Abstract
A series of novel thiochromanone derivatives containing a sulfonyl hydrazone moiety were designed and synthesized. Their structures were determined by 1H-NMR, 13C-NMR, and HRMS. Bioassay results showed that most of the target compounds revealed moderate to good antibacterial activities against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicolaby, and Xanthomonas axonopodis pv. citri. Compound 4i had the best inhibitory activity against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicolaby, and Xanthomonas axonopodis pv. citri, with the EC50 values of 8.67, 12.65, and 10.62 μg/mL, which were superior to those of Bismerthiazol and Thiodiazole-copper. Meanwhile, bioassay results showed that all of the target compounds proved to have lower antifungal activities against Sclerotinia sclerotiorum, Fusarium oxysporum, Gibberella zeae, Rhizoctonia solani, Verticillium dahlia, and Botrytis cinerea than those of Carbendazim.
1. Introduction
Plant bacterial and fungal diseases pose serious threats in agricultural production and cause huge economic losses throughout the world each year [1]. In recent years, crop cultivators continually battle with plant bacterial and fungal diseases that affect their crops. The available traditional pesticides used for plant bacterial and fungal diseases control irrationally posed dangers to living systems, killing not only the target bacteria and fungi, but also affecting beneficial living systems [2]. In order to protect plant bacterial and fungal diseases, commercial agriculture relies heavily on the inputs of chemical pesticides and the resistance of plant bacterial and fungal diseases against pesticides is rapidly becoming a serious problem. Therefore, the development of novel and promising antibacterial and antifungal agents is still an urgent task.
Chromone, a kind of botanical active component with extensive biological activities, is widely found in the secondary metabolites of flowers, roots, stems, and pericarp of many plants [3,4]. As early as the late 19th century, Khellin was extracted from the fruit of the Umbellifera, which was widely distributed in eastern Mediterranean countries, and was used as the first chromone drug in clinical applications [5]. Meanwhile, biological activity results showed that both natural and synthetic chromone compounds had a wide range of biological activities, such as antifungal [6,7], antibacterial [6], anticancer [8], and antiviral [9] activity. Thiochromanone, a kind of chromone compound, is an important substance in the synthesis of various active molecules that are extensively used in the intermediate skeleton of drugs and has attracted more and more attention due to their extensive biological activities, including antiviral [10], antibacterial [11,12], antifungal [11,13,14,15], herbicidal [16,17], and insecticidal [18] activity. Therefore, due to its excellent features of low toxicity and easy to be synthetized and derived, thiochromanone is considered to be a leading compound to develop promising agrochemical candidates, which will become a reality. As an important nitrogen-containing compounds in organic synthesis, sulfonyl hydrazones are widely employed to construct C–C, C–N, and C–S bonds. Compounds with sulfonyl hydrazone structural units present various biological activities, including antifungal [19,20,21], antibacterial [22,23], anticancer [24,25,26], and insecticidal [27] activity. However, there are no reports on the synthesis and bioactivity evaluation of thiochromanone derivatives containing a sulfonyl hydrazone structure.
Motivated by the above-mentioned findings and to discover new active small molecules, in this study, using botanical active component thiochromanone as the lead compound, a series of novel thiochromanone derivatives containing a sulfonyl hydrazone moiety were designed, synthesized, and determined their in vitro antibacterial activities against Xanthomonas oryzae pv. oryzae (Xoo), Xanthomonas oryzae pv. oryzicolaby (Xoc), and Xanthomonas axonopodis pv. citri (Xac), as well as their in vitro antifungal activities against Sclerotinia sclerotiorum (S. sclerotiorum), Fusarium oxysporum (F. oxysporum), Gibberella zeae (Gibberella zeae), Rhizoctonia solani (R. solani), Verticillium dahlia (V. dahlia), and Botrytis cinerea (B. cinerea).
2. Results and Discussion
2.1. Chemistry
Using 4-substituted thiophenol as the raw materials, as shown in Scheme 1, the target compounds 4a–4r were prepared with the yields of 60.0%–84.6%. In the 1H-NMR spectra of 4a–4r, the singlet around δ = 10.62–11.02 ppm indicated the presence of the –NH– group. The singlet in δ = 170.1–170.8 ppm indicated the presence of a C=O group. The 1H-NMR, 13C-NMR, and HRMS data and spectra for all the synthesized compounds are shown in the Supplementary Materials.
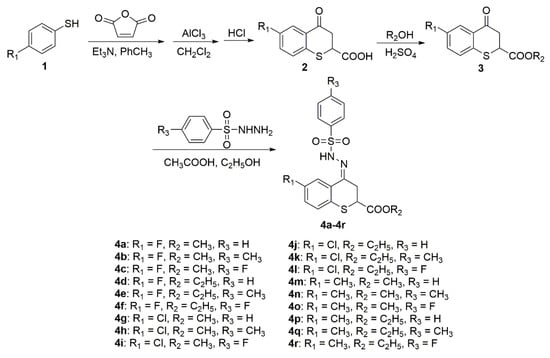
Scheme 1.
Synthesis of compounds 4a–4r.
2.2. Biological Evaluations
In this study, the in vitro antibacterial activities of the target compounds 4a–4r against Xoo, Xoc, and Xac at 200 and 100 μg/mL were determined by the turbidimeter test [28] and the results were statistically analyzed and listed in Table 1. Most of the target compounds revealed moderate to good in vitro antibacterial activities against Xoo, Xoc, and Xac at 200 and 100 μg/mL. In particular, compounds 4g, 4i, 4j, and 4l revealed a 100% inhibition rate against Xoo at 200 μg/mL; notably compound 4i and 4l still achieved a 100% inhibition rate at 100 μg/mL, which were even better than those of Bismerthiazol and Thiodiazole-copper. Meanwhile, Table 1 showed that most of the target compounds proved to have better in vitro antibacterial activity against Xoc at 200 and 100 μg/mL. Among of them, compound 4i exhibited the best inhibitory activity against Xoc at 200 and 100 μg/mL, with the inhibition rates of 96% and 90%, respectively, than those of Bismerthiazol and Thiodiazole-copper. In addition, all the target compounds, except compounds 4n, 4p, and 4q, revealed better inhibitory activity against Xac than those of Bismerthiazol and Thiodiazole-copper; notably, compounds 4g, 4i, and 4l revealed a 100% inhibition rate against Xac at 200 μg/mL.

Table 1.
In vitro antibacterial activities of the target compounds 4a–4r against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicolaby, and Xanthomonas axonopodis pv. citri at 200 and 100 μg/mL.
Based on preliminary bioactivity results, the EC50 values of some of the target compounds against Xoo, Xoc, and Xac were also determined and the results were statistically analyzed and listed in Table 2. As shown in Table 2, compounds 4c, 4f, 4g, 4h, 4i, 4j, and 4l revealed lower EC50 values against Xoo (8–32 μg/mL), Xoc (12–46 μg/mL), and Xac (10–38 μg/mL) than those of Bismerthiazol and Thiodiazole-copper. In particular, compound 4i showed the best in vitro antibacterial activities against Xoo, Xoc, and Xac, with EC50 values of 8, 12 and 10 μg/mL, which were superior to those of Bismerthiazol and Thiodiazole-copper.

Table 2.
The EC50 values of some of the target compounds against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicolaby, and Xanthomonas axonopodis pv. citri.
Meanwhile, the in vitro antifungal activities of the target compounds 4a–4r against S. sclerotiorum, F. oxysporum, G. zeae, R. solani, V. dahlia, and B. cinerea were tested at 50 μg/mL by the mycelial growth rate method [29] and the results were statistically analyzed and listed in Table 3. As shown in Table 3, bioassay results showed that all the target compounds revealed lower antifungal activities against S. sclerotiorum, F. oxysporum, G. zeae, R. solani, V. dahlia, and B. cinerea at 50 μg/mL than those of Carbendazim.

Table 3.
In vitro antifungal activities of the target compounds 4a–4r against Sclerotinia sclerotiorum, Fusarium oxysporum, Gibberella zeae, Rhizoctonia solani, Verticillium dahlia, and Botrytis cinerea at 50 μg/mL.
2.3. Structure-Activity Relationship (SAR) Analysis
As an extension of this approach, the SAR was deduced on the basis of the inhibitory activity values of the antibacterial and antifungal activities shown in Table 1, Table 2 and Table 3. First, compared to the same substituent at R2 and R3, the presence of the –Cl atom at R1 showed better antibacterial and antifungal activities in the order of 4g > 4a and 4k > 4b. Second, compared to the same substituent at R1 and R2, the electron drawing group (–F) at R3 could cause an increase in the antibacterial and antifungal activities following the order 4c > 4a > 4b, 4i > 4g > 4h, and 4o > 4m > 4n. Third, compared to the same substituent at R1 and R3, the smaller substituent groups, such as –CH3, at the R2 could cause an increase in the antibacterial and antifungal activities. The bioactivities of the target compounds followed the order 4a > 4d, 4b > 4e, and 4c > 4f.
3. Materials and Methods
3.1. General Information
The melting points were determined by an uncorrected WRX-4 binocular microscope (Shanghai Yice Tech. Instrument Co., Shanghai, China). 1H-NMR and 13C-NMR spectral analyses were performed on a Bruker DRX-400 NMR spectrometer (Bruker, Rheinstetten, Germany). HRMS data were measured on an Agilent Technologies 6210 LC/MS TOF mass spectrometer (Agilent, Palo Alto, CA, USA). All reagent products from the Chinese Chemical Reagent Company were analytical or chemically pure.
3.2. Chemical Synthesis
3.2.1. Preparation Procedure of Intermediates 2 and 3
A mixture of substituted thiophenols (70 mmol) and a slight excess of maleic anhydride (84 mmol, 1.2 equivalents) in methylbenzene was added to a 250 mL round bottom flask equipped with a magnetic stirrer and reacted at 50 °C for 0.5 h, and then triethylamine (2 drops) was slowly added and stirred at 70 °C for 4 h. The reaction was quenched to room temperature and then the solvent was removed under reduced pressure. After that, the residues were redissolved with dichloromethane (100 mL) with bath ice and then a significant excess of AlCl3 (210 mmol, 3 equivalents) was added. The mixture reaction was stirred in an ice bath for 3–4 h. After the reaction was completed, as determined by TLC, the reaction mixture was diluted with dichloromethane (100 mL) and treated with precooled 5% dilute hydrochloric acid (50 mL). The residues were filtrated, dried under vacuum, and recrystallized from ethanol to give intermediate 2.
A mixture of intermediate 2 (50 mmol), methanol or ethanol (100 mL), and H2SO4 (4 mmol) were added in a 250 mL round bottom flask and reacted under reflux conditions for 6–8 h. Upon completion of reaction (determined by TLC), the mixture was quenched to room temperature and the precipitated residues were filtrated, dried under vacuum, and recrystallized from methanol to give pure intermediate 3.
3.2.2. Preparation Procedure of the Target Compound 4a–4r
A mixture of intermediate 3 (10 mmol), substituted benzenesulfonyl hydrazide (12 mmol, 1.2 equivalents), acetic acid (10 mL), and ethanol (10 mL) was added to a 50 mL round bottom flask equipped with a magnetic stirrer, and reacted under reflux conditions for 2–4 h. Upon completion of the reaction (determined by TLC), the mixture was cooled to room temperature and the precipitated residues was dried under a vacuum and recrystallized from ethanol to give the pure target compounds 4a–4r.
3.3. Bioactivity Evaluation
3.3.1. Bacterial and Fungal Strains
All bacterial and fungal strains used in this study were provided by Guizhou University, China.
3.3.2. In Vitro Antibacterial Activity Test
All the taget compounds (7.5 mg) were dissolved in 150 μL DMSO and 80 and 40 μL of the mixture solution was poured into two 15 mL centrifuge tubes with 0.1% Tween aqueous solution (4 mL), respectively. Next, 1 mL Tween aqueous solution with the testing compounds was added into the test tubes containing 4 mL nutrient broth (NB) mediums (Solarbio, Beijing, China) to prepare 5 mL test solutions with concentrations of 200 and 100 μg/mL, respectively. Finally, 40 μL of precultured NB mediums containing Xoo, Xoc, and Xac, respectively, were added to the test tubes and incubated at 30 °C and 180 rpm for 24–48 h until the bacteria were incubated on reaching the logarithmic growth phase. DMSO served as the negative control, whereas Thiodiazole copper and Bismerthiazol served as the positive controls. The OD595 values of the cultures were monitored on a Multiskan Sky 1530 spectrophotometer (Thermo Scientific, Poland). Three replicates were conducted for each treatment. Inhibition rate I (%) is calculated by the following formula (1), where C is the corrected turbidity value of the untreated NB medium and T is the corrected turbidity value of the treated NB medium.
Inhibition rate I (%) = (C–T)/C × 100
Based on the preliminary bioassays results, five corresponding concentration gradients were prepared, and the antibacterial activities (expressed by EC50) of some of the target compounds against Xoo, Xoc, and Xac were also evaluated and calculated using SPSS 17.0 software (SPSS, Chicago, IL, USA). The experiments were repeated three times for each compound.
3.3.3. In Vitro Antifungal Activity Test
All the taget compounds (5 mg) were dissolved in 1 mL DMSO and then mixed with 90 mL potato dextrose agar (PDA) medium (Solarbio, Beijing, China). After that, the mixed PDA medium were poured into 6 or 9 dishes and then cooled to room temperature to prepare PDA plates. Mycelia dishes of approximately 0.4 cm diameter were cut from culture medium and then picked up with a germfree inoculation needle to the middle of PDA plate aseptically. The inoculated PDA plates were fostered in an incubator at 28 ± 1 °C for 3–4 days. DMSO served as a negative control, whereas Carbendazim acted as a positive control. Three replicates were conducted for each treatment. The inhibition rate I (%) were calculated by the formula (2), where C (cm) represents the diameter of fungi growth on an untreated PDA plate, and T (cm) represents the diameter of fungi on the treated PDA plate.
Inhibition rate I (%) = [(C−T)/(C−0.4)] × 100
3.4. Statistical Analysis
Statistical analysis was conducted by analysis of variance (ANOVA) in SPSS 17.0 software with equal variances assumed (p > 0.05) and equal variances not assumed (p < 0.05). Asterisk (*) shown in Table 1, Table 2 and Table 3 indicated the inhibition rates of the target compounds with significant difference at p < 0.05 compared to those of positive controls (Thiodiazole copper, Bismerthiazol, and Carbendazim).
4. Conclusions
In this study, a total of 16 novel thiochromanone derivatives containing a sulfonyl hydrazone moiety were designed and synthesized. Bioassay results showed that most of the target compounds revealed moderate to good in vitro antibacterial activities against Xoo, Xoc, and Xac, as well as lower in vitro antifungal activities against S. sclerotiorum, F. oxysporum, G. zeae, R. solani, V. dahlia, and B. cinerea. In particular, compound 4i exhibited the best inhibitory activities against Xoo, Xoc, and Xac, with the EC50 values of 8.67, 12.65, and 10.62 μg/mL, which were superior to those of Bismerthiazol and Thiodiazole-copper. The SAR analysis showed that the size and electron-withdrawing property of substituent groups in R1, R2, and R3 is one of the crucial factors to affect the antibacterial and antifungal activities against the tested bacterial and fungal strains used in this study. The SAR study provided a practical tool for guiding the design and synthesis of novel and more promising active small molecules of thiochromanone derivatives containing a sulfonyl hydrazone moiety for controlling plant bacterial and fungal diseases.
Supplementary Materials
The following are available online. The 1H-NMR, 13C-NMR, and HRMS data and spectra for all the synthesized compounds are shown in the Supplementary Materials.
Author Contributions
Methodology, L.Y.; data analysis, J.C., L.X., J.L. and Z.T.; writing—original draft preparation, L.Y. and P.L.; writing—review and editing, S.T.; funding acquisition, L.Y. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2017YFD0200903, Science and Technology Foundation of Guizhou Province, grant number ZK[2 021]137, and Kaili University Doctoral Program, grant number BS201811.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data present in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 4a–4r are available from the authors.
References
- Bhattacharjee, R.; Dey, U. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr. J. Microbiol. Res. 2014, 8, 1479–1762. [Google Scholar]
- Patel, N.; Desai, P.; Patel, N.; Jha, A.; Gautam, H.K. Agronanotechnology for plant fungal disease management: A review. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 71–84. [Google Scholar]
- Saengchantara, S.T.; Wallace, T.W. Chromanols, chromanones, and chromones. Nat. Prod. Rep. 1986, 3, 465–475. [Google Scholar] [CrossRef]
- Wang, A.L.; Zhou, W.C.; Wang, R.R.; Zhang, Y.W.; Zhou, W.M. Synthesis and antifungal activity of 3-substituted-benzylidene-6,7-methylenedioxy-4-chromanone. Chin. J. Pest. Sci. 2013, 15, 622–628. [Google Scholar]
- Yu, G.Y.; Xiao, T. Recent advances in the research of chromone and thiochromone derivatives as antifungal agents. Chin. J. Antibiot. 2011, 36, 481–486. [Google Scholar]
- Abdel Ghani, S.B.; Mugisha, P.J.; Wilcox, J.C.; Gado, E.A.M.; Medu, E.O.; Lamb, A.J.; Brown, R.C.D. Convenient one-pot synthesis of chromone derivatives and their antifungal and antibacterial evaluation. Synth. Commun. 2013, 43, 1549–1556. [Google Scholar] [CrossRef]
- Malefo, M.S.; Ramadwa, T.E.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N.; Sonopo, M.S.; Selepe, M.A. Synthesis and antifungal activity of chromones and benzoxepines from the leaves of Ptaeroxylon Obliq. J. Nat. Prod. 2020, 83, 2508–2517. [Google Scholar] [CrossRef]
- Jo, H.; Seo, S.H.; Na, Y.; Kwon, Y. The synthesis and anticancer activities of chiral epoxy-substituted chromone analogs. Bioorg. Chem. 2019, 84, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zan, N.N.; Huang, M.X.; Jiang, D.H.; Hu, D.Y.; Song, B.A. Design, synthesis and anti-TMV activities of novel chromone derivatives containing dithioacetal moiety. Bioorg. Med. Chem. Lett. 2020, 30, 126945. [Google Scholar] [CrossRef]
- Zhang, D.J.; Ji, X.Y.; Gao, R.M.; Wang, H.Q.; Meng, S.; Zhong, Z.J.; Li, Y.H.; Jiang, J.D.; Li, Z.R. Synthesis and antiviral activities of a novel class of thioflavone and flavonoid analogues. Acta Pharm. Sin. B 2012, 2, 575–580. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdallah, M.A.; Khedr, M.A.; Mahmoud, M.A. Synthesis, antimicrobial activity and molecular docking study of thiazole derivatives. J. Heterocycl. Chem. 2017, 54, 2417–2425. [Google Scholar] [CrossRef]
- Suresh, L.; Kumar, P.S.V.; Poornachandra, Y.; Kumar, C.G.; Babu, N.J.; Chandramouli, G.V.P. An expeditious four-component domino protocol for the synthesis of novel thiazolo[3,2-a]thiochromeno[4,3-d]pyrimidine derivatives as antibacterial and antibiofilm agents. Bioorg. Med. Chem. 2016, 24, 3808–3817. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Feng, J.J.; Li, S.B.; Feng, S.R.; Liu, Z.M.; Song, Y.L.; Qiao, X.Q. Design, synthesis, and antifungal activity of novel thiochromanone derivatives containing 1,3,4-oxadiazole skeleton. Chin. J. Org. Chem. 2019, 39, 1037–1043. [Google Scholar] [CrossRef]
- Li, S.B.; Qi, H.; Zhang, C.C.; Liu, Z.M.; Song, Y.L.; Qiao, X.Q. Synthesis, antifungal activity and molecular docking of (E)-3-(((1,3,4-thiadiazol-2-yl)amino)methylene)-thiochroman-4-ones. Acta Pharm. Sin. 2018, 53, 1518–1525. [Google Scholar]
- Xu, H.; Hu, Y.K.; Guo, M.B.; Huang, A.S.; Su, X.; Guo, C. Design, synthesis and antifungal activity of novel selenochroman-4-one derivatives. Chem. Pap. 2017, 71, 2455–2463. [Google Scholar] [CrossRef]
- Ichiro, N.; Mitsuru, S.; Masashi, S.; Kazuyoshi, K. Preparation of 4-(6-Thiochromancarbonyl) Pyrazole Derivatives as Herbicides. PCT Int. Appl. WO 9401431, 20 January 1994. [Google Scholar]
- Szucs, S.S. Thiono-thiochroman and -dihydrobenzothiophene Compounds as Herbicidal Agents. PCT International Application WO 2001029033, 26 April 2001. [Google Scholar]
- Steiner, G.; Schmidt, T.; Kordes, M.; Von Deyn, W.; Hofmann, M.; Baumann, E.; Puhl, M.; Heffernan, G.; Culbertson, D.L.; Treacy, M.F. Amino-Substituted Benzo(hetero)cyclic Derivatives, particularly 1-alkyl-4-benzo(hetero)Cyclicsubstituted Piperazines, Useful as Insecticides, Acaricides, and Nematocides, and their Preparation, Uses, and Compositions. PCT International Application WO 2004080170, 23 September 2004. [Google Scholar]
- Neumann, D.M.; Cammarata, A.; Backes, G.; Palmer, G.E.; Jursic, B.S. Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. [Google Scholar] [CrossRef]
- Loncle, C.; Brunel, J.M.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004, 39, 1067–1071. [Google Scholar] [CrossRef]
- Li, Y.T.; Lin, J.; Yao, W.Q.; Gao, G.L.; Jing, D.W.; Wu, Y. Discovery of a new fungicide by screening triazole sulfonylhydrazone derivatives and its downy mildew inhibition in cucumber. J. Heterocycl. Chem. 2020, 57, 2128–2138. [Google Scholar] [CrossRef]
- Karacan, M.S.; Zharmukhamedov, S.K.; Mamas, S.; Kupriyanova, E.V.; Shitov, A.V.; Klimov, V.V.; Ozbek, N.; Ozmen, U.; Gunduzalp, A.; Schmitt, F.J.; et al. Screening of novel chemical compounds as possible inhibitors of carbonic anhydrase and photosynthetic activity of photosystem II. J. Photochem. Photobiol. B Biol. 2014, 137, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Gunduzalp, A.B.; Ozmen, U.O.; Cevrimli, B.S.; Mamas, S.; Cete, S. Synthesis, characterization, electrochemical behavior, and antimicrobial activities of aromatic/heteroaromatic sulfonylhydrazone derivatives. Med. Chem. Res. 2014, 23, 3255–3268. [Google Scholar] [CrossRef]
- Loh, W.; Cosby, L.A.; Sartorelli, A.C. Synthesis and antineoplastic activity of phenyl-substituted benzenesulfonylhydrazones of 2-pyridinecarboxaldehyde 1-oxide. J. Med. Chem. 1980, 23, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, G.; Chen, D.; Peng, X.; Chen, Y.L.; Su, Y.; Ji, Y.; Liang, J.; Wang, X.; Chen, L.; et al. Design and optimization of a series of 1-sulfonylpyrazolo[4,3-b]pyridines as selective c-met inhibitors. J. Med. Chem. 2015, 58, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.D.; Rewcastle, G.W.; Frederick, R.; Mawson, C.; Denny, W.A.; Marshall, E.S.; Baguley, B.C.; Chaussade, C.; Jackson, S.P.; Shepherd, P.R. Synthesis, biological evaluation and molecular modeling of sulfonohydrazides as selective PI3K p110a inhibitors. Bioorg. Med. Chem. 2007, 15, 7677–7687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, X.; Zhi, X.Y.; Xiao, X.; Yang, C.; Xu, H. Synthesis and insecticidal activity of novel hydrazone compounds derived from a naturally occurring lignan podophyllotoxin against Mythimna separata (Walker). Bioorg. Med. Chem. Lett. 2014, 24, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Schaad, N.W.; Wang, Z.K.; Di, M.; McBeath, J.; Peterson, G.L.; Bonde, M.R. An improved infiltration technique to test the pathogenicity of Xanthomonas oryzae pv. oryzae in rice seedlings. Seed Sci. Technol. 1996, 24, 449–456. [Google Scholar]
- Tarun, K.C.; Prem, D.J. Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food Chem. 2006, 54, 2129–2133. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).