Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds
Abstract
1. Introduction
2. Nitric Oxide, Its Congeners and Reactive Products
2.1. Formation of NO Redox Congeners
2.2. Nitric Oxide by Reduction of Nitrate and Nitrite: External Sources and Metabolite Recycling
2.3. Properties of the NO Congeners
2.4. Other NO-Derived Reactive Nitrogen Species and Generation of Secondary Radicals
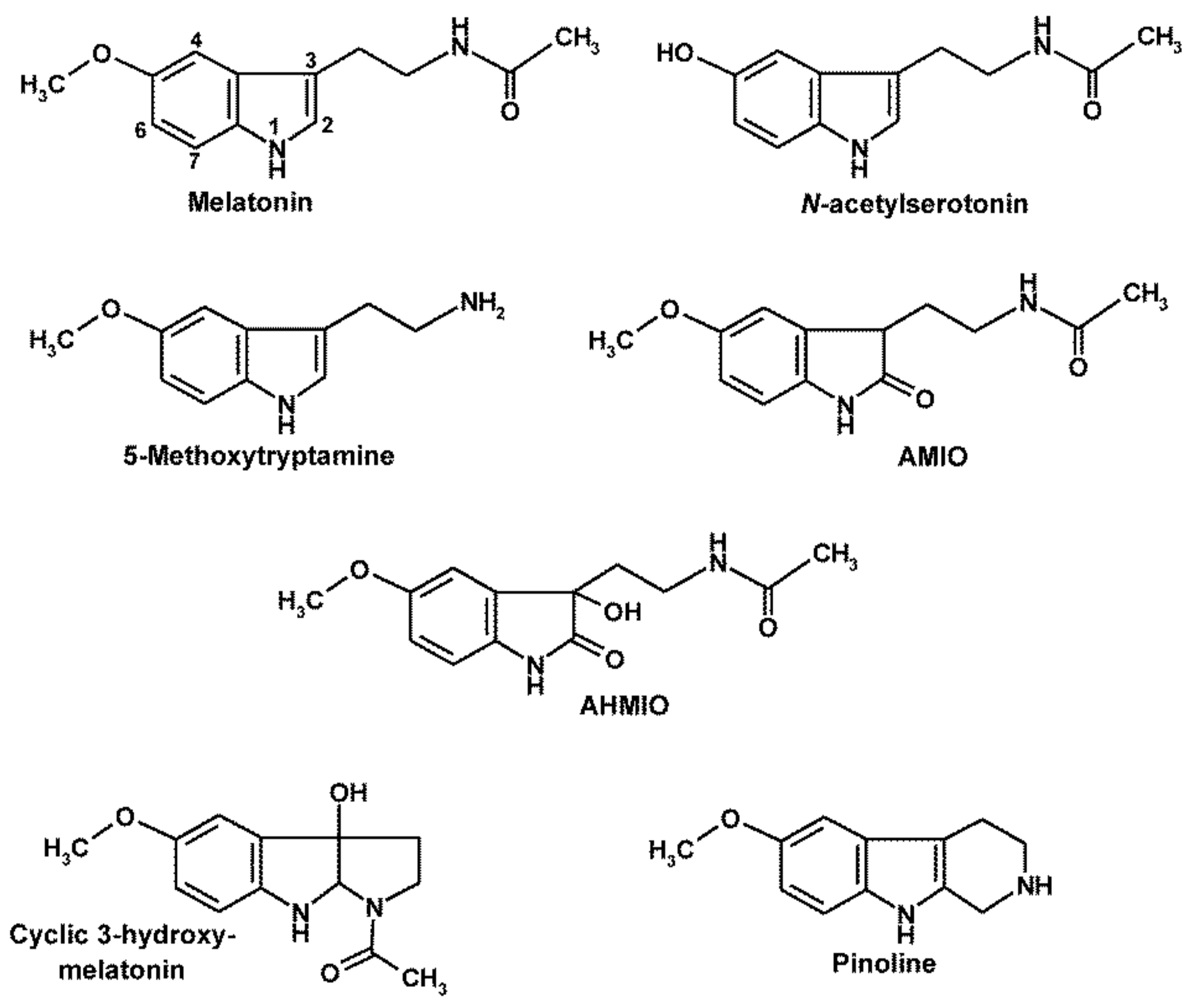
3. Melatonin’s Oxidatively Formed Metabolites
4. Nitrosylation, Nitration and RNS-Mediated Oxidation of Melatonin and Its Metabolites
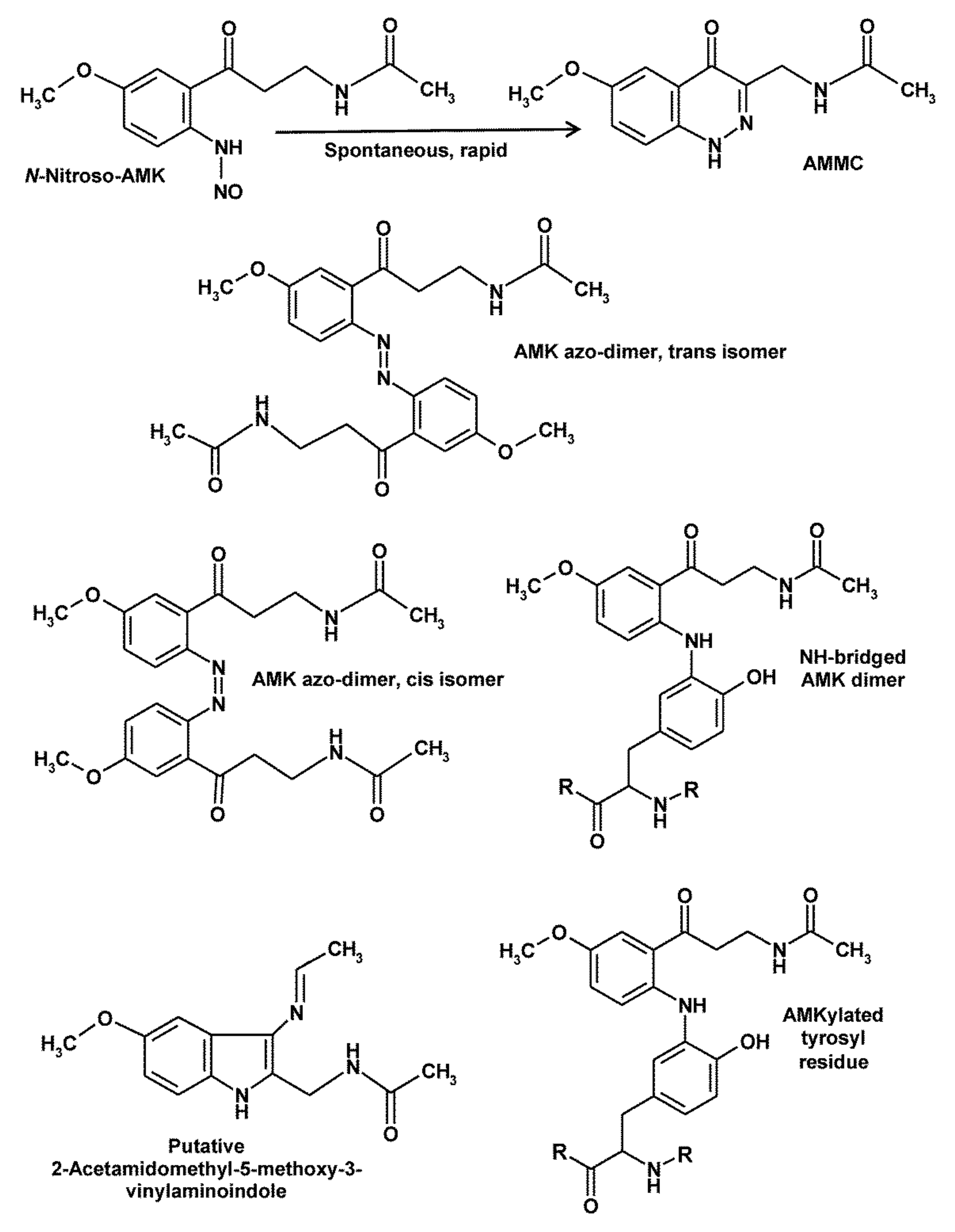
5. Peculiarities of AMK
6. Pathophysiological Relevance of Actions against Nitration, RNS-Mediated Oxidation and Nitrosylation
7. Consequences to Inflammation and Mitochondrial Integrity
8. Conclusions
Funding
Conflicts of Interest
References
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin—Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Divergent importance of chronobiological considerations in high- and low-dose melatonin therapies. Diseases 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Quintela, T.; Gonçalves, I.; Silva, M.; Duarte, A.C.; Guedes, P.; Andrade, K.; Freitas, F.; Talhada, D.; Albuquerque, T.; Tavares, S.; et al. Choroid plexus is an additional source of melatonin in the brain. J. Pineal Res. 2018, 65, e12528. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Chen, L.-D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Tan, D.-X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Entrena, A.; Camacho, M.E.; Carrión, M.D.; López-Cara, L.C.; Velasco, G.; León, J.; Escames, G.; Acuña-Castroviejo, D.; Tapias, V.; Gallo, M.A.; et al. Kynurenamines as neural nitric oxide synthase inhibitors. J. Med. Chem. 2005, 48, 8174–8181. [Google Scholar] [CrossRef]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Ortíz, F.; Ros, E.; Acuña-Castroviejo, D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: Effects of melatonin treatment. Exp. Gerontol. 2006, 41, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; León, J.; Rodríguez, M.I.; Acuña-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pandi-Perumal, S.R.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav. Brain Funct. 2006, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Ortíz, F.; López, A.; García, J.A.; Ros, E.; Acuña-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007, 274, 2135–2147. [Google Scholar] [CrossRef]
- Hardeland, R. Neuroprotection by radical avoidance: Search for suitable agents. Molecules 2009, 14, 5054–5102. [Google Scholar] [CrossRef]
- Hardeland, R.; Coto-Montes, A. New vistas on oxidative damage and aging. Open Biol. J. 2010, 3, 39–52. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, mitochondrial electron flux and leakage: Recent findings and resolution of contradictory results. Adv. Stud. Biol. 2009, 1, 207–230. [Google Scholar]
- Kim, T.-K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell. Endocrinol. 2015, 404, 1–8. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lin, Z.; Li, W.; Reiter, R.J.; Slominski, A.T. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology 2015, 156, 1630–1636. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Fischer, T.W.; Kim, T.-K.; Kleszczyński, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.L.; Schmidt, S.I.; Laatsch, H.; Fotso, S.; Ness, H.; Ressmeyer, A.-R.; Poeggeler, B.; Hardeland, R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acet-amidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 2005, 39, 251–260. [Google Scholar] [CrossRef]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.-X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Omar, S.A.; Webb, A.J. Nitrite reduction and cardiovascular protection. J. Mol. Cell. Cardiol. 2014, 73, 57–69. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid. Med. Cell. Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef] [PubMed]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; Flitney, F.W.; Williams, D.L.H. NO, nitrosonium ions, nitroxide ions and iron-nitrosyls in biology: A chemist’s perspective. Trends Pharmacol. Sci. 1995, 16, 18–22. [Google Scholar] [CrossRef]
- Hardeland, R.; Backhaus, C.; Fadavi, A. Reactions of the NO redox forms NO+, •NO and HNO (protonated NO−) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J. Pineal Res. 2007, 43, 382–388. [Google Scholar] [CrossRef]
- Nagababu, E.; Ramasamy, S.; Abernethy, D.R.; Rifkind, J.M. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem. 2003, 278, 46349–46356. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Kajimura, M.; Yoshimura, Y.; Suematsu, M. Nonendothelial source of nitric oxide in arterioles but not in venules: Alternative source revealed in vivo by diaminofluorescein microfluorography. Circ. Res. 2002, 91, e55–e64. [Google Scholar] [CrossRef]
- Filipović, M.R.; Stanić, D.; Raicević, S.; Spasić, M.; Niketić, V. Consequences of MnSOD interactions with nitric oxide: Nitric oxide dismutation and the generation of peroxynitrite and hydrogen peroxide. Free Radic. Res. 2007, 41, 62–72. [Google Scholar] [CrossRef]
- Fukuto, J.M. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. Br. J. Pharmacol. 2019, 176, 135–146. [Google Scholar] [CrossRef]
- Cheong, E.; Tunbev, V.; Abramson, J.; Salama, G.; Stoyanovsky, D.A. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium 2005, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Wang, W.; Froehlich, J.P.; Huke, S.; Aon, M.A.; Wilson, G.M.; Di Benedetto, G.; O’Rourke, B.; Gao, W.D.; Wink, D.A.; et al. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 2007, 100, 96–104. [Google Scholar] [CrossRef]
- Jon, O.; Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Pinheiro, L.C.; Tanus-Santos, J.E. Mechanisms impairing blood pressure responses to nitrite and nitrate. Nitric Oxide 2019, 85, 35–43. [Google Scholar] [CrossRef]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Fini, H.; Kerman, K. Revisiting the nitrite reductase activity of hemoglobin with differential pulse voltammetry. Anal. Chim. Acta 2020, 1104, 38–46. [Google Scholar] [CrossRef]
- Lim, Y.J.; Foo, T.C.; Yeung, A.W.S.; Tu, X.; Ma, Y.; Hawkins, C.L.; Witting, P.K.; Jameson, G.N.L.; Terentis, A.C.; Thomas, S.R. Human Indoleamine 2,3-Dioxygenase 1 Is an Efficient Mammalian Nitrite Reductase. Biochemistry 2019, 58, 974–986. [Google Scholar] [CrossRef]
- Gherasim, C.; Yadav, P.K.; Kabil, O.; Niu, W.-N.; Banerjee, R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine β-synthase. PLoS ONE 2014, 9, e85544. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant. Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Gattullo, D.; Pagliaro, P.; Marsh, N.A.; Losano, G. New insights into nitric oxide and coronary circulation. Life Sci. 1999, 65, 2167–2174. [Google Scholar] [CrossRef]
- Cherian, L.; Hlatky, R.; Robertson, C.S. Nitric oxide in traumatic brain injury. Brain Pathol. 2004, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp. Gerontol. 2005, 40, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Mónica, F.Z.; Bian, K.; Murad, F. The endothelium-dependent nitric oxide-cGMP pathway. Adv. Pharmacol. 2016, 77, 1–27. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin and the theories of aging: A critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013, 55, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kayahara, M.; Joashi, U.; Mazarakis, N.D.; Sarraf, C.; Edwards, A.D.; Hughes, M.N.; Mehmet, H. Differential induction of apoptosis in Swiss 3T3 cells by nitric oxide and the nitrosonium cation. J. Cell Sci. 1997, 110, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, M.; Felderhoff, U.; Pocock, J.; Hughes, M.N.; Mehmet, H. Nitric oxide (NO•) and the nitrosonium cation (NO+) reduce mitochondrial membrane potential and trigger apoptosis in neuronal PC12 cells. Biochem. Soc. Trans. 1998, 26, S340. [Google Scholar] [CrossRef]
- Kim, S.; Ponka, P. Role of nitric oxide in cellular iron metabolism. Biometals 2003, 16, 125–135. [Google Scholar] [CrossRef]
- Mikhael, M.; Kim, S.F.; Schranzhofer, M.; Soe-Lin, S.; Sheftel, A.D.; Mullner, E.W.; Ponka, P. Iron regulatory protein-independent regulation of ferritin synthesis by nitrogen monoxide. FEBS J. 2006, 273, 3828–3836. [Google Scholar] [CrossRef]
- Wink, D.A.; Miranda, K.M.; Katori, T.; Mancardi, D.; Thomas, D.D.; Ridnour, L.; Espey, M.G.; Feelisch, M.; Colton, C.A.; Fukuto, J.M.; et al. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: A novel redox paradigm. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2264–H2276. [Google Scholar] [CrossRef]
- Paolocci, N.; Katori, T.; Champion, H.C.; St John, M.E.; Miranda, K.M.; Fukuto, J.M.; Wink, D.A.; Kass, D.A. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: Independence from beta-adrenergic signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 5537–5542. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P.; Mancardi, D.; Rastaldo, R.; Penna, C.; Gattullo, D.; Miranda, K.M.; Feelisch, M.; Wink, D.A.; Kass, D.A.; Paolocci, N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003, 34, 33–43. [Google Scholar] [CrossRef]
- Miranda, K.M.; Paolocci, N.; Katori, T.; Thomas, D.D.; Ford, E.; Bartberger, M.D.; Espey, M.G.; Kass, D.A.; Feelisch, M.; Fukuto, J.M.; et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2003, 100, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Feelisch, M.; Fukuto, J.; Chistodoulou, D.; Jourd’heuil, D.; Grisham, M.B.; Vodovotz, Y.; Cook, J.A.; Krishna, M.; DeGraff, W.G.; et al. The cytotoxicity of nitroxyl: Possible implications for the pathophysiological role of NO. Arch. Biochem. Biophys. 1998, 351, 66–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiva, S.; Crawford, J.H.; Ramachandran, A.; Ceaser, E.K.; Hillson, T.; Brookes, P.S.; Patel, R.P.; Darley-Usmar, V.M. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem. J. 2004, 379, 359–366. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Cooper, C.E. Reactions of nitric oxide with mitochondrial cytochrome c: A novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 1998, 332, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; He, N.; Wang, Y.; Kang, Q.; Shen, D.; Yu, F.; Chen, L. Imaging of anti-inflammatory effects of HNO via a near-infrared fluorescent probe in cells and in rat gouty arthritis model. J. Mater. Chem. B 2019, 7, 305–313. [Google Scholar] [CrossRef]
- Peyrot, F.; Houée-Levin, C.; Ducrocq, C. Melatonin nitrosation promoted by NO*2; comparison with the peroxynitrite reaction. Free Radic. Res. 2006, 40, 910–920. [Google Scholar] [CrossRef]
- Kirsch, M.; Groot, H. Detection of N-nitrosomelatonin and other N-nitrosotryptophan derivatives by transnitrosation of APF and DAF-2. J. Pineal Res. 2006, 40, 10–17. [Google Scholar] [CrossRef]
- Nedospasov, A.A. Is N2O3 the main nitrosating intermediate in aerated nitric oxide (NO) solutions in vivo? If so, where, when, and which one? J. Biochem. Mol. Toxicol. 2002, 16, 109–120. [Google Scholar] [CrossRef]
- Basu, S.; Grubina, R.; Huang, J.; Conradie, J.; Huang, Z.; Jeffers, A.; Jiang, A.; He, X.; Azarov, I.; Seibert, R.; et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 2007, 3, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Goetz, B.I.; Shields, H.W.; Basu, S.; Wang, P.; King, S.B.; Hogg, N.; Gladwin, M.T.; Kim-Shapiro, D.B. An electron paramagnetic resonance study of the affinity of nitrite for methemoglobin. Nitric Oxide 2010, 22, 149–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. The underrated carbonate radical (CO3•−)—Detoxification and reduced formation by melatonin. Biomed. J. Sci. Tech. Res. 2017, 1, 264. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Taxon- and site-specific melatonin catabolism. Molecules 2017, 22, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Hardies, L.J.; Weintraub, S.T.; Shepherd, A.M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998, 253, 614–620. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin beyond its classical functions. Open Physiol. J. 2008, 1, 1–23. [Google Scholar] [CrossRef]
- Pähkla, R.; Zilmer, M.; Kullisaar, T.; Rägo, L. Comparison of the antioxidant activity of melatonin and pinoline in vitro. J. Pineal Res. 1998, 24, 96–101. [Google Scholar] [CrossRef]
- Dose, A.; Poeggeler, P.; Schoenke, M.; Zsizsik, B.K.; Hardeland, R. Pinoline [6-methoxy-1,2,3,4-tetrahydro-9H-pyrido-(3,4-b)-indole] as a radical scavenger. I. Scavenging properties and multiplicity of products. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 101–106. [Google Scholar]
- Thuermann, S.; Hardeland, R.; Poeggeler, B. Pinoline [6-methoxy-1,2,3,4-tetra-hydro-9H-pyrido-(3,4-b)-indole] as a radical scavenger. II. Chemiluminescence during oxidation. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 2001; pp. 107–113. [Google Scholar]
- Piñol-Ripoll, G.; Fuentes-Broto, L.; Millán-Plano, S.; Reyes-Gonzáles, M.; Mauri, J.A.; Martínez-Ballarín, E.; Reiter, R.J.; García, J.J. Protective effect of melatonin and pinoline on nitric oxide-induced lipid and protein peroxidation in rat brain homogenates. Neurosci. Lett. 2006, 405, 89–93. [Google Scholar] [CrossRef]
- Diem, S.; Gutsche, B.; Herderich, M. Degradation of tetrahydro-β-carbolines in the presence of nitrite: HPLC-MS analysis of the reaction products. J. Agric. Food Chem. 2001, 49, 5993–5998. [Google Scholar] [CrossRef]
- Herraiz, T.; Galisteo, J. Nitrosative deamination of 2′-deoxyguanosine and DNA by nitrite, and antinitrosating activity of β-carboline alkaloids and antioxidants. Food Chem. Toxicol. 2018, 112, 282–289. [Google Scholar] [CrossRef]
- Burkhardt, S.; Reiter, R.J.; Tan, D.-X.; Hardeland, R.; Cabrera, J.; Karbownik, M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int. J. Biochem. Cell Biol. 2001, 33, 775–783. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.-X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ressmeyer, A.-R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.-X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Ressmeyer, A.-R.; Zelosko, V.; Burkhardt, S.; Poeggeler, B. Metabolites of melatonin: Formation and properties of the methoxylated kynuramines AFMK and AMK. In Recent Advances in Endocrinology and Reproduction: Evolutionary, Biotechnological and Clinical Applications; Haldar, C., Singh, S.S., Eds.; Banaras Hindu University: Varanasi, India, 2004; pp. 21–38. [Google Scholar]
- Silva, S.O.; Rodrigues, M.R.; Carvalho, S.R.Q.; Catalani, L.H.; Campa, A.; Ximenes, V.F. Oxidation of melatonin and its catabolites, N1-acetyl-N2 -formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine, by activated leukocytes. J. Pineal Res. 2004, 37, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.-S.; Hardeland, R.; Reiter, R.J. N1-Acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Collin, F.; Bonnefont-Rousselot, D.; Yous, S.; Marchetti, C.; Jore, D.; Gardès-Albert, M. Online H/D exchange liquid chromatography as a support for the mass spectrometric identification of the oxidation products of melatonin. J. Mass Spectrom. 2009, 44, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Seever, K.; Hardeland, R. Novel pathway for N1-acetyl-5-methoxykynuramine: UVB-induced liberation of carbon monoxide from precursor N1-acetyl-N2-formyl-5-methoxykynuramine. J. Pineal Res. 2008, 44, 450–455. [Google Scholar] [CrossRef]
- Hirata, F.; Hayaishi, O.; Tokuyama, T.; Senoh, S. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 1974, 249, 1311–1313. [Google Scholar] [CrossRef]
- Niu, S.; Li, F.; Tan, D.-X.; Zhang, L.; Idle, J.R.; Gonzalez, F.J.; Ma, X. Analysis of N1-acetyl-N2-formyl-5-methoxykynuramine/N1-acetyl-5-methoxy-kynuramine formation from melatonin in mice. J. Pineal Res. 2010, 49, 106–114. [Google Scholar] [CrossRef]
- Schaefer, M.; Hardeland, R. The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 2009, 46, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Mori, A.; Liburdy, R.; Packer, L. Melatonin and its precursors scavenge nitric oxide. J. Pineal Res. 1999, 27, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, B.; Pompon, D.; Ducrocq, C. Nitrosation of melatonin by nitric oxide and peroxynitrite. J. Pineal Res. 2000, 29, 184–192. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Sáenz, D.A.; Doctorovich, F.; Estrin, D.A.; Rosenstein, R.E. Nitrosation of melatonin by nitric oxide: A computational study. J. Pineal Res. 2001, 31, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Turjanski, A.; Chaia, Z.D.; Doctorovich, F.; Estrin, D.; Rosenstein, R.; Piro, O.E. N-nitrosomelatonin. Acta Crystallogr. C 2000, 56, 682–683. [Google Scholar] [CrossRef]
- Kirsch, M.; de Groot, H. First insights into regiospecific transnitrosation reactions between tryptophan derivatives: Melatonin as an effective target. J. Pineal Res. 2005, 38, 247–523. [Google Scholar] [CrossRef]
- Peyrot, F.; Fernandez, B.O.; Bryan, N.S.; Feelisch, M.; Ducrocq, C. N-Nitroso products from the reaction of indoles with Angeli’s salt. Chem. Res. Toxicol. 2006, 19, 58–67. [Google Scholar] [CrossRef][Green Version]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, H.; Yadav, S.; Bhatla, S.C. Does N-nitrosomelatonin compete with S-nitrosothiols as a long distance nitric oxide carrier in plants? Biochem. Anal. Biochem. 2016, 5, 262. [Google Scholar] [CrossRef]
- Berchner-Pfannschmidt, U.; Tug, S.; Trinidad, B.; Becker, M.; Oehme, F.; Flamme, I.; Fandrey, J.; Kirsch, M. The impact of N-nitrosomelatonin as nitric oxide donor in cell culture experiments. J. Pineal Res. 2008, 45, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and its metabolites as anti-nitrosating and anti-nitrating agents. J. Exp. Integ. Med. 2011, 1, 67–81. [Google Scholar] [CrossRef]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Fillion, B.; Servy, C.; Ducrocq, C. 1-Nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic. Res. 2001, 35, 857–866. [Google Scholar] [CrossRef]
- Peyrot, F.; Grillon, C.; Vergely, C.; Rochette, L.; Ducrocq, C. Pharmacokinetics of 1-nitrosomelatonin and detection by EPR using iron dithiocarbamate complex in mice. Biochem. J. 2005, 387, 473–478. [Google Scholar] [CrossRef] [PubMed]
- De Biase, P.M.; Turjanski, A.G.; Estrin, D.A.; Doctorovich, F. Mechanisms of NO release by N1-nitrosomelatonin: Nucleophilic attack versus reducing pathways. J. Org. Chem. 2005, 70, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; de Groot, H. N-nitrosomelatonin outcompetes S-nitrosocysteine in inhibiting glyceraldehyde 3-phosphate dehydrogenase: First evidence that N-nitrosomelatonin can modify protein function. J. Pineal Res. 2008, 44, 244–249. [Google Scholar] [CrossRef]
- Kirsch, M.; de Groot, H. N-nitrosomelatonin: Synthesis, chemical properties, potential prodrug. J. Pineal Res. 2009, 46, 121–127. [Google Scholar] [CrossRef]
- Suzuki, T.; Mower, H.F.; Friesen, M.D.; Gilibert, I.; Sawa, T.; Ohshima, H. Nitration and nitrosation of N-acetyl-L-tryptophan and tryptophan residues in proteins by various reactive nitrogen species. Free Radic. Biol. Med. 2004, 37, 671–681. [Google Scholar] [CrossRef]
- Peyrot, F.; Ducrocq, C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008, 45, 235–426. [Google Scholar] [CrossRef]
- Lehnig, M.; Kirsch, M. 15N-CIDNP investigations during tryptophan, N-acetyl-L-tryptophan, and melatonin nitration with reactive nitrogen species. Free Radic. Res. 2007, 41, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Squadrito, G.L.; Pryor, W.A. The reaction of melatonin with peroxynitrite: Formation of melatonin radical cation and absence of stable nitrated products. Biochem. Biophys. Res. Commun. 1998, 51, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Uppu, R.M.; Squadrito, G.L.; Pryor, W.A. Acceleration of peroxynitrite oxidations by carbon dioxide. Arch. Biochem. Biophys. 1996, 327, 335–343. [Google Scholar] [CrossRef]
- Hensley, K.; Maidt, M.L.; Yu, Z.; Sang, H.; Markesbery, W.R.; Floyd, R.A. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 1998, 18, 8126–8132. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Lehnig, M. Radical mechanisms of the decomposition of peroxynitrite and the peroxynitrite-CO2 adduct and of reactions with L-tyrosine and related compounds as studied by 15N chemically induced dynamic nuclear polarization. Arch. Biochem. Biophys. 1999, 368, 303–318. [Google Scholar] [CrossRef]
- Santos, C.X.; Bonini, M.G.; Augusto, O. Role of the carbonate radical anion in tyrosine nitration and hydroxylation by peroxynitrite. Arch. Biochem. Biophys. 2000, 377, 146–152. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B.; Niebergall, R.; Zelosko, V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J. Pineal Res. 2003, 34, 17–25. [Google Scholar] [CrossRef]
- Kuesel, J.T.; Hardeland, R.; Pfoertner, H.; Aeckerle, N. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine with carbamoyl phosphate and related compounds. J. Pineal Res. 2010, 48, 47–54. [Google Scholar] [CrossRef]
- Krotzky, M.; Hardeland, R. Metabolism of the melatonin metabolite N1-acetyl-N2-formyl-5-methoxykynuramine in Saccharomyces cerevisiae. Cytologia 2008, 73, 123–128. [Google Scholar] [CrossRef]
- Hardeland, R.; Backhaus, C.; Fadavi, A.; Hess, M. N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: Cyclization to a cinnolinone prevents formation of unstable nitrosamines. J. Pineal Res. 2007, 43, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Heer, C.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the ABTS cation radical: Identification of new oxidation products. Redox Rep. 2006, 11, 15–24. [Google Scholar] [CrossRef]
- Koehler, A. Investigations on the Redox Behavior of the Melatonin Metabolite N1-acetyl-5-methoxykynuramine (AMK). Master’s Thesis, University of Goettingen, Biological Faculty, Goettingen, Germany, 2007. (In German). [Google Scholar]
- Nowak, A.; Rahman, H.; Heer, C.; Schueth, A.; Laatsch, H.; Hardeland, R. Reactions of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK) with the tyrosine side-chain fragment, 4-ethylphenol. Redox Rep. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Nakazawa, H.; Fukuyama, N.; Takizawa, S.; Tsuji, C.; Yoshitake, M.; Ishida, H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic. Res. 2000, 33, 771–784. [Google Scholar] [CrossRef]
- Deng, G.; Vaziri, N.D.; Jabbari, B.; Ni, Z.; Yan, X.X. Increased tyrosine nitration of the brain in chronic renal insufficiency: Reversal by antioxidant therapy and angiotensin-converting enzyme inhibition. J. Am. Soc. Nephrol. 2001, 12, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Barnes, P.J. Nitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary disease. Curr. Allergy Asthma Rep. 2003, 3, 121–129. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Nitrotyrosine and chlorotyrosine: Clinical significance and biological functions in the vascular system. J. Surg. Res. 2006, 133, 143–149. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Ozyurt, V.H.; Cetin, A.E.; Otles, S. Nitration of tyrosine and its effect on DNA hybridization. Biosens. Bioelectron. 2018, 102, 464–469. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, A.; Ahsan, H. Peroxynitrite: Cellular pathology and implications in autoimmunity. J. Immunoass. Immunochem. 2019, 40, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Issoglio, F.M.; Estrin, D.A.; Bartesaghi, S.; Radi, R. 3-Nitrotyrosine and related derivatives in proteins: Precursors, radical intermediates and impact in function. Essays Biochem. 2020, 64, 111–133. [Google Scholar] [CrossRef]
- Ceriello, A.; Quagliaro, L.; D’Amico, M.; Di Filippo, C.; Marfella, R.; Nappo, F.; Berrino, L.; Rossi, F.; Giugliano, D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 2002, 51, 1076–1082. [Google Scholar] [CrossRef]
- Eisele, H.J.; Markart, P.; Schulz, R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: Evidence from human studies. Oxid. Med. Cell. Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef]
- Thomson, L. 3-Nitrotyrosine modified proteins in atherosclerosis. Dis. Markers 2015, 2015, 708282. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Poulin, M.J.; Hemmelgarn, B.R.; Muruve, D.A.; Chirico, E.N.; Faes, C.; Sola, D.Y.; Ahmed, S.B. Cyclooxygenase-2 inhibition limits angiotensin II-induced DNA oxidation and protein nitration in humans. Front. Physiol. 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Proteomics: A new approach to investigate oxidative stress in Alzheimer’s disease brain. Brain Res. 2004, 1000, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Ozben, T. Reactive oxygen and nitrogen species in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Bombeiro, A.L.; D’Império Lima, M.R.; Chadi, G.; Alvarez, J.M. Neurodegeneration and increased production of nitrotyrosine, nitric oxide synthase, IFN-γ and S100β protein in the spinal cord of IL-12p40-deficient mice infected with Trypanosoma cruzi. Neuroimmunomodulation 2010, 17, 67–78. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Sultana, R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic. Res. 2011, 45, 59–72. [Google Scholar] [CrossRef]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.I.; Wang, D.; Leu, F.J.; Chen, C.F.; Chen, H.I. Ischemia and reperfusion of liver induces eNOS and iNOS expression: Effects of a NO donor and NOS inhibitor. Chin. J. Physiol. 2004, 47, 121–127. [Google Scholar] [PubMed]
- Yanagisawa, D.; Kitamura, Y.; Inden, M.; Takata, K.; Taniguchi, T.; Morikawa, S.; Morita, M.; Inubushi, T.; Tooyama, I.; Taira, T.; et al. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J. Cereb. Blood Flow Metab. 2008, 28, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Grosche, A.; Freeman, D.E.; Morton, A.J.; Polyak, M.M.; Matyjaszek, S.A. Effects of ischemia and reperfusion on production of nitrotyrosine, activation of eosinophils, and apoptosis in the large colonic mucosa of horses. Am. J. Vet. Res. 2012, 73, 53–61. [Google Scholar] [CrossRef]
- Gao, L.; Chen, X.; Peng, T.; Yang, D.; Wang, Q.; Lv, Z.; Shen, J. Caveolin-1 protects against hepatic ischemia/reperfusion injury through ameliorating peroxynitrite-mediated cell death. Free Radic Biol. Med. 2016, 95, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tatarkova, Z.; Kovalska, M.; Sivonova, M.K.; Racay, P.; Lehotsky, J.; Kaplan, P. Tyrosine nitration of mitochondrial proteins during myocardial ischemia and reperfusion. J. Physiol. Biochem. 2019, 75, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Torisu, K.; Tomita, K.; Kawai, Y.; Tsuruya, K.; Nakano, T.; Kitazono, T. Arginase 2 is a mediator of ischemia-reperfusion injury in the kidney through regulation of nitrosative stress. Kidney Int. 2020, 98, 673–685. [Google Scholar] [CrossRef]
- Chen, H.; Guan, B.; Wang, B.; Pu, H.; Bai, X.; Chen, X.; Liu, J.; Li, C.; Qiu, J.; Yang, D.; et al. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl. Stroke Res. 2020, 11, 967–982. [Google Scholar] [CrossRef]
- Tuo, J.; Liu, L.; Poulsen, H.E.; Weimann, A.; Svendsen, O.; Loft, S. Importance of guanine nitration and hydroxylation in DNA in vitro and in vivo. Free Radic. Biol. Med. 2000, 29, 147–155. [Google Scholar] [CrossRef]
- Ohshima, H.; Sawa, T.; Akaike, T. 8-Nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: Formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid. Redox Signal. 2006, 8, 1033–1045. [Google Scholar] [CrossRef]
- Kim, J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat. Cell Biol. 2017, 50, 200–206. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Alexander, K.J.; McConville, M.; Williams, K.R.; Luzyanin, K.V.; O’Neil, I.A.; Cosstick, R. Chemistry of the 8-nitroguanine DNA lesion: Reactivity, labelling and repair. Chemistry 2018, 24, 3013–3020. [Google Scholar] [CrossRef]
- Caulfield, J.L.; Wishnok, J.S.; Tannenbaum, S.R. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 1998, 273, 12689–12695. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Wu, S.B.; Chang, C.M. Biological and dietary antioxidants protect against DNA nitration induced by reaction of hypochlorous acid with nitrite. Arch. Biochem. Biophys. 2003, 415, 109–116. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B.; Pappolla, M.A. Mitochondrial actions of melatonin—An endeavor to identify their adaptive and cytoprotective mechanisms. Proc. Saxon Acad. Sci. 2009, 65, 14–31. [Google Scholar]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation: Determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Fernhoff, N.B.; Derbyshire, E.R.; Underbakke, E.S.; Marletta, M.A. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J. Biol. Chem. 2012, 287, 43053–43062. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef]
- Aranda, E.; López-Pedrera, C.; De La Haba-Rodriguez, J.R.; Rodriguez-Ariza, A. Nitric oxide and cancer: The emerging role of S-nitrosylation. Curr. Mol. Med. 2012, 12, 50–67. [Google Scholar] [CrossRef]
- Wang, Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012, 320, 123–129. [Google Scholar] [CrossRef]
- Bignon, E.; Allega, M.F.; Lucchetta, M.; Tiberti, M.; Papaleo, E. Computational structural biology of S-nitrosylation of cancer targets. Front. Oncol. 2018, 8, 272. [Google Scholar] [CrossRef]
- Tan, C.; Li, Y.; Huang, X.; Wei, M.; Huang, Y.; Tang, Z.; Huang, H.; Zhou, W.; Wang, Y.; Hu, J. Extensive protein S-nitrosylation associated with human pancreatic ductal adenocarcinoma pathogenesis. Cell Death Dis. 2019, 10, 914. [Google Scholar] [CrossRef]
- Mishra, D.; Patel, V.; Banerjee, D. Nitric oxide and S-nitrosylation in cancers: Emphasis on breast cancer. Breast Cancer 2020, 14. [Google Scholar] [CrossRef]
- Xu, P.; Ye, S.; Li, K.; Huang, M.; Wang, Q.; Zeng, S.; Chen, X.; Gao, W.; Chen, J.; Zhang, Q.; et al. NOS1 inhibits the interferon response of cancer cells by S-nitrosylation of HDAC2. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Filomeni, G. Exploiting S-nitrosylation for cancer therapy: Facts and perspectives. Biochem. J. 2020, 477, 3649–3672. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Guo, Y.; Song, Y.; Chen, L.; Ruan, Q.; Wang, Y.; Sun, L.; Hu, Y.; Zhou, J.; et al. Regulation of ezrin tension by S-nitrosylation mediates non-small cell lung cancer invasion and metastasis. Theranostics 2019, 9, 2555–2571. [Google Scholar] [CrossRef]
- Ehrenfeld, P.; Cordova, F.; Duran, W.N.; Sanchez, F.A. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide 2019, 87, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.H.; Cheng, R.Y.; Ridnour, L.A.; Glynn, S.A.; Ambs, S.; Wink, D.A. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012, 14, R125. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.E.; Foster, M.W. S-nitrosylation of Ras in breast cancer. Breast Cancer Res. 2012, 14, 113. [Google Scholar] [CrossRef]
- Gupta, A.; Anjomani-Virmouni, S.; Koundouros, N.; Dimitriadi, M.; Choo-Wing, R.; Valle, A.; Zheng, Y.; Chiu, Y.H.; Agnihotri, S.; Zadeh, G.; et al. PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-nitrosylation. Mol. Cell 2017, 65, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, K.; Ma, C.; Li, J.; Zhuo, L.; Huang, W.; Chen, T.; Jiang, Y. PTPS facilitates compartmentalized LTBP1 S-nitrosylation and promotes tumor growth under hypoxia. Mol. Cell 2020, 77, 95–107. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Costa, P.E.; Reis, A.K.; Stern, A. Nitric oxide: Protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed. J. 2015, 38, 380–388. [Google Scholar]
- Switzer, C.H.; Glynn, S.A.; Cheng, R.Y.; Ridnour, L.A.; Green, J.E.; Ambs, S.; Wink, D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Cancer Res. 2012, 10, 1203–1215. [Google Scholar] [CrossRef]
- Basudhar, D.; Somasundaram, V.; de Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric oxide synthase-2-derived nitric oxide drives multiple pathways of breast cancer progression. Antioxid. Redox Signal. 2017, 26, 1044–1058. [Google Scholar] [CrossRef]
- Jin, L.; Cao, Y.; Zhang, T.; Wang, P.; Ji, D.; Liu, X.; Shi, H.; Hua, L.; Yu, R.; Gao, S. Effects of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation and cell survival in glioma cells. Int. J. Mol. Med. 2018, 41, 1339–1348. [Google Scholar] [CrossRef]
- Romagny, S.; Bouaouiche, S.; Lucchi, G.; Ducoroy, P.; Bertoldo, J.B.; Terenzi, H.; Bettaieb, A.; Plenchette, S. S-nitrosylation of cIAP1 switches cancer cell fate from TNFalpha/TNFR1-mediated cell survival to cell death. Cancer Res. 2018, 78, 1948–1957. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Xiao, G.; Shan, H.; Tang, L.; Yi, Y.; Yu, W.; Gu, Y. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Schmit, T.L.; Reagan-Shaw, S.R.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J. Pineal Res. 2011, 50, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics—Evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 2014, 15, 18221–18252. [Google Scholar] [CrossRef]
- Hardeland, R. Sirtuins, melatonin, and the relevance of circadian oscillators. In Sirtuin Biology in Medicine. Targeting New Avenues of Care in Development, Aging, and Disease; Maiese, K., Ed.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2021; pp. 137–151. [Google Scholar]
- Hardeland, R. Dysregulation of sirtuins in cancer. Oncol. Res. Rev. 2019, 2, 1–4. [Google Scholar] [CrossRef]
- Lanoix, D.; Lacasse, A.A.; Reiter, R.J.; Vaillancourt, C. Melatonin: The smart killer: The human trophoblast as a model. Mol. Cell. Endocrinol. 2012, 348, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bizarri, M.; Proietti, S.; Cucina, A.; Reiter, R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: A review. Expert Opin. Ther. Targets 2013, 17, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Dawson, T.M.; Dawson, V.L. Nitric oxide, S-nitrosylation and neurodegeneration. Cell. Mol. Biol. 2005, 51, 247–254. [Google Scholar] [PubMed]
- Chung, K.K. Say NO to neurodegeneration: Role of S-nitrosylation in neurodegenerative disorders. Neurosignals 2006, 15, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.H.; Lee, Y.I.; Ko, H.S.; Savitt, J.M.; Pletnikova, O.; Troncoso, J.C.; Dawson, V.L.; Dawson, T.M.; Chung, K.K. S-nitrosylation of XIAP compromises neuronal survival in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 4900–4905. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Rizza, S.; Montagna, C.; Filomeni, G. Established principles and emerging concepts on the interplay between mitochondrial physiology and S-(de)nitrosylation: Implications in cancer and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 361872. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Sunico, C.R.; Nakamura, T.; Lipton, S.A. Redox regulation of protein function via cysteine S-nitrosylation and its relevance to neurodegenerative diseases. Int. J. Cell Biol. 2012, 2012, 463756. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Kalashnikova, E.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Nutter, A.; et al. S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 2014, 8, 217–228. [Google Scholar] [CrossRef]
- Conway, M.E.; Harris, M. S-nitrosylation of the thioredoxin-like domains of protein disulfide isomerase and its role in neurodegenerative conditions. Front. Chem. 2015, 3, 27. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Sanz-Blasco, S.; Dolatabadi, N.; Parker, J.; Chon, K.; Lee, M.S.; Soussou, W.; McKercher, S.R.; Ambasudhan, R.; Nakamura, T.; et al. Elevated glucose and oligomeric β-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016, 7, 10242. [Google Scholar] [CrossRef] [PubMed]
- Montagna, C.; Rizza, S.; Maiani, E.; Piredda, L.; Filomeni, G.; Cecconi, F. To eat, or NOt to eat: S-nitrosylation signaling in autophagy. FEBS J. 2016, 283, 3857–3869. [Google Scholar] [CrossRef]
- Ni, C.L.; Seth, D.; Fonseca, F.V.; Wang, L.; Xiao, T.S.; Gruber, P.; Sy, M.S.; Stamler, J.S.; Tartakoff, A.M. Polyglutamine tract expansion increases S-nitrosylation of huntingtin and ataxin-1. PLoS ONE 2016, 11, e0163359. [Google Scholar] [CrossRef]
- Wang, Y.; Veremeyko, T.; Wong, A.H.; El Fatimy, R.; Wei, Z.; Cai, W.; Krichevsky, A.M. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer’s disease. Neurobiol. Aging 2017, 51, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fominykh, V.; Vorobyeva, A.; Onufriev, M.V.; Brylev, L.; Zakharova, M.N.; Gulyaeva, N.V. Interleukin-6, S-nitrosothiols, and neurodegeneration in different central nervous system demyelinating disorders: Is there a relationship? J. Clin. Neurol. 2018, 14, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Crunfli, F.; Mazucanti, C.H.; de Moraes, R.C.M.; Costa, A.P.; Rodrigues, A.C.; Scavone, C.; Torrão, A.S. NO-dependent Akt inactivation by S-nitrosylation as a possible mechanism of STZ-induced neuronal insulin resistance. J. Alzheimers Dis. 2018, 65, 1427–1443. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Lenkiewicz, A.M.; Czapski, G.A.; Jęśko, H.M.; Hilgier, W.; Brodzik, R.; Gąssowska-Dobrowolska, M.; Culmsee, C.; Adamczyk, A. Extracellular alpha-synuclein oligomers induce parkin S-nitrosylation: Relevance to sporadic Parkinson’s disease etiopathology. Mol. Neurobiol. 2019, 56, 125–140. [Google Scholar] [CrossRef]
- Wijasa, T.S.; Sylvester, M.; Brocke-Ahmadinejad, N.; Schwartz, S.; Santarelli, F.; Gieselmann, V.; Klockgether, T.; Brosseron, F.; Heneka, M.T. Quantitative proteomics of synaptosome S-nitrosylation in Alzheimer’s disease. J. Neurochem. 2020, 152, 710–726. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Du, X.; Bi, M.; Ma, F.; Xie, J.; Jiang, H. The S-nitrosylation of parkin attenuated the ubiquitination of divalent metal transporter 1 in MPP+-treated SH-SY5Y cells. Sci. Rep. 2020, 10, 15542. [Google Scholar] [CrossRef]
- Montagna, C.; Cirotti, C.; Rizza, S.; Filomeni, G. When S-nitrosylation gets to mitochondria: From signaling to age-related diseases. Antioxid. Redox Signal. 2020, 32, 884–905. [Google Scholar] [CrossRef]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Nakamura, T.; Cieplak, P.; Cho, D.H.; Godzik, A.; Lipton, S.A. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion 2010, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Jiang, X.; Zhai, S.; Xing, D. The essential role of Drp1 and its regulation by S-mitrosylation of parkin in dopaminergic neurodegeneration: Implications for Parkinson’s disease. Antioxid. Redox Signal. 2016, 25, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.D.; Ma, T.; Diao, S.; Zhang, X.; Chen, Y.; Hsu, J.; Lipton, S.A.; Masliah, E.; Xu, H.; Liao, F.F. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol. Neurodegener. 2010, 5, 49. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid. Redox Signal. 2008, 10, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.; Parakh, S.; Atkin, J.D. The role of S-nitrosylation and S-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int. J. Cell Biol. 2013, 2013, 797914. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Oguro, A.; Yagi, E.; Mitani, A.; Kudoh, S.N.; Imaoka, S. Bisphenol A and rotenone induce S-nitrosylation of protein disulfide isomerase (PDI) and inhibit neurite outgrowth of primary cultured cells of the rat hippocampus and PC12 cells. J. Toxicol. Sci. 2020, 45, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.H. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2013, 2013, 781050. [Google Scholar] [CrossRef]
- Yang, L.; Calay, E.S.; Fan, J.; Arduini, A.; Kunz, R.C.; Gygi, S.P.; Yalcin, A.; Fu, S.; Hotamisligil, G.S. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 2015, 349, 500–506. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, Z.; Orwig, A.; Chen, S.; Ding, W.X.; Xu, Y.; Kunz, R.C.; Lind, N.R.L.; Stamler, J.S.; Yang, L. S-nitrosoglutathione reductase dysfunction contributes to obesity-associated hepatic insulin resistance via regulating autophagy. Diabetes 2018, 67, 193–207. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar]
- Hegyi, B.; Bers, D.M.; Bossuyt, J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Tokunaga, E.; Ota, H.; Sugita, H.; Martyn, J.A.; Kaneki, M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 2005, 280, 7511–7518. [Google Scholar] [CrossRef] [PubMed]
- Kaneki, M.; Shimizu, N.; Yamada, D.; Chang, K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid. Redox Signal. 2007, 9, 319–329. [Google Scholar] [CrossRef]
- Pérez-Gallardo, R.V.; Noriega-Cisneros, R.; Esquivel-Gutiérrez, E.; Calderón-Cortés, E.; Cortés-Rojo, C.; Manzo-Avalos, S.; Campos-García, J.; Salgado-Garciglia, R.; Montoya-Pérez, R.; Boldogh, I.; et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J. Bioenerg. Biomembr. 2014, 46, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, J.; Jin, Z.; Yan, L.J. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem. Insights 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visiedo, F.; Santos-Rosendo, C.; Mateos-Bernal, R.M.; Gil-Sánchez, M.D.; Bugatto, F.; Aguilar-Diosdado, M.; Segundo, C.; López-Tinoco, C. Characterization of NO-induced nitrosative status in human placenta from pregnant women with gestational diabetes mellitus. Oxid. Med. Cell. Longev. 2017, 2017, 5629341. [Google Scholar] [CrossRef]
- Yanar, K.; Atayik, M.C.; Simsek, B.; Çakatay, U. Novel biomarkers for the evaluation of aging-induced proteinopathies. Biogerontology 2020, 21, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Goligorsky, M.S.; Rabelink, T. Meeting report: ISN forefronts in nephrology on endothelial biology and renal disease: From bench to prevention. Kidney Int. 2006, 70, 258–264. [Google Scholar] [CrossRef]
- López-Sánchez, L.M.; Corrales, F.J.; Barcos, M.; Espejo, I.; Muñoz-Castañeda, J.R.; Rodríguez-Ariza, A. Inhibition of nitric oxide synthesis during induced cholestasis ameliorates hepatocellular injury by facilitating S-nitrosothiol homeostasis. Lab. Investig. 2010, 90, 116–127. [Google Scholar] [CrossRef]
- Montagna, C.; Di Giacomo, G.; Rizza, S.; Cardaci, S.; Ferraro, E.; Grumati, P.; De Zio, D.; Maiani, E.; Muscoli, C.; Lauro, F.; et al. S-nitrosoglutathione reductase deficiency-induced S-nitrosylation results in neuromuscular dysfunction. Antioxid. Redox Signal. 2014, 21, 570–587. [Google Scholar] [CrossRef]
- Cao, Y.; Gomes, S.A.; Rangel, E.B.; Paulino, E.C.; Fonseca, T.L.; Li, J.; Teixeira, M.B.; Gouveia, C.H.; Bianco, A.C.; Kapiloff, M.S.; et al. S-nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J. Clin. Investig. 2015, 125, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; León, J.; Acuña-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.I.; Escames, G.; López, L.C.; García, J.A.; Ortiz, F.; López, A.; Acuña-Castroviejo, D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J. Endocrinol. 2007, 194, 637–643. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Carretero, M.; Escames, G.; López, L.C.; Maldonado, M.D.; Tan, D.-X.; Reiter, R.J.; Acuña-Castroviejo, D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic. Res. 2007, 41, 15–24. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Escames, G.; López, L.C.; López, A.; García, J.A.; Ortiz, F.; Sánchez, V.; Romeu, M.; Acuña-Castroviejo, D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 2008, 43, 749–756. [Google Scholar] [CrossRef]
- Carretero, M.; Escames, G.; López, L.C.; Venegas, C.; Dayoub, J.C.; García, L.; Acuña-Castroviejo, D. Long-term melatonin administration protects brain mitochondria from aging. J. Pineal Res. 2009, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Y.; Wu, X.; Zhao, G.; Zhang, K.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin and (-)-epigallocatechin-3-gallate: Partners in fighting cancer. Cells 2019, 8, 745. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and neuroinflammation: Encouraging findings vs. fundamental problems. In Pineal Gland: Research Advances and Clinical Challenges; Català, A., Ed.; Nova Science: Hauppauge, NY, USA, 2017; pp. 163–204. [Google Scholar]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef]
- Hardeland, R. Recent findings in melatonin research and their relevance to the CNS. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, melatonin and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Huang, C.C.; Liu, S.C.; Tang, C.H. Reconsidering the Role of Melatonin in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; de Las Heras, N.; Ferder, L.; Lahera, V.; Reiter, R.J.; Manucha, W. Potential effects of melatonin and micronutrients on mitochondrial dysfunction during a cytokine storm typical of oxidative/inflammatory diseases. Diseases 2021, 9, 30. [Google Scholar] [CrossRef]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Dos Santos, M.; Veronese, F.V.; Rezzani, R. NLRP3 inflammasome modulation by melatonin aupplementation in chronic pristane-lnduced lupus nephritis. Int. J. Mol. Sci. 2019, 20, 3466. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Wu, X.; Ji, H.; Wang, Y.; Gu, C.; Gu, W.; Hu, L.; Zhu, L. Melatonin alleviates eadiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxid. Med. Cell. Longev. 2019, 2019, 4087298. [Google Scholar]
- Wu, H.M.; Zhao, C.C.; Xie, Q.M.; Xu, J.; Fei, G.H. TLR2-melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front. Immunol. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ge, J.; Gao, H.; Pan, Y.; Hao, Y.; Li, J. Melatonin attenuates AFB1-induced cardiotoxicity via the NLRP3 signalling pathway. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol. 2020, 34, 101560. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 connection in mouse heart mitochondria during aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Gao, S.; Wu, H.; Xu, W.; Pan, Y.; Fang, Y.; Wang, X.; Zhang, J. Melatonin ameliorates hemorrhagic transformation via suppression of ROS-induced NLRP3 activation after cerebral ischemia in gyperglycemic rats. Oxid. Med. Cell. Longev. 2021, 2021, 6659282. [Google Scholar] [CrossRef]
- Hardeland, R. Noncoding RNAs: Bridging regulation of circadian rhythms and inflammation. Adv. Neuroimmune Biol. 2019, 7, 155–177. [Google Scholar] [CrossRef]
- Sayed, R.K.; Fernández-Ortiz, M.; Fernández-Martínez, J.; Aranda Martínez, P.; Guerra-Librero, A.; Rodríguez-Santana, C.; de Haro, T.; Escames, G.; Acuña-Castroviejo, D.; Rusanova, I. The impact of melatonin and NLRP3 inflammasome on the expression of microRNAs in aged muscle. Antioxidants 2021, 10, 524. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; González Menéndez, P.; Cepas, V.; Tan, D.-X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef]
- Hardeland, R. Brain inflammaging: Roles of melatonin, circadian clocks and sirtuins. J. Clin. Cell. Immunol. 2018, 9, 543. [Google Scholar] [CrossRef]
- Hardeland, R. Extended signaling by melatonin. Cell Cell. Life Sci. J. 2018, 3, 000123. [Google Scholar]
- Xia, Y.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; Ren, W. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef]
- Crespo, E.; Macías, M.; Pozo, D.; Escames, G.; Martín, M.; Vives, F.; Guerrero, J.M.; Acuña-Castroviejo, D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999, 13, 1537–1546. [Google Scholar] [CrossRef]
- Escames, G.; León, J.; Macías, M.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J. 2003, 17, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Acuña-Castroviejo, D.; López, L.C.; Tan, D.-X.; Maldonado, M.D.; Sánchez-Hidalgo, M.; León, J.; Reiter, R.J. Pharmacological utility of melatonin in the treatment of septic shock: Experimental and clinical evidence. J. Pharm. Pharmacol. 2006, 58, 1153–1165. [Google Scholar] [CrossRef]
- Gitto, E.; Marseglia, L.; Manti, S.; D’Angelo, G.; Barberi, I.; Salpietro, C.; Reiter, R.J. Protective role of melatonin in neonatal diseases. Oxid. Med. Cell. Longev. 2013, 2013, 980374. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Marseglia, L.; Reiter, R.J.; Buonocore, G.; Gitto, E. Melatonin and neonatal sepsis: A promising antioxidant adjuvant agent. Am. J. Perinatol. 2017, 34, 1382–1388. [Google Scholar]
- El-Gendy, F.M.; El-Hawy, M.A.; Hassan, M.G. Beneficial effect of melatonin in the treatment of neonatal sepsis. J. Matern. Fetal Neonatal. Med. 2018, 31, 2299–2303. [Google Scholar] [CrossRef]
- Henderson, R.; Kim, S.; Lee, E. Use of melatonin as adjunctive therapy in neonatal sepsis: A systematic review and meta-analysis. Complement. Ther. Med. 2018, 39, 131–136. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: Focus on COVID-19. Melatonin Res. 2020, 3, 120–143. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.-X. Protection by melatonin in respiratory diseases: Valuable information for the treatment of COVID-19. Melatonin Res. 2020, 3, 264–275. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Estimated doses of melatonin for treating deadly virus infections: Focus on COVID-19. Melatonin Res. 2020, 3, 276–296. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Escames, G.; López, L.C.; López, A.; Camacho, E.; Carrión, M.D.; Entrena, A.; Gallo, M.A.; Espinosa, A.; Acuña-Castroviejo, D. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J. Neurosci. Res. 2009, 87, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.-X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardeland, R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules 2021, 26, 4105. https://doi.org/10.3390/molecules26134105
Hardeland R. Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules. 2021; 26(13):4105. https://doi.org/10.3390/molecules26134105
Chicago/Turabian StyleHardeland, Rüdiger. 2021. "Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds" Molecules 26, no. 13: 4105. https://doi.org/10.3390/molecules26134105
APA StyleHardeland, R. (2021). Melatonin, Its Metabolites and Their Interference with Reactive Nitrogen Compounds. Molecules, 26(13), 4105. https://doi.org/10.3390/molecules26134105





