Abstract
Despite the technological importance of urea perhydrate (percarbamide) and sodium percarbonate, and the growing technological attention to solid forms of peroxide, fewer than 45 peroxosolvates were known by 2000. However, recent advances in X-ray diffractometers more than tripled the number of structurally characterized peroxosolvates over the last 20 years, and even more so, allowed energetic interpretation and gleaning deeper insight into peroxosolvate stability. To date, 134 crystalline peroxosolvates have been structurally resolved providing sufficient insight to justify a first review article on the subject. In the first chapter of the review, a comprehensive analysis of the structural databases is carried out revealing the nature of the co-former in crystalline peroxosolvates. In the majority of cases, the coformers can be classified into three groups: (1) salts of inorganic and carboxylic acids; (2) amino acids, peptides, and related zwitterions; and (3) molecular compounds with a lone electron pair on nitrogen and/or oxygen atoms. The second chapter of the review is devoted to H-bonding in peroxosolvates. The database search and energy statistics revealed the importance of intermolecular hydrogen bonds (H-bonds) which play a structure-directing role in the considered crystals. H2O2 always forms two H-bonds as a proton donor, the energy of which is higher than the energy of analogous H-bonds existing in isostructural crystalline hydrates. This phenomenon is due to the higher acidity of H2O2 compared to water and the conformational mobility of H2O2. The dihedral angle H-O-O-H varies from 20 to 180° in crystalline peroxosolvates. As a result, infinite H-bonded 1D chain clusters are formed, consisting of H2O2 molecules, H2O2 and water molecules, and H2O2 and halogen anions. H2O2 can form up to four H-bonds as a proton acceptor. The third chapter of the review is devoted to energetic computations and in particular density functional theory with periodic boundary conditions. The approaches are considered in detail, allowing one to obtain the H-bond energies in crystals. DFT computations provide deeper insight into the stability of peroxosolvates and explain why percarbamide and sodium percarbonate are stable to H2O2/H2O isomorphic transformations. The review ends with a description of the main modern trends in the synthesis of crystalline peroxosolvates, in particular, the production of peroxosolvates of high-energy compounds and mixed pharmaceutical forms with antiseptic and analgesic effects.
1. Introduction
Crystalline peroxosolvates, adducts of hydrogen peroxide, were first introduced by Tanatar who synthesized sodium percarbonate Na2CO3·1.5H2O2 [1] and urea perhydrate (percarbamide) CH4N2O·H2O2 [2]. These compounds are the two most widely used solid peroxocompounds with annual production in the millions of tons [3]. Sustainable, nontoxic, and minimal hazard processing trends combine to intensify the use of hydrogen peroxide in diverse fields and the same trends are responsible for the perpetually growing use of peroxosolvates [3,4,5]. Peroxosolvates are now used for bleaching, disinfection, and oxidation; as chemical reagents in household commodities, cosmetics, pharmaceuticals, and washing powders; in industrial environmental processes such as remediation, bioremediation, and oxygen production; and as explosive ingredients and reagents for chemical synthesis [3,4,5,6]. In general, hydrogen peroxide release from peroxosolvates tends to lower the pH, whereas hydroperoxo- and peroxo-complexes tend to increase the pH [6,7,8,9,10]. Thus, peroxosolvates are considered safer and more economic as aqueous hydrogen peroxide decomposes at high pH.
From an academic point of view, the insight gained from the energetics of peroxosolvates as reflected by single crystal x-ray studies and DFT computations sheds light on the non-redox behavior of hydrogen peroxide in aqueous and biological systems. Selective transmembrane uptake and transport, bioactivation, and detoxification of hydrogen peroxide are all likely to involve non-redox bonding mechanisms, which also determine crystalline peroxosolvate formation and stability in aqueous media [11,12,13]. It is not a mere coincidence that one of the largest classes of peroxosolvates involves amino acids and dipeptides. In fact, the number of structurally resolved amino acid peroxosolvates outweighs the number of the corresponding hydrates. Peroxosolvates of 14 amino acids are structurally characterized, while only six of these amino acids form hydrates according to the Cambridge Structural Database (CSD) [14,15]. The ability to form networks of strong intermolecular hydrogen bonds (H-bonds) both in solution and in the crystalline phase determines the key role that H2O2 plays in ecologically significant and biological processes [16,17,18]. Hydrogen peroxide is formed in living cells in mitochondria [11,19]. H2O2 plays an important role in oxidative stress processes [18,20]. Several integral membrane proteins act as transmembrane channels promoting the hydrogen peroxide transport across cell membranes [21,22,23,24].
The H-bond is the main type of intermolecular interaction in crystalline peroxosolvates. The hydrogen peroxide molecule is capable of forming up to six such bonds: two as a proton donor and four as an acceptor [25]. However, at the moment, only a few crystalline peroxosolvates are known, in which the hydrogen peroxide molecule forms six H-bonds [25,26,27]. Hydrogen peroxide has pronounced acidic properties, which, for example, are manifested in the ability to deprotonate under mild conditions and form ammonium hydroperoxide [28] and metal peroxides [29]. We have previously shown that, due to its pronounced acidic properties, the hydrogen peroxide molecule in peroxosolvates always forms two H-bonds as a proton donor, which are structure-directing [25]. This suggests that the compounds forming stable peroxosolvates should contain proton-acceptor groups, that is, they are Brønsted bases or have amphoteric properties.
CSD [14,15] and the Inorganic Crystal Structure Database (ICSD) [30,31] contain information on 134 peroxosolvates. This is several orders of magnitude less than the number of crystalline hydrates that existed in these databases about twenty years ago [32]. As a result of the analysis of structural databases, the chemical composition and networks of H-bonds in peroxosolvates, in which H2O2 molecules do not directly interact with metal atoms, have been characterized [25]. The study of more than 260 H-bonds in 65 crystal structures showed that hydrogen peroxide always participates as proton donor in two H-bonds and forms from zero to four hydrogen bonds as a proton acceptor.
Taking into account the peroxosolvates synthesized over the past four years, as well as crystals with an H2O2–metal atom contact [33,34,35], the total number of crystalline peroxosolvates is 134. This is two times more than the number of crystal structures analyzed in [25]. A significant number of peroxosolvates (44 adducts) were synthesized and structurally characterized at the Kurnakov Institute of General and Inorganic Chemistry of Russian Academy of Sciences (IGIS RAS) (Figure 1).
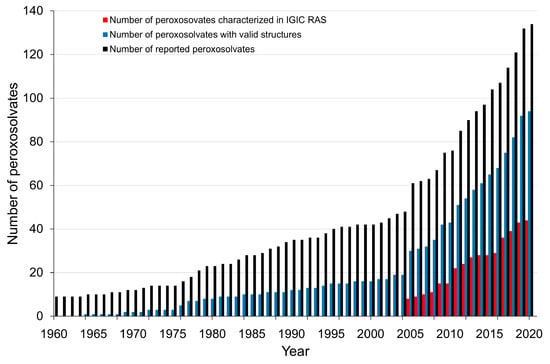
Figure 1.
Cumulative chart of the total number of peroxosolvates in the period 1960–2020.
The search for new peroxosolvates is an active task. Analysis of the composition and structure of known crystalline peroxosolvates made it possible to formulate the main directions of such a search, which are outlined at the end of Section 2.
The presented material is arranged as follows. First, the chemical composition of crystalline peroxosolvates is considered. This made it possible to formulate criteria for the directed synthesis of new stable crystalline H2O2 adducts with certain properties: mixed pharmaceutical forms, high-energy substances, etc. Section 3 is devoted to the dimension and topology of peroxide clusters existing in crystalline peroxosolvates. The main focus is on infinite one-dimensional (1D) chains of H2O2 molecules. Then, approaches based on calculations using density functional theory methods with periodic boundary conditions are considered, which make it possible to obtain the energies of intermolecular H2O2 interactions in organic crystals. The fifth section describes the specific features of the H-bond networks in crystalline peroxosolvates. Particular attention is paid to the analysis of the lengths of H-bonds formed by H2O2 as a proton acceptor, and the types of H2O2 coordination. The review ends with a description of the main modern trends in the synthesis of crystalline peroxosolvates.
2. Chemical Composition of Crystalline Peroxosolvates
All 134 crystalline peroxosolvates known to date can be divided into three main groups depending on the chemical nature of the coformer (Figure 2):
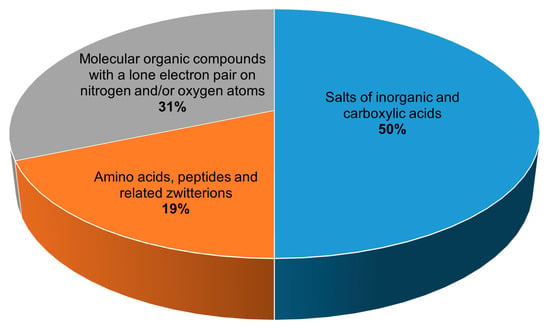
Figure 2.
Distribution of peroxosolvates by the chemical nature of the coformer.
(1) salts of inorganic and carboxylic acids,
(2) amino acids, peptides and related zwitterions, and
(3) molecular compounds with a lone electron pair on nitrogen and/or oxygen atoms.
Intermolecular H-bonds play a structure-directing role in the considered crystals.
The largest number of structurally characterized peroxosolvates, 67 compounds, are adducts of hydrogen peroxide and salts of inorganic and carboxylic acids with various cations. Among the salts of carboxylic acids that form stable peroxosolvates [36,37,38,39,40], oxalates can be distinguished in the composition of six compounds. The salts of inorganic acids that form adducts with hydrogen peroxide are very diverse. In addition to fluorides [41,42,43], chlorides, and bromides [44,45], a number of peroxosolvates of alkali metal and ammonium carbonates are known [46,47,48]. The latter include the commercially demanded sodium peroxocarbonate synthesized by Tanatar [1]. This class of compounds should include peroxosolvates of complex anions, which can formally be attributed to the salts of the corresponding complex acids, for example, peroxovanadates [49,50,51,52,53,54,55,56], peroxoniobates [57,58,59], peroxotantalates [60], uranyl peroxo complexes [61], peroxotellurates [62], and platinum complexes [63,64,65]. Peroxosolvates of metal peroxides [66,67,68,69] can formally belong to the specified class of peroxosolvates of salts of inorganic acids if hydrogen peroxide is considered as a diacid.
The next group in terms of the number of compounds (42 compounds) are peroxosolvates formed by molecular organic compounds with a lone electron pair(s) on the nitrogen and/or oxygen atom(s). The main representatives of this group of crystalline hydrogen peroxide adducts are organophosphorus compounds containing the P=O functional group [49,70,71,72,73,74,75,76] and nitrogen-containing heterocyclic compounds [25,26,77,78,79,80,81,82,83,84], in particular N-oxides [85,86,87,88,89,90,91,92], obtained as a result of the oxidation reaction of the corresponding compounds with hydrogen peroxide. Urea peroxosolvate [2,27] is used as a solid source of hydrogen peroxide, and, along with 1,4-diazabicyclo[2.2.2]octane (DABCO) peroxosolvate [93], is used in organic syntheses to obtain anhydrous hydrogen peroxide solutions.
Separately, it is worth highlighting the third group, which includes amino acids, peptides, and related zwitterions (25 peroxosolvates). Peroxosolvates of a number of proteionogenic l-amino acids (serine, threonine, leucine, isoleucine, tyrosine, glycine, and phenylalanine) [94,95] and non-proteinogenic amino acids (gamma-aminobutyric acid, beta-alanine, and sarcosine) were obtained and structurally characterized at the Kurnakov Institute of General and Inorganic Chemistry RAS [95,96]. The class of zwitterions related to amino acids that form peroxosolvates includes pyridine carboxylic acids: nicotinic, isonicotinic, and picolinic acids [97], as well as 2-aminonicotinic acid [85]. Peroxosolvates of cyclic dipeptides—diglycine, disarcosine, and dialanine—are an example of the nonoxidative interaction of concentrated hydrogen peroxide and a peptide fragment [13].
The CSD analysis revealed the necessary properties of co-former peroxosolvates, which are promising compounds for the synthesis of new crystalline peroxosolvates [25]:
(1) Hydrogen peroxide should not participate in redox reactions with coformers.
(2) They must be sufficiently soluble in protic solvents to carry out the crystallization process.
(3) Coformers should have the ability to form H-bonds, primarily as proton acceptors.
(4) Compounds with pronounced acidic properties do not form peroxosolvates [14], since in such compounds the proton-acceptor groups are protonated.
(5) Coformers should exhibit amphoteric or basic properties. Strong bases deprotonate hydrogen peroxide and form peroxide or hydroperoxide as ionic or complex moieties (ZnO2 [29], NH4+OOH− [28,98], or [Sn(OOH)6]2− [7]).
A significant part of the 134 peroxosolvates available in the structural databases, namely 40 structures, contain incomplete or erroneous data. Some crystal structures contain unlocalized hydrogen atoms [39,51,55,57,99,100,101,102,103] and errors in the O-O bond lengths [56,104] and H-O-O-H and O-O-H angles [105,106] in the hydrogen peroxide molecule. This is due to the fact that during the preparation of these compounds, H2O2 was used as an oxidizing agent or ligand to obtain the corresponding peroxo complexes; therefore, the mass content of hydrogen peroxide in the obtained crystals is low. The chemical composition of 94 crystal structures of peroxosolvates with objectively localized protons and free of structural errors is presented in Supplementary Materials Table S1. These 94 crystal structures are the subject of this review, as they allow one to analyze the topology of H2O2 hydrogen-bonded networks.
3. Dimensions and Topology of Peroxide Clusters in the Crystalline Phase
It was already mentioned above that peroxosolvates exist due to a system of various H-bonds formed by hydrogen peroxide with coformers in crystals. The question arises, can H-bonds between hydrogen peroxide molecules, in addition to H-bonds with organic molecules, be observed in the structures of crystalline peroxosolvates? Furthermore, if so, what are the dimensions and topology of the formed peroxide clusters?
Over the past 10 years, a number of structures have been described containing insular (finite) clusters of two H2O2 molecules in peroxosolvates of peroxovanadates [56,107] and in potassium alumoxalate peroxalate [108]. A linear centrosymmetric cluster of three hydrogen peroxide molecules [72] with the D3 topology in the Infantes–Motherwell notation [109] was obtained. In the potassium peroxocarbonate peroxosolvate, a cyclic cluster consisting of four hydrogen peroxide molecules was found [110]. Somewhat later, another example of a dimeric cluster was found in the structure of tyrosine peroxosolvate [95]. More recently, data on the structures of lidocaine N-oxide and 2-aminonicotinic acid peroxosolvates were published, including stellar pentameric and giant dodecameric clusters of peroxide molecules [85]. The last of these clusters cannot be accurately classified within the Infantes–Motherwell notation and its topology can be seen as a combination of R4 and D5 motifs.
In 1984, the structure of a mixed hydrate of a peroxosolvate with infinite one-dimensional chains of H2O2 molecules with the simplest topology C1 was published. In the chain, some H2O2 molecules were statistically replaced by water molecules due to the phenomenon of mutual isomorphic substitution of peroxide and water molecules, recently studied in the work [25]. Subsequently, the same peroxide–water chains were found in the structures of thymine peroxosolvate hydrates [111] and DABCO [93]. In these three cases, the presence of an impurity of water is explained by the fact that the authors carried out crystallization from dilute solutions of hydrogen peroxide in water. Only in 2017 was anhydrous thymine peroxosolvate (from 98% peroxide) containing “pure peroxide” C1 chains obtained (Figure 3a) [25].
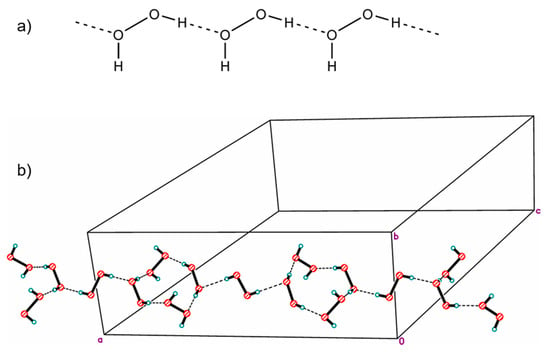
Figure 3.
Infinite one-dimensional chains of hydrogen peroxide molecules: (a) the simplest topology C1 (YAFGEU) and (b) a nontrivial topology, which is a combination of alternating linear and cyclic fragments (SEMXIU). H-bonds are denoted by dotted lines.
Two more examples of “purely peroxide” chains of trivial C1 topology were recently discovered in the structures of phenylserine and pipicolinic acid peroxosolvates [96,112]. An example of the structure of an organic peroxosolvate with infinite peroxide chains of a nontrivial topology was presented in [80], which is a combination of alternating linear and cyclic fragments consisting only of H2O2 molecules (Figure 3b).
It should be noted that hydrogen peroxide tends to form infinite one-dimensional H-bonded chain clusters with halogen anions (Figure 4). This phenomenon was studied in detail in [45], which led to the discovery of the phenomenon of peroxomorphism by the example of crystallization of solvatomorphs with the composition (Ph4As)+Cl−·nH2O2 (n = 1, 1.5, 2) from peroxide solutions of different concentrations (30, 50, and 96%).
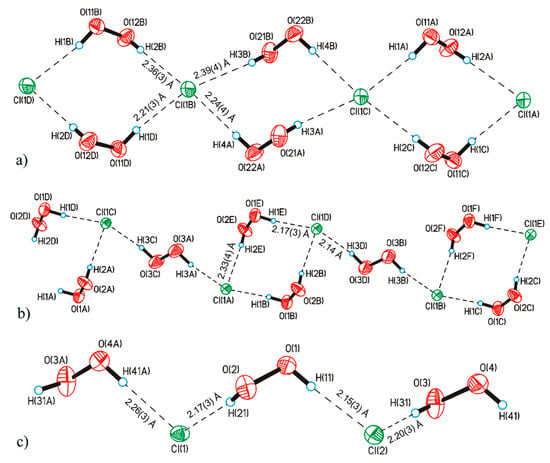
Figure 4.
Structure of hydrogen peroxide clusters formed in crystalline Ph4AsCl·nH2O2: (a) [(H2O2)2(Cl−)1]inf chains at n = 2; (b) [(H2O2)3(Cl−)2]inf chains at n = 1.5; (c) [(H2O2)1(Cl−)1]inf chains at n = 1. O-H···Cl− bonds are denoted by dotted lines. Distances H···Cl− are presented. Reproduced from the work in [45] with permission from The Royal Society of Chemistry.
4. The Energies of Intermolecular Interactions of H2O2 in Organic Crystals: Calculations by the Kohn–Sham Methods with Periodic Boundary Conditions
Various theoretical approaches/methods are used to describe the structure and properties of organic crystals: calculations in the cluster approximation [113], methods using empirical force fields (molecular dynamics) [114,115], and calculations by the Kohn–Sham methods with periodic boundary conditions (periodic DFT) [116,117]. Calculations in the cluster approximation can deduce certain properties of an organic crystal: the energy of the crystal lattice [118], the mobility of charges in organic semiconductors [119,120], the chemical shift of the nucleus [121]. This requires knowledge of the structure of the crystal under study, that is, the cif file. Empirical force field parameters have limited transferability [122]. Simultaneous calculation of various properties of an organic crystal (energy of intermolecular interactions, enthalpy of sublimation, low-frequency IR and Raman spectra, etc.) requires the use of periodic DFT methods [123,124]. There are a number of methods and programs for performing this type of calculation [125,126]. In computational methods of solid-state physics, approaches that utilize basic sets of plane waves are usually used [117], and in solid state chemistry, basic sets of the Gaussian type are used [116]. The advantages and disadvantages of these approaches in calculating organic crystals are discussed in [124,125,127].
In the theoretical study of crystals, much attention is paid to assessing the energy of intermolecular interactions. In contrast to the gas phase, such a calculation in a solid is a non-trivial problem, which involves the “isolation” of the energy (enthalpy) of a specific intermolecular interaction from the energy of the crystal lattice (enthalpy of sublimation) [128]. In most cases, empirical approaches are used that relate the energy of intermolecular interaction with one or another parameter of the electron density at the bond critical point [129,130,131]. In this case, the calculated values of the electron density, the values of the parameters retrieved from the precise X-ray diffraction data, and hybrid approaches are used [132]. (Calculation of the electron density characteristics in plane wave basis is not trivial due to the use of pseudopotentials [133].) The approaches indicated above are often used to assess intermolecular interactions of various natures in various crystals [134,135,136], which gives rise to well-founded criticism [137,138].
Intermolecular interactions of H2O2 in crystalline peroxosolvates are mainly due to conventional H-bonds. The energy or enthalpy of these bonds can be estimated from the spectroscopic [139] and metric [140] characteristics of H-bonds in crystals, that is, without invoking the electron density parameters.
The H-bond enthalpy (−ΔHHB) can be estimated as previously shown [140]:
−ΔHHB [kJ/mol] = 0.134·R(H···B)−3.05.
Here, the R(H···B) is the H···B distance (nm), and B = O, N. The main limitation of this approach is the problem of accurate experimental determination of the position of hydrogen atoms. (As noted above, many papers have been published in which the position of hydrogen atoms in crystalline peroxosolvates has not been determined.) For this it is necessary to use the neutron diffraction method. We note that the number of crystals with H-bonds studied by this method is very limited [141]; in particular, this method was used to study the H2O2 crystal [142]. The exact values of the H···B distances can be computed using the periodic DFT methods [117,125].
The −ΔHHB value can be evaluated using Equation (2) [139]:
here Δν = ν(OHfree) − ν(OH); ν(OHfree) and ν(OH) are the frequencies of free and H-bonded OH group stretching vibrations, respectively [133]. They can be determined both experimentally and by periodic DFT calculations.
−ΔHHB [kJ/mol] = 1.386·(Δν [cm−1] − 40)0.5,
The H-bond energy, EHB, is calculated as [129]
EHB [kJ/mol] = 1124·Gb [a.e.].
Here, Gb is local electronic kinetic energy density at the bond critical point. It can be calculated by periodic DFT methods or obtained using Kirzhnitz’s approximation from experimental data [143].
Comparison of enthalpies/energies of intermolecular H-bonds in crystals of organic molecules, in particular, crystalline peroxosolvates, obtained using approximations (1), (2), and (3) was carried out in a number of papers [13,144,145,146]. Significant differences in the calculated values are observed only for short (strong) H-bonds [147], which are caused by the contribution of the covalent component to the energy of these bonds [148,149]. These approaches yield energies/enthalpies of weak and moderate hydrogen bonds that are in good agreement with each other. Thus, Equation (3) gives reasonable values of the energies of intermolecular interactions driven by the electrostatic factor, that is, for weak and moderate H-bonds and nonconventional H-bonds. It can be recommended for evaluating the energy of intermolecular H-bonds in crystals.
There are also other schemes that make it possible to accurately calculate the enthalpy [139] and energy [143] of intermolecular H-bonds in crystals. However, the first approach uses the integral intensity of the stretching vibration of the O-H group, which is extremely difficult to measure experimentally for an arbitrary organic crystal, and the second approach is limited to the O-H···O fragment.
5. Examples of H-bond Networks: Average Distances, Types of Coordination
In currently known crystalline peroxosolvates, the number of hydrogen peroxide molecules in the asymmetric unit of the crystal structures varies within wide range: ¼ [65], ½ [92], 1 [144], 1½ [150], 2 [45], 3 [96], and 6 [85]. The following general rules were formulated [25]. (1) Hydrogen peroxide acts as a proton donor in two H-bonds. (2) H2O2 forms from 0 to 4 hydrogen bonds as a proton acceptor (Figure 5); however, there are crystals in which these bonds are absent (Figure 6). (3) The total number of H-bonds varies from two to six. In most crystals, H2O2 forms one or two H-bonds as a proton acceptor, while three or four such H-bonds are realized very rarely (Figure 5). According to work [151], this is explained by the insufficient amount of acidic protons in most organic coformers. Urea perhydrate [27] has long been known as the only crystal in which the H2O2 molecule interacts through six H-bonds with the surrounding organic coformers. In recent years, two additional crystals have been structurally characterized at the Kurnakov Institute of General and Inorganic Chemistry RAS in which H2O2 also forms the maximum number of H-bonds: melamine peroxosolvate C3H6N6 H2O2 [25] and 2-aminobenzimidazole peroxosolvate 2(C7H7N3) H2O2 [26] (Figure 5). To search for new stable and inexpensive peroxosolvates with a high active oxygen content (Section 6), the organic coformers of these crystals must have a ratio of proton-donor groups to groups with lone electron pairs equal to 2 [26]. Note that the coformers of melamine and 2-aminobenzimidazole peroxosolvates, as well as urea, have an NH2 group, that is, this rule is fulfilled.
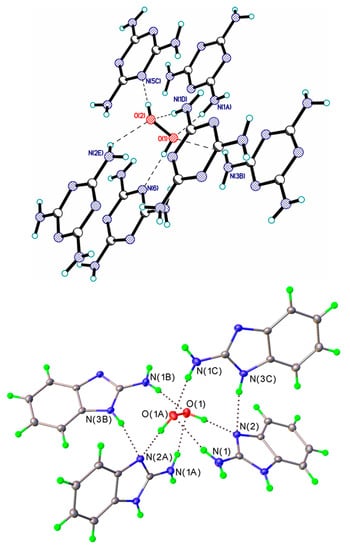
Figure 5.
Crystals with the maximum number of H-bonds. H-bonds are denoted by dotted lines. Top panel: melamine peroxosolvate. Adapted with permission from the authors of [25]. Copyright 2017 American Chemical Society. Bottom panel—2-aminobenzimidazole peroxosolvate. Reproduced from the work in [26] with permission from The Royal Society of Chemistry.
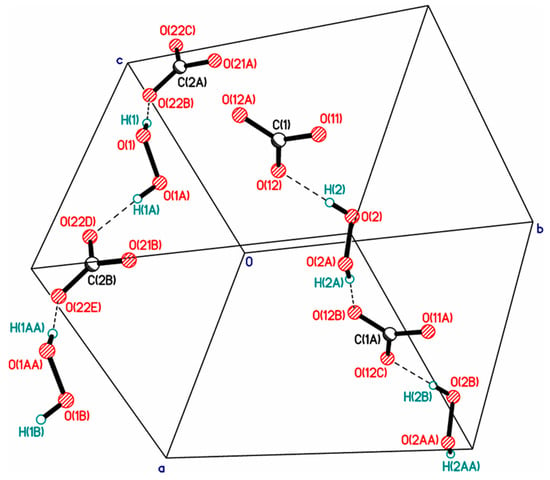
Figure 6.
Fragment of the crystal structure of ammonium carbonate peroxosolvate, in which the H2O2 molecule forms only two H-bonds, as a proton donor; they are given by dotted lines. Reproduced with permission from the work in [48]. Copyright 2012 International Union of Crystallography.
Let us begin to consider the networks of H-bonds formed by H2O2 in crystalline peroxosolvates with H-bonds as a proton donor, as they are realized in all crystals. [25,26]. Moderate O-H···O− bonds are usually formed in amino acid peroxosolvates [85,94,95,96,152]; here, O− denotes the oxygen of the CO2− group of the amino acid zwitterion. These are the so-called charge-assisted H-bonds [153,154]. Distances d(O···O−) range from 2.604 to 2.776 Å [95], and the angle O-H···O− is usually more than 160°. The indicated values of the O···O− distances practically do not differ from the distances in the neutral O-H···O bonds in hydrogen peroxide dihydrate [155] and phenylserine peroxosolvate [96]. A different situation is realized in the case of anionic chains of hydrogen peroxide [25] (Figure 7). These chains are stabilized by short (strong) H-bonds [156]. The d(O···O−) distance is ~2.53 Å [98], and the O-H···O− angle is 178° [28].
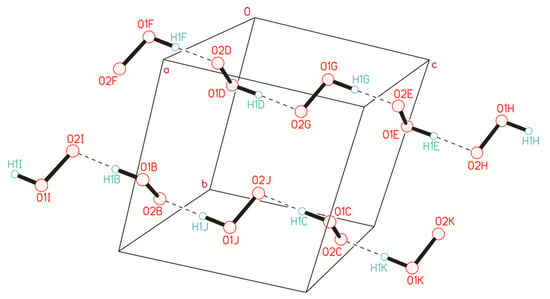
Figure 7.
Infinite ionic chains in the NH4+OOH− crystal, consisting of HOO− fragments interacting through short (strong) H-bonds, which are given by dashed lines. Reproduced with permission from J. Chem. Phys. 133, 16 (2010). Copyright 2004 AIP Publishing [28].
The features of the considered H-bonds in perhydrates are clearly manifested when comparing the networks of H-bonds in crystalline peroxosolvates and amino acid hydrates. Currently, nine crystals of natural amino acid peroxosolvates containing 26 H-bonds as a proton donor have been characterized (Table 1) and a large number of α-amino acid hydrates (not necessarily natural) containing 221 such H-bonds. The average value of the distance d(O···O−) in peroxosolvates of amino acids is 2.67 Å (Table 1), which is much less than the analogous value in hydrates—2.80 Å [14,15]. This is due to the presence in hydrates of a large number of H-bonds with distances d(O···O−) > 2.8 Å (Figure 8), while in peroxosolvates the maximum value of this distance is 2.776 Å (Table 1). The d(O···O) distances in “neutral” H-bonds in amino acid peroxosolvates practically do not differ from the corresponding d(O···O−) values in “charge-assisted” H-bonds [94,95].

Table 1.
H-bonds of H2O2 molecules as proton donors in crystalline peroxosolvates of amino acids and non-proteinogenic amino acids [95]: the number of H-bonds formed by one H2O2 molecule and O···O− distances d(O···O−).
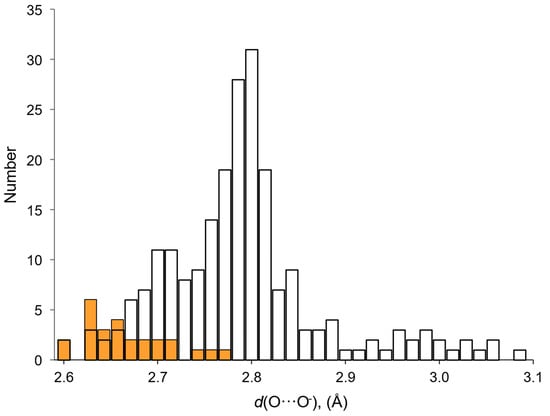
Figure 8.
Distribution of O···O− distances d(O···O−) in H-bonds formed by hydrogen peroxide and water molecules as proton donors in amino acid crystals. White bars—crystalline hydrates; orange bars—peroxosolvates.
In mixed halogen-peroxide chains having O–H···Cl− and O–H···Br− bonds (Figure 4), the shortest O-H···Cl−/O-H···Br− distances are 3.02/3.19 Å [45]. According to the data in Table 4 of the review [157], such a value of d(O···Cl−) corresponds to the shortest O-H···Cl− bonds in crystal hydrates.
Thus, the distances d(O···X), where X = O, O−, Cl−, etc., in the intermolecular H-bonds formed by H2O2 molecules as proton donors are systematically shorter than similar bonds formed by water molecules. According to Equation (1), this means that the enthalpy of H-bonds formed by H2O2 is greater than the values of −ΔHHB for bonds formed by water molecules. Calculations of the enthalpies/energies of H-bonds by Equations (2) and (3) confirm this conclusion [13,146,158]. This phenomenon can be explained by the higher acidity of H2O2 in comparison with water, see introduction, and the conformational mobility of the H2O2 molecule. In contrast to water in crystalline peroxosolvates, the dihedral angle between OH groups in H2O2 is usually ~90° [142].
Hydrogen peroxide is the simplest nonplanar molecule, and its geometry is determined by the H-O-O-H torsion angle. In the gas phase, according to IR and microwave spectroscopy data, the most stable conformation is characterized by a torsion angle of 119.8° [159]. The H2O2 molecule easily rotates around the central O–O bond with cis and trans barriers 7.0 and 1.1 kcal/mol [160]. In crystalline hydrogen peroxide, the torsion angle is, according to X-ray diffraction and neutron diffraction data, 90.2(6) and 94(2)°, respectively [142,161]. The experimentally found values of torsion angles in crystalline peroxosolvates, according to the latest version of CSD, occupy the entire range of values from 0 to 180° with maxima of about 95 and 180° (Figure 9). According to this figure, some peroxosolvates have a HOOH torsion of about 180°. This phenomenon can be explained by the effects of crystal packing, see below.
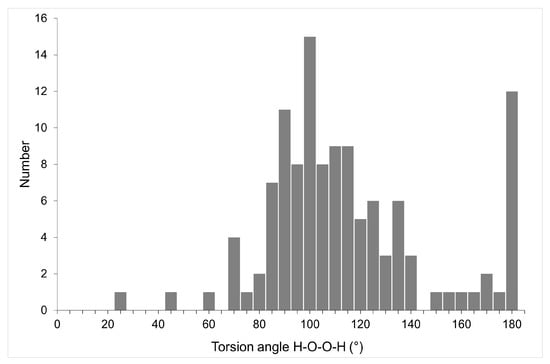
Figure 9.
Distribution of H-O-O-H torsion angles in crystalline peroxosolvates.
It should be noted that the state of these torsion angles in the crystalline phases is somewhat different from the gaseous and liquid phases, as hydrogen peroxide in crystals can be located both in general positions (without symmetry elements) and in special positions (at the centers of inversion i, 2-fold rotation axis and mirror planes m). Axes 2 do not impose restrictions on the values of the H-O-O-H angles; planes m are not realized in the structures of organic peroxosolvates. In nine structures (CAZHUH, GADOXP10, KUMRER, VAYGUY01, BAFJUQ, VAYMAJ, YUHTAW, ZUWCIG, and KELXEH) peroxide molecules lie on crystallographic centers i with rigidly fixed torsion angles of 180°.
The number, type, and strength of H-bonds formed by the H2O2 molecule in peroxosolvates as a proton acceptor are larger and more diverse than the analogous characteristics/properties of H-bonds, in which H2O2 acts as a proton donor. This is due to two reasons: First, the number of “acceptor” H-bonds can vary from 0 to 4 (Figure 5 and Figure 6). Second, the type and strength of such H-bonds are determined by the nature and charge of the proton-donor group of the coformer. In the case of natural amino acids, the considered H-bonds are mainly due to the interaction of the lone electron pair H2O2 with the NH3+ group of the amino acid (Table 2). Distances d(O···N+) vary from ~2.64 [162] to ~2.83 Å [25], however, both “short” and “long” O···HN+ bonds can be highly nonlinear, with the O···H-N+ angle less than 140°. Usually, one or two H-bonds are formed, while peroxosolvates of natural amino acids with three H-bonds or without H-bonds between the H2O2 and NH3+ group of the amino acid (Figure 10) are much less common (Table 2). The latter case is realized when H2O2 molecules form endless chains of H-bonds (Figure 10). As a result, the crystal may lack H-bonds between the H2O2 and NH3+ group of the amino acid [96].

Table 2.
H-bonds of H2O2 molecules as proton acceptors in crystalline peroxosolvates of amino acids and non-proteinogenic amino acids [95]: the number of H-bonds formed by one H2O2 molecule and nature of the proton donor group.
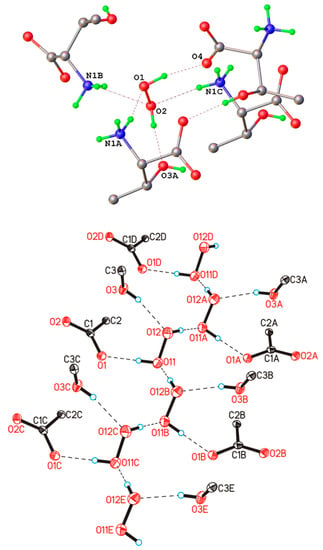
Figure 10.
H-bond networks in amino acid peroxosolvates. Upper panel: l-threonine peroxosolvate: three H-bonds between the H2O2 molecule and the NH3+ groups. (Reproduced from the work in [95] with permission from The Royal Society of Chemistry) Lower panel: phenylserine peroxosolvate: no H-bonds between the H2O2 molecule and the NH3+ groups. The phenyl and amino groups of phenylserine are omitted. (Reproduced from the work in [96] with permission from The Royal Society of Chemistry). H-bonds are shown with dashed lines.
The NH2 groups of the co-former usually form “neutral” H-bonds with hydrogen peroxide. These bonds are relatively weak [157] and are characterized by distances d(N···O) > 3.0 Å [25,27]. In the presence of “peptide” groups, such as –CONH [13] or the NH-group of picolinic acid [97], the formation of “neutral” H-bonds between these groups and hydrogen peroxide is possible (Figure 11). In a number of peroxosolvates of natural amino acids, neutral O···H-O bonds are realized, where, for example, O-H is the hydroxyl group of l-threonine (Figure 10).
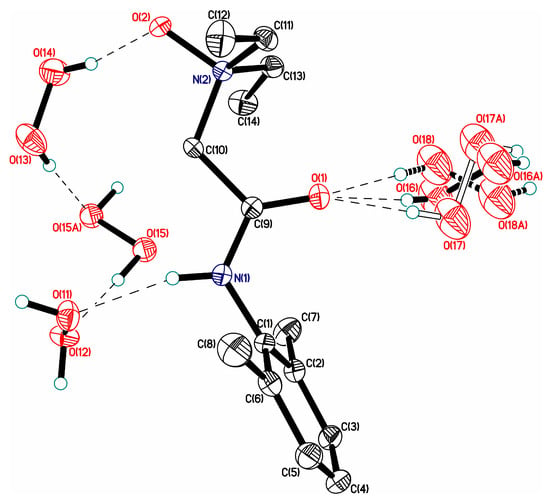
Figure 11.
Neutral H-bonds between the NH group and hydrogen peroxide in the crystal of the peroxosolvate lidocaine N-oxide C14H22N2O2·3H2O2. H-bonds are given by dotted lines. Reprinted with permission from the authors of [85]. Copyright 2017 WileyVCH Verlag GmbH & Co. KGaA.
In the literature, much attention is paid to bifurcated H-bonds, see Section 3.4 in [157]. In contrast to the C=O and P=O groups, which quite often form such bonds [163,164], the oxygen atoms of hydrogen peroxide rarely participate in bifurcate H-bonds. Two examples of such H-bonds are shown in Figure 12. On the other hand, coformers with P=O and N=O groups and not containing active (acidic) hydrogen atoms form bifurcated H-bonds with H2O2 molecules [71,86] (Figure 13 and Figure 14). In these structures, H2O2 molecules usually form only two H-bonds, as proton donors. However, H2O2 clusters are also realized, in which some H2O2 molecules interact with each other, with one of the hydrogen peroxide molecules acting as a proton acceptor [72,85]. At the moment, there is one example of a bifurcate H-bond formed by a hydrogen peroxide molecule and a nitro group 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazoisowurtzitane [84] in one of the polymorphs of the crystalline peroxosolvate of this molecule (Figure 15).
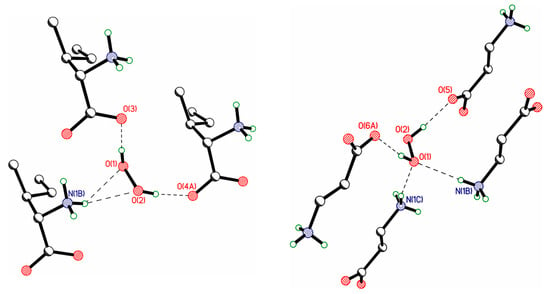
Figure 12.
Bifurcate H-bonds, which are realized in isoleucine (TANDET, left panel) and β-alanine (TANDAP, right panel) peroxosolvates.
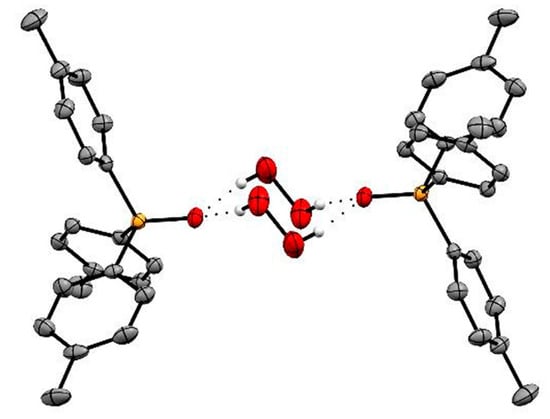
Figure 13.
Single crystal structure of (p-Tol3PO·H2O2)2. Reproduced from the work in [71] with permission from The Royal Society of Chemistry.
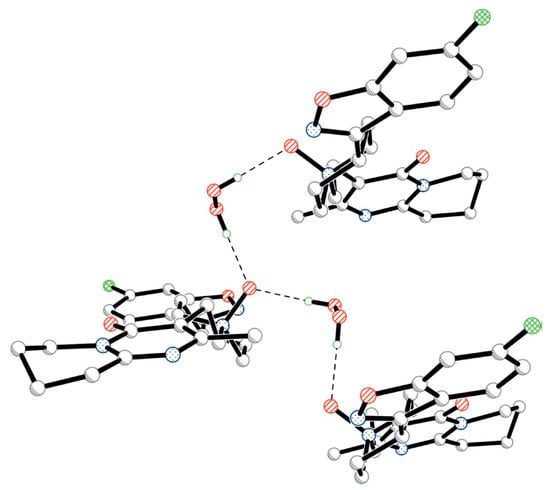
Figure 14.
Fragment of the crystal structure of risperidone N-oxide, illustrating the interaction of hydrogen peroxide with coformer molecules through O-H···O bonds (dashed lines). H atoms attached to C atoms have been omitted for clarity. Reproduced with permission from the authors of [86]. Copyright 2005 International Union of Crystallography.
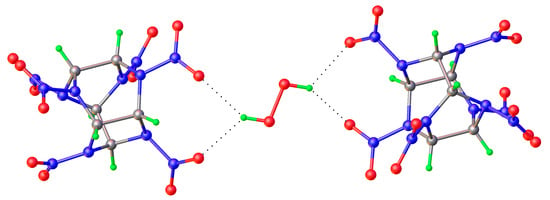
Figure 15.
Bifurcate H-bond in 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazoisowurtzitane peroxosolvate [84].
In organic crystals, nonconventional O···H-C [128] bonds often arise. In crystalline peroxosolvates, such bonds are rarely formed [165].
Note that in the case of coformers, the size of which significantly exceeds the size of a hydrogen peroxide molecule, H-bonds can arise between the coformers (Figure 10, upper panel). This increases the already wide variety of H-bond networks found in crystalline peroxosolvates.
In addition to the formation of H-bonds with anions of inorganic and organic acids, hydrogen peroxide molecules form contacts with alkali metals, enabling additional stabilization of the peroxosolvate. The contact distances between the alkali metal (Li, Na, K, Rb, and Cs) and the oxygen atom of the H2O2 molecule are shown in Table S1. When searching for distances, the following distance constraints were used: Li-O (2.5 Å), Na-O (3.0 Å), K/Rb/Cs-O (3.5 Å). Analysis of similar contacts between an alkali metal and an oxygen atom of a water molecule in hydrates according to CSD data did not reveal significant differences between the values of the analyzed distances in peroxosolvates and hydrates. The average contact distances in hydrates and peroxolvates were 1.97, 2.42, 2.88, 3.06, and 3.22 Å for Li, Na, K, Rb, and Cs, respectively. The Zn-O distance in the only structurally characterized molecular zinc complex ZnII(H2O2) is 2.172 Å, while the hydrogen peroxide molecule also participates in the formation of two H-bonds as a proton donor [35]. Thus, hydrogen peroxide can be considered as a weak ligand, which manifests its coordination properties in a number of crystal structures of peroxosolvates due to relatively short contacts between oxygen atoms and the above alkali metal cations.
6. Trends and Prospects
6.1. High-Energy Substances
High-energy substances are a class of compounds that can generate large amounts of heat due to the extremely rapid exothermic decomposition reaction caused by external influences. Typical representatives of these compounds are nitroamines, for example, cyclotrimethylenetrinitramine [92] or nitro derivatives of triazole, for example, 1-methyl-3,5-dinitro-1,2,4-triazole [77], azasydnones [166], etc. High-energy density (i.e., high self-healing potential) in most high-energy substances usually leads to a decrease in stability and increased sensitivity to external influences. The main challenge in the production of new high-energy substances is to simultaneously achieve high energy density and stability to ensure safe production, storage and transportation. A promising method for solving this problem is the creation of two-component energy crystals, each component of which is a high-energy compound. The production of such crystals is difficult due to the poor “controllability” of the self-assembly process of the two components due to various intermolecular interactions (H-bonds, π-stacking, etc.). The synthesis of new two-component high-energy compounds requires the development of approaches and rules that make it possible to predict the self-assembly of molecules by isolating certain intermolecular interactions that form a structural motif in a crystal.
The concepts of “host” and “guest” (receptor and substrate), or the “key-and-lock” principle, underlie molecular recognition in supramolecular chemistry [167]. The effect of limited cavities in macrocyclic compounds—hosts (cyclodextrin, calixarene, cucurbituril, etc.)—allows them to recognize well small guest molecules with high binding energies [168]. The key-lock principle allows “controlling” the intermolecular interaction of various components and, thus, creating new high-energy substances.
In [92], two-dimensional (2D) porous host materials were used to create energetic materials by trapping H2O2 (guest). Obviously, the sizes of cavities in porous 2D materials should correspond to the sizes of molecules with oxidizing properties. 2,4,6-Triamino-5-nitropyrimidine-1,3-dioxide (CM-102) has the ability to form H-bonds, primarily as proton acceptors. It was used as an energetic host, which has a 2D layered structure with cavities (Figure S1A in [92]). The H2O molecules are located between the ICM-102 layers, forming a sandwich structure (Figure S1A in [92]). It was found that H2O2 molecules replace water molecules in crystal hydrate CM-102 and are located in cavities, forming a graphite-like layered structure (Figure 2C in [92]). The peroxide molecule is crosswise disordered, and both components lie at the center of the inversion (Figure 16). H2O2 interacts with surrounding host molecules through O-H···O bonds with distances ~2.66 Å and N-H···O bonds with distances from 1.922 to 2.299 Å. The density of the obtained crystal was 1.915 g/cm3, that is, significantly higher than the density of crystalline hydrate CM-102, 1.845 g/cm3.
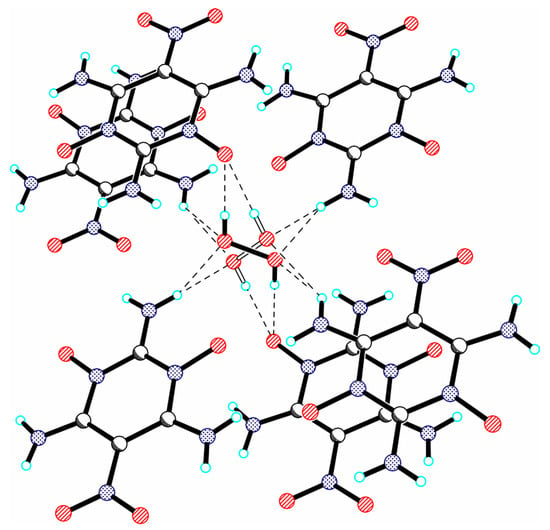
Figure 16.
Fragment of the crystal structure of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide peroxosolvate, illustrating the interaction of H2O2 with surrounding host molecules through O-H···O and N-H···O bonds (dashed lines).
A very important characteristic of high-energy substances is the so-called “oxygen index” [77]. It characterizes the mass percentage of oxygen available to oxidize conformer to neutral molecules. Usually energy materials are characterized by negative oxygen index values, that is, in most fuel cells there is an oxidant deficiency compared to the content of high-energy substances. Multicomponent crystallization can increase the oxygen index. Therefore, the preparation of crystalline peroxosolvates of high-energy substances is one of the ways to improve the value of the oxygen index [84]. The effect of polymorphism of peroxosolvates on the properties of high-energy compounds—azoles—was studied in [77]. 5,5’-Dinitro-2H,2H’-3,3’-bi-1,2,4-triazole (DNT) has been investigated because of its high detonation velocity and low impact sensitivity. DNT is characterized by an improved oxygen index (Figure 17). This compound has not found widespread use, in part because it does not form solvates under traditional crystallization conditions [169]. In [77], crystalline DNT hydrates were obtained; then, two crystalline peroxosolvates of this compound were synthesized. In both peroxosolvates, the H2O2 molecule forms four H-bonds with neighboring DNT molecules (Figure 18). The oxygen index in these peroxosolvates is −30%, which is significantly higher than the corresponding value for anhydrous DNT (−35%). Crystallization of peroxosolvates is an effective strategy for improving the performance of high-energy hydrate-prone materials.
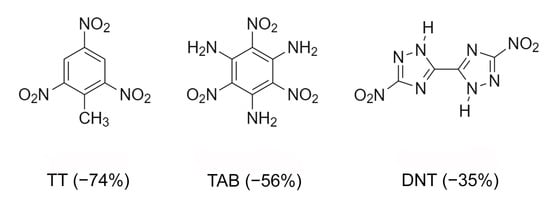
Figure 17.
Chemical structure of 2,4,6-trinitrotoluene (TT), 2,4,6-triamino-1,3,5-trinitrobenzene (TAB) and DNT. Oxygen index of these compounds is given in parenthesis.
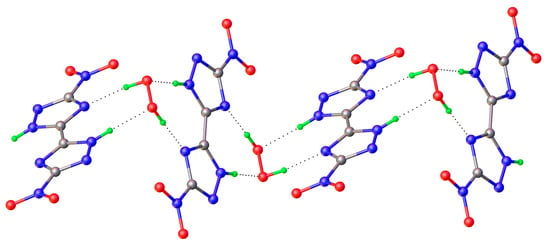
Figure 18.
H-bond network in DNT crystal solvates [77].
The above approach was successfully used in [170] for the synthesis of a new H2O2-adduct of ammonium cyclopentazolate NH4N5∙½H2O2. This crystalline peroxosolvate is characterized by a high oxygen index (−22.86%) and a high calculated velocity and detonation pressure (8938 m/s and 26.37 GPa). H2O2 forms four H-bonds with the surrounding ions. The O-H···N bonds are almost linear (163°) and very short (2.811 Å) [170].
6.2. Mixed Pharmaceutical Forms. Antiseptic and Analgesic Effect
Pharmaceutical co-crystals and solvates are promising systems to improve the solubility of active pharmaceutical ingredients (API) [165,171]. H2O2 is green reagent, which is safe for human consumption. Therefore, hydrogen peroxide can be used as effective coformer.
Miconazole was chosen as the topical API (Figure 19). This compound is commonly used as a nitrate salt in ointments and powders, or in anhydrous form in gels and tablets. It also exists in the hydrated form from which the crystalline miconazole peroxosolvate was derived. There are two API molecules per H2O2 molecule in this crystal (Figure 19). H2O2 forms two N···H-O bonds with imidazole nitrogen atoms of two neighboring miconazole molecules with N...H distances equal to 1.75 and 1.81 Å. The corresponding values in the crystal hydrate of miconazole are slightly higher, 1.87 and 1.83 Å (see Section 5). Oxygen atoms of H2O2 and water molecules in both crystals do not form classic H-bonds, however, they interact with CH-groups of miconazole molecules through O···H-C bonds. The O···H distances are 2.23–2.50 Å for the peroxosolvate and 2.26–2.47 Å for the crystalline hydrate. The solubility of miconazole peroxosolvate in phosphate–citrate buffer at pH 4 was ~230 μg/mL, that is, significantly higher than the solubility of miconazole and its crystal hydrate, ~200 and ~160 μg/mL, respectively [165].
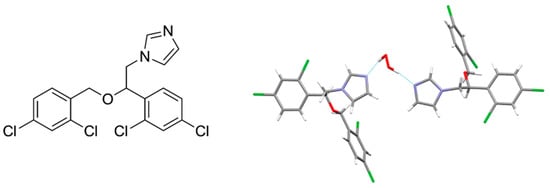
Figure 19.
Chemical structure of miconazole (left). Asymmetric cell of the crystal structure of miconazole peroxosolvate [165].
7. Conclusions
Water forms stable crystalline hydrates with almost all known classes of compounds. Hydrogen peroxide has significantly more pronounced acidic properties compared to water. Therefore, compounds with pronounced acidic properties do not form peroxosolvates. Compounds that can enter into redox reactions with hydrogen peroxide should also be excluded from the list of potential peroxosolvate coformers. These considerations may explain why the number of perhydrates is smaller than the number of known hydrates, but they cannot explain why the number of known crystalline peroxosolvates is several orders of magnitude smaller than that of known crystalline hydrates, which suggests that the chemistry of this class of compounds is seriously understudied.
A review of all currently known peroxosolvates allows us to unambiguously confirm the following general conclusions: (1) Every hydrogen peroxide molecule always participates as proton donor in two H-bonds; (2) Coformers should exhibit amphoteric or basic properties.
Analysis of structural databases revealed the nature of the molecules (coformers) that are prone to forming peroxosolvates. These are (a) salts of inorganic and carboxylic acids; (b) amino acids, peptides, and related zwitterions; and (c) molecular compounds with a lone electron pair on nitrogen and/or oxygen atoms. A promising method for the preparation of crystalline peroxosolvates is the replacement of a water molecule with H2O2 from structurally related solvates using a high concentration (>80%) of H2O2. Peroxosolvates can be obtained from aqueous hydrogen peroxide of low concentration (<30%) if their crystal structure meets one of the following requirements:
(1) H2O2 molecule forms at least five H-bonds with the surrounding molecules; (2) torsion angle of hydrogen peroxide molecule is close to 180°. Urea perhydrate and percabonate are fine demonstrations of these criteria.
Supplementary Materials
The following are available online. Table S1: Structure features of peroxosolvates with localized protons, Table S2: Refcodes of proton disordered peroxosolvates.
Author Contributions
Conceptualization, A.V.C., P.V.P. and M.V.V.; writing and visualization, A.G.M., A.V.C., P.V.P., O.L. and M.V.V.; supervision, M.V.V. and O.L.; project administration, M.V.V.; funding acquisition, M.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out with the financial support of D. Mendeleev University of Chemical Technology. The work was supported by the Russian Scientific Found (grants 18-03-00973, 18-03-01107, and 20-03-00449). The publication was carried out within the State Assignment on Fundamental Research to the Kurnakov Institute of General and Inorganic Chemistry.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanatar, S. Percarbonate. Ber. Dtsch. Chem. Ges. 1899, 32, 1544–1546. [Google Scholar] [CrossRef]
- Tanatar, S. Double Compounds of Hydrogen Peroxide with Organic Substances. J. Russ. Phys. Chem. Soc. 1906, 40L, 376–380. [Google Scholar]
- Jakob, H.; Leininger, S.; Lehmann, T.; Jacobi, S.; Gutewort, S. Peroxo Compounds, Inorganic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–33. [Google Scholar]
- Goti, A.; Cardona, F. Hydrogen Peroxide in Green Oxidation Reactions: Recent Catalytic Processes. In Green Chemical Reactions; Springer: Dordrecht, The Netherlands, 2008; pp. 191–212. [Google Scholar]
- Jones, C.W. Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 1–264. [Google Scholar]
- Schumb, W.C.; Satterfield, C.N.; Wentworth, R.L. Hydrogen peroxide; Reinhold Publishing Corporation: New York, NY, USA, 1955; pp. 1–759. [Google Scholar]
- Churakov, A.V.; Sladkevich, S.; Lev, O.; Tripol’skaya, T.A.; Prikhodchenko, P.V. Cesium Hydroperoxostannate: First Complete Structural Characterization of a Homoleptic Hydroperoxocomplex. Inorg. Chem. 2010, 49, 4762–4764. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Tripol’skaya, T.A.; Popov, V.S.; Mokrushin, A.S.; Krut’ko, D.P.; Prikhodchenko, P.V.; Lev, O. H2O2 Induced Formation of Graded Composition Sodium-doped Tin Dioxide and Template-free Synthesis of Yolk–shell SnO2 Particles and their Sensing Application. Dalton Trans. 2017, 46, 16171–16179. [Google Scholar] [CrossRef] [PubMed]
- Wolanov, Y.; Lev, O.; Churakov, A.V.; Medvedev, A.G.; Novotortsev, V.M.; Prikhodchenko, P.V. Preparation of Pure Hydrogen Peroxide and Anhydrous Peroxide Solutions from Crystalline Serine Perhydrate. Tetrahedron 2010, 66, 5130–5133. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Churakov, A.V.; Vener, M.V.; Tripol’skaya, T.A.; Cohen, S.; Lev, O.; Prikhodchenko, P.V. Potassium, Cesium, and Ammonium Peroxogermanates with Inorganic Hexanuclear Peroxo Bridged Germanium Anion Isolated from Aqueous Solution. Inorg. Chem. 2015, 54, 8058–8065. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane Transport of Hydrogen Peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Churakov, A.V.; Grishanov, D.A.; Medvedev, A.G.; Mikhaylov, A.A.; Tripol’skaya, T.A.; Vener, M.V.; Navasardyan, M.A.; Lev, O.; Prikhodchenko, P.V. Cyclic Dipeptide Peroxosolvates: First Direct Evidence for Hydrogen Bonding Between Hydrogen Peroxide and a Peptide Backbone. CrystEngComm 2019, 21, 4961–4968. [Google Scholar] [CrossRef]
- Cambridge Structural Database. Ver. 5.41. 2020. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 December 2020).
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Miller, E.W.; Tulyathan, O.; Isacoff, E.Y.; Chang, C.J. Molecular Imaging of Hydrogen Peroxide Produced for Cell Signaling. Nat. Chem. Biol. 2007, 3, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Encrenaz, T.; Greathouse, T.K.; Richter, M.J.; Bézard, B.; Fouchet, T.; Lefèvre, F.; Montmessin, F.; Forget, F.; Lebonnois, S.; Atreya, S.K. Simultaneous Mapping of H2O and H2O2 on Mars from Infrared High-resolution Imaging Spectroscopy. Icarus 2008, 195, 547–556. [Google Scholar] [CrossRef]
- Sies, H. Role of Metabolic H2O2 Generation: Redox Signaling and Oxidative Stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen Peroxide as a Signal Controlling Plant Programmed Cell Death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 Mediates Hydrogen Peroxide Uptake to Regulate Downstream Intracellular Signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial Aquaporin-8-mediated Hydrogen Peroxide Transport is Essential for Teleost Spermatozoon Motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide Across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Chernyshov, I.Y.; Vener, M.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Lev, O.; Churakov, A.V. Peroxosolvates: Formation Criteria, H2O2 Hydrogen Bonding, and Isomorphism with the Corresponding Hydrates. Cryst. Growth Des. 2017, 17, 214–220. [Google Scholar] [CrossRef]
- Churakov, A.V.; Grishanov, D.A.; Medvedev, A.G.; Mikhaylov, A.A.; Vener, M.V.; Navasardyan, M.A.; Tripol’skaya, T.A.; Lev, O.; Prikhodchenko, P.V. Stabilization of Hydrogen Peroxide by Hydrogen Bonding in the Crystal Structure of 2-aminobenzimidazole Perhydrate. CrystEngComm 2020, 22, 2866–2872. [Google Scholar] [CrossRef]
- Fritchie, C.J.; McMullan, R.K. Neutron Diffraction Study of the 1:1 Urea:Hydrogen Peroxide Complex at 81 K. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1981, 37, 1086–1091. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Lev, O.; Medvedev, A.G.; Tripol’skaya, T.A.; Vener, M.V. A Model Proton-Transfer System in the Condensed Phase: NH4+OOH−, a Crystal with Short Intermolecular H-bonds. J. Chem. Phys. 2010, 133, 164506–164515. [Google Scholar] [CrossRef] [PubMed]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.G.; Pedahzur, R.; Lev, O. Zinc Dioxide Nanoparticulates: A Hydrogen Peroxide Source at Moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef] [PubMed]
- Inorganic Crystal Structure Database. Ver. 4.2.0. 2019. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 1 December 2020).
- Belsky, A.; Hellenbrandt, M.; Karen, V.L.; Luksch, P. New Developments in the Inorganic Crystal Structure Database (ICSD): Accessibility in Support of Materials Research and Design. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H. The Cambridge Structural Database: A Quarter of a Million Crystal Structures and Rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.F.; Pedersen, B. The Crystal Structure of Sodium Oxalate Perhydrate Na2C2O4·H2O2. Acta Chem. Scand. 1964, 18, 1454–1468. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Jones, W. Potassium Hydrogen Phthalate Hemiperhydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 1128–1130. [Google Scholar] [CrossRef]
- Wallen, C.M.; Bacsa, J.; Scarborough, C.C. Hydrogen Peroxide Complex of Zinc. J. Am. Chem. Soc. 2015, 137, 14606–14609. [Google Scholar] [CrossRef]
- Pedersen, B.F. The Crystal Structure of Ammonium Oxalate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 746–754. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Larsen, T.K.; Soling, H.; Torbjörnsson, L.; Werner, P.-E.; Junggren, U.; Lamm, B.; Samuelsson, B. The Crystal Structure of Lithium Oxalate Monoperhydrate, Li2C2O4·H2O2. Acta Chem. Scand. 1969, 23, 1871–1877. [Google Scholar] [CrossRef][Green Version]
- Pedersen, B.F.; Kvick, Å. Neutron Diffraction Study of Potassium Oxalate Monoperhydrate at 123 K. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 21–23. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Seip, H.M.; Santesson, J.; Holmberg, P.; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. The Crystal Structure of Potassium and Rubidium Oxalate Monoperhydrates, K2C2O4·H2O2 and Rb2C2O4·H2O2. Acta Chem. Scand. 1967, 21, 779–790. [Google Scholar] [CrossRef][Green Version]
- Adams, J.M.; Pritchard, R.G. The Crystal Structure of Guanidinium Oxalate Dihydrate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 2438–2440. [Google Scholar] [CrossRef]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-ray and Neutron-diffraction Study of KF·2H2O2. Kristallografiya 1976, 21, 929–936. [Google Scholar]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Fykin, L.E.; Zavodnik, V.E. X-ray and Neutron-diffraction Studies of RbF·H2O2 Crystals. Kristallografiya 1977, 22, 982–987. [Google Scholar]
- Sarin, V.A.; Dudarev, V.Y.; Dobrynina, T.A.; Zavodnik, V.E. X-ray structural Investigation of NH4F·H2O2 Crystals. Kristallografiya 1979, 24, 824–825. [Google Scholar]
- Pritchard, R.G.; Begum, Z.; Lau, Y.F.; Austin, J. Structures of Na9[SO4]4X·2H2O2, where X = Cl or Br, in which the Halide Anions Orchestrate Extended Orientation Sequences of H2O2 Solvate Molecules. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 663–668. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Howard, J.A.K. The Preparation and Crystal Structures of Novel Perhydrates Ph4X+Hal−·nH2O2: Anionic Hydrogen-bonded Chains Containing Hydrogen Peroxide. CrystEngComm 2005, 7, 664–669. [Google Scholar] [CrossRef]
- Carrondo, M.A.A.F.; De, C.T.; Griffith, W.P.; Jones, D.P.; Skapski, A.C. X-ray Crystal Structure of the Industrial Bleaching Agent ‘Sodium Percarbonate’[Sodium Carbonate–Hydrogen Peroxide (2/3)]. J. Chem. Soc. Dalton Trans. 1977, 2323–2327. [Google Scholar] [CrossRef]
- Pritchard, R.G.; Islam, E. Sodium Percarbonate between 293 and 100 K. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.G.; Mikhaylov, A.A.; Churakov, A.V.; Prikhodchenko, P.V.; Lev, O. Ammonium and Caesium Carbonate Peroxosolvates: Supramolecular Networks Formed by Hydrogen Bonds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, i20–i24. [Google Scholar] [CrossRef] [PubMed]
- Thierbach, D.; Huber, F.; Preut, H. Structure of Triphenylphosphine Oxide Hemiperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 974–977. [Google Scholar] [CrossRef]
- Stomberg, R.; Klemets, R.; Lundström, I.; Fontell, K.; Nielsen, C.J.; Urso, F.; Weidlein, J.; Zingaro, R.A. The Crystal Structures of Potassium Bis(oxalato)oxoperoxovanadate(V) Hemihydrate, K3[VO(O2)(C2O4)2]·½H2O, and Potassium Bis(oxalato)dioxovanadate(V) Trihydrate, K3[VO2(C2O4)2]·3H2O. Acta Chem. Scand. 1986, 40a, 168–176. [Google Scholar] [CrossRef]
- Won, T.-J.; Barnes, C.L.; Schlemper, E.O.; Thompson, R.C. Two Crystal Structures Featuring the Tetraperoxovanadate(V) Anion and a Brief Reinvestigation of Peroxovanadate Equilibria in Neutral and Basic Solutions. Inorg. Chem. 1995, 34, 4499–4503. [Google Scholar] [CrossRef]
- Szentivanyi, H.; Stomberg, R.; Hämäläinen, R.; Kohl, F.X.; Seip, R. The Crystal Structure of 2,2′-Bipyridinium(1+) mu-Hydrogen-bis[(2,2′-bipyridine)oxodiperoxovanadate](1-)-x-hydrogen peroxide-(6-x)-water, (Hbipy)[H{VO(O2)2bipy}2]·xH2O2·(6-x)H2O, x ~= 0.5, at −100 degrees C. Acta Chem. Scand. 1984, 38, 101–107. [Google Scholar] [CrossRef]
- Campbell, N.J.; Capparelli, M.V.; Griffith, W.P.; Skapski, A.C. On the Existence of Triperoxo Vanadium Complexes. X-ray Crystal Structures of K3[VO(O2)2(C2O4]·H2O2 and of (NH4)[VO(O2)2(bipy)]·4H2O. Inorg. Chim. Acta 1983, 77, L215–L216. [Google Scholar] [CrossRef]
- Schwendt, P.; Ahmed, M.; Marek, J. Complexation between Vanadium (V) and Phenyllactate: Synthesis, spectral Studies and Crystal Structure of (NEt4)(NH4)3[V2O2(O2)2(R-3-phlact)2][V2O2(O2)2(S-3-phlact)2]·6H2O, [3-phlact=3-phenyllactato(2−)]. Inorg. Chim. Acta 2005, 358, 3572–3580. [Google Scholar] [CrossRef]
- Shao, M.; Dong, X.U.N.; Tang, Y. Crystal Structure Investigation of Vanadyl Complexes of Tridentate Ligand. (II)—Synthese and Crystal Structure of 1-(2-pyridylazo)-2-naphtholato-dioxovanadium(V) Dimer [VO2(C15)H10N3O)]2(H2O2)(CHCl3)2 and Pyridine-(1-(2-pyridylazo)-2-naphtolato)peroxo Oxovanadium(V) VO(O2)(C15H10N3O)(C5H5N). Sci. Sin. Ser. B (Engl. Ed.) 1988, 31, 789–799. [Google Scholar] [CrossRef]
- Šimuneková, M.; Šimunek, J.; Chrappová, J.; Schwendt, P.; Žák, Z.; Pavelčík, F. Dinucleating Role of a Strong Hydrogen Bond in Crystal Structure of [N(C4H9)4]{[VO(HO2)(O2)(phen)][VO(O2)2(phen)]}·3H2O2·H2O. Inorg. Chem. Commun. 2012, 24, 125–128. [Google Scholar] [CrossRef]
- Mathern, G.; Weiss, R. Structure des Complexes Peroxydiques des Métaux de Transition. II. Structure Cristalline du Triperoxo-(o-phénanthroline)niobate de Potassium à Trois Molécules d’Eau et de son Perhydrate KNb(O2)3(C12H8N2)·3H2O et KNb(O2)3(C12H8N2)·3H2O·H2O2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 1582–1597. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Mathieu, B.; Declercq, J.-P.; Devillers, M. Spectroscopic and Structural Characterizations of Novel Water-Soluble Peroxo[polyaminocarboxylato bis(N-oxido)]niobate(V) Complexes. Eur. J. Inorg. Chem. 2003, 2003, 737–743. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Devillers, M. Homo- and Heterobimetallic Niobium(V) and Tantalum(V) Peroxo-tartrate Complexes and Their Use as Molecular Precursors for Nb−Ta Mixed Oxides. Inorg. Chem. 2005, 44, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Bayot, D.; Tinant, B.; Devillers, M. Spectroscopic and Structural Characterizations of Novel Water-Soluble Tetraperoxo and Diperoxo[polyaminocarboxylato bis(N-oxido)]tantalate(V) Complexes. Inorg. Chem. 2004, 43, 5999–6005. [Google Scholar] [CrossRef]
- Qiu, J.; Vlaisavljevich, B.; Jouffret, L.; Nguyen, K.; Szymanowski, J.E.S.; Gagliardi, L.; Burns, P.C. Cation Templating and Electronic Structure Effects in Uranyl Cage Clusters Probed by the Isolation of Peroxide-Bridged Uranyl Dimers. Inorg. Chem. 2015, 54, 4445–4455. [Google Scholar] [CrossRef]
- Mikhaylov, A.A.; Medvedev, A.G.; Churakov, A.V.; Grishanov, D.A.; Prikhodchenko, P.V.; Lev, O. Peroxide Coordination of Tellurium in Aqueous Solutions. Chem. Eur. J. 2016, 22, 2980–2986. [Google Scholar] [CrossRef]
- Mühle, C.; Peters, E.-M.; Jansen, M. New Hydrogen Peroxide Adducts of Alkali Metal Tetracyanoplatinates A2[Pt(CN)4]·H2O2 (A = K, Rb, Cs). Z. Naturforsch. B 2009, 64, 111–115. [Google Scholar] [CrossRef]
- Khodadad, P.; Rodier, N. Trans-Diammine-trans-dichloro-trans-dihydroxoplatine(IV) di(peroxyde d’hydrogène). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 2219–2220. [Google Scholar] [CrossRef]
- Barnard, C.F.J.; Hydes, P.C.; Griffiths, W.P.; Mills, O.S. A Stable Platinum Complex Perhydrate Adduct: Crystal Stucture of cis,trans-[PtCl2(OH)2(2-NH2Pr)2]·0.5H2O2 and water and N,N-dimethylacetamide adducts. J. Chem. Res. Synop. 1983, 302–303. [Google Scholar]
- Vannerberg, N.G. On the System SrO2-H2O-H2O2. I. The Crystal Structure of α -SrO2·2H2O2 and β -SrO2·2H2O2. Ark. Kemi 1958, 13, 29–41. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. I. Investigation of the Existing Phases and their Preparation. Ark. Kemi 1959, 14, 147–149. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. II The Structure of BaO2·H2O2. Ark. Kemi 1959, 14, 149–159. [Google Scholar]
- Vannerberg, N.G. On the System BaO2-H2O-H2O2. III. The Crystal Structure of α-BaO2, β-BaO2, and γ-BaO2·2H2O2 and BaO2·H2O2·2H2O. Ark. Kemi 1959, 14, 125–145. [Google Scholar]
- Arp, F.F.; Ahn, S.H.; Bhuvanesh, N.; Blümel, J. Selective Synthesis and Stabilization of Peroxides via Phosphine Oxides. New J. Chem. 2019, 43, 17174–17181. [Google Scholar] [CrossRef]
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide Adducts of Triarylphosphine Oxides. Dalton Trans. 2019, 48, 14312–14325. [Google Scholar] [CrossRef]
- Ahn, S.H.; Cluff, K.J.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide and Di(hydroperoxy)propane Adducts of Phosphine Oxides as Stoichiometric and Soluble Oxidizing Agents. Angew. Chem. Int. Ed. 2015, 54, 13341–13345. [Google Scholar] [CrossRef]
- Hilliard, C.R.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Synthesis, Purification, and Characterization of Phosphine Oxides and their Hydrogen Peroxide Adducts. Dalton Trans. 2012, 41, 1742–1754. [Google Scholar] [CrossRef]
- Čermák, J.; Kvíčalová, M.; Šabata, S.; Blechta, V.; Vojtíšek, P.; Podlaha, J.; Shaw, B.L. Diphosphinoazines (Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2 with R Groups of Various Sizes and Complexes {[(Z,Z)-R2PCH2C(But)=NN=C(But)CH2PR2]-[η3-CH2C(CH3)=CH2PdCl]2}. Inorg. Chim. Acta 2001, 313, 77–86. [Google Scholar] [CrossRef]
- Neda, I.; Kaukorat, T.; Fischer, A.; Jones, P.G.; Schmutzler, R. Oxidationsreaktionen an 2-[2-(N,N-Dimethylamino)ethyl-methylamino]-1,3,5-trimethyl-1,3,5-triaza-2λ3-phosphorinan-4,6-dion; Hydrolyse und Thermolyse eines Perfluorpinakolylsubstituierten Spirophosphorans. J. Fluor. Chem. 1994, 69, 35–40. [Google Scholar] [CrossRef]
- Sevcik, R.; Necas, M.; Novosad, J. The Synthesis and Characterization of Three Oxidized Derivatives of bis(diphenylphosphino)pyridine and their Sn(IV) Complexes. Polyhedron 2003, 22, 1585–1593. [Google Scholar] [CrossRef]
- Wiscons, R.A.; Bellas, M.K.; Bennion, J.C.; Matzger, A.J. Detonation Performance of Ten Forms of 5,5′-Dinitro-2H,2H′-3,3′-bi-1,2,4-triazole (DNBT). Cryst. Growth Des. 2018, 18, 7701–7707. [Google Scholar] [CrossRef]
- Laus, G.; Schwärzler, A.; Bentivoglio, G.; Hummel, M.; Kahlenberg, V.; Wurst, K.; Kristeva, E.; Schütz, J.; Kopacka, H.; Kreutz, C.; et al. Synthesis and Crystal Structures of 1-Alkoxy-3-alkylimidazolium Salts Including Ionic Liquids, 1-Alkylimidazole 3-oxides and 1-Alkylimidazole Perhydrates. Z. Naturforsch. B 2008, 63, 447–464. [Google Scholar] [CrossRef]
- Jakob, F.; Herdtweck, E.; Bach, T. Synthesis and Properties of Chiral Pyrazolidines Derived from (+)-Pulegone. Chem. Eur. J. 2010, 16, 7537–7546. [Google Scholar] [CrossRef] [PubMed]
- Navasardyan, M.A.; Bezzubov, S.I.; Kuz’mina, L.G.; Prikhodchenko, P.V.; Churakov, A.V. Crystal Structure of 2,3,5,6-tetrakis(pyridin-2-yl)pyrazine Hydrogen Peroxide 4.75-solvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Churakov, A.V.; Chetina, O.V.; Howard, J.A.K. Dicyclohexylamine Hydrogen Peroxide Hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o3503–o3505. [Google Scholar] [CrossRef]
- Serra, M.A.; Dorner, B.K.; Silver, M.E. Structure of an Adenine-hydrogen Peroxide Adduct. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 1957–1960. [Google Scholar] [CrossRef]
- Kersten, K.M.; Breen, M.E.; Mapp, A.K.; Matzger, A.J. Pharmaceutical Solvate Formation for the Incorporation of the Antimicrobial Agent Hydrogen Peroxide. Chem. Commun. 2018, 54, 9286–9289. [Google Scholar] [CrossRef]
- Bennion, J.C.; Chowdhury, N.; Kampf, J.W.; Matzger, A.J. Hydrogen Peroxide Solvates of 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane. Angew. Chem. Int. Ed. 2016, 55, 13118–13121. [Google Scholar] [CrossRef]
- Grishanov, D.A.; Navasardyan, M.A.; Medvedev, A.G.; Lev, O.; Prikhodchenko, P.V.; Churakov, A.V. Hydrogen Peroxide Insular Dodecameric and Pentameric Clusters in Peroxosolvate Structures. Angew. Chem. Int. Ed. 2017, 56, 15241–15245. [Google Scholar] [CrossRef]
- Ravikumar, K.; Sridhar, B.; Manjunatha, S.G.; Thomas, S. Risperidone N-oxide Hydrogen Peroxide Methanol Solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, o2515–o2517. [Google Scholar] [CrossRef]
- Kay Hon, P.; Mak, T.C.W. Isolation and Crystal Structures of 1,3 Molecular Complexes of TriethylenediamineN,N’-dioxide with Hydrogen Peroxide and Water. J. Crystallogr. Spectrosc. Res. 1987, 17, 419–429. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Medvedev, A.G.; Mikhaylov, A.A. Crystal Structure of (Z)-N-benzylidene-1-phenylmethanamine Oxide Hydrogen Peroxide Monosolvate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Lynch, W.; Padgett, C.W. 2,2′-Disulfanediylbis(pyridine N-oxide)–hydrogen Peroxide (1/1). IUCrData 2018, 3, x180320. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Timosheva, N.V.; Day, R.O.; Holmes, R.R. Pseudoheptacoordination and Pseudohexacoordination in Tris(2-N,N-dimethylbenzylamino)phosphane. Inorg. Chem. 2002, 41, 5235–5240. [Google Scholar] [CrossRef]
- Mak, T.C.W.; Lam, Y.-S. Hexamethylenetetramine Oxide–hydrogen Peroxide–water (1:1:1). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1732–1735. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Huang, C.; Qi, X.; Wang, K.; Liu, Y.; Zhang, Q. Hunting for Advanced High-energy-density Materials with Well-balanced Energy and Safety through an Energetic Host–guest Inclusion Strategy. J. Mater. Chem. A 2019, 7, 19248–19257. [Google Scholar] [CrossRef]
- Laus, G.; Kahlenberg, V.; Wurst, K.; Lörting, T.; Schottenberger, H. Hydrogen Bonding in the Perhydrate and Hydrates of 1,4-diazabicyclo[2.2.2]octane (DABCO). CrystEngComm 2008, 10, 1638–1644. [Google Scholar] [CrossRef]
- Churakov, A.V.; Prikhodchenko, P.V.; Howard, J.A.K.; Lev, O. Glycine and L-serine Crystalline Perhydrates. Chem. Commun. 2009, 4224–4226. [Google Scholar] [CrossRef]
- Prikhodchenko, P.V.; Medvedev, A.G.; Tripol’skaya, T.A.; Churakov, A.V.; Wolanov, Y.; Howard, J.A.K.; Lev, O. Crystal Structures of Natural Amino Acid Perhydrates. CrystEngComm 2011, 13, 2399–2407. [Google Scholar] [CrossRef]
- Navasardyan, M.A.; Grishanov, D.A.; Tripol’skaya, T.A.; Kuz’mina, L.G.; Prikhodchenko, P.V.; Churakov, A.V. Crystal Structures of Non-proteinogenic Amino Acid Peroxosolvates: Rare Example of H-bonded Hydrogen Peroxide Chains. CrystEngComm 2018, 20, 7413–7416. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhailov, A.A.; Prikhodchenko, P.V.; Tripol’skaya, T.A.; Lev, O.; Churakov, A.V. Crystal Structures of Pyridinemonocarboxylic Acid Peroxosolvates. Russ. Chem. Bull. 2013, 62, 1871–1876. [Google Scholar] [CrossRef]
- Tegenfeldt, J.; Olovsson, I. Hydrogen Bond Studies. X. The Crystal Structure of Ammonium Hydrogenperoxide. Acta Crystallogr. 1966, 21, 934–942. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyromellitate Triperhydrate. Inorg. Chim. Acta 1979, 34, L225–L227. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyromellitate Trihydrate Monoperhydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 2781–2785. [Google Scholar] [CrossRef]
- Adams, J.M.; Ramdas, V. The Crystal Structure of Guanidinium Pyrophosphate Monoperhydrate Sesquihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 2150–2156. [Google Scholar] [CrossRef]
- Churakov, A.V.; Legurova, E.A.; Dutov, A.A.; Prikhodchenko, P.V.; Tripol’skaya, T.A. Peroxide Derivatives of Heteropoly Compounds with Keggin Anions [PW12O40]3− and [SiW12O40]4−: Synthesis and Structure. Russ. J. Inorg. Chem. 2008, 53, 1187–1192. [Google Scholar] [CrossRef]
- Farkens, M.; Meyer, T.G.; Neda, I.; Sonnenburg, R.; Müller, C.; Fischer, A.K.; Jones, P.G.; Schmutzler, R. Zur Chemie der l,3,5-Triaza-2-phosphinan-4,6-dione. Teil VI. Darstellung von 1,3,5-Triaza-2 λ3-, 1,3,5-Triaza-2 λ4- und 1,3,5-Triaza-2 λ5-phosphinan-4,6-dionen / Chemistry of the 1,3,5-Triaza-2-phosphinane-4,6-diones. Part VI. Synthesis of 1,3,5-Triaza-2 λ3-, 1,3,5-Triaza-2 λ4- and 1,3,5-Triaza-2 λ5-phosphinan-4,6-diones. Z. Naturforsch. B 1994, 49, 145–164. [Google Scholar] [CrossRef]
- Schölkopf, T.; Van, N.-D.; Schleid, T. Rb2[B12(OH)12]·2H2O and Rb2[B12(OH)12]·2H2O2: Hydrate and perhydrolate of rubidium dodecahydroxo-closo-dodecaborate. Inorg. Chim. Acta 2011, 374, 181–186. [Google Scholar] [CrossRef]
- Fidalgo, E.G.; Neels, A.; Stoeckli-Evans, H.; Süss-Fink, G. New Iso and Heteropolyoxomolybdates: Synthesis and Molecular Structure of the Anions [Mo(VI)8O26(OH)]5−, [Has(III)As(V)Mo(V)Mo(VI)8O34]6− and [HAs(III)As(V)Mo(V)Mo(VI)8O34{Co(C5H5N)2(H2O)3}]4−. Polyhedron 2002, 21, 1921–1928. [Google Scholar] [CrossRef]
- Sousa, D.P.; Bigelow, J.O.; Sundberg, J.; Que, L.; McKenzie, C.J. Caught! Crystal Trapping of a Side-on Peroxo Bound to Cr(IV). Chem. Commun. 2015, 51, 2802–2805. [Google Scholar] [CrossRef]
- Stomberg, R.; Szentivanyi, H.; Hämäläinen, R.; Kohl, F.X.; Seip, R. The Crystal Structure of 2,2′-Bipyridinium(1+) (2,2′-Bipyridine)oxodiperoxovanadate(1-)-(3+x)-hydrogen peroxide-(2-x)-water, (C10H9N2)[VO(O2)2(C10H8N2)]·(3+x)H2O2·(2-x)H2O, x = 0.4, at -100 degrees C. Acta Chem. Scand. 1984, 38a, 121–128. [Google Scholar] [CrossRef]
- Chohan, S.; Pritchard, R.G. Tripotassium tris(oxalato-κ 2 O,O′)aluminate bis(hydrogen peroxide) Hydrate, the First Example of a Cyclic Hydrogen-bonded H2O2 Dimer. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, m187–m189. [Google Scholar] [CrossRef] [PubMed]
- Infantes, L.; Motherwell, S. Water Clusters in Organic Molecular Crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Hinrichs, F.; Adam, A. Ein neues Salz der Monoperoxokohlensäure: K2(O2)CO2·3.5H2O2. Z. Anorg. Allg. Chem. 2011, 637, 426–429. [Google Scholar] [CrossRef]
- Churakov, A.V.; Howard, J.A.K. Thymine Hydrogen Peroxide 0.55-solvate 0.45-hydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, o4483. [Google Scholar] [CrossRef]
- Navasardyan, M.A.; Grishanov, D.A.; Prikhodchenko, P.V.; Churakov, A.V. DL-Piperidinium-2-carboxylate bis(hydrogen peroxide): Unusual Hydrogen-bonded Peroxide Chains. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1331–1335. [Google Scholar] [CrossRef]
- Zubatyuk, R.I.; Sinelshchikova, A.A.; Enakieva, Y.Y.; Gorbunova, Y.G.; Tsivadze, A.Y.; Nefedov, S.E.; Bessmertnykh-Lemeune, A.; Guilard, R.; Shishkin, O.V. Insights into the Crystal Packing of Phosphorylporphyrins based on the Topology of their Intermolecular Interaction Energies. CrystEngComm 2014, 16, 10428–10438. [Google Scholar] [CrossRef]
- Colombo, V.; Presti, L.L.; Gavezzotti, A. Two-component Organic Crystals without Hydrogen Bonding: Structure and Intermolecular Interactions in Bimolecular Stacking. CrystEngComm 2017, 19, 2413–2423. [Google Scholar] [CrossRef]
- Masunov, A.E.; Torres, K.; Dyakov, A.A.; Yushina, I.D.; Bartashevich, E.V. First-Principles Crystal Engineering of Nonlinear Optical Materials. II. Effect of Halogen Bonds on the Structure and Properties of Triiodobenzenes. J. Phys. Chem. C 2018, 122, 22622–22631. [Google Scholar] [CrossRef]
- Evarestov, R.A. Quantum Chemistry of Solids; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–734. [Google Scholar]
- Deringer, V.L.; George, J.; Dronskowski, R.; Englert, U. Plane-Wave Density Functional Theory Meets Molecular Crystals: Thermal Ellipsoids and Intermolecular Interactions. Acc. Chem. Res. 2017, 50, 1231–1239. [Google Scholar] [CrossRef]
- Červinka, C.; Fulem, M.; Růžička, K. CCSD(T)/CBS Fragment-based Calculations of Lattice Energy of Molecular Crystals. J. Chem. Phys. 2016, 144, 064505. [Google Scholar] [CrossRef] [PubMed]
- Basilevsky, M.V.; Odinokov, A.V.; Komarova, K.G. Charge-Transfer Mobility Parameters in Photoelectronic Devices: The Advanced Miller–Abrahams Computation. J. Phys. Chem. B 2015, 119, 7430–7438. [Google Scholar] [CrossRef] [PubMed]
- Sosorev, A.Y. Role of Intermolecular Charge Delocalization and its Dimensionality in Efficient band-like Electron Transport in Crystalline 2,5-difluoro-7,7,8,8-tetracyanoquinodimethane (F2-TCNQ). Phys. Chem. Chem. Phys. 2017, 19, 25478–25486. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, I.Y.; Vener, M.V.; Shenderovich, I.G. Local-structure Effects on 31P NMR Chemical Shift Tensors in Solid State. J. Chem. Phys. 2019, 150, 144706. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; Wiley: Chichester, UK, 2004; pp. 1–562. [Google Scholar]
- Chernyshov, I.Y.; Vener, M.V.; Feldman, E.V.; Paraschuk, D.Y.; Sosorev, A.Y. Inhibiting Low-Frequency Vibrations Explains Exceptionally High Electron Mobility in 2,5-Difluoro-7,7,8,8-tetracyanoquinodimethane (F2-TCNQ) Single Crystals. J. Phys. Chem. Lett. 2017, 8, 2875–2880. [Google Scholar] [CrossRef]
- Voronin, A.P.; Surov, A.O.; Churakov, A.V.; Parashchuk, O.D.; Rykounov, A.A.; Vener, M.V. Combined X-ray Crystallographic, IR/Raman Spectroscopic, and Periodic DFT Investigations of New Multicomponent Crystalline Forms of Anthelmintic Drugs: A Case Study of Carbendazim Maleate. Molecules 2020, 25, 2386. [Google Scholar] [CrossRef]
- Korlyukov, A.A.; Antipin, M.Y. Structural Studies of Crystals of Organic and Organoelement Compounds Using Modern Quantum Chemical Calculations within the Framework of the Density Functional Theory. Russ. Chem. Rev. 2012, 81, 105–129. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef]
- Tosoni, S.; Tuma, C.; Sauer, J.; Civalleri, B.; Ugliengo, P. A Comparison Between Plane Wave and Gaussian-type Orbital Basis Sets for Hydrogen Bonded Systems: Formic Acid as a Test Case. J. Chem. Phys. 2007, 127, 154102. [Google Scholar] [CrossRef]
- Melikova, S.M.; Voronin, A.P.; Panek, J.; Frolov, N.E.; Shishkina, A.V.; Rykounov, A.A.; Tretyakov, P.Y.; Vener, M.V. Interplay of π-stacking and Inter-stacking Interactions in Two-component Crystals of Neutral Closed-shell Aromatic Compounds: Periodic DFT Study. RSC Adv. 2020, 10, 27899–27910. [Google Scholar] [CrossRef]
- Mata, I.; Alkorta, I.; Espinosa, E.; Molins, E. Relationships Between Interaction Energy, Intermolecular Distance and Electron Density Properties in Hydrogen Bonded Complexes under External Electric Fields. Chem. Phys. Lett. 2011, 507, 185–189. [Google Scholar] [CrossRef]
- Kuznetsov, M.L. Can Halogen Bond Energy be Reliably Estimated from Electron Density Properties at Bond Critical Point? The Case of the (A)nZ—Y⋯X − (X, Y = F, Cl, Br) interactions. Int. J. Quantum Chem. 2019, 119, e25869. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Korlyukov, A.A.; Nelyubina, Y.V. Quantum Chemical Methods in Charge Density Studies from X-ray Diffraction Data. Russ. Chem. Rev. 2019, 88, 677–716. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Tsirelson, V.G. Hydrogen Bonds and O⋯O Interactions in Proton-ordered Ices. DFT Computations with Periodic Boundary Conditions. Chem. Phys. Lett. 2010, 500, 272–276. [Google Scholar] [CrossRef]
- Borissova, A.O.; Korlyukov, A.A.; Antipin, M.Y.; Lyssenko, K.A. Estimation of Dissociation Energy in Donor−Acceptor Complex AuCl·PPh3 via Topological Analysis of the Experimental Electron Density Distribution Function. J. Phys. Chem. A 2008, 112, 11519–11522. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Yushina, I.D.; Stash, A.I.; Tsirelson, V.G. Halogen Bonding and Other Iodine Interactions in Crystals of Dihydrothiazolo(oxazino)quinolinium Oligoiodides from the Electron-Density Viewpoint. Cryst. Growth Des. 2014, 14, 5674–5684. [Google Scholar] [CrossRef]
- Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Structure-directing sulfur⋯metal Noncovalent Semicoordination Bonding. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 436–449. [Google Scholar] [CrossRef]
- Dem’yanov, P.; Polestshuk, P. A Bond Path and an Attractive Ehrenfest Force Do Not Necessarily Indicate Bonding Interactions: Case Study on M2X2 (M=Li, Na, K; X=H, OH, F, Cl). Chem. Eur. J. 2012, 18, 4982–4993. [Google Scholar] [CrossRef]
- Shahbazian, S. Why Bond Critical Points Are Not “Bond” Critical Points. Chem. Eur. J. 2018, 24, 5401–5405. [Google Scholar] [CrossRef]
- Iogansen, A.V. Direct Proportionality of the Hydrogen Bonding Energy and the Intensification of the Stretching ν(XH) Vibration in Infrared Spectra. Spectrochim. Acta Part A 1999, 55, 1585–1612. [Google Scholar] [CrossRef]
- Rozenberg, M.; Loewenschuss, A.; Marcus, Y. An Empirical Correlation Between Stretching Vibration Redshift and Hydrogen Bond Length. Phys. Chem. Chem. Phys. 2000, 2, 2699–2702. [Google Scholar] [CrossRef]
- Yukhnevich, G.V. Relationship Between the Lengths of Covalent and Intermolecular Bonds in X-H⋯Y bridges. Crystallogr. Rep. 2010, 55, 377–380. [Google Scholar] [CrossRef]
- Busing, W.R.; Levy, H.A. Crystal and Molecular Structure of Hydrogen Peroxide: A Neutron-Diffraction Study. J. Chem. Phys. 1965, 42, 3054–3059. [Google Scholar] [CrossRef]
- Vener, M.V.; Levina, E.O.; Astakhov, A.A.; Tsirelson, V.G. Specific Features of the Extra Strong Intermolecular Hydrogen Bonds in Crystals: Insights from the Theoretical Charge Density Analysis. Chem. Phys. Lett. 2015, 638, 233–236. [Google Scholar] [CrossRef]
- Medvedev, A.G.G.; Shishkina, A.V.V.; Prikhodchenko, P.V.V.; Lev, O.; Vener, M.V.V. The Applicability of the Dimeric Heterosynthon Concept to Molecules with Equivalent Binding Sites. A DFT Study of Crystalline Urea–H2O2. RSC Adv. 2015, 5, 29601–29608. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Vener, M.V.; Zvereva, E.E.; Fei, Z.; Scopelliti, R.; Brandenburg, J.G.; Siankevich, S.; Dyson, P.J. Quantification of Conventional and Nonconventional Charge-Assisted Hydrogen Bonds in the Condensed and Gas Phases. J. Phys. Chem. Lett. 2015, 6, 4431–4436. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Chernyshov, I.Y.; Vener, M.V.; Lev, O.; Prikhodchenko, P.V. Effect of Aluminum Vacancies on the H2O2 or H2O Interaction with a gamma-AlOOH Surface. A Solid-state DFT Study. Int. J. Quantum Chem. 2019, 119, e25920. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular Hydrogen Bond Energies in Crystals Evaluated Using Electron Density Properties: DFT Computations with Periodic Boundary Conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef]
- Vener, M.V.; Manaev, A.V.; Egorova, A.N.; Tsirelson, V.G. QTAIM Study of Strong H-Bonds with the O−H···A Fragment (A = O, N) in Three-Dimensional Periodical Crystals. J. Phys. Chem. A 2007, 111, 1155–1162. [Google Scholar] [CrossRef]
- Grabowski, S.J. What Is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- McKillop, A.; Sanderson, W.R. Sodium perborate and sodium percarbonate: Cheap, Safe and Versatile Oxidising Agents for Organic Synthesis. Tetrahedron 1995, 51, 6145–6166. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Kapustin, E.A.; Minkov, V.S.; Boldyreva, E.V. Oxidative Stress of H2O2 on N,N-dimethylglycine: Formation of Perhydrate Crystals and More. CrystEngComm 2014, 16, 10165–10168. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009; pp. 1–318. [Google Scholar]
- Nelyubina, Y.V.; Antipin, M.Y.; Lyssenko, K.A. Hydrogen Bonds between Zwitterions: Intermediate between Classical and Charge-Assisted Ones. A Case Study. J. Phys. Chem. A 2009, 113, 3615–3620. [Google Scholar] [CrossRef]
- Olovsson, I.; Templeton, D.H.; Rundqvist, S.; Varde, E.; Westin, G. The Crystal Structure of Hydrogen Peroxide Dihydrate. Acta Chem. Scand. 1960, 14, 1325–1332. [Google Scholar] [CrossRef]
- Biliškov, N.; Kojić-Prodić, B.; Mali, G.; Molćanov, K.; Stare, J. A Partial Proton Transfer in Hydrogen Bond O−H···O in Crystals of Anhydrous Potassium and Rubidium Complex Chloranilates. J. Phys. Chem. A 2011, 115, 3154–3166. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Vener, M.V.; Medvedev, A.G.; Churakov, A.V.; Prikhodchenko, P.V.; Tripol’skaya, T.A.; Lev, O. H-Bond Network in Amino Acid Cocrystals with H2O or H2O2. The DFT Study of Serine–H2O and Serine–H2O2. J. Phys. Chem. A 2011, 115, 13657–13663. [Google Scholar] [CrossRef]
- Redington, R.L.; Olson, W.B.; Cross, P.C. Studies of Hydrogen Peroxide: The Infrared Spectrum and the Internal Rotation Problem. J. Chem. Phys. 1962, 36, 1311–1326. [Google Scholar] [CrossRef]
- Oelfke, W.C.; Gordy, W. Millimeter-Wave Spectrum of Hydrogen Peroxide. J. Chem. Phys. 1969, 51, 5336–5343. [Google Scholar] [CrossRef]
- Savariault, J.M.; Lehmann, M.S. Experimental Determination of the Deformation Electron Density in Hydrogen Peroxide by Combination of X-ray and Neutron Diffraction Measurements. J. Am. Chem. Soc. 1980, 102, 1298–1303. [Google Scholar] [CrossRef]
- Minkov, V.S.; Kapustin, E.A.; Boldyreva, E.V. Betaine 0.77-perhydrate 0.23-hydrate and Common Structural Motifs in Crystals of Amino Acid Perhydrates. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Rozas, I.; Alkorta, I.; Elguero, J. Bifurcated Hydrogen Bonds: Three-Centered Interactions. J. Phys. Chem. A 1998, 102, 9925–9932. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P═O Moiety as an Ambidextrous Hydrogen Bond Acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Serushkina, O.V.; Muravyev, N.V.; Meerov, D.B.; Miroshnichenko, E.A.; Kon’kova, T.S.; Suponitsky, K.Y.; Vener, M.V.; Sheremetev, A.B. Azasydnone—Novel “Green” Building Block for Designing High Energetic Compounds. J. Mater. Chem. A 2018, 6, 18669–18676. [Google Scholar] [CrossRef]
- Lehn, J.M. Cryptates: Inclusion Complexes of Macropolycyclic Receptor Molecules. Pure Appl. Chem. 1978, 50, 871–892. [Google Scholar] [CrossRef]
- Liu, Z.; Nalluri, S.K.M.; Stoddart, J.F. Surveying Macrocyclic Chemistry: From Flexible Crown Ethers to Rigid Cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M. Nitrogen-Rich Bis-1,2,4-triazoles-A Comparative Study of Structural and Energetic Properties. Chem. Eur. J. 2012, 18, 16742–16753. [Google Scholar] [CrossRef]
- Luo, J.; Xia, H.; Zhang, W.; Song, S.; Zhang, Q. A Promising Hydrogen Peroxide Adduct of Ammonium Cyclopentazolate as a Green Propellant Component. J. Mater. Chem. A 2020, 8, 12334–12338. [Google Scholar] [CrossRef]
- Manin, A.N.; Voronin, A.P.; Shishkina, A.V.; Vener, M.V.; Churakov, A.V.; Perlovich, G.L. Influence of Secondary Interactions on the Structure, Sublimation Thermodynamics, and Solubility of Salicylate: 4-Hydroxybenzamide Cocrystals. Combined Experimental and Theoretical Study. J. Phys. Chem. B 2015, 119, 10466–10477. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).