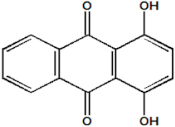
Abstract
Solubility of several anthraquinone derivatives in supercritical carbon dioxide was readily available in the literature, but correcting ability of the existing models was poor. Therefore, in this work, two new models have been developed for better correlation based on solid–liquid phase equilibria. The new model has five adjustable parameters correlating the solubility isotherms as a function of temperature. The accuracy of the proposed models was evaluated by correlating 25 binary systems. The proposed models observed provide the best overall correlations. The overall deviation between the experimental and the correlated results was less than 11.46% in averaged absolute relative deviation (AARD). Moreover, exiting solubility models were also evaluated for all the compounds for the comparison purpose.
1. Introduction
Supercritical fluid (SCF) applications in process industry have gained a lot of momentum. The proper application solely depends on exact information on solubility, therefore, the estimation of solubility of a variety of substances in supercritical fluids has taking place in recent literature [1]. Among various supercritical fluids, carbon dioxide is one that has more attention due to its interesting and easily attainable critical properties [1]. Dyeing industry and pharmaceutical industry require solubility data, but the data are limited and available at particular specified temperatures and pressures [1]. Measuring solubility at each and every point would be a tedious task, therefore modeling is a must [2]. The present study is concerned about the modeling of anthraquinone derivatives in supercritical carbon dioxide. Anthraquinone derivatives are majorly used in dyeing industries—the exact prediction of solubility data are very much essential for the development of supercritical dyeing process. There are five frameworks through which solubility data are analyzed [3]. Out of five approaches, thermodynamic frameworks based on solid–gas equilibrium criteria and solid–liquid equilibrium criteria are very successful [4]. Solid–gas equilibrium approach requires critical, chemical, and physical information for the modeling. The availability of such information is very rare; therefore, solid–gas equilibrium approach entirely depends on group contribution methods for those necessary properties. Therefore, the solid–gas equilibrium approach would be purely lies on the accuracy of the predicted properties. Sometimes these properties may not be real and the corresponding correlation may not be appropriate. Therefore, we need to look for an alternative correlating approach under thermodynamic framework for better correlation purpose; under such circumstance, the solid–liquid equilibrium criteria approach may be useful in correlating the solubility [5,6,7]. In the present work we aimed at the development of new solubility models for the anthraquinone derivative which will be useful for supercritical dyeing process. In this work, we proposed two new models based on solid–liquid equilibrium criteria. Further, important exiting solubility models are also evaluated for all the compounds for the comparison purpose. The following section deals with existing solubility models considered in this study.
2. Existing Solubility Models
2.1. Empirical Models
2.1.1. Chrastil Model
Chrastil et al. [8] proposed a semi empirical model based on solvate complex theory and have related the solubility of solute to density of supercritical fluid as follows:
where is the solute solubility in kg·m−3, k is the association number, is constant, and presents the function of enthalpy of solvation and vaporization. Equation (1) can be rewritten [9] to be mole fraction terms as follows:
2.1.2. Adachi and Lu Model
Adachi and Lu (1983) [10] modified Chrastil’s equation by considering the quantity k to be density-dependent and the model can be written as:
where is the solute solubility in the mole fraction, the to are parameters constant.
2.1.3. Mitra–Wilson Model
Mitra and Wilson (1991) [11] developed an empirical model for solubility of solute as a function of temperature and pressure:
Here, P is the pressure system used in atm, and to are the constant parameters.
2.1.4. Keshmiri Model
Keshmiri et al. (2014) [12] proposed the possible linear relationship between ln and as the following expression:
where T and P are the temperature and pressure system used, respectively. The to are constant parameters.
2.1.5. Khansary Model
Subsequently, Khansary et al. (2015) [13] also developed a model relationship between ln and as:
The to are constant parameters.
2.1.6. Bian Model
Bian et al. (2016) [14] found a model with five constant parameters with relationship between solubility of solute ( and density, , and obtained the following model:
where to are the model parameters.
2.1.7. Garlapati and Madras Model
Garlapati and Madras [2] proposed an empirical model and related solute solubility to density of supercritical fluid as:
where to are constant parameters.
2.1.8. Reddy Model
Reddy et al. (2018) [15] proposed an empirical model based on degrees of freedom analysis as:
where and are reduced pressure of carbon dioxide ( and reduced temperature of carbon dioxide (, respectively. The - are model constants. The and are critical pressure ( = 7.387 MPa) and critical temperature ( = 304.12 K), respectively.
2.2. Solid–Liquid Equilibrium Criteria Model
The behavior of solid solute in the liquid phase is determined by a ratio of the fugacity between pure liquid solute and the solid state at pressure (P) and temperature (T), which have reported elsewhere [4,16,17,18]. Moreover, the activity of substance obtained from the melting temperature and the melting enthalpy of compound. The activity coefficient of the substance can be represented by the regular solution model together with theory of Flory Huggins [6,17,19]. The solubility representation of the solute in ScCO2 is expressed by
where , and are the enthalpy of melting, melting temperature, and molar volume of the solute, respectively. These data are presented in Table 1. is the molar volume of ScCO2. and are estimated by Jain et al. method [20] and by Fedors method [21], respectively. The solubility parameter of ScCO2 ( is calculated by Giddings method [22],
where is critical pressure ( = 7.387 MPa), is the reduced density of CO2, it can be calculated by the density of ScCO2, , is obtained from website of NIST Web Book [23]. By assumption that the ScCO2 density depends on the solubility parameter of the solid solute (), the correlation can be expressed as:
where is the density of ScCO2 in (mol/m3) obtained from website of NIST Web Book [23], a, b, and c are adjustable parameters.

Table 1.
Physical properties of the compounds.
3. New Models
3.1. Model 1
In solid–liquid equilibrium criteria, the supercritical phase is generally assumed as an expanded liquid consisting of infinite dissolved solute. At equilibrium, the solubility is expressed as [2,13,14]
In Equation (13), is solute activity coefficient at infinite dilution in supercritical fluid and , are fugacity of solute in solid phase and supercritical fluid phase, respectively. From thermodynamics, pure solid to pure liquid fugacity ratio is expressed [24] as
In Equation (14), is known as difference in heat capacity between that of solid state minus liquid state, R is well known as universal gas constant. Combining Equations (13) and (14) gives Equation (15) for solubility for a special case where is constant.
In Equation (15), the quantities and are constants for a given substance, therefore the exponential term in Equation (15) is written only in terms of temperature as
In Equation (16), , and .
The required activity coefficients in Equation (16) can be obtained from van Laar equation [24] as
Equation (17) combined with Equation (16) would give the new model as
Equation (18) represents the five parameter model derived based on solid and liquid phase equilibrium criterion and van Laar model for activity coefficient. In Equation (18), , , , and are constants.
3.2. Model 2
In this model, the solid–liquid equilibrium criteria are the same as that of model 1. In place of pure solid to pure liquid fugacity ratio, a second order polynomial in temperature is considered [25]. The consideration may be justified from the actual expression for the fugacity ratio [26,27], which is
Equation (19) gross form is a polynomial in temperature. The polynomial term (for temperature dependence) in literature is also observed with the work presented by Nordström and Rasmuson [25], who fitted the solubility of salicylamide in various solvents at normal pressures. Therefore, fugacity ratio in this work is expressed as a second order polynomial in terms of temperature as exp (A + B/T + C/T2). Therefore, the final expression for solubility is
The required activity coefficients in Equation (20) can be obtained from van Laar equation as in Equation (17). Equation (17) combined with Equation (20) would give the new model as
Equation (21) represents the five parameter model derived based on solid and liquid phase equilibrium criterion and van Laar model for activity coefficient. In Equation (21) A, B, C, A12, and A21 are constants.
4. Methodology
We used fminsearch algorithm which uses the Nelder–Mead simplex as described by Lagarias et al. [28] built in MATLAB software (R2019b) student version to fit models and experimental data collected from literature. Furthermore, we also inspected the quality of modeling through various entities such as correlation coefficient (R2), adjusted R2 (Adj. R2), root mean square deviation (RMSE), sum of squares due to error (SSE), and the overall average absolute relative deviation (AARD) between experimental data and calculated results. The R2, Adj. R2, SSE, and RMSE are evaluated using the following formulas [29]
In Equation (22), and represent the mole fraction of calculated and experimental solubility’s values, respectively. is the global mean value of experimental data in mole fraction.
Statistical comparison of models is essential to ensure the success of the new model. In order to this achieve this, the well-known Akaike’s Information Criterion (AIC) proposed by Akaike [30,31] has been used. AIC is expressed as
In Equation (27), K is number of parameter constants, N is number of data points, SSE is the sum of squares due to error. Importantly, AIC is number of adjustable parameters of the individual model.
5. Results and Discussion
In this study, we propose two new solid–liquid equilibrium criteria models to correlate solubility of solid in supercritical carbon dioxide. The accuracy of the proposed models is evaluated by correlating 25 anthraquinone derivative compounds available in the literature. The correlating ability of the new models are evaluated in terms of: AARD, R2, Adj. R2, SSE, and RMSE. There are more than 25 models available in literature [29] for correlating solubility of solids in supercritical fluids. However, for comparison purposes, we have considered Chrastil model, Adachi and Lu model, Mitra—Wilson model, Keshmiri et al. model, Khansary et al. model, Garlapati and Madras model, Reddy et al., model, and one existing three parameters solid–liquid equilibrium model. These are grouped as three parameter models and five parameter models. Table 2 shows the information of the 25 anthraquinone derivatives considered in this study. Table 2 shows the solubility range and references [4,16,17,18,32,33,34,35,36,37,38] from which the data are obtained. Table 1 shows the physical properties such as melting point, melting enthalpy, and molar volume of the solutes. For some compounds, these properties are not available and for such compounds we have used the Jain et al. method [20] and Fedors method [21] for evaluating the melting enthalpy and solute molar volumes, respectively. The constant parameters of literature models considered, Chrastil, Adachi-Lu, Mitra—Wilson, Keshmiri et al., Khansary et al., Bian et al., Garlapati—Madras, and Reddy et al., are listed in the Supplementary Materials (Tables S1–S8). Table 3 shows the correlation results of the three parameter solid–liquid equilibrium model. Table 4 shows the correlation constants of the new model 1. Table 5 shows the correlation constants of the new model 2. Table 6 shows the overall mean statistical parameters of various solubility models. From Table 6, it is clear that the proposed models show the lowest AARD. The new model 1 shows an overall AARD% of 6.538 and the second model (new model 2) shows an overall AARD% of 6.377. The two models proposed in this work are observed to perform the correlation on a par. Although they look different in functional form, their correlation ability is matching well. This correlating matching ability may be attributed to its oneness in their functional form.

Table 2.
Solubility information of the compounds.

Table 3.
Correlation results of the three parameter solid–liquid equilibrium model (Equations (10)–(12)).

Table 4.
Correlation constants of the new model 1 (Equation (18)).

Table 5.
Correlation constants of the new model 2 (Equation (21)).

Table 6.
Overall mean statistical parameters of solubility models.
To know the efficacy of the proposed models, further analysis is carried out with paired t-test and Akaike’s Information Criterion (AIC). Table 7 shows the paired t-test (paired t-test, p < 0.05) results for AARD, R2, and Adj. R2. From the results, it is clear that AARDs of the new models are statistically significant. Table 7 shows the paired t-test results for SSE and RMSE. From the results, it is clear that SSEs of the new models are not statistically significant. R2, Adj.R2, and RMSE are showing mixed results and hence we could not infer any statistical meaning from them such as significant or not significant. Table 8 shows AIC information of the proposed models and literature models. From Table 4 and Table 5, the new models are significantly different at 95% confidence level (paired t-test, p < 0.05). Table 8 of AIC information shows that among all models, the new models are having lower AIC values. The AIC value for the new model 1 is −730.59, and for the new model 2 is −1177.56. The lower AIC value indicates the goodness of the new models and we conclude that those models are superior to other models considered in the work.

Table 7.
a. Paired t-test results for averaged absolute relative deviation (AARD), R2 and Adj.R2, b. Paired t-test results for sum of squares due to error (SSE) and RMSE.

Table 8.
AIC information of the proposed models and literature models.
From new model 1 constants, one can calculate melting temperature and melting enthalpies. The back calculations are a bit tricky and we need to use a root finding method to calculate melting temperature and then melting enthalpy is estimated. The calculated values are reported in Table 9. It is observed that the melting temperature is much lower than actual values (Table 1); whereas the melting enthalpies for few compounds (Compound numbers 9, 11, 20, and 24) are magnitude wise matching with the computed values reported in Table 1 (Jain et al. method). This disparity may be attributed to use of approximate empirical expression for the fugacity ratio for the development of the solubility expression. Probably, exact expression would give better results and this is out of the scope of the present work.

Table 9.
Computed Tm and from new model 1.
To illustrate the ability of the proposed models, solubility data of 1-amino-2,3-dimethyl-9,10-anthraquinone in supercritical carbon dioxide were selected as illustrated in Figure 1, Figure 2, Figure 3 and Figure 4, respectively. In another illustration, we selected Red 15 (1-amino-4-hydroxyanthraquinone) to show the goodness of the new model 1 and the new model 2 (Figure 5 and Figure 6). In Figure 7, the global mean AARD% values of all models is depicted. In terms of global mean AARD, the overall order for the ability correlating of the models is: new model 2 > new model 1 > Adachi and Lu > Garlapati and Madras > Keshmiri et al. > Chrastil > Khansary et al. > Bian et al. > Mitra and Wilson > SLE model > Reddy et al.
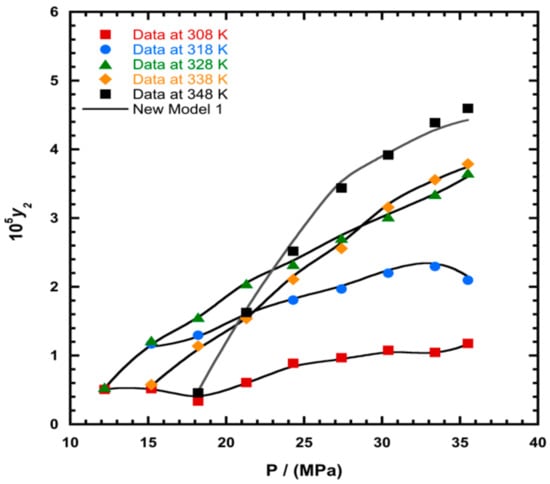
Figure 1.
Plot of mole fraction (y2) as a function of pressure (P/MPa) for 1-amino-2,3-dimethyl-9,10-anthraquinone, the solid line represents the proposed model 1 (Equation (18)).
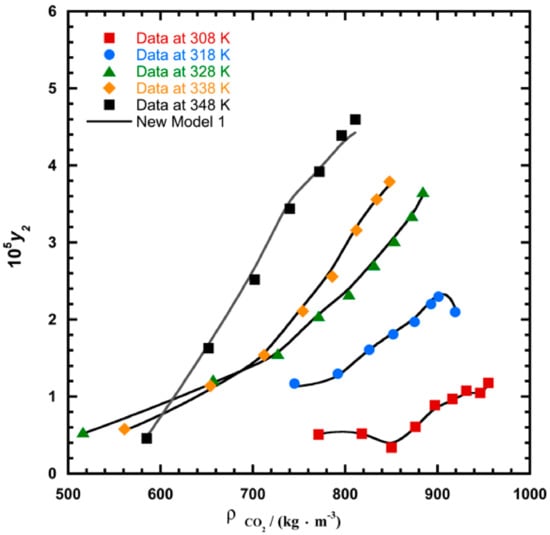
Figure 2.
Plot of mole fraction (y2) as a function of density /(kg.m−3) for 1-amino-2,3-dimethyl-9,10-anthraquinone, the solid line represents the proposed model 1 (Equation (18)).
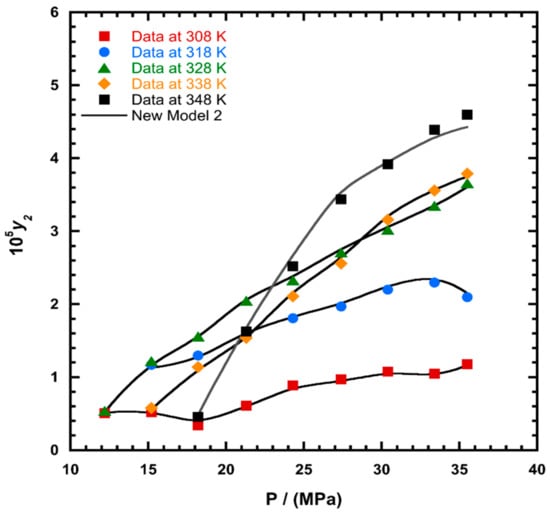
Figure 3.
Plot of mole fraction (y2) as a function of pressure (P/MPa) for 1-amino-2,3-dimethyl-9,10-anthraquinone, the solid line represents the proposed model 2 (Equation (21)).
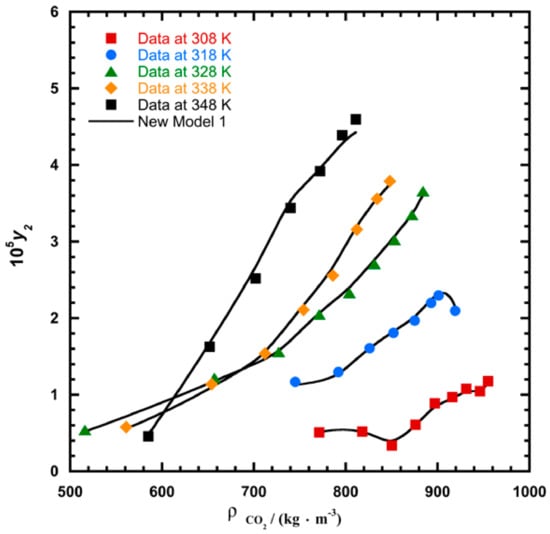
Figure 4.
Plot of mole fraction (y2) as a function of density /(kg.m−3) for 1-amino-2,3-dimethyl-9,10-anthraquinone, the solid line represents the proposed model 2 (Equation (21)).
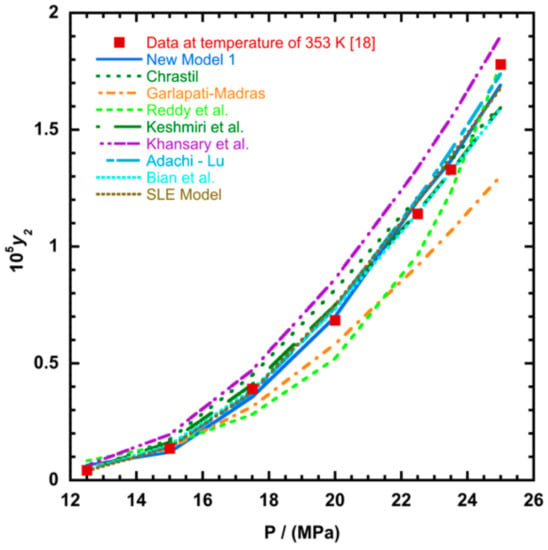
Figure 5.
Plot of mole fraction solubility of Red 15 (1-amino-4-hydroxyanthraquinone) against pressure (P/MPa), the solid line presents the new model 1 (Equation (18)).
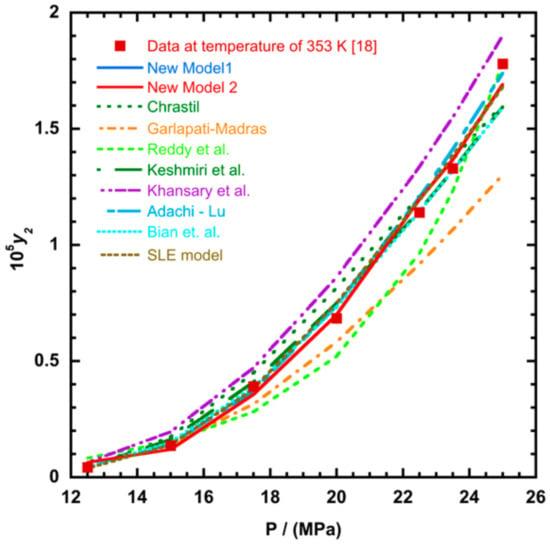
Figure 6.
Plot of mole fraction solubility of Red 15 (1-amino-4-hydroxyanthraquinone) against pressure (P/MPa), the solid lines present the new model 1 (Equation (18)) and the new model 2 (Equation (21)), respectively.
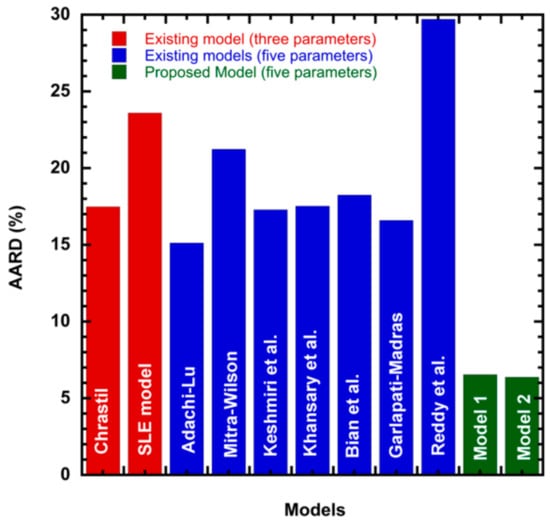
Figure 7.
Global mean AARD% of literature models and the proposed model.
6. Conclusions
The new models developed in this study may be useful in correlating solubility data of any compound in supercritical fluid. A comparison between the proposed models and some specific literature models (particularly three and five parameters constants) was made to correlate the solubility of 25 anthraquinone derivatives. The results showed that the proposed models exhibited excellent agreement with those experimental data in the literature and that the proposed models are superior to all of the other models considered in the present work with AARD of 6.538% for new model 1, and AARD of 6.377% for new model 2. The new models of this work can be used for modeling solubility of any other system.
Supplementary Materials
The following are available online, Table S1: Correlation Constants of Chrastil’s model, Table S2: Correlation Constants of Adachi-Lu model, Table S3: Correlation Constants of Mitra -Wilson model, Table S4: Correlation Constants of Keshmiri et al. model, Table S5: Correlation Constants of Khansary et al. model, Table S6: Correlation Constants of Bian et al. model, Table S7: Correlation Constants of Garlapati – Madras model, Table S8: Correlation Constants of Reddy et al. model.
Author Contributions
Conceptualization, R.S.A. and C.G.; methodology, R.S.A., C.G., and K.T.; software, R.S.A. and C.G.; validation, R.S.A., C.G., and K.T.; investigation, R.S.A.; writing—original draft preparation, R.S.A. and C.G.; writing—review and editing, R.S.A., C.G., and K.T.; supervision, C.G., and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Directorate of Research and Community Services, Directorate General of Strengthening Research and Development, Ministry of Research Technology and Higher Education, Republic Indonesia.
Data Availability Statement
Datas are available from the authors.
Acknowledgments
Research Grant sponsored by Directorate of Research and Community Services, Directorate General of Strengthening Research and Development, Ministry of Research Technology and Higher Education, Republic Indonesia (No. B/87/E3/RA.00/2020).
Conflicts of Interest
The authors declare no competing interests.
References
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420005995. [Google Scholar]
- Garlapati, C.; Madras, G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta 2010, 500, 123–127. [Google Scholar] [CrossRef]
- Sodeifian, G.; Garlapati, C.; Hazaveie, S.M.; Sodeifian, F. Solubility of 2,4,7-Triamino-6-phenylpteridine (Triamterene, Diuretic Drug) in Supercritical Carbon Dioxide: Experimental Data and Modeling. J. Chem. Eng. Data 2020, 65, 4406–4416. [Google Scholar] [CrossRef]
- Alwi, R.S.; Tamura, K. Measurement and Correlation of Derivatized Anthraquinone Solubility in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2015, 60, 3046–3052. [Google Scholar] [CrossRef]
- Kramer, A.; Thodos, G. Solubility of 1-hexadecanol and palmitic acid in supercritical carbon dioxide. J. Chem. Eng. Data 1988, 33, 230–234. [Google Scholar] [CrossRef]
- Iwai, Y.; Koga, Y.; Fukuda, T.; Arai, Y. Correlation of Solubilities of High-Boiling Components in Supercritical Carbon Dioxide Using a Solution Model. J. Chem. Eng. Jpn. 1992, 25, 757–760. [Google Scholar] [CrossRef]
- Su, C.-S.; Chen, Y.-P. Correlation for the solubilities of pharmaceutical compounds in supercritical carbon dioxide. Fluid Phase Equilib. 2007, 254, 167–173. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Sridar, R.; Bhowal, A.; Garlapati, C. A new model for the solubility of dye compounds in supercritical carbon dioxide. Thermochim. Acta 2013, 561, 91–97. [Google Scholar] [CrossRef]
- Adachi, Y.; Lu, B.C.-Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilib. 1983, 14, 147–156. [Google Scholar] [CrossRef]
- Mitra, S.; Wilson, N.K. An Empirical Method to Predict Solubility in Supercritical Fluids. J. Chromatogr. Sci. 1991, 29, 305–309. [Google Scholar] [CrossRef]
- Keshmiri, K.; Vatanara, A.; Yamini, Y. Development and evaluation of a new semi-empirical model for correlation of drug solubility in supercritical CO2. Fluid Phase Equilib. 2014, 363, 18–26. [Google Scholar] [CrossRef]
- Asgarpour Khansary, M.; Amiri, F.; Hosseini, A.; Hallaji Sani, A.; Shahbeig, H. Representing solute solubility in supercritical carbon dioxide: A novel empirical model. Chem. Eng. Res. Des. 2015, 93, 355–365. [Google Scholar] [CrossRef]
- Bian, X.-Q.; Zhang, Q.; Du, Z.-M.; Chen, J.; Jaubert, J.-N. A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid Phase Equilib. 2016, 411, 74–80. [Google Scholar] [CrossRef]
- Reddy, T.A.; Srividya, R.; Garlapati, C. A new empirical model to correlate solubility of pharmaceutical compounds in supercritical carbon dioxide. J. Appl. Sci. Eng. Methodol. 2018, 4, 575–590. [Google Scholar]
- Alwi, R.S.; Tanaka, T.; Tamura, K. Measurement and correlation of solubility of anthraquinone dyestuffs in supercritical carbon dioxide. J. Chem. Thermodyn. 2014, 74. [Google Scholar] [CrossRef]
- Tamura, K.; Alwi, R.S.; Tanaka, T.; Shimizu, K. Solubility of 1-aminoanthraquinone and 1-nitroanthraquinone in supercritical carbon dioxide. J. Chem. Thermodyn. 2017, 104. [Google Scholar] [CrossRef]
- Tamura, K.; Alwi, R.S. Solubility of anthraquinone derivatives in supercritical carbon dioxide. Dye. Pigment. 2015, 113. [Google Scholar] [CrossRef]
- Tamura, K.; Fukamizu, T. Representation of solubilities of phenylthioanthraquinone in supercritical carbon dioxide using Hansen solubility parameter. Fluid Phase Equilib. 2019, 489, 68–74. [Google Scholar] [CrossRef]
- Jain, A.; Yang, G.; Yalkowsky, S.H. Estimation of Melting Points of Organic Compounds. Ind. Eng. Chem. Res. 2004, 43, 7618–7621. [Google Scholar] [CrossRef]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Giddings, J.C.; Myers, M.N.; McLaren, L.; Keller, R.A. High Pressure Gas Chromatography of Nonvolatile Species. Science 1968, 162, 67–73. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology. U.S. Department of Commerce. Available online: https://webbook.nist.gov/chemistry (accessed on 28 September 2020).
- Prausnitz, J.M.; Lichtenthaler, R.N.; de Azvedo, E.G. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Nordström, F.L.; Rasmuson, Å.C. Solubility and Melting Properties of Salicylic Acid. J. Chem. Eng. Data 2006, 51, 1668–1671. [Google Scholar] [CrossRef]
- Kramer, A.; Thodos, G. Adaptation of the Flory-Huggins theory for modeling supercritical solubilities of solids. Ind. Eng. Chem. Res. 1988, 27, 1506–1510. [Google Scholar] [CrossRef]
- Lee, J.W.; Min, J.M.; Bae, H.K. Solubility Measurement of Disperse Dyes in Supercritical Carbon Dioxide. J. Chem. Eng. Data 1999, 44, 684–687. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder—Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Reddy, T.A.; Garlapati, C. Dimensionless Empirical Model to Correlate Pharmaceutical Compound Solubility in Supercritical Carbon Dioxide. Chem. Eng. Technol. 2019, 42, 2621–2630. [Google Scholar] [CrossRef]
- Akaike, H. Likelihood of a model and information criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Joung, S.N.; Shin, H.Y.; Park, Y.H.; Yoo, K.-P. Measurement and correlation of solubility of disperse anthraquinone and azo dyes in supercritical carbon dioxide. Korean J. Chem. Eng. 1998, 15, 78–84. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, M.W.; Bae, H.K. Measurement and correlation of dye solubility in supercritical carbon dioxide. Fluid Phase Equilib. 2000, 173, 277–284. [Google Scholar] [CrossRef]
- Coelho, J.P.; Stateva, R.P. Solubility of red 153 and blue 1 in supercritical carbon dioxide. J. Chem. Eng. Data 2011, 56, 4686–4690. [Google Scholar] [CrossRef]
- Ferri, A.; Banchero, M.; Manna, L.; Sicardi, S. An experimental technique for measuring high solubilities of dyes in supercritical carbon dioxide. J. Supercrit. Fluids 2004, 30, 41–49. [Google Scholar] [CrossRef]
- Fat’hi, M.R.; Yamini, Y.; Sharghi, H.; Shamsipur, M. Solubilities of Some 1,4-Dihydroxy-9,10-anthraquinone Derivatives in Supercritical Carbon Dioxide. J. Chem. Eng. Data 1998, 43, 400–402. [Google Scholar] [CrossRef]
- Shamsipur, M.; Karami, A.R.; Yamini, Y.; Sharghi, H. Solubilities of Some Aminoanthraquinone Derivatives in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2003, 48, 71–74. [Google Scholar] [CrossRef]
- Shamsipur, M.; Karami, A.R.; Yamini, Y.; Sharghi, H. Solubilities of some 1-hydroxy-9,10-anthraquinone derivatives in supercritical carbon dioxide. J. Supercrit. Fluids 2004, 32, 47–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).