1. Introduction
Voice prostheses (VPs) are indwelling silicone valves that are used for speech rehabilitation in patients with laryngeal malignancies after laryngectomy [
1]. Microbial biofilm formation on VPs is a major problem that requires its frequent replacement and increases the risk of chest infection and pneumonia [
2]. Microbial growth is promoted in the environment of the esophagus where they are exposed to food, saliva, liquids, humidity, and temperature close to 37 °C [
1].
Candida albicans is the predominant fungal species and
Staphylococcus aureus is the most frequent bacterial colonizer found on VPs [
2]. In addition,
C. albicans better colonizes surfaces in CO
2-rich environments such as those provided by exhaled breath, and the fungi can also penetrate the silicone of the device, eventually rendering it completely unusable [
1,
2]. Due to fungal and bacterial proclivity for growth in such a favorable environment, the median VP lifespan is reported to range between 60–92 days, depending on the device [
3,
4].
A number of approaches have been proposed to increase the VPs lifespan, such as the incorporation of silver oxide (Blom Singer Advantage) and Teflon-like fluoroplastic (Provox ActiValve) within the VPs material to prevent microbial colonization [
1,
5,
6]. Although the Blom Singer Advantage and Provox ActiValve showed reduced
Candida biofilm formation in comparison to the unmodified Provox 2 and Provox Vega after repeated exposures to the fungi, still biofilms were formed on these devices [
5]. Attempts have also been made to coat the silicone VPs with gold or titanium to create a
Candida-resistant surface [
7]. However,
Candida biofilm was formed on the gold-coated VPs after 1 month use by the patients [
7]. De Prijck et al. [
8] covalently bound antimicrobial quaternary ammonium compounds dimethylamino-ethyl methacrylate and polyethylenimine to the silicon surfaces, and observed that it is possible to prevent
Candida biofilm formation after one time exposure to the fungi for 24 h. Other approaches for decontamination of oropharyngeal yeast include amphotericin B lozenges and buccal adhesive slow-release tablets containing miconazole [
1]. However, once biofilms are formed, it is extremely difficult to eradicate them since the microbes in biofilms are much less sensitive to antimicrobial drugs when compared to planktonic growth [
9]. Hence, it is important to develop new strategies for the prevention of initial microbial colonization on the VPs. The prevention and control of biofilm formation is not only beneficial for the lifespan of the prosthesis, but also for overall patient health and for his quality of life.
Herein we propose a unique method for VP coating using a sustained-release varnish (SRV) containing the broad-spectrum antifungal drug clotrimazole (CTZ), that forms a thin film coating on the VPs. SRV is a pharmaceutical technology that has the advantage of delivering the drug in controlled dosages over a long period of time, thereby providing a more consistent antimicrobial environment [
10]. Such varnishes embedded with different active agents have previously been shown to provide long-term antimicrobial activities in other medical systems including oral care [
10] and catheter-associated urinary tract infections [
11,
12].
CTZ is a well-tolerated fungistatic drug which acts by targeting the biosynthesis of ergosterol, an essential component of the fungal plasma membrane [
13]. In addition, CTZ and its related miconazole have been documented to exert antibacterial activity toward certain
Staphylococcus species [
14,
15], although at 20–40 times higher concentrations than those needed for the fungistatic effect. In previous studies, a formulation of SRV-CTZ was demonstrated to relieve oral stomatitis and reduce the
Candida level when applied to the upper denture [
16]. Herein we demonstrate that this SRV may be utilized to protect voice prosthesis from
C. albicans infection and biofilm formation, thereby extending the lifespan of the device.
3. Discussion
Microbial biofilm formation on medical devices, including VPs, is a major health problem resulting in replacement of the device involving additional procedures, risks, cost, and recovery [
18]. Fungal biofilm formation of VP is a common cause requiring its removal, cleaning and/or installment of a new prosthesis. Fungal infections of VPs, especially during the initial period following its insertion, are a major cause of device failure, often necessitating replacement. This may also cause local or systemic inflammation. Therefore, there is a compelling need for the development of a method to inhibit the initial biofilm formation on VPs, without being reliant on patient compliance. Many strategies have been examined during the years to prevent fungal infection and biofilm formation such as coating the surfaces with antibiotics or antiseptics, or, alternatively, coating the surfaces with a material that displays low adhesive properties [
18]. Despite all of these efforts, the lifespan of VPs may be limited to approximately 2–3 months. Our present approach of applying a coating on the VPs using a sustained release delivery system containing the highly potent antifungal drug CTZ holds great clinical potential. The particular advantages associated with the use of this SRV include: (1) prolonged duration of drug concentration in the targeted area; (2) controlled dosages over time; (3) low dosage per time; (4) easy application by the user; (5) no reliance on patient compliance; and (6) improved microbial and clinical outcome.
CTZ was chosen to be the drug incorporated into the SRV because of its proven antifungal properties [
13]. Its low solubility in water makes it an ideal drug for the sustained release device. Another advantage is that CTZ is stable at 37 °C, obviating the concern that the drug may degrade while the VP is in place. Sustained antifungal effect was observed as the CTZ continued to be released from the SRV-coated VPs in sufficient amounts to inhibit fungal growth of
C. albicans at least for the test period of 60 days. Significant inhibition was still observed after 60 days of exposure to
C. albicans, implying that the protective effect of the SRV could be even longer, delaying the colonization of the VP, which at the end dictates the useful lifespan of the implant. Moreover, the amount of the drug retained in the film is sufficient to hinder biofilm formation on the prosthesis. It is likely that the antibiofilm effect of SRV-CTZ is caused by the antifungal activity of CTZ. Further studies need to be performed to determine whether CTZ has a direct antibiofilm activity. We observed that CTZ prevented yeast–hyphae transition (
Figure 5), which might contribute to the antibiofilm activity since the hyphal form is the major virulence factor. The important point is that our technique allows the protection of the VPs from
Candida biofilm formation. The dual activity of preventing the growth of fungi in the vicinity of the VPs together with the hindrance of fungal biofilm formation on the VPs for a long period of time, is crucial for the maintenance of the VPs and for improving the health of the patient. Since the throat microflora is heterogeneous, future studies should be conducted that analyze the efficacy of SRV-CTZ on mixed microflora of fungi and bacteria before proceeding to clinical trials.
Another concern is the appearance of drug-resistant fungi. There are fungal species that are intrinsic resistant to azoles [
13]. However, the most prevalent fungal colonizer of VPs is the azole-sensitive
C. albicans that is the commensal yeast found on the mucosal surfaces of the oral cavity and gastrointestinal tract [
2]. Long-term treatment with azole drugs has not usually led to drug resistance (The SCCNFP/0706/03 Report), since the resistance is not transferred between pathogenic fungi and the mechanisms of resistance differ from those seen in bacteria. In our experimental design, the daily transfer of SRV-CTZ coated VP pieces from an old
C. albicans culture to a new
C. albicans culture resulting in passive transfer of the old cultures to the new one, did not lead to the appearance of CTZ-resistant fungi even after 60 days. Importantly, our study is a proof of principle that incorporating an antifungal drug into a sustained release varnish has the potential to prevent
C. albicans biofilm formation and thus is expected to prolong the lifetime of the device. Further studies should focus on incorporating additional drugs to broaden both the spectrum of antifungal activity as well as antibacterial activity.
The results of this study suggest that SRV-CTZ-coated VPs offers a flexible platform for designing coatings to protect VP surfaces from fungal infection and biofilm formation. Pharmaceutical formulations similar to the proposed SRVs of this type have been used in clinical trials in the oral cavity of human and in catheter-associated urinary tract infections in dogs [
10,
11,
12]. In these studies, the active component of the SRV was either chlorhexidine or thiazolidinedione-8. More so, similar SRV-CTZ preparations have been clinically tested as a pharmaceutical indication for oral candidiasis, supplementing the traditional local therapy [
16,
19]. In the study of Czerninski et al. [
19], the SRV-CTZ was applied on the buccal side of the oral cavity, and the CTZ concentration in the saliva was found to be above the minimum inhibitory concentration (MIC) during the test period of 5 h. In a subsequent study [
16], SRV-CTZ was applied on denture stomatitis patients and compared to CTZ troches (Oralten). After 14 days, the SRV-CTZ treated patients showed significant lower levels of
Candida on the denture surfaces and in saliva, and had a better compliance to the medication [
16]. These studies relied on sufficient release of CTZ to the surroundings to suppress
Candida infection. These studies also showed that the SRV-CTZ was well tolerated. In the present study, we utilized a similar sustained release varnish to show that coating VPs with SRV-CTZ could also prevent
C. albicans biofilm formation on the device for a long time period, besides providing a protective surrounding. Given the current platform, the recommendation for preventive therapy with daily antifungal mouthwashes might become redundant and needs re-evaluation. Furthermore, CTZ-coating of voice prosthesis is expected to potentially improve the treatment outcomes as it eliminates the factor of patient compliance.
5. Materials and Methods
5.1. Voice Prosthesis
The Blom-Singer classic indwelling VPs (InHealth Technology, Carpinteria, CA, USA) were cut into 5–6 pieces of similar sizes and sterilized by immersing them in 70% alcohol overnight, and then dried aseptically.
5.2. Clotrimazole and Placebo Varnishes
The SRV-CTZ was composed of 1.2 g Clotrimazole (CTZ, Sigma), 0.9 g ethylcellulose (Ashland Specialty Ingredients, Wilmington, USA), 0.9 g Klucel EF (Ashland Specialty Ingredients, Switzerland), and 12 mL ethanol [
16]. The dry film contained 40% (
w/
w) CTZ. The placebo varnish contained 0.9 g ethyl cellulose, 0.9 g Klucel EF, and 12 mL ethanol.
5.3. Coating of Voice Prosthesis Pieces
Sterile VP pieces were coated by immersing them in the SRV and drying them at room temperature to allow for the formation of a film on the surface of each piece. This process was repeated twice. The pieces were allowed to dry completely for 3 days before use. The average SRV coating of the pieces was 10–12 mg containing 4–4.8 mg of CTZ. The resulting film adhered well to the VPs and remained attached to the material throughout the tested period of 60 days.
5.4. Fungi Culture Conditions
Candida albicans SC5314 (ATCC MYA-2876) from a frozen vial was seeded on potato dextrose agar (PDA) (Acumedia, Neogen, MI, USA) plates and incubated at room temperature. C. albicans colonies were picked up daily from the agar plates and suspended in RPMI-1640 medium (Sigma, Ronkonkoma, NY USA), and used for the assays described below.
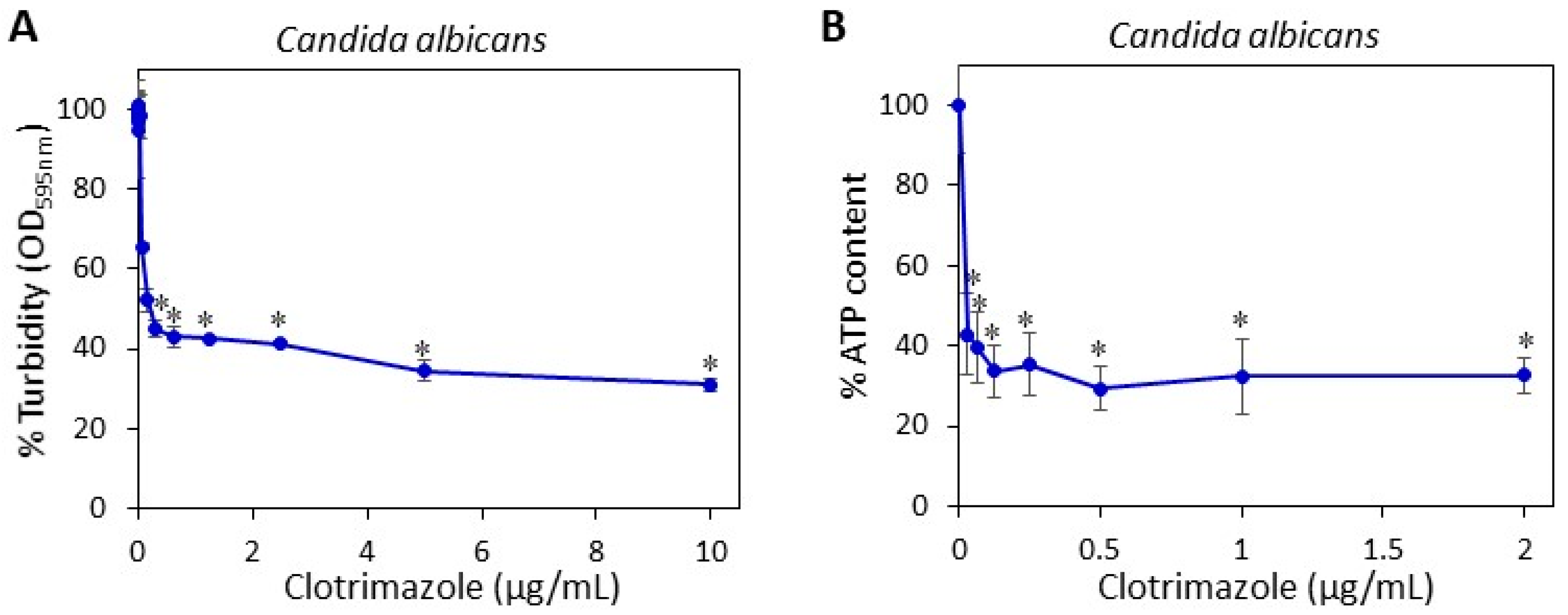
5.5. Determining the Minimum Fungistatic Concentration of Clotrimazole in Microdilution Assay
Two hundred microliters of a suspension of
C. albicans in RPMI with an optical density (OD) of 0.3 at 600 nm were seeded in 96 flat-bottom transparent wells (Corning) in the presence of increasing concentrations of CTZ or an equivalent concentration of ethanol that served as the control. The OD at 600 nm was measured after a 24 h incubation in Infinite M200PRO plate reader (Tecan, Trading AG, Männedorf, Switzerland) [
20]. The percentage of fungi in the samples was calculated using the following formula: (OD
CTZ/OD
PL) × 100, where OD
CTZ is the average OD
600 nm of the CTZ-treated samples and OD
PL the average OD
600 nm of the control samples.
5.6. Agar-Based Activity Assay
PDA plates were seeded daily with 200 µL of freshly prepared C. albicans suspension at an OD600 nm of 0.3. Two pieces of CTZ-coated VPs and two pieces of placebo-coated VPs were transferred daily to C. albicans-coated agar plates that were incubated at 37 °C for 24 h. The clearance zone was calculated by [(d1/2) × (d2/2) × π] − [(d3/2)2 × π], where d1 and d2 are the two diameters of the clearance zone and d3 is the diameter of the VPs.
5.7. Planktonic-Based Activity Assay
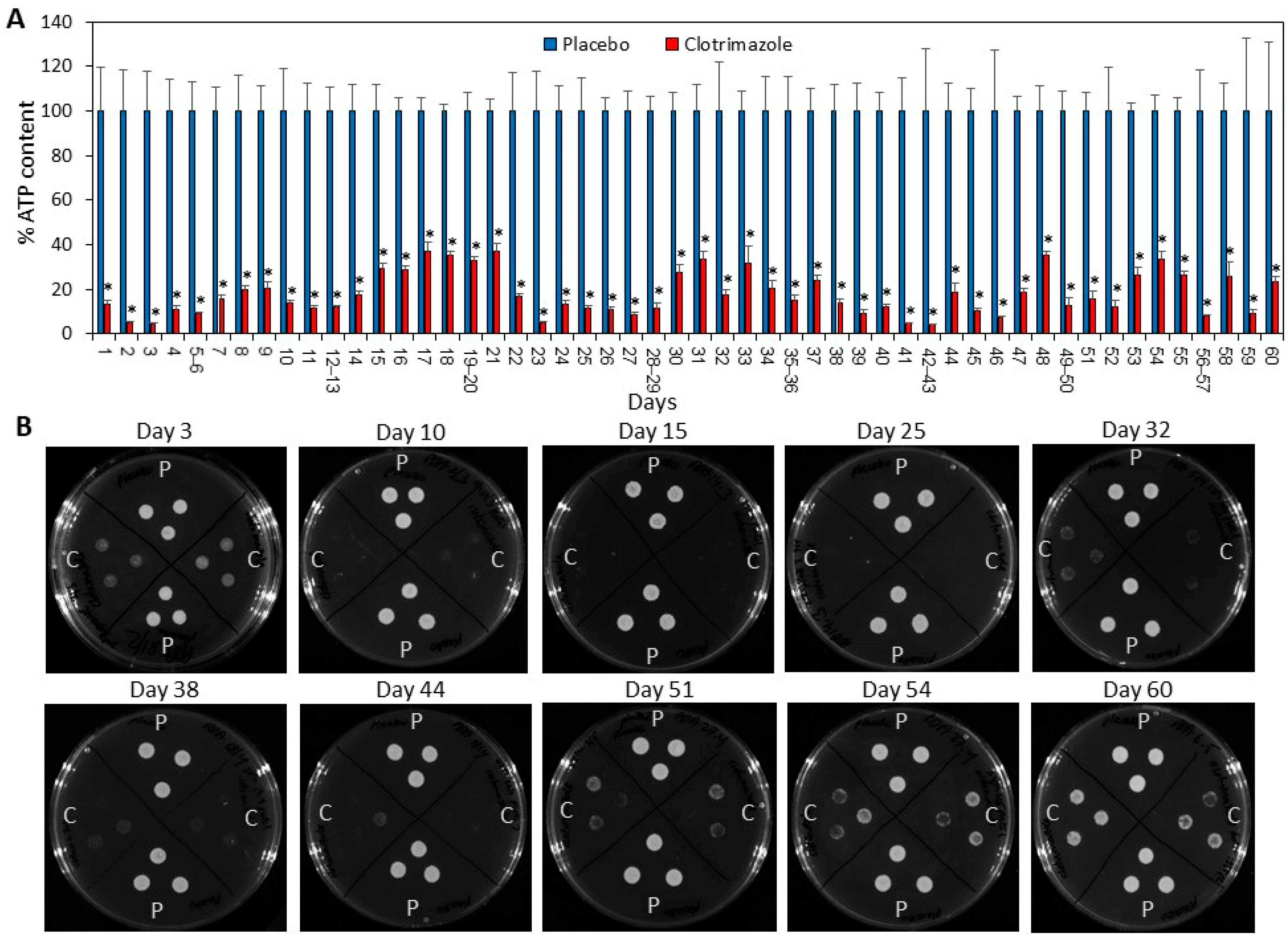
Five pieces of CTZ-coated VPs and five pieces of placebo-coated VPs were transferred daily to 1 mL freshly prepared C. albicans suspension at an OD600 nm of 0.025 in 48-flat-bottomed tissue culture wells (Corning) and incubated at 37 °C for 24 h, except for the weekends where the incubation endured for 48 h. Following each incubation period, the coated VPs were transferred from the old culture to a fresh culture. This was repeated for 14 or 60 days. One piece of each kind (treated and placebo) of coated SRV-VPs was taken for spinning disk confocal microscopy (SDCM) and high-resolution scanning electron microscopy (HR-SEM) for studying biofilm formation (see below) after 14 days of exposure to C. albicans. The other VP pieces were used for continued daily incubation with fresh C. albicans suspension for 60 days. Daily analysis of the C. albicans load in the wells were performed using the drop agar plate method and by measuring the relative ATP content using the BacTiterGlo microbial cell viability assay (Promega Corporation, Madison, WI, USA) (see below), where the ATP content in the placebo group was set to 100%.
5.8. Drop Agar Plate Method
To study the ability of the C. albicans to grow from the samples exposed to SRV-CTZ or SRV-placebo-coated VPs in the planktonic-based activity assay, the fungi in the wells were suspended to a homogenous suspension, and three drops of 10 µL were plated from each well on PDA plates, that were incubated at 37 °C for 24 h.
5.9. BacTiterGlo Microbial Cell Viability Assay
To determine the relative amount of live
C. albicans in the media of the planktonic-based activity assay, 100 µL of the homogeneous fungal suspension from each well were transferred to white 96-well flat-bottomed microplates, to which 100 µL of the BacTiter
Glo reagent (Promega Corporation, Madison, WI, USA) was added [
21]. After 20 min of shaking at room temperature, the luminescence was measured in an Infinite M200PRO plate reader (Tecan, Männedorf, Switzerland). The percentage of viable fungi in the SRV-CTZ group in comparison to SRV-placebo group was calculated using the following formula: (Lum
CTZ/Lum
PL) × 100, where Lum
CTZ is the average luminescence of the samples from the SRV-CTZ group and Lum
PL the average luminescence of the samples from the SRV-placebo group.
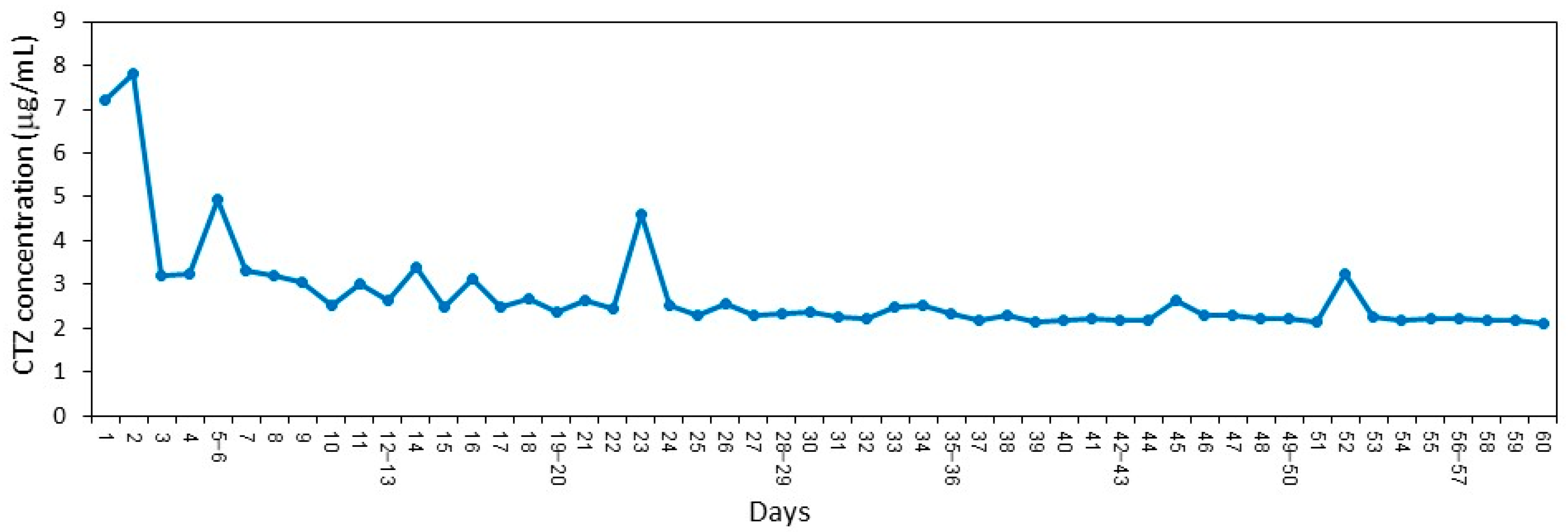
5.10. Determination of the Daily Release of Clotrimazole from SRV-CTZ-coated VPs
SRV-CTZ-coated VP was daily incubated in 1 mL sterile PBS for 24 h at 37 °C for a period of 60 days. The daily release of CTZ into PBS was determined by high-performance liquid chromatography (HPLC) according to the method of de Bruijn et al. [
22]. Next, 100 µL of each sample were automatically injected into a HPLC column packed with Inertsil ODS-80A (5µm particle size; 150 mm (length) × 4.6 mm (internal diameter); GL Science, Tokyo, Japan) using the HP 1090 series HPLC system (Hewlett Packard, Palo Alto, CA, USA). The mobile phase was composed of water–acetonitrile–tetrahydrofuran–ammonium hydroxide–trimethylamine at a ratio of 45:50.2:2.5:0.1:0.1 (
v/
v), pH = 6.0. The column effluent was monitored by UV at a wavelength of 206 nm, and the clotrimazole concentration in the samples were determined according to a standard curve made from known clotrimazole concentrations.
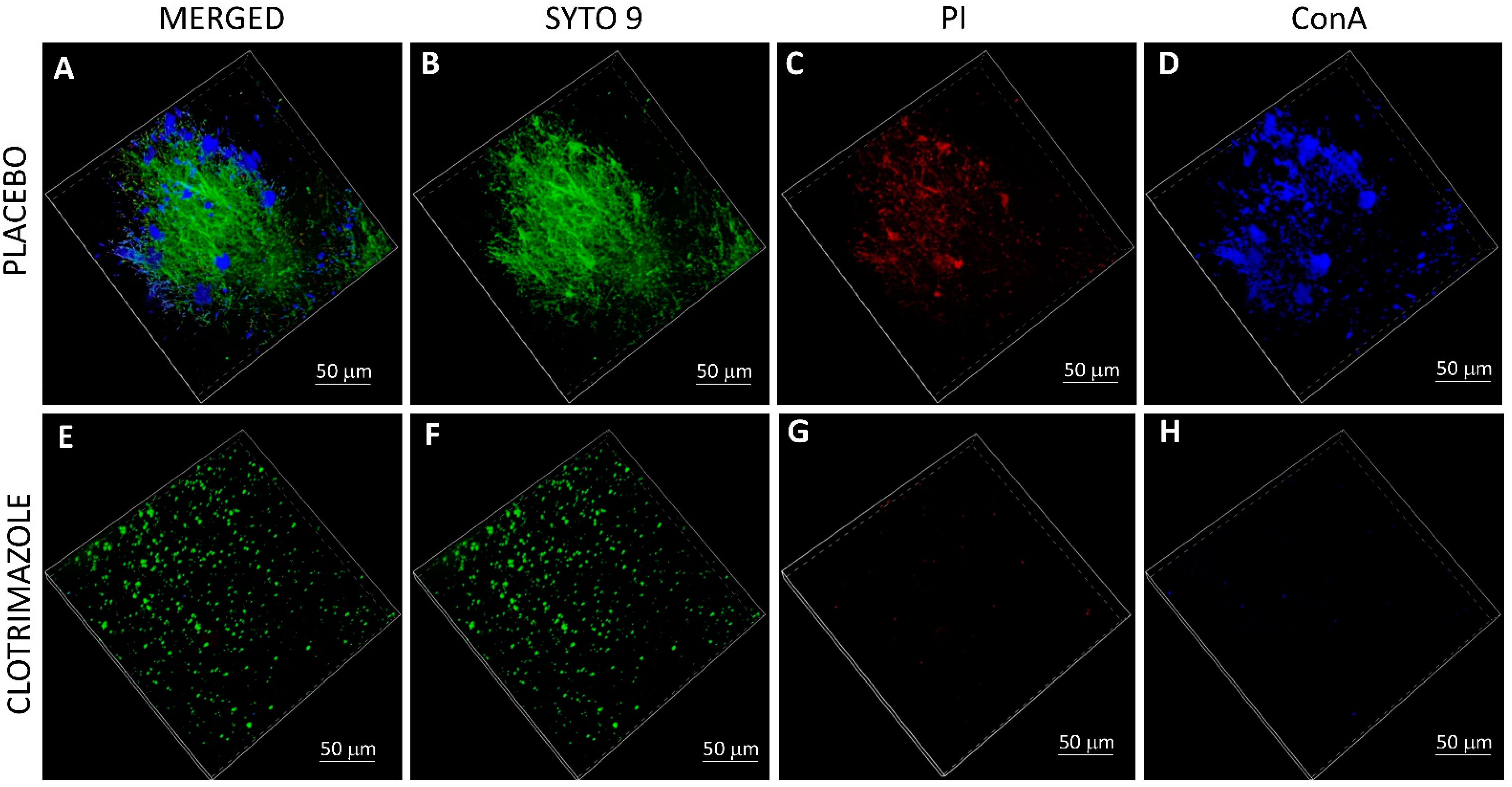
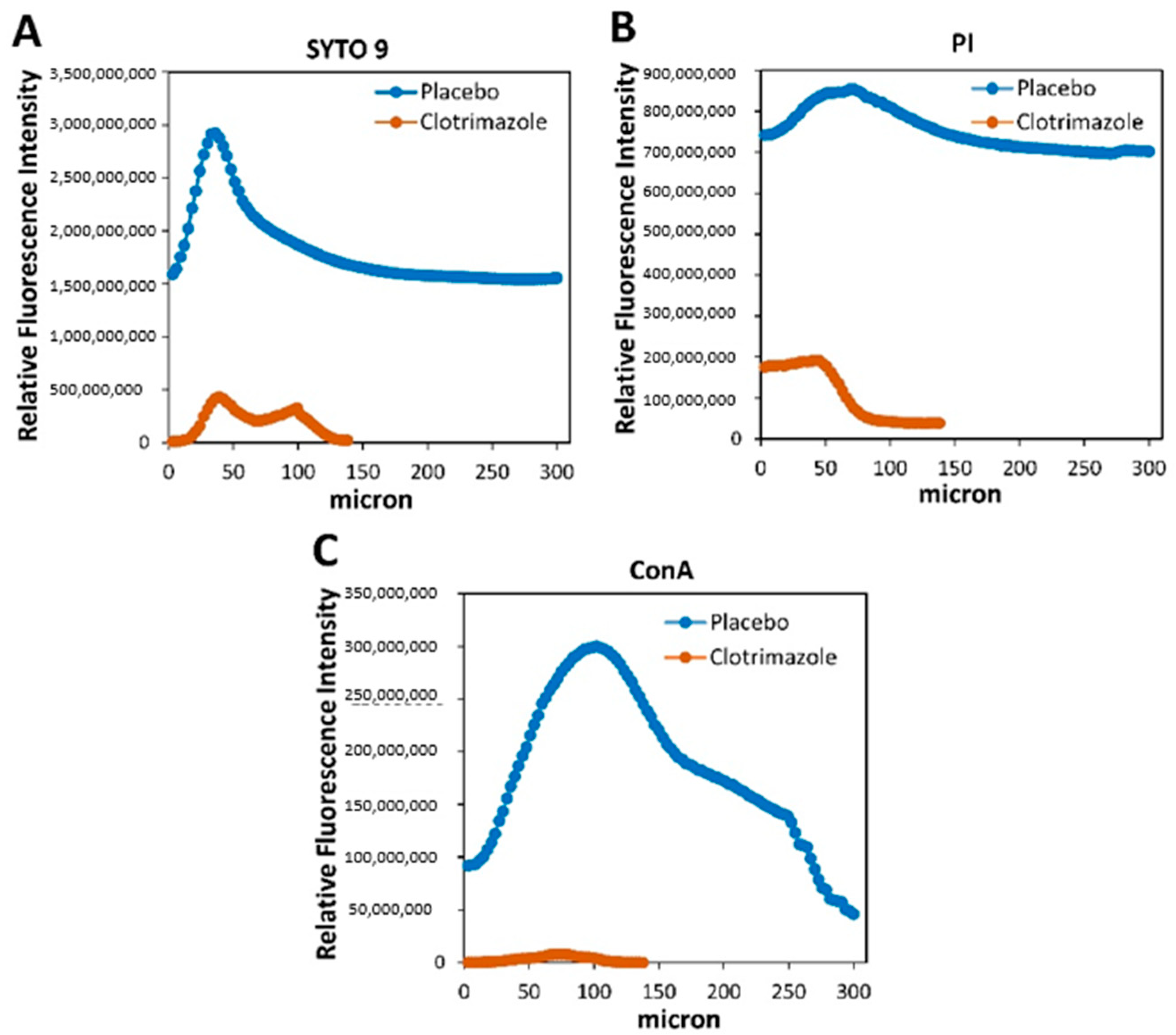
5.11. Live/Dead SYTO 9/Propidium Iodide (PI) Staining and EPS Staining of VPs
After 14 days of exposure to
C. albicans in the planktonic-based activity assay, the SRV-VPs were washed twice in sterile PBS, and stained with 3.25 µM SYTO 9 green fluorescent nucleic acid stain (Invitrogen, Life Technologies Corporation, Eugene, OR, USA), 2.5 µg/mL propidium iodide (PI; Sigma, St. Louis, MO, USA) and 20 µg/mL AlexaFluor
647-conjugated ConA (Invitrogen, Life Technologies Corporation, Eugene, OR, USA) for 20 min at room temperature in the dark [
20]. Thereafter, the samples were washed twice in PBS, fixed in 4% paraformaldehyde in PBS for 1 h, and kept in 50% glycerol in DDW until visualizing the biofilms under a spinning disk confocal microscope (Nikon Yokogawa W1 Spinning Disk, Tokyo, Japan, with 50 µm pinholes; SDCM). The biofilm depth was assessed by capturing optical cross-sections at 3 μm intervals from the bottom of the biofilm to its top. The SYTO 9 green fluorescence dye, which enters both live and dead bacteria, was visualized using 488 nm excitation and 515 nm emission filters. The PI red fluorescence dye, which only penetrates dead bacteria, was measured using 543 nm excitation and 570 nm emission filters. AlexaFluor
647-ConA staining of EPS was measured using 638 nm excitation wavelength and 680 nm emission filter. Thus, live bacteria fluoresce green light, while dead bacteria fluoresce both green and red light. Three-dimensional images of the formed biofilms were reconstructed using the NIS-Element AR software. This software was also used to analyze the fluorescence intensity of SYTO 9, PI and EPS staining in each captured layers of the biofilms. The staining on SRV-CTZ-coated VP was compared to that of SRV-placebo-coated VP.
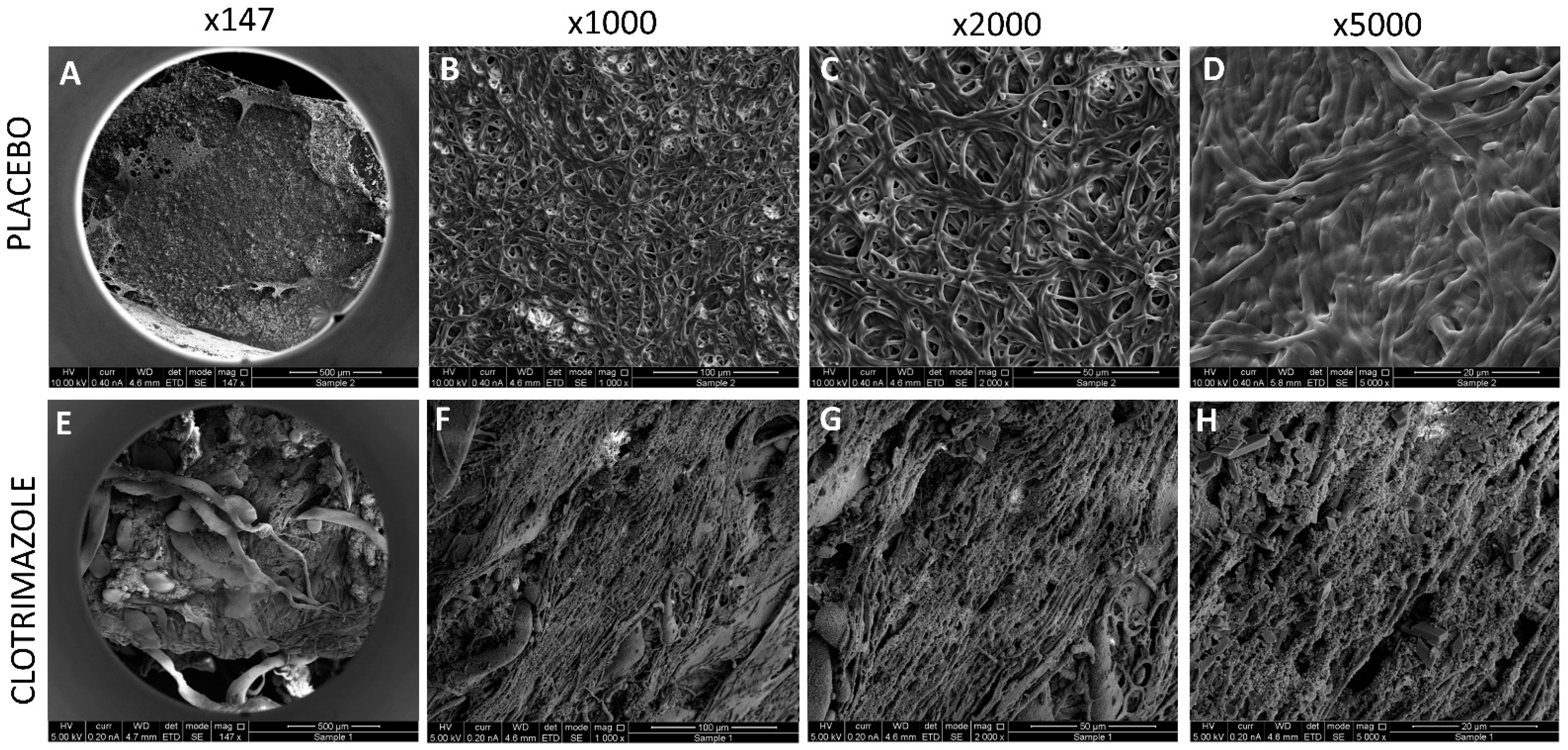
5.12. HR-SEM Imaging of VPs
After 14 days of exposure to C. albicans in the planktonic-based activity assay, the VPs were washed twice in sterile DDW, and fixed in 4% paraformaldehyde in DDW for 1 h. Then the samples were washed again in DDW, dried, and coated with iridium and visualized using a FEI Magellan 400 L High-Resolution Scanning Electron Microscope (HR-SEM; FEI Company, Hillsboro, OR, USA) at 200×–10,000× magnification.
5.13. Statistical Analysis
The data are expressed as the average ± standard deviation. The number of coated pieces used in each experiment varied from 2–5. Statistical analysis was performed using the Microsoft excel software. Student's t-test was used to compare clotrimazole-coated VPs with placebo-coated VPs, with a P value less than 0.05 considered significant.