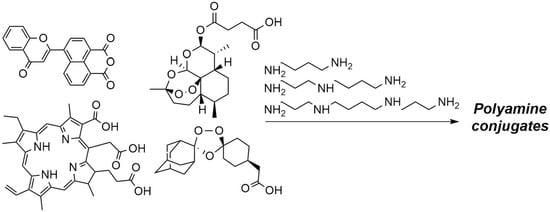
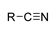Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications
Abstract
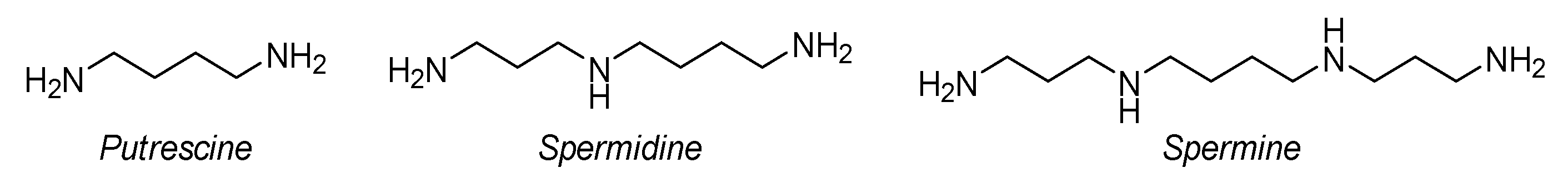
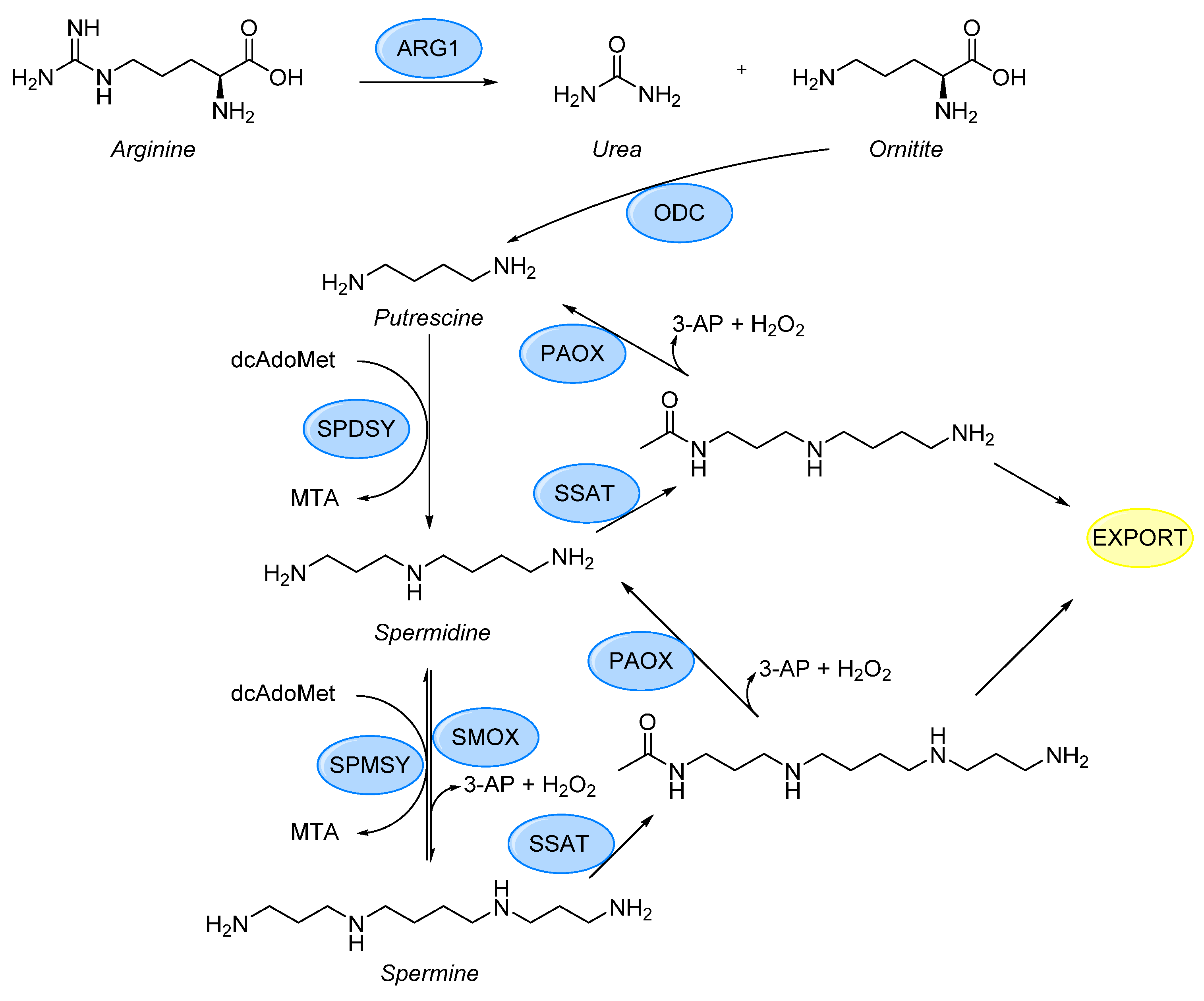
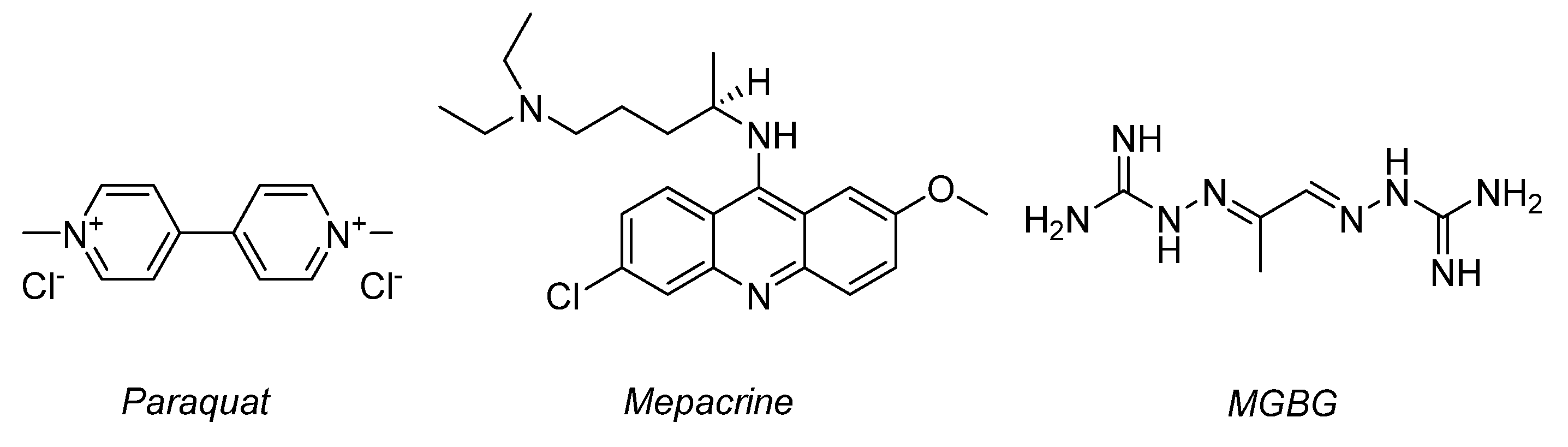
:1. Introduction
2. Methods for Polyamines Synthesis
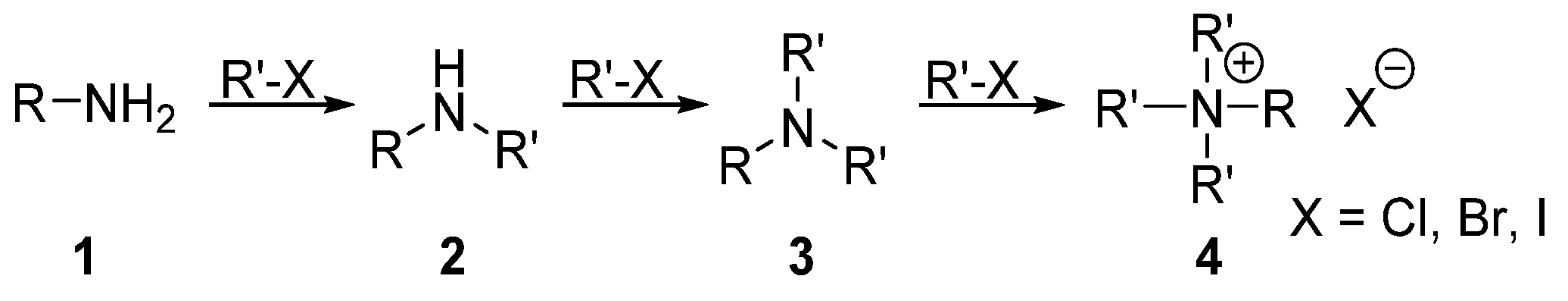
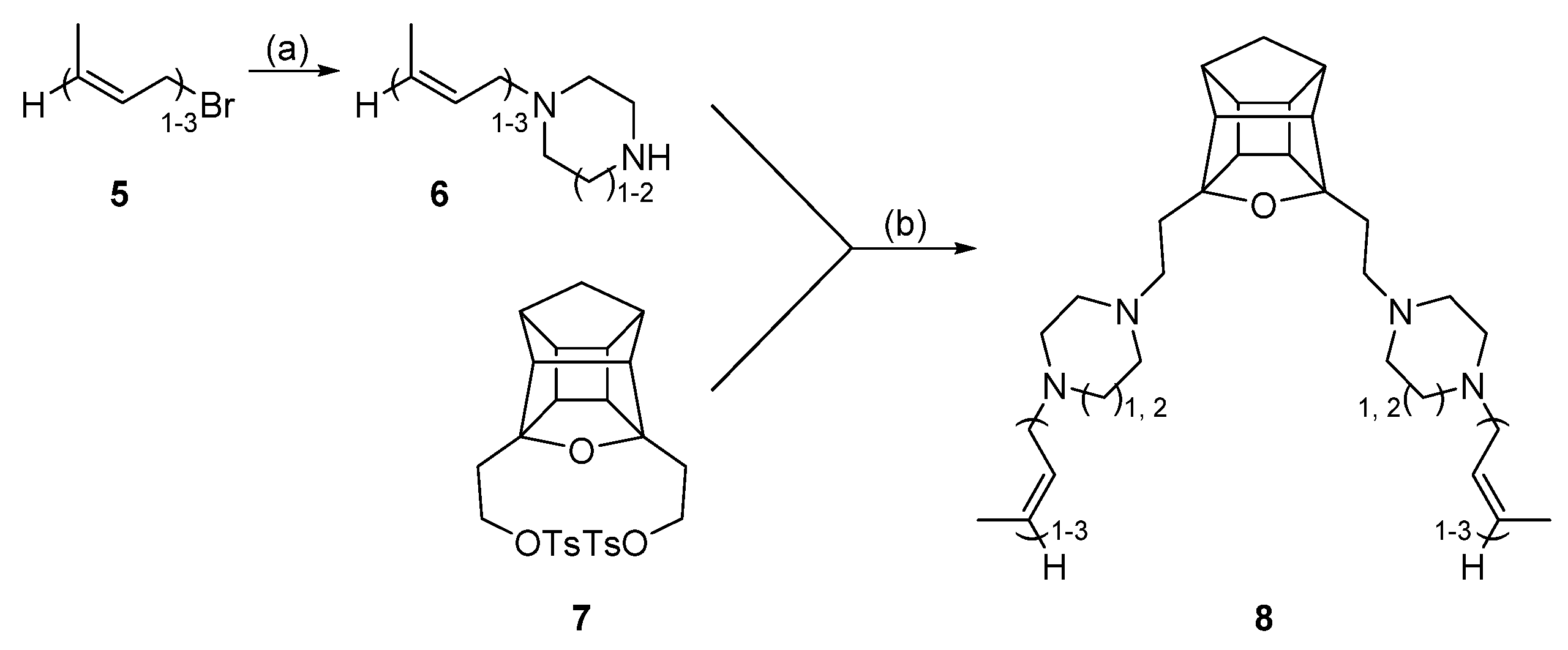
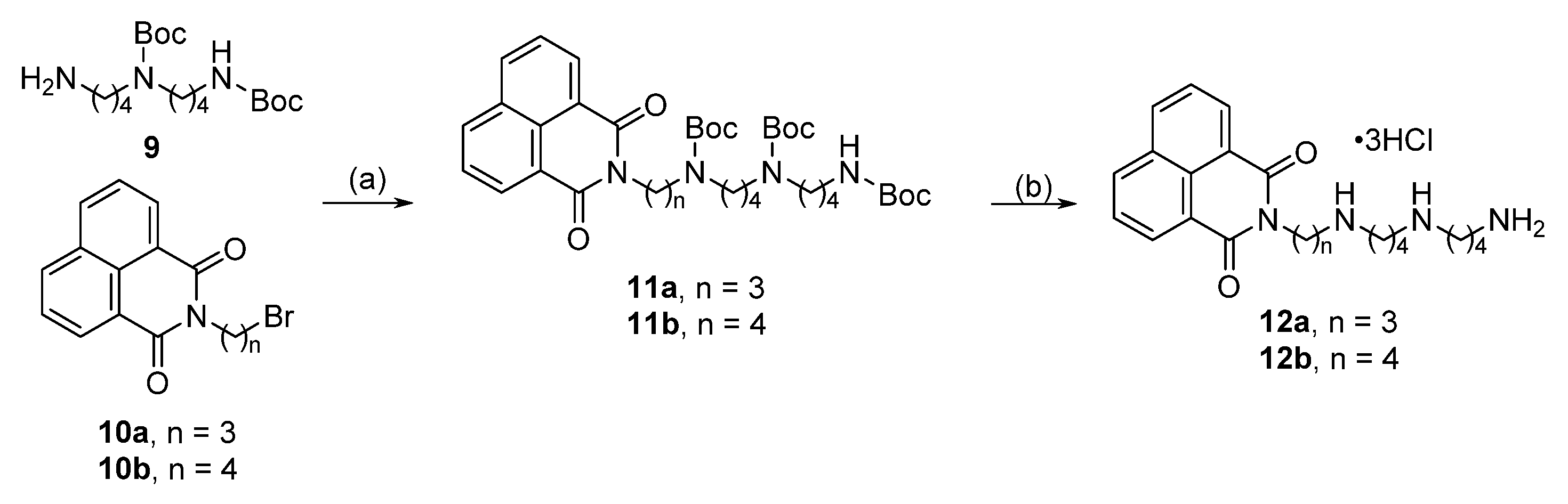
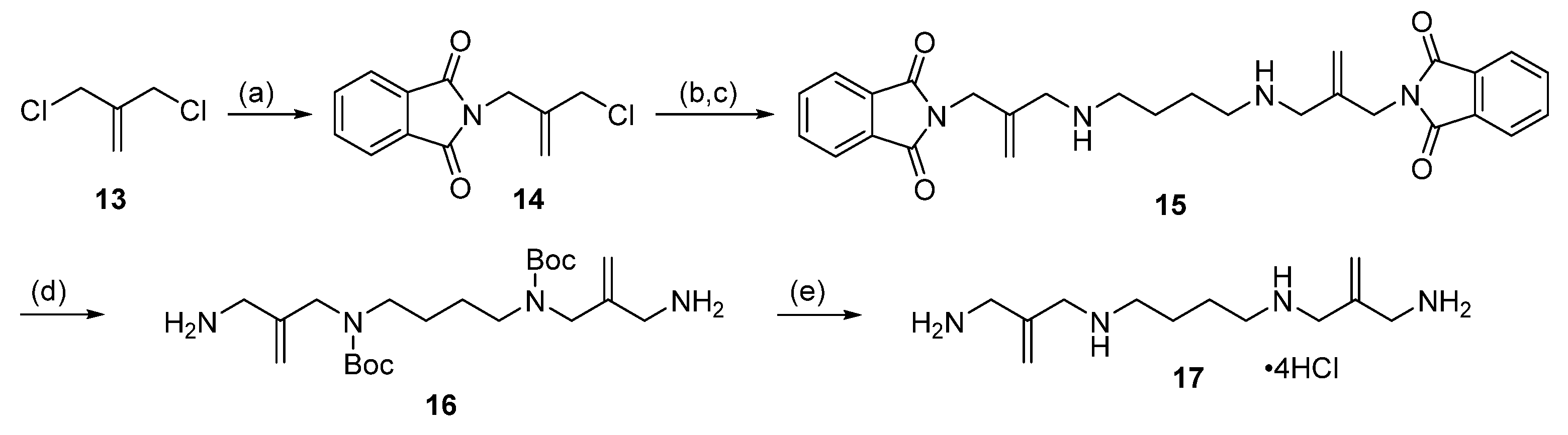
2.1. Alkylation
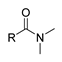
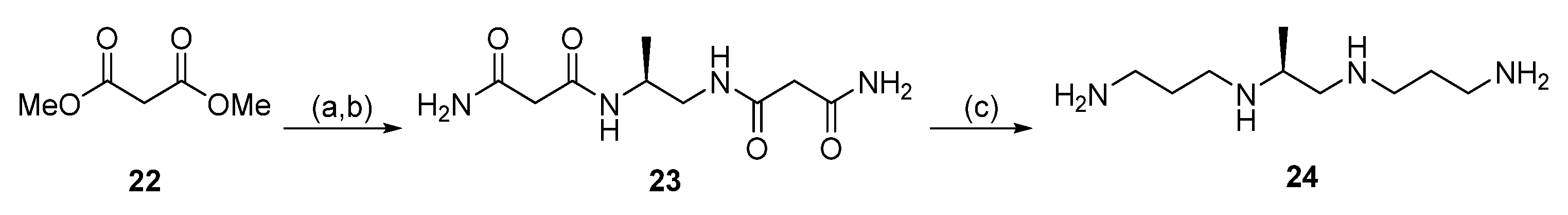
2.2. Acylation
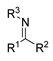
2.3. Imine Formation
2.4. Michael Reaction
2.5. Mitsunobu Reaction
2.6. Fukuyama Amine Synthesis
2.7. Solid-Phase Synthesis
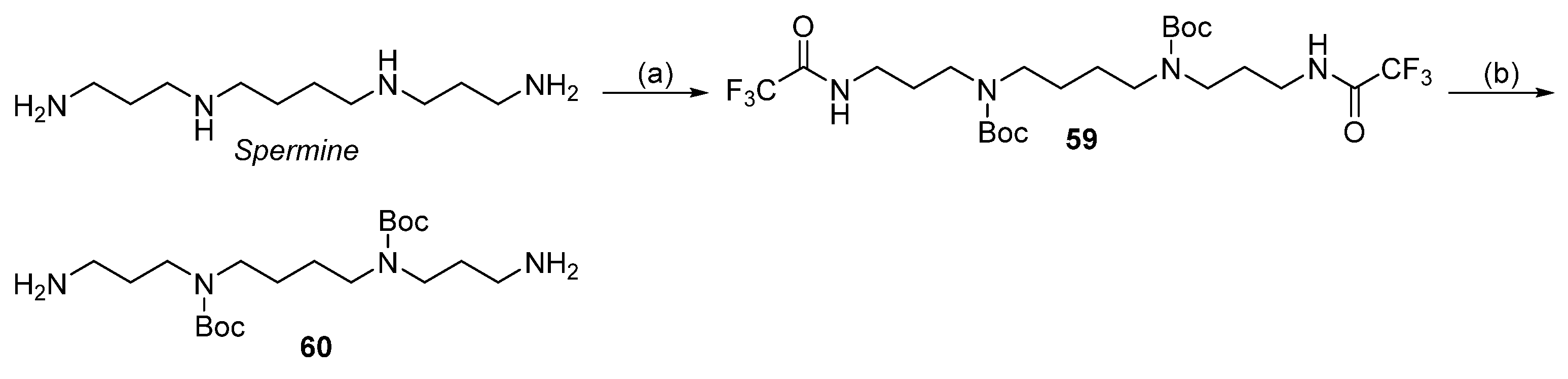
2.8. Regioselective Protection of Amino Groups
2.9. Cleavage of the Nitrogen-Containing Cycle
2.10. Opening of the Oxygen-Containing Cycle
3. Reduction
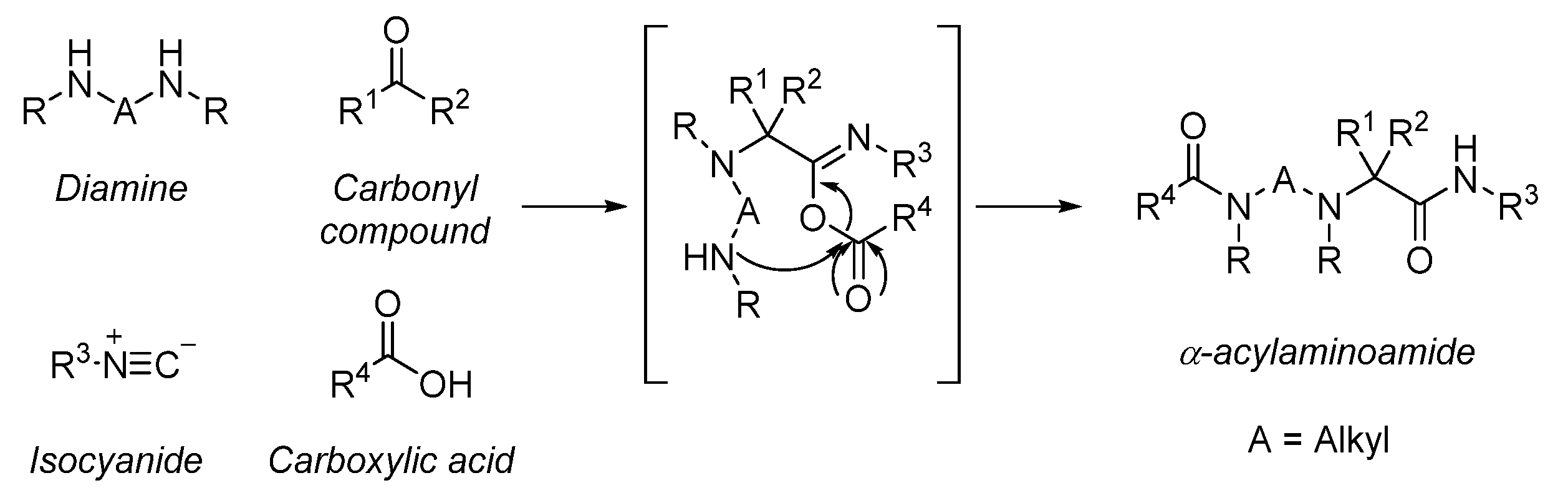
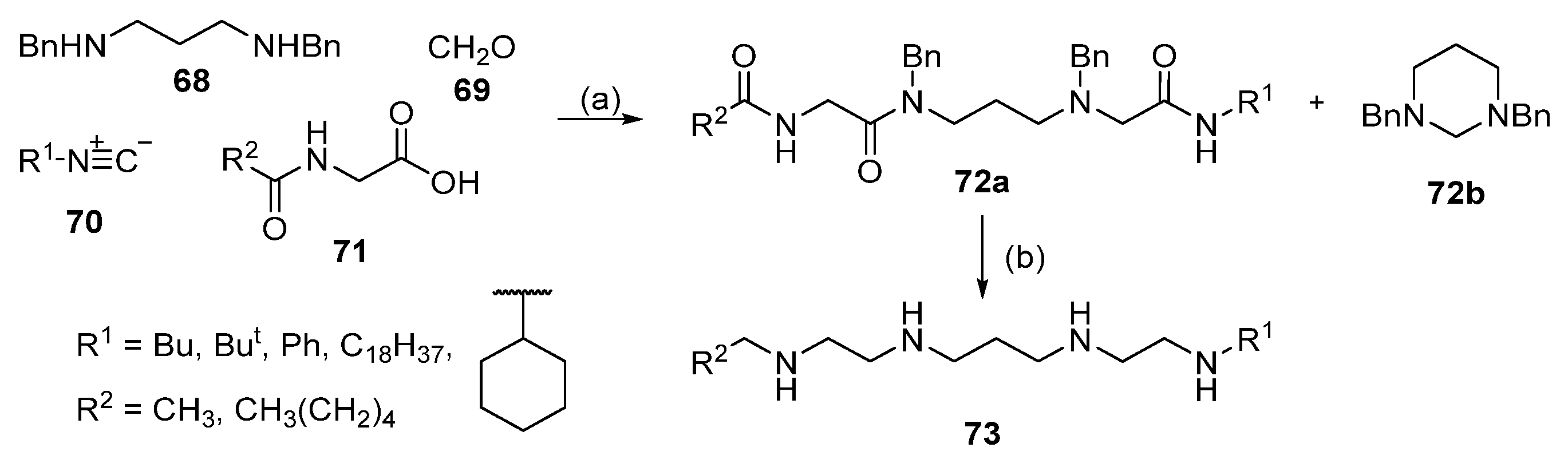
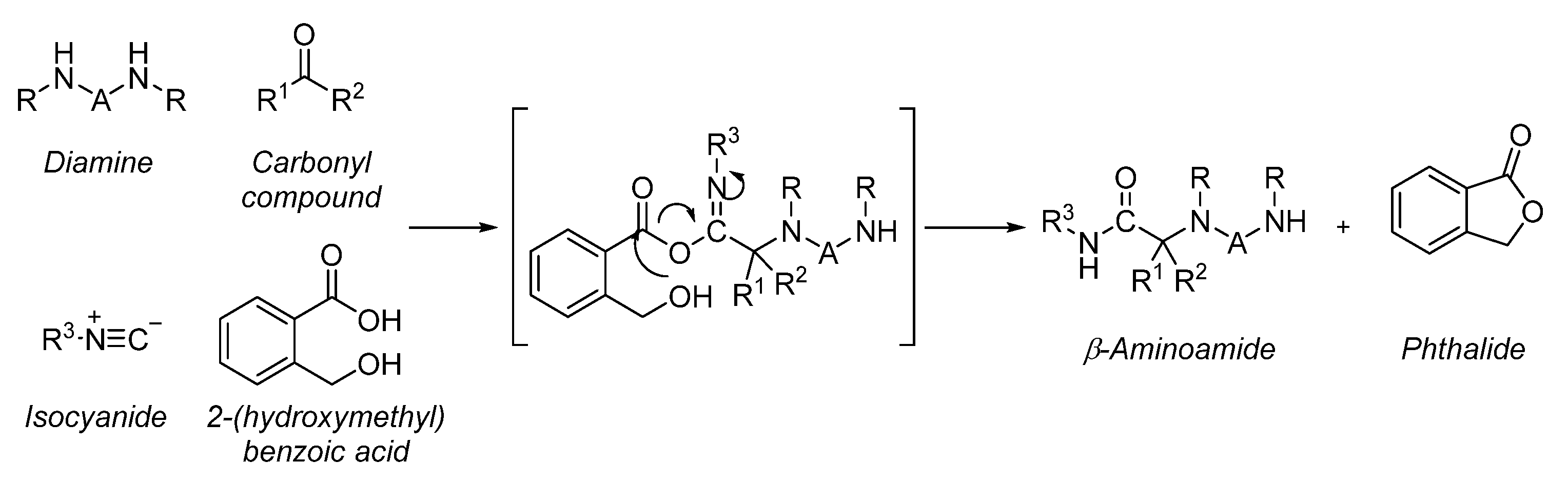
4. Multicomponent Ugi Reaction
5. Synthesis of Polyamines for Clinical Trials
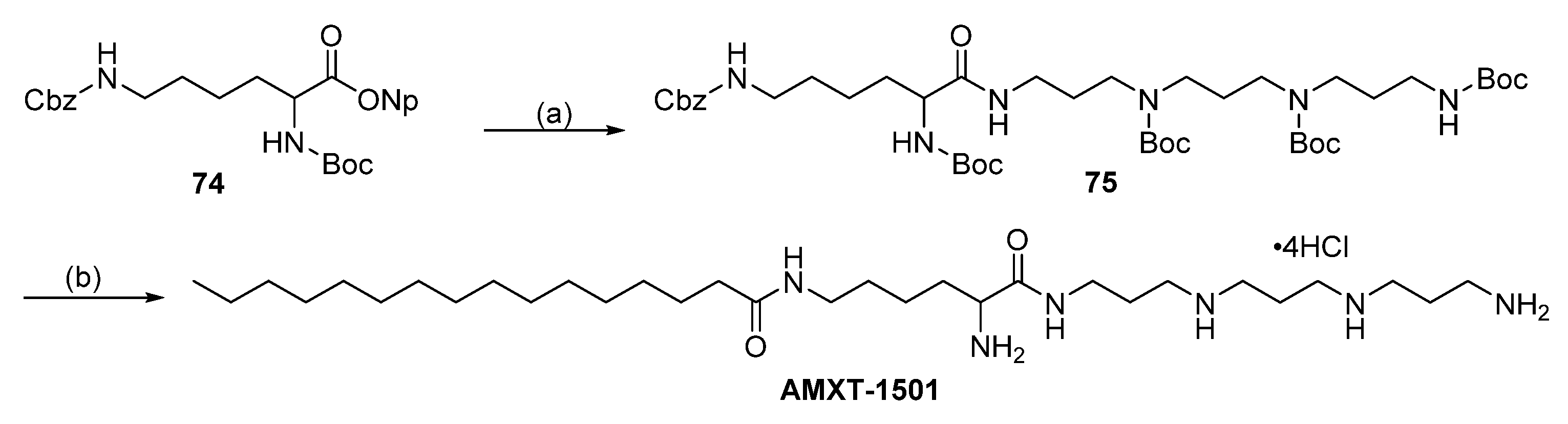
5.1. AMXT-1501
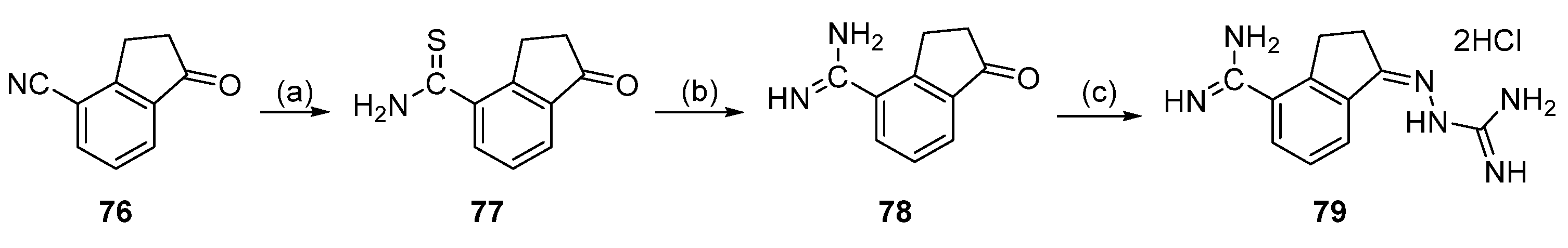
5.2. SAM486A
6. Synthesis of Polyamines Conjugates and Their Biological Activity
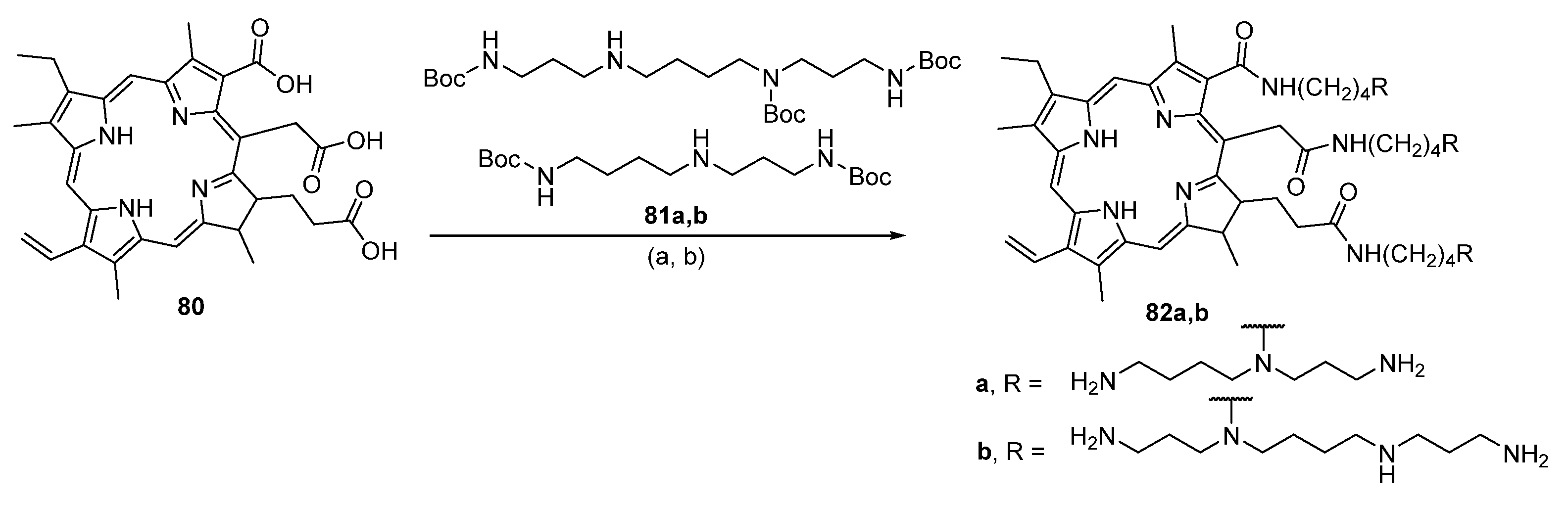
6.1. Conjugates with Porphyrins (Chlorin e6)
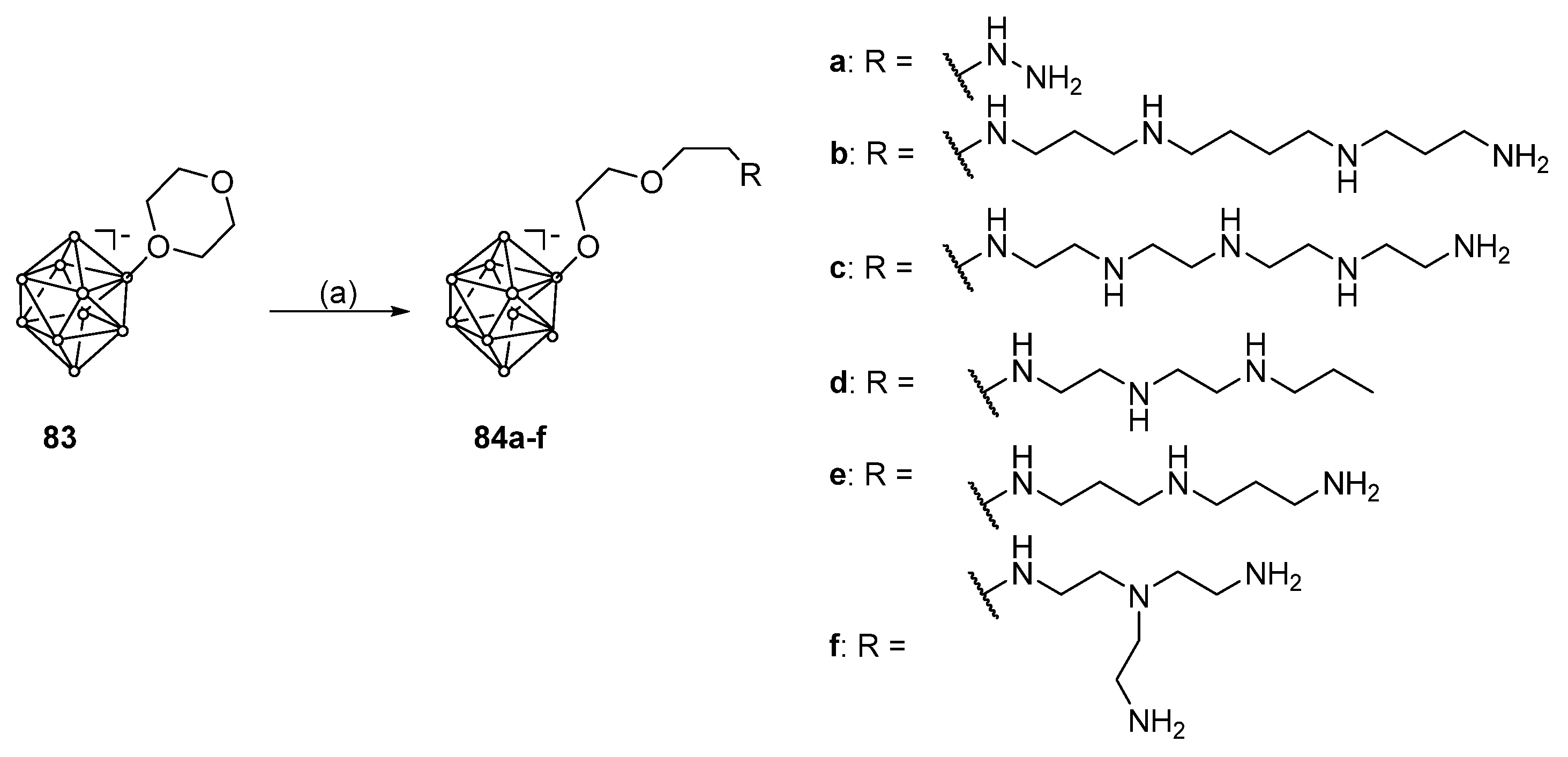
6.2. Conjugates with Boron Cluster
6.3. Conjugates with Flavonoids
6.4. Conjugates with Artesunate and Trioxolane
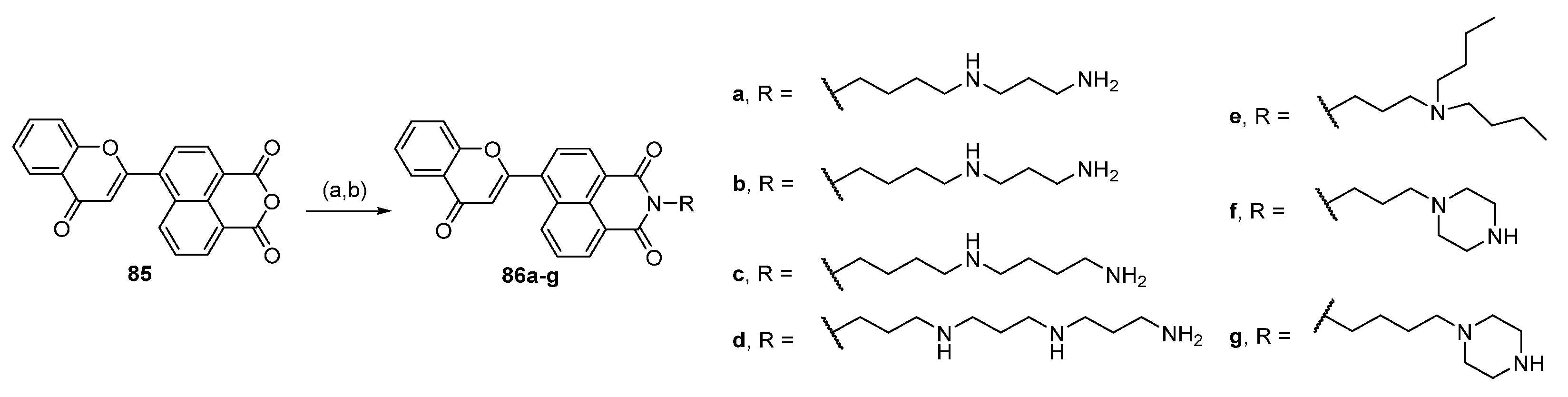
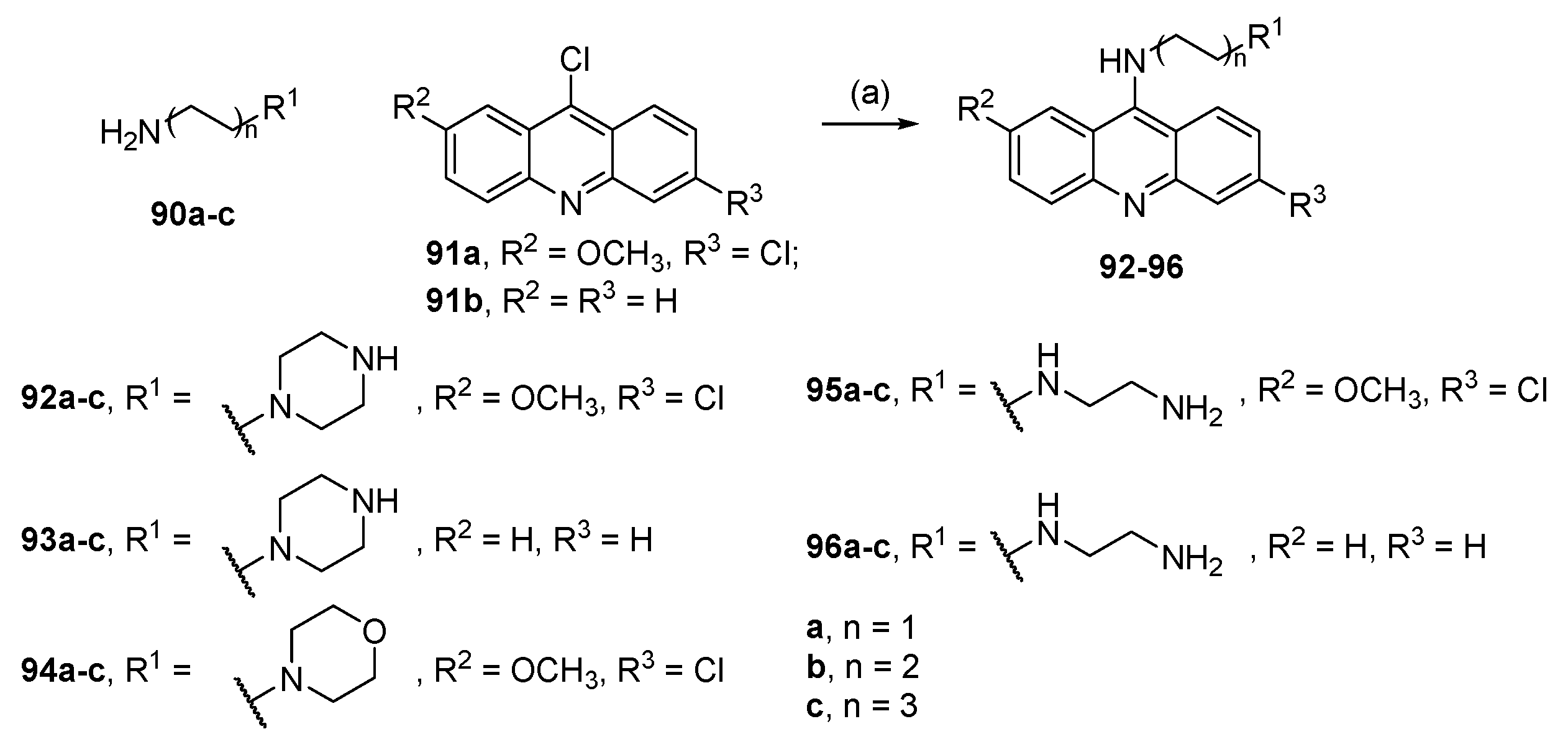
6.5. Conjugates with Acridine
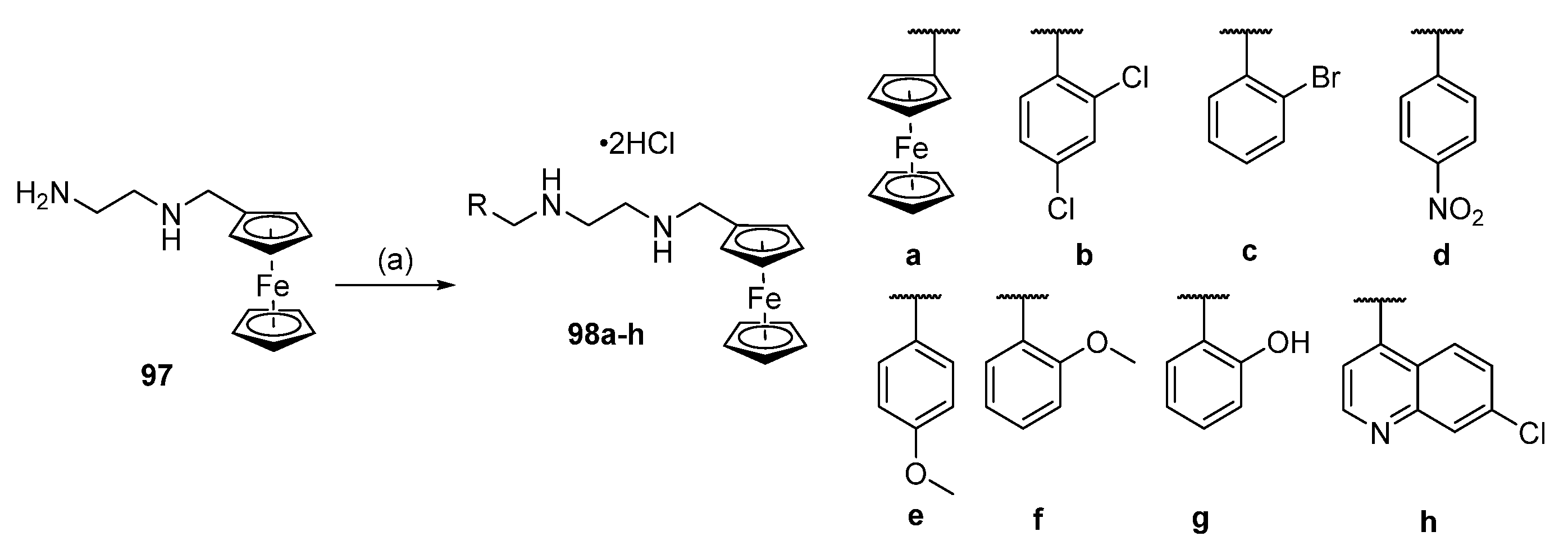
6.6. Conjugates with Ferrocene
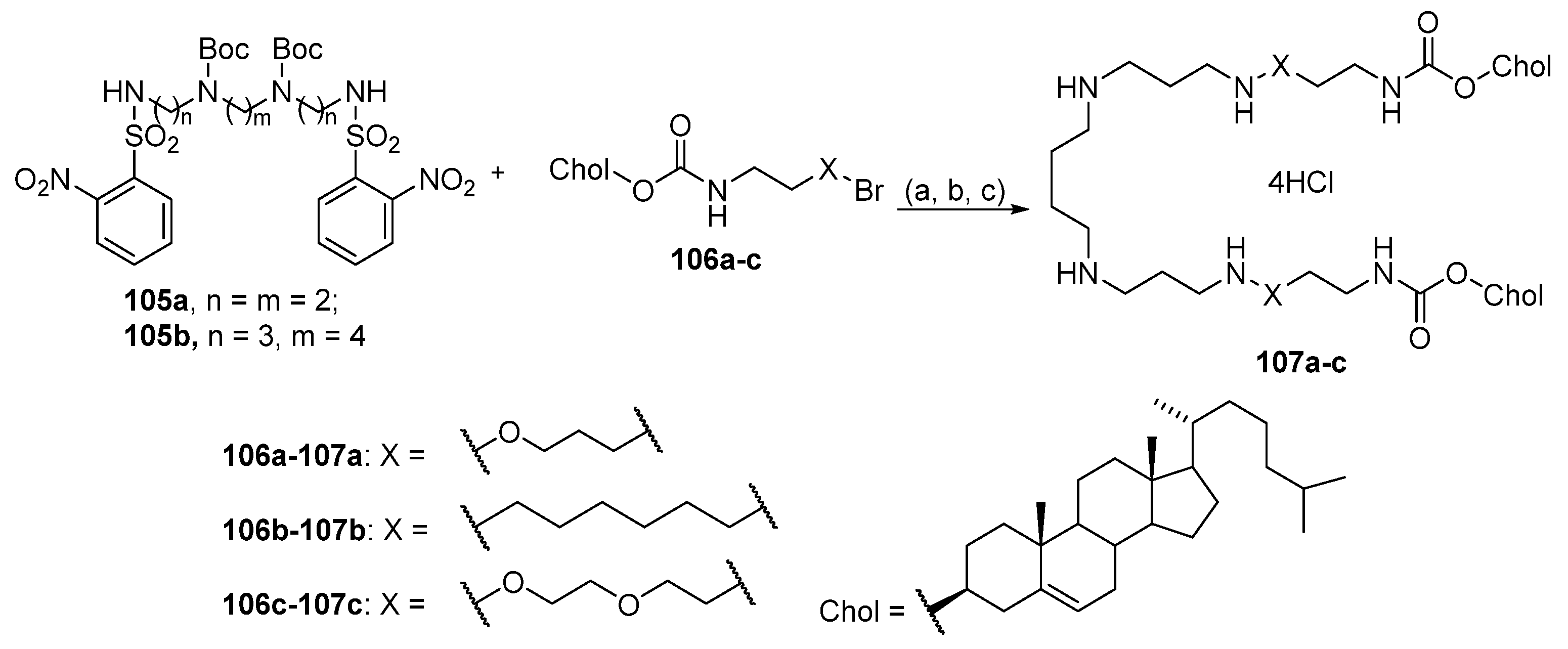
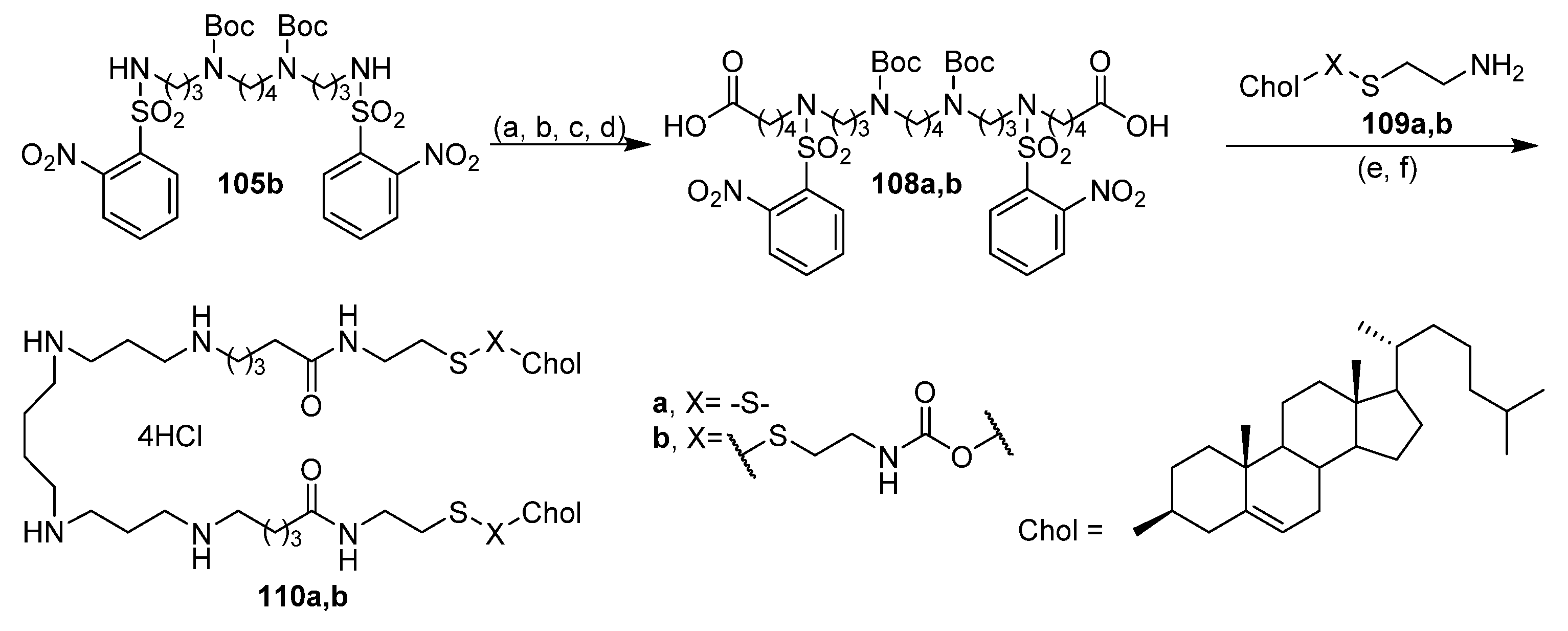
6.7. Lipophilic Polyamines
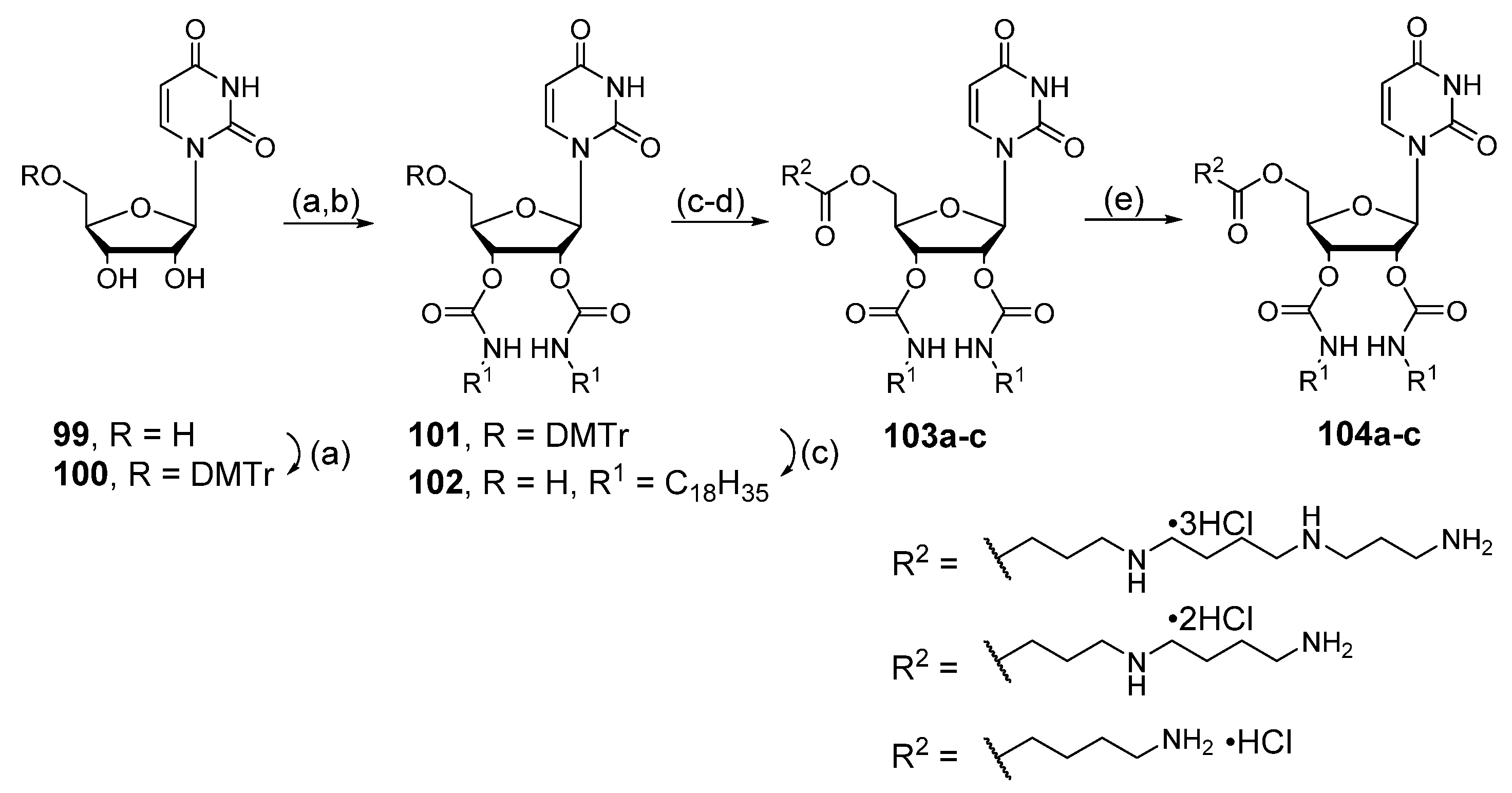
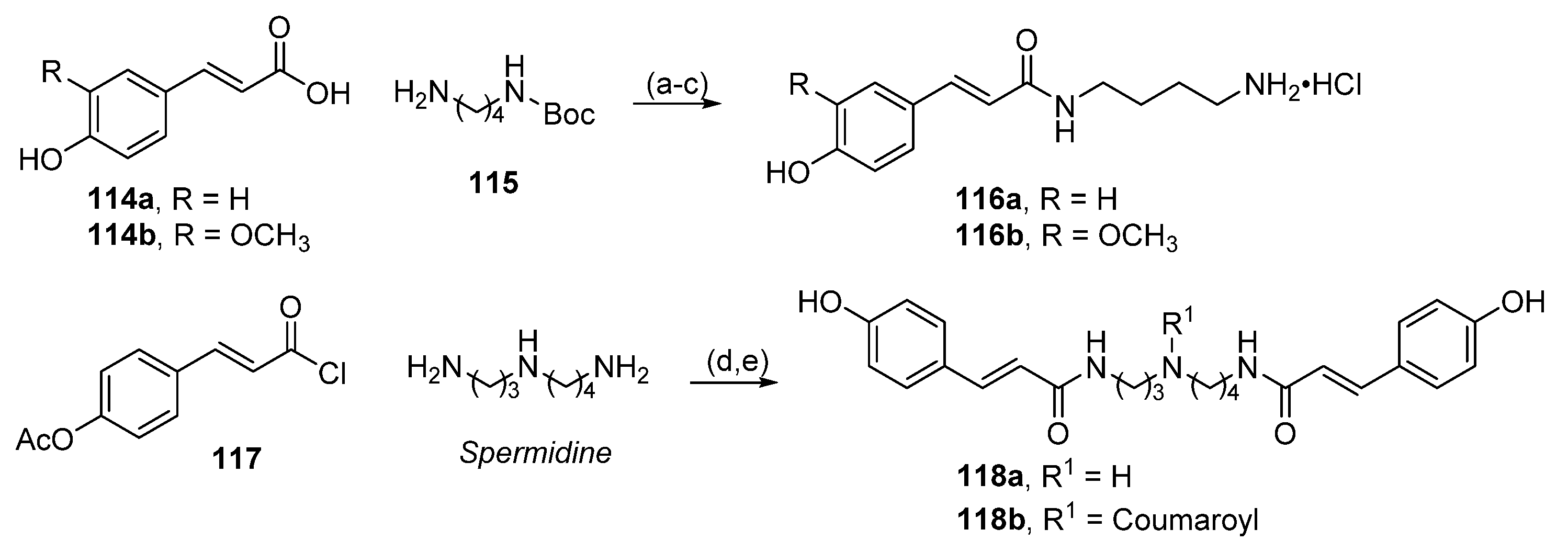
6.8. Conjugates with Hydroxycinnamic Acids
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pegg, A.E.; Casero, R.A. Current Status of the Polyamine Research Field. In Polyamines; Pegg, A.E., Casero, R.A., Eds.; Humana Press: New York, NY, USA, 2011; pp. 3–35. ISBN 978-1-61779-033-1. [Google Scholar]
- Minois, N. Molecular Basis of the ‘Anti-Aging’ Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology 2014, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and Implications in Human Diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of Cellular Function by Polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A. Targeting Polyamine Metabolism for Cancer Therapy and Prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terui, Y.; Yoshida, T.; Sakamoto, A.; Saito, D.; Oshima, T.; Kawazoe, M.; Yokoyama, S.; Igarashi, K.; Kashiwagi, K. Polyamines Protect Nucleic Acids against Depurination. Int. J. Biochem. Cell Biol. 2018, 99, 147–153. [Google Scholar] [CrossRef]
- Kurata, H.T.; Akrouh, A.; Li, J.W.; Marton, L.J.; Nichols, C.G. Scanning the Topography of Polyamine Blocker Binding in an Inwardly Rectifying Potassium Channel. J. Biol. Chem. 2013, 288, 6591–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, N.; Raul, F. Polyamines and Apoptosis. J. Cell. Mol. Med. 2005, 9, 623–642. [Google Scholar] [CrossRef]
- Casero, R.A.; Marton, L.J. Targeting Polyamine Metabolism and Function in Cancer and Other Hyperproliferative Diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef]
- Chen, J.; Ni, H.; Meng, Z.; Wang, J.; Huang, X.; Dong, Y.; Sun, C.; Zhang, Y.; Cui, L.; Li, J.; et al. Supramolecular Trap for Catching Polyamines in Cells as an Anti-Tumor Strategy. Nat. Commun. 2019, 10, 3546. [Google Scholar] [CrossRef]
- de Silva, T.R.M. Polyamine-Based Anticancer Strategies: The Role of Modified Polyamines. PhD Thesis, University of Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and Membrane Transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Novita Sari, I.; Setiawan, T.; Seock Kim, K.; Toni Wijaya, Y.; Won Cho, K.; Young Kwon, H. Metabolism and Function of Polyamines in Cancer Progression. Cancer Lett. 2021, 519, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Persson, L. Polyamine Homoeostasis. Essays Biochem. 2009, 46, 11–24. [Google Scholar] [CrossRef]
- Damiani, E.; Wallace, H.M. Polyamines; Methods in Molecular Biology; Alcázar, R., Tiburcio, A.F., Eds.; Springer: New York, NY, USA, 2018; Volume 1694, ISBN 978-1-4939-7397-2. [Google Scholar]
- Goyal, L.; Supko, J.G.; Berlin, J.; Blaszkowsky, L.S.; Carpenter, A.; Heuman, D.M.; Hilderbrand, S.L.; Stuart, K.E.; Cotler, S.; Senzer, N.N.; et al. Phase 1 Study of N 1,N 11-Diethylnorspermine (DENSPM) in Patients with Advanced Hepatocellular Carcinoma. Cancer Chemother. Pharmacol. 2013, 72, 1305–1314. [Google Scholar] [CrossRef]
- Xie, Y.; Murray-Stewart, T.; Wang, Y.; Yu, F.; Li, J.; Marton, L.J.; Casero, R.A.; Oupický, D. Self-Immolative Nanoparticles for Simultaneous Delivery of MicroRNA and Targeting of Polyamine Metabolism in Combination Cancer Therapy. J. Control. Release 2017, 246, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacker, A.; Marton, L.J.; Sobolewski, M.; Casero, R.A. In Vitro and in Vivo Effects of the Conformationally Restricted Polyamine Analogue CGC-11047 on Small Cell and Non-Small Cell Lung Cancer Cells. Cancer Chemother. Pharmacol. 2008, 63, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A.; Maris, J.M.; Lock, R.; Kolb, E.A.; Gorlick, R.; Keir, S.T.; Carol, H.; Morton, C.L.; Reynolds, C.P.; Kang, M.H.; et al. Initial Testing (Stage 1) of the Polyamine Analog PG11047 by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2011, 57, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Murray-Stewart, T.; Ferrari, E.; Xie, Y.; Yu, F.; Marton, L.J.; Oupicky, D.; Casero, R.A. Biochemical Evaluation of the Anticancer Potential of the Polyamine-Based Nanocarrier Nano 11047. PLoS ONE 2017, 12, e0175917. [Google Scholar] [CrossRef] [Green Version]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine Metabolism and Cancer: Treatments, Challenges and Opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.R.; Graminski, G.F.; Weeks, R.S.; Chen, Y.; O’Brien, T.G. Lipophilic Lysine−Spermine Conjugates Are Potent Polyamine Transport Inhibitors for Use in Combination with a Polyamine Biosynthesis Inhibitor. J. Med. Chem. 2009, 52, 1983–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuksa, V.; Buchan, R.; Kong Thoo Lin, P. Synthesis of Polyamines, Their Derivatives, Analogues and Conjugates. Synthesis 2000, 2000, 1189–1207. [Google Scholar] [CrossRef]
- Onajole, O.K.; Govender, K.; Govender, P.; van Helden, P.D.; Kruger, H.G.; Maguire, G.E.M.; Muthusamy, K.; Pillay, M.; Wiid, I.; Govender, T. Pentacyclo-Undecane Derived Cyclic Tetra-Amines: Synthesis and Evaluation as Potent Anti-Tuberculosis Agents. Eur. J. Med. Chem. 2009, 44, 4297–4305. [Google Scholar] [CrossRef] [PubMed]
- Onajole, O.K.; Govender, P.; van Helden, P.D.; Kruger, H.G.; Maguire, G.E.M.; Wiid, I.; Govender, T. Synthesis and Evaluation of SQ109 Analogues as Potential Anti-Tuberculosis Candidates. Eur. J. Med. Chem. 2010, 45, 2075–2079. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Ma, H.X.; Xie, S.Q.; Wang, X.; Zhao, J.; Wang, C.J.; Gao, W.Y. Synthesis, DNA Binding and Topoisomerase Inhibition of Mononaphthalimide Homospermidine Derivatives. Chin. Chem. Lett. 2008, 19, 509–512. [Google Scholar] [CrossRef]
- Grigorenko, N.A.; Khomutov, M.A.; Simonian, A.R.; Kochetkov, S.N.; Khomutov, A.R. Synthesis of 2,11-Bis(Methylidene)Spermine, a New Inhibitor of Spermine Oxidase. Russ. J. Bioorg. Chem. 2016, 42, 423–427. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J.R.J. Advanced Organic Chemistry, 5th ed.; Advanced Organic Chemistry; Springer: Boston, MA, USA, 2007; ISBN 978-0-387-44897-8. [Google Scholar]
- Jagu, E.; Pomel, S.; Diez-Martinez, A.; Ramiandrasoa, F.; Krauth-Siegel, R.L.; Pethe, S.; Blonski, C.; Labruère, R.; Loiseau, P.M. Synthesis and in Vitro Antikinetoplastid Activity of Polyamine–Hydroxybenzotriazole Conjugates. Bioorg. Med. Chem. 2017, 25, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Astorga, C.; Alfonso, I.; Rebolledo, F.; Gotor*, V. Chemoenzymatic Syntheses of Polyamines and Tetraazamacrocycles. Synth. Commun. 2002, 32, 2441–2452. [Google Scholar] [CrossRef]
- Nagarajan, S.; Ganem, B. Chemistry of Naturally Occurring Polyamines. 11. Unsaturated Spermidine and Spermine Derivatives. J. Org. Chem. 1987, 52, 5044–5046. [Google Scholar] [CrossRef]
- Edwards, M.L.; Stemerick, D.M.; McCarthy, J.R. Stereospecific Synthesis of Secondary Amines by the Mitsunobu Reaction. Tetrahedron Lett. 1990, 31, 3417–3420. [Google Scholar] [CrossRef]
- Drake, C.R.; Aissaoui, A.; Argyros, O.; Thanou, M.; Steinke, J.H.G.; Miller, A.D. Examination of the Effect of Increasing the Number of Intra-Disulfide Amino Functional Groups on the Performance of Small Molecule Cyclic Polyamine Disulfide Vectors. J. Control. Release 2013, 171, 81–90. [Google Scholar] [CrossRef]
- Lizzi, F.; Veronesi, G.; Belluti, F.; Bergamini, C.; López-Sánchez, A.; Kaiser, M.; Brun, R.; Krauth-Siegel, R.L.; Hall, D.G.; Rivas, L.; et al. Conjugation of Quinones with Natural Polyamines: Toward an Expanded Antitrypanosomatid Profile. J. Med. Chem. 2012, 55, 10490–10500. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis; Wuts, P.G.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118057, ISBN 9781118905074. [Google Scholar]
- Oliver, M.; Jorgensen, M.R.; Miller, A. The Facile Solid-Phase Synthesis of Cholesterol-Based Polyamine Lipids. Tetrahedron Lett. 2004, 45, 3105–3107. [Google Scholar] [CrossRef]
- Malamas, A.S.; Gujrati, M.; Kummitha, C.M.; Xu, R.; Lu, Z.-R. Design and Evaluation of New PH-Sensitive Amphiphilic Cationic Lipids for SiRNA Delivery. J. Control. Release 2013, 171, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Lu, Z.-R. Design, Synthesis and Evaluation of Spermine-Based PH-Sensitive Amphiphilic Gene Delivery Systems: Multifunctional Non-Viral Gene Carriers. Sci. China Chem. 2011, 54, 359–368. [Google Scholar] [CrossRef]
- Salmon-Chemin, L.; Lemaire, A.; De Freitas, S.; Deprez, B.; Sergheraert, C.; Davioud-Charvet, E. Parallel Synthesis of a Library of 1,4-Naphthoquinones and Automated Screening of Potential Inhibitors of Trypanothione Reductase from Trypanosoma Cruzi. Bioorg. Med. Chem. Lett. 2000, 10, 631–635. [Google Scholar] [CrossRef]
- Muth, A.; Madan, M.; Archer, J.J.; Ocampo, N.; Rodriguez, L.; Phanstiel, O. Polyamine Transport Inhibitors: Design, Synthesis, and Combination Therapies with Difluoromethylornithine. J. Med. Chem. 2014, 57, 348–363. [Google Scholar] [CrossRef]
- Mensah, E.A.; Nguyen, H.M. Nickel-Catalyzed Stereoselective Formation of α-2-Deoxy-2-Amino Glycosides. J. Am. Chem. Soc. 2009, 131, 8778–8780. [Google Scholar] [CrossRef]
- Miller, K.A.; Suresh Kumar, E.V.K.; Wood, S.J.; Cromer, J.R.; Datta, A.; David, S.A. Lipopolysaccharide Sequestrants: Structural Correlates of Activity and Toxicity in Novel Acylhomospermines. J. Med. Chem. 2005, 48, 2589–2599. [Google Scholar] [CrossRef] [Green Version]
- Petukhov, I.A.; Maslov, M.A.; Morozova, N.G.; Serebrennikova, G.A. Synthesis of Polycationic Lipids Based on Cholesterol and Spermine. Russ. Chem. Bull. 2010, 59, 260–268. [Google Scholar] [CrossRef]
- Ilies, M.A.; Seitz, W.A.; Johnson, B.H.; Ezell, E.L.; Miller, A.L.; Thompson, E.B.; Balaban, A.T. Lipophilic Pyrylium Salts in the Synthesis of Efficient Pyridinium-Based Cationic Lipids, Gemini Surfactants, and Lipophilic Oligomers for Gene Delivery. J. Med. Chem. 2006, 49, 3872–3887. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Rao, K.V.; Raj, K.S.; Prasad, A.R. Indium Tribromide Catalyzed Aminolysis of Aziridines: An Efficient Synthesis of Vicinal-Diamines. Synthesis 2002, 2002, 1061–1064. [Google Scholar] [CrossRef]
- Marti, A.; Richter, L.; Schneider, C. Iron-Catalyzed Ring-Opening of Meso-Aziridines with Amines. Synlett 2011, 2011, 2513–2516. [Google Scholar] [CrossRef]
- Peruncheralathan, S.; Henze, M.; Schneider, C. Scandium Triflate Catalyzed Aminolysis of Meso -Aziridines. Synlett 2007, 2007, 2289–2291. [Google Scholar] [CrossRef]
- Smith, M.B. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-37179-3. [Google Scholar]
- Reis Corrales, R.N.; Pinheiro, L.S.; Coimbra, E.S.; Da Silva, A.D.; Le Hyaric, M. Synthesis and Antileishmanial Activity of Lipophilic Aromatic Aminoalcohols. Sci. World J. 2010, 10, 1067–1072. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Yu, S.; Ye, S.; Zou, W.; Zhang, H.; Chen, J. Highly Regioselective Ring-Opening of Epoxides with Amines: A Metal- and Solvent-Free Protocol for the Synthesis of β-Amino Alcohols. Chem. Commun. 2020, 56, 2256–2259. [Google Scholar] [CrossRef] [PubMed]
- Manickam, M.; Boggu, P.R.; Pillaiyar, T.; Nam, Y.J.; Abdullah, M.; Lee, S.J.; Kang, J.S.; Jung, S.H. Design, Synthesis and Anticancer Activity of 2-Amidomethoxy-1,4-Naphthoquinones and Its Conjugates with Biotin/Polyamine. Bioorg. Med. Chem. Lett. 2021, 31, 127685. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.; Tinnis, F.; Slagbrand, T.; Trillo, P.; Adolfsson, H. Chemoselective Reduction of Carboxamides. Chem. Soc. Rev. 2016, 45, 6685–6697. [Google Scholar] [CrossRef]
- Velásquez, A.M.A.; Francisco, A.I.; Kohatsu, A.A.N.; Silva, F.A.D.J.; Rodrigues, D.F.; Teixeira, R.G.D.S.; Chiari, B.G.; de Almeida, M.G.J.; Isaac, V.L.B.; Vargas, M.D.; et al. Synthesis and Tripanocidal Activity of Ferrocenyl and Benzyl Diamines against Trypanosoma Brucei and Trypanosoma Cruzi. Bioorg. Med. Chem. Lett. 2014, 24, 1707–1710. [Google Scholar] [CrossRef]
- Neuse, E.W. Macromolecular Ferrocene Compounds as Cancer Drug Models. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–31. [Google Scholar] [CrossRef]
- Pirali, T.; Callipari, G.; Ercolano, E.; Genazzani, A.A.; Giovenzana, G.B.; Tron, G.C. A Concise Entry into Nonsymmetrical Alkyl Polyamines. Org. Lett. 2008, 10, 4199–4202. [Google Scholar] [CrossRef]
- Simmons, B.J.; Hoffmann, M.; Hwang, J.; Jackl, M.K.; Garg, N.K. Nickel-Catalyzed Reduction of Secondary and Tertiary Amides. Org. Lett. 2017, 19, 1910–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe, G.; Charette, A.B. Highly Chemoselective Metal-Free Reduction of Tertiary Amides. J. Am. Chem. Soc. 2008, 130, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Chaikin, S.W.; Brown, W.G. Reduction of Aldehydes, Ketones and Acid Chlorides by Sodium Borohydride. J. Am. Chem. Soc. 2002, 71, 122–125. [Google Scholar] [CrossRef]
- Erb, W.; Kadari, L.; Al-Mekhlafi, K.; Roisnel, T.; Dorcet, V.; Radha Krishna, P.; Mongin, F. Functionalization of 3-Iodo-N,N-Diisopropylferrocene-Carboxamide, a Pivotal Substrate to Open the Chemical Space to 1,3-Disubstituted Ferrocenes. Adv. Synth. Catal. 2020, 362, 832–850. [Google Scholar] [CrossRef] [Green Version]
- Lew, W.; Escarpe, P.A.; Mendel, D.B.; Sweeny, D.J.; Kim, C.U. Stereospecific Synthesis of a GS 4104 Metabolite: Determination of Absolute Sterochemistry and Influenza Neuraminidase Inhibitory Activity. Bioorg. Med. Chem. Lett. 1999, 9, 2811–2814. [Google Scholar] [CrossRef]
- Itsuno, S. Boron Hydride Reduction. ACS Symp. Ser. 2016, 1236, 241–274. [Google Scholar] [CrossRef]
- Hanada, S.; Tsutsumi, E.; Motoyama, Y.; Nagashima, H. Practical Access to Amines by Platinum-Catalyzed Reduction of Carboxamides with Hydrosilanes: Synergy of Dual Si−H Groups Leads to High Efficiency and Selectivity. J. Am. Chem. Soc. 2009, 131, 15032–15040. [Google Scholar] [CrossRef] [PubMed]
- Gutsulyak, D.V.; Nikonov, G.I. Chemoselective Catalytic Hydrosilylation of Nitriles. Angew. Chem. 2010, 122, 7715–7718. [Google Scholar] [CrossRef]
- Pelletier, G.; Bechara, W.S.; Charette, A.B. Controlled and Chemoselective Reduction of Secondary Amides. J. Am. Chem. Soc. 2010, 132, 12817–12819. [Google Scholar] [CrossRef]
- Ugi, I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Giovenzana, G.B.; Tron, G.C.; Di Paola, S.; Menegotto, I.G.; Pirali, T. A Mimicry of Primary Amines by Bis-Secondary Diamines as Components in the Ugi Four-Component Reaction. Angew. Chem. 2006, 118, 1117–1120. [Google Scholar] [CrossRef]
- La Spisa, F.; Feo, A.; Mossetti, R.; Tron, G.C. An Efficient Synthesis of Symmetric and Unsymmetric Bis-(β-Aminoamides) via Ugi Multicomponent Reaction. Org. Lett. 2012, 14, 6044–6047. [Google Scholar] [CrossRef]
- Samal, K.; Zhao, P.; Kendzicky, A.; Yco, L.P.; McClung, H.; Gerner, E.; Burns, M.; Bachmann, A.S.; Sholler, G. AMXT-1501, a Novel Polyamine Transport Inhibitor, Synergizes with DFMO in Inhibiting Neuroblastoma Cell Proliferation by Targeting Both Ornithine Decarboxylase and Polyamine Transport. Int. J. Cancer 2013, 133, 1323–1333. [Google Scholar] [CrossRef]
- Burns, M.R.; Wood, S.J.; Miller, K.A.; Nguyen, T.; Cromer, J.R.; David, S.A. Lysine–Spermine Conjugates: Hydrophobic Polyamine Amides as Potent Lipopolysaccharide Sequestrants. Bioorg. Med. Chem. 2005, 13, 2523–2536. [Google Scholar] [CrossRef]
- Salmon-Chemin, L.; Buisine, E.; Yardley, V.; Kohler, S.; Debreu, M.-A.; Landry, V.; Sergheraert, C.; Croft, S.L.; Krauth-Siegel, R.L.; Davioud-Charvet, E. 2- and 3-Substituted 1,4-Naphthoquinone Derivatives as Subversive Substrates of Trypanothione Reductase and Lipoamide Dehydrogenase from Trypanosoma c Ruzi: Synthesis and Correlation between Redox Cycling Activities and in Vitro Cytotoxicity. J. Med. Chem. 2001, 44, 548–565. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, F.; Muñoz-Uribe, M.; Pérez-Laines, J.; Ascencio-Leal, L.; Rivera-Dictter, A.; Martin-Martin, A.; Burgos, R.A.; Alarcon, P.; López-Muñoz, R. Searching for Drug Synergy Against Cancer Through Polyamine Metabolism Impairment: Insight Into the Metabolic Effect of Indomethacin on Lung Cancer Cells. Front. Pharmacol. 2020, 10, 1670. [Google Scholar] [CrossRef]
- Millward, M.J.; Joshua, A.; Kefford, R.; Aamdal, S.; Thomson, D.; Hersey, P.; Toner, G.; Lynch, K. Multi-Centre Phase II Trial of the Polyamine Synthesis Inhibitor SAM486A (CGP48664) in Patients with Metastatic Melanoma. Investig. New Drugs 2005, 23, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Sarrazy, V.; Sol, V.; Le Morvan, C.; Granet, R.; Alves, S.; Krausz, P. DNA Photocleavage by Porphyrin–Polyamine Conjugates. Bioorg. Med. Chem. 2009, 17, 767–776. [Google Scholar] [CrossRef]
- Garcia, G.; Sol, V.; Lamarche, F.; Granet, R.; Guilloton, M.; Champavier, Y.; Krausz, P. Synthesis and Photocytotoxic Activity of New Chlorin–Polyamine Conjugates. Bioorg. Med. Chem. Lett. 2006, 16, 3188–3192. [Google Scholar] [CrossRef]
- Matveev, E.Y.; Akimov, S.S.; Kubasov, A.S.; Nichugovskii, A.I.; Nartov, A.S.; Retivov, V.M.; Zhizhin, K.Y.; Kuznetsov, N.T. Reaction of the [B10H9O2C4H8]− Anion with C-Nucleophiles. Russ. J. Inorg. Chem. 2017, 62, 808–813. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Matveev, E.Y.; Turyshev, E.S.; Polyakova, I.N.; Nichugovskiy, A.I.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis and Stability Studies of Derivatives of the 2-Sulfanyl-Closo-Decaborate Anion [2-B10H9SH]2−. Inorg. Chim. Acta 2018, 477, 277–283. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Matveev, E.Y.; Klyukin, I.N.; Nichugovskiy, A.I.; Zhizhin, K.Y.; Kuznetsov, N.T. Silver(I) Complexes with Substituted Derivatives of the Boron Cluster Anions as Ligands. Inorg. Chim. Acta 2020, 510, 119749–119755. [Google Scholar] [CrossRef]
- Matveev, E.Y.; Limarev, I.P.; Nichugovskii, A.I.; Bykov, A.Y.; Zhizhin, K.Y.; Kuznetsov, N.T. Derivatives of Closo-Decaborate Anion with Polyamines. Russ. J. Inorg. Chem. 2019, 64, 977–983. [Google Scholar] [CrossRef]
- Dai, F.; Li, Q.; Wang, Y.; Ge, C.; Feng, C.; Xie, S.; He, H.; Xu, X.; Wang, C. Design, Synthesis, and Biological Evaluation of Mitochondria-Targeted Flavone–Naphthalimide–Polyamine Conjugates with Antimetastatic Activity. J. Med. Chem. 2017, 60, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhai, Y.; Luo, W.; Zhu, Z.; Zhang, X.; Xie, S.; Hong, C.; Wang, Y.; Su, Y.; Zhao, J.; et al. Synthesis and Biological Properties of Polyamine Modified Flavonoids as Hepatocellular Carcinoma Inhibitors. Eur. J. Med. Chem. 2016, 121, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, X.; Zhao, J.; Xie, S.; Wang, C. Nonhematotoxic Naphthalene Diimide Modified by Polyamine: Synthesis and Biological Evaluation. J. Med. Chem. 2012, 55, 3502–3512. [Google Scholar] [CrossRef]
- Pearce, A.N.; Kaiser, M.; Copp, B.R. Synthesis and Antimalarial Evaluation of Artesunate-Polyamine and Trioxolane-Polyamine Conjugates. Eur. J. Med. Chem. 2017, 140, 595–603. [Google Scholar] [CrossRef]
- Yu, X.-M.; Ramiandrasoa, F.; Guetzoyan, L.; Pradines, B.; Quintino, E.; Gadelle, D.; Forterre, P.; Cresteil, T.; Mahy, J.-P.; Pethe, S. Synthesis and Biological Evaluation of Acridine Derivatives as Antimalarial Agents. ChemMedChem 2012, 7, 587–605. [Google Scholar] [CrossRef]
- Puchkov, P.A.; Maslov, M.A. Lipophilic Polyamines as Promising Components of Liposomal Gene Delivery Systems. Pharmaceutics 2021, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Kowouvi, K.; Alies, B.; Gendrot, M.; Gaubert, A.; Vacher, G.; Gaudin, K.; Mosnier, J.; Pradines, B.; Barthelemy, P.; Grislain, L.; et al. Nucleoside-Lipid-Based Nanocarriers for Methylene Blue Delivery: Potential Application as Anti-Malarial Drug. RSC Adv. 2019, 9, 18844–18852. [Google Scholar] [CrossRef] [Green Version]
- Allain, V.; Bourgaux, C.; Couvreur, P. Self-Assembled Nucleolipids: From Supramolecular Structure to Soft Nucleic Acid and Drug Delivery Devices. Nucleic Acids Res. 2012, 40, 1891–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.P.; Yi, J.W.; Bang, E.-K.; Jeon, E.M.; Kim, B.H. Synthesis and Efficient SiRNA Delivery of Polyamine-Conjugated Cationic Nucleoside Lipids. Medchemcomm 2011, 2, 505. [Google Scholar] [CrossRef] [Green Version]
- Puchkov, P.A.; Perevoshchikova, K.A.; Kartashova, I.A.; Luneva, A.S.; Kabilova, T.O.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Polycationic Amphiphiles Based on Triethylenetetramine and Their Transfection Efficacy. Russ. J. Bioorg. Chem. 2017, 43, 561–569. [Google Scholar] [CrossRef]
- Puchkov, P.A.; Kartashova, I.A.; Shmendel, E.V.; Luneva, A.S.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Spacer Structure and Hydrophobicity Influences Transfection Activity of Novel Polycationic Gemini Amphiphiles. Bioorg. Med. Chem. Lett. 2017, 27, 3284–3288. [Google Scholar] [CrossRef] [PubMed]
- Puchkov, P.A.; Shmendel, E.V.; Luneva, A.S.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. Design, Synthesis and Transfection Efficiency of a Novel Redox-Sensitive Polycationic Amphiphile. Bioorg. Med. Chem. Lett. 2016, 26, 5911–5915. [Google Scholar] [CrossRef] [PubMed]
- Puchkov, P.A.; Shmendel, E.V.; Andreeva, V.D.; Morozova, N.G.; Zenkova, M.A.; Maslov, M.A. A Novel Disulfide-Containing Polycationic Amphiphile: 1,28-Di[(Cholest-5-En-3β-Yl)Disulfanyl]-4,25-Dioxo-3,8,12,17,21,26-Hexaazaoctacosane Tetrahydrochloride. Molbank 2018, 2018, M981. [Google Scholar] [CrossRef] [Green Version]
- Puchkov, P.A.; Shmendel, E.V.; Luneva, A.S.; Zenkova, M.A.; Maslov, M.A. Position of Disulfide Bond Determines the Properties of Novel Stimuli-Responsive Cationic Lipids. ChemistrySelect 2020, 5, 4509–4514. [Google Scholar] [CrossRef]
- Perevoshchikova, K.A.; Nichugovskiy, A.I.; Isagulieva, A.K.; Morozova, N.G.; Ivanov, I.V.; Maslov, M.A.; Shtil, A.A. Synthesis of Novel Lipophilic Tetraamines with Cytotoxic Activity. Mendeleev Commun. 2019, 29, 616–618. [Google Scholar] [CrossRef]
- Castro, B.M.; Fedorov, A.; Hornillos, V.; Delgado, J.; Acuña, A.U.; Mollinedo, F.; Prieto, M. Edelfosine and Miltefosine Effects on Lipid Raft Properties: Membrane Biophysics in Cell Death by Antitumor Lipids. J. Phys. Chem. B 2013, 117, 7929–7940. [Google Scholar] [CrossRef]
- Ausili, A.; Martínez-Valera, P.; Torrecillas, A.; Gómez-Murcia, V.; de Godos, A.M.; Corbalán-García, S.; Teruel, J.A.; Gómez Fernández, J.C. Anticancer Agent Edelfosine Exhibits a High Affinity for Cholesterol and Disorganizes Liquid-Ordered Membrane Structures. Langmuir 2018, 34, 8333–8346. [Google Scholar] [CrossRef]
- Plyavnik, N.V.; Kramareva, T.V.; Serebrennikova, G.A. Synthesis of Cationic Alkyl Glycerolipids with Heterocyclic Nitrogen-Containing Bases as Polar Domains. Russ. J. Bioorg. Chem. 2011, 37, 492–498. [Google Scholar] [CrossRef]
- Kyselka, J.; Bleha, R.; Dragoun, M.; Bialasová, K.; Horáčková, Š.; Schätz, M.; Sluková, M.; Filip, V.; Synytsya, A. Antifungal Polyamides of Hydroxycinnamic Acids from Sunflower Bee Pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. [Google Scholar] [CrossRef] [PubMed]

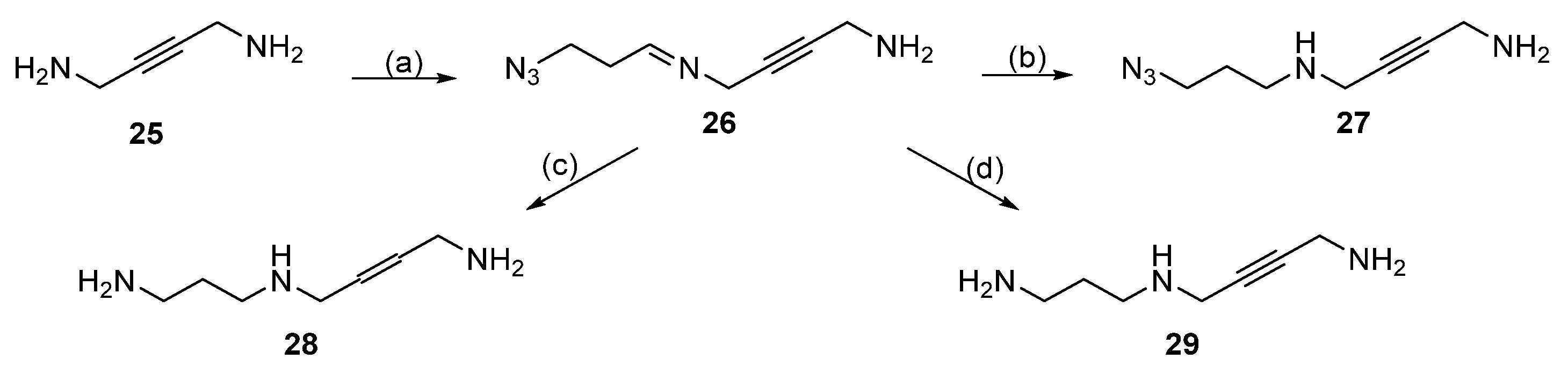
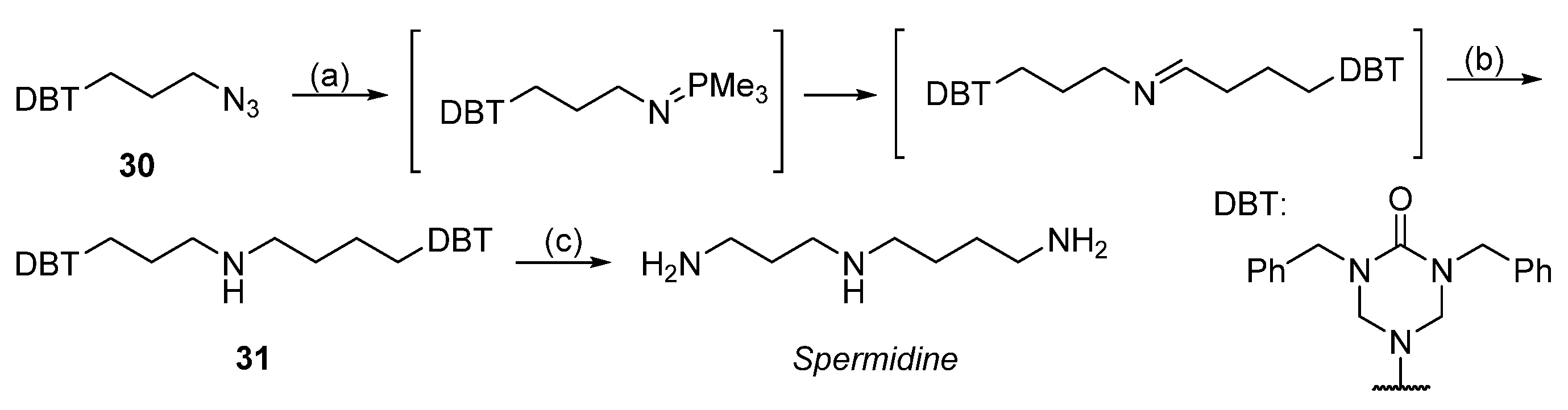
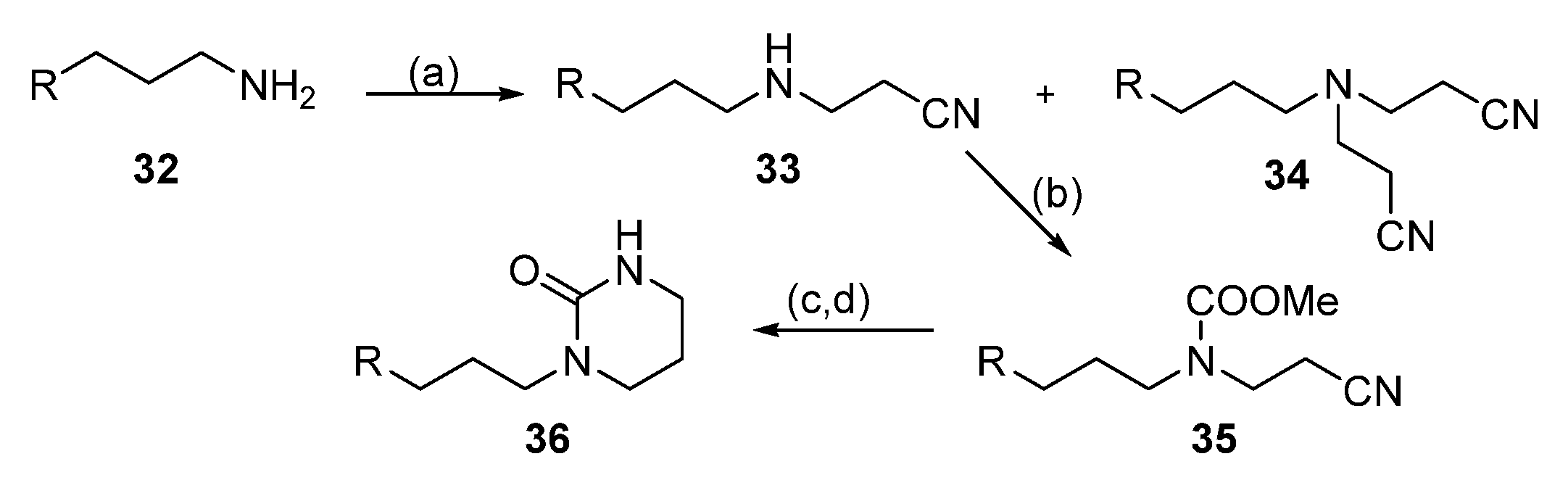
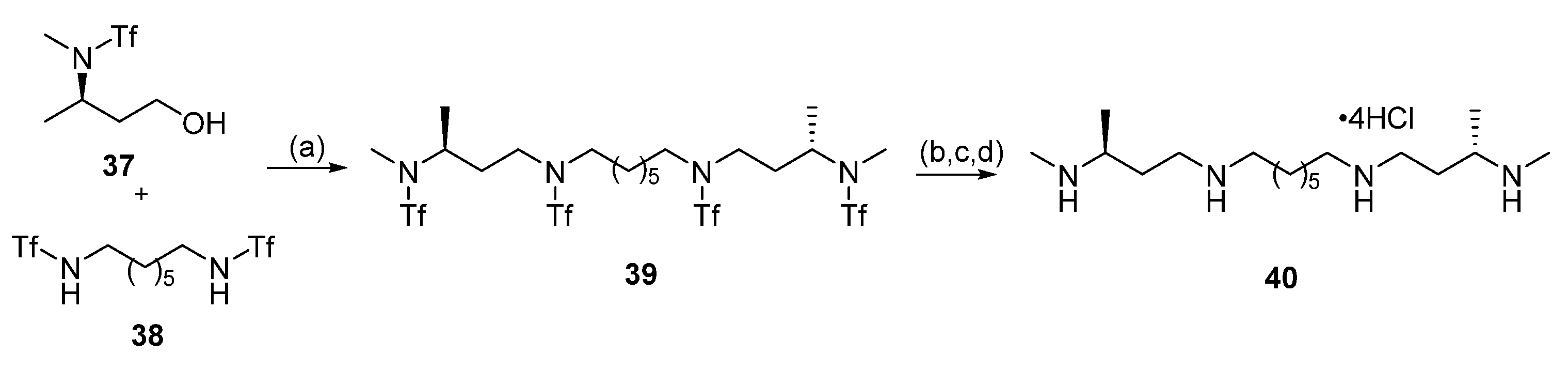
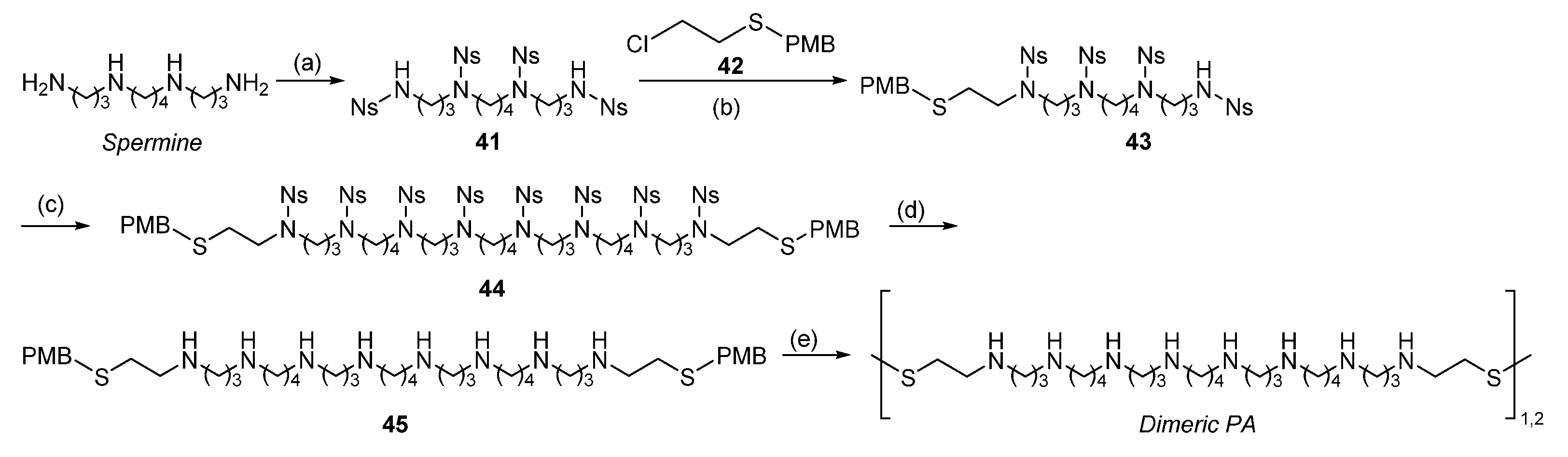
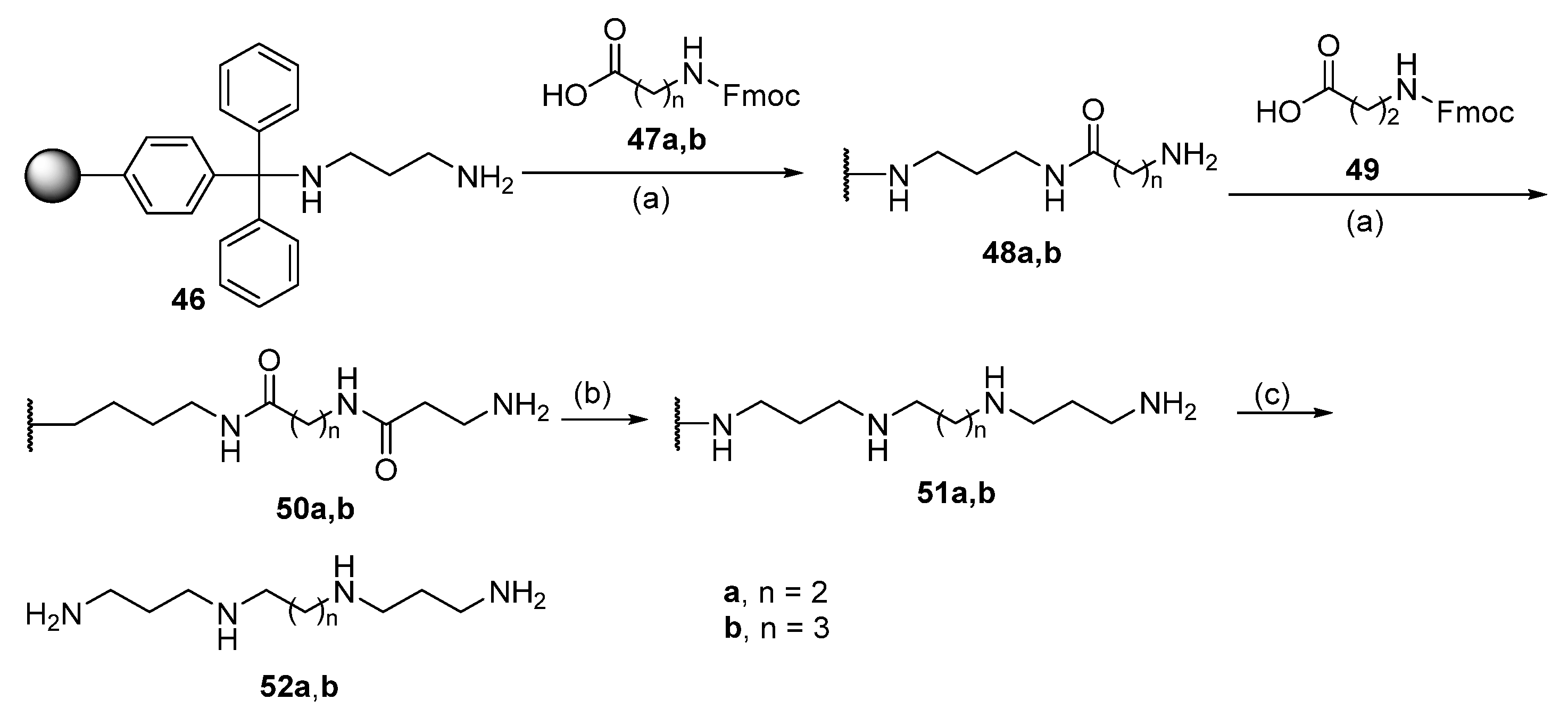

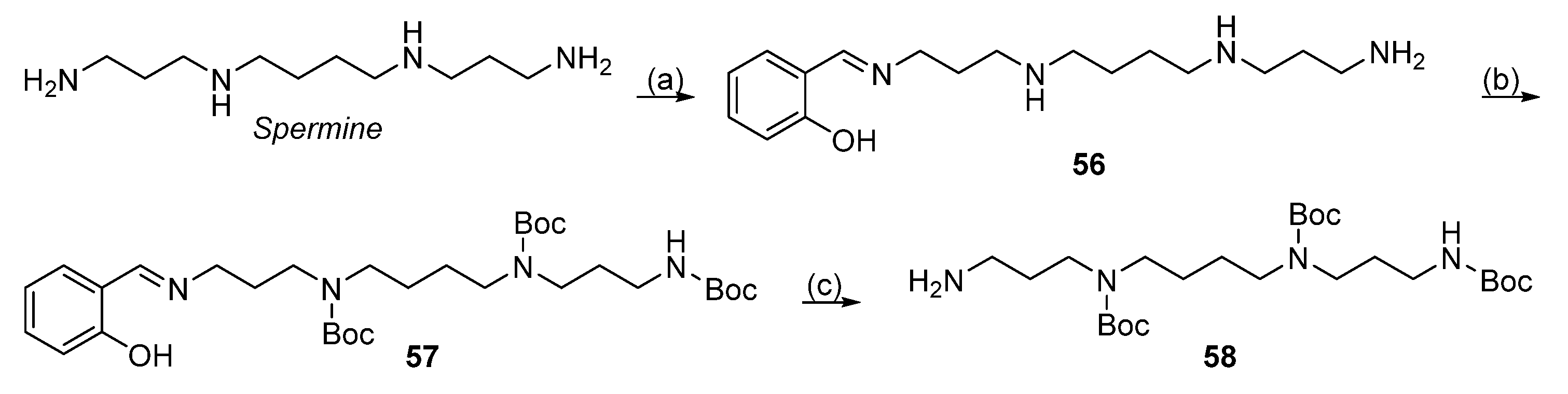
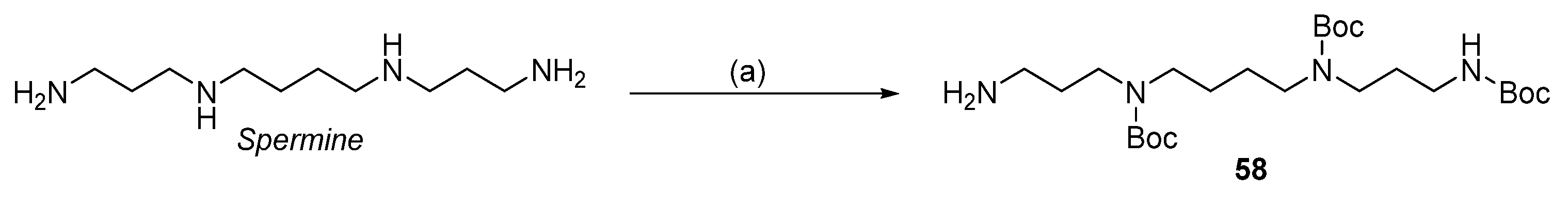
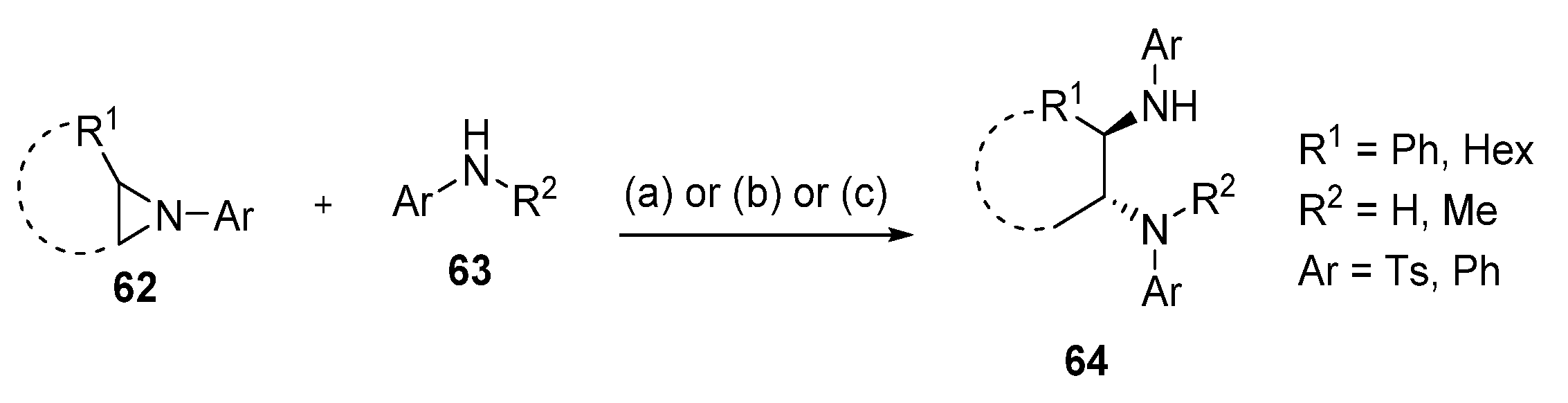

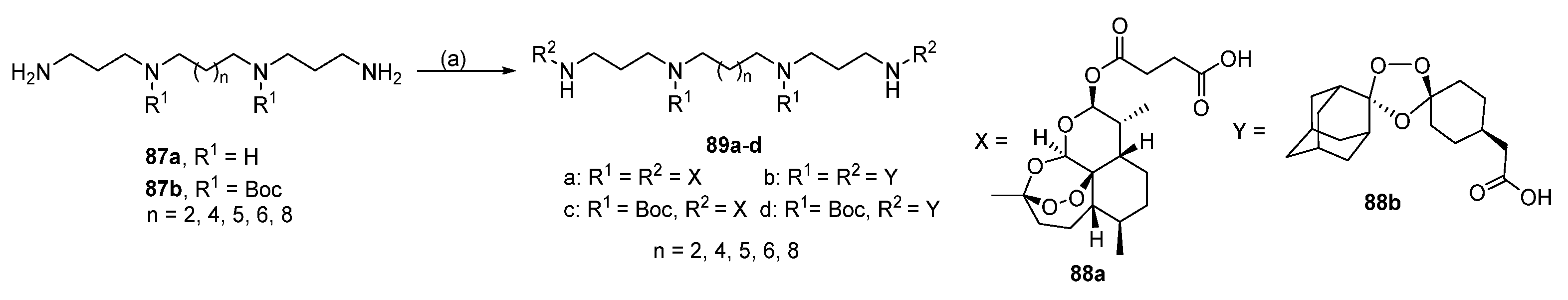

| Reagent | Trityl Ester | 2-Chlorotrityl Ester |
|---|---|---|
| 0.5M 1H-tetrazole in MeCN | 30 min | >1 h |
| AcOH, H2O, 4:1 (v/v) | <5 min | 15 min |
| 2.5% Cl2CHCO2H, CH2Cl2 | <1 min | <1 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichugovskiy, A.; Tron, G.C.; Maslov, M. Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications. Molecules 2021, 26, 6579. https://doi.org/10.3390/molecules26216579
Nichugovskiy A, Tron GC, Maslov M. Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications. Molecules. 2021; 26(21):6579. https://doi.org/10.3390/molecules26216579
Chicago/Turabian StyleNichugovskiy, Artemiy, Gian Cesare Tron, and Mikhail Maslov. 2021. "Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications" Molecules 26, no. 21: 6579. https://doi.org/10.3390/molecules26216579
APA StyleNichugovskiy, A., Tron, G. C., & Maslov, M. (2021). Recent Advances in the Synthesis of Polyamine Derivatives and Their Applications. Molecules, 26(21), 6579. https://doi.org/10.3390/molecules26216579