| β-phellandrene | 1025 | 1029 | 28.1 | 24.6 | 23.3 | 23.8 | 15.6 | 18.3 | 8 | 15.6 | 20.6 | 18 | 12.6 | 24 |
| terpinolene | 1086 | 1083 | 2.5 | 1.1 | 1.1 | 1.6 | 1.2 | 1.5 | 1 | 1.4 | 1.8 | 1.5 | 1.2 | 1.7 |
| n-decanal | 1201 | 1205 | 1.3 | 1.3 | 0.9 | 0.9 | 1.1 | 1.7 | 0.6 | 0.7 | 0.6 | 0.8 | 0.6 | |
| α-copaene | 1374 | 1373 | 2 | 2 | 1.6 | 1.7 | 2.3 | 2 | 2.3 | 1.7 | 1.3 | 1.6 | 2 | 1.2 |
| β-elemene | 1389 | 1387 | 2.7 | 6.3 | 5.9 | 6 | 7.1 | 8.3 | 5.2 | 4.3 | 5 | 6.3 | 3.2 | |
| methyl eugenol | 1403 | 1399 | 0.4 | 0.1 | 0.3 | 0.4 | 0.2 | |||||||
| dodecanal | 1408 | 1409 | 0.6 | 0.7 | 0.5 | 0.5 | 0.7 | 1 | 0.9 | 0.4 | 0.4 | 0.3 | 0.5 | 0.4 |
| β-caryophyllene | 1417 | 1415 | 2.2 | 2.9 | 2.2 | 2.5 | 3.2 | 2.9 | 1 | 1.2 | 0.8 | 0.8 | 1 | 0.8 |
| β-ylangene | 1419 | 1419 | 2.6 | 2 | 2 | 2 | 2.3 | 1.8 | ||||||
| β-copaene | 1430 | 1426 | 2.1 | 1.2 | 1.6 | 1.5 | 2.3 | 1.4 | 3.2 | 2.8 | 2.3 | 2.4 | 2.7 | 3.2 |
| α-neo-clovene | 1452 | 1447 | 0.6 | 1.1 | 0.6 | 0.6 | 0.9 | 0.8 | 1 | 0.9 | 1 | 1.1 | 0.9 | 1 |
| alloaromadendrene | 1458 | 1456 | 0.5 | 0.8 | 1.1 | 0.6 | 0.7 | 1.2 | 0.7 | 0.6 | 0.5 | 0.5 | 0.6 | 1 |
| γ-muurolene | 1478 | 1474 | 1.5 | 2 | 1.4 | 1.4 | 2 | 1.5 | 1.6 | 1.1 | 0.8 | 0.9 | 1.2 | 0.8 |
| germacrene D | 1484 | 1481 | 6.2 | 8.4 | 6.6 | 7.3 | 9.1 | 8.4 | 8.8 | 8 | 6.8 | 7 | 7.9 | 5.9 |
| (E)-muurola-4 (14),5-diene | 1493 | 1489 | 0.6 | 0.6 | 0.5 | 0.7 | 0.7 | 0.7 | 1.5 | 0.9 | 0.7 | 0.7 | 0.9 | 0.4 |
| epi-Cubebol | 1493 | 1494 | 0.8 | 1.5 | 1.8 | 1.8 | 2.2 | 2 | 1.2 | 1.1 | 1.1 | 1.4 | 1.4 | 1.1 |
| α-muurolene | 1500 | 1496 | 1.4 | 1.6 | 1.2 | 1.5 | 2 | 1.8 | 2.3 | 1.7 | 1.4 | 1.5 | 1.9 | 1.2 |
| δ-cadinene | 1522 | 1515 | 3.6 | 3.5 | 2.1 | 3.3 | 4.6 | 3.6 | 5.5 | 6.2 | 5.2 | 4.3 | 4.7 | 5.4 |
| cubebol | 1514 | 1518 | 2.8 | 4.5 | 7.5 | 6.5 | 6.5 | 6.7 | 4 | 3.5 | 3.7 | 5.4 | 3.9 | 1.8 |
| cis-nerolidol | 1531 | 1521 | 0.4 | 0.8 | 1.5 | 1.7 | 1 | 0.4 | 1.2 | |||||
| elemol | 1548 | 1548 | 4.6 | 1 | 6 | 2.4 | 8.8 | 0.5 | 15 | 13.2 | 11.2 | 13.3 | 12.7 | 8.8 |
| elemicin | 1555 | 1555 | 2.5 | 4 | 8 | |||||||||
| germacrene D-4-ol | 1574 | 1575 | 0.6 | 0.4 | 0.4 | 0.4 | 0.3 | 1 | 1.4 | 0.9 | 0.3 | 0.9 | ||
| junenol | 1618 | 1603 | 0.6 | 1 | 0.9 | 1.1 | 1.5 | 1.4 | 1.2 | 0.6 | 0.7 | 1 | 0.6 | |
| 1.10-di-epi-Cubenol | 1618 | 1623 | 0.8 | 0.9 | 0.7 | 0.9 | 1.4 | 1 | 1.6 | 1.2 | 0.7 | 0.9 | 1.5 | 0.9 |
| epi-α-Cadinol | 1638 | 1633 | 0.4 | 0.7 | 1 | 0.7 | 1.9 | 0.6 | 0.8 | |||||
| epi-α-muurolol | 1640 | 1639 | 1 | 0.6 | 0.4 | 0.3 | 0.7 | 0.6 | 1.9 | 0.9 | 0.6 | 1.3 | 1.9 | 1 |
| α-Muurolol | 1644 | 1642 | 0.5 | 0.5 | 0.6 | 0.5 | 1 | 0.6 | 1 | 0.7 | 0.9 | 1.2 | 0.9 | |
| α-Cadinol | 1652 | 1651 | 0.4 | 1 | 0.8 | 0.5 | 2 | 1 | 1.9 | 2.9 | 2 | 1.7 | 2 | 3.4 |
| Monoterpene hydrocarbons | 54.8 | 44.4 | 44.4 | 42.9 | 26.4 | 34.4 | 16.8 | 26.91 | 39.5 | 34.7 | 27.3 | 44.6 | ||
| Oxygenated monoterpenes | 1.3 | 1.3 | 0.9 | 0.9 | 1.1 | 1.7 | 0.6 | 0.7 | 0.6 | 0.8 | 0.6 | |||
| Sesquiterpenes Hydrocarbons | 23.4 | 30.4 | 24.8 | 27.1 | 27.8 | 31.4 | 38.8 | 32.3 | 27.1 | 27.8 | 32.4 | 25.9 | ||
| Oxygenated sesquiterpenes | 11.5 | 11 | 19.7 | 15.1 | 25.9 | 14.9 | 28.9 | 27.2 | 24.3 | 27.5 | 26.3 | 21.4 | ||
| Phenylpropanoids | 0.4 | 2.6 | 0.3 | 4.4 | 0.2 | 8 | ||||||||
| Others | 0.6 | 0.7 | 0.5 | 0.5 | 0.7 | 1 | 0.9 | 0.4 | 0.4 | 0.3 | 0.5 | 0.4 | ||
| Totals | 92 | 90.4 | 90.6 | 90.9 | 82.1 | 91.4 | 85.4 | 87.41 | 92 | 90.9 | 87.3 | 92.9 |
| Essential Oil | Concentration (μg·mL−1) | Mortality (%) | LC50 (μg·mL−1) |
|---|---|---|---|
| 50 | 100 | ||
| JulF | 25 | 80 | 17.66 ± 0.33 |
| 10 | 10 | ||
| 5 | 0 | ||
| 50 | 100 | ||
| JulD | 25 | 100 | 14.45 ± 0.25 |
| 10 | 20 | ||
| 5 | 0 | ||
| 100 | 100 | ||
| SetD | 50 | 80 | 35.11 ± 0.93 |
| 25 | 40 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| NovD | 50 | 100 | 26.32 ± 0.00 |
| 25 | 90 | ||
| 10 | 0 | ||
| 50 | 100 | ||
| JanF | 25 | 70 | 18.29 ± 0.00 |
| 10 | 10 | ||
| 5 | 0 | ||
| 100 | 100 | ||
| JanD | 50 | 100 | 23.37 ± 2.55 |
| 25 | 100 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MarF | 50 | 100 | 26.87 ± 0.47 |
| 25 | 80 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MarD | 50 | 100 | 21.90 ± 0.00 |
| 25 | 100 | ||
| 10 | 0 | ||
| 100 | 80 | ||
| MayF | 50 | 50 | 51.55 ± 2.12 |
| 25 | 40 | ||
| 10 | 0 | ||
| 100 | 100 | ||
| MayD | 50 | 80 | 33.07 ± 3.80 |
| 25 | 50 | ||
| 10 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, K.d.S.M.; Feitosa, B.d.S.; Cruz, J.N.; Ferreira, O.O.; Franco, C.d.J.P.; Cascaes, M.M.; Oliveira, M.S.d.; Andrade, E.H.d.A. Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules 2021, 26, 7359. https://doi.org/10.3390/molecules26237359
Mesquita KdSM, Feitosa BdS, Cruz JN, Ferreira OO, Franco CdJP, Cascaes MM, Oliveira MSd, Andrade EHdA. Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules. 2021; 26(23):7359. https://doi.org/10.3390/molecules26237359
Chicago/Turabian StyleMesquita, Késsia do Socorro Miranda, Bruna de Souza Feitosa, Jorddy Neves Cruz, Oberdan Oliveira Ferreira, Celeste de Jesus Pereira Franco, Márcia Moraes Cascaes, Mozaniel Santana de Oliveira, and Eloisa Helena de Aguiar Andrade. 2021. "Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach" Molecules 26, no. 23: 7359. https://doi.org/10.3390/molecules26237359
APA StyleMesquita, K. d. S. M., Feitosa, B. d. S., Cruz, J. N., Ferreira, O. O., Franco, C. d. J. P., Cascaes, M. M., Oliveira, M. S. d., & Andrade, E. H. d. A. (2021). Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules, 26(23), 7359. https://doi.org/10.3390/molecules26237359










